- 1Department of Physical Medicine and Rehabilitation, Harvard Medical School, Boston, MA, United States
- 2Spaulding Rehabilitation Hospital and Spaulding Research Institute, Charlestown, MA, United States
- 3MassGeneral Hospital for Children™ Sport Concussion Program, Boston, MA, United States
- 4Home Base, A Red Sox Foundation and Massachusetts General Hospital Program, Charlestown, MA, United States
- 5Hunter New England Local Health District, Sports Concussion Program, Newcastle, NSW, Australia
- 6Priority Research Centre for Stroke and Brain Injury, School of Medicine and Public Health, University of Newcastle, Newcastle, NSW, Australia
Background: There are no validated or agreed upon criteria for diagnosing chronic traumatic encephalopathy (CTE) in a living person. In recent years, it has been proposed that anger dyscontrol represents a behavioral clinical phenotype of CTE. This is the first study to examine the specificity of the diagnostic research criteria for traumatic encephalopathy syndrome (TES, the clinical condition proposed to be CTE) in men from the US general population who have anger dyscontrol problems. It was hypothesized that a substantial percentage of these men would meet the research criteria for TES.
Methods: Data from 4,139 men who participated in the National Comorbidity Survey Replication, an in-person survey that examined the prevalence and correlates of mental disorders in the United States, were included in this study. Men who were diagnosed with intermittent explosive disorder in the past year were the clinical sample of interest (n = 206; 5.0% of all men in the database), and the remaining men were used as a comparison sample. They were classified as meeting the research criteria for TES if they presented with the purported supportive clinical features of CTE (e.g., impulsivity/substance abuse, anxiety, apathy, suicidality, headache).
Results: In this sample of men from the general population with intermittent explosive disorder, 27.3% met a conservative definition of the proposed research criteria for CTE (i.e., traumatic encephalopathy syndrome). If one assumes the delayed-onset criterion is present, meaning that the men in the sample are compared to former athletes or military veterans presenting with mental health problems years after retirement, then 65.0% of this sample would meet the research criteria for TES.
Conclusions: These results have important implications. Using conservative criteria, at least one in four men from the general population, who have serious anger control problems, will meet the symptom criteria for TES. If one considers former athletes and military veterans with anger control problems who present many years after retirement and who experienced a documented decline in their mental health, nearly two-thirds will meet these research criteria. More research is needed to examine risks for misdiagnosing TES and to determine whether anger dyscontrol is a clinical phenotype of CTE.
Introduction
There is tremendous interest in chronic traumatic encephalopathy (CTE). In the twentieth century, CTE was considered to be a neurological disorder affecting a subgroup of long-career boxers (1, 2), and the clinical features were usually described as reflecting rather obvious chronic brain damage and cognitive impairment (3, 4). Varying degrees of neurological hard signs, such as abnormal reflexes and hemiparesis, and extrapyramidal signs, such as slurred or dysarthric speech, gait abnormalities, and tremor, were described (2, 4–11). The extent to which CTE is static or progressive or whether its course reflects two or more different clinical conditions has never been clear (1, 2, 7, 10, 12–17), and many authors conceptualized CTE as a progressive parkinsonian-like neurological disorder, and others have not.
In its modern form, CTE is considered to be a postmortem neuropathological diagnosis (18, 19), with the defining pathological feature being the accumulation of hyperphosphorylated tau (p-tau), in a patchy distribution at the depths of the cortical sulci around small vessels (18, 19). This specific neuropathology has been identified after death in the brains of young athletes (20, 21), active NFL players (20, 21), former collegiate athletes from multiple sports (20), retired boxers (20), retired professional hockey players (20), retired NFL players (18, 20, 21), and military veterans (22). P-tau accumulates in the brain in normal aging and in numerous neurodegenerative diseases (23–28), but researchers have asserted that it does not accumulate in a patchy distribution in the depths of sulci in association with aging or other diseases (18, 19). However, this assertion remains in doubt because CTE neuropathology has been identified in some people from the general population with no known exposure to repetitive neurotrauma and in association with substance abuse, temporal lobe epilepsy, multiple system atrophy, amyotrophic lateral sclerosis, and other neurodegenerative diseases (29–36).
The extent to which the neuropathology of CTE causes specific clinical symptoms and problems is unclear (19, 37); there is no agreed-upon way to diagnose CTE in a living person, and there is major interest in developing and validating clinical diagnostic criteria. At present, validated diagnostic criteria do not exist, although several sets have been proposed (13, 38–40). Preliminary proposed research criteria for “traumatic encephalopathy syndrome (TES)” (39) include three core features of CTE: (i) “cognitive,” (ii) “behavioral” (i.e., anger dyscontrol), and (iii) “mood” (i.e., depression or hopelessness). These core features are used to define diagnostic “subtypes” or “variants” according to the research criteria. In addition to a subtype, two supportive features must be present [i.e., impulsivity, anxiety, apathy, paranoia, suicidality, headache, motor signs, a progressive clinical course, or a delayed onset of symptoms (e.g., after retirement from sport)].
A major gap in the literature is that there are very few published studies relating to the specificity of the proposed research criteria for TES (41, 42). This is the first study to examine the research criteria for TES (39) in men from the US general population who have intermittent explosive disorder (IED). We chose to study these men from the general population because the “behavioral” subtype of TES is defined as follows: “Being described as emotionally explosive (e.g., having a “short fuse” or being “out of control”), physically violent, and/or verbally violent, as reported by self or informant, by history of treatment, or by clinician's report. A formal diagnosis of IED would meet this criterion but is not necessary (39). We hypothesized that a substantial percentage of these men from the general population would meet the proposed research criteria for TES.
Methods
Participants
The National Comorbidity Survey Replication (NCS-R), conducted between February 2001 and April 2003 (43, 44), examined the prevalence and correlates of mental disorders in the United States (45–49). The interview for this survey was conducted in the homes of a nationally representative sample of adult respondents (N = 9,282, 4,139 men and 5,143 women) (44). The clinical sample of interest was obtained by applying a filter to the publicly available NCS-R database selecting all “male” participants meeting the criteria for a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) IED in the past year (i.e., variable = “D_IED12”). This filter resulted in the inclusion of 206 men; an incidence rate of 5.0% of all men in the database. The mean age of this sample was 32.9 years (median = 30.5, SD = 12.1, interquartile range = 23–41, range = 18–83). Their race was reported as follows: white = 65.5%, African Americans = 12.6%, Hispanic = 11.2%, Asian = 2.4%, and all other races = 8.3%. Their level of education was as follows: 0–11 years = 22.3%, 12 years = 33.0%, 13–15 years = 30.6%, and 16 or more years = 14.1%. Their employment status was as follows: employed = 73.8%, unemployed = 1.9%, and not in the labor force = 23.8%. Their relationship status was described as 56.3% married, 32.0% as never married, and 11.7% as divorced, separated, or widowed. The remaining 3,933 men were considered to be a sample representing the general population. The mean age of this sample was 44.4 years (median = 43.0, SD = 17.0, interquartile range = 31–56, range = 18–93). Their race was reported as follows: white = 74.8%, African Americans = 10.8%, Hispanic = 9.4%, Asian = 2.0%, and all other races = 3.0%. Their level of education was as follows: 0–11 years = 15.4%, 12 years = 28.9%, 13–15 years = 27.9%, and 16 or more years = 27.8%. Their employment status was as follows: employed = 72.1%, unemployed = 6.8%, not in the labor force = 20.5%, and missing = 0.5%. Their relationship status was described as 62.4% married, 22.2% never married, and 15.4% divorced, separated, or widowed.
