Combined systemic inflammatory immunity index and prognostic nutritional index scores as a screening marker for sarcopenia in patients with locally advanced gastric cancer
- 1The Third Department of Surgery, The Fourth Hospital of Hebei Medical University, Shijiazhuang, China
- 2Hebei Key Laboratory of Precision Diagnosis and Comprehensive Treatment of Gastric Cancer, Shijiazhuang, China
- 3AMITA Health Saint Joseph Hospital Chicago, Chicago, IL, United States
- 4Newham University Hospital, London, United Kingdom
- 5Radiation Oncology, Mayo Clinic, Rochester, MN, United States
Background: Sarcopenia is associated with poor clinical outcomes in patients with locally advanced gastric cancer (LAGC). Currently, the diagnostic criteria for sarcopenia are complex and laborious. Increased evidence suggests the inflammatory state of the body is closely associated with the development of sarcopenia. The systemic immune-inflammatory index (SII) and the prognostic nutritional index (PNI) are representative blood indicators of the status of the systemic inflammatory response, but the clinical significance of the combined testing of these two indicators remains unclear. We aimed to develop a simple and practical risk score (SII-PNI score) to screen patients with LAGC for sarcopenia on admission for early diagnosis.
Methods: We registered a prospective clinical study from January 2011 to May 2016 involving 134 patients with LAGC undergoing radical surgical resection. All patients followed the definition of sarcopenia in the Asian Working Group on Sarcopenia (AWGS) guidelines and were divided into sarcopenia and non-sarcopenia groups. SII-PNI score 0–2 was scored as 2 for high SII (≥432.9) and low PNI ( ≤ 49.5); score 1, either high SII or low PNI; score 0, no high SII or low PNI.
Results: All patients underwent radical surgery, including 31 patients (23.13%) with sarcopenia according to AWGS criteria. The SII-PNI score was significantly lower in the non-sarcopenic patients than in the sarcopenic patients (p < 0.001). Logistic multivariate analysis showed that the SII-PNI score predicted an independent prognostic factor for sarcopenia (p < 0.001). Patients with high SII-PNI scores had significantly worse prognosis than those with low SII-PNI scores (p < 0.001). The SII-PNI score was an independent prognostic factor for predicting overall survival and disease-free survival (p = 0.016, 0.023).
Conclusion: Peripheral blood parameters SII-PNI scores accurately identify sarcopenia in patients with LAGC and could be used as potential systemic markers.
12pt
Introduction
Gastric cancer is a common malignant tumor of the digestive tract, ranking fifth in incidence and fourth in mortality among malignant tumors (1). Most patients with gastric cancer are at an advanced stage at initial diagnosis and despite advances in current anti-tumor treatments including surgery, chemotherapy, targeted and immunotherapy, the overall prognosis remains poor with only slight improvement (2–4). Apart from the prognosis of patients with gastric cancer, which is closely related to tumor-specific factors (pathological type, tumor stage, etc.), the nutritional status and skeletal muscle mass (SMM) of patients are also important factors (5, 6).
Cancer cachexia is a syndrome of progressive loss of skeletal muscle mass, anorexia, systemic inflammation, and other metabolic abnormalities that lead to decreased bodily function (7, 8). Sarcopenia is an age-related syndrome characterized by progressive loss of skeletal muscle mass and function (8, 9). Numerous studies have shown that sarcopenia is not only high in the elderly population, but also has a high incidence in cancer patients (10, 11). The incidence of sarcopenia in patients with gastric cancer is as high as 28.8%, and especially in patients with locally advanced gastric cancer (LAGC) may be higher (12, 13). Concomitantly, sarcopenia in patients with LAGC has received widespread attention in recent years, as it is not only associated with increased adverse outcomes such as post-operative complications, but also has a detrimental effect on long-term survival (14–16). Thus, early diagnosis of sarcopenia in clinical practice is an important part of the management of LAGC patients. Currently, based on international consensus (17–19), the diagnosis of sarcopenia requires muscle mass [e.g., computed tomography (CT), dual-energy x-ray absorptiometry (DXA)], muscle strength [e.g., handgrip strength (HGS) and walking speed]. However, these complex procedures in a busy clinical setting limit sarcopenia screening and are not readily available. Therefore, there is an urgent need for a simple, reproducible, accurate and cost-effective biomarker to screen for and predict sarcopenia.
In recent years, an increasing number of studies have found that inflammatory factors in the body promote muscle atrophy, stimulate protein catabolism and inhibit muscle synthesis (20, 21). A meta-analysis by Bano et al. (22) of 17 studies included on the relationship between sarcopenia and the inflammatory response showed that C-reactive protein (CRP) levels were significantly higher in those with sarcopenia than in those in the non-sarcopenic group. The same results were obtained in two other studies, which confirmed that high levels of inflammatory factors were negatively associated with muscle strength and mass (23, 24). Growing evidence indicated that the inflammatory response is closely related to the occurrence and development of sarcopenia (25, 26). The increase of inflammatory cytokines in the body promotes the increase of muscle metabolism, which will further induce the imbalance of muscle protein synthesis and catabolism (27, 28). The systemic inflammatory immunity index (SII) is a new inflammatory index based on peripheral blood neutrophil, lymphocyte and platelet counts that adequately reflects the balanced relationship between immunity and inflammation (29, 30). A recent cross-sectional study that included 4,224 elderly patients found that higher SII levels were associated with an increased prevalence of sarcopenia (31). Meanwhile, Bullock et al. found that the prognostic nutritional index (PNI) calculated by peripheral blood albumin and lymphocyte count can be seen as a marker of inflammation rather than nutrition, which can be used as a tool for screening malnutrition and sarcopenia (32). Nonetheless, the value of SII combined with PNI in screening for sarcopenia in patients with LAGC has not been reported.
