- 1Department of Medical Genetics, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Phytochemistry Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 3Department of Pharmacognosy, College of Pharmacy, Hawler Medical University, Erbil, Iraq
- 4Urology and Nephrology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 5Institute of Human Genetics, Jena University Hospital, Jena, Germany
- 6Skull Base Research Center, Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
AFAP1-AS1 is a long non-coding RNA which partakes in the pathoetiology of several cancers. The sense protein coding gene from this locus partakes in the regulation of cytophagy, cell motility, invasive characteristics of cells and metastatic ability. In addition to acting in concert with AFAP1, AFAP1-AS1 can sequester a number of cancer-related miRNAs, thus affecting activity of signaling pathways involved in cancer progression. Most of animal studies have confirmed that AFAP1-AS1 silencing can reduce tumor volume and invasive behavior of tumor cells in the xenograft models. Moreover, statistical analyses in the human subjects have shown strong correlation between expression levels of this lncRNA and clinical outcomes. In the present work, we review the impact of AFAP1-AS1 in the carcinogenesis.
Introduction
Actin filament-associated protein 1 antisense RNA 1 (AFAP1-AS1, NC_000004.12) is a long non-coding RNA (lncRNA) which contributes in the pathoetiology of several cancers (1). It is transcribed from AFAP1 gene locus on 4p16.1. It has two alternatively spliced variants. Its second exon overlaps with exons 14-16 of AFAP1 gene. The motor fiber-associated protein encoded by AFAP1 has been shown to organize a platform for joining a number of tumor-related proteins such as SRC and protein kinase C (2). This platform can influence the organization and activity of actin filaments, therefore participating in cytophagy, cell motility, invasive characteristics of cells and metastatic ability (3). Both AFAP1 and FAP1-AS1 participate in the carcinogenesis through modulation of related signaling pathways. AFAP1 has acknowledged roles in the pathogenesis of a number of cancers, namely breast (4) and prostate cancer (5), yet its expression has been found to decreased in gastric cancer samples (6). AFAP1-AS1 is mainly regarded as an oncogenic lncRNA (1). However, the oncogenic effect of this lncRNA is not necessarily exerted through AFAP1-dependent routes. A number of deletion type copy-number variants (CNVs) have been identified in AFAP1-AS1 coding gene through application of whole genome sequencing (7). AFAP1-AS1 has been shown to affect several aspects of carcinogenesis through modulation of expression of cancer-related miRNAs. Since it has been shown to be dysregulated in diverse types of cancer, this lncRNA is a putative marker for a wide variety of cancers. Functional impacts of AFAP1-AS1 in the carcinogenesis have been appraised through knock-down and over-expression studies in cell lines and animal models. Moreover, the impact of AFAP1-AS1 deregulation has been assessed in human samples. In the present review, we discuss the role of AFAP1-AS1 in the carcinogenesis based on the evidence from these three types of studies.
Cell Line Studies
Lung Cancer
AFAP1-AS1 has been found to be over-expressed in non-small cell lung cancer (NSCLC) cells H1975, PC-9, A549, and SPCA-1 compared with the human non-tumorigenic lung epithelial cell line BEAS-2B. Functional studies in these cells have confirmed the ability of this lncRNA in binding with and sequestering miR-139-5p, a down-regulated miRNA in NSCLC samples. AFAP1-AS1 silencing and miR-139-5p up-regulation could similarly inhibit proliferation, colony forming ability and chemoresistance of NSCLC cells, while increasing their apoptosis. The sequestering impact of AFAP1-AS1 on miR-139-5p leads to up-regulation of RRM2, a protein which has been demonstrated to increase chemoresistance of NSCLC cells via activation of EGFR/AKT pathway (8). Another study in NSCLC has shown up-regulation of FAP1-AS1 parallel with down-regulation of IL-12 and up-regulation of IL-10 and IFN-γ. Functionally, AFAP1-AS1 has been shown to induce activity of IRF7, RIG-I-like receptor signals and Bcl-2. Cumulatively, AFAP1-AS1 enhances migration and invasive properties of NSCLC cells through activating IRF7 and the RIG-I-like receptor signaling pathway (9). Moreover, the interaction between AFAP1-AS1 and EZH2 and subsequent recruitment of EZH2 to the promoter of p21 has been shown to repress expression of p21 in this type of cancer (10). AFAP1-AS1 has also been shown to enhance expression of AFAP1 in lung cancer cells. Expression of AFAP1-AS1 in lung cancer cells is regulated through CpG methylation marks in its promoter, since the DNA methyltransferase inhibitor agent decitabine has been demonstrated to activate AFAP1-AS1 expression. AFAP1-AS1 has been reported to increase expression levels of pro-invasive genes PPP1R13L, VASP and SPTAN1, while decreasing expression levels of a number of anti-metastatic genes such as STAT1, NF1, and FBN2 (11). Figure 1 summarizes the mentioned routes of participation of AFAP1-As1 in the pathogenesis of lung cancer.
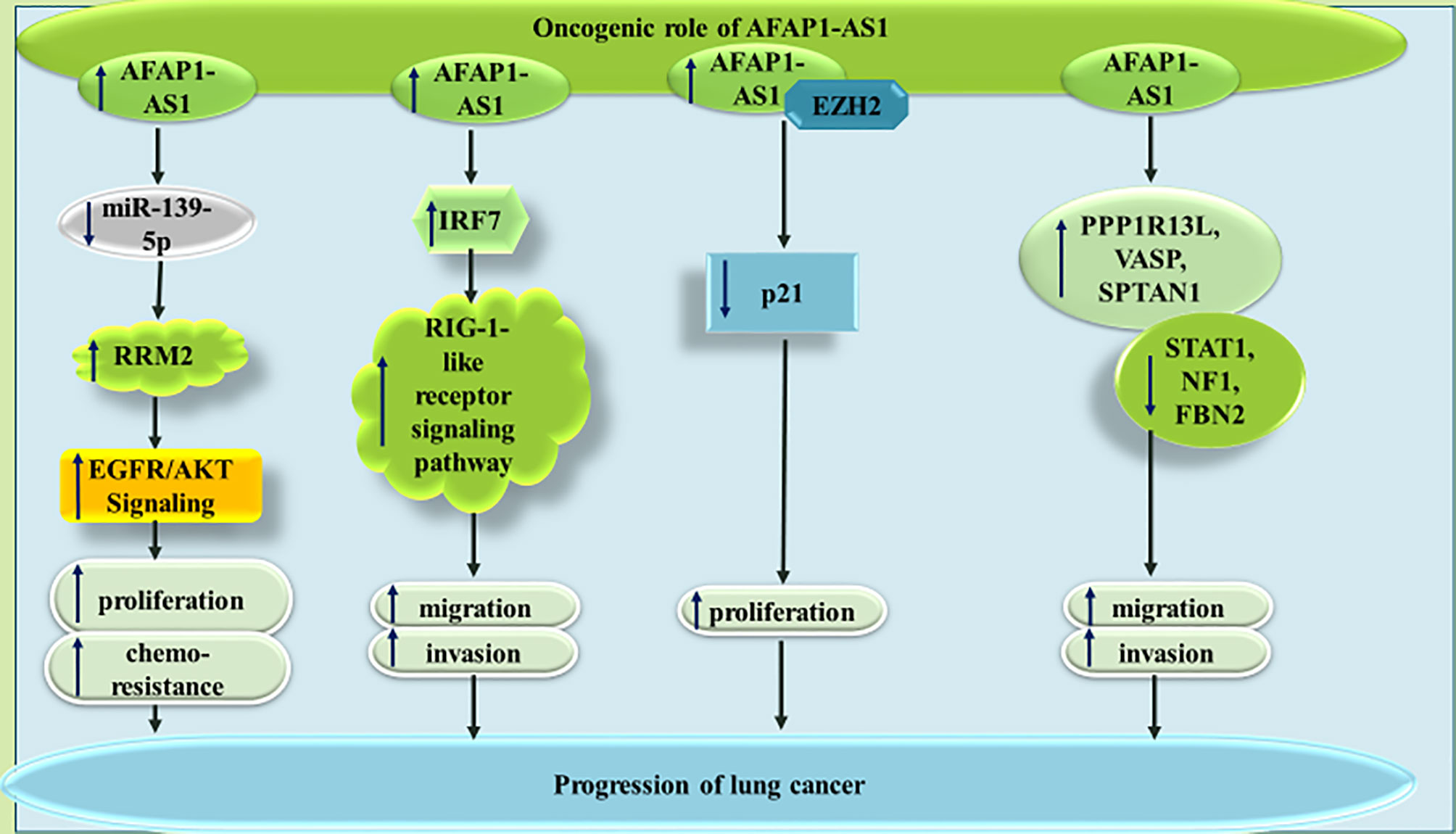
Figure 1 The oncogenic role of AFAP1-AS1 in lung cancer through modulation of expressions of RRM2, IRF7, p21 and PPP1R13L. The effects of AFAP1-AS1 on RRM2 expression is mediated through sponging miR-139-5p. This mode of action results in enhancement of cell proliferation, migration and invasiveness.
