Influence of Comorbidities and Airway Clearance on Mortality and Outcomes of Patients With Severe Bronchiectasis Exacerbations in Taiwan
- 1Department of Thoracic Medicine, Chang Gung Memorial Hospital, Taipei, Taiwan
- 2College of Medicine, Chang Gung University, Taoyuan, Taiwan
- 3Department of Thoracic Medicine, New Taipei City Municipal TuCheng Hospital, Chang Gung Medical Foundation, New Taipei City, Taiwan
- 4Department of Respiratory Care, New Taipei City Municipal TuCheng Hospital, Chang Gung Medical Foundation, New Taipei City, Taiwan
- 5Center for Big Data Analytics and Statistics, Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 6Division of Pulmonary and Critical Care, Department of Internal Medicine, Saint Paul's Hospital, Taoyuan, Taiwan
- 7Biomedical Research Unit, Experimental Studies, National Heart and Lung Institute, Imperial College London, Royal Brompton Hospital, London, United Kingdom
Bronchiectasis is characterized by systemic inflammation and multiple comorbidities. This study aimed to investigate the clinical outcomes based on the bronchiectasis etiology comorbidity index (BACI) score in patients hospitalized for severe bronchiectasis exacerbations. We included non-cystic fibrosis patients hospitalized for severe bronchiectasis exacerbations between January 2008 and December 2016 from the Chang Gung Research Database (CGRD) cohort. The main outcome was the 1-year mortality rate after severe exacerbations. We used the Cox regression model to assess the risk factors of 1-year mortality. Of 1,235 patients who were hospitalized for severe bronchiectasis exacerbations, 641 were in the BACI < 6 group and 594 in the BACI ≥ 6 group. The BACI ≥ 6 group had more previous exacerbations and a lower FEV1. Pseudomonas aeruginosa (19.1%) was the most common bacterium, followed by Klebsiella pneumoniae (7.5%). Overall, 11.8% of patients had respiratory failure and the hospital mortality was 3.0%. After discharge, compared to the BACI < 6 group, the BACI ≥ 6 group had a significantly higher cumulative incidence of respiratory failure and mortality in a 1-year follow-up. The risk factors for 1-year mortality in a multivariate analysis include age [hazard ratio (HR) 4.38, p = 0.01], being male (HR 4.38, p = 0.01), and systemic corticosteroid usage (HR 6.35, p = 0.001), while airway clearance therapy (ACT) (HR 0.50, p = 0.010) was associated with a lower mortality risk. An increased risk of respiratory failure and mortality in a 1-year follow-up after severe exacerbations was observed in bronchiectasis patients with multimorbidities, particularly older age patients, male patients, and patients with a history of systemic corticosteroid use. ACT could effectively improve the risk for 1-year mortality.
Introduction
Bronchiectasis is characterized by permanent dilatation of the bronchi and airway inflammation (1), thus leading to the excess mucus secretion that can make the lungs more vulnerable to infection. Infection and exacerbations are associated with respiratory failure and mortality in bronchiectasis (2, 3). Patients with frequent exacerbations, particularly those experiencing three or more exacerbations per year, had worse quality of life, more frequent hospitalizations, and increased mortality over 5 years (4).
The potential risk factors such as hospital admissions and quality of life score have been utilized to predict the risk of death in patients with bronchiectasis (1, 2, 5–7). The Bronchiectasis Severity Index (BSI) scoring system, including physical characteristics, radiological severity, sputum microbiology, dyspnea score, and a history of exacerbation, has been used to predict mortality from bronchiectasis (2). Pneumonia leads to hospitalization and the deterioration of physiological functions in patients with bronchiectasis (8, 9). Respiratory failure is the major cause of death in bronchiectasis (10, 11). Bronchiectasis is characterized by systemic inflammation and multiple comorbidities, thus all-cause related death is higher in patients with bronchiectasis compared to those without bronchiectasis (7, 12). More recently, the bronchiectasis etiology comorbidity index (BACI) scoring system based on 13 comorbidities has been developed and validated to stratify the risk of mortality and hospital admissions in an European cohort (1). We recently used the BACI to assess the severity of bronchiectasis from different etiologies (13), but have not yet analyzed the clinical treatment outcome and mortality of patients with bronchiectasis hospitalized for pneumonia based on BACI scores to stratify their severity.
The long-term accumulation of mucus in the airway of patients with bronchiectasis facilitates bacterial colonization and recurrent lower respiratory tract infection, thus contributing to pneumonia or hospitalization (14). Besides antibiotics, mucoactive drugs and airway clearance therapy (ACT) are also used as an adjuvant treatment of pneumonia in bronchiectasis (15). ACT may facilitate expectoration and improves airway clearance and quality of life (16–18). Although between 40 and 59% of patients with bronchiectasis receive ACT in Europe, the USA, and India (19–21), the role of ACT and mucoactive drugs in improving hospitalization outcomes or in reducing the risk of exacerbation-related readmission in bronchiectasis is still unknown (22).
Although the BACI score has been shown to be a predictor of the risk for mortality (1), it is not known whether this index could be used to stratify disease severity in clinical trials or database studies. In this study, we used the BACI to stratify disease severity and to investigate the factors that may influence the mortality of patients hospitalized for severe exacerbations in a bronchiectasis cohort in Taiwan.
Methods
Data Source
In this study, we used the Chang Gung Research Database (CGRD) to construct a multi-institutional bronchiectasis cohort. The CGRD provides the electronic medical records collection from the Chang Gung Memorial Hospital system. The CGRD includes 6.1% of outpatients and 10.2% of hospitalized patients in Taiwan (23). The locations of Chang Gung Memorial Hospital system (three medical centers including Linkou, Taipei, and Kaohsiung branches) and four regional hospitals (Taoyuan, Chiayi, Keelung, and Yunlin branches) and more detailed information about CGRD has been earlier reported (23, 24). The Institutional Review Board of Chang Gung Memorial Hospital approved this study (IRB number: 201800712B0C502).
Bronchiectasis Cohort
This cohort included adult patients (age ≥ 18 years) with diagnoses of bronchiectasis in the CGRD between January 2008 and December 2016. Patients with at least two bronchiectasis diagnoses [International Classification of Diseases, 9th Clinical Modification (ICD-9-CM) 494.0 or 494.1, or 10th Revision (ICD-10) J47)] in outpatient visits or one diagnosis from the hospitalization record were collected in the cohort. The inclusion criteria of this study were patients with severe exacerbations, which were defined as emergency room (ER) visits or hospitalizations for bronchiectasis-related infective exacerbations (25). Infective exacerbations were defined as the requirement for antibiotics for deterioration in respiratory symptoms (26), and pneumonia was defined by ICD-9-CM codes (481, 482, 483, 485, and 486) with antibiotic use more than 1 week as described earlier (27–29). The diagnosis of bronchiectasis for our enrolled subjects was made by clinical symptoms, history, and the image study of high-resolution CT (HRCT) which was confirmed by a radiologist and a pulmonary specialist. In the cohort, 70% of patients with bronchiectasis from 2002 to 2008 and 90% of patients with bronchiectasis from 2009 to 2016 had a chest CT scan. Therefore, a portion of bronchiectasis diagnosis was based on a clinical history and chest x-ray. About 1,204 patients with bronchiectasis by the ICD code who did not undergo HRCT were excluded. The BACI score was calculated for each subject based on comorbidities obtained from CGRD diagnoses (ICD-9-CM and ICD-10) [1]. Therefore, patients with bronchiectasis were divided into the BACI ≥ 6 group (high risk) and the BACI < 6 group (low and intermediate risk) as previously described (1).
