- 1Department of Radiation Oncology, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, China
- 2Department of Pathology, Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, China
Background: Preoperative radiotherapy followed by radical surgery is the standard treatment for locally advanced rectal cancer; however, its long-term survival benefit remains controversial. This study aimed to determine the relationship between pretreatment carcinoembryonic antigen (CEA) levels and the long-term prognosis of preoperative radiotherapy in locally advanced rectal cancer (LARC) patients.
Methods: Data of LARC patients who underwent surgery between 2011 and 2015 were identified from the Surveillance, Epidemiology, and End Results (SEER) database, and patients were accordingly divided into surgery (S) group and radiotherapy followed by surgery (RT+S) group. The primary outcomes were cancer-specific survival (CSS) and cancer-specific mortality (CSM). CSS was evaluated using Kaplan-Meier analysis, while CSM was evaluated using a competitive risk model. Subgroup analysis was also conducted, which was stratified by pretreatment CEA levels.
Results: A total of 2,760 patients were eligible for this study, including 350 (12.7%) patients in the S group and 2,410 (87.3%) in the RT+S group. There were no significant differences in the CSS and CSM rates at 1, 3, and 4 years between the S and RT+S groups before and after PSM (all p > 0.05). Pretreatment CEA levels were independently associated with CSS and CSM after adjusting for age, sex, stage, pathological factors, and treatment factors (all p < 0.05). Subgroup analysis showed that preoperative radiotherapy would benefit patients with elevated CEA in terms of CSS and CSM (both p < 0.05) but not those patients with normal CEA (both p > 0.05). Further analysis showed that preoperative radiotherapy was an independent protective factor for CSS and CSM in patients with elevated CEA levels (both p < 0.05).
Conclusions: Pretreatment CEA level may be considered a potential biomarker to screen LACR patients who would benefit from preoperative radiotherapy in terms of long-term prognosis.
Introduction
Preoperative radiotherapy, either long-course radiotherapy (LCRT) or short-course radiotherapy (SCRT), is the standard neoadjuvant strategy for locally advanced rectal cancer (LARC) (1, 2). In the recent decade, the proportion of LARC patients receiving preoperative radiotherapy has been as high as 59.8% in the USA (3), although direct surgery is still preferred in some areas, such as Japan (4). With the advent of preoperative radiotherapy, the rate of sphincter preservation is increasing, this being mainly due to the significant downstaging effect (5, 6). However, as a hard endpoint of treatment, the long-term survival benefit of preoperative radiotherapy remains controversial, regardless of LCRT and SCRT (7–11).
Carcinoembryonic antigen (CEA) is a routine screening and diagnostic index of colorectal cancer and is a widely used screening marker for postoperative recurrence (12–15). CEA levels, both before and after surgery, have been identified as important risk factors for long-term prognosis as well as for dynamic changes in CEA levels (16, 17). Moreover, CEA levels have been found to be associated with the response rate of neoadjuvant treatment (12, 18). However, there are no reports on the use of CEA in guiding the management of preoperative radiotherapy in terms of long-term prognosis. In the current study, we selected LARC patients diagnosed between 2011 and 2015 in the Surveillance, Epidemiology, and End Results (SEER) database to identify the long-term survival benefit of preoperative radiotherapy and to determine the relationship between the pretreatment CEA level and the prognosis of patients receiving preoperative radiotherapy.
Materials and Methods
Ethics Statement
Since we gained an official permit to access the research data of the SEER database (ID: 22032-Nov2019) and all the analyses in the current study were conducted under the rules of the SEER database, neither informed consent nor ethical approval was required for this study.
Data Source
Patients aged 18 years or older who were diagnosed with rectal adenocarcinoma by pathology (2011 to 2015) were identified using the World Health Organization’s International Classification of Disease (ICD), 3rd edition (8140, 8144, 8210, 8211, 8213, 8221, 8255, 8261, 8263). Data on age, sex, marital status, insurance, pretreatment serum CEA level, tumor size, tumor differentiation, tumor deposits (TD), perineural invasion (PNI) status, circumferential resection margin (CRM), number of dissected lymph nodes (LND), tumor stage, node stage, surgery, radiation before surgery, chemotherapy, and survival (survival time and cause of death) were extracted from the SEER database.
Patient Selection
Patients were eligible if they: (1) underwent radical surgery and were diagnosed with rectal cancer by pathology, (2) staged at T3–4NanyM0 or TanyN+M0, and (3) received chemotherapy. Patients were excluded from this study if they met one of the following criteria: (1) receipt of postoperative radiotherapy, (2) multiple cancers, (3) survival month ≤1 month, or (4) unknown clinical data. Based on whether patients received preoperative radiotherapy or not, they were divided into surgery (S) and radiotherapy +surgery (RT+S) groups.
Variable Definition and Stratification
Variables were categorized according to the 8th American Joint Committee on Cancer guidelines or based on published studies: age at diagnosis (≤65 years, >65 years), sex (male or female), marital status (unmarried, married, other), insurance (no, yes), CEA level (normal, elevated), tumor size (≤5 cm, >5 cm), tumor differentiation (I/II, III/IV), TD (negative, positive), PNI (absent, present), CRM (negative, positive), number of LND (<12 or ≥12), stage (II, III), T stage (T1-2, T3, T4), N stage (N0, N1, N2), and survival (months).
Outcome Definition
The endpoints of this study were cancer-specific survival (CSS) and cancer-specific mortality (CSM). CSS was defined as the time from the date of diagnosis to the date of death from rectal cancer or the latest follow-up. CSM was defined as cumulative mortality from the date of diagnosis to the date of death from rectal cancer or at the latest follow-up.
Propensity Score Matching
Propensity score matching (PSM) analysis was performed to reduce selection bias. Briefly, baseline characteristics between the two groups were matched using the 1:1 nearest-neighbor matching method with a standard deviation of 0.2.
Statistical Analyses
The chi-square (χ2) test or Fisher’s test was used for comparisons between the two groups. The Kaplan–Meier (K-M) method was used for comparison of CSS analysis between the two groups using a log-rank test. A multivariate Cox regression model was used to identify the independent risk factors for CSS.
In the competitive-risk analysis, death from other causes was recognized as a competitive event of cancer-specific death. Gray’s test was used to determine the intergroup difference in the CSM, and the subdistribution proportional hazards model was used to perform multivariate analysis of CSM.
All statistical tests were conducted using RStudio (version 1.3.1073) in this study, including packages of xlsx, Table 1, survival, Survminer, MatchIt, cmprsk, and plyr. All tests were two-sided, and statistical significance was set at p < 0.05.