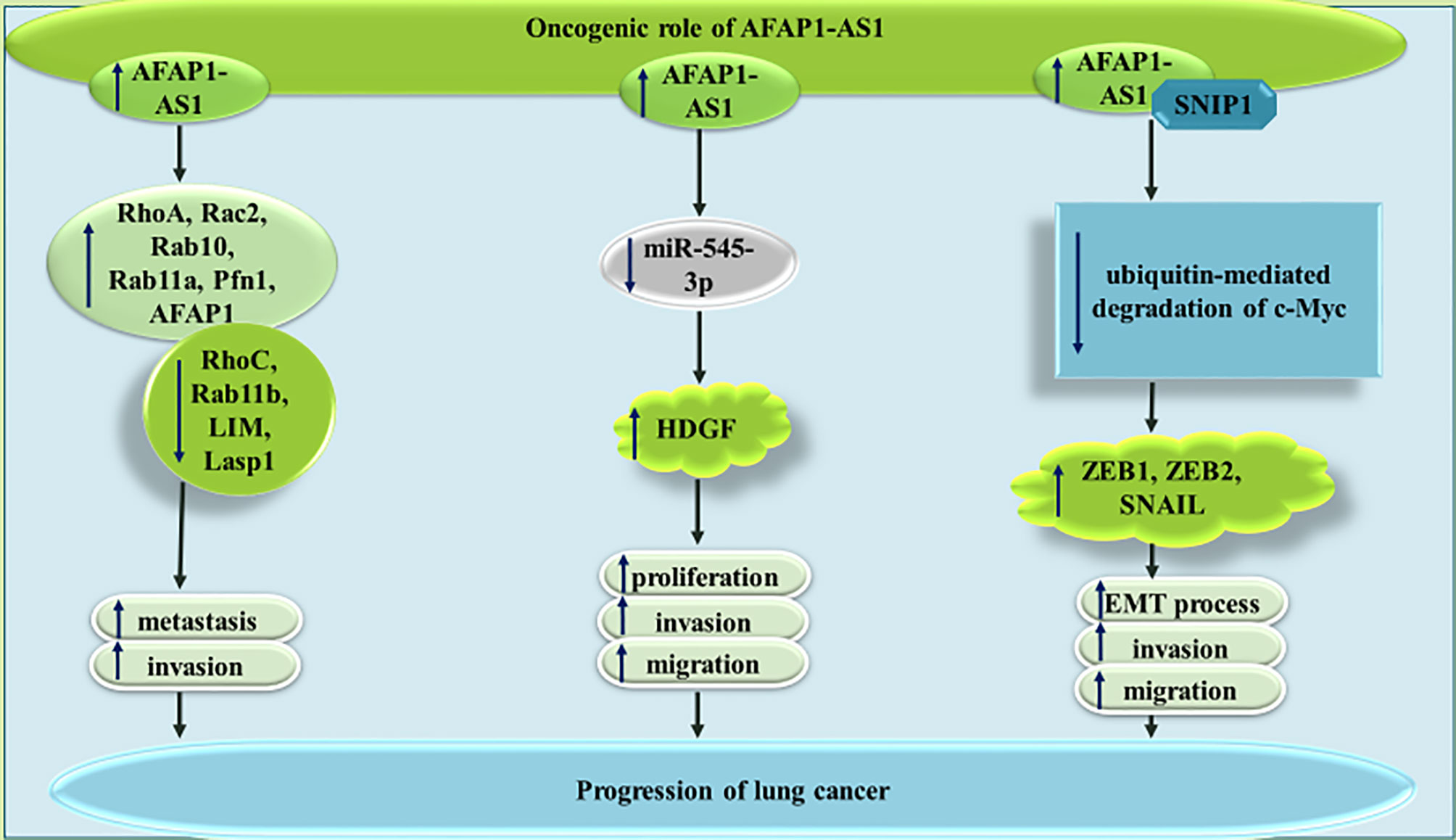
AFAP1-AS1 can also affect lung cancer through a variety of other mechanisms being summarized in Figure 2. For instance, AFAP1-AS1 has been shown to regulate expression of numerous members of the small GTPase proteins as well as those participating in the actin cytokeratin signaling. Thus, the promoting effect of AFAP1-AS1 on cancer metastasis is most probably exerted through modulation of actin filament integrity (12). GTPases harmonize several cellular processes, such as cell polarity, migration, and cell cycle transition, thus they can participate in the pathogenies of cancer (13). Moreover, cytokeratins as members of intermediate filament protein family have been shown to affect carcinogenesis. They can also been used as cancer biomarkers (14).
AFAP1-AS1 can also enhance expression of HDGF through decreasing miR-545-3p levels in lung cancer cells. Thus, AFAP1-AS1 silencing could inhibit progression of lung cancer through influencing activity of miR-545-3p/HDGF axis (15). Finally, AFAP1-AS1 can interact with Smad nuclear interacting protein 1 (SNIP1), a protein which suppresses ubiquitination and subsequent destruction of c-Myc. This function of AFAP1-AS1 leads to over-expression of c-Myc, increase in ZEB1, ZEB2, and SNAIL levels, and enhancement of epithelial to mesenchymal transition (EMT) (16).
Breast Cancer
In breast cancer cells, AFAP1-AS1 silencing could decrease proliferation and migratory potential, and increase cell apoptosis. miR-497-5p has been recognized as a target of AFAP1-AS1 in breast cancer cells. Since this miRNA targets SEPT2, AFAP1-AS1 up-regulation results in up-regulation of SEPT2 (17). miR-145 is another target of AFAP1-AS1 in triple negative breast cancer cells (TNBC) MDA-MB-231 breast cancer cells. According to the results of luciferase reporter assay, miR-145 can directly target MTH1. Thus, the effects of AFAP1-AS1 in enhancement of proliferation and invasiveness of TNBC are exerted through miR-145/MTH1 axis (18). Moreover, in this type of cancer, AFAP1-AS1 can sequester miR-2110 to enhance expression of Sp1 (19). AFAP1-AS1 has also been shown to enhance EMT of TNBC cells via influencing Wnt/β-catenin signaling (20). Finally, AFAP1-AS1 has been found to have significant over-expression in trastuzumab-resistant breast cancer cells versus responsive cells. Expression of this lncRNA has been enhanced by H3K27ac at its promoter. Most notably, trastuzumab resistant cells have been shown to secrete AFAP1-AS1 into exosomes, thus disseminating trastuzumab resistance in other cells. The impact of exosomal AFAP1-AS1 in induction of trastuzumab resistance is exerted via its interaction with AUF1 and subsequent induction of ERBB2 translation (21). Figure 3 depicts the impact of AFAP1-AS1 in carcinogenesis and therapy resistance of breast cancer cells.
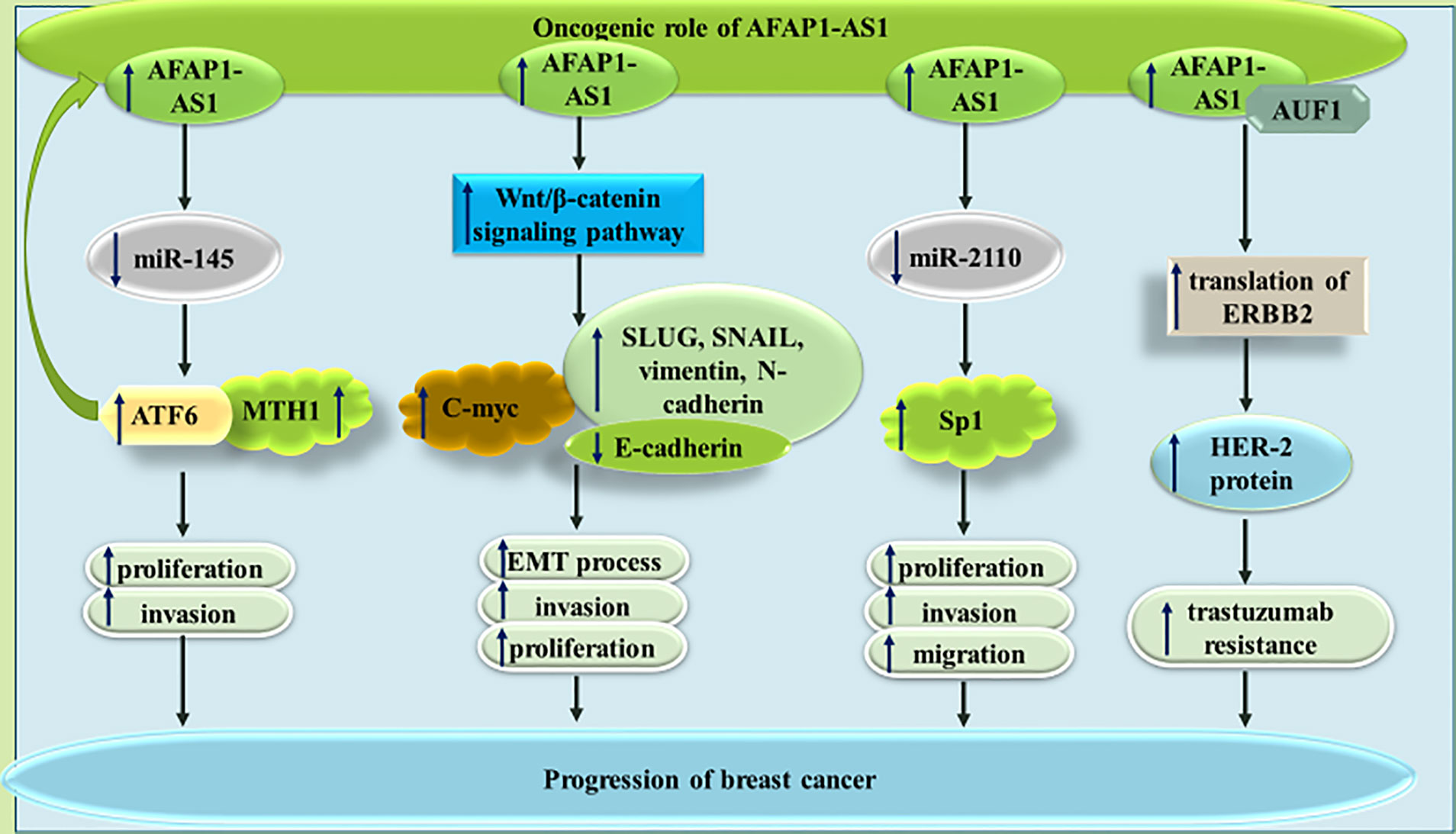
Figure 3 The impact of AFAP1-AS1 in breast cancer progression and resistance to therapy. In addition to increasing cell proliferation and invasion, this lncRNA can increase expression of Her-2 protein, thus increasing resistance to trastuzumab.
Osteosarcoma
In MNNG/HOS and U2OS osteosarcoma cells, AFAP1-AS1 has been found to promote tumorigenesis via influencing RhoC/ROCK1/p38MAPK/Twist1 cascade (22). The AFAP1-AS1-mediated increase in Twist1 can enhance expression of N-cadherin and Vimentin, while diminishing E-cadherin levels, thus promoting EMT of osteosarcoma cells (22). Moreover, AFAP1-AS1 can sequester miR-497 and miR-4695-5p in these cells, therefore increasing expressions of IGF1R and TCF4, respectively (23, 24). The latter can activate Wnt-β catenin pathway and increase both proliferation and invasive abilities of osteosarcoma cells (24). Figure 4 depicts the oncogenic role of AFAP1-AS1 in osteosarcoma.