Outcomes
The main outcomes were the rates of respiratory failure and mortality at 1 year after severe exacerbations. The secondary outcome was in-hospital mortality. Acute respiratory failure (ICD-9-CM: 518.81, ICD-10: J96.0) was defined as the acute onset of respiratory failure during hospitalization with the need for bi-level positive airway pressure or invasive mechanical ventilator use (30). Mild exacerbation was defined as the requirement for antibiotics in a clinic for the deterioration in respiratory symptoms (26).
Clinical Measurements
The CGRD included inpatient and outpatient clinical data. We retrieved demographic data, image, and microbiology and pulmonary function reports. The total number of affected lobes was identified in CT reports (<2 lobes affected, 2 lobes, or ≥3 lobes affected, lingual lobe as a separate lobe) (5). We collected sputum microbiology reports during hospitalization and within 1 year after hospitalization. Forced expiratory volume in 1 s (FEV1) and forced vital capacity <80% predicted value were retrieved from a pulmonary function test, which was performed with a spirometer according to the American Thoracic Society and the European Respiratory Society criteria (31). For etiologies of bronchiectasis patients, as previously described (13), clinicians mostly would review the history of pulmonary TB and pneumonia, and the sputum culture for infection workup was performed, and the possible comorbidities, such as asthma, COPD, and GERD, were evaluated. The COPD was defined by one inpatient or two outpatient codes (491, 492, 496, and 493.2). Clinicians might take an immune or autoimmune survey if the signs of immunodeficiency and connective tissue diseases were present. When primary ciliary dyskinesia was suspected, nasal mucociliary clearance was measured by using the saccharin test. A1-Antitrypsin was evaluated when HRCT revealed the presence of emphysema affecting the lower lobes. Sweat tests were requested if the signs and symptoms suggestive of cystic fibrosis were present.
Medical treatment included antibiotics, systemic corticosteroids, and inhalation medication. ACT was a hospital-based program, including postural drainage and intermittent positive pressure breathing (IPPB), to provide short-term mechanical ventilation via a mouthpiece for assistance in clearing mucus from the lungs. The IPPB used was a device of Bird-Mark respirator (Bird Products Corporation, Springs, CA, USA) that delivered the inspiratory pressure at 20–30 cm H2O. The frequency of 10–20 times per min was set to maximize the patient's comfort. Both treatments lasted 30 min per session and were given two times daily (morning and late afternoon) during hospitalization. A physiotherapist or respiratory therapists supervised and trained the patients to complete the treatment session. After discharge, the ACT treatment of an IPPB device and postural drainage were conducted once and 1 h each per week in the outpatient clinic. All therapies had medical records. Postural drainage was performed at least four times per week and lasted at least 60 min per session at home. The physiotherapist would call out to remind the patients. The CGRD database is based on real-world clinical practice, and clinicians prescribed ACT for patients with bronchiectasis according to clinical symptoms. The duration of ACT in a clinic was dependent on patients' consent and physicians' prescription.
Statistical Analysis
Descriptive statistics (mean and SD) were calculated for all continuous variables. Independent t-test and χ2 test were used to compare the baseline and main outcome difference of two study groups. A log-rank test was used to compare the survival between the two BACI groups. Univariate and multivariate Cox regression models were used to identify the independent factors that were associated with 1-year mortality and respiratory failure. The analysis was done with the SAS version software; p < 0.05 was considered to be statistically significant.
Results
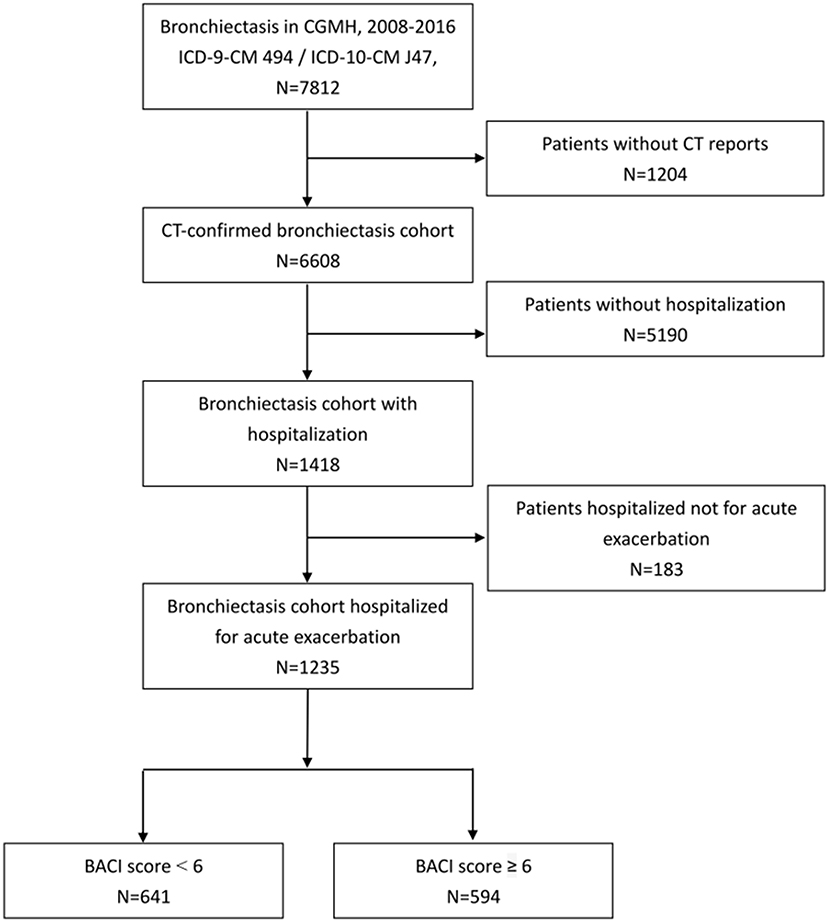
A total of 7,812 adult patients who had at least two ICD claims (ICD-9-CM 494.0 or 494.1) in outpatient visits or any one ICD claim from hospitalization were included in our bronchiectasis cohort from 2008 to 2016. We excluded the patients without HRCT reports (n = 1,204) and those without hospitalization (n = 5,190) (Figure 1). A total of 1,235 patients with bronchiectasis hospitalized for pneumonia were enrolled (641 in the BACI < 6 group and 594 in the BACI ≥ 6 group).