The NCS-R Protocol
Researchers from the Survey Research Center of the Institute for Social Research at the University of Michigan conducted the survey using laptop computer-assisted personal interviews. The core diagnostic assessment, conducted with 9,282 respondents, included the following modules: household listing, screening, depression, mania, irritable depression, panic disorder, specific phobia, social phobia, agoraphobia, generalized anxiety disorder, IED, suicidality, services, and pharmacoepidemiology. The diagnoses were derived from the World Mental Health Survey Initiative Version of the World Health Organization Composite International Diagnostic Interview, a fully structured lay-administered diagnostic interview that generates both International Classification of Diseases, 10th Revision (50) and DSM-IV (51) diagnoses. The NCS-R database is publicly available, and we accessed it at http://www.icpsr.umich.edu/icpsrweb/ICPSR/studies/20240.
Research Criteria for Traumatic Encephalopathy Syndrome
The research criteria for “traumatic encephalopathy syndrome” (39) include three proposed core features of CTE: (i) “cognitive,” (ii) “behavioral” (i.e., anger dyscontrol), and (iii) “mood” (i.e., depression or hopelessness). We selected a sample of men who would definitively meet the core criterion for “behavioral” in that they were diagnosed with DSM-IV IED within the past 12 months. The “supportive features” for a diagnosis of the syndrome include impulsivity, anxiety, apathy, paranoia, suicidality, headache, motor signs, a documented decline in functioning or a progression of symptoms, or a delayed onset—such as having problems at least 2 years after the end of a career in contact sports. Two or more supportive features must be present. For the present study, we selected five of the nine supportive features, available in the NCS-R database, for the primary analyses (i.e., impulsivity, anxiety, apathy, suicidality, and headache). Other supportive features, such as paranoia, motor signs, decline in functioning, and delayed onset of symptoms were deemed to be less reliable or missing variables in the NCS-R database. The neurotrauma exposure criterion for TES is broad and diverse and includes any one of the following: (i) four of more concussions; (ii) two or more moderate or severe TBIs; (iii) involvement in “high exposure” contact sports (e.g., American football, ice hockey, lacrosse, rugby, wrestling, and soccer) for a minimum of 6 years, including at least 2 years at the college level (or higher); (iv) military service (including, but not limited to, combat exposure to blast and other explosions as well as non-combat exposure to explosives, or to combatant training or breaching training); or (v) history of any other significant exposure to repetitive hits to the head (including, but not limited to, domestic abuse, head banging, and vocational activities such as door breaching by police). This criterion could not be applied because the information was not available in the NCS-R database.
The rates of screening positively for TES are presented in two ways. First, the rate at which the men meet two of the five supportive criteria is presented. Second, the rate at which the men meet one of the five supportive criteria is presented because this simulates the normal clinical situation in which the delayed-onset criterion and/or the decline in functioning criterion would be met. The “delayed onset” criterion requires “delayed onset of clinical features after significant head impact exposure, usually at least 2 years and in many cases several years after the period of maximal exposure,” and the “documented decline” criterion requires a “progressive decline in function and/or a progression in symptoms” (39). Any former athlete or military veteran who met the neurotrauma exposure criteria and retired in their 20s or 30s, for example, who had mental health or neurological problems consistent with “TES” anytime between the ages of 40 years and the time of their death would meet the delayed-onset criterion.