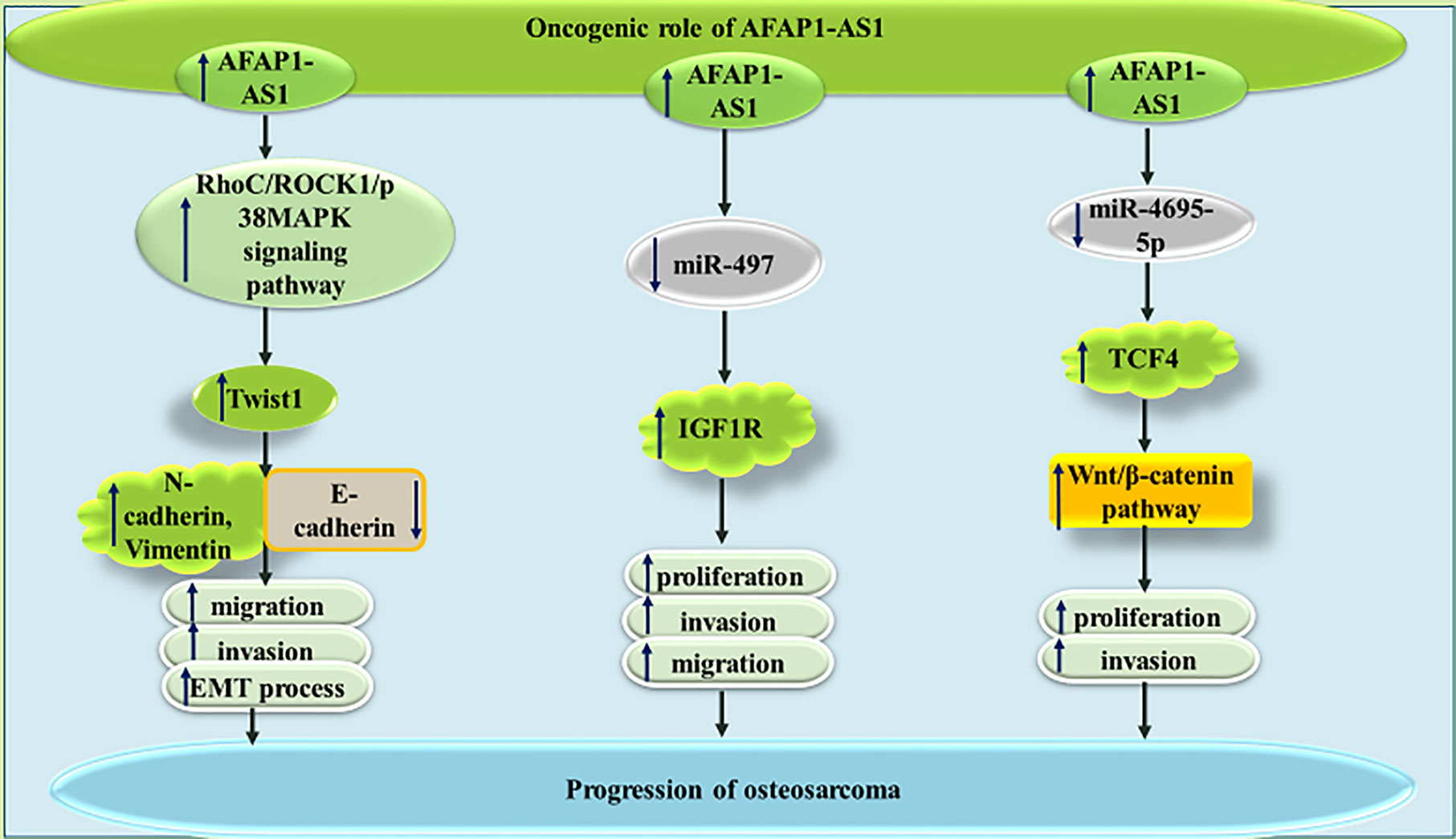
Figure 4 The oncogenic role of AFAP1-AS1 in osteosarcoma is exerted through modulation of RhoC/ROCK1/p38MAPK/Twist1 cascade as well as sponging miR-497 and miR-4695-5p.
Gastric Cancer
Similarly, AFAP1-AS1 has an oncogenic role in gastric cancer. AFAP1-AS1 silencing has significantly suppressed proliferation and cell cycle transition in this kind of cancer. Besides, reduction in the levels of this lncRNA can inhibit invasive capacity through affecting EMT (25). Down-regulation of KLF2 is another mechanism by which AFAP1-AS1 enhances proliferative and migratory aptitudes of gastric cancer cells (26). AFAP1-AS1 silencing in gastric cancer cells has led to a significant increase in the levels of Bax, cleaved PARP, Caspase 3, and Caspase 9, while decreasing Bcl-2 level. AFAP1-AS1 silencing has also reduced p-AKT levels and enhanced expression of PTEN in gastric cancer cells. Taken together, AFAP1-AS1 regulates proliferation and apoptotic processes in gastric cancer cell through PTEN/p-AKT cascade (27). AFAP1-AS1 can also promote proliferation and metastatic ability of gastric cancer cell through sequestering miR-155-5p and enhancing expression of FGF7 (28). Figure 5 shows the oncogenic role of AFAP1-AS1 in gastric cancer.
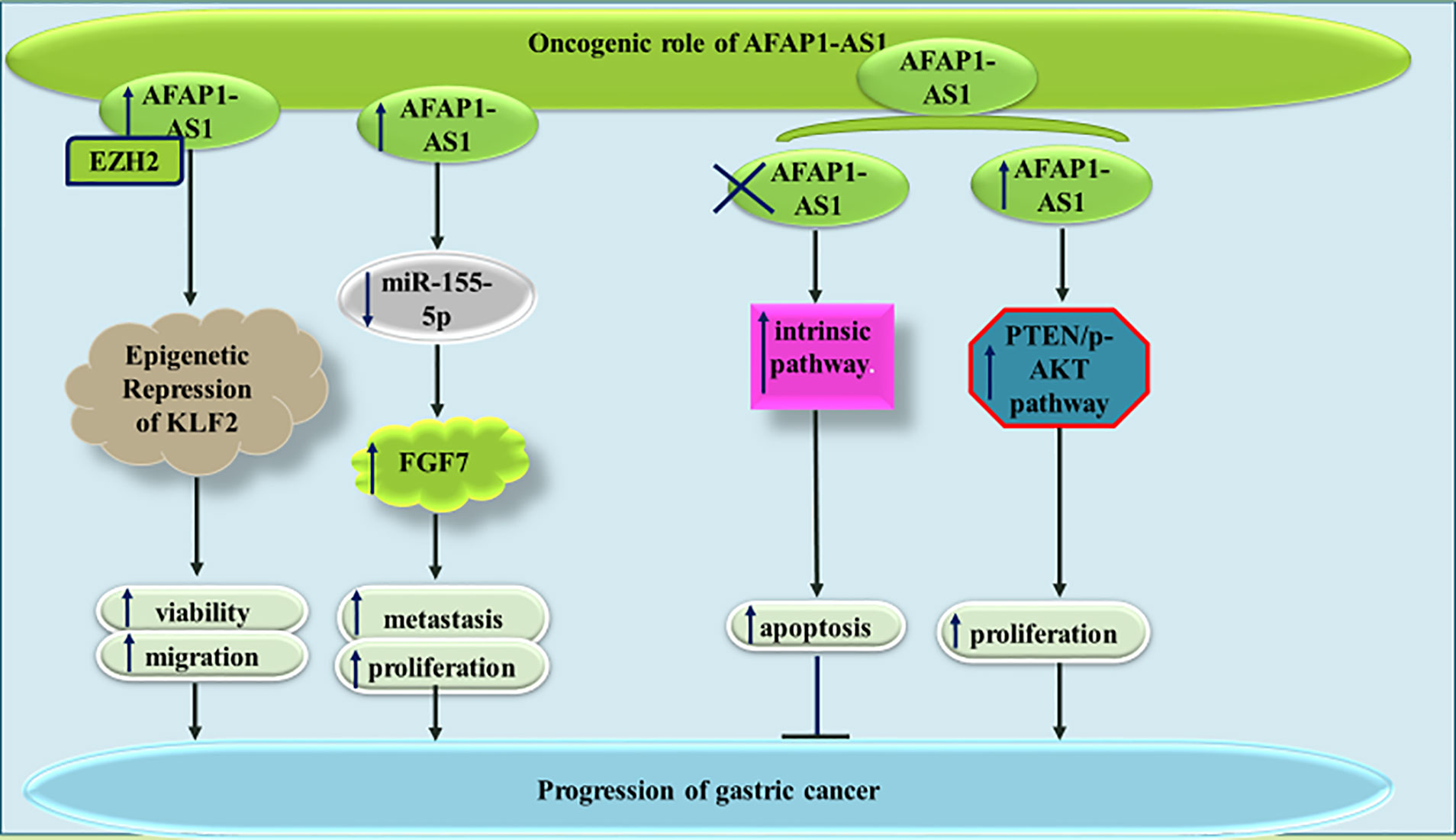
Figure 5 The oncogenic role of AFAP1-AS1 in gastric cancer is exerted through repression of KLF2, sponging miR-155-5p and enhancing activity of PTEN/p-AKT pathway.
Esophageal Cancer
AFAP1-AS1 have also been shown to bind with miR-26a, therefore influencing expression of its target gene, i.e. ATF2. Exosomes originated from M2 macrophages have higher expression of AFAP1-AS1 and ATF2 and reduced expression of miR-26a, compared with M1 macrophages. These exosomes could transfer AFAP1-AS1 to esophageal cancer cells, thus downregulating miR-26a and enhancing ATF2 levels in the recipeint cells. These expression changes affect phenotype of esophageal cancer cells (29). The regulatory role of AFAP1-AS1 on miR-498/VEGFA axis is another mechanism of participation of this lncRNA in the pathetiology of esophageal cancer (30).
