Effect of Enteral Immunonutrition in Patients Undergoing Surgery for Gastrointestinal Cancer: An Updated Systematic Review and Meta-Analysis
- 1The Second Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, China
- 2Department of General Surgery, HwaMei Hospital, University of Chinese Academy of Sciences, Ningbo, China
Background: The efficacy of enteral immunonutrition (EIN) in patients undergoing gastrointestinal cancer surgery remains debatable. This meta-analysis aimed to investigate the effectiveness of EIN administration in patients undergoing surgery for gastrointestinal cancer.
Methods: From January 2000 to January 2022, PubMed, EMBASE, Cochrane Library, and Web of Science were thoroughly searched for randomized controlled trials (RCTs) with EIN versus standard diet or no supplement in patients undergoing surgery for gastrointestinal cancer. Overall complications and infectious complications were the primary outcomes. The secondary results were non-infectious complications, mortality, length of hospital stay, and enteral nutrition-related complications.
Results: Thirty-five studies reporting 3,692 patients undergoing surgery for gastrointestinal cancer (including gastric cancer, colorectal cancer, esophageal cancer, periampullary cancer, or pancreatic cancer) were included. Compared with the control group, EIN group had a significantly decreased incidence of overall complications (RR = 0.79, p < 0.001). Infectious complications in patients who received EIN were considerably lower than in the control group (RR = 0.66, p < 0.001). Compared to the control group, the incidence of surgical site infection, abdominal abscess, anastomotic leakage, bacteremia, duration of systemic inflammatory response syndrome (SIRS), and duration of antibiotic therapy was significantly lower in the specific infectious complications treated with EIN. Still, there was no significant difference between the two groups with other infectious complications. Moreover, a substantial shortening in the length of hospital stay was shown in EIN group compared with the control group. Still, no significant effect of EIN was demonstrated in non-infectious complicatios and mortality. The enteral nutrition-related complications had no significant difference between two groups.
Conclusions: EIN is safe and effective in reducing overall complications, infectious complications, and hospital stay in patients undergoing gastrointestinal cancer surgery (including gastric cancer, colorectal cancer, esophageal cancer, periampullary cancer, or pancreatic cancer).
Introduction
Gastrointestinal cancers are among the most frequent tumors and a leading cause of cancer death worldwide (1). Compared to other cancer types, gastrointestinal cancer patients have higher malnutrition rates, with the risk of malnutrition reaching up to 80% (2), with a higher risk of upper gastrointestinal cancer (3). Surgery is an essential treatment for gastrointestinal tumors (4–9). Patients undergoing gastrointestinal cancer surgery are at a high risk of poor postoperative outcomes (10, 11). Preoperative malnutrition is an independent risk factor for postoperative complications following gastrointestinal surgery (12–18).
Therefore, nutritional support is essential for patients with gastrointestinal cancer, particularly those undergoing surgery. Enteral immunonutrition (EIN) with specific nutrients such as arginine, glutamine, omega-3 fatty acids, and nucleotides is typically supplemented in formulations (19). EIN can improve nutrition status and enhance immune function (20–24). Some published clinical studies suggested that perioperative EIN administration, enriched with at least two of the immunonutrition nutrients, is beneficial for reducing complications after major abdominal surgery, particularly in malnourished patients (23–27). However, not all studies could draw a similar conclusion; some suggested that EIN does not significantly reduce postoperative complications, mortality, and length of hospital stay (28–30).
There is currently no comprehensive systematic review of the efficacy of perioperative EIN administration in patients undergoing gastrointestinal cancer surgery in literature. Thus, a meta-analysis was conducted to assess the effect of EIN administration vs. control on postoperative outcomes in patients undergoing surgery for gastrointestinal cancer (including gastric cancer, colorectal cancer, esophageal cancer, periampullary cancer, or pancreatic cancer). To fully demonstrate the role of EIN, the study defined EIN as containing at least two or more nutrients, including arginine, glutamine, omega-3 fatty acids, and nucleotides.
Methods
Search Strategy
PRISMA 2020 statement: an updated guideline for reporting systematic reviews was used to conduct this systematic review and meta-analysis (31). This meta-analysis investigated was comprehensively conducted in PubMed, EMBASE, Cochrane Library, and Web of Science to search for studies published between January 2000 and January 2022, assessing the impact of EIN on postoperative outcomes, such as complications, in patients undergoing surgery for gastrointestinal cancer. The medical subject heading terms listed below were used and adjusted to meet the requirements of various databases: (immunonutrition OR immune-enhancing nutrition OR immune-enhanced nutrition OR immune-modulating nutrition OR immune nutrition OR immunological nutrition OR glutamine OR omega 3 fatty acid OR ω-3 fatty acid OR n 3 oil OR n 3 fatty acid OR n 3 polyunsaturated fatty acid OR alpha-linolenic acid OR docosahexaenoic acid OR eicosapentaenoic acid OR arginine OR nucleotides) AND (gastrointestinal neoplasm OR gastrointestinal tract cancer OR gastrointestinal cancer OR esophageal neoplasm OR esophagus neoplasm OR esophagus cancer OR esophageal cancer OR intestinal neoplasm OR intestines neoplasm OR intestines cancers OR intestinal cancer OR cecal neoplasm OR cecal cancer OR colorectal neoplasm OR colorectal tumor OR colorectal cancer OR colorectal carcinoma OR duodenal neoplasm OR duodenal cancer OR duodenum cancer OR ileal neoplasm OR ileal cancer OR jejunal neoplasm OR jejunal cancer OR jejunum cancer OR pancreatic neoplasm OR pancreas cancer OR pancreatic cancer OR stomach neoplasm OR gastric neoplasm OR gastric cancer OR stomach cancer). To avoid missing information that might be needed, limitations were not set for the type of specific complications. Relevant bibliographies of identified articles were hand-searched.
Selection and Exclusion Criteria
The “PICOS” principles were used to develop inclusion and exclusion criteria. There were no restrictions on age, gender, comorbidities, surgical method, or cancer diagnostic criteria. The studies were included if they met the following criteria: (a) participants: patients with gastrointestinal cancer and underwent surgery; (b) intervention: EIN; (c) control: standard diet (an isocaloric and isonitrogenous enteral nutrition supplement) or no supplement (a normal diet without supplements); (d) outcomes: at least one investigated postoperative outcomes, such as complications, mortality, and length of hospital stay; (e) study design: randomized controlled trials (RCTs).
Studies that met any of the following exclusion criteria were excluded: (a) study intervention contained only one component of EIN; (b) articles were not published in English; (c) the data was unavailable. If there are multiple publications from the same trial, the updated or informative article would be used. Two investigators screened titles and abstracts for potentially eligible articles and then retrieved the full text for further selection based on the selection and exclusion criteria.
Data Extraction
Two investigators extracted data from eligible RCTs independently using a predefined standardized form. Author, year, country, total size, tumor types, time of administration, duration of intervention, EIN composition, infectious complications, non-infectious complications, mortality, length of hospital stay, enteral nutrition-related adverse effects, and the like were among the information gathered. The corresponding authors of studies, or national registry databases used as a data source in the original studies, were consulted for additional information if required. Consensus and discussion were used to resolve any discrepancies.
Quality Assessment
For assessing the quality of RCTs, the Cochrane Collaboration's tool (32) was used. Random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other biases were among the domains of bias examined. Bias risk was classified into low risk, unclear risk, and high risk.
Statistical Analysis
Revman version 5.3 (the Cochrane Collaboration) was used for statistical analysis. A random-effects model was used to assess the postoperative outcomes of gastrointestinal cancer patients undergoing surgery who received EIN or a control group, considering the differences in patient baselines, tumor types, immunonutrition components, and intervention duration. The risk ratio (RR) and 95% confidence interval (95% CI) were applied to analyze dichotomous data. Concurrently, the mean difference (MD) and 95% CI were utilized for the result analysis of continuous data. A two-sided test was used to determine statistical significance, and p ≤ 0.05 indicated a statistically significant difference. The chi-squared test and I2 test were used to quantify study heterogeneity, classified as low, moderate, high, or severe, corresponding to I2 <25%, 25–50%, 50–75%, and >75% (33), respectively. Sensitivity analysis was used to investigate the impact of each study on the overall meta-analysis. The funnel plot identified potential publication bias and the specific causes of publication bias.