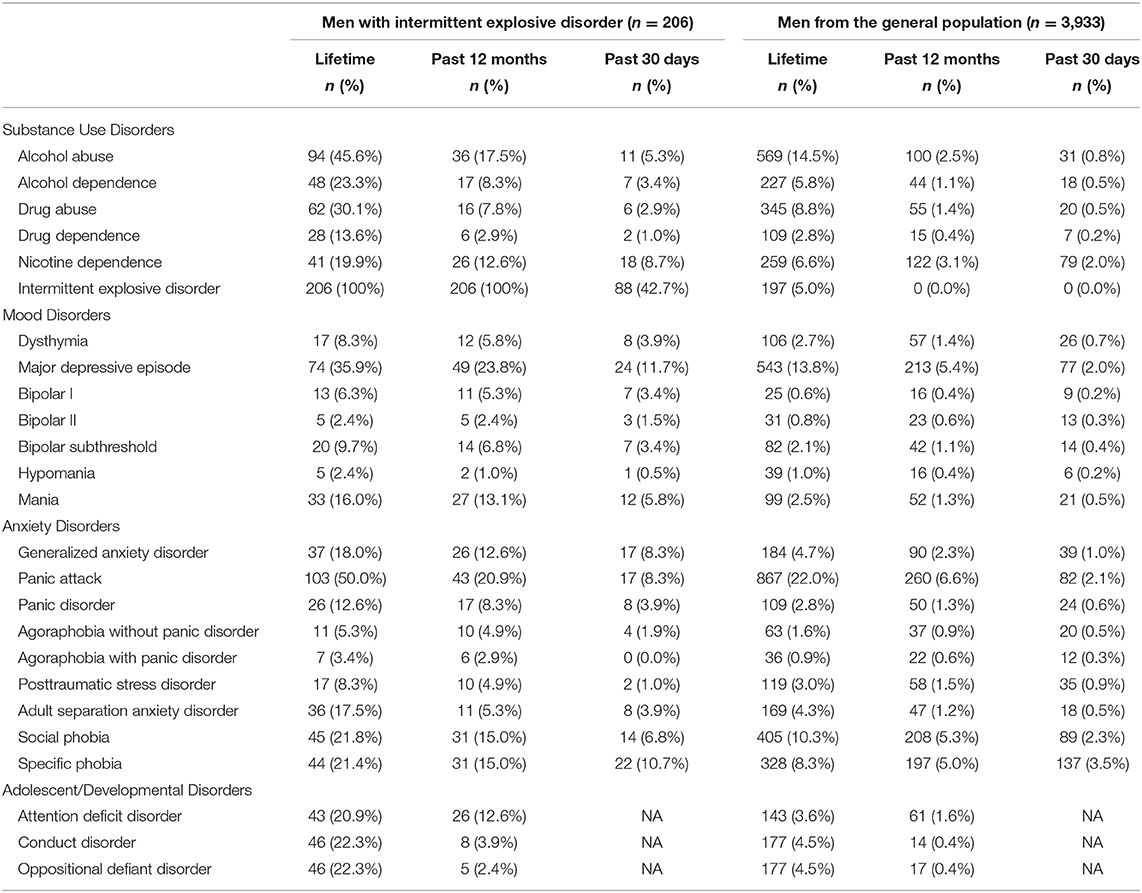
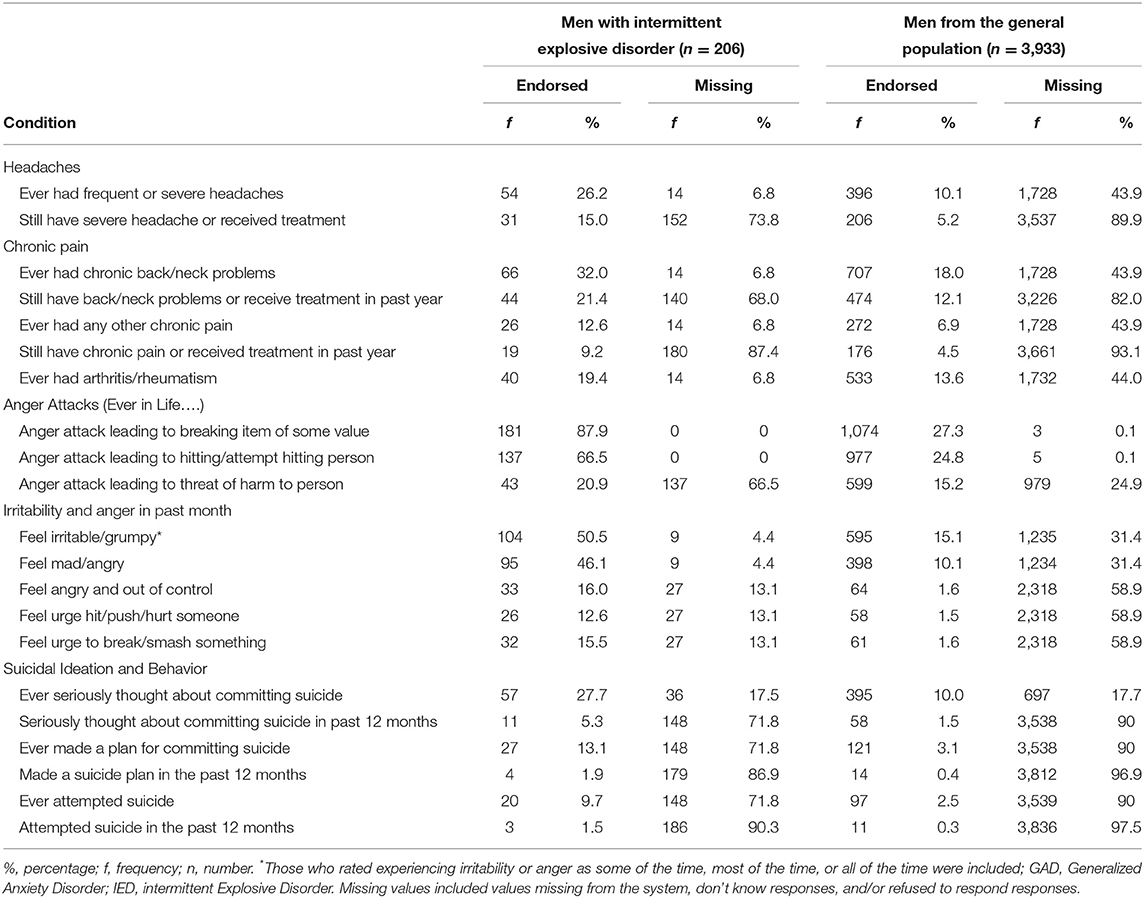
Results
The prevalence of IED in men in the National Comorbidity Survey Replication was 9.7% for lifetime, 5.0% for past year, and 2.1% for past 30 days. Lifetime diagnoses of major mental disorders, and mental disorders experienced over the past year and past 30 days, stratified by group, are presented in Table 1. Lifetime experiences with headaches, suicidality, anger, and chronic pain, stratified by group, are presented in Table 2. Compared to men in the general population, those with IED have a greater lifetime history of many disorders, including but not limited to attention-deficit/hyperactivity disorder [20.9 vs. 3.6%; χ2 = 135.5, p < 0.001, relative risk (RR) = 5.7, 95% confidence interval (CI) = 4.1–7.9], conduct disorder and oppositional defiant disorder (both 22.3 vs. 4.5%; χ2 = 122.1, p < 0.001, RR = 5.0, 95% CI = 3.6–6.7), alcohol abuse (45.6 vs. 14.5%; χ2 = 141.3, p < 0.001, RR = 3.2, 95% CI = 2.6–3.7), drug abuse (30.1 vs. 8.8%; χ2 = 100.4, p < 0.001, RR = 3.4, 95% CI = 2.7–4.3), major depressive episode (35.9 vs. 13.8%; χ2 = 75.5, p < 0.001, RR = 2.6, 95% CI = 2.1–3.2), mania (16.0 vs. 2.5%; χ2 = 115.6, p < 0.001, RR = 6.4, 95% CI = 4.3–9.3), generalized anxiety disorder (18.0 vs. 4.7%; χ2 = 72.2, p < 0.001, RR = 4.1, 95% CI = 2.9–5.7), panic disorder (12.6 vs. 2.8%; χ2 = 68.3, p < 0.001, RR = 3.8, 95% CI = 2.7–5.3), adult separation anxiety disorder (17.5 vs. 4.3%), social phobia (i.e., social anxiety disorder, 21.8 vs. 10.3%; χ2 = 26.9, p < 0.001, RR = 2.1, 95% CI = 1.6–2.8), and a specific phobia (21.4 vs. 8.3%; χ2 = 40.6, p < 0.001, RR = 2.6, 95% CI = 1.9–3.4). Over the past 12 months, those with IED were 6.9 times more likely to meet criteria for DSM–IV alcohol abuse disorder (17.5 vs. 2.5%; χ2 = 137.4, p < 0.001, RR = 6.9, 95% CI = 4.7–9.9), and 4.4 times more likely to meet criteria for DSM–IV major depressive episode (23.8 vs. 5.4%; χ2 = 111.4, p < 0.001, RR = 4.4, 95% CI = 3.3–5.8) than men from the general population.
The percentages of men with IED meeting supportive criteria for TES are presented in Table 3. “Impulsivity,” as reflected by a diagnosis of alcohol abuse or drug abuse in the past year, was present in 20.4%. Problems with anxiety were present in 49.0%, and suicidality was present in 5.3%. Apathy was reported by 10.7%. A significant problem with headaches was reported by 15.0%. Overall, 27.3% met the criteria for two or more supportive features for the syndrome and thus when combined with their diagnosis of IED would meet the clinical criteria for TES. We could not apply the criteria relating to delayed onset (e.g., anger control problems in a middle-aged man who played college football) or progressive worsening of symptoms (i.e., over at least a 1-year duration). Assuming that one of those two criteria was met, then only one additional criterion from Table 3 would be necessary to meet research criteria for the syndrome. The proportion of the IED sample who met one or more of the criteria was 65.0%.