Other Types of Cancers
In prostate cancer cells, AFAP1-AS1 has been shown to promote sequester miR-195-5p (31) and miR-512-3p (32), thus affecting malignnat behavious of these cells.
A number of other miRNAs, namely miR-423-5p (33), miR-320a (34), miR-107 (35) and miR-384 (36) have been found to be sequestered by AFAP1-AS1 in different cancer tissues (Figure 6).
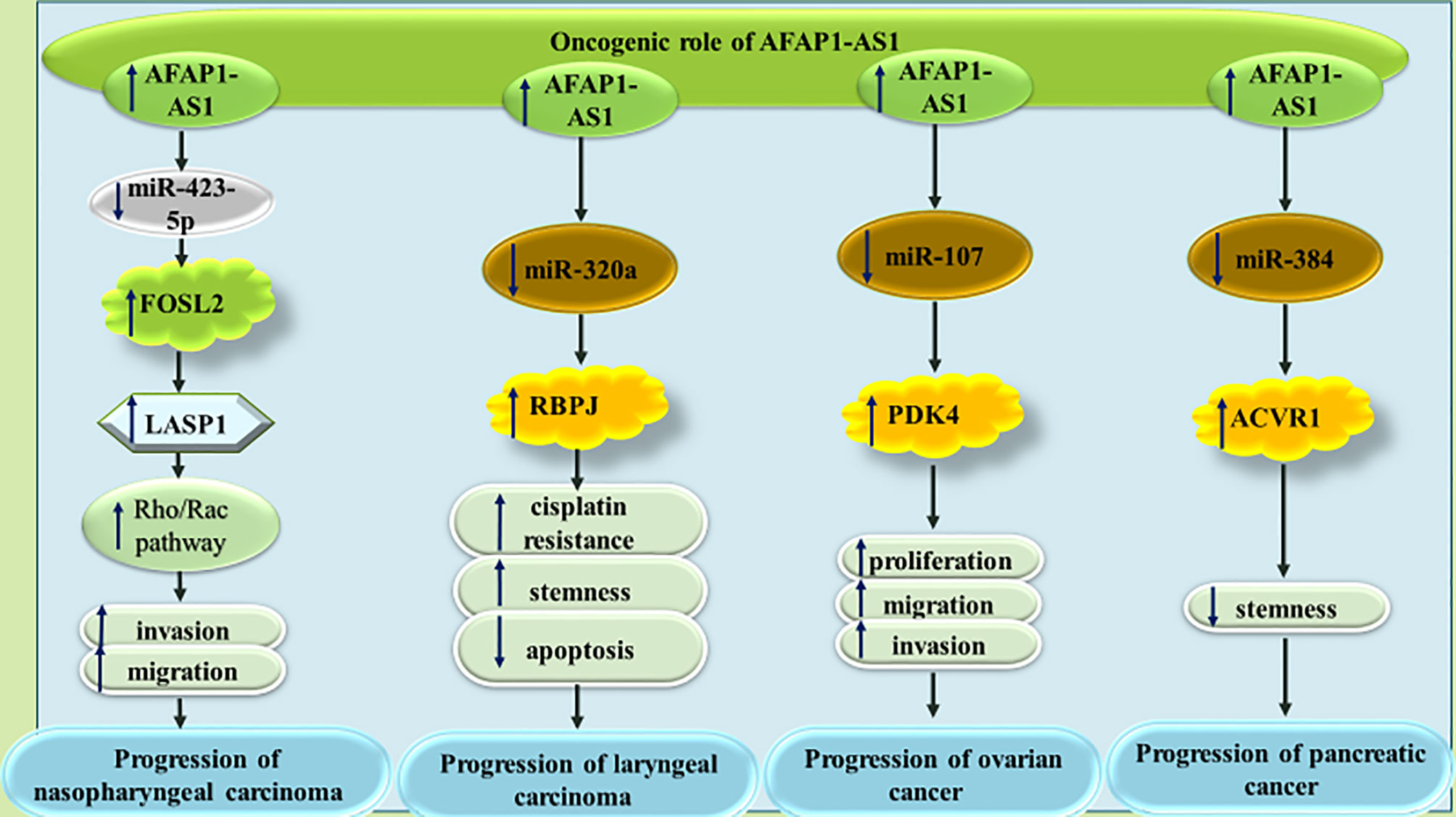
Figure 6 The oncogenic role of AFAP1-AS1 in nasopharyngeal carcinoma, laryngeal carcinoma, ovarian cancer and pancreatic cancer. In all types of mentioned cancers, AFAP1-AS1 can act as molecular sponge for tumor suppressor miRNAs.
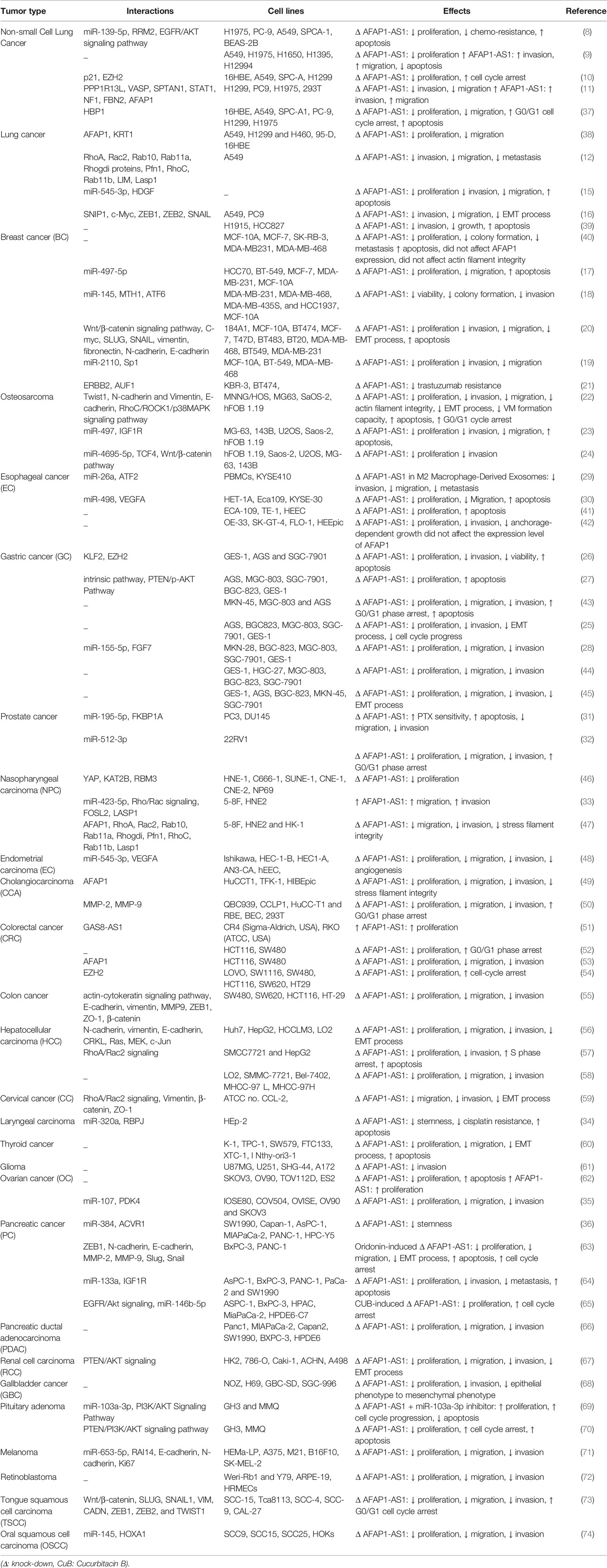
Table 1 summarizes the results of studies which appraised oncogenic roles of AFAP1-AS1 in different tissues.
Animal Studies
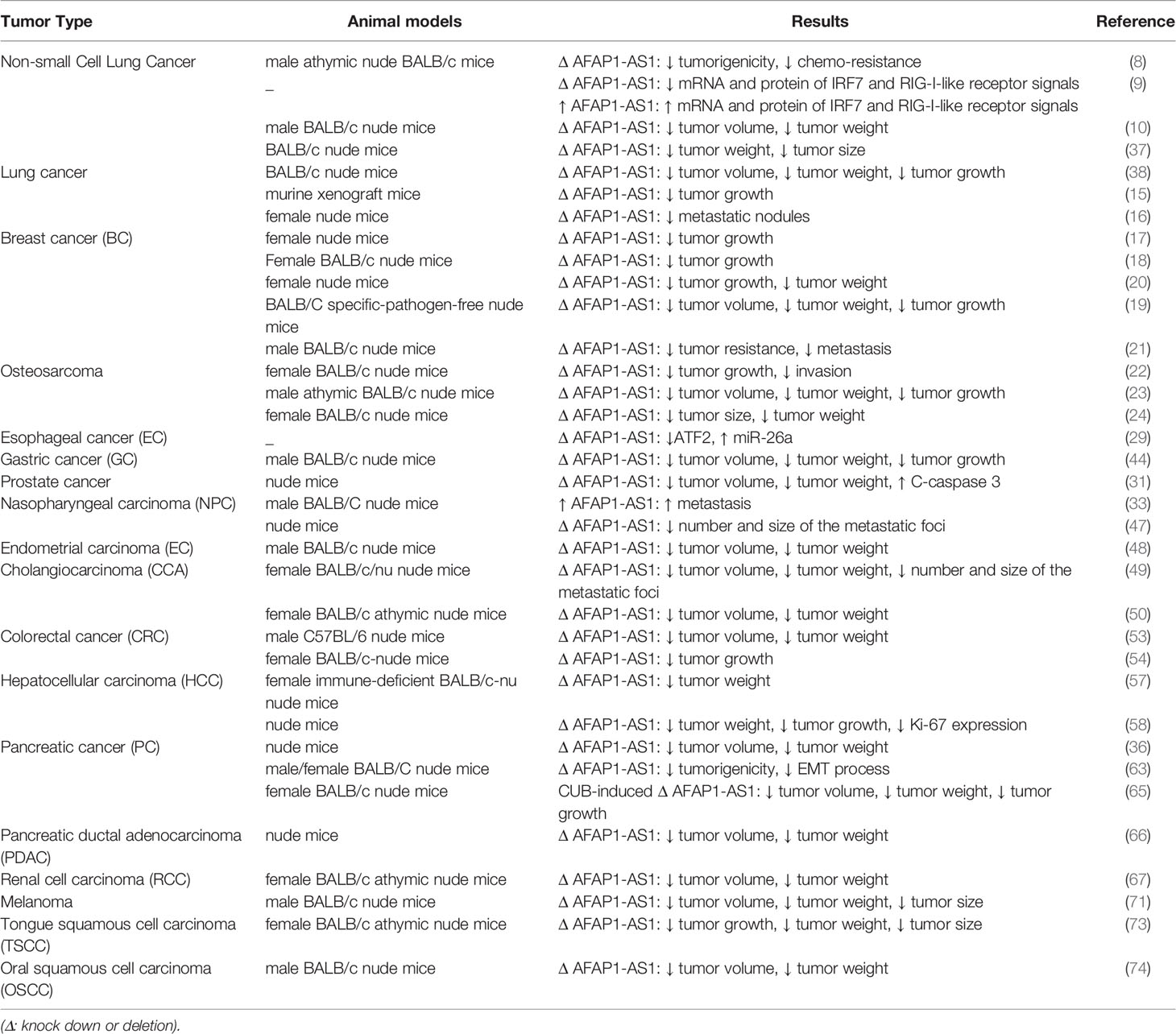
Investigations, particularly those conducted in BALB/c nude mice models have verified the oncogenic roles of AFAP1-AS1 in different types of cancers. AFAP1-AS1 knock-down has consistently led to significant reduction in tumor size/weight, attenuation of tumor growth rate and enhancement of response of cancer cells to therapeutic modalities (Table 2). In NSCLC, AFAP1-As1 silencing not only reduces tumorigenicity, but also confers chemosensitivity (8). Moreover, its silencing can affect IRF7 and RIG-I-like receptor signals (9). In breast cancer, AFAP1-AS1 down-regulation can affect trastuzumab resistance (21).
