- 1Human Cancer Genomic Research, Research Center, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia
- 2Department of Surgery, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia
- 3Department of Pathology, King Faisal Specialist Hospital and Research Centre, Riyadh, Saudi Arabia
Background: Prophylactic central lymph node dissection (PCLND) for adult patients with papillary thyroid carcinoma (PTC) is still a matter of debate. Data on incidence, risk and benefits of PCLND in Middle Eastern patients is lacking. Therefore, we aimed to identify the incidence and predictive clinico-pathological and molecular marker of PCLND in adult patients with clinically node negative (cN0) Middle Eastern PTC.
Methods: This retrospective study included 942 adult Middle Eastern patients with cN0 PTC who underwent total thyroidectomy (TT) or TT+PCLND. Clinico-pathological associations of central lymph node metastasis (CLNM) were assessed. Multivariate analysis was performed using logistic regression and Cox proportional hazards model.
Results: 213 patients underwent PCLND and 38.0% (81/213) had positive CLNM. Multivariate analysis demonstrated age ≤55 years (Odds Ratio (OR) = 7.38; 95% Confidence Interval (CI) = 1.59 – 34.31; p = 0.0108), tumor bilaterality (OR = 3.01; 95% CI = 1.01 – 9.21; p = 0.0483), lymphovascular invasion (OR = 2.92; 95% CI = 1.18 – 7.23; p = 0.0206) and BRAF mutation (OR = 3.24; 95% CI = 1.41 – 7.49; p = 0.0058) were independent predictors of CLNM in adult PTC. Furthermore, patients who underwent PCLND showed significant association with improved recurrence-free survival (RFS; p = 0.0379). Multivariate analysis demonstrated that PCLND was an independent predictor of improved recurrence-free survival.
Conclusions: cN0 Middle Eastern PTC patients treated with PCLND showed a significantly better prognosis. PCLND was effective in improving RFS in Middle Eastern PTC patients and should be encouraged for patients with potential risk factors for CLNM.
Introduction
During the past two decades, the incidence of thyroid cancer has continually increased (1, 2). Papillary thyroid carcinoma (PTC) accounts for the majority of thyroid cancers (3). PTC has become the second most prevalent carcinoma among women in Saudi Arabia (4). Most PTC patients have an excellent prognosis (5). However, despite this favorable outcome, PTC is prone to spread to the central lymph node (CLN). In fact, 20 – 40% of PTC patients have been reported to have CLN metastasis (CLNM) even in clinically node negative patients (6–9).
The incidence of occult node metastasis is high and reaches up to 90% according to several reports (10–13). Furthermore, CLNM have been reported to increase the risk of recurrence and disease-specific mortality (14–16). Recurrence in PTC remains a big challenge and affects the optimal therapeutic strategy for PTC patients. Although central lymph node dissection (CLND) is recommended for patients who are suspected of CLNM in preoperative evaluation, there are still no clear guidelines for CLND for patients with clinically node negative (cN0) PTC.
In surgical treatments, prophylactic CLND (PCLND) for patients with PTC is still a controversial issue. Some studies have shown that PCLND could be beneficial in terms of reducing locoregional recurrence and thus improving the disease-free survival. In addition, PCLND could also help in more precise staging of PTC, since nodal positivity could convert patients from cN0 to pN1a, leading many patients over 55 years of age from AJCC stage I to AJCC stage III, which in turn changes the therapeutic choices (17–20). However, other studies consider the risk of postoperative complications (such as laryngeal nerve injury and hypoparathyroidism) following PCLND to outweigh the potential benefits, and thus do not recommend the routine use of PCLND in PTC (21–24). Although the American Thyroid Association (ATA) Guidelines suggest PCLND be considered, especially for PTC patients who present with advanced tumors (T3 and T4) (25), the endocrine community is still debating the guidelines suggestion data.
However, the benefits of PCLND in Middle Eastern patients with cN0 PTC has not been fully explored. Therefore, we conducted this retrospective study on a large cohort of Middle Eastern PTC to identify both the incidence and the predictive parameters of CLNM in cN0 PTC patients. We then assessed, for the first time, the benefit of PCLND with clinically node negative PTC in reducing the incidence of recurrent disease and improving patient outcome. Data from this study may give additional guidance on the surgical and therapeutic decision for PTC patients from Middle Eastern ethnicity.