Results
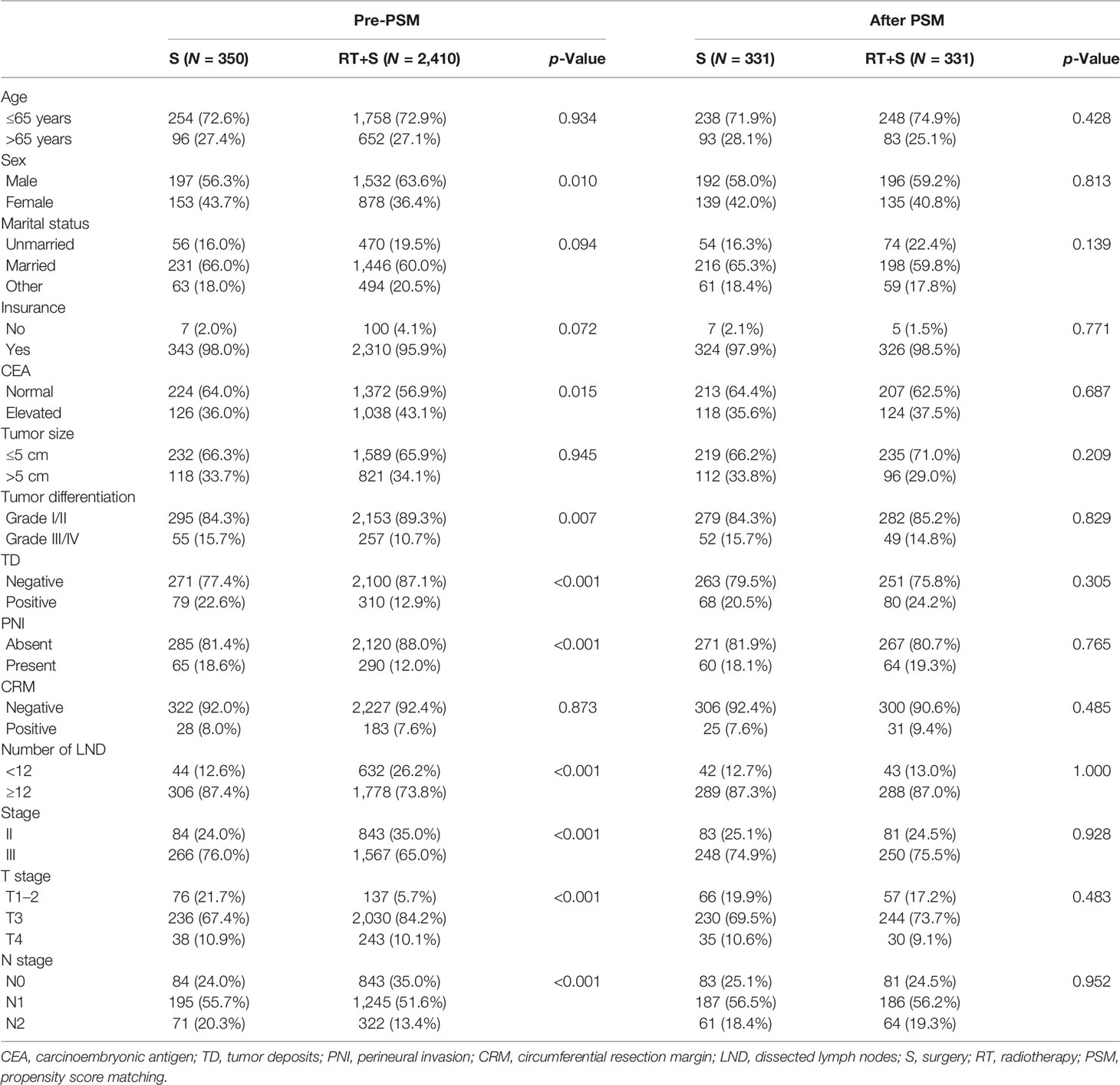
Patients’ Characteristics
A total of 2,760 patients were eligible for this study, including 350 (12.7%) patients in the S group and 2,410 (87.3%) in the RT+S group. The baseline characteristics between the S and RT+S groups were unparalleled, as depicted in Table 1. Briefly, the proportions of males, elevated CEA level, LND <12, and T3 in the RT+S group were all higher than those in the S group (all p < 0.05, Table 1), while the rates of tumor differentiation grades III/IV, TD, PNI, stage III, and N1/2 were lower in the RT+S group than in the S group (all p < 0.05, Table 1). However, the baseline characteristics between the two groups were comparable after 1:1 PSM (all p > 0.05, Table 1).
Effect of Preoperative Radiotherapy on CSS and CSM in LARC Patients
Before PSM, there were no significant differences in the CSS rates at 1, 3, and 4 years between the RT+S and S groups (98.02% vs. 95.78%, p = 0.078; 90.63% vs. 87.51%, p = 0.224; 84.57% vs. 82.94%, p = 0.374, respectively; Figure 1A), as well as the 1-, 3-, and 4-year CSM rates (1.97% vs. 4.20%, p = 0.068; 9.21% vs. 12.28%, p = 0.189; 15.03% vs. 16.61%, p = 0.364, respectively; Figure 2A). After PSM, the CSS rates at 1, 3, and 4 years in the RT+S group were higher than those in the S group, but there were still no statistical differences between the two groups (97.94% vs. 96.27%, P = 0.203; 93.58% vs. 88.64%, p = 0.134; 88.36% vs. 83.68%, p = 0.279, respectively; Figure 1B). Similar findings were observed in terms of the 1-, 3-, and 4-year CSM rates (2.05% vs. 3.72%, p = 0.194; 6.38% vs. 11.18%, p = 0.110; 11.53% vs. 15.88%, p = 0.255, respectively; Figure 2B).
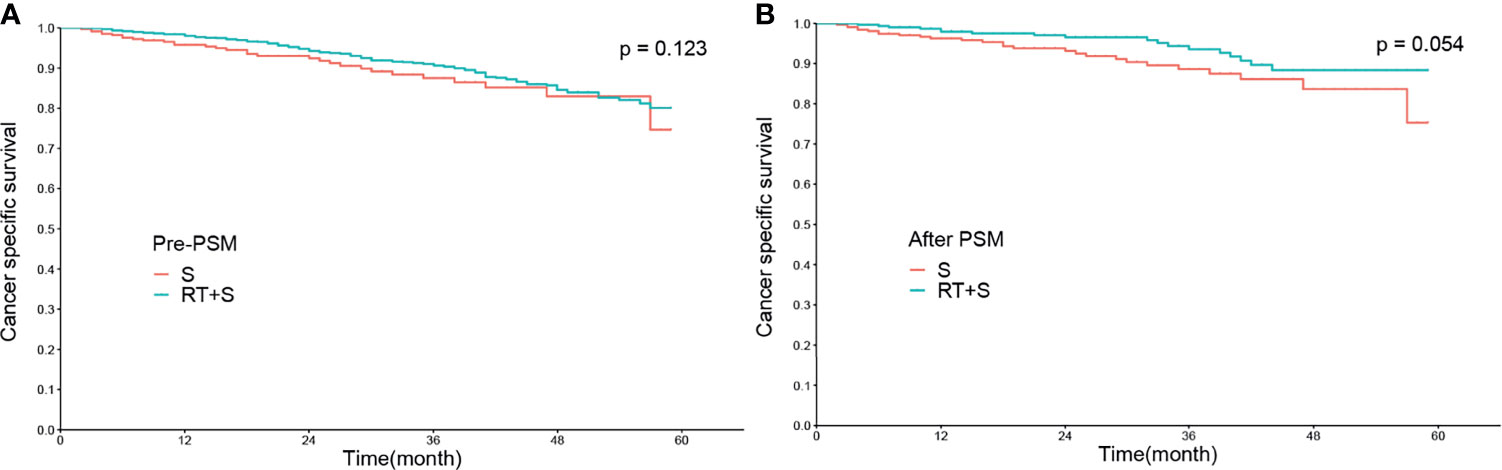
Figure 1 Cancer-specific survival of locally advanced rectal cancer before PSM (A) and after PSM (B). S, surgery; RT, radiotherapy; PSM, propensity score matching.