Based on the above reasons, we are wondering whether we can explore the prediction of muscle mass in LAGC patients by SII combined with PNI and determine the optimal cut-off value for SII combined with PNI to predict sarcopenia and its impact on the prognosis of LAGC patients.
Materials and methods
Patients and study design
This study is a prospective observational study that included 290 LAGC patients receiving surgery in the Fourth Hospital of Hebei Medical University from January 2011 and May 2016. This study is approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University (Approval Number: 20111214029). Inclusion criteria were as follows: (I) pathology conformed the gastric cancer diagnosis; (II) age ≥ 18 years; (III) no other anti-tumor therapy before surgery; (IV) the Eastern Cooperative Oncology Group (ECOG) activity status score was ≤ 2 points. The exclusion criteria were as follows: (I) residue of cancer cells in surgical margin (R1/R2 resection); (II) incomplete clinical data; (III) combined with other tumor history or hematological diseases; (IV) preoperative combined infection leads to abnormal blood routine results; (V) the presence of metal implants in the lumbar spine prevents measurement of skeletal muscle at L3 level.
Laboratory measurements
Peripheral venous blood was collected from all patients on an empty stomach within 1 week before surgery. Peripheral blood neutrophil, lymphocyte, and platelet counts in LAGC patients were analyzed by the method in our previous study (29, 30). The specific operation process is as follows: using an automatic hematology analyzer (Beckman Coulter LH750) to measure and analyze the counts of neutrophils, platelets and lymphocytes, and the albumin level using an automatic hematology analyzer (Beckman Coulter AU5800) assay analysis. The SII was defined as follows: SII = P × N/L, where P, N, and L were the platelet, neutrophil, and lymphocyte counts, respectively (29, 30, 33). Similarly, PNI is calculated by albumin plus 5 times lymphocyte count (34).
Measurement of muscle mass and strength
Preoperative abdominal and pelvic CT images of all enrolled LAGC patients were analyzed, and skeletal muscle area at the L3 level of the lumbar spine was measured. The images of each patient were uploaded to the picture archiving and communication system (PACS, SIEMENS SOMATOM) for processing. The following tissue Hounsfield Units (HU) thresholds were used: skeletal muscle attenuation ranged from −29 to 150 HU (35). The software evaluates and measures the pixel area of the corresponding area of skeletal muscle attenuation to obtain the skeletal muscle area (SMA). The skeletal muscle index (SMI) was then calculated by dividing the SMA by the square of the patient's height. Meanwhile, all patients were assessed for handgrip strength (HGS) using a Camry dynamometer (EH101; Xiangshan Company, Guangdong, China) before surgery. Two measurements were made alternately, and the maximum value was used for further analysis.
Diagnostic criteria for sarcopenia
The definition of sarcopenia in this study was assessed using the Asian Working Group on Sarcopenia (AWGS) (18) consensus. We used the following cut-off values to define sarcopenia: SMI <40.8 cm2/m2 in men and SMI <34.9 cm2/m2 in women as muscle loss, and HGS <26 kg in men and HGS <18 kg in women as sarcopenia.
Follow-up of participants
Overall survival (OS) was defined as the time interval from the start of initial recruitment to cancer-related death or final contact. Disease-free survival (DFS) is defined as the time from the start of randomization until disease recurrence or death of the patient due to disease progression. All patients were recommended to have an enhanced CT scan of the abdomen and pelvis every 3 months for the first 3 years post-operatively and every 6 months for the 4th to 5th post-operative years. Follow-up methods mainly included telephone encounters, outpatient visits, and hospitalization.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp, Armonk, NY, USA) and MedCalc Statistical Software v15.2 (MedCalc Software bvba, Ostend, Belgium). Categorical variables were expressed as numbers and proportions, using the X2-test or Fisher's exact test. Continuous variables were expressed as mean with standard deviation (SD) or median with interquartile range (IQR). All continuous data in this study were non-normally distributed according to the Kolmogorov-Smirnov test, so the Wilcoxon rank-sum test was used to compare differences between groups. Scatter plots and Pearson's correlation coefficient were used to assess linear correlations. Multivariate logistic regression was used to analyze the effect of various factors on the prevalence of sarcopenia. The subject receiver operating characteristic (ROC) curve and area under the curve (AUC) were used to assess the ability of the SII and PNI to screen for sarcopenia. Kaplan-Meier curves were performed to estimate OS and DFS, and a log-rank test was used to compare the difference between groups. Cox regression models were used to identify independent prognostic factors. A p-value <0.05 was considered to show statistical significance.
Results
Patients characteristics
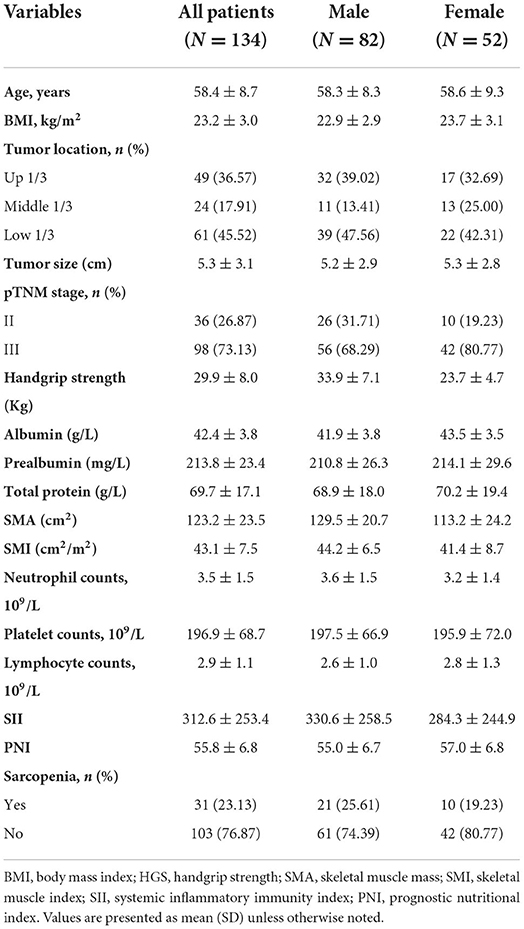
A total of 134 patients with LAGC were included according to the inclusion and exclusion criteria of this study (Figure 1). The patients' characteristics are presented in Table 1. The mean age was 58.4 years were enrolled in this study and a total of 82 male (61.19%) patients with LAGC. According to the AWGS consensus definition of sarcopenia, there were 21 sarcopenia patients (25.61%) out of 82 men, and 10 sarcopenia patients (19.23%) out of 52 women among the patients. The median SII before radical surgery for all patients was 217.3, ranged from 59.1 to 1,051.2 and in addition for PNI the median value was 55.9, ranged from 38.9 to 71.6. Meanwhile, the two systemic indices SII and PNI had close negative correlation (r = −0.531, p < 0.001; Figure 2). As shown in Figure 3, SII had a negative correlation with SMA (r = −0.364; p < 0.001), SMI (r = −0.389; p < 0.001), and HGS (r = −0.371; p < 0.001), while PNI had a positive correlation with SMA (r = 0.232; p = 0.007), SMI (r = 0.259; p = 0.003), and HGS (r = 0.210; p = 0.015).