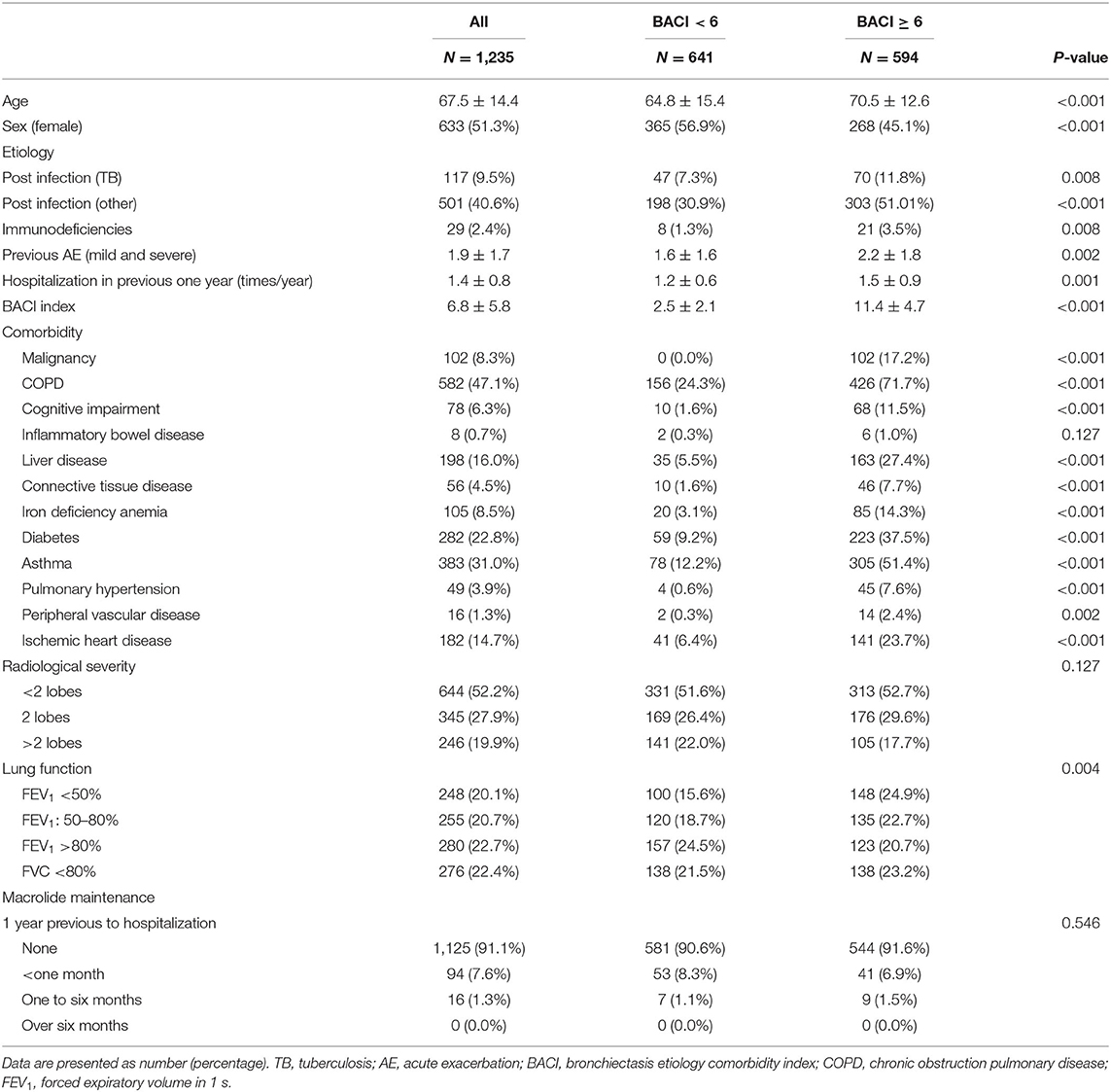
The characteristics of the hospitalized patients with bronchiectasis are summarized in Table 1. As compared to the BACI < 6 group, the BACI ≥ 6 group include older age, more male subjects, a lower FEV1 at baseline, and a higher frequency of acute exacerbations and hospitalizations in the previous year (Table 1). Of 110 patients who have used macrolide, 94 of them have used it for <1 month and none used macrolide more than 6 months before hospitalization. After discharge, 121 of 177 patients used macrolide for <1 month and only 14 used macrolide as Non-TB mycobacteria (NTM) treatment for more than 6 months. The distribution of macrolide usage in the BACI ≥ 6 and BACI < 6 groups was similar. The airway microbiological status of previous year is listed in Supplementary Table 1. Thus, bronchiectasis patients with a higher BACI score not only manifested more severe comorbidities but also had a worse baseline clinical status. During hospitalization, 857 patients had sputum cultures. Pseudomonas aeruginosa (19.1%) was the most common bacterium, followed by Klebsiella pneumoniae (7.5%) and Haemophilus influenzae (5.5%). In the BACI ≥ 6 group, the rate of P. aeruginosa infection was high. A higher proportion of patients in the BACI ≥ 6 group received ACT during hospitalization than those in the BACI < 6 group (52.9 vs. 45.8%, p = 0.014). In addition, a greater proportion of patients with bronchiectasis in the BACI ≥ 6 group received ACT for longer than 7 days during hospitalization compared to those in the BACI < 6 group (14.8 vs. 8.9%, p = 0.043). Patients in the BACI ≥ 6 group took more systemic corticosteroids than those in the BACI < 6 group (50.7 vs. 29.6%, 9.7 ± 10.6 vs. 6.9 ± 8.4 days, p < 0.001). The hospital stays were shorter (11.8 ± 9.3 days) for bronchiectasis patients in the BACI < 6 group than those in the BACI ≥ 6 group (13.8 ± 11.1 days, p = 0.001). The two groups exhibited the same in-hospital respiratory failure and mortality rate (Table 2).
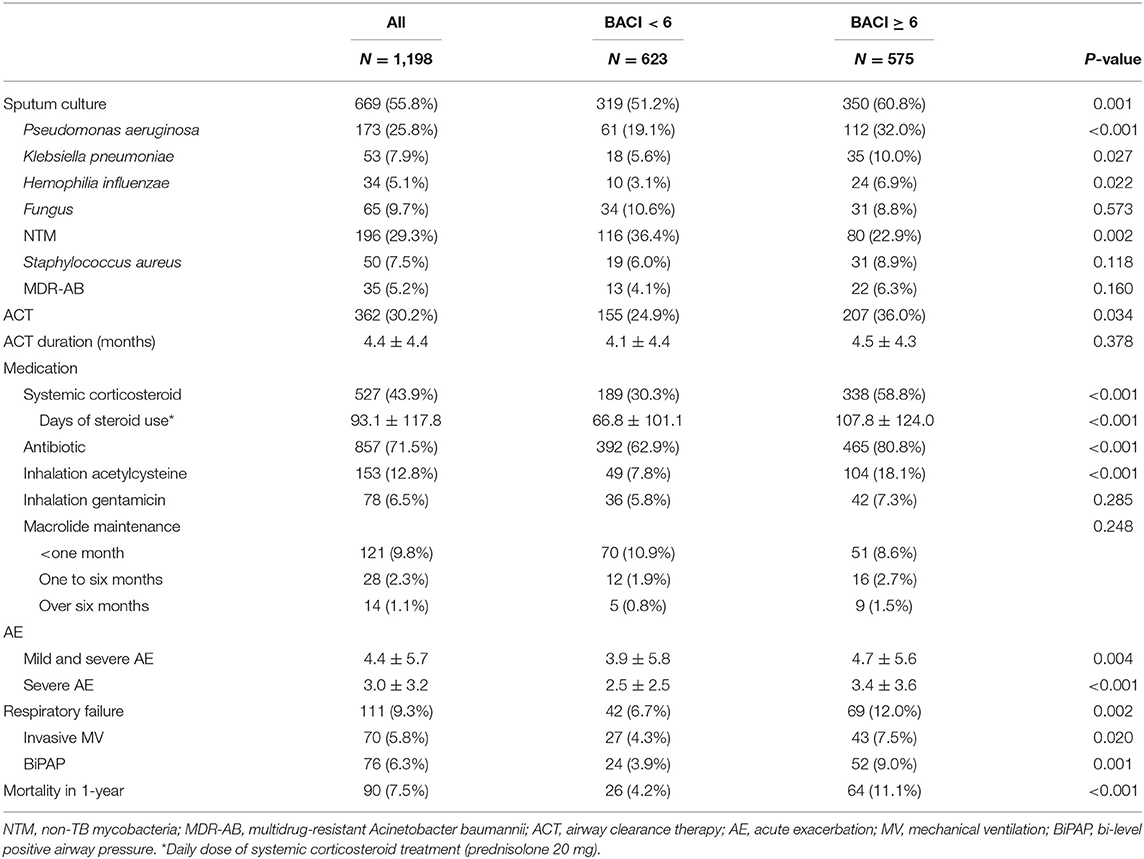
The clinical outcomes of a 1-year follow-up after discharge are shown in Table 3. During the 1-year follow-up period, 669 patients had sputum cultures. NTM (29.3%) was the most common pathogen, followed by P. aeruginosa (25.8%), fungus (9.7%), and K. pneumoniae (7.9%) in bronchiectasis patients with higher comorbidities. Compared to patients with bronchiectasis in the BACI < 6 group, a higher proportion of patients in the BACI ≥ 6 group received ACT at home during a 1-year follow-up (36.0 vs. 24.9%, p = 0.034). During the period of 1-year follow-up, patients with bronchiectasis in the BACI ≥ 6 group took more systemic corticosteroids, antibiotics, and nebulized acetylcysteine. In addition, the rates of acute exacerbation (4.7 vs. 3.9%, p = 0.004), respiratory failure (12.0 vs. 6.7%, p = 0.002), and 1-year mortality (11.1 vs. 4.2%, p < 0.001) were higher in patients with bronchiectasis in the BACI ≥ 6 compared to those in the BACI < 6 group during a 1-year follow-up. The cumulative incidence of respiratory failure and mortality was significantly higher in the BACI ≥ 6 group during a 1-year follow-up (Figures 2A,B). The outcomes of the BACI = 0 group are listed in Supplementary Table 2. The BACI = 0 group (n = 248) had the lowest risk of 1-year mortality and respiratory failure (Supplementary Figure 1).