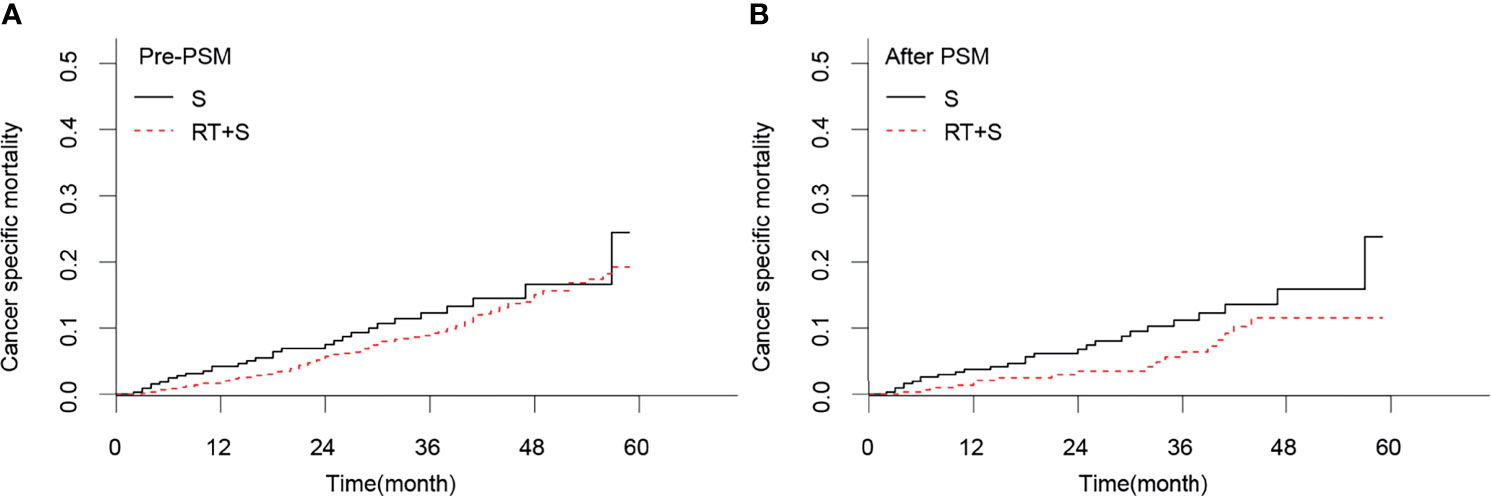
Figure 2 Cancer-specific mortality of locally advanced rectal cancer before PSM (A) and after PSM (B). S, surgery; RT, radiotherapy; PSM, propensity score matching.
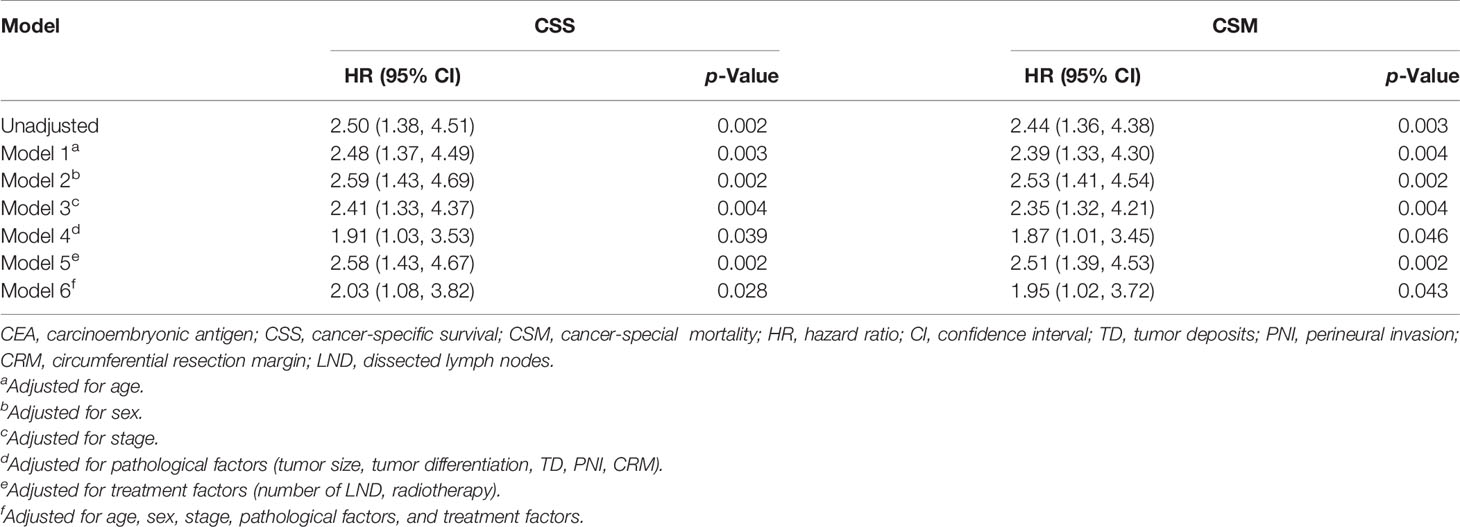
Effect of Pretreatment CEA Level on CSS and CSM in LARC Patients
In the matched cohort, time-dependent coefficient analysis showed a strong association between CEA and CSS (unadjusted hazard ratio (HR) = 2.50, 95% confidence interval (CI) = 1.38–4.51, p = 0.002). Age-adjusted HR for CSS among elevated CEA patients compared with normal CEA patients was 2.48 (95% CI = 1.37–4.49, p = 0.003). Further adjustments for sex (model 2), stage (model 3), pathological factors (model 4), and treatment factors (model 5) showed similar results (all p < 0.05, Table 2). Furthermore, these associations were not attenuated after adjustment for all five factor groups (model 6). Likewise, pretreatment CEA level was also found to be an independent risk factor of CSM regardless of the model (all p < 0.05, Table 2).