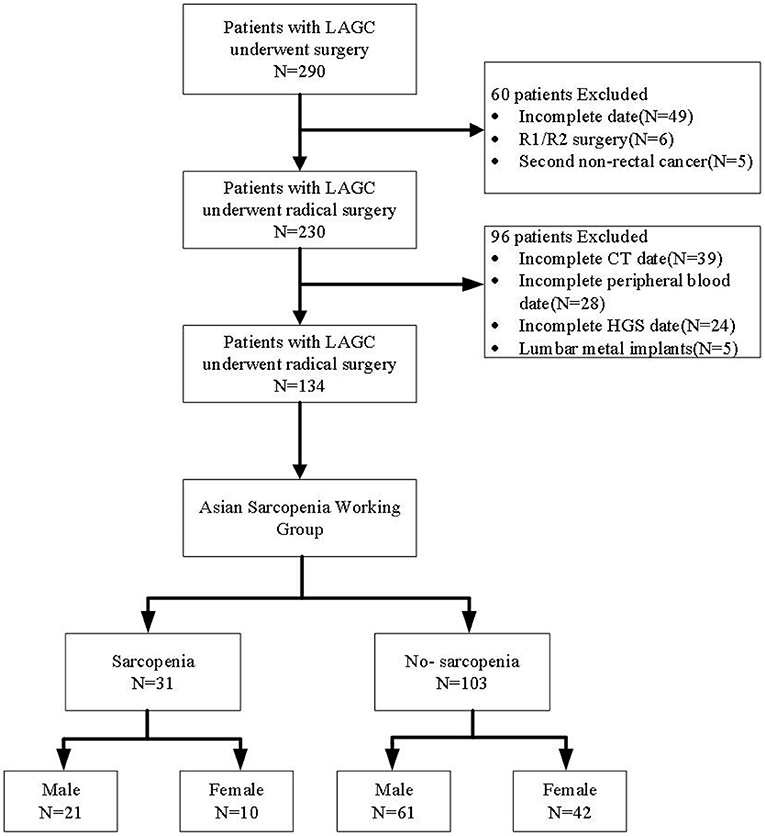
Figure 1. Flow diagram participants included in the study. LAGC, locally advanced gastric cancer; HGS, handgrip strength.
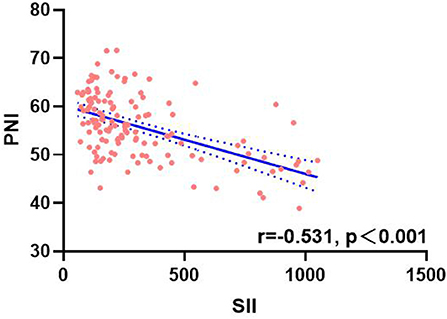
Figure 2. Correlation analysis between SII and PNI. SII, systemic inflammatory immunity index; PNI, prognostic nutritional index.
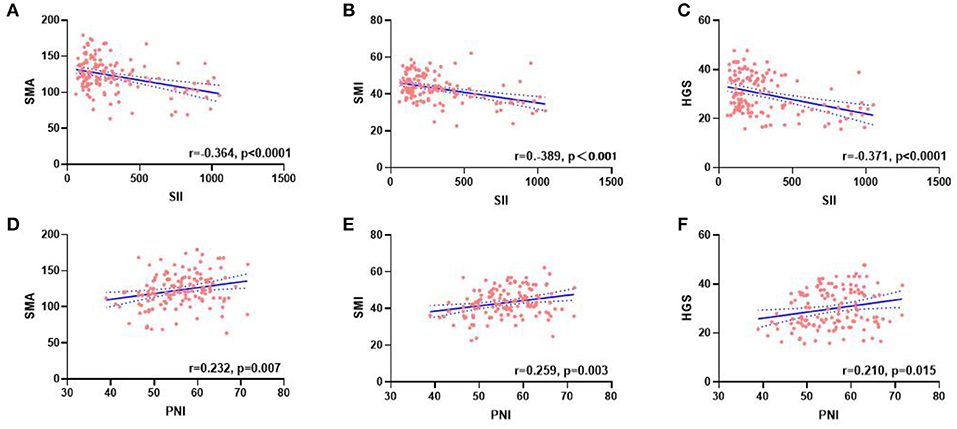
Figure 3. Correlation between the SII (A–C), PNI (D–F), and SMA, SMI, and HGS. SII, systemic inflammatory immunity index; PNI, prognostic nutritional index; SMA, skeletal muscle area; SMI, skeletal muscle mass index; HGS, handgrip strength.