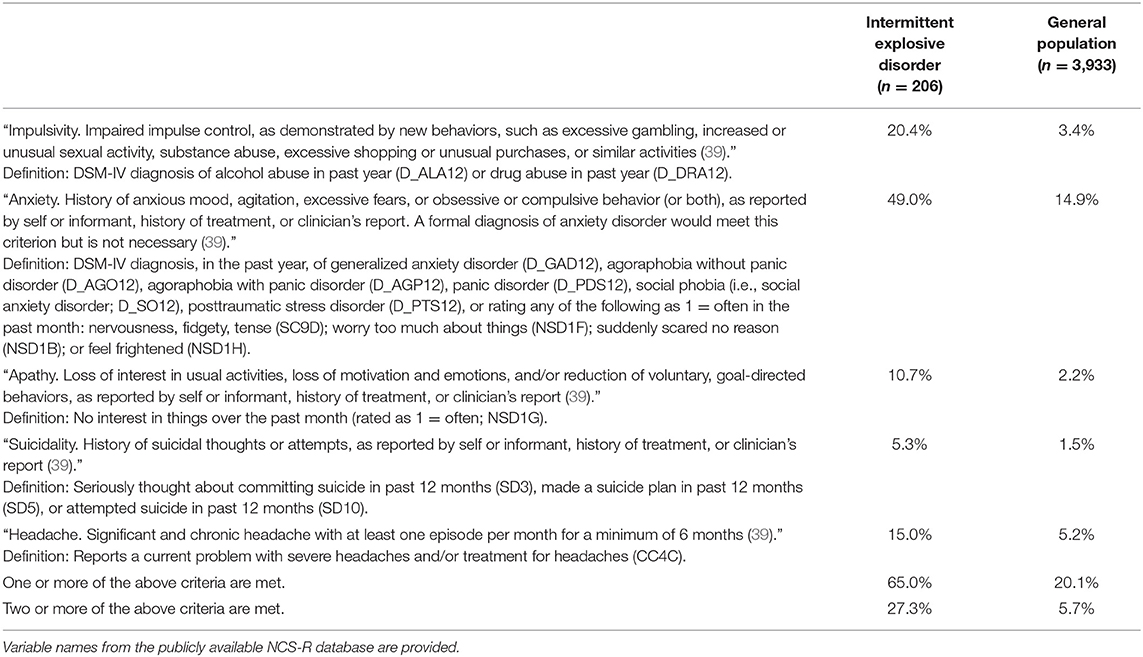
Table 3. Percentage of men meeting research criteria for supportive features of traumatic encephalopathy syndrome.
Discussion
This is the first study to examine a proposed set of research criteria for the diagnosis of TES (39) in a sample of men from the general population with IED. There were three primary important findings. First, anger attacks are common in men in the general population, with one in four men reporting them at some point during their lifetime (Table 2). Second, in the general population, the lifetime prevalence of IED is 9.7% in the present study, and men with this disorder have a high lifetime prevalence of other disorders that have been proposed to be clinical features of CTE and TES, such as alcohol abuse (45.6%), drug abuse (30.1%), and major depressive episode (35.9%). In other words, men in the US general population with severe anger control problems are likely to experience other symptoms, problems, and disorders that researchers have proposed to be characteristic of CTE and TES (Tables 1, 2). Finally, the rate of meeting the symptom criteria for TES in men from the US general population who have serious anger control problems, during the past year, is high (Table 3). The average age of this sample was 33, 50% were between the ages of 23 and 41 years, and 25% were older than 41 years. In other words, a large percentage of this sample was of a similar age of men who have retired from contact or collision sports or retired from the military. As such, if we assume the “delayed onset” supportive feature is met, then only one additional supportive feature is necessary to diagnose TES. As such, approximately two of three men from the general population, who have serious anger control problems, meet the proposed research diagnostic criteria for TES.
It is essential to appreciate that anger dyscontrol and aggressive behavior are complex and multifactorial in causation. There are many reasons why former athletes or military veterans might have anger control problems. Temperamental (52–54) and personality (55, 56) factors have been linked to risk of anger dyscontrol and aggression. Adverse events in childhood, such as abuse and neglect, have been associated with increased risk of future anger control problems (57–59). Men who had abusive or aggressive fathers are statistically more likely to also be abusive or aggressive (57). Some boys might choose certain high contact, collision, or combat sports in part due to innate aggressiveness (60, 61). As such, a certain degree of anger dyscontrol and aggressiveness may represent longstanding behavioral and personality characteristics in some former athletes, as has been speculated by authors writing about former boxers (2, 4, 5, 8, 62–64). These longstanding characteristics could be amplified or exacerbated by life stress, depression, anxiety, substance abuse, chronic cumulative brain damage, and a number of neurological and neurodegenerative diseases. Life stress (65), financial problems (66), marital problems (66), and substance abuse (67, 68) are all associated with anger control problems. Military veterans with posttraumatic stress disorder frequently have anger control problems (69–71). Men who develop a depressive disorder are also at risk of having anger attacks (72, 73). Anger attacks in men with depression have been assumed to be related to the depressive disorder, as opposed to reflecting a primary underlying IED. People with TBIs sometimes develop problems with anger dyscontrol and aggressiveness, particularly after sustaining a single severe TBI (74, 75). However, the associations between cumulative mild injuries to the brain and anger or aggression are not well-understood. Finally, problems with anger and aggression can occur as a result of a neurological disorder, such as a stroke (76–78), or during the course of a neurodegenerative disease, such as Alzheimer disease (79, 80).
Modern researchers studying CTE have emphasized psychiatric and behavioral problems as being common (39), and those who are younger and who have less neuropathology have been conceptualized as being more likely to have this proposed “mood” or “behavioral” subtype or phenotype of TES and CTE (81). The mechanisms by which small amounts of p-tau in specific brain regions drive complex changes in behavior, such as depression and anger control problems, have not been studied in a meaningful way and are unknown. Nonetheless, modern researchers seem to suggest that virtually any clinical or psychosocial problem present prior to death in someone who has CTE neuropathology in their brain identified on postmortem examination must have that problem as a direct result of the CTE neuropathology. For example, the two leading research groups in the United States have asserted that clinical features of CTE include (i) depression and anxiety (18, 82, 83); (ii) suicidality (18, 81, 83–87); (iii) poor financial decisions, financial problems, and bankruptcy (82); (iv) gambling (39); (v) excessive shopping or unusual purchases (39); (vi) marital problems, separation, and divorce (87); and (vii) substance abuse (39). This approach to defining clinical features represents association by assertion, or circulus in probando, as opposed to being based on empirical research or rigorous clinicopathological correlation.
In their review of all known cases of CTE, published in 2009, McKee et al. (20) documented that 17 of the 41 cases (41.5%) published in the twentieth century had a personal history of aggression or violence. We reexamined the 41 case studies, and without question, some former boxers believed to have CTE had documented anger control problems and violent behavior during their lifetime (4–6, 8, 10, 64). In contrast, other former boxers were described as showing euphoria (6, 15), a child-like demeanor (4), or “fatuous cheerfulness” (6). The demographic and clinical characteristics of the 17 cases with aggressive behavior are summarized in Table 4. According to the new proposed criteria for TES, a former boxer (or contact sport athlete) could be diagnosed as having the behavioral variant of TES if he had developed clinical problems “at least 2 years after a period of maximal exposure” (39) and had any one of the following problems: excessive gambling, unusual sexual activity, excessive shopping or unusual purchases, anxiety, excessive fears, obsessive-compulsive disorder, any anxiety disorder, or suicidality. To our knowledge, there was never a case that matched any of those characteristics in the twentieth century, based on our review of the case information presented in the tables in McKee et al. and our review of the six published studies that reported the case histories of those 17 people. As seen in Table 4, the aggressive behavior and volatility displayed by these former boxers generally co-occurred with other obvious neurological signs of brain damage, such as dysarthric speech, gait problems, and parkinsonism, and virtually all had cognitive impairment or dementia (2, 4, 5, 10, 64). Moreover, authors sometimes noted that aggressive behavior seemed to be a longstanding problem (4, 5, 10), perhaps contributing to their chosen career of boxing.