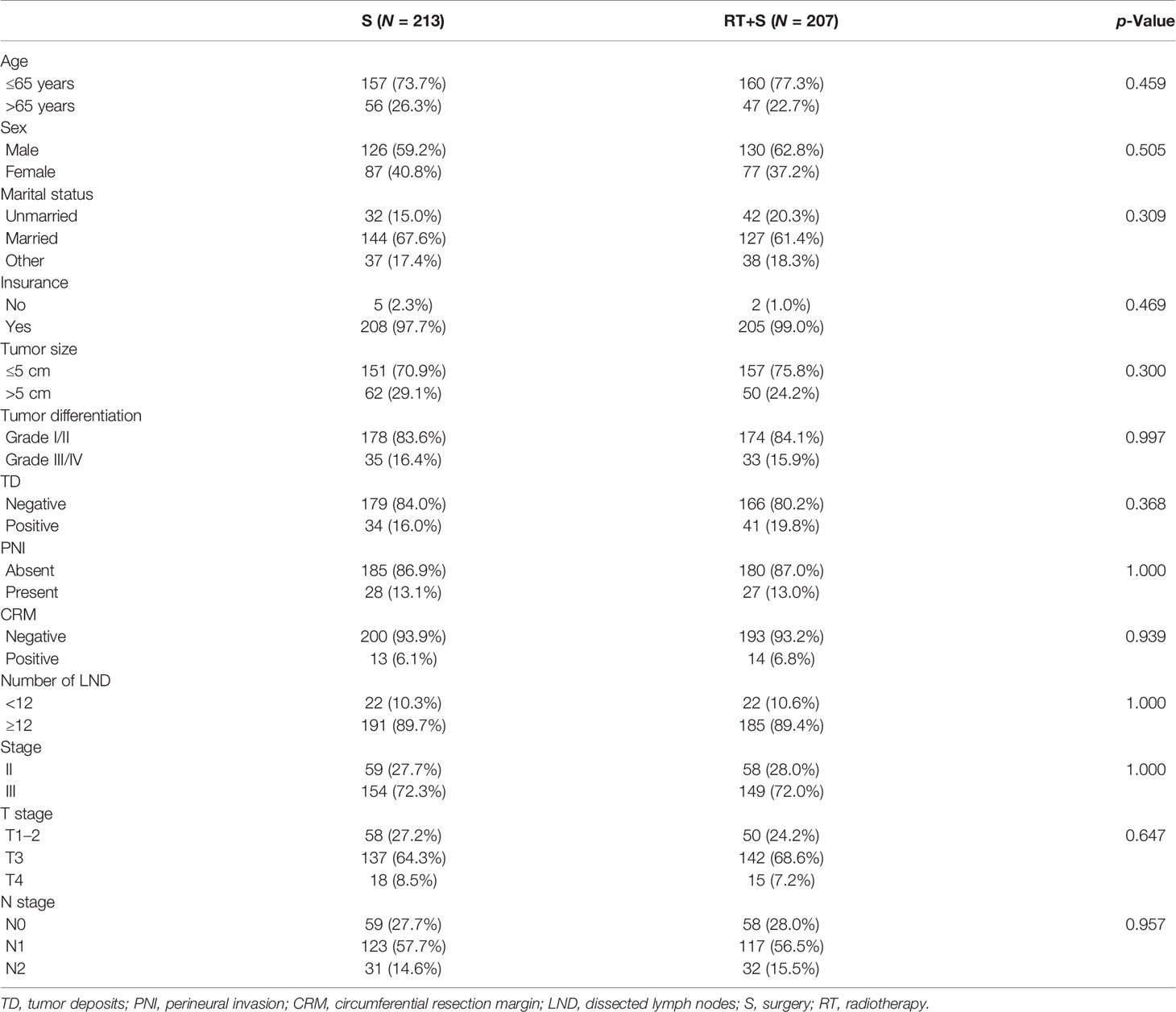
Effect of Preoperative Radiotherapy on CSS and CSM in Normal CEA Subgroup
In the matched cohort, 420 patients had normal CEA levels, including 213 patients in the S group and 207 in the RT+S group. Of note, there were no significant differences between the S and RT+S groups in terms of baseline characteristics (all p < 0.05, Table 3). K-M survival analysis showed that there was no significant difference in the median CSS between the two groups (HR = 0.73, 95% CI = 0.30–1.77, p = 0.490, Figure 3A). A similar finding was observed in CSM (HR = 0.74, 95% CI = 0.31–1.78, p = 0.500, Figure 4A).
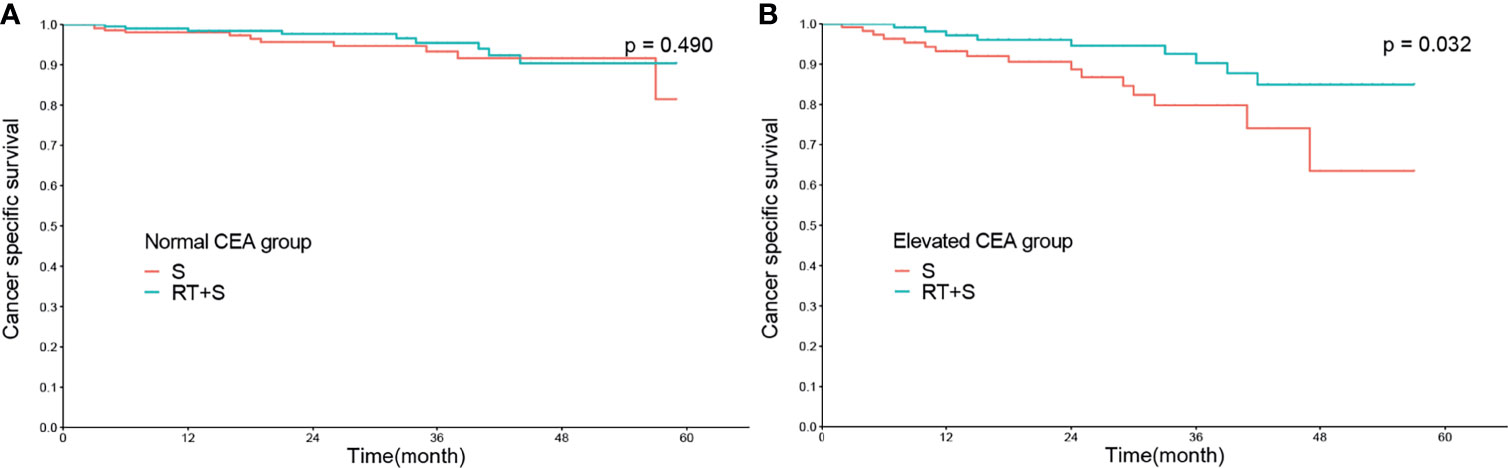
Figure 3 Cancer-specific survival of locally advanced rectal cancer in normal CEA group (A) and elevated CEA group (B). CEA, carcinoembryonic antigen; S, surgery; RT, radiotherapy.
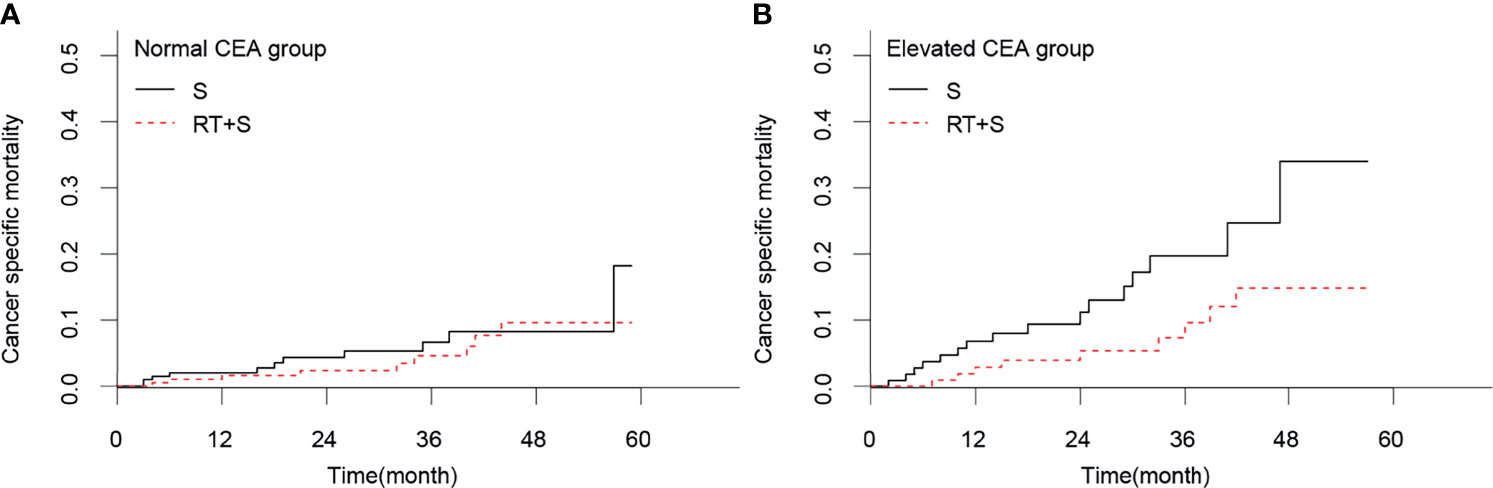
Figure 4 Cancer-specific mortality of locally advanced rectal cancer in normal CEA group (A) and elevated CEA group (B). CEA, carcinoembryonic antigen; S, surgery; RT, radiotherapy.