Optimal cut-off values for SII and PNI values for pre-surgical diagnosis of sarcopenia
The mean SII values were significantly lower in the non-sarcopenic patients than in the sarcopenic patients (215.7 ± 148.0 vs. 634.7 ± 266.0, p < 0.001) (Figure 4A). However, patients in the non-sarcopenic group had higher mean PNI values than those in the sarcopenic group (57.1 ± 6.0 vs. 51.6 ± 7.6, p < 0.001) (Figure 4B). We used the Youden-Index to maximize the “sensitivity + specificity-1” value to calculate serum SII and PNI cut-off values of 432.9 (AUC = 0.930, 95%CI: 0.882–0.978, p < 0.001, sensitivity: 0.774, specificity: 0.951) and 49.5 (AUC = 0.726, 95%CI: 0.609–0.844, p < 0.001, sensitivity: 0.903, specificity: 0.548), respectively (Figure 5). Patients were divided into three groups according to the optimal cut-off values for SII and PNI: high SII (≥432.9) and low PNI ( ≤ 49.5) were defined as a score of 2 (n = 17, 12.69%); either high SII or low PNI was defined as a score of 1 (n = 21, 15.67%); and a score of 0 (n = 96, 71.64%) with no high SII or low PNI.
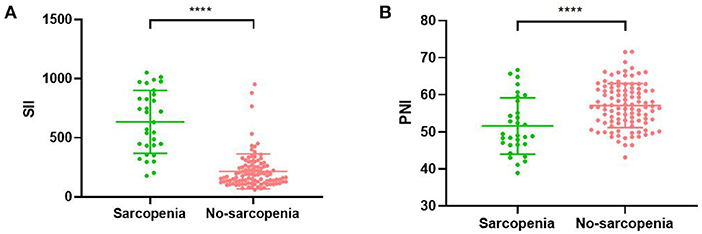
Figure 4. Relationship between sarcopenia and the SII (A)/PNI (B). SII, systemic inflammatory immunity index; PNI, prognostic nutritional index; **** <0.001.
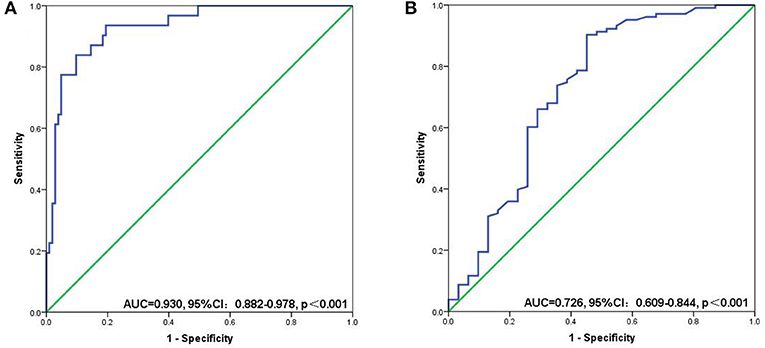
Figure 5. ROC curves for discriminating patients with sarcopenia and those with non-sarcopenia according to values of the SII (A) and PNI (B). SII, systemic inflammatory immunity index; PNI, prognostic nutritional index.
Associations between SII-PNI score and sarcopenia
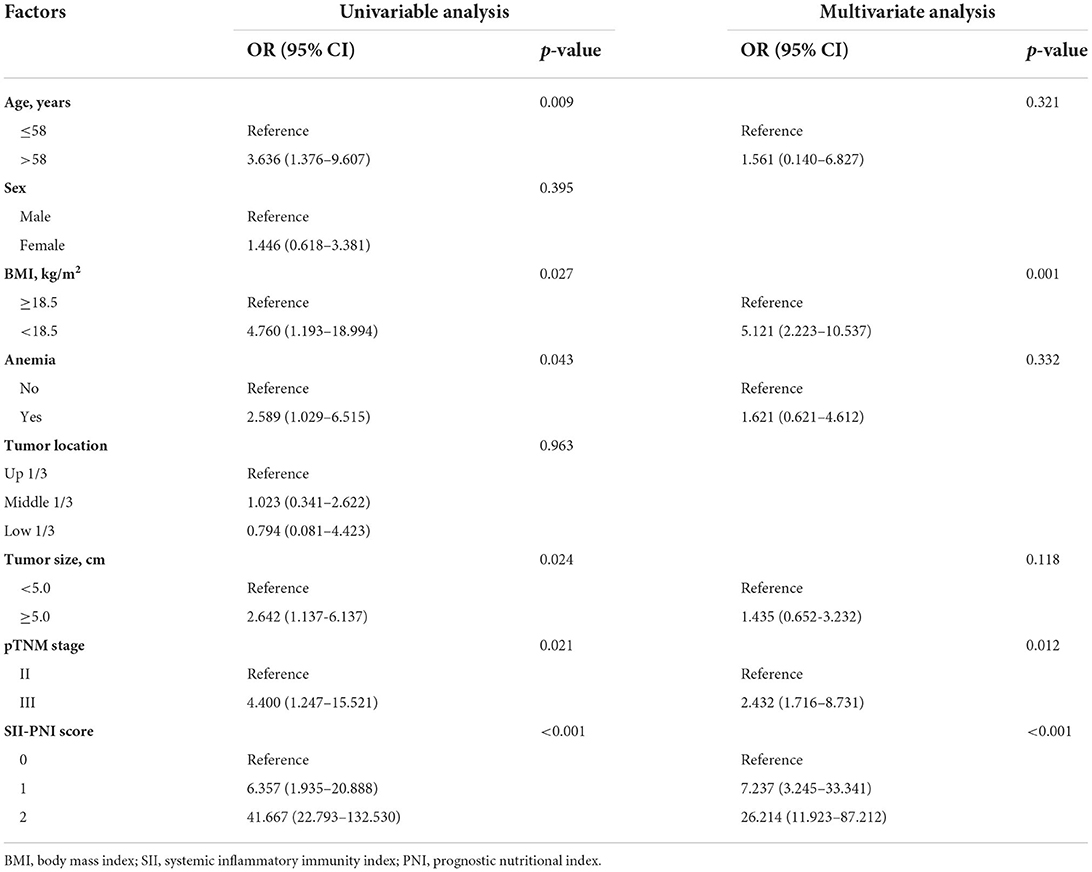
Seven patients (7.29%) with SII-PNI score of 0 were diagnosed as sarcopenia, while 7 patients (33.33%) with SII-PNI score of 1 and 17 patients (100.00%) with SII-PNI score of 2 were diagnosed as sarcopenia, and the differences among the three groups were significant (p < 0.001). To further analyze the association between the SII-PNI score and the presence of sarcopenia, we used logistic regression analysis to identify predictors of sarcopenia. Univariate analysis showed that SII-PNI score, age, BMI, preoperative hemoglobin, tumor size, and pTNM stage were risk factors for sarcopenia. After adjusting for age, anemia, and tumor size, multivariate analysis showed that SII-PNI score (OR = 26.214, 95%CI: 11.923–87.212, p < 0.001) was still an independent predictor of increased risk of sarcopenia (Table 2).