Results
Eligible Studies
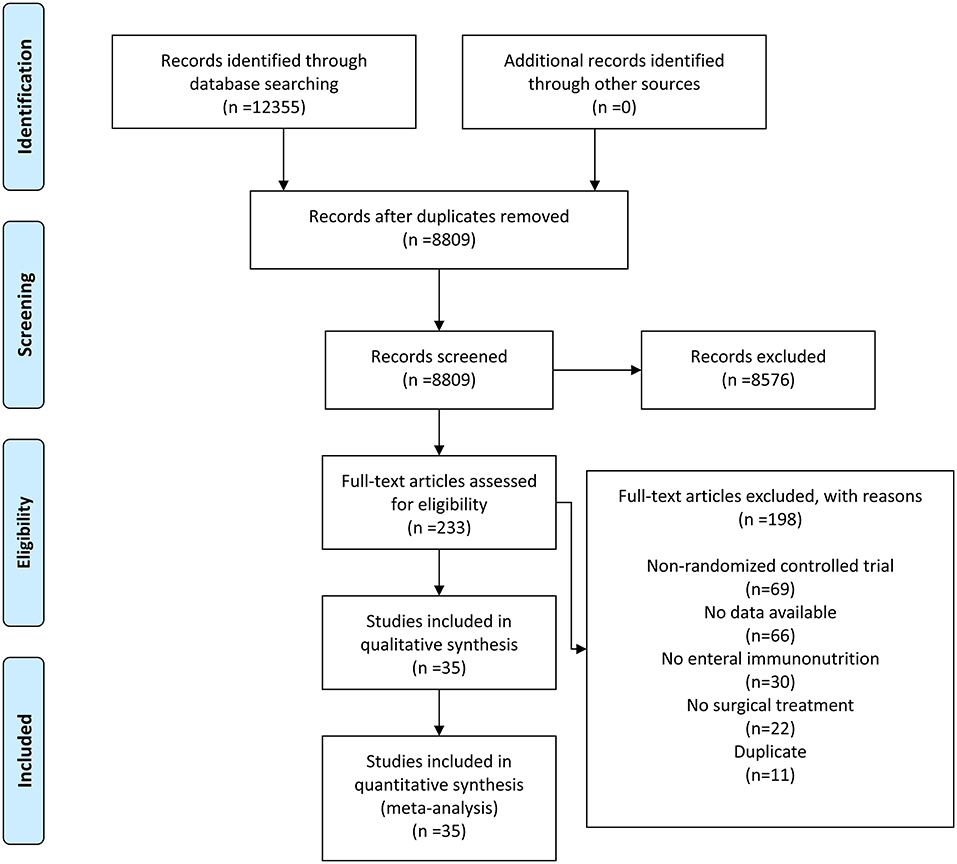
The flowchart for the search strategy is displayed in Figure 1. After excluding duplicates and irrelevant records, we identified 233 articles on EIN and gastrointestinal cancers from 12,355 records published between January 2000 and January 2022. By examining the full texts, 198 articles were excluded for non-RCT, no data available, no EIN, no surgical treatment, and duplicate, leaving 35 eligible articles for the final quantitative analysis (23, 24, 26–28, 30, 34–55).
Study Characteristics and Quality Assessment
Table 1 and Supplementary Table 1 summarize the detailed characteristics of included studies. Nine studies were carried out from Japan (25, 28, 38, 44, 48, 53, 56, 58, 59), six from Poland (26, 30, 39–41, 52), four from Italy (24, 34, 37, 49), four from China (23, 43, 46, 54), three from Spain (45, 50, 51), two from England (22, 36), two from Switzerland (35, 42), two from Turkey (27, 55), one from Denmark (57), one from Korea (47), and one from Australia (29). A total of 3,692 patients undergoing surgery for gastrointestinal cancer were included in the 35 studies. According to the intervention period, 21 preoperative groups, 11 postoperative groups, and 12 perioperative groups. According to the type of control, 26 groups were on a standard diet, and 18 were no supplement. Seven of the tumor types were gastric cancers (24, 26, 28, 43–46), seven were colorectal cancers (47–52, 59), five were esophageal cancers (25, 29, 53, 54, 58), three were periampullary cancers (including pancreatic cancer) (22, 56, 57), and others were mixed types (23, 27, 30, 34–42, 55). In addition, malnutrition rates before intervention were reported in 20 of the 35 studies, with all participants well-nourished in four studies (25, 37, 42, 48), all participants malnourished in four studies (34, 40, 41, 55), and patients in the remaining 12 studies were mixed (22, 24, 28–30, 35, 39, 45, 47, 49, 56, 58).
Overall complications and infectious complications were the primary outcome measures. Non-infectious complications, mortality, length of hospital stay, and enteral nutrition-related complications were the secondary outcome measures.
The quality of each study was appraised through the Cochrane Collaboration's tool. Supplementary Figures 1, 2 present the quality assessment of studies.
Results of Meta-Analysis
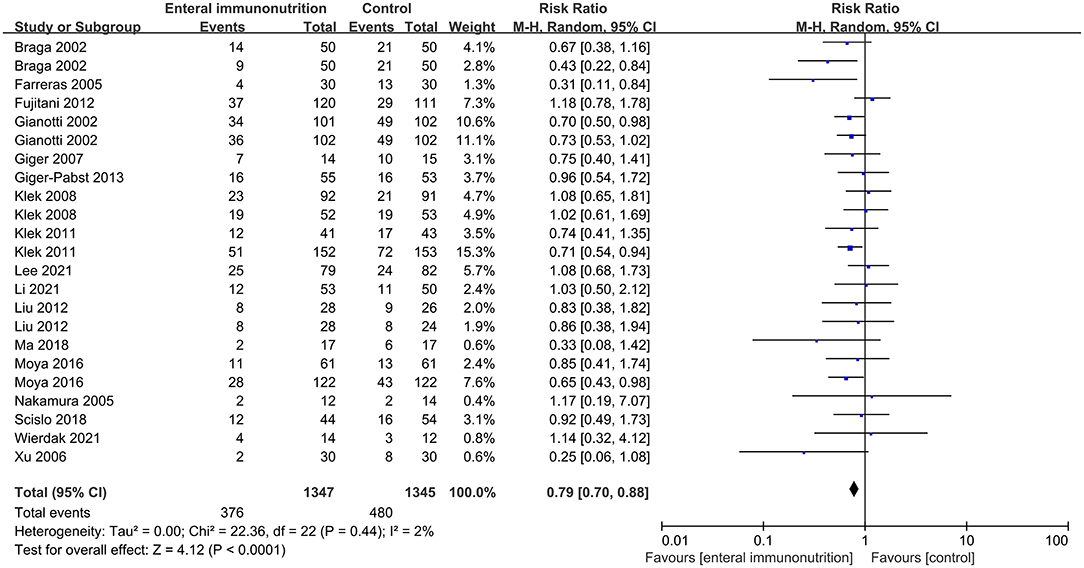
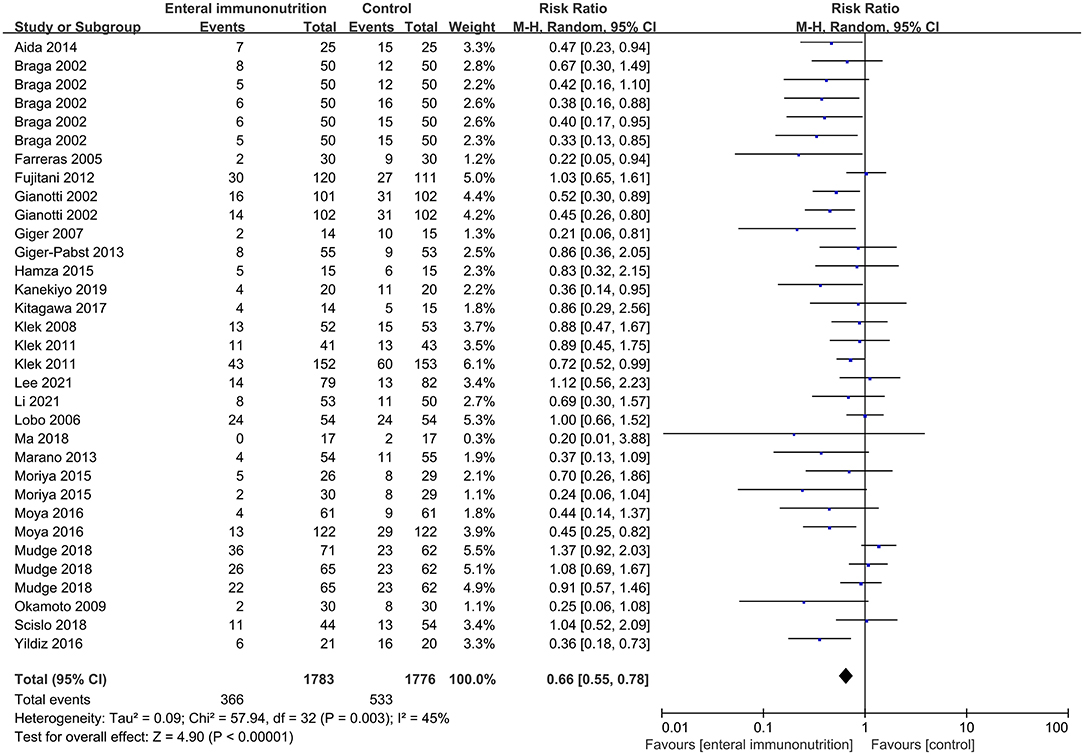
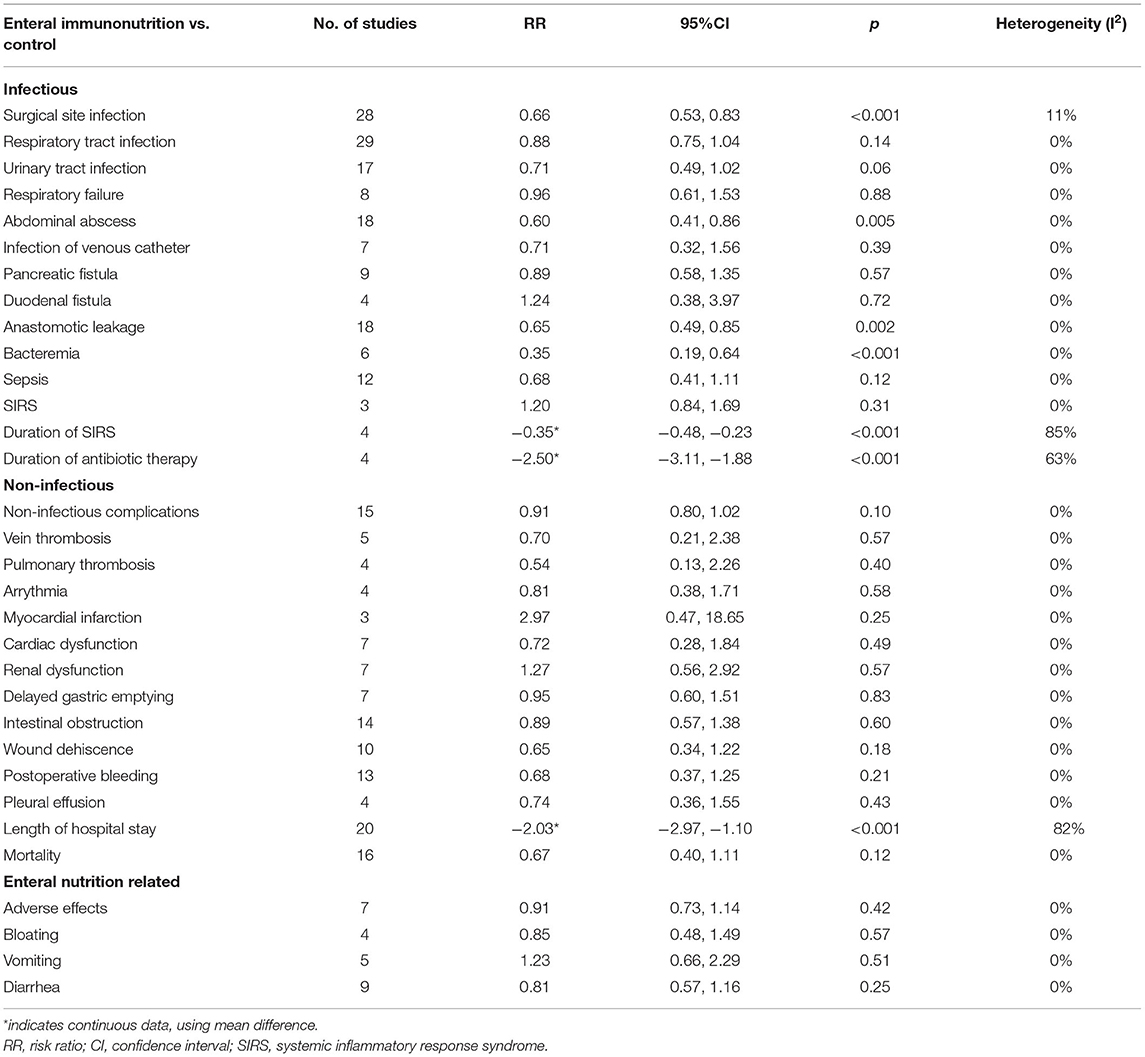
In this study, all 20 studies provided relevant data for the overall complications of 1,347 patients in EIN group vs. 1,345 patients in the control group (23, 28, 30, 34, 35, 37–43, 45, 46, 55). Compared with the control group, EIN group had a significantly decreased incidence of overall complications, and the pooled RR was 0.79 (95% CI: 0.70–0.88; p < 0.001; I2 = 2%; Figure 2). Then, the subgroup analyses of infectious, non-infectious, length of hospital stay, mortality, and enteral nutrition-related were performed. Among 26 studies that reported the data (24, 27, 30, 34–37, 40–45, 55, 59), the incidence of infectious complications was significantly lower in patients with EIN administration than in the control group, and the pooled RR was 0.66 (95% CI: 0.55–0.78; p < 0.001; I2 = 45%; Figure 3). When compared to patients in the control group, surgical site infection, abdominal abscess, anastomotic leakage, bacteremia, duration of systemic inflammatory response syndrome (SIRS), and duration of antibiotic therapy were significantly lower in the specific infectious complications treated with EIN administration. However, there was no significant difference between the two groups with other infectious complications, such as respiratory tract infection, urinary tract infection, and respiratory failure. Furthermore, when compared to the control group, EIN group had a significantly shorter length of hospital stay. Still, there was no significant effect of EIN on non-infectious complications or mortality. There was no significant difference in enteral nutrition-related complications between the two groups. Table 2 contains more specific information.
Analysis of Specific Cancer
Gastric Cancer