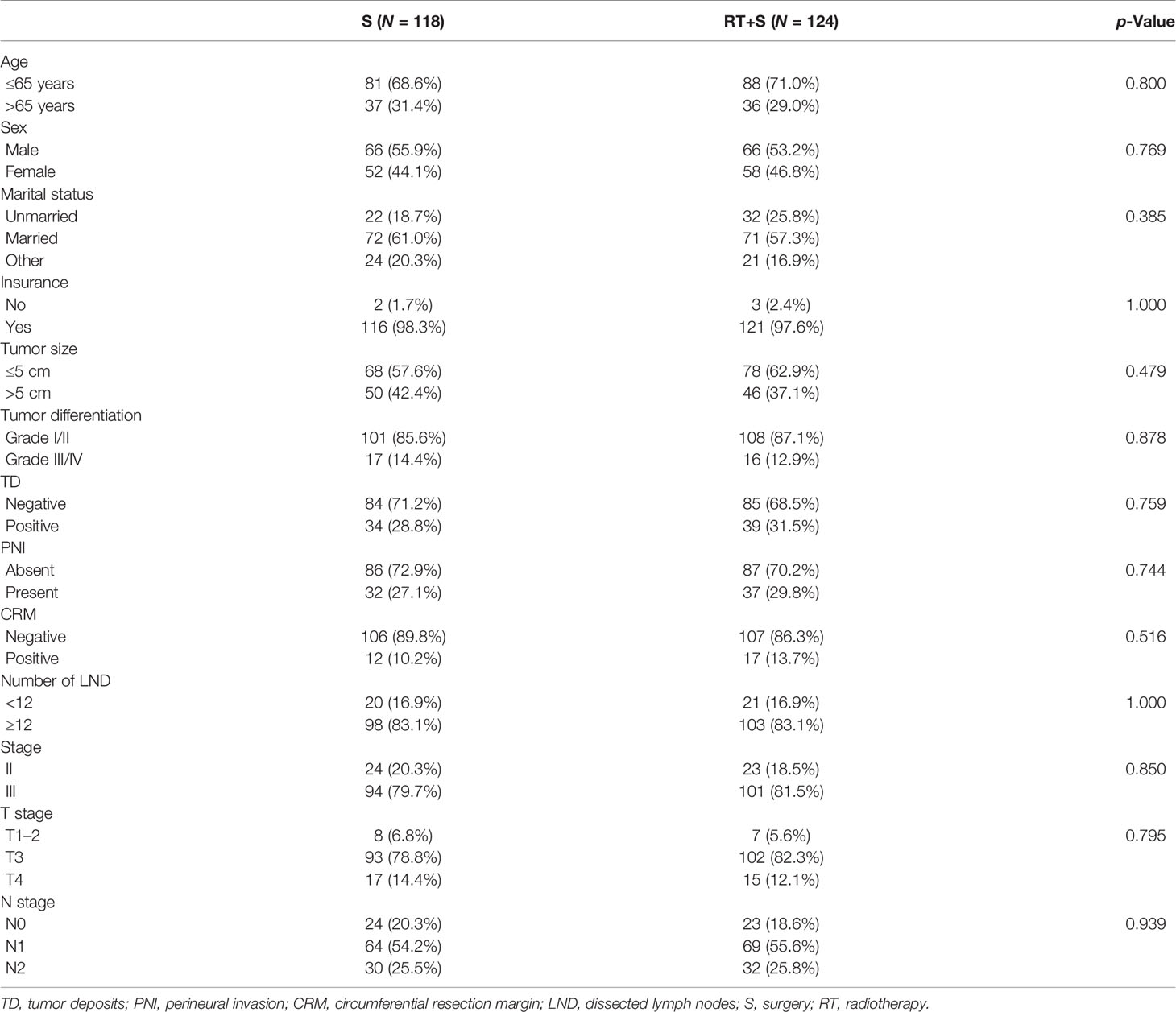
Effect of Preoperative Radiotherapy on CSS and CSM in Elevated CEA Subgroup
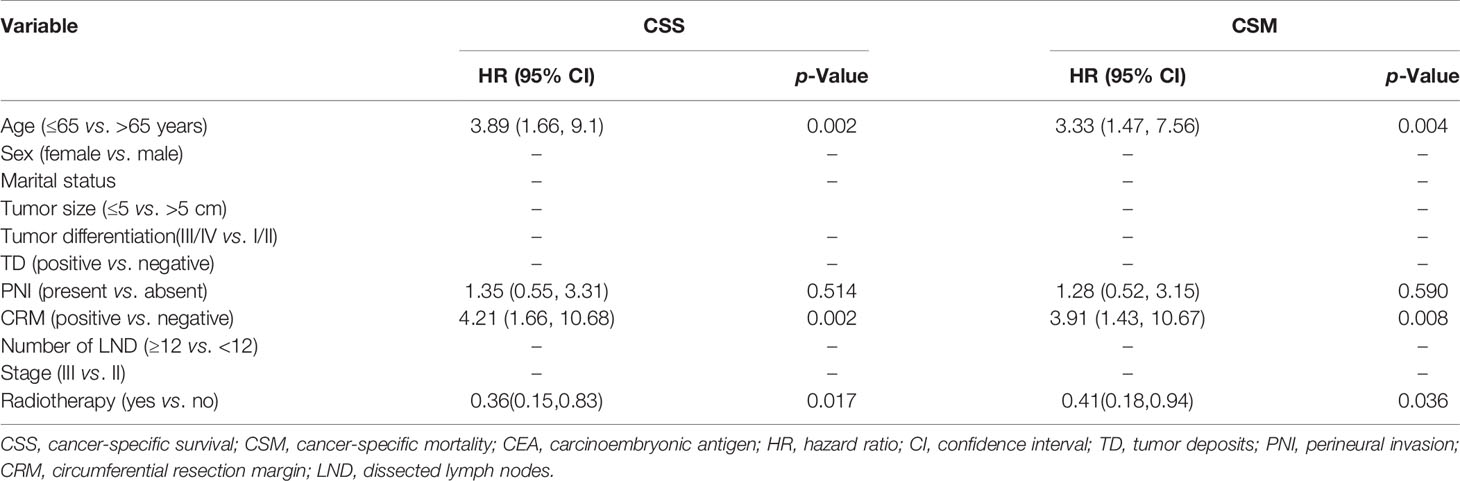
In the matched cohort, 242 patients were present with pre-treatment elevated CEA levels, including 118 patients in the S group and 124 in the RT+S group. Likewise, no significant differences were observed between the S and RT+S groups in terms of baseline characteristics (all p < 0.05, Table 4). The pooled HR for the median CSS was in favor of the RT+S group as compared with the S group (HR = 0.41, 95% CI = 0.18–0.92, p = 0.032, Figure 3B), with elevated survival rates at 1, 3, and 4 years (97.12% vs. 93.22%, 90.24% vs. 79.79%, 84.91% vs. 63.51%, respectively). Similar differences were also observed between the two groups (HR = 0.42, 95% CI = 0.19-0.94, p = 0.032; Figure 4B). Furthermore, multivariate analysis in the elevated CEA subgroup showed that preoperative radiotherapy was an independent protective factor for CSS and CSM (CSS: HR = 0.36, 95% CI = 0.15–0.83, p = 0.017; CSM: HR = 0.41, 95% CI = 0.18–0.94, p = 0.036, respectively; Table 5).