Materials and Methods
Patient Selection
Nine hundred and forty-two PTC patients diagnosed between 1988 and 2018 at King Faisal Specialist Hospital and Research Centre (Riyadh, Saudi Arabia) were included in the study. Inclusion criteria were: 1) PTC confirmed by fine needle aspiration cytology; 2) no evidence of lymph node metastasis at either clinical examination or on ultrasound; and 3) more than 18 years of age. The Institutional Review Board of the hospital approved this study and the Research Advisory Council (RAC) provided waiver of consent under project RAC # 221 1168 and # 2110 031.
Clinico-Pathological Data
Patients underwent total thyroidectomy with (n = 213) or without (n = 729) PCLND. PCLND was performed in patients with clinically uninvolved central neck lymph nodes (cN0) who had advanced primary tumors (T3 or T4) or clinically involved lateral neck nodes (cN1b), or if the information could be used to plan further steps in therapy, in accordance with the 2015 ATA guidelines (25). Baseline clinico-pathological data were collected from case records and have been summarized in Table 1. Clinico-pathological data included patients’ age at diagnosis, gender, histologic subtype, tumor laterality, focality, extrathyroidal extension, lymphovasular invasion, tumor size, central lymph node status, stage and tumor recurrence. Staging of PTC was performed using the eighth edition of AJCC staging system. Only structural recurrence (local, regional or distant) was considered for analysis. Recurrence was defined as any newly detected tumor (local or distant) or metastatic regional lymph node (LN) based on ultrasound and/or imaging studies in patients who had been previously free of disease following initial treatment. Risk categories were defined based on 2015 ATA guidelines (25).
BRAF and TERT Mutation Analysis