Supplementary Table 2 presents the results of gastric cancer. Seven articles (24, 26, 28, 43–46), including 670 patients, reported information related to gastric cancer. The incidence of overall complications, non-infectious, length of hospital stay, mortality, and enteral nutrition-related complications had no significant statistical difference between the two groups. SIRS duration was significantly reduced when compared to the control group when EIN was administered, but other infectious complications did not differ significantly between the two groups.
Colorectal Cancer
The outcomes of colorectal cancer are presented in Supplementary Table 3. The incidence of overall complications and non-infectious between the experimental and control group represented a non-significant difference. When compared to the control, EIN administration resulted in a significant reduction in the length of hospital stay. In the infectious subgroup, compared to the control, EIN administration reduced the incidence of infectious complications and surgical site infection statistically significantly, but no significant effects were seen for other infectious complications.
Esophageal Cancer
There was no significant difference in postoperative outcomes, including infectious and length of hospital stay, between EIN administration and controls in esophageal cancer patients. The details are presented in Supplementary Table 4.
Periampullary Cancer (Including Pancreatic Cancer)
The results of periampullary cancer (including pancreatic cancer) are presented in Supplementary Table 5. In the infectious subgroup, compared to the control, the incidence of infectious complications and surgical site infection was significantly lower in patients with EIN, but there was no difference between the two groups for other infectious complications. There was no significant difference in non-infectious complications between the two groups.
Analysis of Different Intervention Periods
Preoperative
Supplementary Table 6 shows the results of preoperative nutrition. In terms of overall complications, non-infectious complications, mortality, and enteral nutrition-related complications, EIN administration had no significant effect compared to the control. Preoperative EIN administration significantly reduced the incidence of infectious complications, anastomotic leakage, bacteremia, duration of SIRS, and duration of antibiotic therapy compared to the control, but other infectious complications showed no significant difference between the two groups. Moreover, the length of hospital stay was significantly shortened in the experimental group compared with the control group.
Postoperative
The outcomes of postoperative nutrition are presented in Supplementary Table 7. The incidence of overall complications was significantly lower with EIN administration compared with the control, and the pooled RR was 0.80 (95% CI: 0.66–0.96; p = 0.02; I2 = 0%). There was no significant difference between the experimental and control groups in non-infectious, length of hospital stay, mortality, or enteral nutrition-related complications. When compared to the control, the incidence of surgical site infection and bacteremia was significantly lower with postoperative EIN administration, but other infectious complications showed no significant difference between the two groups.
Perioperative
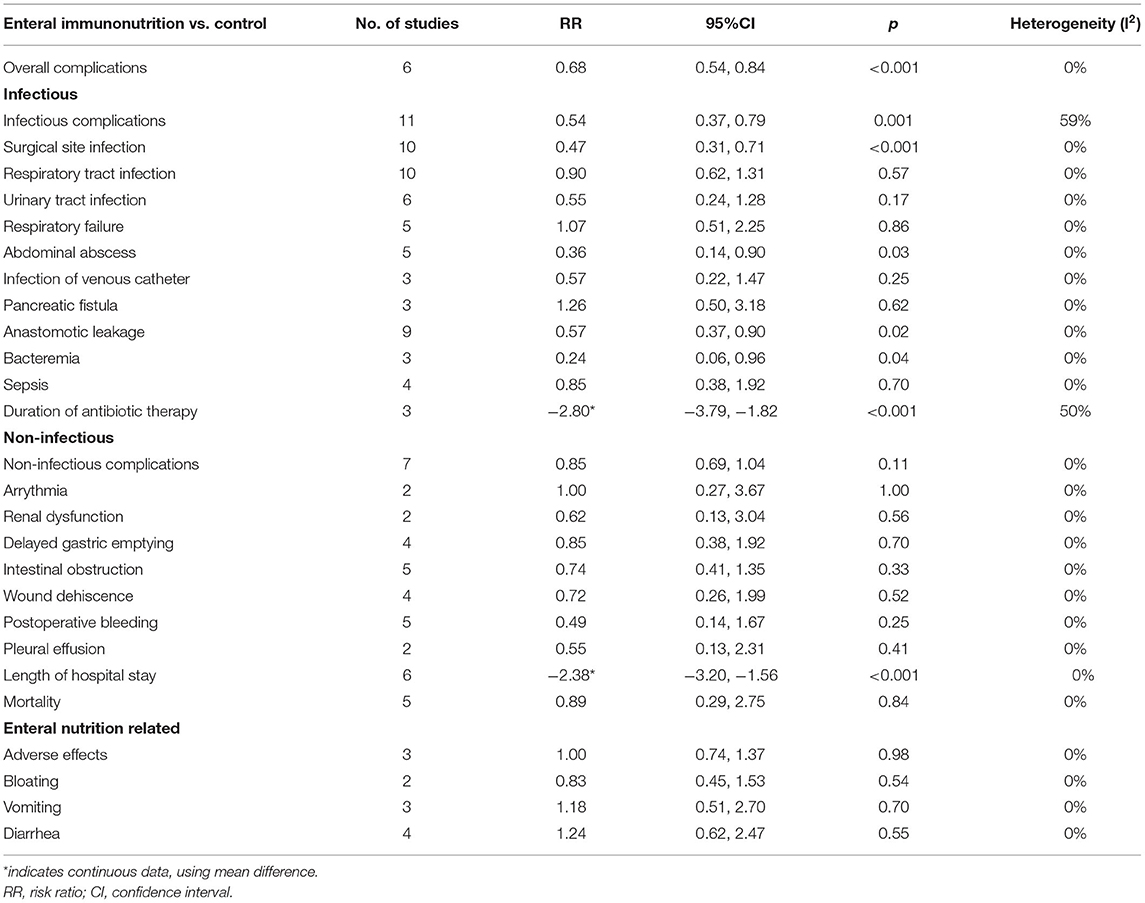
The outcomes of perioperative nutrition are presented in Table 3. The incidence of overall complications was significantly reduced with EIN administration compared with the control, and the pooled RR was 0.68 (95% CI: 0.54–0.84; p < 0.001; I2 = 0%). There was a significant reduction in the incidence of infectious complications, surgical site infection, abdominal abscess, anastomotic leakage, bacteremia, and duration of antibiotic therapy with perioperative EIN administration compared to the control group, but no significant difference was demonstrated in other infectious complications between the two groups. In comparison to the control, there was no significant effect of EIN on non-infectious, mortality or enteral nutrition-related complications, but the length of hospital stay was significantly reduced.