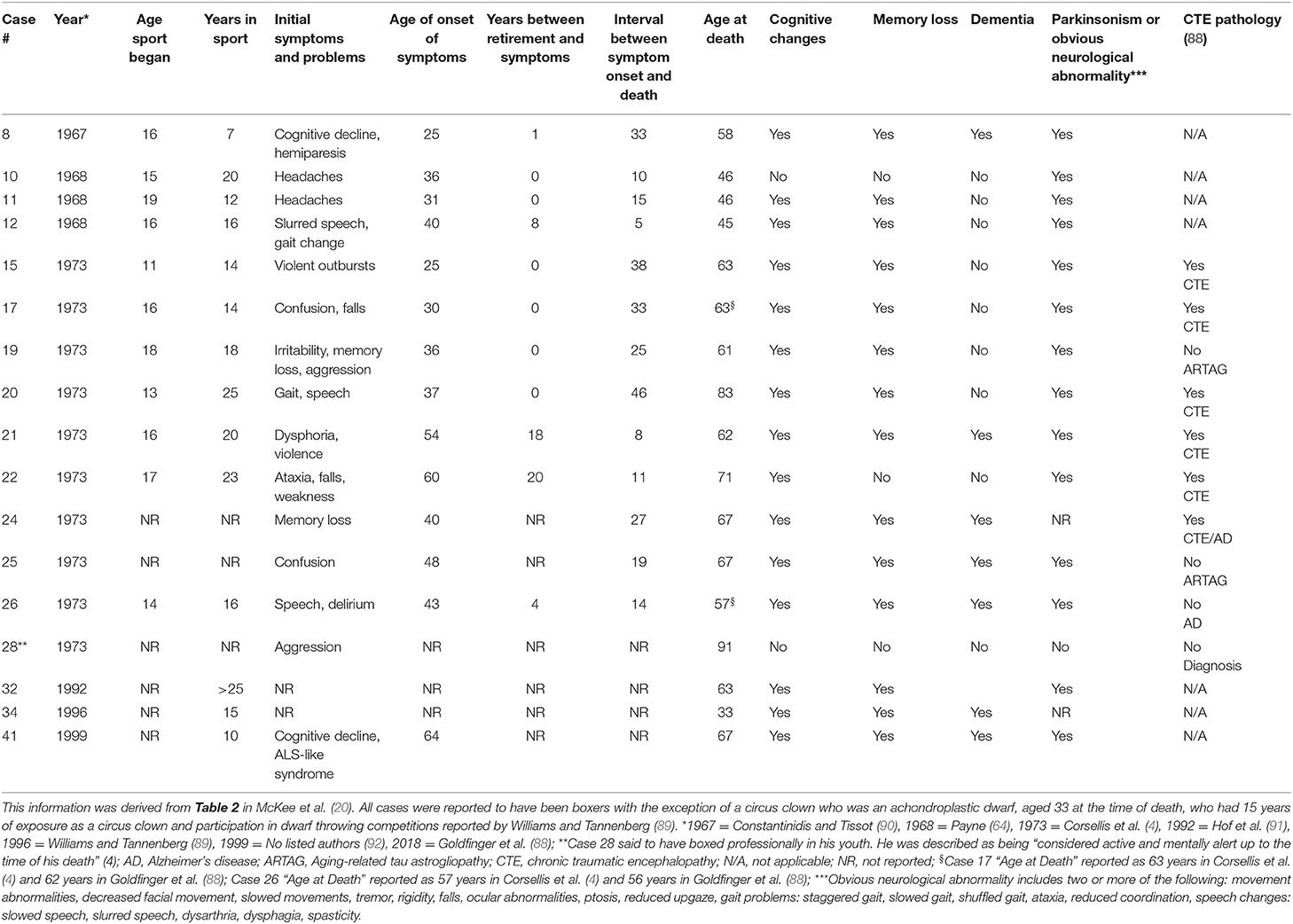
Table 4. Twentieth century case studies of presumed CTE with a history of violence or aggressiveness (n = 17) from McKee et al. (20).
Our study has three important limitations. First, we have no information on the subjects' concussion history, and it is likely that some of the men in our case series experienced one or more concussions during the course of their lives because concussions are very common in men in the general population (93, 94). Second, we were unable to study the “exposure history” criterion in the research definition of TES. It is possible that some of the men included in this study would have met the exposure criterion. It is important to appreciate that all former professional soccer players, hockey players, boxers, and American or Canadian football players meet the exposure criteria for repetitive neurotrauma. The exposure criterion is very inclusive and simply requires that the person played one or more sports (e.g., boxing, American football, ice hockey, lacrosse, rugby, wrestling, or soccer), for a minimum of 6 years [with 2 at the college level (or equivalent) or higher], which resulted in “multiple impacts to the head” that can be concussions or “subconcussive trauma” (i.e., with no clinical symptoms) (39). Moreover, military service or police training involving exposure to blasts, explosives, combat, or breaching is listed as a source of exposure sufficient to meet criteria. It is very likely that some men who participated in the NCS-R study played contact sports, at least at the high school level. No information relating to lifetime history of sports participation was available in the NCS-R database. Finally, we were not able to align precisely the results of the NCS-R interviews on to the research criteria for TES, which are broader and more inclusive than what we could study. We did not include two categories of supportive features: (i) paranoia and (ii) motor signs. Moreover, within the category of impulsivity, we did not include “excessive gambling,” “increased or unusual sexual activity,” or “excessive shopping or unusual purchases.” If we had more variables that aligned with all the supportive features criteria, the rate of identifying TES in this sample would have been greater.
In conclusion, we examined the proposed research criteria for the behavioral subtype of TES in a large sample of men with serious anger control problems who were selected from a nationally representative sample of men from the US general population who underwent a thorough in-person psychiatric interview yielding DSM-IV diagnoses. Using liberal criteria, we discovered that approximately two of three of these men could be identified as having TES. Researchers have not established a clinicopathological correlation between anger control problems and the region-specific accumulation of hyperphosphorylated tau believed to characterize CTE (19), so researchers and clinicians should not assume that anger control problems in a former athlete or military veteran are caused by CTE neuropathology. Anger control problems described in the case histories of boxers in the twentieth century were not considered to be a “behavioral” phenotype or subtype of CTE, nor were they described as a core clinical feature. More research is needed to examine risks for misdiagnosing TES, which appear considerable based on the results of this study. In addition, more research is needed to determine whether anger dyscontrol is a clinical phenotype of CTE.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: http://www.icpsr.umich.edu/icpsrweb/ICPSR/studies/20240.
Ethics Statement
The studies involving human participants were reviewed and approved by University of Michigan. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
GI and AG contributed to the conception and design of the study. GI analyzed the database. All authors drafted and revised the manuscript and approved the submitted version.
Funding
This study was funded in part by the National Football League for a program of research entitled The Spectrum of Concussion: Predictors of Clinical Recovery, Treatment and Rehabilitation, and Possible Long-Term Effects. Unrestricted philanthropic support was provided by ImPACT Applications, Inc., the Mooney-Reed Charitable Foundation, Spaulding Research Institute, and the National Rugby League. AG was funded by an NHMRC Early Career Fellowship and a Postgraduate Award from the Australian-American Fulbright Commission.