The relationship between SII-PNI score and prognosis
All patients were followed up, and the median follow-up time was 55.7 months (range: 13.6–80.8 months). The 5-year OS of the whole group of patients was 52.99%, and the 5-year DFS was 47.76%. Further subgroup analysis found that the 5-year OS (61.17% vs. 25.81%, p < 0.001) and DFS (56.31% vs. 19.35%, p < 0.001) of non-sarcopenic patients were better than those of sarcopenic patients, and the difference was statistically significant (Figures 6A,B). Furthermore, based on the different SII-PNI scores, we found that the 5-year OS and DFS of patients with 0 score were 61.46% and 57.29%, respectively, while the 5-year OS of patients with 1 score and 2 score were 42.86% and 17.65%, respectively, and the 5-year DFS were 33.33% and 11.76%, respectively, and there were significant differences in 5-year OS and DFS among the three groups (all p < 0.05) (Figures 6C,D). The result of Cox regression model revealed that SII-PNI scores was an independent prognostic factor of OS (adjusted HR = 2.422, 95%CI: 1.022–6.727, p = 0.016) and DFS (adjusted HR = 2.62, 95%CI: 1.341–5.247, p = 0.023) in patients with LAGC.
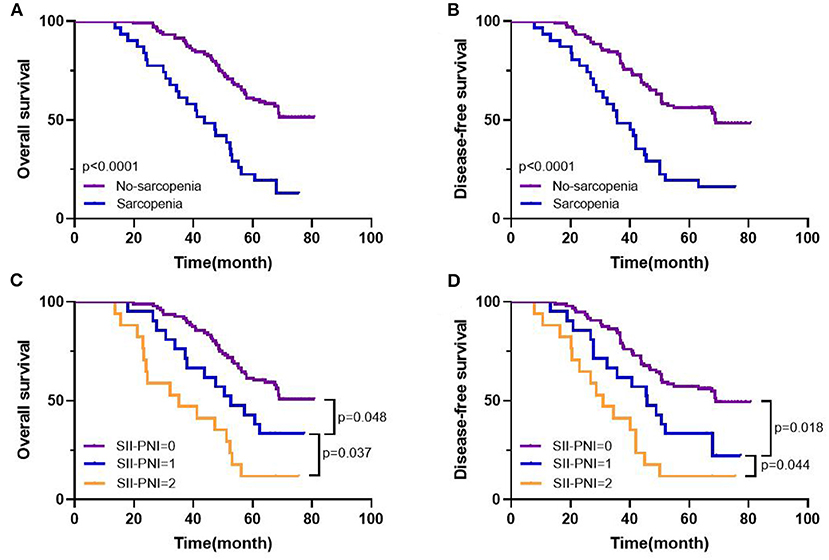
Figure 6. Kaplan-Meier survival curves in patients with LAGC. 5-year overall survival (A), disease-free survival (B) based on sarcopenia status; 5-year overall survival (C), disease-free survival (D) based on SII-PNI score.
Discussion
Our study showed that SII was negatively correlated with muscle mass and hand grip strength in LAGC patients, while PNI was positively correlated. Furthermore, both indicators performed satisfactorily and comparably in predicting sarcopenia. Moreover, we calculated separate cut-off values for the SII and PNI diagnoses of sarcopenia, and based on these cut-off values the two indicators were combined to form a new SII-PNI score. As the SII-PNI score increased, the possibility of sarcopenia is also greater, indicating that SII-PNI score has high predictive value in predicting sarcopenia. These results suggest that the SII-PNI score can be used as a surrogate biomarker to assess sarcopenia in patients with LAGC, with a score of 2 essentially determining that sarcopenia will develop in patients. Alternatively, based on the results of the survival curves, we found that the SII-PNI score can also predict 5-year OS and DFS in LAGC patients and can also be used as a predictive marker for survival prognosis.
Numerous studies have found that the essence of sarcopenia is the decline of muscle fibers and the degradation of proteins, which is closely associated with increased inflammation, oxidative stress damage, mitochondrial dysfunction, abnormal cellular autophagy and dysregulation of muscle mass regulatory factors (36, 37). Previous studies on markers of sarcopenia have mostly focused on serum creatinine and cystatin C, but also on related inflammatory mediators such as C-reactive protein (CRP) (23), tumor necrosis factor-alpha (TNF-α) (38), and interleukins (IL) (22). Currently, screening for these markers requires additional cost to the patient and redundant targeted testing. As a highly accessible and reproducible biomarker, the association of PNI nutritional status indicators and SII inflammatory indicators with prognosis in patients with various tumors, including gastric cancer, has received increasing attention (39–41). However, few studies have focused on the relationship between SII and PNI on sarcopenia.