Analysis of Control Groups
Standard Diet
Supplementary Table 8 presents the results of the standard diet as the control. The incidence of overall complications was significantly reduced in the experimental group compared with the control group, and the pooled RR was 0.78 (95% CI: 0.66–0.92; p = 0.003; I2 = 4%). In comparison to the control, there was no significant effect of EIN on non-infectious, mortality or enteral nutrition-related complications, but the length of hospital stay was significantly reduced. In the infectious subgroup, compared to the control, the incidence of infectious complications, surgical site infection, abdominal abscess, bacteremia, and duration of SIRS was significantly lower in patients with EIN, but other infectious complications were not significantly different between the two groups.
No Supplement
Supplementary Table 9 presents the results of no supplement as the control. Compared with the control, there was a significant reduction with EIN administration in the incidence of overall complications, and the pooled RR was 0.80 (95% CI: 0.67–0.94; p = 0.009; I2 = 8%). In comparison to the control, there was no significant effect of EIN on non-infectious, mortality or enteral nutrition-related complications, but the length of hospital stay was significantly reduced. In the infectious subgroup, compared to the control, the incidence of infectious complications, surgical site infection, abdominal abscess, anastomotic leakage, bacteremia, duration of SIRS, and duration of antibiotic therapy was significantly lower in EIN patients, but other infectious complications were not significantly different between the two groups.
Analysis of Nutriture
Malnourished
Supplementary Table 10 presents the results of malnourished patients. All four studies (34, 40, 41, 55), including 572 participants, provided relevant data for malnourished patients. The incidence of overall complications in malnourished patients showed a significant reduction in EIN group vs. the control group, and the pooled RR was 0.67 (95% CI: 0.54–0.84; p < 0.001; I2 = 0%). In EIN group, there was a significant reduction in the incidence of infectious complications and bacteremia when compared to the control group, but there was no significant difference between the two groups for other infectious complications. There was no significant effect of EIN on non-infectious complications when compared to the control, but the length of hospital stay and mortality were significantly lower.
Well-Nourished
Supplementary Table 11 presents the results of well-nourished patients. All four studies (25, 37, 42, 48), including 520 participants, provided relevant data for malnourished patients. The incidence of overall complications in well-nourished patients showed a significant reduction in EIN group vs. the control group, and the pooled RR was 0.75 (95% CI: 0.60–0.93; p = 0.01; I2 = 0%). Compared with the control, no significant effect of EIN was seen for non-infectious, mortality, and enteral nutrition-related complications, but the length of hospital stay was significantly shortened. Compared with the control, there was a significant reduction in the incidence of infectious complications, surgical site infection, abdominal abscess, and anastomotic leakage in EIN group, but other infectious complications showed no significant difference between the two groups.
Sensitivity Analysis and Publication Bias
The funnel charts for the studies reporting overall complications and infectious compilations were roughly symmetrical, indicating that no studies had a significant publication bias (Supplementary Figures 3, 4). Sensitivity analysis revealed that the outcomes of all studies were consistent.
Discussion
Patients with gastrointestinal cancers often suffer from malnutrition (2, 3), which is associated with impaired cellular and humoral immune function and changes in inflammatory responses (12–18). Therefore, perioperative nutritional support is critical. However, the benefits of EIN in terms of clinical outcomes and immune markers remain debatable. Given this, we conducted a systematic review and meta-analysis. This meta-analysis included patients undergoing surgery for different gastrointestinal cancers, primarily gastric, colorectal, esophageal, periampullary (including pancreatic), and mixed types. The EIN was defined as containing at least two or more nutrients, including arginine, glutamine, omega-3 fatty acids, and nucleotides. In the included studies, most of the nutritional formulations were a combination of arginine, ω-3 fatty acids, glutamine; some were a combination of arginine, ω-3 fatty acids, glutamine; and a few were arginine, glutamine, or arginine, ω-3 fatty acids, RNA, glutamine, or arginine, ω-3 fatty acids. The duration of administration ranges from a minimum of 3 days to a maximum of 21 days. The dose of EIN also differed.
The results of this systematic review and meta-analysis showed that, in patients undergoing surgery for gastrointestinal cancer, compared with standard diet or no supplement, EIN administration effectively reduced the incidence of overall complications, infectious complications, and length of hospital stay, but not in reducing the incidence of non-infectious complications or mortality. Moreover, the incidence of enteral nutrition-related complications had no significant association with EIN administration. In infectious complications, EIN could reduce the risk of surgical site infection, abdominal abscess, anastomotic leakage, bacteremia, duration of SIRS, and duration of antibiotic therapy. However, EIN's effects on infectious complications were limited. EIN was not associated with the incidence of respiratory tract infection, urinary tract infection, respiratory failure, infection of the venous catheter, pancreatic fistula, duodenal fistula, sepsis, and SIRS.
The intestinal tract has both physiological and immune barriers (60). The physiological barrier is formed by the tight junctions between the epithelial cells and the epithelial cells (60). Gut-associated lymphoid tissue, which includes peyer patches, intraepithelial lymphocytes, and lamina propria lymphocytes, functions as an immune barrier in the intestine (60). Surgery can damage the defense mechanism, change the intestinal flora, and lead to various postoperative complications (10, 11, 61).
To a certain extent, EIN can reduce the occurrence of infectious complications. This could be because EIN boosts immune response and reduces inflammation in gastrointestinal surgery. Both Li and Chen confirmed that CD4 cell counts and the CD4/CD8 ratio were eventually higher in EIN group compared to the control group in gastric cancer patients undergoing gastrectomy (21, 62). Concurrently, TNF-α levels were significantly lower (21, 62). Additionally, specific nutrients in EIN play their respective roles in immune response and anti-infection. Arginine therapy could markedly increase intestinal IgA levels, stimulate lymphocyte function, and improve wound healing (63–65). Glutamine is essential for cellular immunity, maintaining gut barrier function, and synthesizing the endogenous antioxidant glutathione (63, 64, 66). Omega-3 fatty acids reduce responsiveness to cytokines and the systemic inflammatory response by affecting membrane phospholipids composition to produce the lipid mediators with lower bio-activity, stabilize NFkB/IkB complex, and act as agonists for peroxisomal proliferators-activated receptors (63, 64). Ribonucleic acid (RNA) can stimulate T lymphocytes' maturation and phenotypic expression (66). In short, EIN is primarily composed of arginine, glutamine, omega-3 fatty acids, and nucleotides, and it has the potential to reduce overall complications, particularly infectious complications, via several pathways.
Anastomotic leakage, one of the most severe complications in gastrointestinal surgery, is associated with a prolonged hospital stay and increased risk of morbidity and mortality (67–75). Normal anastomosis healing is divided into four stages: hemostasis, inflammation, proliferative, and remodeling. Numerous gastrointestinal aerobic and anaerobic bacteria and the role of increased loads of collagenases and matrix metalloproteinase will lead to the occurrence of infectious complications during the anastomosis healing process (71). Besides, malnutrition is a significant risk factor for developing anastomotic leakage (76–79). Thus, appropriate nutritional support is essential to prevent anastomotic leakage. Therefore, on the one hand, EIN contributes to improving the nutritional status of patients; on the other hand, EIN conduces to maintain the gut-associated lymphoid tissue function, stimulates tissue growth after infection, and thus modulates dysfunction of the intestinal barrier, promotes wound healing, and achieves the effect of reducing anastomotic leakage (63, 64, 66, 80). Yildiz et al. found that EIN reduced the incidence of anastomotic leakage undergoing gastrointestinal surgery (27). Our meta-analysis reached a consistent conclusion.