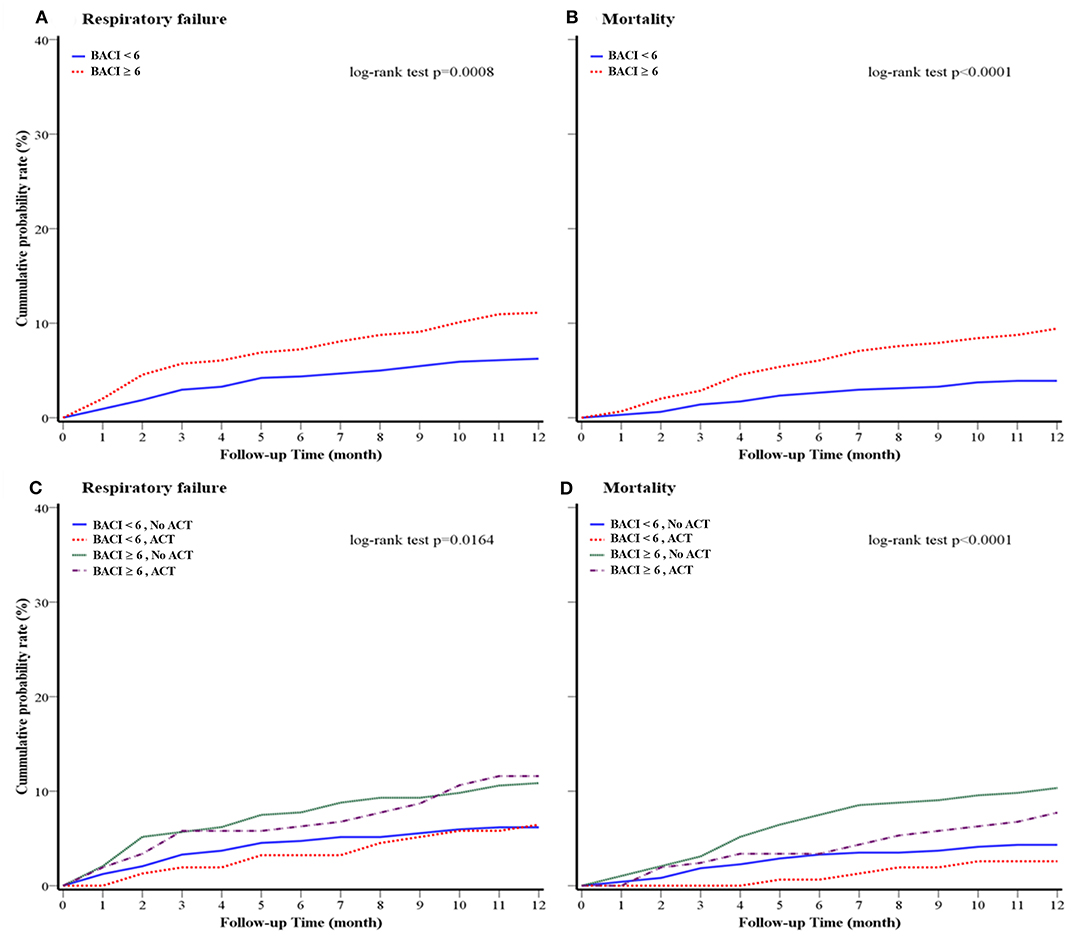
Figure 2. Kaplan–Meier survival curves for (A) 1-year respiratory failure and (B) 1-year overall mortality of the cohort (BACI groups); (C) 1-year respiratory failure and (D) 1-year mortality of the cohort (BACI and ACT groups) after a 1-year follow-up. An indicative value of p was shown. ACT, airway clearance therapy; BACI, bronchiectasis etiology comorbidity index.
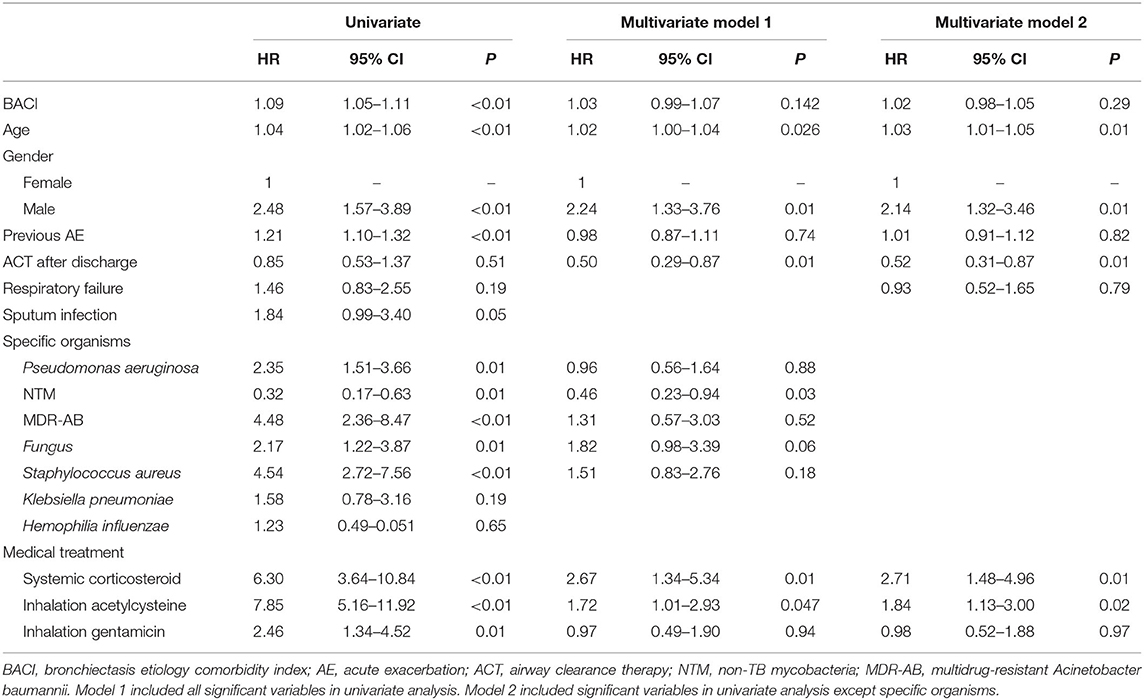
The risk factors for increased hospital mortality were older age and multi-drug-resistant Acinetobacter baumannii (MDR-AB) infection (age: HR 1.05, p = 0.03; MDR-AB: HR 6.36, p = 0.01) (Supplementary Table 3 in the Online supplement). ACT (HR 0.25, p = 0.02) was associated with a lower risk of mortality, while the systemic use of corticosteroids (HR 4.38, p = 0.01) was associated with an increased risk of hospital mortality. The risk factors for 1-year mortality with a multivariate analysis included age (HR 4.38, p = 0.01), being male (HR 4.38, p = 0.01), and systemic corticosteroid use (HR 6.35, p = 0.001), while ACT (HR 0.50, p = 0.010) could decrease the risk for 1-year mortality (Table 4). The 1-year respiratory failure hazard comparing bronchiectasis with high comorbidities to bronchiectasis with low comorbidities was 2.07 (95% CI: 1.34–5.34) for systemic corticosteroids, 1.69 (95% CI: 1.00–2.83) for nebulized gentamicin, and 0.59 (95% CI: 0.37–0.96) for ACT, which was a low risk for 1-year respiratory failure (Table 5). More importantly, the long-term cumulative incidence of respiratory failure and mortality was significantly reduced by ACT in the BACI ≥ 6 group in a 1-year follow-up (Figures 2C,D). However, the influence of ACT on the one-year respiratory failure and mortality in the BACI < 6 group was not obvious.