Conflict of Interest
GI serves as a scientific advisor for BioDirection, Inc., Sway Operations, LLC, and Highmark, Inc. He has a clinical and consulting practice in forensic neuropsychology, including expert testimony, involving individuals who have sustained mild TBIs (including athletes). He has received research funding from several test publishing companies, including ImPACT Applications, Inc., CNS Vital Signs, and Psychological Assessment Resources (PAR, Inc.). He has received research funding as a principal investigator from the National Football League, and salary support as a collaborator from the Harvard Integrated Program to Protect and Improve the Health of National Football League Players Association Members. He acknowledges unrestricted philanthropic support from ImPACT Applications, Inc., the Heinz Family Foundation, and the Mooney-Reed Charitable Foundation. AG has a clinical practice in neuropsychology involving individuals who have sustained sport-related concussion (including current and former athletes). He has been a contracted concussion consultant to Rugby Australia since July 2016. He has received travel funding from the Australian Football League (AFL) to present at the Concussion in Football Conference in 2013 and 2017. Previous grant funding includes the NSW Sporting Injuries Committee, the Brain Foundation (Australia), and the Hunter Medical Research Institute (HMRI), supported by Jennie Thomas, and the HMRI, supported by Anne Greaves. He is currently funded through an NHMRC Early Career Fellowship, and Hunter New England Local Health District, Research, Innovation and Partnerships Health Research & Translation Center and Clinical Research Fellowship Scheme, an Australian-American Fulbright Commission Postdoctoral Award, and the University of Newcastle's Priority Research Center for Stroke and Brain Injury.
The handling editor declared a past co-authorship with one of the authors (AG).
References
1. Martland HS. Punch drunk. J Am Med Assoc. (1928) 19:1103–7. doi: 10.1001/jama.1928.02700150029009
2. Roberts A. Brain Damage in Boxers: A Study of Prevalence of Traumatic Encephalopathy Among Ex-Professional Boxers. London: Pitman Medical Scientific Publishing Co. (1969).
3. Jordan B. Chronic traumatic brain injury associated with boxing. Semin Neurol. (2000) 20:179–85. doi: 10.1055/s-2000-9826
4. Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol Med. (1973) 3:270–303. doi: 10.1017/S0033291700049588
5. Mawdsley C, Ferguson FR. Neurological disease in boxers. Lancet. (1963) 2:795–801. doi: 10.1016/S0140-6736(63)90498-7
6. Critchley M. Medical aspects of boxing, particularly from a neurological standpoint. Br Med J. (1957) 1:357–62. doi: 10.1136/bmj.1.5015.357
7. Parker HL. Traumatic encephalopathy (‘Punch Drunk') of professional pugilists. J Neurol Psychopathol. (1934) 15:20–8. doi: 10.1136/jnnp.s1-15.57.20
8. Harvey PK, Davis JN. Traumatic encephalopathy in a young boxer. Lancet. (1974) 2:928–9. doi: 10.1016/S0140-6736(74)91133-7
9. Jedlinski J, Gatarski J, Szymusik A. Chronic posttraumatic changes in the central nervous system in pugilists. Pol Med J. (1970) 9:743–52.
10. Johnson J. Organic psychosyndromes due to boxing. Br J Psychiatry. (1969) 115:45–53. doi: 10.1192/bjp.115.518.45
11. Sercl M, Jaros O. The mechanisms of cerebral concussion in boxing and their consequences. World Neurol. (1962) 3:351–8.
13. Victoroff J. Traumatic encephalopathy: review and provisional research diagnostic criteria. NeuroRehabilitation. (2013) 32:211–24. doi: 10.3233/NRE-130839
14. Courville CB. Punch drunk. Its pathogenesis and pathology on the basis of a verified case. Bull Los Angeles Neurol Soc. (1962) 27:160–8.
15. Critchley M. Punch-drunk syndromes: the chronic traumatic encephalopathy of boxers. In: Maloine, editor. Hommage a Clovis Vincent. Strasbourg: Imprimerie Alascienne (1949). p. 131–45.
16. Grahmann H, Ule G. Diagnosis of chronic cerebral symptoms in boxers (dementia pugilistica & traumatic encephalopathy of boxers). Psychiatr Neurol. (1957) 134:261–83. doi: 10.1159/000138743
17. Mendez MF. The neuropsychiatric aspects of boxing. Int J Psychiatry Med. (1995) 25:249–62. doi: 10.2190/CUMK-THT1-X98M-WB4C
18. McKee AC, Stein TD, Nowinski CJ, Stern RA, Daneshvar DH, Alvarez VE, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain J Neurol. (2013) 136:43–64. doi: 10.1093/brain/aws307
19. McKee AC, Cairns NJ, Dickson DW, Folkerth RD, Keene CD, Litvan I, et al. The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. (2016) 131:75–86. doi: 10.1007/s00401-015-1515-z
20. McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. (2009) 68:709–35. doi: 10.1097/NEN.0b013e3181a9d503
21. Mez J, Daneshvar DH, Kiernan PT, Abdolmohammadi B, Alvarez VE, Huber BR, et al. Clinicopathological evaluation of chronic traumatic encephalopathy in players of American Football. JAMA. (2017) 318:360–70. doi: 10.1001/jama.2017.16687
22. Goldstein LE, Fisher AM, Tagge CA, Zhang XL, Velisek L, Sullivan JA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med. (2012) 4:134ra60. doi: 10.1126/scitranslmed.3004862
23. Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol. (2011) 70:960–9. doi: 10.1097/NEN.0b013e318232a379
24. von Bergen M, Barghorn S, Biernat J, Mandelkow EM, Mandelkow E. Tau aggregation is driven by a transition from random coil to beta sheet structure. Biochim Biophys Acta. (2005) 1739:158–66. doi: 10.1016/j.bbadis.2004.09.010
25. Alonso AD, Cohen LS, Corbo C, Morozova V, ElIdrissi A, Phillips G, et al. Hyperphosphorylation of tau associates with changes in its function beyond microtubule stability. Front Cell Neurosci. (2018) 12:338. doi: 10.3389/fncel.2018.00338
26. Kovacs GG, Ferrer I, Grinberg LT, Alafuzoff I, Attems J, Budka H, et al. Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta Neuropathol. (2016) 131:87–102. doi: 10.1007/s00401-015-1509-x
27. Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol. (2014) 128:755–66. doi: 10.1007/s00401-014-1349-0
28. Morsch R, Simon W, Coleman PD. Neurons may live for decades with neurofibrillary tangles. J Neuropathol Exp Neurol. (1999) 58:188–97. doi: 10.1097/00005072-199902000-00008
29. Noy S, Krawitz S, Del Bigio MR. Chronic traumatic encephalopathy-like abnormalities in a routine neuropathology service. J Neuropathol Exp Neurol. (2016) 75:1145–54. doi: 10.1093/jnen/nlw092
30. Ling H, Holton JL, Shaw K, Davey K, Lashley T, Revesz T. Histological evidence of chronic traumatic encephalopathy in a large series of neurodegenerative diseases. Acta Neuropathol. (2015) 130:891–3. doi: 10.1007/s00401-015-1496-y
31. Puvenna V, Engeler M, Banjara M, Brennan C, Schreiber P, Dadas A, et al. Is phosphorylated tau unique to chronic traumatic encephalopathy? Phosphorylated tau in epileptic brain and chronic traumatic encephalopathy. Brain Res. (2016) 1630:225–40. doi: 10.1016/j.brainres.2015.11.007