Developing surgical site infection involves many factors such as microbial characteristics, patient characteristics, and surgical characteristics (81). Surgical site infection is mainly caused by endogenous infection (81), among which anastomotic leakage is a crucial cause of surgical site infection (82). During anastomotic leakage, abscess formation and septic complications caused by intraperitoneal spillage of feculent material and considerable bowel leakage could cause the direct or hematogenous spread of the infected surgical site (82). Our study found that EIN reduced the incidence of surgical site infection, probably because EIN can maintain the number of gut-associated lymphoid tissue cells and IgA levels in the intestinal lumen, thus maintaining the intestinal immune barrier and preventing the transfer of bacteria from the intestinal tract, playing a role in fighting infection to some extent (60, 66).
The abdominal abscess may be secondary to anastomosis leaks or be caused by a distant blood spread of infection (83). For example, abdominal abscess after pancreaticoduodenectomy is likely the consequence of pancreatic fistula or leakage (84). Developing abdominal abscesses depends on bacterial contamination, the virulence of the bacteria, and the patient's resistance and defense system (83). In our study, EIN administration significantly reduced the incidence of the abdominal abscess. EIN may play a role in preserving the intestinal mucosal barrier, preventing bacteria from spreading, and boosting the immune system (66).
In patients undergoing surgery for gastrointestinal cancer, compared with a standard diet or no supplement, EIN cannot reduce the incidence of any non-infectious complications included in this study, such as pulmonary thrombosis, vein thrombosis, delayed gastric emptying, and intestinal obstruction. Although EIN can maintain some intestinal function, postoperative intestinal peristalsis is influenced by various factors. Postoperative peritoneal irritation or inflammation causes sympathetic nerve excitation, inhibiting gastrointestinal motility (85–88). In addition, the release of cytokines and other inflammatory mediators during inflammation reduces gastrointestinal motility (85–88). Another critical factor is the use of opioids. Opioids act upon μ-opioid receptors in the myenteric and submucosal neurons in the gut (85–88). These elements can cause intestinal obstruction and delayed gastric emptying. Furthermore, EIN was not linked to thrombosis. Three factors contribute to venous thrombosis: vein damage, blood stasis, and hypercoagulability (89). In the surgical setting, venous stasis is considered one of the significant triggers of thrombosis (90, 91). Prolonged operative time and general anesthesia-induced vasodilation lead to potential venous stasis, which induces pulmonary thrombosis and vein thrombosis (91). However, Zhang et al.'s meta-analysis demonstrated that perioperative EIN reduced postoperative non-infectious complications in patients undergoing gastrointestinal cancer surgery, which may be due to perioperative EIN could ameliorate splanchnic microperfusion and oxygenation and increase immune response (92).
EIN administration was not associated with an increase in the incidence of enteral nutrition-related complications, indicating that EIN was well tolerated. Our study discovered that EIN could reduce the length of hospital stay in gastrointestinal cancer patients undergoing surgery, most likely due to EIN's ability to reduce the occurrence of anastomotic leakage, surgical site infection, and other complications, which may be risk factors for length of hospital stay (93). In addition, EIN improves patients' nutrition to prevent the prolonged length of hospital stay. Nevertheless, EIN did not reduce mortality. Our results are consistent with Wong et al. Their meta-analysis demonstrated that EIN reduced the length of hospital stay but cannot reduce the incidence of mortality in patients undergoing upper gastrointestinal surgery (94). Various factors, such as characteristics of the disease, the patient's preoperative condition, operation type, and postoperative complications, are associated with mortality after gastrointestinal surgery (95, 96). In addition, EIN's anti-infection effect is also limited, so it is challenging to decrease postoperative mortality across a single measure.
EIN appeared to be more effective in patients with colorectal cancer in analyzing specific cancers. When compared to the control, EIN significantly reduced the incidence of infectious complications, surgical site infection, and length of hospital stay in colorectal cancer. Intestinal bacteria reside mainly in the lower gastrointestinal tract (97), and infectious complications of the lower gastrointestinal tract have a relatively high incidence (98, 99), so EIN may have a more significant improvement effect on postoperative infection for colorectal cancer. Moreover, the inadequate sample size in subgroups and variation in amount and duration of EIN administration could contribute to this. Further studies are required in the subgroup of specific cancers. In the analysis of the intervention period, perioperative EIN outperformed preoperative or postoperative in reducing the incidence of infections and could also shorter the length of hospital stay. This is consistent with the conclusion of Song et al. (100), which further confirmed that perioperative EIN administration is the optimum option for patients undergoing surgery for gastrointestinal cancer. When compared to the standard diet in the control group, EIN was more effective in reducing the incidence of postoperative complications when no supplement was used, implying the importance of nutrition supplements. When specific nutritional conditions were examined, EIN was found to reduce overall complications, some infectious complications, and length of hospital stay in well-nourished and malnourished patients compared to controls. It is worth noting that the mortality was significantly decreased in malnourished groups with EIN administration, which seems that EIN was more efficient for malnourished patients. Due to malnutrition being a significant risk factor for postoperative complications (101–103), EIN can significantly improve postoperative complications by improving the nutritional status of malnourished patients. The likely reason is that EIN helps malnourished patients reduce inflammation, accelerate wound healing, prevent severe complications, and thus reduce mortality (60). Nevertheless, most studies have failed to prove that EIN reduces mortality in surgical patients (94, 104, 105). Regrettably, we did not have sufficient data for further analysis of the effect of EIN in the malnourished and well-nourished group for postoperative complications. As a result, the impact of EIN on mortality remains to be further studied, and more randomized trials are warranted to focus on the effect of EIN on postoperative complications in people with different nutritional statuses.
Strengths and Limitations
There are several limitations to the current systematic analysis that should be considered. First, this study includes unavoidable heterogeneity, such as variations in operation, disease severity, duration of intervention, and definition of complications. Second, some subgroup analyses used small sample sizes, which reduced the credibility of the results. Furthermore, some problems remain to be solved, such as the best formula, ratio, and amount of EIN and the influence of EIN on postoperative outcomes of patients with different types of gastrointestinal tumors. This systematic review and meta-analysis, on the other hand, thoroughly examined the effect of EIN on postoperative outcomes in patients undergoing surgery for gastrointestinal cancers, including subgroup analysis of specific tumor types, EIN administration period, control group type, and patient nutrition.
Conclusion
According to this systematic review and meta-analysis, EIN is safe and beneficial for reducing overall complications, infectious complications, and length of hospital stay, but it has no efficacy for reducing non-infectious complications in patients undergoing surgery for gastrointestinal cancer (including gastric cancer, colorectal cancer, esophageal cancer, periampullary cancer, or pancreatic cancer). In terms of infectious complications, EIN primarily minimizes the incidence of surgical site infection, abdominal abscess, anastomotic leakage, bacteremia, SIRS duration, and antibiotic therapy duration. Therefore, perioperative EIN administration is recommended for malnourished patients undergoing surgery for gastrointestinal cancer, especially for patients with colorectal cancer. Overall, more well-designed and large-scale RCTs are required to clarify the unanswered questions and further evaluate the effect of EIN in patients undergoing gastrointestinal cancer surgery to provide reasonable theoretical guidelines for clinical practice.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
JC designed the research process. JS and SD searched the database for corresponding articles and drafted the meta-analysis. ZL and WD extracted useful information from the articles above. JHo used statistical software for analysis. JHu polished this article. All authors had read and approved the manuscript and ensured that this was the case.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.941975/full#supplementary-material
Supplementary Figure 1. Risk of bias summary of studies included.
Supplementary Figure 2. Risk of bias graph of studies included.
Supplementary Figure 3. Funnel plot of meta-analysis of overall complications.
Supplementary Figure 4. Funnel plot of meta-analysis of infectious complications.
Supplementary Table 1. Characteristics of all studies included in the meta-analysis.
Supplementary Table 2. Analysis of gastric cancer outcomes.
Supplementary Table 3. Analysis of colorectal cancer outcomes.
Supplementary Table 4. Analysis of esophageal cancer outcomes.
Supplementary Table 5. Analysis of periampullary cancer (including pancreatic cancer) outcomes.
Supplementary Table 6. Analysis of preoperative nutrition outcomes.
Supplementary Table 7. Analysis of postoperative nutrition outcomes.
Supplementary Table 8. Analysis of standard diet as control groups.
Supplementary Table 9. Analysis of no supplement as control groups.
Supplementary Table 10. Analysis of malnourished patients outcomes.
Supplementary Table 11. Analysis of well-nourished patients outcomes.