The overall mortality from hospitalization to a 1-year follow-up was higher in the BACI ≥ 6 group (13.3%, n = 79, p = 0.0001) than in the BACI <6 group (n = 43, 6.7%, Figure 3). Pneumonia/respiratory failure was the main cause of death, followed by malignancy in both groups. The mortality rates of pneumonia/respiratory failure (5.1% vs 1.9%, p = 0.0025) and malignancy (3.4% vs 1.2%, p = 0.013) were lower in the BACI < 6 group than in the BACI ≥ 6 group. The existence of the other causes of death, including cardiovascular disease, diabetes, chronic liver disease, and chronic renal disease, showed no difference between the two groups. It seems that pneumonia and higher comorbidities of malignancy may contribute to mortality in the BACI ≥ 6 group.
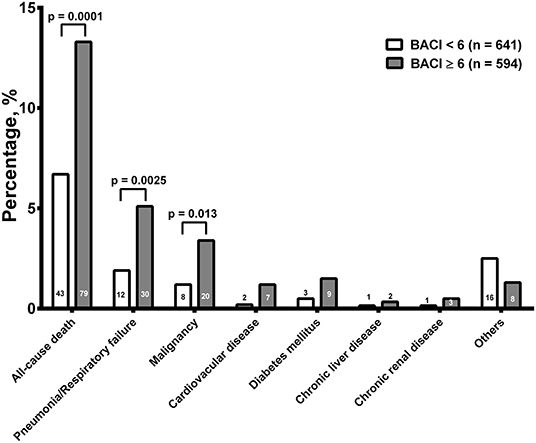
Figure 3. The overall mortality from hospitalization to a 1-year follow-up. The numbers of death and significance are indicated. BACI, bronchiectasis etiology comorbidity index.
Discussion
Our study has used the BACI scoring system to characterize clinical features and comorbidities and to evaluate the risk factor for mortality of patients with bronchiectasis hospitalized for pneumonia in a bronchiectasis cohort in Taiwan. Patients with bronchiectasis in the BACI ≥ 6 group had more previous exacerbations and a worsening of baseline airflow obstruction. Among patients with bronchiectasis, the BACI ≥ 6 group exhibited significantly more frequent acute exacerbations, hospitalizations, and higher rates of respiratory failure and mortality in 1 year after discharge from hospitalization. In the multivariate model, old age, being male, and the use of systemic corticosteroids and nebulized acetylcysteine were independent risk factors for 1-year mortality. More importantly, our results further indicate that ACT can reduce the risk of respiratory failure and mortality during a 1-year follow-up in patients with bronchiectasis hospitalized for pneumonia.
Several indexes such as BSI, FACED, E-FACED, and BACI have been developed, and their scores have been used as the predictors of mortality in bronchiectasis (1, 2, 5, 32). BSI, which incorporates multiple clinical parameters, has become a major tool for evaluating bronchiectasis severity in research studies and clinical trials. Comorbidities have been reported to be associated with an increased risk of mortality in bronchiectasis (1, 12, 33). BACI includes 13 comorbidities that confer a high risk of mortality in bronchiectasis and can be used as a clinical predictive tool independently or with the BSI (10). Thus, our study revealed that patients with high-risk comorbidities (BACI ≥ 6) had a worse baseline physiological state and exhibited significantly more acute exacerbations, hospitalizations, and higher rates of respiratory failure and mortality 1 year after discharge. Those patients hospitalized for pneumonia had a higher incidence of P. aeruginosa infection (22.8%). In a 1-year follow-up, we showed that chronic bronchial infection with P. aeruginosa was also dominant in bronchiectasis patients with high comorbidities. Notably, bronchiectasis patients with P. aeruginosa infection have worse clinical outcomes, such as increased inflammation, greater impairment of the lung function, more exacerbations, and mortality (34, 35). MDR pathogens are difficult to treat, often requiring a combination of antibiotic regimens. A Spanish cohort study reported that MDR pathogens were isolated in 24.5% of patients hospitalized for bronchiectasis exacerbations, and the common pathogens were MDR P. aeruginosa and extended-spectrum betalactamase Enterobacteriaceae (36). Our results indicated that P. aeruginosa and MDR-AB infection are associated with increased mortality in a hospital and a 1-year follow-up in bronchiectasis patients with high comorbidities (Table 4; Supplementary Table 3 in the Online Supplement). Our results would remind physicians to pay increased attention toward a targeted treatment approach to bronchiectasis patients with high comorbidities who were hospitalized for pneumonia with the aim of avoiding respiratory failure and the risk of death.
In our cohort study, NTM infection was the most common pathogen in chronic airway infection of bronchiectasis during a 1-year follow-up after hospitalization. Individuals susceptible of more comorbidities who developed NTM pulmonary disease had increased mortality (37). However, our high comorbidity bronchiectasis with NTM infection was associated with less mortality in a 1-year follow-up after discharge, which is compatible with another report that the bronchiectasis cohort with NTM infection did not show increased mortality relative to the matched control group without NTM infection (12). It might be suggested that the long-term use of macrolide antibiotics, an important drug to treat NTM infection, had a protective effect on the mortality of bronchiectasis patients with NTM infection (38). The number of patients with bronchiectasis and NTM infection in our cohort was too small to draw definite conclusions. There is no evidence-based treatment strategy available for bronchiectasis patients with low or high comorbidities in combination with NTM infection, thus supporting the need for a prospective study to be undertaken. Macrolide maintenance has been reported to prevent acute exacerbation and decrease the frequency of infectious exacerbations using the EMBRACE study and BAT trial (39, 40). Only limited subjects used macrolide for maintenance therapy over 6 months (main usage for NTM treatment). Therefore, the benefit of macrolide maintenance therapy on the outcomes could not be found. A prospective study or the recruitment of more subjects is necessary to evaluate the impact of macrolide maintenance therapy on the outcome of bronchiectasis.