Discussion
The question whether preoperative radiotherapy can bring long-term survival benefit to LARC patients has been troubling the minds of surgeons and radiotherapists for a long time (7–11). In the current study, we found that pretreatment CEA level was a robust risk factor for prognosis after adjusting for confounding factors in different models. Furthermore, we also found that only a subgroup of LARC patients with elevated pretreatment CEA levels will benefit from preoperative radiotherapy in terms of CSS and CSM.
Preoperative radiotherapy followed by radical surgery has been preferred prevalently mainly due to its advantage on downstaging, pathological complete response (pCR), sphincter preservation and superior to adjuvant radiotherapy in prevention of local recurrence (19–21), although it can (1) increase the risk of surgical complications (22, 23), (2) bring radiation-related toxicity (24, 25), and (3) cannot improve the long-term prognosis of LARC patients when compared with adjuvant radiotherapy (20, 26, 27). In this study, 87.3% of patients received preoperative radiotherapy from 2011 to 2015 in the SEER database with a satisfactory 4-year CSS rate of 88.36%, indicating the importance of standardization treatment. Furthermore, in the recent decade, more neoadjuvant strategies have been explored with inspiring results in trials of FOWARC, RAPDIO, PRODIGEL 23, and IWWD, which attach more importance to preoperative radiotherapy (7, 9, 28, 29). The pCR rate is reported to range from 16.1% to 30% (9, 30–32), and patients with pCR generally have a better prognosis (33–35). However, the long-term survival benefit of preoperative radiotherapy has rarely been identified in previous reports (Table 6) (5–7, 9, 21, 28, 30, 36–38). In this study, we found that preoperative radiotherapy did not improve CSS in LARC patients before and after PSM (both p > 0.05). Additionally, we applied a competitive risk model to identify the true effect size of preoperative radiotherapy on long-term prognosis, since the rates of competition events were as high as 25% before PSM and as high as 21% after PSM. However, the results of the competitive risk model were highly consistent with the results of traditional K-M analysis, which indicated that noncancer-related mortality may have little effect on the conclusion of the study. Nonetheless, preoperative radiotherapy could not benefit LARC patients in terms of CSM before and after PSM (both p > 0.05). The reasons for this occurrence may be as follows: (1) in the era of neoadjuvant treatment followed by total mesorectal excision (TME), distant metastasis but not local recurrence is the decisive factor for long-term prognosis (7, 39); otherwise, (2) in the matched cohort, apparent survival differences are observed between the RT+S and S groups both in terms of CSS and CSM but with a margin p-value (p = 0.054, p = 0.059, respectively), which indicates that a larger sample size may be needed to avoid false-negative results.
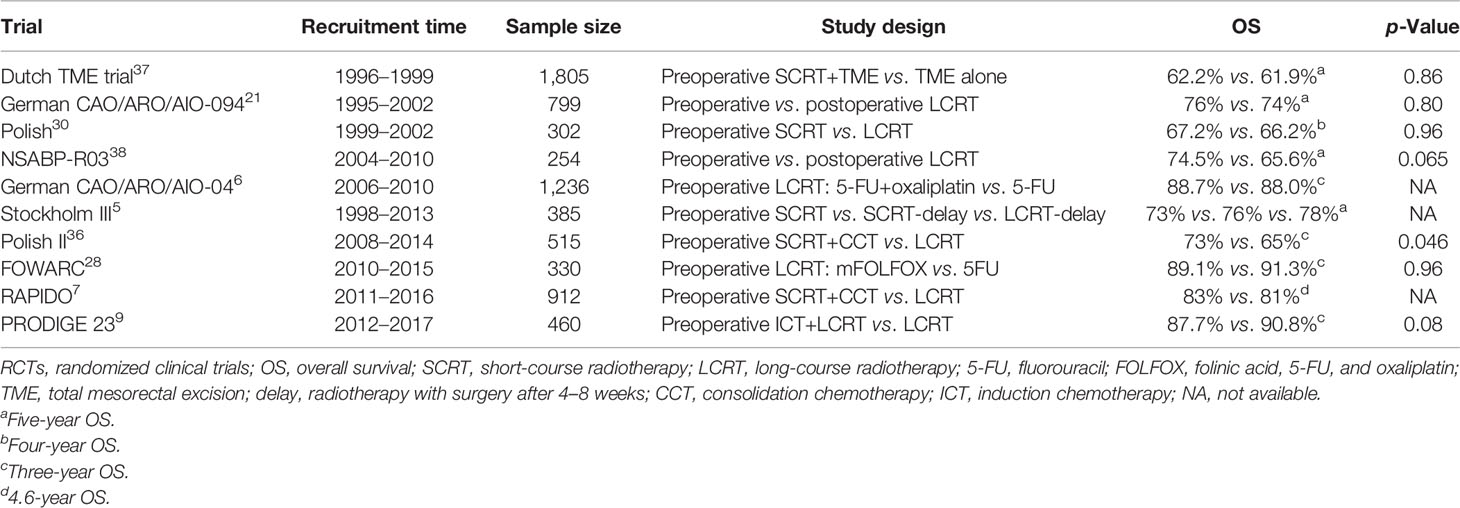
Table 6 Overall survival of locally advanced rectal cancer patients associated with neoadjuvant radiotherapy in phase III RCTs.
As a tumor-associated antigen, CEA has been used as a specific marker for the early diagnosis of colorectal cancer, however, its specificity is far from satisfactory (40, 41). In the current study, only 1,164 (42.6%) patients presented with elevated pre-treatment CEA levels. Nevertheless, the pretreatment CEA level was found to be an independent risk factor for both CSS and CSM, and it was reconfirmed in different models by adjusting for age, sex, tumor characteristics, and treatment factors (all p < 0.05), which indicated that pretreatment CEA level was a robust prognostic factor of long-term survival, and there may be an interaction between pre-treatment CEA level and preoperative treatment on long-term prognosis.
The relationship between CEA level and preoperative radiotherapy has been explored, however, pretreatment CEA level is not an indicator of preoperative radiotherapy. Pretreatment CEA level has been identified as a predictive biomarker of neoadjuvant treatment response, as well as post-treatment CEA levels and dynamic changes in CEA levels (18). Furthermore, CEA is associated with radiation sensitivity; tumors with normal CEA are sensitive to radiation, while tumors with elevated CEA levels are resistant to radiation (12). In the current study, subgroup analysis stratified by pretreatment CEA level showed that preoperative radiotherapy would only benefit patients with elevated CEA levels. The reasons for this may be as follows: (1) preoperative radiotherapy could reduce the risk of local recurrence, which is an important risk factor for long-term prognosis; (2) the compliance of patients with elevated CEA is higher than those with normal CEA, who are much more likely to receive a full course of chemotherapy; and (3) more intensive postoperative monitoring would be conducted in patients with elevated CEA levels, indicating a more timely intervention for early recurrence/metastasis. This finding suggested that pretreatment CEA could also be used as a potential biomarker to screen patients who would enjoy the long-term survival benefit of preoperative radiotherapy.
However, there are several limitations to the current study. First, selection bias is difficult to avoid in a retrospective analysis, although a well-designed PSM was conducted in our study. Second, data on preoperative radiotherapy, including clinical target volume and radiation regimen, are unavailable in the SEER database, which would weaken the conclusion of the current study. Third, data on chemotherapy, such as regimen and courses, are also unavailable, which is one of the most important risk factors for long-term prognosis. Hence, in the present study, we excluded all those patients who had not received adjuvant chemotherapy to decrease the effect of adjuvant chemotherapy on long-term prognosis. Finally, the receipt rate of preoperative radiotherapy varies from region to region, which indicates that our conclusion needed further validation using either data outside of the USA or multicenter randomized clinical trials accordingly.