Abbreviations
EIN, enteral immunonutrition; RCT, randomized controlled trial; RR, risk ratio; CI, confidence interval; MD, mean difference; SIRS, systematic inflammatory response syndrome; RNA, ribonucleic acid.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Garla P, Waitzberg DL, Tesser A. Nutritional therapy in gastrointestinal cancers. Gastroenterol Clin North Am. (2018) 47:231–42. doi: 10.1016/j.gtc.2017.09.009
3. Shaw C. Management of diet in gastrointestinal cancer. Proc Nutr Soc. (2021) 80:65–72. doi: 10.1017/S0029665120007041
4. Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. (2021) 24:1–21. doi: 10.1007/s10120-020-01042-y
5. Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun. (2021) 41:747–95. doi: 10.1002/cac2.12193
6. Wang FH, Shen L, Li J, Zhou ZW, Liang H, Zhang XT, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun. (2019) 39:10. doi: 10.1186/s40880-019-0349-9
7. Stahl M. Is there any role for surgery in the multidisciplinary treatment of esophageal cancer? Ann Oncol. (2010) 21 (Suppl. 7):vii283–5. doi: 10.1093/annonc/mdq294
8. Khorana AA, Mangu PB, Berlin J, Engebretson A, Hong TS, Maitra A, et al. Potentially curable pancreatic cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. (2016) 34:2541–56. doi: 10.1200/JCO.2016.67.5553
9. Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. (2018) 23:1–34. doi: 10.1007/s10147-017-1101-6
10. Szakmany T, Ditai J, Kirov M, Protsenko D, Osinaike B, Venara A, et al. In-hospital clinical outcomes after upper gastrointestinal surgery: data from an international observational study. Eur J Surg Oncol. (2017) 43:2324–32. doi: 10.1016/j.ejso.2017.08.002
11. Jakobson T, Karjagin J, Vipp L, Padar M, Parik AH, Starkopf L, et al. Postoperative complications and mortality after major gastrointestinal surgery. Medicina. (2014) 50:111–7. doi: 10.1016/j.medici.2014.06.002
12. Hennessey DB, Burke JP, Ni-Dhonochu T, Shields C, Winter DC, Mealy K. Preoperative hypoalbuminemia is an independent risk factor for the development of surgical site infection following gastrointestinal surgery: a multi-institutional study. Ann Surg. (2010) 252:325–9. doi: 10.1097/SLA.0b013e3181e9819a
13. Mosquera C, Koutlas NJ, Edwards KC, Strickland A, Vohra NA, Zervos EE, et al. Impact of malnutrition on gastrointestinal surgical patients. J Surg Res. (2016) 205:95–101. doi: 10.1016/j.jss.2016.05.030
14. Wang X, Naito Y, Nakatani H, Ida M, Kawaguchi M. Prevalence of undernutrition in surgical patients and the effect on length of hospital stay. J Anesth. (2022) 36:89–95. doi: 10.1007/s00540-021-03013-8
15. Ho JW, Wu AH, Lee MW, Lau SY, Lam PS, Lau WS, et al. Malnutrition risk predicts surgical outcomes in patients undergoing gastrointestinal operations: results of a prospective study. Clin Nutr. (2015) 34:679–84. doi: 10.1016/j.clnu.2014.07.012
16. Seretis C, Kaisari P, Wanigasooriya K, Shariff U, Youssef H. Malnutrition is associated with adverse postoperative outcome in patients undergoing elective colorectal cancer resections. J Buon. (2018) 23:36–41.
17. Hu WH, Cajas-Monson LC, Eisenstein S, Parry L, Cosman B, Ramamoorthy S. Preoperative malnutrition assessments as predictors of postoperative mortality and morbidity in colorectal cancer: an analysis of ACS-NSQIP. Nutr J. (2015) 14:91. doi: 10.1186/s12937-015-0081-5
18. Lohsiriwat V. The influence of preoperative nutritional status on the outcomes of an enhanced recovery after surgery (ERAS) programme for colorectal cancer surgery. Tech Coloproctol. (2014) 18:1075–80. doi: 10.1007/s10151-014-1210-4
19. Mariette C. Immunonutrition. J Visceral Surg. (2015) 152:S14–S7. doi: 10.1016/S1878-7886(15)30005-9
20. Luo Z, Wang J, Zhang Z, Li H, Huang L, Qiao Y, et al. Efficacy of early enteral immunonutrition on immune function and clinical outcome for postoperative patients with gastrointestinal cancer. J Parenter Enteral Nutr. (2018) 42:758–65. doi: 10.1177/0148607117715439
21. Li K, Xu Y, Hu Y, Liu Y, Chen X, Zhou Y. Effect of enteral immunonutrition on immune, inflammatory markers and nutritional status in gastric cancer patients undergoing gastrectomy: a randomized double-blinded controlled trial. J Invest Surg. (2020) 33:950–9. doi: 10.1080/08941939.2019.1569736
22. Hamza N, Darwish A, O'Reilly DA, Denton J, Sheen AJ, Chang D, et al. Perioperative enteral immunonutrition modulates systemic and mucosal immunity and the inflammatory response in patients with periampullary cancer scheduled for pancreaticoduodenectomy: a randomized clinical trial. Pancreas. (2015) 44:41–52. doi: 10.1097/MPA.0000000000000222
23. Xu J, Zhong Y, Jing D, Wu Z. Preoperative enteral immunonutrition improves postoperative outcome in patients with gastrointestinal cancer. World J Surg. (2006) 30:1284–9. doi: 10.1007/s00268-005-0756-8
24. Marano L, Porfidia R, Pezzella M, Grassia M, Petrillo M, Esposito G, et al. Clinical and immunological impact of early postoperative enteral immunonutrition after total gastrectomy in gastric cancer patients: a prospective randomized study. Ann Surg Oncol. (2013) 20:3912–8. doi: 10.1245/s10434-013-3088-1
25. Kanekiyo S, Takeda S, Iida M, Nishiyama M, Kitahara M, Shindo Y, et al. Efficacy of perioperative immunonutrition in esophageal cancer patients undergoing esophagectomy. Nutrition. (2019) 59:96–102. doi: 10.1016/j.nut.2018.08.006
26. Scislo L, Pach R, Nowak A, Walewska E, Gadek M, Brandt P, et al. The impact of postoperative enteral immunonutrition on postoperative complications and survival in gastric cancer patients - randomized clinical trial. Nutr Cancer. (2018) 70:453–9. doi: 10.1080/01635581.2018.1445770
27. Yildiz SY, Yazicioglu MB, Tiryaki C, Ciftci A, Boyacioglu Z. The effect of enteral immunonutrition in upper gastrointestinalsurgery for cancer: a prospective study. Turk J Med Sci. (2016) 46:393–400. doi: 10.3906/sag-1411-102
28. Fujitani K, Tsujinaka T, Fujita J, Miyashiro I, Imamura H, Kimura Y, et al. Prospective randomized trial of preoperative enteral immunonutrition followed by elective total gastrectomy for gastric cancer. Br J Surg. (2012) 99:621–9. doi: 10.1002/bjs.8706
29. Mudge LA, Watson DI, Smithers BM, Isenring EA, Smith L, Jamieson GG, et al. Multicentre factorial randomized clinical trial of perioperative immunonutrition versus standard nutrition for patients undergoing surgical resection of oesophageal cancer. Br J Surg. (2018) 105:1262–72. doi: 10.1002/bjs.10923
30. Klek S, Kulig J, Sierzega M, Szybinski P, Szczepanek K, Kubisz A, et al. The impact of immunostimulating nutrition on infectious complications after upper gastrointestinal surgery: a prospective, randomized, clinical trial. Ann Surg. (2008) 248:212–20. doi: 10.1097/SLA.0b013e318180a3c1
31. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
32. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
33. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
34. Braga M, Gianotti L, Nespoli L, Radaelli G, Di Carlo V. Nutritional approach in malnourished surgical patients: a prospective randomized study. Arch Surg. (2002) 137:174–80. doi: 10.1001/archsurg.137.2.174
35. Giger U, Buchler M, Farhadi J, Berger D, Husler J, Schneider H, et al. Preoperative immunonutrition suppresses perioperative inflammatory response in patients with major abdominal surgery-a randomized controlled pilot study. Ann Surg Oncol. (2007) 14:2798–806. doi: 10.1245/s10434-007-9407-7
36. Lobo DN, Williams RN, Welch NT, Aloysius MM, Nunes QM, Padmanabhan J, et al. Early postoperative jejunostomy feeding with an immune modulating diet in patients undergoing resectional surgery for upper gastrointestinal cancer: a prospective, randomized, controlled, double-blind study. Clin Nutr. (2006) 25:716–26. doi: 10.1016/j.clnu.2006.04.007
37. Gianotti L, Braga M, Nespoli L, Radaelli G, Beneduce A, Di Carlo V. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology. (2002) 122:1763–70. doi: 10.1053/gast.2002.33587