BRAF and TERT mutation data was assessed in our laboratory by Sanger sequencing and has been published by us previously (26, 27).
Follow-Up and Study Endpoint
Patients were regularly followed by both physical examinations and imaging studies to identify tumor recurrence. The median follow-up was 9.1 years (range 1.0 – 30.1 years). Recurrence-free survival (RFS) was defined as the time (in months) from date of initial surgery to the occurrence of any tumor recurrence (local, regional or distant). In case of no recurrence, date of last follow-up was the study endpoint for RFS.
Statistical Analysis
The associations between clinico-pathological variables was performed using contingency table analysis and Chi square tests. Mantel-Cox log-rank test was used to evaluate recurrence-free survival. Survival curves were generated using the Kaplan-Meier method. Logistic regression and Cox proportional hazards model were used for multivariate analysis. Clinico-pathological variables adjusted in multivariate analysis were age, sex, histology, laterality, focality, extrathyroidal extension, lymphovascular invasion, pT, pN, stage, BRAF mutation and TERT mutation. Two-sided tests were used for statistical analyses with a limit of significance defined as p value < 0.05. All data analyses were performed using the JMP14.0 (SAS Institute, Inc., Cary, NC) software package.
Results
Patient and Tumor Characteristics
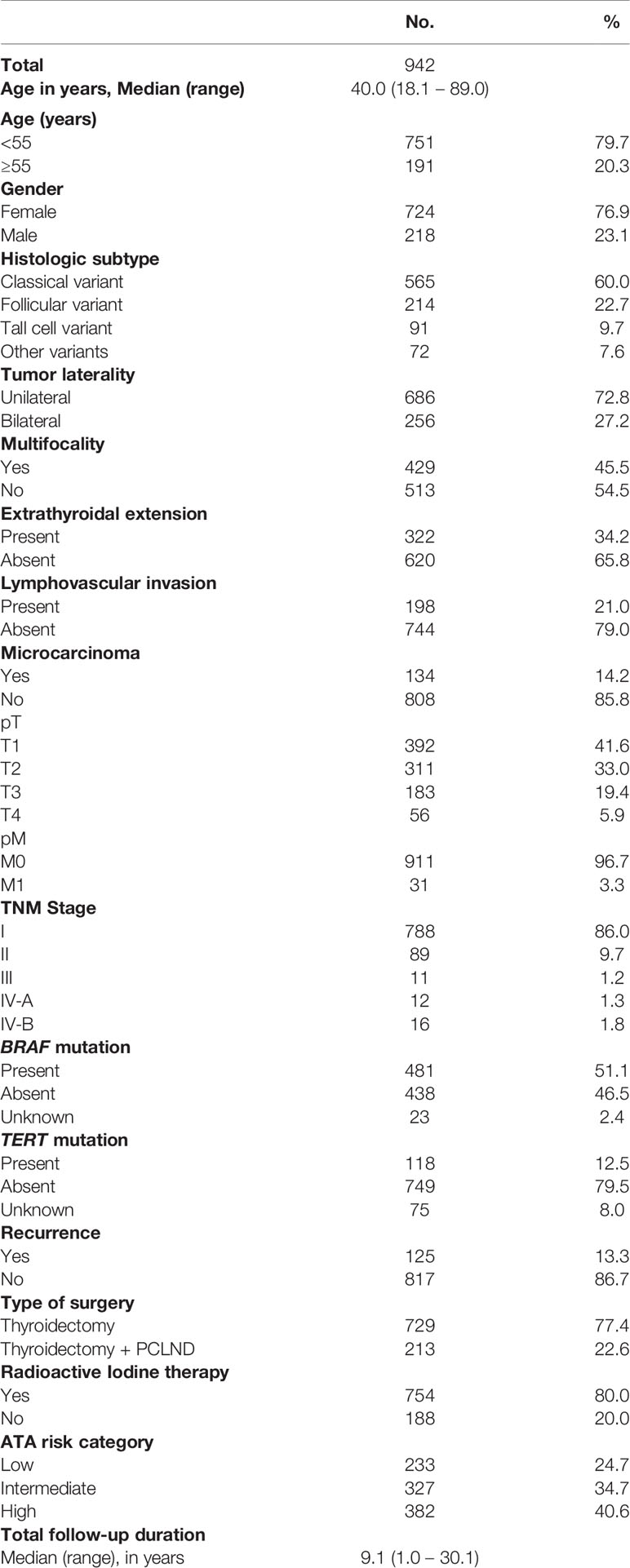
Median age of the study cohort was 40.0 years (range = 18.1 – 89.0 years), with a male: female ratio of 1:3.3. Classical variant PTC was the predominant histologic subtype, accounting for 60.0% (565/942) of all cases, followed by follicular variant (22.7%; 214/942) and tall cell variant (9.7%; 91/942). Extrathyroidal extension was noted in 34.2% (322/942) of cases and lymphovascular invasion in 21.0% (198/942). 