To the best of our knowledge, this is the first analysis of the association of the SII-PNI score with sarcopenia. A previous retrospective study found that sarcopenia was significantly associated with neutrophil-to-lymphocyte Ratio (NLR) in elderly patients with esophageal squamous cell carcinoma (ESCC), and both were independent prognostic factors in ESCC patients (21). Another multicentre prospective study found that NLR, PNI, SII and platelet lymphocyte ratio (PLR) were all good predictors of sarcopenia, with ALI [BMI (kg/m2) × albumin (g/dl)/NLR] being the best predictor (20, 21). These studies have shown that all of these indicators based on inflammation can be used as markers to predict sarcopenia. The present study combined the strengths and weaknesses of markers from previous studies, combining neutrophil, platelet and lymphocyte counts and albumin levels to form the SII-PNI score, and based on this score found that patients with higher scores were more likely to develop sarcopenia.
Recent studies have found that high levels of inflammatory factors promote muscle atrophy, stimulate increased proteolytic metabolism and inhibit muscle synthesis, so inflammatory factors are negatively associated with muscle strength and mass (20, 21, 42–44). Similarly, other studies have found that increased inflammatory factors can also lead to insulin resistance and muscle wastage through activation of the ubiquitin-proteasome protein hydrolysis pathway, and that muscle wastage itself further exacerbates insulin resistance (45). Meanwhile, a number of studies have found that the inflammatory response is also an independent risk factor for the prognosis of patients with LAGC (46, 47). A high inflammatory response leads to the release of a series of inflammatory mediators from tumor cells, causing oxidative damage and DNA mutation, which in turn alters the tumor microenvironment and promotes the proliferation and migration of tumor cells (47, 48). The systemic inflammatory response will aggravate malnutrition and decline in body function of patients with malignant tumors, which will promote poor prognosis of patients with malignant tumors (29, 30). It has been reported that muscle hormones secreted by myocytes can inhibit the growth of tumor cells, and that muscle hormone expression may be reduced in sarcopenia, leading to tumor proliferation and recurrence (49). In addition, the systemic inflammatory response will release more pro-inflammatory cytokines and growth factors, resulting in profound catabolic effects on the body's metabolism, ultimately leading to increased muscle breakdown (50). Low muscularity could contribute to local inflammation in the muscle, leading to further breakdown and driving systemic inflammation (8). In conclusion, systemic inflammation, tumor invasion and proliferation and sarcopenia are closely related, forming a vicious circle. In our study, there were significant differences in prognosis between patients with different SII-PNI scores, and also between patients with and without sarcopenia. Systemic inflammation and malnutrition are unavoidable problems for cancer patients. Therefore, there is an urgent need to enhance the patient's comprehensive treatment to prevent muscle wastage and improve physical condition, stamina and quality of life.
It is noteworthy that a few limitations of current research also exist. First, this was a prospective with a small sample size and the selection of LAGC patients may have been biased, which would have limited the generalizability of the results. Second, this study did not include patients with LAGC from other centers to validate the diagnostic efficacy of the SII-PNI score for sarcopenia. Given this, there is an urgent need for a larger, multicentre prospective study to explore the predictive power of the SII-PNI score for sarcopenia to consolidate our findings.
Conclusions
In conclusion, this study suggests that the SII-PNI score may be a simple, cost-effective, and efficient screening tool for sarcopenia in LAGC patients. Furthermore, a higher SII-PNI score is associated with poorer 5 year OS and DFS, indicating its promising prognostic value for long-term survival. However, further studies with larger sample size and different patient groups are required to validate these findings.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study is registered with ClinicalTrials.gov, Registry Number NCT01516944, and approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University (Approval Number: 20111214029). The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conception and design and administrative support: QZha. Provision of study materials or patients: PD, PY, YT, HG, and JL. Collection and assembly of data: PD, PY, YT, and HG. Data analysis and interpretation: PD, CS, SC, and QZha. All authors writing and gave final approval of the manuscript.
Funding
This work was supported by the Cultivating Outstanding Talents Project of Hebei Provincial Government Fund (No. 2019012), the Hebei Public Health Committee County-Level Public Hospitals suitable health technology promotion and storage project (No. 2019024), and the Hebei University Science and Technology Research Project (No. ZD2019139).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Ilson DH. Advances in the treatment of gastric cancer: 2019. Curr Opin Gastroenterol. (2019) 35:551–4. doi: 10.1097/MOG.0000000000000577