Clinical Studies
Except from a single low-sample size study in gastric cancer which reported down-regulation of AFAP1-AS1 in tumoral tissues versus nearby samples (6), other studies consistently reported over-expression of AFAP1-AS1 in different neoplastic tissues compared with non-neoplastic tissues of the same origin (Table 3). Even in the mentioned study, levels of AFAP1-AS1 were higher in patients who showed lymphatic or vascular invasion in comparison with those without these properties (6). Moreover, different statistical methods have been applied to assess correlations between expression level of AFAP1-AS1 and clinical outcomes, all of them reporting significant impact of up-regulation of this lncRNA on increasing malignant behaviors of tumors and decreasing patients’ survival. In pancreatic cancer, up-regulation of AFAP1-AS1 has been associated with lymph node involvement, perineural invasion, and poor clinical outcome. An in silico analysis of TCGA data of breast cancer patients has revealed AFAP1-AS1, as a differentially expressed lncRNA in basal tumors whose expression levels are associated with poor survival. Expression of this lncRNA has also been associated with hormone receptors status, HER2 expression, and PAM50 classification (81).
Tissue levels of AFAP1-AS1 could be used as a prognostic biomarker with the areas under ROC curves values of 0.86 and 0.93 for forecasting cancer progression in the periods of 6 and 12 months, respectively (66).
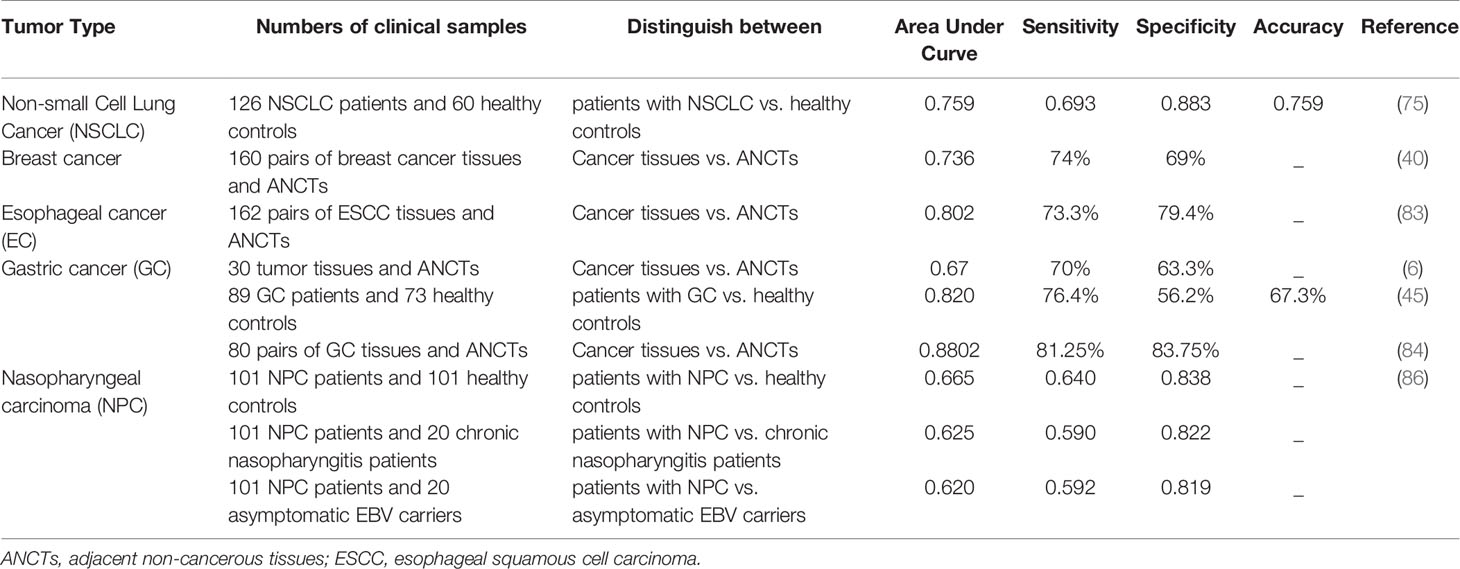
The ability of tissue levels of AFAP1-AS1 or its circulatory levels in differentiation of patients’ samples from control samples has been appraised in different types of cancers (Table 4). For instance, Li et al. have shown that over-expression of AFAP1-AS1 in serum samples of patients with NSCLC compared with normal controls can be used to distinguish these two sets of samples with an area under the curve (AUC) of 0.759. Combination of expression levels of this lncRNA with those of cyfra21-1 has increased AUC value to 0.860. Moreover, AFAP1-AS1 over-expression has been more prominent in patients with distant or lymph node metastasis, advanced clinical stage, and greater tumor burden (75). Serum levels of AFAP1-AS1 have also been shown to separate gastric cancer patients from controls with higher AUC value compared with conventional markers, i.e. CEA and CA19-9. Notably, serum levels of AFAP1-AS1 have been shown to be reduced following surgical treatment of patients (45).
Discussion
AFAP1-AS1 has been found to be up-regulated in almost all kinds of malignant tissues. This lncRNA has multiple effects in the carcinogenesis process, most of them being exerted through AFAP1-independent manners. Most notably, AFAP1-AS1 can sequester a number of tumor suppressor miRNAs, thus releasing the targets of these miRNAs from inhibitory effects of miRNAs. miR-139-5p, miR-545-3p, miR-497-5p, miR-145, miR-2110, miR-4695-5p, miR-26a, miR-498, miR-155-5p, miR-195-5p, miR-512-3p, miR-423-5p, miR-545-3p, miR‐320a, miR-107, miR-384, miR-133a, miR‐146b‐5p, miR-103a-3p and miR-653-5p are among miRNAs which have been found to be sequestered by AFAP1-AS1 through functional studies in different types of cancer cells. Notably, the interaction between AFAP1-AS1 and miR-497 has been verified in breast cancer and osteosarcoma. Moreover, similar interaction has been verified between this lncRNA and miR-145 in breast cancer and oral squamous cell carcinoma.
In fact, AFAP1-AS1 has multiple binding sites for miRNAs, thus regulating expression of a wide array of miRNAs. It is not clear whether binding of this lncRNA with a certain miRNA affects its interactions with other miRNAs. The crosstalk between AFAP1-AS1 and miRNAs can regulate activity of signaling pathways, angiogenic processes as well as EMT.
AFAP1-AS1 can indirectly influence activity of some cancer-related pathways such as EGFR/AKT, Wnt/β-catenin, PTEN/p-AKT, RhoA/Rac2 and PI3K/AKT. The effects of this lncRNA on Wnt/β-catenin, EGF/AKT and PI3K/AKT are mediated through sponging miR-4695-5p, miR-139-5p and miR-103a-3p, respectively. However, its effects on other pathways might be exerted in an independent manner from miRNAs sponging.
Lung cancer, nasopharyngeal carcinoma, colorectal cancer and cholangiocarcinoma are among cancers in which the interaction between AFAP1-AS1 and AFAP1 has been verified. However, the results of these studies are conflicting. For instance, AFAP1-AS1 silencing has been shown to increase expression of AFAP1 in a single study in lung cancer cells (12), while another study in this type of cancer has shown its effect on enhancement of expression of AFAP1 (11). Moreover, in a single study in MCF-7 breast cancer cells, AFAP1-AS1 silencing has not affected AFAP1 levels or actin filament integrity (40). Therefore, future studies are needed to elaborate the mechanistical impacts of AFAP1/AFAP1-AS1 interactions.
AFAP1-AS1 can affect response of cancer cells to a variety of anti-cancer modalities ranging from conventional chemotherapeutics to targeted therapeutics such as trastuzumab. Therefore, measurement of expression levels of this lncRNA can guide clinical oncologists to find the most appropriate therapeutic option for each patient. AFAP1-AS1 can also affect EMT and stemness of cancer cells, thus promoting their metastatic ability and increasing the propensity to tumor recurrence.