45.5% (429/942) of PTCs were multifocal and 27.2% (256/942) were bilateral. 80.0% (754/942) of PTC patients in our cohort received radioactive iodine (RAI) therapy. Tumor recurrence was noted in 13.3% (125/942) of the entire cohort (Table 1). However, among the 213 patients who underwent PCLND, tumor recurrence was seen in only 8.5% (18/213) (Table 2).
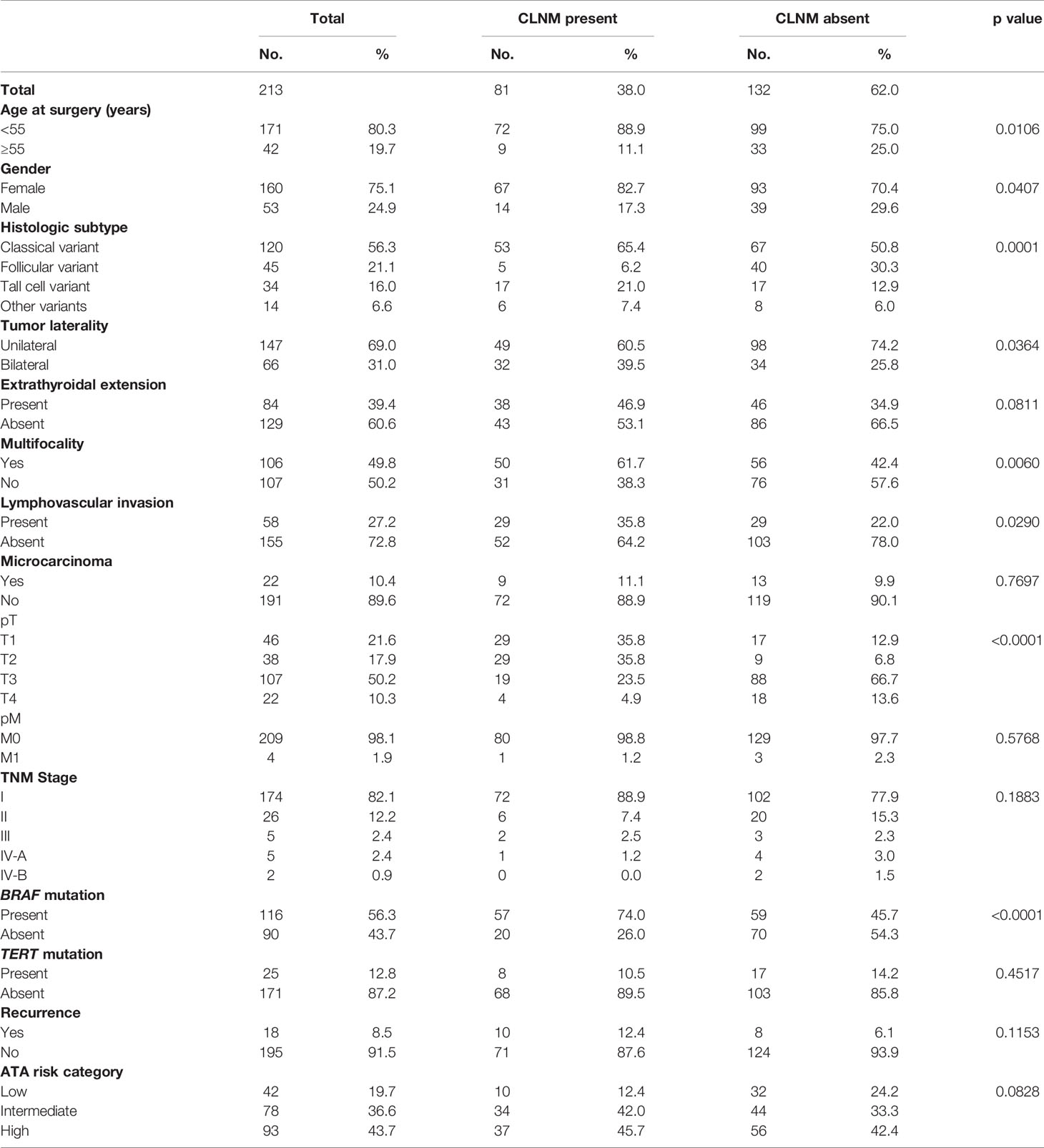
Table 2 Clinico-pathological characteristics stratified by central lymph node metastasis (CLNM) status in adult cN0 PTC patients who underwent prophylactic central lymph node dissection.
Clinico-Pathological Associations of CLNM
Of the 213 PTC patients that underwent PCLND, CLNM was noted in 38.0% (81/213) of cases. Presence of CLNM in these patients was significantly associated with adverse clinico-pathological risk factors, such as tall cell variant (p = 0.0001), bilateral tumors (p = 0.0364), multifocality (p = 0.0060), lymphovascular invasion (p = 0.0290) and BRAF mutation (p < 0.0001). Interestingly, we also found that CLNM was associated with younger age (< 55 years; p = 0.0106) and female sex (p = 0.0407). CLNM was also found to be more frequent in PTCs with extrathyroidal extension, although the difference was not statistically significant (p = 0.0811) (Table 2).
Risk Factors Predicting CLNM
We analyzed the clinico-pathological risk factors that could independently predict CLNM in patients undergoing PCLND. Using multivariate logistic regression analysis, we found age < 55 years (Odds ratio (OR) = 7.38; 95% Confidence interval (CI) = 1.59 – 34.31; p = 0.0108), bilateral tumors (OR = 3.01; 95% CI = 1.01 – 9.21; p = 0.0483), lymphovascular invasion (OR = 2.92; 95% CI = 1.18 – 7.23; p = 0.0206) and BRAF mutation (OR = 3.24; 95% CI = 1.41 – 7.49; p = 0.0058) to be independent predictors for CLNM (Table 3).