3. Le DT, Ott PA, Korytowsky B, Le H, Le TK, Zhang Y, et al. Real-world treatment patterns and clinical outcomes across lines of therapy in patients with advanced/metastatic gastric or gastroesophageal junction cancer. Clin Colorectal Cancer. (2020) 19:32–8.e3. doi: 10.1016/j.clcc.2019.09.001
4. Bonelli P, Borrelli A, Tuccillo FM, Silvestro L, Palaia R, Buonaguro FM. Precision medicine in gastric cancer. World J Gastrointest Oncol. (2019) 11:804–29. doi: 10.4251/wjgo.v11.i10.804
5. Matsunaga T, Satio H, Miyauchi W, Shishido Y, Miyatani K, Murakami Y, et al. Impact of skeletal muscle mass in patients with recurrent gastric cancer. World J Surg Oncol. (2021) 19:170. doi: 10.1186/s12957-021-02283-6
6. Kanazawa Y, Yamada T, Kakinuma D, Matsuno K, Ando F, Fujita I, et al. Skeletal muscle mass depletion after gastrectomy negatively affects the prognosis of patients with gastric cancer. Anticancer Res. (2020) 40:4271–9. doi: 10.21873/anticanres.14429
7. Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. (2011) 12:489–95. doi: 10.1016/S1470-2045 (10)70218-7
8. Peixoto da Silva S, Santos JMO, Costa E Silva MP, Gil da Costa RM, Medeiros R. Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia. J Cachexia Sarcopenia Muscle. (2020) 11:619–35. doi: 10.1002/jcsm.12528
9. He Y, Xie W, Li H, Jin H, Zhang Y, Li Y. Cellular senescence in sarcopenia: possible mechanisms and therapeutic potential. Front Cell Dev Biol. (2022) 9:793088. doi: 10.3389/fcell.2021.793088
10. Zhang G, Li X, Sui C, Zhao H, Zhao J, Hou Y, et al. Incidence and risk factor analysis for sarcopenia in patients with cancer. Oncol Lett. (2016) 11:1230–4. doi: 10.3892/ol.2015.4019
11. Buentzel J, Heinz J, Bleckmann A, Bauer C, Röver C, Bohnenberger H, et al. Sarcopenia as prognostic factor in lung cancer patients: a systematic review and meta-analysis. Anticancer Res. (2019) 39:4603–12. doi: 10.21873/anticanres.13640
12. Kuwada K, Kuroda S, Kikuchi S, Yoshida R, Nishizaki M, Kagawa S, et al. Clinical impact of sarcopenia on gastric cancer. Anticancer Res. (2019) 39:2241–9. doi: 10.21873/anticanres.13340
13. Seto Y. Sarcopenia, muscle quality, and gastric cancer surgery. Ann Gastroenterol Surg. (2021) 5:402–3. doi: 10.1002/ags3.12485
14. Chen F, Chi J, Liu Y, Fan L, Hu K. Impact of preoperative sarcopenia on postoperative complications and prognosis of gastric cancer resection: a meta-analysis of cohort studies. Arch Gerontol Geriatr. (2022) 98:104534. doi: 10.1016/j.archger.2021.104534
15. Fukuda Y, Yamamoto K, Hirao M, Nishikawa K, Nagatsuma Y, Nakayama T, et al. Sarcopenia is associated with severe postoperative complications in elderly gastric cancer patients undergoing gastrectomy. Gastric Cancer. (2016) 19:986–93. doi: 10.1007/s10120-015-0546-4
16. Zhuang CL, Huang DD, Pang WY, Zhou CJ, Wang SL, Lou N, et al. Sarcopenia is an independent predictor of severe postoperative complications and long-term survival after radical gastrectomy for gastric cancer: analysis from a large-scale cohort. Medicine. (2016) 95:e3164. doi: 10.1097/MD.0000000000003164
17. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:601. doi: 10.1093/ageing/afz046
18. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300−7.e2. doi: 10.1016/j.jamda.2019.12.012
19. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences International working group on sarcopenia. J Am Med Dir Assoc. (2011) 12:249–56. doi: 10.1016/j.jamda.2011.01.003
20. Ruan GT, Ge YZ, Xie HL, Hu CL, Zhang Q, Zhang X, et al. Association between systemic inflammation and malnutrition with survival in patients with cancer sarcopenia-a prospective multicenter study. Front Nutr. (2022) 8:811288. doi: 10.3389/fnut.2021.811288
21. Peng H, Tan X. The prognostic significance of sarcopenia and the neutrophil-to-lymphocyte ratio in elderly patients with esophageal squamous cell carcinoma. Cancer Manag Res. (2021) 13:3209–18. doi: 10.2147/CMAR.S302274
22. Bano G, Trevisan C, Carraro S, Solmi M, Luchini C, Stubbs B, et al. Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas. (2017) 96:10–5. doi: 10.1016/j.maturitas.2016.11.006
23. Shokri-Mashhadi N, Moradi S, Heidari Z, Saadat S. Association of circulating C-reactive protein and high-sensitivity C-reactive protein with components of sarcopenia: a systematic review and meta-analysis of observational studies. Exp Gerontol. (2021) 150:111330. doi: 10.1016/j.exger.2021.111330
24. Can B, Kara O, Kizilarslanoglu MC, Arik G, Aycicek GS, Sumer F, et al. Serum markers of inflammation and oxidative stress in sarcopenia. Aging Clin Exp Res. (2017) 29:745–52. doi: 10.1007/s40520-016-0626-2
25. Budui SL, Rossi AP, Zamboni M. The pathogenetic bases of sarcopenia. Clin Cases Miner Bone Metab. (2015) 12:22–6. doi: 10.11138/ccmbm/2015.12.1.022
26. Jo E, Lee SR, Park BS, Kim JS. Potential mechanisms underlying the role of chronic inflammation in age-related muscle wasting. Aging Clin Exp Res. (2012) 24:412–22. doi: 10.3275/8464
27. Livshits G, Kalinkovich A. Inflammaging as a common ground for the development and maintenance of sarcopenia, obesity, cardiomyopathy and dysbiosis. Ageing Res Rev. (2019) 56:100980. doi: 10.1016/j.arr.2019.100980
28. Lin JX, Lin JP, Xie JW, Wang JB, Lu J, Chen QY, et al. Prognostic value and association of sarcopenia and systemic inflammation for patients with gastric cancer following radical gastrectomy. Oncologist. (2019) 24:e1091–101. doi: 10.1634/theoncologist.2018-0651
29. Ding P, Guo H, Sun C, Yang P, Kim NH, Tian Y, et al. Combined systemic immune-inflammatory index (SII) and prognostic nutritional index (PNI) predicts chemotherapy response and prognosis in locally advanced gastric cancer patients receiving neoadjuvant chemotherapy with PD-1 antibody sintilimab and XELOX: a prospective study. BMC Gastroenterol. (2022) 22:121. doi: 10.1186/s12876-022-02199-9