Mucoactive treatments, inhaled antibiotics, and ACT for bronchiectasis are discussed (41). Among bronchiectasis patients with high comorbidities, inhaled mucoactive agents increased the risk of 1-year mortality and respiratory failure and inhalation gentamycin was associated with a worsened 1-year respiratory failure rate, while in those treated with ACT, there was a decrease in the risk of 1-year mortality and respiratory failure. Recommendations for the use of inhaled mucoactive agents in bronchiectasis are indeed weak (15). ACT is beneficial in stable bronchiectasis in improving sputum expectoration, relieving respiratory symptoms, providing a better quality of life, and maintaining the lung function (16–18). Limited studies have investigated the role of ACT in reducing acute exacerbation, hospitalization, or mortality exclusively in patients with non-cystic fibrosis bronchiectasis. In our study, ACT was associated with a reduced risk of in-hospital mortality and of respiratory failure and mortality during a 1-year follow-up in the high-risk comorbidity group (BACI ≥ 6). In contrast, Basavaraj et al. (20) reported that patients with bronchiectasis who used ACTs continuously up to a 1-year follow-up had more frequent chronic infection with P. aeruginosa, exacerbations, and hospitalizations and had greater odds for exacerbations in a 1-year follow-up compared with those who did not use ACTs. They found that 58% of patients who used ACTs at baseline did not use ACTs in a 1-year follow-up. In our study, a similar portion of patients with bronchiectasis who used ACTs in hospitalization, but 66% of patients in the BACI ≥ 6 group and 54% of patients in the BACI < 6 group still used ACTs in a 1-year follow-up. It is possible that the higher long-term usage of ACTs in bronchiectasis patients with higher comorbidities may contribute to better clinical outcomes, in terms of mortality and severe exacerbations. There is a particular need to establish that ACTs are effective in which phenotype of patients with bronchiectasis. The modalities of ACT may have a different impact of acute exacerbations on bronchiectasis (16, 42). In our hospital, the ACTs included positioning and gravity-assisted drainage to facilitate sputum removal, and an IPPB device, which may have the effect of augmentation of lung volumes and the prevention of early airway closure during expiration (43), thus helping sputum clearance (44, 45). This means that multiple modalities of ACTs may have different physiological effects on airway clearance and achieve the different clinical outcomes. Currently, there are no similar studies to describe the clinical characteristics or investigate short-term and long-term clinical outcomes of ACT in bronchiectasis with acute exacerbation. Because our study shows the evidence provided by the database, further trials are needed to consolidate the beneficial effects of ACT in exacerbation or in stable bronchiectasis. In addition, nebulized gentamycin may reduce the bacterial burden in the airways and sputum volume, and may decrease neutrophil-related airway inflammation (46, 47). However, our study did not show that inhaled gentamycin had a positive impact on the improvement of 1-year mortality and respiratory failure in bronchiectasis patients with high comorbidities. The role of ACT in combination with mucoactive agents should be investigated in future trials.
Clinically, short-term corticosteroid treatment is often used to control bronchiectasis exacerbations but sometimes is also prescribed to decrease airway inflammation and attenuate the bronchiectasis progression (48). A Cochrane review indicated that corticosteroids may have short-term benefits, but there is lack of evidence for routine use recommendation (49). In our cohort study, bronchiectasis patients with high comorbidities hospitalized for pneumonia and presenting with the use of systemic corticosteroids had a greater risk for mortality (HR: 4.38) in hospitalization as well as mortality (HR: 2.71) and respiratory failure (HR: 2.52) in a 1-year follow-up compared to those with low comorbidities. Corticosteroids may promote the cell immunity dysregulation that could lead to an increase in infections and airway inflammation that could worsen clinical outcomes, such as exacerbations and mortality (50, 51). The long-term use of inhaled corticosteroids has been associated with an increased risk of pneumonia and mortality in adults with chronic obstructive pulmonary disease (52). Our study does not support the widespread use of systemic corticosteroids in bronchiectasis patients with high comorbidities.
Although administrative databases such as the CGRD provide valuable clinical information, they often do not include all the parameters necessary to calculate disease severity scores used in clinical practice. Therefore, the claims-based severity index is developed not to replace the existing clinical scales but rather to provide an alternative tool. In this study, BACI is used to stratify the disease severity. Although CGRD includes demographic information, diagnoses, examinations, laboratory tests, and medications, the clinical scores such as BSI and FACED could not be calculated due to an incomplete record of clinical parameters. Though BACI previously describes the comorbidity of bronchiectasis, our study might provide evidence that BACI could be used to accurately stratify the risk of hospital and 1-year follow-up mortality in CGRD. Future database studies could use the BACI to classify patients with bronchiectasis and evaluate the clinical outcome.
The limitations of this study are as follows. First, because there was no standard protocol of screening etiology or comorbidities in bronchiectasis in CGMH, some comorbidities may be underestimated in the CGRD database. Second, not all patients had sputum culture performed during hospitalization and we did not routinely check sputum culture in the clinic during a 1-year follow-up. Third, ACT included mainly IPPB and postural drainage, so we could not evaluate or compare the outcomes with other ACT regimens such as positive expiratory pressure devices, high-frequency chest wall oscillation, or other modalities. Fourth, because CGRD is a hospital-based database, we could not exactly identify patients previously treated with home oxygen and/or home non-invasive ventilation. Fifth, because this was an observational study from a multi-institution database, treatment selection bias may exist when evaluating the effect of inhalation medicine or ACT in bronchiectasis. Multicenter randomized trials will be needed to better define the optimal regime and duration of treatment (long-term maintenance therapy or repeated short-term therapy), and to compare the effectiveness and safety of different inhaled antibiotics and between inhaled and systemic antibiotic therapies.
In conclusion, BACI stratifies the risk of mortality and respiratory failure in patients with bronchiectasis who admitted for the treatment of pneumonia, making it a good measure of bronchiectasis severity or adjusting for the risk of outcomes in future claims-based studies. ACT reduced the risk of respiratory failure and mortality in a 1-year follow-up. Future trials or database studies are required to validate our observations from this cohort.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Chang Gung Memorial Hospital approved this study (IRB number: 201800712B0C502). Written informed consent is not required for this retrospective database study. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
H-YH, H-CL, and C-HW: conceptualization. F-TC and C-YL: investigation. Y-TH, Y-TL, and H-CL: methodology. Y-CH: data curation. Y-TH: validation. H-YH and C-HW: writing—original draft preparation. KC and C-HW: writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Chang Gung Memorial Hospital Research Project Grant (CMRPG3H0931; CMRPG3K2061), Saint Paul's Hospital Research Project (SPMRP-U1-3001), and the Maintenance Project of the Center for Big Data Analytics and Statistics (Grant CLRPG3D0046) at Chang Gung Memorial Hospital.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the statistical assistance and wish to acknowledge the support of the Center for Big Data Analytics and Statistics at Chang Gung Memorial Hospital for study design and monitor, data analysis, and interpretation.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.812775/full#supplementary-material
Appendix A. Supplementary data.