Conclusion
Based on our results, we conclude that pretreatment CEA level may be considered a potential biomarker to screen LACR patients who would benefit from preoperative radiotherapy in terms of long-term prognosis.
Data Availability Statement
The dataset analyzed in this study from SEER can be obtained from: https://seer.cancer.gov/data/.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
LW, XZ, HL, LS, GC, and JW contributed to conception and design. XZ and HL conducted data collection and analyzed the data. LW, XZ, and HL interpreted the data. LW, XZ, and HL drafted the manuscript. LS, GC, and JW contributed to the critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the Science and Technology Program of Fujian Province, China (No. 2019L3018 and 2019YZ016006); the Fujian Province Finance Department Project (No. (2019)827).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the SEER database for providing valuable and public datasets.
References
1. Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2018) 16(7):874–901. doi: 10.6004/jnccn.2018.0061
2. Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, et al. Rectal Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann Oncol (2017) 28(suppl_4):iv22–40. doi: 10.1093/annonc/mdx224
3. Zhao F, Wang J, Yu H, Cheng X, Li X, Zhu X, et al. Neoadjuvant Radiotherapy Improves Overall Survival for T3/4N+M0 Rectal Cancer Patients: A Population-Based Study of 20300 Patients. Radiat Oncol (2020) 15(1):49. doi: 10.1186/s13014-020-01497-4
4. Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2016 for the Treatment of Colorectal Cancer. Int J Clin Oncol (2018) 23(1):1–34. doi: 10.1007/s10147-017-1101-6
5. Erlandsson J, Lörinc E, Ahlberg M, Pettersson D, Holm T, Glimelius B, et al. Tumour Regression After Radiotherapy for Rectal Cancer - Results From the Randomised Stockholm III Trial. Radiother Oncol (2019) 135:178–86. doi: 10.1016/j.radonc.2019.03.016
6. Rödel C, Graeven U, Fietkau R, Hohenberger W, Hothorn T, Arnold D, et al. Oxaliplatin Added to Fluorouracil-Based Preoperative Chemoradiotherapy and Postoperative Chemotherapy of Locally Advanced Rectal Cancer (the German CAO/ARO/AIO-04 Study): Final Results of the Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol (2015) 16(8):979–89. doi: 10.1016/S1470-2045(15)00159-X
7. Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, et al. Short-Course Radiotherapy Followed by Chemotherapy Before Total Mesorectal Excision (TME) Versus Preoperative Chemoradiotherapy, TME, and Optional Adjuvant Chemotherapy in Locally Advanced Rectal Cancer (RAPIDO): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2021) 22(1):29–42. doi: 10.1016/S1470-2045(20)30555-6
8. Cammà C, Giunta M, Fiorica F, Pagliaro L, Craxì A, Cottone M. Preoperative Radiotherapy for Resectable Rectal Cancer: A Meta-Analysis. JAMA (2000) 284(8):1008–15. doi: 10.1001/jama.284.81008
9. Conroy T, Bosset JF, Etienne PL, Rio E, François É, Mesgouez-Nebout N, et al. Neoadjuvant Chemotherapy With FOLFIRINOX and Preoperative Chemoradiotherapy for Patients With Locally Advanced Rectal Cancer (UNICANCER-PRODIGE 23): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2021) 22(5):702–15. doi: 10.1016/S1470-2045(21)00079-6
10. Keller DS, Berho M, Perez RO, Wexner SD, Chand M. The Multidisciplinary Management of Rectal Cancer. Nat Rev Gastroenterol Hepatol (2020) 17(7):414–29. doi: 10.1038/s41575-020-0275-y
11. Rahbari NN, Elbers H, Askoxylakis V, Motschall E, Bork U, Büchler MW, et al. Neoadjuvant Radiotherapy for Rectal Cancer: Meta-Analysis of Randomized Controlled Trials. Ann Surg Oncol (2013) 20(13):4169–82. doi: 10.1245/s10434-013-3198-9
12. Lee JH, Kim DY, Kim S, Cho HM, Shim BY, Kim TH, et al. Carcinoembryonic Antigen has Prognostic Value for Tumor Downstaging and Recurrence in Rectal Cancer After Preoperative Chemoradiotherapy and Curative Surgery: A Multi-Institutional and Case-Matched Control Study of KROG 14-12. Radiother Oncol (2015) 116(2):202–8. doi: 10.1016/j.radonc.2015.07.049
13. Li Z, Li C, Pu H, Pang X, Wang Y, Zhang D, et al. Trajectories of Perioperative Serum Carcinoembryonic Antigen and Colorectal Cancer Outcome: A Retrospective, Multicenter Longitudinal Cohort Study. Clin Transl Med (2021) 11(2):e293. doi: 10.1002/ctm2.293
14. Liemburg GB, Brandenbarg D, Berger MY, Duijts SFA, Holtman GA, de Bock GH, et al. Diagnostic Accuracy of Follow-Up Tests for Detecting Colorectal Cancer Recurrences in Primary Care: A Systematic Review and Meta-Analysis. Eur J Cancer Care (Engl) (2021) 30(5):e13432. doi: 10.1111/ecc.13432
15. Nicholson BD, Shinkins B, Mant D. Blood Measurement of Carcinoembryonic Antigen Level for Detecting Recurrence of Colorectal Cancer. JAMA (2016) 316(12):1310–1. doi: 10.1001/jama.2016.11212
16. Konishi T, Shimada Y, Hsu M, Tufts L, Jimenez-Rodriguez R, Cercek A, et al. Association of Preoperative and Postoperative Serum Carcinoembryonic Antigen and Colon Cancer Outcome. JAMA Oncol (2018) 4(3):309–15. doi: 10.1001/jamaoncol
17. Nakamura Y, Shida D, Tanabe T, Takamizawa Y, Imaizumi J, Ahiko Y, et al. Prognostic Impact of Preoperatively Elevated and Postoperatively Normalized Carcinoembryonic Antigen Levels Following Curative Resection of Stage I-III Rectal Cancer. Cancer Med (2020) 9(2):653–62. doi: 10.1002/cam4.2758
18. Cheong C, Shin JS, Suh KW. Prognostic Value of Changes in Serum Carcinoembryonic Antigen Levels for Preoperative Chemoradiotherapy Response in Locally Advanced Rectal Cancer. World J Gastroenterol (2020) 26(44):7022–35. doi: 10.3748/wjg.v26.i44.7022
19. Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, et al. Chemotherapy With Preoperative Radiotherapy in Rectal Cancer. N Engl J Med (2006) 355(11):1114–23. doi: 10.1056/NEJMoa060829
20. Park JH, Yoon SM, Yu CS, Kim JH, Kim TW, Kim JC. Randomized Phase 3 Trial Comparing Preoperative and Postoperative Chemoradiotherapy With Capecitabine for Locally Advanced Rectal Cancer. Cancer (2011) 117(16):3703–12. doi: 10.1002/cncr.25943
21. Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, et al. Preoperative Versus Postoperative Chemoradiotherapy for Rectal Cancer. N Engl J Med (2004) 351(17):1731–40. doi: 10.1056/NEJMoa040694
22. Jonker FH, Tanis PJ, Coene PP, van der Harst E. Impact of Neoadjuvant Radiotherapy on Complications After Hartmann Procedure for Rectal Cancer. Dis Colon Rectum (2015) 58(10):931–7. doi: 10.1097/DCR.0000000000000432