30. Ding P, Yang P, Sun C, Tian Y, Guo H, Liu Y, et al. Predictive effect of systemic immune-inflammation index combined with prognostic nutrition index score on efficacy and prognosis of neoadjuvant intraperitoneal and systemic paclitaxel combined with apatinib conversion therapy in gastric cancer patients with positive peritoneal lavage cytology: a prospective study. Front Oncol. (2022) 11:791912. doi: 10.3389/fonc.2021.791912
31. Zhao WY, Zhang Y, Hou LS, Xia X, Ge ML, Liu XL, et al. The association between systemic inflammatory markers and sarcopenia: results from the West China Health and Aging Trend Study (WCHAT). Arch Gerontol Geriatr. (2021) 92:104262. doi: 10.1016/j.archger.2020.104262
32. Bullock AF, Greenley SL, McKenzie GAG, Paton LW, Johnson MJ. Relationship between markers of malnutrition and clinical outcomes in older adults with cancer: systematic review, narrative synthesis and meta-analysis. Eur J Clin Nutr. (2020) 74:1519–35. doi: 10.1038/s41430-020-0629-0
33. Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W, et al. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. (2014) 20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442
34. Takechi H, Fujikuni N, Tanabe K, Hattori M, Amano H, Noriyuki T, et al. Using the preoperative prognostic nutritional index as a predictive factor for non-cancer-related death in post-curative resection gastric cancer patients: a retrospective cohort study. BMC Gastroenterol. (2020) 20:256. doi: 10.1186/s12876-020-01402-z
35. Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol 1985. (1998) 85:115–22. doi: 10.1152/jappl.1998.85.1.115
36. Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, et al. Sarcopenia: aging-related loss of muscle mass and function. Physiol Rev. (2019) 99:427–511. doi: 10.1152/physrev.00061.2017
37. Wiedmer P, Jung T, Castro JP, Pomatto LCD, Sun PY, Davies KJA, et al. Sarcopenia - molecular mechanisms and open questions. Ageing Res Rev. (2021) 65:101200. doi: 10.1016/j.arr.2020.101200
38. Thoma A, Lightfoot AP. NF-kB and inflammatory cytokine signalling: role in skeletal muscle atrophy. Adv Exp Med Biol. (2018) 1088:267–79. doi: 10.1007/978-981-13-1435-3_12
39. Zhang X, Fang H, Zeng Z, Zhang K, Lin Z, Deng G, et al. Preoperative prognostic nutrition index as a prognostic indicator of survival in elderly patients undergoing gastric cancer surgery. Cancer Manag Res. (2021) 13:5263–73. doi: 10.2147/CMAR.S316437
40. Zengin A, Bag YM, Aydin MC, Kocaaslan H, Kaplan K, Sumer F, et al. Is prognostic nutritional index an indicator for postoperative 90-day mortality in laparoscopic gastric cancer surgery? Nutr Cancer. (2021) 15:1–7. doi: 10.1080/01635581.2021.2002920
41. Cao X, Xue J, Yang H, Han X, Zu G. Association of clinical parameters and prognosis with the pretreatment systemic immune-inflammation index (SII) in patients with gastric cancer. J Coll Physicians Surg Pak. (2021) 31:83–8. doi: 10.29271/jcpsp.2021.01.83
42. Baracos VE. Regulation of skeletal-muscle-protein turnover in cancer-associated cachexia. Nutrition. (2000) 16:1015–8. doi: 10.1016/S0899-9007 (00)00407-X
43. Guttridge DC, Mayo MW, Madrid LV, Wang CY, Baldwin AS Jr. NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. (2000) 289:2363–6. doi: 10.1126/science.289.5488.2363
44. Hotamisligil GS. The role of TNFalpha and TNF receptors in obesity and insulin resistance. J Intern Med. (1999) 245:621–5. doi: 10.1046/j.1365-2796.1999.00490.x
45. Wang X, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology. (2006) 147:4160–8. doi: 10.1210/en.2006-0251
46. Hirahara T, Arigami T, Yanagita S, Matsushita D, Uchikado Y, Kita Y, et al. Combined neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predicts chemotherapy response and prognosis in patients with advanced gastric cancer. BMC Cancer. (2019) 19:672. doi: 10.1186/s12885-019-5903-y
47. Gambardella V, Castillo J, Tarazona N, Gimeno-Valiente F, Martínez-Ciarpaglini C, Cabeza-Segura M, et al. The role of tumor-associated macrophages in gastric cancer development and their potential as a therapeutic target. Cancer Treat Rev. (2020) 86:102015. doi: 10.1016/j.ctrv.2020.102015
48. Oya Y, Hayakawa Y, Koike K. Tumor microenvironment in gastric cancers. Cancer Sci. (2020) 111:2696–707. doi: 10.1111/cas.14521
49. Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. (2012) 8:457–65. doi: 10.1038/nrendo.2012.49
Keywords: systemic immune-inflammatory index, prognostic nutritional index, locally advanced gastric cancer, sarcopenia, marker
Citation: Ding P, Lv J, Sun C, Chen S, Yang P, Tian Y, Zhou Q, Guo H, Liu Y and Zhao Q (2022) Combined systemic inflammatory immunity index and prognostic nutritional index scores as a screening marker for sarcopenia in patients with locally advanced gastric cancer. Front. Nutr. 9:981533. doi: 10.3389/fnut.2022.981533
Received: 29 June 2022; Accepted: 27 July 2022;
Published: 15 August 2022.
Edited by:
Gabriela Salim de Castro, University of São Paulo, BrazilReviewed by:
Tolga Olmez, Ministry of Health, TurkeyDaniela Caetano Gonçalves, Federal University of São Paulo, Brazil
Ana Flávia Marçal Pessoa, University of São Paulo, Brazil
Copyright © 2022 Ding, Lv, Sun, Chen, Yang, Tian, Zhou, Guo, Liu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qun Zhao, zhaoqun@hebmu.edu.cn
†These authors have contributed equally to this work
 Ping'an Ding
Ping'an Ding Jingxia Lv1†
Jingxia Lv1†  Chenyu Sun
Chenyu Sun Shuya Chen
Shuya Chen Qin Zhou
Qin Zhou Qun Zhao
Qun Zhao