32. Fournier CN, Gearing M, Upadhyayula SR, Klein M, Glass JD. Head injury does not alter disease progression or neuropathologic outcomes in ALS. Neurology. (2015) 84:1788–95. doi: 10.1212/WNL.0000000000001522
33. Gao AF, Ramsay D, Twose R, Rogaeva E, Tator C, Hazrati LN. Chronic traumatic encephalopathy-like neuropathological findings without a history of trauma. Int J Pathol Clin Res. (2017) 3:50. doi: 10.23937/2469-5807/1510050
34. Koga S, Dickson DW, Bieniek KF. Chronic traumatic encephalopathy pathology in multiple system atrophy. J Neuropathol Exp Neurol. (2016) 75:963–70. doi: 10.1093/jnen/nlw073
35. Bieniek KF, Blessing MM, Heckman MG, Diehl NN, Serie AM, Paolini MA II, et al. Association between contact sports participation and chronic traumatic encephalopathy: a retrospective cohort study. Brain Pathol. (2019). doi: 10.1111/bpa.12757
36. Iverson GL, Luoto TM, Karhunen PJ, Castellani RJ. Mild chronic traumatic encephalopathy neuropathology in people with no known participation in contact sports or history of repetitive neurotrauma. J Neuropathol Exp Neurol. (2019) 78:615–25. doi: 10.1093/jnen/nlz045
37. Iverson GL, Keene CD, Perry G, Castellani RJ. The need to separate chronic traumatic encephalopathy neuropathology from clinical features. J Alzheimers Dis. (2018) 61:17–28. doi: 10.3233/JAD-170654
38. Jordan BD. The clinical spectrum of sport-related traumatic brain injury. Nat Rev Neurol. (2013) 9:222–30. doi: 10.1038/nrneurol.2013.33
39. Montenigro PH, Baugh CM, Daneshvar DH, Mez J, Budson AE, Au R, et al. Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res Ther. (2014) 6:68. doi: 10.1186/s13195-014-0068-z
40. Reams N, Eckner JT, Almeida AA, Aagesen AL, Giordani B, Paulson H, et al. A clinical approach to the diagnosis of traumatic encephalopathy syndrome: a review. JAMA Neurol. (2016) 73:743–9. doi: 10.1001/jamaneurol.2015.5015
41. Laffey M, Darby AJ, Cline MG, Teng E, Mendez MF. The utility of clinical criteria in patients with chronic traumatic encephalopathy. NeuroRehabilitation. (2018) 43:431–41. doi: 10.3233/NRE-182452
42. Iverson GL, Gardner AJ. Risk for misdiagnosing chronic traumatic encephalopathy in men with depression. J Neuropsychiatry Clin Neurosci. (2020) 32:139–46. doi: 10.1176/appi.neuropsych.19010021
43. Kessler RC, Merikangas KR. The national comorbidity survey replication (NCS-R): background and aims. Int J Methods Psychiatr Res. (2004) 13:60–8. doi: 10.1002/mpr.166
44. Kessler RC, Berglund P, Chiu WT, Demler O, Heeringa S, Hiripi E, et al. The US National Comorbidity Survey Replication (NCS-R): design and field procedures. Int J Methods Psychiatr Res. (2004) 13:69–92. doi: 10.1002/mpr.167
45. Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. (2003) 289:3095–105. doi: 10.1001/jama.289.23.3095
46. Kessler RC, Brandenburg N, Lane M, Roy-Byrne P, Stang PD, Stein DJ, et al. Rethinking the duration requirement for generalized anxiety disorder: evidence from the National Comorbidity Survey Replication. Psychol Med. (2005) 35:1073–82. doi: 10.1017/S0033291705004538
47. Borges G, Angst J, Nock MK, Ruscio AM, Walters EE, Kessler RC. A risk index for 12-month suicide attempts in the National Comorbidity Survey Replication (NCS-R). Psychol Med. (2006) 36:1747–57. doi: 10.1017/S0033291706008786
48. Kessler RC, Hwang I, LaBrie R, Petukhova M, Sampson NA, Winters KC, et al. DSM-IV pathological gambling in the National Comorbidity Survey Replication. Psychol Med. (2008) 38:1351–60. doi: 10.1017/S0033291708002900
49. Angst J, Cui L, Swendsen J, Rothen S, Cravchik A, Kessler RC, et al. Major depressive disorder with subthreshold bipolarity in the National Comorbidity Survey Replication. Am J Psychiatry. (2010) 167:1194–201. doi: 10.1176/appi.ajp.2010.09071011
50. World Health Organization. International Statistical Classification of Diseases and Related Health Problems. 10th ed. Geneva: World Health Organization (1992).
51. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC (1994).
52. Vitaro F, Barker ED, Boivin M, Brendgen M, Tremblay RE. Do early difficult temperament and harsh parenting differentially predict reactive and proactive aggression? J Abnorm Child Psychol. (2006) 34:685–95. doi: 10.1007/s10802-006-9055-6
53. Li DP, Zhang W, Li DL, Wang YH, Zhen SJ. The effects of parenting styles and temperament on adolescent aggression: examining unique, differential, and mediation effects. Acta Psychol Sin. (2012) 44:211–25. doi: 10.3724/SP.J.1041.2012.00211
54. Fox NA, Calkins SD. Pathways to aggression and social withdrawal: interactions among temperament, attachments, and regulation. In: Rubin KH, Asendorph JB, editors. Social Withdrawal, Inhibition, and Shyness in Childhood. New York, NY: Psychology Press (2014). p. 81–100.
55. Gardner BO, Boccaccini MT, Bitting BS, Edens JF. Personality Assessment Inventory scores as predictors of misconduct, recidivism, and violence: a meta-analytic review. Psychol Assess. (2015) 27:534–44. doi: 10.1037/pas0000065
56. Collins K, Bell R. Personality and aggression: the dissipation-rumination scale. Personal Individual Differ. (1997) 22:751–5. doi: 10.1016/S0191-8869(96)00248-6
57. Capaldi DM, Clark S. Prospective family predictors of aggression toward female partners for at-risk young men. Dev Psychol. (1998) 34:1175–88. doi: 10.1037/0012-1649.34.6.1175
58. Widom CS, Czaja S, Dutton MA. Child abuse and neglect and intimate partner violence victimization and perpetration: a prospective investigation. Child Abuse Negl. (2014) 38:650–63. doi: 10.1016/j.chiabu.2013.11.004
59. Nicholas KB, Bieber SL. Parental abusive versus supportive behaviors and their relation to hostility and aggression in young adults. Child Abuse Negl. (1996) 20:1195–211. doi: 10.1016/S0145-2134(96)00115-9
60. Baird SM, McGannon KR. Mean (ing) to me: a symbolic interactionist approach to aggression in sport psychology. Quest. (2009) 61:377–96. doi: 10.1080/00336297.2009.10483622
61. Keeler LA. The differences in sport aggression, life aggression, and life assertion among adult male and female collision, contact, and non-contact sport athletes. J Sport Behav. (2007) 30:57–76.
63. Neubuerger KT, Sinton DW, Denst J. Cerebral atrophy associated with boxing. AMA Arch Neurol Psychiatry. (1959) 81:403–8. doi: 10.1001/archneurpsyc.1959.02340160001001
65. Aseltine RH Jr, Gore S, Gordon J. Life stress, anger and anxiety, and delinquency: an empirical test of general strain theory. J Health Soc Behav. (2000) 41:256–75. doi: 10.2307/2676320
66. Davis GD, Mantler J. The consequences of financial stress for individuals, families and society. Ottawa: Carlton University; Department of Psychology; Centre for Research on Stress, Coping and Well-Being (2004).