From a prognostic point of view, AFAP1-AS1 levels have been associated with tumor depth, tumor differentiation, TNM stage and other determinants of patients’ survival, thus could be used as markers for prediction of clinical outcomes of patients with a variety of malignant conditions. Diagnostic application of AFAP1-AS1 has been appraised in several types of cancers, with the best results being obtained from studies in gastric and esophageal cancers.
Cumulatively, AFAP1-AS1 is a prototype of cancer-related lncRNAs that regulates carcinogenesis not only through modification of expression of its sense transcript, but also through a variety of other methods such as miRNA sequestering and epigenetically affecting expression of tumor suppressor genes.
Author Contributions
SG-F and BH wrote the draft and revised it. MT designed and supervised the study. TK and MM collected the data and designed the figures and tables. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ji D, Zhong X, Jiang X, Leng K, Xu Y, Li Z, et al. The Role of Long Non-Coding RNA AFAP1-AS1 in Human Malignant Tumors. Pathol Res Pract (2018) 214(10):1524–31. doi: 10.1016/j.prp.2018.08.014
2. Baisden JM, Qian Y, Zot HM, Flynn DC. The Actin Filament-Associated Protein AFAP-110 Is an Adaptor Protein That Modulates Changes in Actin Filament Integrity. Oncogene (2001) 20(44):6435–47. doi: 10.1038/sj.onc.1204784
3. Liu F-T, Xue Q-Z, Zhu P-Q, Luo H-L, Zhang Y, Hao T. Long Noncoding RNA AFAP1-AS1, a Potential Novel Biomarker to Predict the Clinical Outcome of Cancer Patients: A Meta-Analysis. OncoTargets Ther (2016) 9:4247. doi: 10.2147/OTT.S107188
4. Dorfleutner A, Stehlik C, Zhang J, Gallick GE, Flynn DC. AFAP-110 Is Required for Actin Stress Fiber Formation and Cell Adhesion in MDA-MB-231 Breast Cancer Cells. J Cell Physiol (2007) 213(3):740–9. doi: 10.1002/jcp.21143
5. Zhang J, Park SI, Artime MC, Summy JM, Shah AN, Bomser JA, et al. AFAP-110 Is Overexpressed in Prostate Cancer and Contributes to Tumorigenic Growth by Regulating Focal Contacts. J Clin Invest (2007) 117(10):2962–73. doi: 10.1172/JCI30710
6. Esfandi F, Taheri M, Namvar A, Kholghi Oskooei V, Ghafouri−Fard S. AFAP1 and its Naturally Occurring Antisense RNA Are Downregulated in Gastric Cancer Samples. Biomed Rep (2019) 10(5):296–302. doi: 10.3892/br.2019.1207
7. Wong L-P, Ong RT-H, Poh W-T, Liu X, Chen P, Li R, et al. Deep Whole-Genome Sequencing of 100 Southeast Asian Malays. Am J Hum Genet (2013) 92(1):52–66. doi: 10.1016/j.ajhg.2012.12.005
8. Huang N, Guo W, Ren K, Li W, Jiang Y, Sun J, et al. LncRNA AFAP1-AS1 Supresses miR-139-5p and Promotes Cell Proliferation and Chemotherapy Resistance of Non-Small Cell Lung Cancer by Competitively Upregulating RRM2. Front Oncol (2019) 9:1103. doi: 10.3389/fonc.2019.01103
9. Tang X-D, Zhang D-D, Jia L, Ji W, Zhao Y-S. lncRNA AFAP1-AS1 Promotes Migration and Invasion of Non-Small Cell Lung Cancer via Up-Regulating IRF7 and the RIG-I-Like Receptor Signaling Pathway. Cell Physiol Biochem (2018) 50(1):179–95. doi: 10.1159/000493967
10. Yin D, Lu X, Su J, He X, De W, Yang J, et al. Long Noncoding RNA AFAP1-AS1 Predicts a Poor Prognosis and Regulates Non–Small Cell Lung Cancer Cell Proliferation by Epigenetically Repressing P21 Expression. Mol Cancer (2018) 17(1):1–12. doi: 10.1186/s12943-018-0836-7
11. He J, Wu K, Guo C, Zhou J-K, Pu W, Deng Y, et al. Long Non-Coding RNA AFAP1-AS1 Plays an Oncogenic Role in Promoting Cell Migration in Non-Small Cell Lung Cancer. Cell Mol Life Sci (2018) 75(24):4667–81. doi: 10.1007/s00018-018-2923-8
12. Zeng Z, Bo H, Gong Z, Lian Y, Li X, Li X, et al. AFAP1-AS1, a Long Noncoding RNA Upregulated in Lung Cancer and Promotes Invasion and Metastasis. Tumor Biol (2016) 37(1):729–37. doi: 10.1007/s13277-015-3860-x
13. Clayton NS, Ridley AJ. Targeting Rho GTPase Signaling Networks in Cancer. Front Cell Dev Biol (2020) 8:222. doi: 10.3389/fcell.2020.00222
14. Barak V, Goike H, Panaretakis KW, Einarsson R. Clinical Utility of Cytokeratins as Tumor Markers. Clin Biochem (2004) 37(7):529–40. doi: 10.1016/j.clinbiochem.2004.05.009
15. Sun J, Min H, Yu L, Yu G, Shi Y, Sun J. The Knockdown of LncRNA AFAP1-AS1 Suppressed Cell Proliferation, Migration, and Invasion, and Promoted Apoptosis by Regulating miR-545-3p/Hepatoma-Derived Growth Factor Axis in Lung Cancer. Anti-Cancer Drugs (2020) 32(1):11–21. doi: 10.1097/CAD.0000000000001003
16. Zhong Y, Yang L, Xiong F, He Y, Tang Y, Shi L, et al. Long Non-Coding RNA AFAP1-AS1 Accelerates Lung Cancer Cells Migration and Invasion by Interacting With SNIP1 to Upregulate C-Myc. Signal Transduction Targeted Ther (2021) 6(1):1–13. doi: 10.1038/s41392-021-00562-y
17. Cai B, Wang X, Qa B, Li P, Xue Q, Zhang J, et al. LncRNA AFAP1-AS1 Knockdown Represses Cell Proliferation, Migration, and Induced Apoptosis in Breast Cancer by Downregulating SEPT2 via Sponging miR-497-5p. Cancer Biother Radiopharmaceut (2020). doi: 10.1089/cbr.2020.3688
18. Zhang X, Zhou Y, Mao F, Lin Y, Shen S, Sun Q. lncRNA AFAP1-AS1 Promotes Triple Negative Breast Cancer Cell Proliferation and Invasion via Targeting miR-145 to Regulate MTH1 Expression. Sci Rep (2020) 10(1):1–11. doi: 10.1038/s41598-020-64713-x
19. Zhang X, Li F, Zhou Y, Mao F, Lin Y, Shen S, et al. Long Noncoding RNA AFAP1-AS1 Promotes Tumor Progression and Invasion by Regulating the miR-2110/Sp1 Axis in Triple-Negative Breast Cancer. Cell Death Dis (2021) 12(7):1–11. doi: 10.1038/s41419-021-03917-z
20. Zhang K, Liu P, Tang H, Xie X, Kong Y, Song C, et al. AFAP1-AS1 Promotes Epithelial-Mesenchymal Transition and Tumorigenesis Through Wnt/β-Catenin Signaling Pathway in Triple-Negative Breast Cancer. Front Pharmacol (2018) 9:1248. doi: 10.3389/fphar.2018.01248
21. Han M, Gu Y, Lu P, Li J, Cao H, Li X, et al. Exosome-Mediated lncRNA AFAP1-AS1 Promotes Trastuzumab Resistance Through Binding With AUF1 and Activating ERBB2 Translation. Mol Cancer (2020) 19(1):1–18. doi: 10.1186/s12943-020-1145-5
22. Shi D, Wu F, Mu S, Hu B, Zhong B, Gao F, et al. LncRNA AFAP1-AS1 Promotes Tumorigenesis and Epithelial-Mesenchymal Transition of Osteosarcoma Through RhoC/ROCK1/p38MAPK/Twist1 Signaling Pathway. J Exp Clin Cancer Res (2019) 38(1):1–12. doi: 10.1186/s13046-019-1363-0
23. Fei D, Zhang X, Lu Y, Tan L, Xu M, Zhang Y. Long Noncoding RNA AFAP1-AS1 Promotes Osteosarcoma Progression by Regulating miR-497/IGF1R Axis. Am J Trans Res (2020) 12(5):2155. doi: 10.1038/s41419-021-03917-z
24. Li R, Liu S, Li Y, Tang Q, Xie Y, Zhai R. Long Noncoding RNA AFAP1−AS1 Enhances Cell Proliferation and Invasion in Osteosarcoma Through Regulating Mir−4695−5p/TCF4−β−Catenin Signaling. Mol Med Rep (2018) 18(2):1616–22. doi: 10.3892/mmr.2018.9131
25. Feng Y, Zhang Q, Wang J, Liu P. Increased lncRNA AFAP1-AS1 Expression Predicts Poor Prognosis and Promotes Malignant Phenotypes in Gastric Cancer. Eur Rev Med Pharmacol Sci (2017) 21(17):3842–9.
26. Yuan X, Li J, Cao Y, Jie Z, Zeng Y. Long Non-Coding RNA AFAP1-AS1 Promotes Proliferation and Migration of Gastric Cancer by Downregulating KLF2. Eur Rev Med Pharmacol Sci (2020) 24(2):673–80.