References
1. McDonnell MJ, Aliberti S, Goeminne PC, Restrepo MI, Finch S, Pesci A, et al. Comorbidities and the risk of mortality in patients with bronchiectasis: an international multicentre cohort study. Lancet Respir Med. (2016) 4:969–79. doi: 10.1016/S2213-2600(16)30320-4
2. Chalmers JD, Goeminne P, Aliberti S, McDonnell MJ, Lonni S, Davidson J, et al. The bronchiectasis severity index. An international derivation and validation study. Am J Respir Crit Med. (2014) 189:576–85. doi: 10.1164/rccm.201309-1575OC
3. Menendez R, Mendez R, Polverino E, Rosales-Mayor E, Amara-Elori I, Reyes S, et al. Factors associated with hospitalization in bronchiectasis exacerbations: a one-year follow-up study. Respir Res. (2017) 18:176. doi: 10.1186/s12931-017-0659-x
4. Chalmers JD, Aliberti S, Filonenko A, Shteinberg M, Goeminne PC, Hill AT, et al. Characterization of the “frequent exacerbator phenotype” in bronchiectasis. Am J Respir Crit Med. (2018) 197:1410–20. doi: 10.1164/rccm.201711-2202OC
5. Martinez-Garcia MA, de Gracia J, Vendrell Relat M, Giron RM, Maiz Carro L, de la Rosa Carrillo D, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J. (2014) 43:1357–67. doi: 10.1183/09031936.00026313
6. Hsieh MH, Fang YT, Chung FT, Lee CS, Chang YC, Liu YZ, et al. Distance-saturation product of the 6-minute walk test predicts mortality of patients with non-cystic fibrosis bronchiectasis. J Thorac Dis. (2017) 9:3168–76. doi: 10.21037/jtd.2017.08.53
7. Sin S, Yun SY, Kim JM, Park CM, Cho J, Choi SM, et al. Mortality risk and causes of death in patients with non-cystic fibrosis bronchiectasis. Respir Res. (2019) 20:271. doi: 10.1186/s12931-019-1243-3
8. Cole PJ. Inflammation: a two-edged sword–the model of bronchiectasis. Eur J Respir Dis Suppl. (1986) 147:6–15.
9. Sanchez-Munoz G, Lopez-de-Andres A, Hernandez-Barrera V, Pedraza-Serrano F, Jimenez-Garcia R, Lopez-Herranz M, et al. Hospitalizations for community-acquired and non-ventilator-associated hospital-acquired pneumonia in Spain: influence of the presence of aronchiectasis. A retrospective database study. J Clin Med. (2020) 9:2339. doi: 10.3390/jcm9082339
10. Goeminne PC, Nawrot TS, Ruttens D, Seys S, Dupont LJ. Mortality in non-cystic fibrosis bronchiectasis: a prospective cohort analysis. Respir Med. (2014) 108:287–96. doi: 10.1016/j.rmed.2013.12.015
11. Keistinen T, Saynajakangas O, Tuuponen T, Kivela SL. Bronchiectasis: an orphan disease with a poorly-understood prognosis. Eur Respir J. (1997) 10:2784–7. doi: 10.1183/09031936.97.10122784
12. Choi H, Yang B, Kim YJ, Sin S, Jo YS, Kim Y, et al. Increased mortality in patients with non cystic fibrosis bronchiectasis with respiratory comorbidities. Sci Rep. (2021) 11:7126. doi: 10.1038/s41598-021-86407-8
13. Huang HY, Chung FT, Lo CY, Lin HC, Huang YT, Yeh CH, et al. Etiology and characteristics of patients with bronchiectasis in Taiwan: a cohort study from 2002 to 2016. BMC Pulm Med. (2020) 20:45. doi: 10.1186/s12890-020-1080-7
14. Whitters D, Stockley R. Immunity and bacterial colonisation in bronchiectasis. Thorax. (2012) 67:1006–13. doi: 10.1136/thoraxjnl-2011-200206
15. Polverino E, Goeminne PC, McDonnell MJ, Aliberti S, Marshall SE, Loebinger MR, et al. European respiratory society guidelines for the management of adult bronchiectasis. Eur Respir J. (2017) 50:1700629. doi: 10.1183/13993003.00629-2017
16. Murray MP, Pentland JL Hill AT. A randomised crossover trial of chest physiotherapy in non-cystic fibrosis bronchiectasis. Eur Respir J. (2009) 34:1086–92. doi: 10.1183/09031936.00055509
17. Lee AL, Burge AT, Holland AE. Airway clearance techniques for bronchiectasis. Cochrane Database Syst Rev. (2015) 11:CD008351. doi: 10.1002/14651858.CD008351.pub3
18. O'Neill K, O'Donnell AE, Bradley JM. Airway clearance, mucoactive therapies and pulmonary rehabilitation in bronchiectasis. Respirology. (2019) 24:227–37. doi: 10.1111/resp.13459
19. Dhar R, Singh S, Talwar D, Mohan M, Tripathi SK, Swarnakar R, et al. Bronchiectasis in India: results from the European multicentre bronchiectasis audit and research collaboration (EMBARC) and respiratory research network of India registry. Lancet Glob Health. (2019) 7:e1269–79. doi: 10.1016/S2214-109X(19)30327-4
20. Basavaraj A, Choate R, Addrizzo-Harris D, Aksamit TR, Barker A, Daley CL, et al. Airway clearance techniques in bronchiectasis: analysis from the united states bronchiectasis and non-TB mycobacteria research registry. Chest. (2020) 158:1376–84. doi: 10.1016/j.chest.2020.06.050
21. Spinou A, Chalmers JD. Using airway clearance techniques in bronchiectasis: halfway there. Chest. (2020) 158:1298–300. doi: 10.1016/j.chest.2020.07.062
22. Spinou A, Chalmers JD. Respiratory physiotherapy in the bronchiectasis guidelines: is there a loud voice we are yet to hear? Eur Respir J. (2019) 54:1901610. doi: 10.1183/13993003.01610-2019
23. Shao SC, Chan YY, Kao Yang YH, Lin SJ, Hung MJ, et al. The chang gung research database-A multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol Drug Saf. (2019) 28:593–600. doi: 10.1002/pds.4713
24. Tsai MS, Lin MH, Lee CP, Yang YH, Chen WC, Chang GH, et al. Chang gung research database: a multi-institutional database consisting of original medical records. Biomed J. (2017) 40:263–9. doi: 10.1016/j.bj.2017.08.002
25. Pasteur MC, Bilton D, Hill AT, British Thoracic Society Bronchiectasis non-CF Guideline Group. British thoracic society guideline for non-CF bronchiectasis. Thorax. (2010) 65(Suppl. 1):i1–58. doi: 10.1136/thx.2010.136119
26. Hill AT, Haworth CS, Aliberti S, Barker A, Blasi F, Boersma W, et al. Pulmonary exacerbation in adults with bronchiectasis: a consensus definition for clinical research. Eur Respir J. (2017) 49:1700051. doi: 10.1183/13993003.00051-2017
27. Lin CF, Chang YH, Chi NF, Chuang MT, Chien LN. Effect of early percutaneous coronary intervention on one-year risk of pneumonia and pneumonia-related adverse outcomes in patients with acute myocardial infarction. EuroIntervention. (2018) 13:1705–13. doi: 10.4244/EIJ-D-16-00840
28. Tsai TL, Wei JC, Wu YT, Ku YH, Lu KL, Wang YH. The association between usage of colchicine and pneumonia: a nationwide population-based cohort study. Front Pharmacol. (2019) 10:908. doi: 10.3389/fphar.2019.00908