38. Nakamura K, Kariyazono H, Komokata T, Hamada N, Sakata R, Yamada K. Influence of preoperative administration of omega-3 fatty acid-enriched supplement on inflammatory and immune responses in patients undergoing major surgery for cancer. Nutrition. (2005) 21:639–49. doi: 10.1016/j.nut.2005.03.001
39. Klek S, Kulig J, Sierzega M, Szczepanek K, Szybinski P, Scislo L, et al. Standard and immunomodulating enteral nutrition in patients after extended gastrointestinal surgery–a prospective, randomized, controlled clinical trial. Clin Nutr. (2008) 27:504–12. doi: 10.1016/j.clnu.2008.04.010
40. Klek S, Sierzega M, Szybinski P, Szczepanek K, Scislo L, Walewska E, et al. The immunomodulating enteral nutrition in malnourished surgical patients – a prospective, randomized, double-blind clinical trial. Clin Nutr. (2011) 30:282–8. doi: 10.1016/j.clnu.2010.10.001
41. Klek S, Sierzega M, Szybinski P, Szczepanek K, Scislo L, Walewska E, et al. Perioperative nutrition in malnourished surgical cancer patients – a prospective, randomized, controlled clinical trial. Clin Nutr. (2011) 30:708–13. doi: 10.1016/j.clnu.2011.07.007
42. Giger-Pabst U, Lange J, Maurer C, Bucher C, Schreiber V, Schlumpf R, et al. Short-term preoperative supplementation of an immunoenriched diet does not improve clinical outcome in well-nourished patients undergoing abdominal cancer surgery. Nutrition. (2013) 29:724–9. doi: 10.1016/j.nut.2012.10.007
43. Ma C, Tsai H, Su W, Sun L, Shih Y, Wang J. Combination of arginine, glutamine, and omega-3 fatty acid supplements for perioperative enteral nutrition in surgical patients with gastric adenocarcinoma or gastrointestinal stromal tumor (GIST): a prospective, randomized, double-blind study. J Postgrad Med. (2018) 64:155–63. doi: 10.4103/jpgm.JPGM_693_17
44. Okamoto Y, Okano K, Izuishi K, Usuki H, Wakabayashi H, Suzuki Y. Attenuation of the systemic inflammatory response and infectious complications after gastrectomy with preoperative oral arginine and omega-3 fatty acids supplemented immunonutrition. World J Surg. (2009) 33:1815–21. doi: 10.1007/s00268-009-0140-1
45. Farreras N, Artigas V, Cardona D, Rius X, Trias M, Gonzalez JA. Effect of early postoperative enteral immunonutrition on wound healing in patients undergoing surgery for gastric cancer. Clin Nutr. (2005) 24:55–65. doi: 10.1016/j.clnu.2004.07.002
46. Liu H, Ling W, Shen ZY, Jin X, Cao H. Clinical application of immune-enhanced enteral nutrition in patients with advanced gastric cancer after total gastrectomy. J Dig Dis. (2012) 13:401–6. doi: 10.1111/j.1751-2980.2012.00596.x
47. Lee SY, Lee J, Park HM, Kim CH, Kim HR. Impact of preoperative immunonutrition on the outcomes of colon cancer surgery: results from a randomized controlled trial. Ann Surg. (2021). doi: 10.1097/SLA.0000000000005140
48. Horie H, Okada M, Kojima M, Nagai H. Favorable effects of preoperative enteral immunonutrition on a surgical site infection in patients with colorectal cancer without malnutrition. Surg Today. (2006) 36:1063–8. doi: 10.1007/s00595-006-3320-8
49. Braga M, Gianotti L, Vignali A, Carlo VD. Preoperative oral arginine and n-3 fatty acid supplementation improves the immunometabolic host response and outcome after colorectal resection for cancer. Surgery. (2002) 132:805–14. doi: 10.1067/msy.2002.128350
50. Moya P, Miranda E, Soriano-Irigaray L, Arroyo A, Aguilar MD, Bellon M, et al. Perioperative immunonutrition in normo-nourished patients undergoing laparoscopic colorectal resection. Surg Endosc. (2016) 30:4946–53. doi: 10.1007/s00464-016-4836-7
51. Moya P, Soriano-Irigaray L, Ramirez JM, Garcea A, Blasco O, Blanco FJ, et al. Perioperative standard oral nutrition supplements versus immunonutrition in patients undergoing colorectal resection in an enhanced recovery (ERAS) protocol: a multicenter randomized clinical trial (SONVI study). Medicine. (2016) 95:e3704. doi: 10.1097/MD.0000000000003704
52. Wierdak M, Surmiak M, Milian-Ciesielska K, Rubinkiewicz M, Rzepa A, Wysocki M, et al. Immunonutrition changes inflammatory response in colorectal cancer: results from a pilot randomized clinical trial. Cancers. (2021) 13:1444. doi: 10.3390/cancers13061444
53. Sakurai Y, Masui T, Yoshida I, Tonomura S, Shoji M, Nakamura Y, et al. Randomized clinical trial of the effects of perioperative use of immune-enhancing enteral formula on metabolic and immunological status in patients undergoing esophagectomy. World J Surg. (2007) 31:2150–7. doi: 10.1007/s00268-007-9170-8
54. Li XK, Cong ZZ, Wu WJ, Xu Y, Zhou H, Wang GM, et al. Enteral immunonutrition versus enteral nutrition for patients undergoing esophagectomy: a randomized controlled trial. Ann Palliat Med. (2021) 10:1351–61. doi: 10.21037/apm-20-1399
55. Gunerhan Y, Koksal N, Sahin UY, Uzun MA, Ekşioglu-Demiralp E. Effect of preoperative immunonutrition and other nutrition models on cellular immune parameters. World J Gastroenterol. (2009) 15:467–72. doi: 10.3748/wjg.15.467
56. Aida T, Furukawa K, Suzuki D, Shimizu H, Yoshidome H, Ohtsuka M, et al. Preoperative immunonutrition decreases postoperative complications by modulating prostaglandin E2 production and T-cell differentiation in patients undergoing pancreatoduodenectomy. Surgery. (2014) 155:124–33. doi: 10.1016/j.surg.2013.05.040
57. Gade J, Levring T, Hillingso J, Hansen CP, Andersen JR. The effect of preoperative oral immunonutrition on complications and length of hospital stay after elective surgery for pancreatic cancer–a randomized controlled trial. Nutr Cancer. (2016) 68:225–33. doi: 10.1080/01635581.2016.1142586
58. Kitagawa H, Namikawa T, Yatabe T, Munekage M, Yamasaki F, Kobayashi M, et al. Effects of a preoperative immune-modulating diet in patients with esophageal cancer: a prospective parallel group randomized study. Langenbecks Arch Surg. (2017) 402:531–8. doi: 10.1007/s00423-016-1538-5
59. Moriya T, Fukatsu K, Ueno C, Hashiguchi Y, Maeshima Y, Omata J, et al. Effects of preoperative use of an immune-enhancing diet on postoperative complications and long-term outcome: a randomized clinical trial in colorectal cancer surgery in Japanese patients. Gastroenterol Hepatol. (2015) 2:1–8. doi: 10.3968/5459
60. Fukatsu K. Role of nutrition in gastroenterological surgery. Ann Gastroenterol Surg. (2019) 3:160–8. doi: 10.1002/ags3.12237
61. Guyton K, Alverdy JC. The gut microbiota and gastrointestinal surgery. Nat Rev Gastroenterol Hepatol. (2017) 14:43–54. doi: 10.1038/nrgastro.2016.139
62. Chen DW, Wei Fei Z, Zhang YC, Ou JM, Xu J. Role of enteral immunonutrition in patients with gastric carcinoma undergoing major surgery. Asian J Surg. (2005) 28:121–4. doi: 10.1016/S1015-9584(09)60275-X
63. Grimble RF. Immunonutrition. Curr Opin Gastroenterol. (2005) 21:216–22. doi: 10.1097/01.mog.0000153360.90653.82
64. Grimm H, Kraus A. Immunonutrition–supplementary amino acids and fatty acids ameliorate immune deficiency in critically ill patients. Langenbecks Arch Surg. (2001) 386:369–76. doi: 10.1007/s004230100241
65. Fan J, Meng Q, Guo G, Xie Y, Li X, Xiu Y, et al. Effects of early enteral nutrition supplemented with arginine on intestinal mucosal immunity in severely burned mice. Clin Nutr. (2010) 29:124–30. doi: 10.1016/j.clnu.2009.07.005
66. McClave SA, Lowen CC, Snider HL. Immunonutrition and enteral hyperalimentation of critically ill patients. Dig Dis Sci. (1992) 37:1153–61. doi: 10.1007/BF01296554
67. Fabbi M, Hagens ERC, van Berge Henegouwen MI, Gisbertz SS. Anastomotic leakage after esophagectomy for esophageal cancer: definitions, diagnostics, and treatment. Dis Esophagus. (2021) 34:doaa039. doi: 10.1093/dote/doaa039
68. Markar SR, Arya S, Karthikesalingam A, Hanna GB. Technical factors that affect anastomotic integrity following esophagectomy: systematic review and meta-analysis. Ann Surg Oncol. (2013) 20:4274–81. doi: 10.1245/s10434-013-3189-x