27. Guo J-Q, Li S-J, Guo G-X. Long Noncoding RNA AFAP1-AS1 Promotes Cell Proliferation and Apoptosis of Gastric Cancer Cells via PTEN/p-AKT Pathway. Digestive Dis Sci (2017) 62(8):2004–10. doi: 10.1007/s10620-017-4584-0
28. Ma H-W, Xi D-Y, Ma J-Z, Guo M, Ma L, Ma D-H, et al. Long Noncoding RNA AFAP1-AS1 Promotes Cell Proliferation and Metastasis via the miR-155-5p/FGF7 Axis and Predicts Poor Prognosis in Gastric Cancer. Dis Markers (2020) 2020. doi: 10.1155/2020/8140989
29. Mi X, Xu R, Hong S, Xu T, Zhang W, Liu M. M2 Macrophage-Derived Exosomal lncRNA AFAP1-AS1 and microRNA-26a Affect Cell Migration and Metastasis in Esophageal Cancer. Mol Ther-Nucleic Acids (2020) 22:779–90. doi: 10.1016/j.omtn.2020.09.035
30. Shen W, Yu L, Cong A, Yang S, Wang P, Han G, et al. Silencing lncRNA AFAP1-AS1 Inhibits the Progression of Esophageal Squamous Cell Carcinoma Cells via Regulating the miR-498/VEGFA Axis. Cancer Manage Res (2020) 12:6397. doi: 10.2147/CMAR.S254302
31. Leng W, Liu Q, Zhang S, Sun D, Guo Y. LncRNA AFAP1-AS1 Modulates the Sensitivity of Paclitaxel-Resistant Prostate Cancer Cells to Paclitaxel via miR-195-5p/FKBP1A Axis. Cancer Biol Ther (2020) 21(11):1072–80. doi: 10.1080/15384047.2020.1829266
32. Wang K, Sun H, Sun T, Qu H, Xie Q, Lv H, et al. Long Non-Coding RNA AFAP1-AS1 Promotes Proliferation and Invasion in Prostate Cancer via Targeting miR-512-3p. Gene (2020) 726:144169. doi: 10.1016/j.gene.2019.144169
33. Lian Y, Xiong F, Yang L, Bo H, Gong Z, Wang Y, et al. Long Noncoding RNA AFAP1-AS1 Acts as a Competing Endogenous RNA of miR-423-5p to Facilitate Nasopharyngeal Carcinoma Metastasis Through Regulating the Rho/Rac Pathway. J Exp Clin Cancer Res (2018) 37(1):1–17. doi: 10.1186/s13046-018-0918-9
34. Yuan Z, Xiu C, Song K, Pei R, Miao S, Mao X, et al. Long Non-Coding RNA AFAP1-AS1/miR-320a/RBPJ Axis Regulates Laryngeal Carcinoma Cell Stemness and Chemoresistance. J Cell Mol Med (2018) 22(9):4253–62. doi: 10.1111/jcmm.13707
35. Liu B, Yan L, Chi Y, Sun Y, Yang X. Long Non-Coding RNA AFAP1-AS1 Facilitates Ovarian Cancer Progression by Regulating the miR-107/PDK4 Axis. J Ovarian Res (2021) 14(1):1–11. doi: 10.1186/s13048-021-00808-x
36. Wu X-B, Feng X, Chang Q-M, Zhang C-W, Wang Z-F, Liu J, et al. Cross-Talk Among AFAP1-AS1, ACVR1 and microRNA-384 Regulates the Stemness of Pancreatic Cancer Cells and Tumorigenicity in Nude Mice. J Exp Clin Cancer Res (2019) 38(1):1–15. doi: 10.1186/s13046-019-1051-0
37. Yu S, Yang D, Ye Y, Liu P, Chen Z, Lei T, et al. Long Noncoding RNA Actin Filament-Associated Protein 1 Antisense RNA 1 Promotes Malignant Phenotype Through Binding With Lysine-Specific Demethylase 1 and Repressing HMG Box-Containing Protein 1 in Non-Small-Cell Lung Cancer. Cancer Sci (2019) 110(7):2211–25. doi: 10.1111/cas.14039
38. Peng B, Liu A, Yu X, Xu E, Dai J, Li M, et al. Silencing of lncRNA AFAP1-AS1 Suppressed Lung Cancer Development by Regulatory Mechanism in Cis and Trans. Oncotarget (2017) 8(55):93608. doi: 10.18632/oncotarget.20549
39. Zhuang Y, Jiang H, Li H, Dai J, Liu Y, Li Y, et al. Down-Regulation of Long Non-Coding RNA AFAP1-AS1 Inhibits Tumor Cell Growth and Invasion in Lung Adenocarcinoma. Am J Trans Res (2017) 9(6):2997.
40. Liu Y, Li Q, Hosen MR, Zietzer A, Flender A, Levermann P, et al. Atherosclerotic Conditions Promote the Packaging of Functional microRNA-92a-3p Into Endothelial Microvesicles. Circ Res (2019) 124(4):575–87. doi: 10.1161/CIRCRESAHA.118.314010
41. Luo HL, Huang MD, Guo JN, Fan RH, Xia XT, He JD, et al. AFAP1-AS1 Is Upregulated and Promotes Esophageal Squamous Cell Carcinoma Cell Proliferation and Inhibits Cell Apoptosis. Cancer Med (2016) 5(10):2879–85. doi: 10.1002/cam4.848
42. Wu W, Bhagat TD, Yang X, Song JH, Cheng Y, Agarwal R, et al. Hypomethylation of Noncoding DNA Regions and Overexpression of the Long Noncoding RNA, AFAP1-AS1, in Barrett’s Esophagus and Esophageal Adenocarcinoma. Gastroenterology (2013) 144(5):956–66. e4. doi: 10.1053/j.gastro.2013.01.019
43. Li Z, Ding Z, Rong D, Tang W, Cao H. Overexpression of lncRNA AFAP1−AS1 Promotes Cell Proliferation and Invasion in Gastric Cancer. Oncol Lett (2019) 18(3):3211–7. doi: 10.3892/ol.2019.10640
44. Lai Z, Lin P, Weng X, Su J, Chen Y, He Y, et al. MicroRNA-574-5p Promotes Cell Growth of Vascular Smooth Muscle Cells in the Progression of Coronary Artery Disease. Biomed Pharmacother (2018) 97:162–7. doi: 10.1016/j.biopha.2017.10.062
45. Liu W, Li Y, Zhang Y, Shen X, Su Z, Chen L, et al. Circulatinglong Non-Coding RNA FEZF1-AS1 and AFAP1-AS1 Serve as Potential Diagnostic Biomarkers for Gastric Cancer. Pathol-Res Pract (2020) 216(1):152757. doi: 10.1016/j.prp.2019.152757
46. Fang M, Zhang M, Wang Y, Wei F, Wu J, Mou X, et al. Long Noncoding RNA AFAP1-AS1 Is a Critical Regulator of Nasopharyngeal Carcinoma Tumorigenicity. Front Oncol (2020) 10:2510. doi: 10.3389/fonc.2020.601055
47. Bo H, Gong Z, Zhang W, Li X, Zeng Y, Liao Q, et al. Upregulated Long Non-Coding RNA AFAP1-AS1 Expression Is Associated With Progression and Poor Prognosis of Nasopharyngeal Carcinoma. Oncotarget (2015) 6(24):20404. doi: 10.18632/oncotarget.4057
48. Zhong Y, Wang Y, Dang H, Wu X. LncRNA AFAP1-AS1 Contributes to the Progression of Endometrial Carcinoma by Regulating miR-545-3p/VEGFA Pathway. Mol Cell Probes (2020) 53:101606. doi: 10.1016/j.mcp.2020.101606
49. Shi X, Zhang H, Wang M, Xu X, Zhao Y, He R, et al. LncRNA AFAP1-AS1 Promotes Growth and Metastasis of Cholangiocarcinoma Cells. Oncotarget (2017) 8(35):58394. doi: 10.18632/oncotarget.16880
50. Lu X, Zhou C, Li R, Deng Y, Zhao L, Zhai W. Long Noncoding RNA AFAP1-AS1 Promoted Tumor Growth and Invasion in Cholangiocarcinoma. Cell Physiol Biochem (2017) 42(1):222–30. doi: 10.1159/000477319
51. Zhao Y, Chu Y, Sun J, Song R, Li Y, Xu F. LncRNA GAS8-AS Inhibits Colorectal Cancer (CRC) Cell Proliferation by Downregulating lncRNA AFAP1-As1. Gene (2019) 710:140–4. doi: 10.1016/j.gene.2019.05.040
52. Wang F, Ni H, Sun F, Li M, Chen L. Overexpression of lncRNA AFAP1-AS1 Correlates With Poor Prognosis and Promotes Tumorigenesis in Colorectal Cancer. Biomed Pharmacother (2016) 81:152–9. doi: 10.1016/j.biopha.2016.04.009
53. Han X, Wang L, Ning Y, Li S, Wang Z. Long Non-Coding RNA AFAP1-AS1 Facilitates Tumor Growth and Promotes Metastasis in Colorectal Cancer. Biol Res (2016) 49(1):1–7. doi: 10.1186/s40659-016-0094-3
54. Tang J, Zhong G, Wu J, Chen H, Jia Y. Long Noncoding RNA AFAP1-AS1 Facilitates Tumor Growth Through Enhancer of Zeste Homolog 2 in Colorectal Cancer. Am J Cancer Res (2018) 8(5):892.
55. Bo H, Fan L, Li J, Liu Z, Zhang S, Shi L, et al. High Expression of lncRNA AFAP1-AS1 Promotes the Progression of Colon Cancer and Predicts Poor Prognosis. J Cancer (2018) 9(24):4677. doi: 10.7150/jca.26461
56. Abdul S, Majid A, Wang J, Liu Q, Sun M-Z, Liu S. Bidirectional Interaction of lncRNA AFAP1-AS1 and CRKL Accelerates the Proliferative and Metastatic Abilities of Hepatocarcinoma Cells. J Advanced Res (2020) 24:121–30. doi: 10.1016/j.jare.2020.03.010
57. Zhang J-Y, Weng M-Z, Song F-B, Xu Y-G, Liu Q, Wu J-Y, et al. Long Noncoding RNA AFAP1-AS1 Indicates a Poor Prognosis of Hepatocellular Carcinoma and Promotes Cell Proliferation and Invasion via Upregulation of the RhoA/Rac2 Signaling. Int J Oncol (2016) 48(4):1590–8. doi: 10.3892/ijo.2016.3385
58. Lu X, Zhou C, Li R, Liang Z, Zhai W, Zhao L, et al. Critical Role for the Long Non-Coding RNA AFAP1-AS1 in the Proliferation and Metastasis of Hepatocellular Carcinoma. Tumor Biol (2016) 37(7):9699–707. doi: 10.1007/s13277-016-4858-8
59. Bo H, Fan L, Gong Z, Liu Z, Shi L, Guo C, et al. Upregulation and Hypomethylation of lncRNA AFAP1−AS1 Predicts a Poor Prognosis and Promotes the Migration and Invasion of Cervical Cancer. Oncol Rep (2019) 41(4):2431–9. doi: 10.3892/or.2019.7027
60. Dai W, Tian Y, Jiang B, Chen W. Down-Regulation of Long Non-Coding RNA AFAP1-AS1 Inhibits Tumor Growth, Promotes Apoptosis and Decreases Metastasis in Thyroid Cancer. Biomed Pharmacother (2018) 99:191–7. doi: 10.1016/j.biopha.2017.12.105
61. Wang Y, Lan Q. Long Non-Coding RNA AFAP1-AS1 Accelerates Invasion and Predicts Poor Prognosis of Glioma. Eur Rev Med Pharmacol Sci (2018) 22(16):5223–9.