29. Yang LC, Suen YJ, Wang YH, Lin TC, Yu HC, Chang YC. The association of periodontal treatment and decreased pneumonia: a nationwide population-based cohort study. Int J Environ Res Public Health. (2020) 17:356. doi: 10.3390/ijerph17010356
30. Phua J, Ang YL, See KC, Mukhopadhyay A, Santiago EA, Dela Pena EG, et al. Noninvasive and invasive ventilation in acute respiratory failure associated with bronchiectasis. Intensive Care Med. (2010) 36:638–47. doi: 10.1007/s00134-009-1743-6
31. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. (2005) 26:319–38. doi: 10.1183/09031936.05.00034805
32. Martinez-Garcia MA, Athanazio RA, Giron R, Maiz-Carro L, de la Rosa D, Olveira C, et al. Predicting high risk of exacerbations in bronchiectasis: the E-FACED score. Int J Chron Obstruct Pulmon Dis. (2017) 12:275–84. doi: 10.2147/COPD.S121943
33. Huang HY, Sheng TF, Lin CW, Wang TW, Lo CY, Chung FT, et al. Oxygen desaturation during the 6-min walk test as a risk for osteoporosis in non-cystic fibrosis bronchiectasis. BMC Pulm Med. (2019) 19:28. doi: 10.1186/s12890-019-0794-x
34. Chai YH, Xu JF. How does pseudomonas aeruginosa affect the progression of bronchiectasis? Clin Microbiol Infect. (2020) 26:313–8. doi: 10.1016/j.cmi.2019.07.010
35. Finch S, McDonnell MJ, Abo-Leyah H, Aliberti S, Chalmers JD. A comprehensive analysis of the impact of pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Ann Am Thorac Soc. (2015) 12:1602–11. doi: 10.1513/AnnalsATS.201506-333OC
36. Menendez R, Mendez R, Polverino E, Rosales-Mayor E, Amara-Elori I, et al. Risk factors for multidrug-resistant pathogens in bronchiectasis exacerbations. BMC Infect Dis. (2017) 17:659. doi: 10.1186/s12879-017-2754-5
37. Mourad A, Baker AW, Stout JE. Reduction in expected survival associated with nontuberculous mycobacterial pulmonary disease. Clin Infect Dis. (2020) 72:e552–7. doi: 10.1093/cid/ciaa1267
38. Chalmers JD, Boersma W, Lonergan M, Jayaram L, Crichton ML, Karalus K, et al. Long-term macrolide antibiotics for the treatment of bronchiectasis in adults: an individual participant data meta-analysis. Lancet Respir Med. (2019) 7:845–54. doi: 10.1016/S2213-2600(19)30191-2
39. Wong C, Jayaram L, Karalus N, Eaton T, Tong C, Hockey H, et al. Azithromycin for prevention of exacerbations in non-cystic fibrosis bronchiectasis (EMBRACE): a randomised, double-blind, placebo-controlled trial. Lancet. (2012) 380:660–7. doi: 10.1016/S0140-6736(12)60953-2
40. Altenburg J, de Graaff CS, Stienstra Y, Sloos JH, van Haren EH, Koppers RJ, et al. Effect of azithromycin maintenance treatment on infectious exacerbations among patients with non-cystic fibrosis bronchiectasis: the BAT randomized controlled trial. JAMA. (2013) 209:1251–9. doi: 10.1001/jama.2013.1937
41. Hill AT, Sullivan AL, Chalmers JD, De Soyza A, Elborn SJ, Floto AR, et al. British thoracic society guideline for bronchiectasis in adults. Thorax. (2019) 74:1–69. doi: 10.1136/thoraxjnl-2018-212463
42. Munoz G, de Gracia J, Buxo M, Alvarez A, Vendrell M. Long-term benefits of airway clearance in bronchiectasis: a randomised placebo-controlled trial. Eur Respir J. (2018) 51:1701926. doi: 10.1183/13993003.01926-2017
43. Garrard CS, Shah M. The effects of expiratory positive airway pressure on functional residual capacity in normal subjects. Crit Care Med. (1978) 6:320–2. doi: 10.1097/00003246-197809000-00004
44. Fouts JB, Brashear JE. Intermittent positive-pressure breathing. A critical appraisal. Postgrad Med. (1976) 59:103–7. doi: 10.1080/00325481.1976.11714357
45. Graham WG, Bradley DA. Efficacy of chest physiotherapy and intermittent positive-pressure breathing in the resolution of pneumonia. N Engl J Med. (1978) 299:624–7. doi: 10.1056/NEJM197809212991203
46. Lin HC, Cheng HF, Wang CH, Liu CY, Yu CT, Kuo HP. Inhaled gentamicin reduces airway neutrophil activity and mucus secretion in bronchiectasis. Am J Respir Crit Care Med. (1997) 155:2024–9. doi: 10.1164/ajrccm.155.6.9196111
47. Chalmers JD, Smith MP, McHugh BJ, Doherty C, Govan JR, Hill AT. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med. (2012) 186:657–65. doi: 10.1164/rccm.201203-0487OC
48. Henkle E, Aksamit TR, Barker AF, Curtis JR, Daley CL, Anne Daniels ML, et al. Pharmacotherapy for non-cystic fibrosis bronchiectasis: results from an NTM Info & research patient survey and the bronchiectasis and NTM research registry. Chest. (2017) 152:1120–7. doi: 10.1016/j.chest.2017.04.167
49. Kapur N, Petsky HL, Bell S, Kolbe J, Chang AB. Inhaled corticosteroids for bronchiectasis. Cochrane Database Syst Rev. (2018) 5:CD000996. doi: 10.1002/14651858.CD000996.pub3
50. Sabroe I, Postma D, Heijink I, Dockrell DH. The yin and the yang of immunosuppression with inhaled corticosteroids. Thorax. (2013) 68:1085–7. doi: 10.1136/thoraxjnl-2013-203773
51. Choi H, Lee H, Ryu J, Chung SJ, Park DW, Sohn JW, et al. Bronchiectasis and increased mortality in patients with corticosteroid-dependent severe asthma: a nationwide population study. Ther Adv Respir Dis. (2020) 14:1753466620963030. doi: 10.1177/1753466620963030
Keywords: bronchiectasis, BACI index, severe exacerbation, airway clearance therapy, mortality
Citation: Huang H-Y, Chung F-T, Lin C-Y, Lo C-Y, Huang Y-T, Huang Y-C, Lai Y-T, Gan S-T, Ko P-C, Lin H-C, Chung KF and Wang C-H (2022) Influence of Comorbidities and Airway Clearance on Mortality and Outcomes of Patients With Severe Bronchiectasis Exacerbations in Taiwan. Front. Med. 8:812775. doi: 10.3389/fmed.2021.812775
Received: 10 November 2021; Accepted: 08 December 2021;
Published: 21 January 2022.
Edited by:
Rodrigo Torres-Castro, University of Chile, ChileReviewed by:
Tjip S. van der Werf, University of Groningen, NetherlandsPieter Goeminne, AZ Nikolaas, Belgium
Copyright © 2022 Huang, Chung, Lin, Lo, Huang, Huang, Lai, Gan, Ko, Lin, Chung and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chun-Hua Wang, wchunhua@ms7.hinet.net
 Hung-Yu Huang
Hung-Yu Huang Fu-Tsai Chung
Fu-Tsai Chung Chun-Yu Lin1,2
Chun-Yu Lin1,2  Yu-Tung Huang
Yu-Tung Huang Po-Chuan Ko
Po-Chuan Ko Kian Fan Chung
Kian Fan Chung Chun-Hua Wang
Chun-Hua Wang