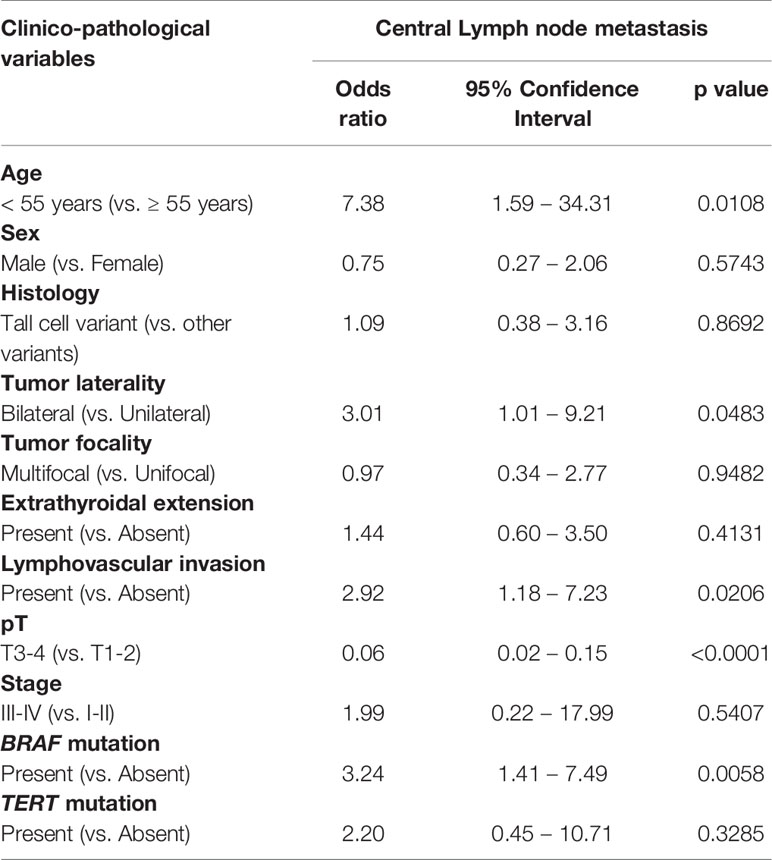
Table 3 Independent predictors of central lymph node metastasis by multivariate logistic regression analysis.
We next to sought to determine if the combination of BRAF mutation with any of the other independent clinical parameters leads to better prediction of CLNM. We found that BRAF + age < 55 years had a better predictive value compared to age alone (OR – 3.91 vs. 2.67). Similarly, BRAF + bilateral tumors had a better predictive value compared to bilateral tumors alone (OR – 2.52 vs. 1.88) and BRAF + lymphovascular invasion had a better predictive value compared to lymphovascular invasion alone (OR – 3.97 vs 1.98) (Table 4).
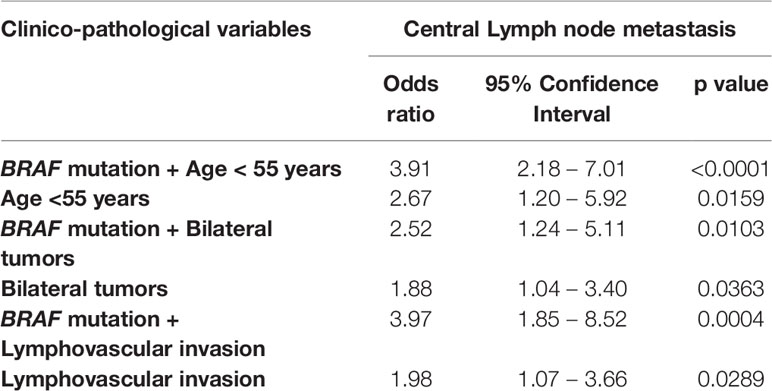
Table 4 Combination of BRAF mutation and other risk factors as predictors of central lymph node metastasis.