62. Yang S, Lin R, Si L, Cui M, Zhang X, Fan L. Expression and Functional Role of Long Non-Coding RNA AFAP1-AS1 in Ovarian Cancer. Eur Rev Med Pharmacol Sci (2016) 20(24):5107–12.
63. Lou S, Xu J, Wang B, Li S, Ren J, Hu Z, et al. Downregulation of lncRNA AFAP1-AS1 by Oridonin Inhibits the Epithelial-to-Mesenchymal Transition and Proliferation of Pancreatic Cancer Cells. Acta Biochim Biophys Sin (2019) 51(8):814–25. doi: 10.1093/abbs/gmz071
64. Chen B, Li Q, Zhou Y, Wang X, Zhang Q, Wang Y, et al. The Long Coding RNA AFAP1-AS1 Promotes Tumor Cell Growth and Invasion in Pancreatic Cancer Through Upregulating the IGF1R Oncogene via Sequestration of miR-133a. Cell Cycle (2018) 17(16):1949–66. doi: 10.1080/15384101.2018.1496741
65. Zhou J, Liu M, Chen Y, Xu S, Guo Y, Zhao L. Cucurbitacin B Suppresses Proliferation of Pancreatic Cancer Cells by ceRNA: Effect of miR-146b-5p and lncRNA-AFAP1-As1. J Cell Physiol (2019) 234(4):4655–67. doi: 10.1002/jcp.27264
66. Ye Y, Chen J, Zhou Y, Fu Z, Zhou Q, Wang Y, et al. High Expression of AFAP1-AS1 Is Associated With Poor Survival and Short-Term Recurrence in Pancreatic Ductal Adenocarcinoma. J Trans Med (2015) 13(1):1–11. doi: 10.1186/s12967-015-0490-4
67. Mu Z, Dong D, Wei N, Sun M, Wang W, Shao Y, et al. Silencing of lncRNA AFAP1-AS1 Inhibits Cell Growth and Metastasis in Clear Cell Renal Cell Carcinoma. Oncol Res (2019) 27(6):653. doi: 10.3727/096504018X15420748671075
68. Ma F, Wang S-H, Cai Q, Zhang M-D, Yang Y, Ding J. Overexpression of LncRNA AFAP1-AS1 Predicts Poor Prognosis and Promotes Cells Proliferation and Invasion in Gallbladder Cancer. Biomed Pharmacother (2016) 84:1249–55. doi: 10.1016/j.biopha.2016.10.064
69. Tang H, Zhu D, Zhang G, Luo X, Xie W. AFAP1-AS1 Promotes Proliferation of Pituitary Adenoma Cells Through miR-103a-3p to Activate PI3K/AKT Signaling Pathway. World Neurosurg (2019) 130:e888–e98. doi: 10.1016/j.wneu.2019.07.032
70. Tang H, Hou B, Ye Z, Ling C, Guo Y. Knockdown of Long Non-Coding RNA AFAP1-AS1 Inhibits Growth and Promotes Apoptosis in Pituitary Adenomas. Int J Clin Exp Pathol (2018) 11(3):1238.
71. Liu F, Hu L, Pei Y, Zheng K, Wang W, Li S, et al. Long Non-Coding RNA AFAP1-AS1 Accelerates the Progression of Melanoma by Targeting miR-653-5p/RAI14 Axis. BMC Cancer (2020) 20(1):1–11. doi: 10.1186/s12885-020-6665-2
72. Hao F, Mou Y, Zhang L, Wang S, Yang Y. LncRNA AFAP1-AS1 Is a Prognostic Biomarker and Serves as Oncogenic Role in Retinoblastoma. Biosci Rep (2018) 38(3):BSR20180384. doi: 10.1042/BSR20180384
73. Wang Z-Y, Hu M, Dai M-H, Xiong J, Zhang S, Wu H-J, et al. Upregulation of the Long Non-Coding RNA AFAP1-AS1 Affects the Proliferation, Invasion and Survival of Tongue Squamous Cell Carcinoma via the Wnt/beta-Catenin Signaling Pathway (Retraction of Vol 17, Art No 3, 2018). 4 CRINAN ST, LONDON N1 9XW, ENGLAND: BMC CAMPUS (2019).
74. Li M, Yu D, Li Z, Zhao C, Su C, Ning J. Long Non−Coding RNA AFAP1−AS1 Facilitates the Growth and Invasiveness of Oral Squamous Cell Carcinoma by Regulating the Mir−145/HOXA1 Axis. Oncol Rep (2021) 45(3):1094–104. doi: 10.3892/or.2020.7908
75. Li W, Li N, Kang X, Shi K. Circulating Long Non-Coding RNA AFAP1-AS1 Is a Potential Diagnostic Biomarker for Non-Small Cell Lung Cancer. Clin Chim Acta (2017) 475:152–6. doi: 10.1016/j.cca.2017.10.027
76. Leng X, Ding X, Wang S, Fang T, Shen W, Xia W, et al. Long Noncoding RNA AFAP1−AS1 Is Upregulated in NSCLC and Associated With Lymph Node Metastasis and Poor Prognosis. Oncol Lett (2018) 16(1):727–32. doi: 10.3892/ol.2018.8784
77. Deng J, Liang Y, Liu C, He S, Wang S. The Up-Regulation of Long Non-Coding RNA AFAP1-AS1 Is Associated With the Poor Prognosis of NSCLC Patients. Biomed Pharmacother (2015) 75:8–11. doi: 10.1016/j.biopha.2015.07.003
78. Wang M, Ma X, Zhu C, Guo L, Li Q, Liu M, et al. The Prognostic Value of Long non Coding RNAs in non Small Cell Lung Cancer: A Meta-Analysis. Oncotarget (2016) 7(49):81292. doi: 10.18632/oncotarget.13223
79. Sui J, Li Y-H, Zhang Y-Q, Li C-Y, Shen X, Yao W-Z, et al. Integrated Analysis of Long Non-Coding RNA−associated ceRNA Network Reveals Potential lncRNA Biomarkers in Human Lung Adenocarcinoma. Int J Oncol (2016) 49(5):2023–36. doi: 10.3892/ijo.2016.3716
80. Wei Y, Zhang X. Transcriptome Analysis of Distinct Long Non-Coding RNA Transcriptional Fingerprints in Lung Adenocarcinoma and Squamous Cell Carcinoma. Tumor Biol (2016) 37(12):16275–85. doi: 10.1007/s13277-016-5422-2
81. Rodrigues de Bastos D, Nagai MA. In Silico Analyses Identify lncRNAs: WDFY3-AS2, BDNF-AS and AFAP1-AS1 as Potential Prognostic Factors for Patients With Triple-Negative Breast Tumors. PloS One (2020) 15(5):e0232284. doi: 10.1371/journal.pone.0232284
82. Dianatpour A, Faramarzi S, Geranpayeh L, Mirfakhraie R, Motevaseli E, Ghafouri-Fard S. Expression Analysis of AFAP1-AS1 and AFAP1 in Breast Cancer. Cancer biomark (2018) 22(1):49–54. doi: 10.3233/CBM-170831
83. Zhou XL, Wang WW, Zhu WG, Yu CH, Tao GZ, Wu QQ, et al. High Expression of Long Non-Coding RNA AFAP1-AS1 Predicts Chemoradioresistance and Poor Prognosis in Patients With Esophageal Squamous Cell Carcinoma Treated With Definitive Chemoradiotherapy. Mol Carcinogenesis (2016) 55(12):2095–105. doi: 10.1002/mc.22454
84. Zhao H, Zhang K, Wang T, Cui J, Xi H, Wang Y, et al. Long Non−Coding RNA AFAP1−Antisense RNA 1 Promotes the Proliferation, Migration and Invasion of Gastric Cancer Cells and Is Associated With Poor Patient Survival. Oncol Lett (2018) 15(6):8620–6. doi: 10.3892/ol.2018.8389
85. Tang Y, He Y, Shi L, Yang L, Wang J, Lian Y, et al. Co-Expression of AFAP1-AS1 and PD-1 Predicts Poor Prognosis in Nasopharyngeal Carcinoma. Oncotarget (2017) 8(24):39001. doi: 10.18632/oncotarget.16545
Keywords: AFAP1-AS1, cancer, biomarker, expression, ncRNA
Citation: Ghafouri-Fard S, Khoshbakht T, Hussen BM, Taheri M and Mokhtari M (2021) A Review on the Role of AFAP1-AS1 in the Pathoetiology of Cancer. Front. Oncol. 11:777849. doi: 10.3389/fonc.2021.777849
Received: 15 September 2021; Accepted: 09 November 2021;
Published: 29 November 2021.
Edited by:
Shiv K. Gupta, Mayo Clinic, United StatesReviewed by:
Rezvan Noroozi, Jagiellonian University, PolandAmin Safa, Complutense University of Madrid, Spain
Copyright © 2021 Ghafouri-Fard, Khoshbakht, Hussen, Taheri and Mokhtari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Taheri, mohammad_823@yahoo.com; Majid Mokhtari, majimokh@gmail.com
 Soudeh Ghafouri-Fard
Soudeh Ghafouri-Fard Tayybeh Khoshbakht
Tayybeh Khoshbakht Bashdar Mahmud Hussen3
Bashdar Mahmud Hussen3 Mohammad Taheri
Mohammad Taheri