23. Musters GD, Buskens CJ, Bemelman WA, Tanis PJ. Perineal Wound Healing After Abdominoperineal Resection for Rectal Cancer: A Systematic Review and Meta-Analysis. Dis Colon Rectum (2014) 57(9):1129–39. doi: 10.1097/DCR.0000000000000303
24. Brændengen M, Tveit KM, Bruheim K, Cvancarova M, Berglund Å, Glimelius B. Late Patient-Reported Toxicity After Preoperative Radiotherapy or Chemoradiotherapy in Nonresectable Rectal Cancer: Results From a Randomized Phase III Study. Int J Radiat Oncol Biol Phys (2011) 81(4):1017–24. doi: 10.1016/j.ijrobp.2010.07.007
25. Kim SH, Kim JH, Jung SH. Late Complications After Proctectomy in Rectal Cancer Patients Who Underwent Radiotherapy. World J Surg (2014) 38(9):2471–6. doi: 10.1007/s00268-014-2577-0
26. Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, et al. Fluorouracil-Based Adjuvant Chemotherapy After Preoperative Chemoradiotherapy in Rectal Cancer: Long-Term Results of the EORTC 22921 Randomised Study. Lancet Oncol (2014) 15(2):184–90. doi: 10.1016/S1470-2045(13)70599-0
27. Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, et al. Preoperative Versus Postoperative Chemoradiotherapy for Locally Advanced Rectal Cancer: Results of the German CAO/ARO/AIO-94 Randomized Phase III Trial After a Median Follow-Up of 11 Years. J Clin Oncol (2012) 30(16):1926–33. doi: 10.1200/JCO.2011.40.1836
28. Deng Y, Chi P, Lan P, Wang L, Chen W, Cui L, et al. Neoadjuvant Modified FOLFOX6 With or Without Radiation Versus Fluorouracil Plus Radiation for Locally Advanced Rectal Cancer: Final Results of the Chinese FOWARC Trial. J Clin Oncol (2019) 37(34):3223–33. doi: 10.1200/JCO.18.02309
29. Fernandez LM, São Julião GP, Figueiredo NL, Beets GL, van der Valk MJM, Bahadoer RR, et al. Conditional Recurrence-Free Survival of Clinical Complete Responders Managed by Watch and Wait After Neoadjuvant Chemoradiotherapy for Rectal Cancer in the International Watch & Wait Database: A Retrospective, International, Multicentre Registry Study. Lancet Oncol (2021) 22(1):43–50. doi: 10.1016/S1470-2045(20)30557-X
30. Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M. Long-Term Results of a Randomized Trial Comparing Preoperative Short-Course Radiotherapy With Preoperative Conventionally Fractionated Chemoradiation for Rectal Cancer. Br J Surg (2006) 93(10):1215–23. doi: 10.1002/bjs.5506
31. Rödel C, Liersch T, Becker H, Fietkau R, Hohenberger W, Hothorn T, et al. Preoperative Chemoradiotherapy and Postoperative Chemotherapy With Fluorouracil and Oxaliplatin Versus Fluorouracil Alone in Locally Advanced Rectal Cancer: Initial Results of the German CAO/ARO/AIO-04 Randomised Phase 3 Trial. Lancet Oncol (2012) 13(7):679–87. doi: 10.1016/S1470-2045(12)70187-0
32. Zhu J, Liu A, Sun X, Liu L, Zhu Y, Zhang T, et al. Multicenter, Randomized, Phase III Trial of Neoadjuvant Chemoradiation With Capecitabine and Irinotecan Guided by UGT1A1 Status in Patients With Locally Advanced Rectal Cancer. J Clin Oncol (2020) 38(36):4231–9. doi: 10.1200/JCO.20.01932
33. Capirci C, Valentini V, Cionini L, De Paoli A, Rodel C, Glynne-Jones R, et al. Prognostic Value of Pathologic Complete Response After Neoadjuvant Therapy in Locally Advanced Rectal Cancer: Long-Term Analysis of 566 ypCR Patients. Int J Radiat Oncol Biol Phys (2008) 72(1):99–107. doi: 10.1016/j.ijrobp.2007.12.019
34. Díaz-González JA, Calvo FA, Cortés J, García-Sabrido JL, Gómez-Espí M, Del Valle E, et al. Prognostic Factors for Disease-Free Survival in Patients With T3-4 or N+ Rectal Cancer Treated With Preoperative Chemoradiation Therapy, Surgery, and Intraoperative Irradiation. Int J Radiat Oncol Biol Phys (2006) 64(4):1122–8. doi: 10.1016/j.ijrobp.2005.09.020
35. Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, et al. Long-Term Outcome in Patients With a Pathological Complete Response After Chemoradiation for Rectal Cancer: A Pooled Analysis of Individual Patient Data. Lancet Oncol (2010) 11(9):835–44. doi: 10.1016/S1470-2045(10)70172-8
36. Ciseł B, Pietrzak L, Michalski W, Wyrwicz L, Rutkowski A, Kosakowska E, et al. Long-Course Preoperative Chemoradiation Versus 5 × 5 Gy and Consolidation Chemotherapy for Clinical T4 and Fixed Clinical T3 Rectal Cancer: Long-Term Results of the Randomized Polish II Study. Ann Oncol (2019) 30(8):1298–303. doi: 10.1093/annonc/mdz186
37. Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative Radiotherapy Combined With Total Mesorectal Excision for Resectable Rectal Cancer. N Engl J Med (2001) 345(9):638–46. doi: 10.1056/NEJMoa010580
38. Roh MS, Colangelo LH, O’Connell MJ, Yothers G, Deutsch M, Allegra CJ, et al. Preoperative Multimodality Therapy Improves Disease-Free Survival in Patients With Carcinoma of the Rectum: NSABP R-03. J Clin Oncol (2009) 27(31):5124–30. doi: 10.1200/JCO.2009.22.0467
39. Frambach P, Pucciarelli S, Perin A, Zuin M, Toppan P, Maretto I, et al. Metastatic Pattern and New Primary Tumours After Neoadjuvant Therapy and Surgery in Rectal Cancer. Colorectal Dis (2018) 20(12):O326–o334. doi: 10.1111/codi.14427
40. Hammarström S. The Carcinoembryonic Antigen (CEA) Family: Structures, Suggested Functions and Expression in Normal and Malignant Tissues. Semin Cancer Biol (1999) 9(2):67–81. doi: 10.1006/scbi.1998.0119
Keywords: locally advanced rectal cancer, preoperative radiotherapy, carcinoembryonic antigen, SEER, biomarker
Citation: Wang L, Zhong X, Lin H, Shao L, Chen G and Wu J (2021) The Correlation Between Survival Benefit of Preoperative Radiotherapy and Pretreatment Carcinoembryonic Antigen Level in Locally Advanced Rectal Cancer. Front. Oncol. 11:735882. doi: 10.3389/fonc.2021.735882
Received: 15 July 2021; Accepted: 17 September 2021;
Published: 07 October 2021.
Edited by:
Sunil Krishnan, Mayo Clinic Florida, United StatesReviewed by:
Olivera Ivanov, University of Novi Sad, SerbiaAaron Bush, Mayo Clinic Florida, United States
Copyright © 2021 Wang, Zhong, Lin, Shao, Chen and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junxin Wu, junxinwufj@aliyun.com; Gang Chen, naichengang@126.com
†These authors have contributed equally to this work
 Lei Wang
Lei Wang Xiaohong Zhong1†
Xiaohong Zhong1† Huaqin Lin
Huaqin Lin Lingdong Shao
Lingdong Shao Junxin Wu
Junxin Wu