Prognostic Impact of PCLND
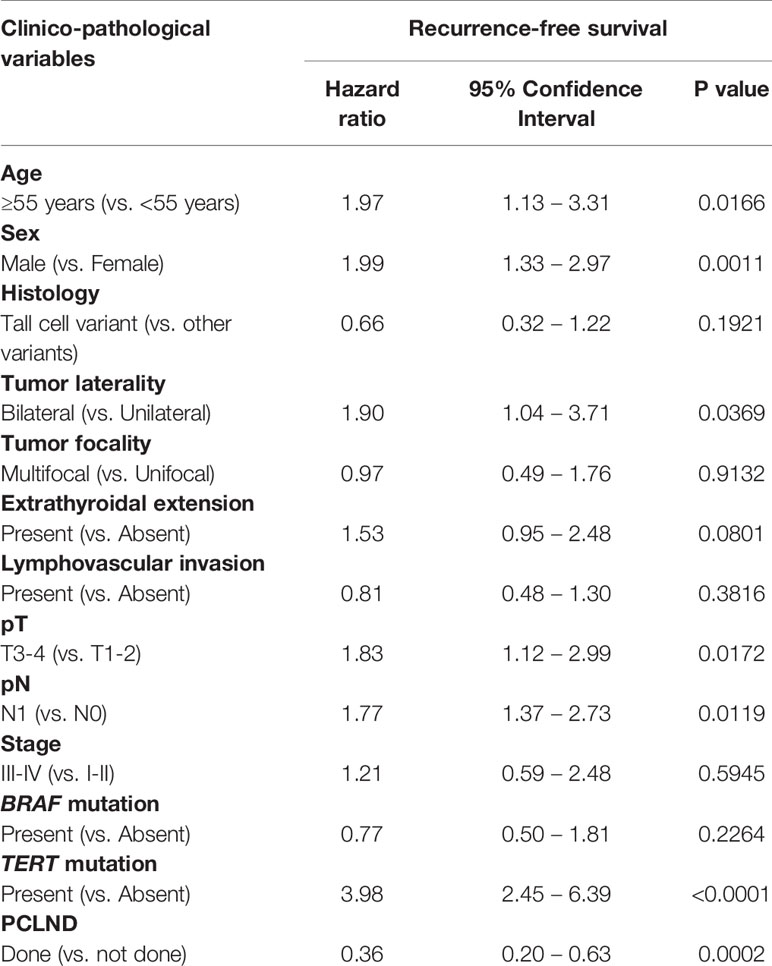
We found that cN0 patients undergoing PCLND had a significantly reduced recurrence rate compared to patients who did not undergo PCLND (8.5% vs. 14.7%; p = 0.0138). To determine whether performing PCLND truly had an impact on recurrence, we analyzed the recurrence-free survival using Kaplan Meier curves and multivariate Cox proportional hazards model. Kaplan Meier curves showed that patients undergoing PCLND had a significantly better recurrence-free survival compared to those who did not (p = 0.0379; Figure 1). On multivariate analysis, PCLND was found to be an independent predictor of improved recurrence-free survival (Hazard ratio = 0.36; 95% confidence interval = 0.20 – 0.63; p = 0.0002) (Table 5).
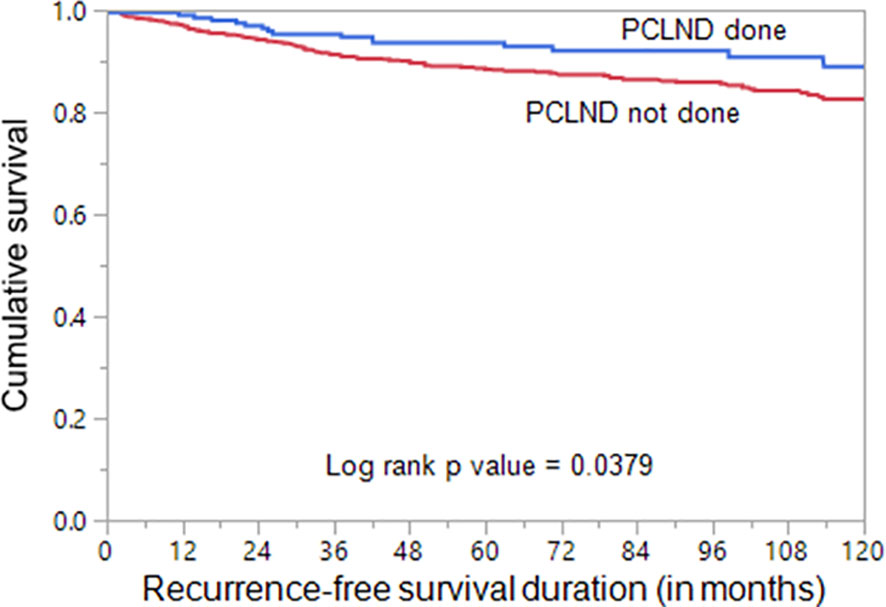
Figure 1 Recurrence-free survival analysis of prophylactic central lymph node dissection (PCLND) in clinically node negative (cN0) PTC. Kaplan Meier survival plot showing statistically significant improved recurrence-free survival in patients undergoing PCLND compared to those that did not (p = 0.0379).