69. Morse BC, Simpson JP, Jones YR, Johnson BL, Knott BM, Kotrady JA. Determination of independent predictive factors for anastomotic leak: analysis of 682 intestinal anastomoses. Am J Surg. (2013) 206:950–5. doi: 10.1016/j.amjsurg.2013.07.017
70. Girard E, Messager M, Sauvanet A, Benoist S, Piessen G, Mabrut JY, et al. Anastomotic leakage after gastrointestinal surgery: diagnosis and management. J Visc Surg. (2014) 151:441–50. doi: 10.1016/j.jviscsurg.2014.10.004
71. Chadi SA, Fingerhut A, Berho M, DeMeester SR, Fleshman JW, Hyman NH, et al. Emerging trends in the etiology, prevention, and treatment of gastrointestinal anastomotic leakage. J Gastrointest Surg. (2016) 20:2035–51. doi: 10.1007/s11605-016-3255-3
72. Sprenger T, Beissbarth T, Sauer R, Tschmelitsch J, Fietkau R, Liersch T, et al. Long-term prognostic impact of surgical complications in the German Rectal Cancer Trial CAO/ARO/AIO-94. Br J Surg. (2018) 105:1510–8. doi: 10.1002/bjs.10877
73. Lawler J, Choynowski M, Bailey K, Bucholc M, Johnston A, Sugrue M. Meta-analysis of the impact of postoperative infective complications on oncological outcomes in colorectal cancer surgery. BJS Open. (2020) 4:737–47. doi: 10.1002/bjs5.50302
74. Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg. (2011) 253:890–9. doi: 10.1097/SLA.0b013e3182128929
75. Ha GW, Kim JH, Lee MR. Oncologic impact of anastomotic leakage following colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg Oncol. (2017) 24:3289–99. doi: 10.1245/s10434-017-5881-8
76. Parthasarathy M, Greensmith M, Bowers D, Groot-Wassink T. Risk factors for anastomotic leakage after colorectal resection: a retrospective analysis of 17 518 patients. Colorectal Dis. (2017) 19:288–98. doi: 10.1111/codi.13476
77. Kingham TP, Pachter HL. Colonic anastomotic leak: risk factors, diagnosis, and treatment. J Am Coll Surg. (2009) 208:269–78. doi: 10.1016/j.jamcollsurg.2008.10.015
78. Suragul W, Rungsakulkij N, Vassanasiri W, Tangtawee P, Muangkaew P, Mingphruedhi S, et al. Predictors of surgical site infection after pancreaticoduodenectomy. BMC Gastroenterol. (2020) 20:201. doi: 10.1186/s12876-020-01350-8
79. Sugiura T, Uesaka K, Ohmagari N, Kanemoto H, Mizuno T. Risk factor of surgical site infection after pancreaticoduodenectomy. World J Surg. (2012) 36:2888–94. doi: 10.1007/s00268-012-1742-6
80. Jablonska B, Mrowiec S. The role of immunonutrition in patients undergoing pancreaticoduodenectomy. Nutrients. (2020) 12:2547. doi: 10.3390/nu12092547
81. Anderson DJ. Surgical site infections. Infect Dis Clin North Am. (2011) 25:135–53. doi: 10.1016/j.idc.2010.11.004
82. Poon JT, Law WL, Wong IW, Ching PT, Wong LM, Fan JK, et al. Impact of laparoscopic colorectal resection on surgical site infection. Ann Surg. (2009) 249:77–81. doi: 10.1097/SLA.0b013e31819279e3
83. Akcam FZ, Ceylan T, Kaya O, Ceylan E, Tarhan OR. Etiology, treatment options and prognosis of abdominal abscesses: a tertiary hospital experience. J Infect Dev Ctries. (2020) 14:59–65. doi: 10.3855/jidc.11277
84. Schulick RD. Complications after pancreaticoduodenectomy: intraabdominal abscess. J Hepatobiliary Pancreat Surg. (2008) 15:252–6. doi: 10.1007/s00534-007-1302-x
85. Kehlet H, Holte K. Review of postoperative ileus. Am J Surg. (2001) 182 (5A Suppl.):3S−10S. doi: 10.1016/S0002-9610(01)00781-4
86. Mattei P, Rombeau JL. Review of the pathophysiology and management of postoperative ileus. World J Surg. (2006) 30:1382–91. doi: 10.1007/s00268-005-0613-9
87. Wattchow D, Heitmann P, Smolilo D, Spencer NJ, Parker D, Hibberd T, et al. Postoperative ileus-An ongoing conundrum. Neurogastroenterol Motil. (2021) 33:e14046. doi: 10.1111/nmo.14046
88. Boeckxstaens GE, de Jonge WJ. Neuroimmune mechanisms in postoperative ileus. Gut. (2009) 58:1300–11. doi: 10.1136/gut.2008.169250
89. Mannucci PM, Poller L. Venous thrombosis and anticoagulant therapy. Br J Haematol. (2001) 114:258–70. doi: 10.1046/j.1365-2141.2001.02961.x
90. Brill A. Multiple facets of venous thrombosis. Int J Mol Sci. (2021) 22:3853. doi: 10.3390/ijms22083853
91. Osaki T, Saito H, Fukumoto Y, Kono Y, Murakami Y, Shishido Y, et al. Risk and incidence of perioperative deep vein thrombosis in patients undergoing gastric cancer surgery. Surg Today. (2018) 48:525–33. doi: 10.1007/s00595-017-1617-4
92. Zhang Y, Gu Y, Guo T, Li Y, Cai H. Perioperative immunonutrition for gastrointestinal cancer: a systematic review of randomized controlled trials. Surg Oncol. (2012) 21:e87–95. doi: 10.1016/j.suronc.2012.01.002
93. Mujagic E, Marti WR, Coslovsky M, Soysal SD, Mechera R, von Strauss M, et al. Associations of hospital length of stay with surgical site infections. World J Surg. (2018) 42:3888–96. doi: 10.1007/s00268-018-4733-4
94. Wong CS, Aly EH. The effects of enteral immunonutrition in upper gastrointestinal surgery: a systematic review and meta-analysis. Int J Surg. (2016) 29:137–50. doi: 10.1016/j.ijsu.2016.03.043
95. Sorensen LT, Malaki A, Wille-Jorgensen P, Kallehave F, Kjaergaard J, Hemmingsen U, et al. Risk factors for mortality and postoperative complications after gastrointestinal surgery. J Gastrointest Surg. (2007) 11:903–10. doi: 10.1007/s11605-007-0165-4
96. Zhuo ZG, Luo J, Song H, Alai GH, Shen X, Lin YD. Is immunonutrition superior to standard enteral nutrition in reducing postoperative complications in patients undergoing esophagectomy? A meta-analysis of randomized controlled trials. J Buon. (2021) 26:204–10.
97. Husebye E. The pathogenesis of gastrointestinal bacterial overgrowth. Chemotherapy. (2005) 51 (Suppl.) 1:1–22. doi: 10.1159/000081988
98. Lam A, Fleischer B, Alverdy J. The biology of anastomotic healing-the unknown overwhelms the known. J Gastrointest Surg. (2020) 24:2160–6. doi: 10.1007/s11605-020-04680-w
99. Sorensen LT, Hemmingsen U, Kallehave F, Wille-Jorgensen P, Kjaergaard J, Moller LN, et al. Risk factors for tissue and wound complications in gastrointestinal surgery. Ann Surg. (2005) 241:654–8. doi: 10.1097/01.sla.0000157131.84130.12
100. Song GM, Tian X, Zhang L, Ou YX, Yi LJ, Shuai T, et al. Immunonutrition support for patients undergoing surgery for gastrointestinal malignancy: preoperative, postoperative, or perioperative? A Bayesian network meta-analysis of randomized controlled trials. Medicine. (2015) 94:e1225. doi: 10.1097/MD.0000000000001225
101. Lobo DN, Gianotti L, Adiamah A, Barazzoni R, Deutz NEP, Dhatariya K, et al. Perioperative nutrition: recommendations from the ESPEN expert group. Clin Nutr. (2020) 39:3211–27. doi: 10.1016/j.clnu.2020.03.038
102. Weimann A, Braga M, Carli F, Higashiguchi T, Hubner M, Klek S, et al. ESPEN practical guideline: clinical nutrition in surgery. Clin Nutr. (2021) 40:4745–61. doi: 10.1016/j.clnu.2021.03.031
103. Weimann A, Braga M, Carli F, Higashiguchi T, Hubner M, Klek S, et al. ESPEN guideline: clinical nutrition in surgery. Clin Nutr. (2017) 36:623–50. doi: 10.1016/j.clnu.2017.02.013
104. Guan H, Chen S, Huang Q. Effects of enteral immunonutrition in patients undergoing pancreaticoduodenectomy: a meta-analysis of randomized controlled trials. Ann Nutr Metab. (2019) 74:53–61. doi: 10.1159/000495468
Keywords: enteral immunonutrition (EIN), gastrointestinal cancer, surgery, complications, meta-analysis
Citation: Shen J, Dai S, Li Z, Dai W, Hong J, Huang J and Chen J (2022) Effect of Enteral Immunonutrition in Patients Undergoing Surgery for Gastrointestinal Cancer: An Updated Systematic Review and Meta-Analysis. Front. Nutr. 9:941975. doi: 10.3389/fnut.2022.941975
Received: 12 May 2022; Accepted: 09 June 2022;
Published: 29 June 2022.
Edited by:
Clelia Madeddu, University of Cagliari, ItalyReviewed by:
Susmita Barman, University of Nebraska Medical Center, United StatesShin Hamada, Tohoku University, Japan
Copyright © 2022 Shen, Dai, Li, Dai, Hong, Huang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingjie Chen, zlcnmyyy@163.com
†These authors have contributed equally to this work and share first authorship
 Jingyi Shen
Jingyi Shen Senjie Dai1†
Senjie Dai1†  Jingjie Chen
Jingjie Chen