67. Zarshenas L, Baneshi M, Sharif F, Moghimi Sarani E. Anger management in substance abuse based on cognitive behavioral therapy: an interventional study. BMC Psychiatry. (2017) 17:375. doi: 10.1186/s12888-017-1511-z
68. Reilly PM, Shopshire MS. Anger management for substance abuse and mental health clients: cognitive behavioral therapy manual. J Drug Addit Educ Eradic. (2002) 10:198–238. doi: 10.1037/e436412005-001
69. Jakupcak M, Conybeare D, Phelps L, Hunt S, Holmes HA, Felker B, et al. Anger, hostility, and aggression among Iraq and Afghanistan War veterans reporting PTSD and subthreshold PTSD. J Trauma Stress. (2007) 20:945–54. doi: 10.1002/jts.20258
70. Taft CT, Creech SK, Murphy CM. Anger and aggression in PTSD. Curr Opin Psychol. (2017) 14:67–71. doi: 10.1016/j.copsyc.2016.11.008
71. Gonzalez OI, Novaco RW, Reger MA, Gahm GA. Anger intensification with combat-related PTSD and depression comorbidity. Psychol Trauma. (2016) 8:9–16. doi: 10.1037/tra0000042
72. Painuly N, Sharan P, Mattoo SK. Relationship of anger and anger attacks with depression: a brief review. Eur Arch Psychiatry Clin Neurosci. (2005) 255:215–22. doi: 10.1007/s00406-004-0539-5
73. Painuly NP, Grover S, Gupta N, Mattoo SK. Prevalence of anger attacks in depressive and anxiety disorders: implications for their construct? Psychiatry Clin Neurosci. (2011) 65:165–74. doi: 10.1111/j.1440-1819.2010.02177.x
74. Rao V, Rosenberg P, Bertrand M, Salehinia S, Spiro J, Vaishnavi S, et al. Aggression after traumatic brain injury: prevalence and correlates. J Neuropsychiatry Clin Neurosci. (2009) 21:420–9. doi: 10.1176/jnp.2009.21.4.420
75. Cole WR, Gerring JP, Gray RM, Vasa RA, Salorio CF, Grados M, et al. Prevalence of aggressive behaviour after severe paediatric traumatic brain injury. Brain Inj. (2008) 22:932–9. doi: 10.1080/02699050802454808
76. Kim JS, Choi S, Kwon SU, Seo YS. Inability to control anger or aggression after stroke. Neurology. (2002) 58:1106–8. doi: 10.1212/WNL.58.7.1106
77. Santos CO, Caeiro L, Ferro JM, Albuquerque R, Luisa Figueira M. Anger, hostility and aggression in the first days of acute stroke. Eur J Neurol. (2006) 13:351–8. doi: 10.1111/j.1468-1331.2006.01242.x
78. Paradiso S, Robinson RG, Arndt S. Self-reported aggressive behavior in patients with stroke. J Nerv Ment Dis. (1996) 184:746–53. doi: 10.1097/00005053-199612000-00005
79. Zahodne LB, Ornstein K, Cosentino S, Devanand DP, Stern Y. Longitudinal relationships between Alzheimer disease progression and psychosis, depressed mood, and agitation/aggression. Am J Geriatr Psychiatry. (2015) 23:130–40. doi: 10.1016/j.jagp.2013.03.014
80. Ballard C, Corbett A. Agitation and aggression in people with Alzheimer's disease. Curr Opin Psychiatry. (2013) 26:252–9. doi: 10.1097/YCO.0b013e32835f414b
81. Stern RA, Daneshvar DH, Baugh CM, Seichepine DR, Montenigro PH, Riley DO, et al. Clinical presentation of chronic traumatic encephalopathy. Neurology. (2013) 81:1122–9. doi: 10.1212/WNL.0b013e3182a55f7f
82. Omalu B, Bailes J, Hamilton RL, Kamboh MI, Hammers J, Case M, et al. Emerging histomorphologic phenotypes of chronic traumatic encephalopathy in American athletes. Neurosurgery. (2011) 69:173–83; discussion: 83. doi: 10.1227/NEU.0b013e318212bc7b
83. Baugh CM, Stamm JM, Riley DO, Gavett BE, Shenton ME, Lin A, et al. Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav. (2012) 6:244–54. doi: 10.1007/s11682-012-9164-5
84. Gavett BE, Stern RA, McKee AC. Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin Sports Med. (2011) 30:179–88, xi. doi: 10.1016/j.csm.2010.09.007
85. Stern RA, Riley DO, Daneshvar DH, Nowinski CJ, Cantu RC, McKee AC. Long-term consequences of repetitive brain trauma: chronic traumatic encephalopathy. PM R. (2011) 3:S460–7. doi: 10.1016/j.pmrj.2011.08.008
86. Omalu BI, Bailes J, Hammers JL, Fitzsimmons RP. Chronic traumatic encephalopathy, suicides and parasuicides in professional American athletes: the role of the forensic pathologist. Am J Forensic Med Pathol. (2010) 31:130–2. doi: 10.1097/PAF.0b013e3181ca7f35
87. Omalu B. Chronic traumatic encephalopathy. Progress Neurol Surg. (2014) 28:38–49. doi: 10.1159/000358761
88. Goldfinger MH, Ling H, Tilley BS, Liu AKL, Davey K, Holton JL, et al. The aftermath of boxing revisited: identifying chronic traumatic encephalopathy pathology in the original Corsellis boxer series. Acta Neuropathol. (2018) 136:973–4. doi: 10.1007/s00401-018-1926-8
89. Williams DJ, Tannenberg AE. Dementia pugilistica in an alcoholic achondroplastic dwarf. Pathology. (1996) 28:102–4. doi: 10.1080/00313029600169653
90. Constantinidis J, Tissot R. Generalized Alzheimer's neurofibrillary lesions without senile plaques (Presentation of one anatomo-clinical case). Schweizer Arch Neurol Neurochir Psychiatr. (1967) 100:117–30.
91. Hof PR, Bouras C, Buee L, Delacourte A, Perl DP, Morrison JH. Differential distribution of neurofibrillary tangles in the cerebral cortex of dementia pugilistica and Alzheimer's disease cases. Acta Neuropathol. (1992) 85:23–30. doi: 10.1007/BF00304630
92. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 12-1999. A 67-year-old man with three years of dementia. N Engl J Med. (1999) 340:1269–77. doi: 10.1056/NEJM199904223401609
93. Nguyen R, Fiest KM, McChesney J, Kwon CS, Jette N, Frolkis AD, et al. The international incidence of traumatic brain injury: a systematic review and meta-analysis. Can J Neurol Sci. (2016) 43:774–85. doi: 10.1017/cjn.2016.290
Keywords: athletes, anger, depression, suicidal ideation, suicide, brain concussion, brain injuries, chronic traumatic encephalopathy
Citation: Iverson GL and Gardner AJ (2020) Risk for Misdiagnosing Chronic Traumatic Encephalopathy in Men With Anger Control Problems. Front. Neurol. 11:739. doi: 10.3389/fneur.2020.00739
Received: 06 October 2019; Accepted: 16 June 2020;
Published: 24 July 2020.
Edited by:
Denes V. Agoston, Karolinska Institutet (KI), SwedenReviewed by:
Joseph Bleiberg, National Intrepid Center of Excellence (NICoE), United StatesMatthew J. Robson, University of Cincinnati, United States
Copyright © 2020 Iverson and Gardner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Grant L. Iverson, giverson@mgh.harvard.edu
 Grant L. Iverson
Grant L. Iverson Andrew J. Gardner
Andrew J. Gardner