Discussion
PTC is common in Middle Eastern population with favorable long term prognosis (4, 28, 29). The fundamental therapy of PTC is surgery. When macroscopic nodal disease is present, either detected on imaging preoperatively or identified intraoperatively, lymph node dissection is the ideal management (25, 30, 31) due to the heightened risk of recurrence. However, the management of microscopic lymph node involvement, which is present in subset of patients, is unclear (32–34). The indications for prophylactic central neck dissection in patients with cN0 remains a subject of debate in PTC surgical management (12, 20, 35). The prevalence and the predictors of central nodal metastasis of cN0 Middle Eastern PTC have not been explored. Therefore, we aimed in this work to evaluate the incidence and the predictive factors of CNLM of cN0 in Middle Eastern PTC and focused our attention on the effect of PCNLD in patient’s prognosis.
In this study, we included 942 adult PTC patients with clinically uninvolved lymph nodes (cN0). PCLND was performed in 22.6% (213/942) patients while thyroidectomy alone was performed in 77.4% (729/942) of the PTC patients. Among those who underwent PCLND, 38% (81/213) of PTC patients were found to have CLNM by histopathology post-operative result, which implies that occult metastatic lymph nodes would have remained undetected if PCLND was not performed. This is of great clinical value for PTC patients from Middle Eastern ethnicity where recurrence rate is relatively high (36–38). Even in this cohort, which included only cN0 PTCs, recurrence rate was 13%. Presence of occult LNM and incomplete CLND could be attributed to this high recurrence rate. The recurrence rate dropped to 8.5% in patients who underwent PCNLD. Thus, we attempted to estimate the risk factors of CLNM and identify accurate predictors of CNLM in this population.
The age factor was found to have great impact on lymph node metastasis in PTC. Previous study has shown that CLNM is significantly higher in younger (<45 years) patients (39). Another recent study conducted on Chinese PTC cohort suggested patients ≤ 35 years old are more vulnerable to CLNM (8). In our study, we used the age cut off of 55 years according to the recent AJCC criteria, and found that age was predictive of occult CLNM, with patients aged < 55 were more likely to have occult CLNM (p = 0.0106). Moreover, we found that age was an independent risk factor for CLNM in cN0 adult patients with PTC (OR = 7.38; 95% CI = 1.59 – 34.31; p = 0.0108). Gender was previously reported to influence the LN metastasis, with male patients considered to have higher risk for CLNM than female patients (8, 9, 40). The risk differences might possibly be explained by the different levels of hormone in females and males. However, our study showed opposite result where CLNM is higher in female PTC patients. However, gender was not an independent predictive factor for CLNM in multivariate analysis.
Among the clinico-pathological markers that were assessed before and during surgery, we found that tumor bilaterality and lymphovascular invasion were independent predictors for CLNM in cN0 PTC. In agreement with our finding, lymphovascular invasion has been reported to adversely influence PTC biological behavior with regards to CLNM and survival (41–43). While some previous reports have documented the association between tumor bilaterality and LNM as well as local-regional recurrence (44–46), others did not consider bilateral PTC as an independent prognostic factor (47).
Advances in translational medicine have highlighted the prognostic impact of novel genetic alterations in PTC. We sought to investigate the usefulness of these molecular markers (TERT and BRAF mutations) as predictors for CLNM, since alterations of both genes are common in PTC and known to be associated with PTC aggressiveness, recurrence and metastasis (27, 48–50). In our study, we could not find association between TERT mutations and CLNM in cN0 PTC. However, we found BRAF mutation to be an independent predictor of occult CLNM (OR = 3.24; 95% CI = 1.41 – 7.49; p = 0.0058). Interestingly, we found that combining BRAF mutation with any of the other independent clinical parameters identified in this study, could increase the prediction power for CLNM. This could help in more accurate stratification of PTC patient into distinct risk groups and help in better therapeutic approach and outcome.
Considering the ATA risk stratification for structural recurrence, patients with CLNM were more often classified in the intermediate and high risk group. This is consistent with Medas et al. (17), where CLNM was significantly associated with ATA intermediate risk. This might be attributed to the PCLND which usually allows better assessment of LN and more accurate staging. Importantly, patients who underwent PCLND showed significantly better recurrence free survival. Moreover, multivariate analysis showed that PCLND was an independent predictor of better RFS regardless of other clinico-pathological variables. This is of high clinical importance since it shows the benefit from aggressive surgery in modifying the outcome of the disease and significantly improved the recurrence free survival in Middle Eastern PTC patients, where recurrence rate is relatively high. In concordance with our findings, two previous meta-analyses conducted on PTC patients from different ethnicity have shown that PCLND is beneficial in reducing risk of recurrence (51, 52).
There are several limitations of this study. Firstly, it is a retrospective single institute study, so selection bias should be considered. Secondly, this study lacks information about post-surgical risk of hypothyroidism and laryngeal nerve injury. Thirdly, all the patients included were from Middle Eastern ethnicity, so applying conclusions to other races should be done with caution.
In summary, CLNM occurred in 38.0% of cN0 adult PTC patients undergoing PCLND; multivariate logistic regression analysis revealed age, tumor bilaterality, lymphovascular invasion and BRAF mutations as independent risk factors for CLNM in adult PTC patients. In addition, our work succeeded in demonstrating better prognosis in patients who underwent PCLND and performing PCLND was an independent marker to improve the RFS. We believe that, in adult Middle Eastern patients with these risk factors, PCLND should be performed in order to reduce the risk of CLNM and to improve patients’ outcome.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Research Advisory Council, King Faisal Specialist Hospital and Research Centre. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
SP and AS analyzed the clinical data, designed and wrote the manuscript. SP performed statistical analysis. FD and SA performed clinical data abstraction. SA-S and FA-D contributed samples and analyzed clinical data. KA-K designed, implemented the study, wrote and critically reviewed the manuscript. This is to confirm that all authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Padmanaban Annaiyappanaidu and Nabil Siraj for their technical assistance.
References
1. Pereira M, Williams VL, Hallanger Johnson J, Valderrabano P. Thyroid Cancer Incidence Trends in the United States: Association With Changes in Professional Guideline Recommendations. Thyroid (2020) 30(8):1132–40. doi: 10.1089/thy.2019.0415
2. Kitahara CM, Sosa JA. The Changing Incidence of Thyroid Cancer. Nat Rev Endocrinol (2016) 12(11):646–53. doi: 10.1038/nrendo.2016.110
3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA: Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
4. Alrawaji, Alshahrani, Alzahrani, Alomran, Almadouj, Alshehri, et al. Cancer Incidence Report Saudi Arabia 2015. Council SH, editor. Riyadh: Saudi Cancer Registry (2018).
5. Shi X, Liu R, Basolo F, Giannini R, Shen X, Teng D, et al. Differential Clinicopathological Risk and Prognosis of Major Papillary Thyroid Cancer Variants. J Clin Endocrinol (2016) 101(1):264–74. doi: 10.1210/jc.2015-2917
6. Viola D, Materazzi G, Valerio L, Molinaro E, Agate L, Faviana P, et al. Prophylactic Central Compartment Lymph Node Dissection in Papillary Thyroid Carcinoma: Clinical Implications Derived From the First Prospective Randomized Controlled Single Institution Study. J Clin Endocrinol Metab (2015) 100(4):1316–24. doi: 10.1210/jc.2014-3825
7. Wang Y, Nie F, Wang G, Liu T, Dong T, Sun Y. Value of Combining Clinical Factors, Conventional Ultrasound, and Contrast-Enhanced Ultrasound Features in Preoperative Prediction of Central Lymph Node Metastases of Different Sized Papillary Thyroid Carcinomas. Cancer Manage Res (2021) 13:3403. doi: 10.2147/CMAR.S299157
8. Jiang L-H, Yin K-X, Wen Q-L, Chen C, Ge M-H, Tan Z. Predictive Risk-Scoring Model for Central Lymph Node Metastasis and Predictors of Recurrence in Papillary Thyroid Carcinoma. Sci Rep (2020) 10(1):1–9. doi: 10.1038/s41598-019-55991-1
9. Sun W, Lan X, Zhang H, Dong W, Wang Z, He L, et al. Risk Factors for Central Lymph Node Metastasis in CN0 Papillary Thyroid Carcinoma: A Systematic Review and Meta-Analysis. PloS One (2015) 10(10):e0139021. doi: 10.1371/journal.pone.0139021
10. Barczyński M, Konturek A, Stopa M, Nowak W. Prophylactic Central Neck Dissection for Papillary Thyroid Cancer. J Br Surg (2013) 100(3):410–8. doi: 10.1002/bjs.8985
11. Alvarado R, Sywak MS, Delbridge L, Sidhu SB. Central Lymph Node Dissection as a Secondary Procedure for Papillary Thyroid Cancer: Is There Added Morbidity? Surgery (2009) 145(5):514–8. doi: 10.1016/j.surg.2009.01.013
12. Moreno MA, Edeiken-Monroe BS, Siegel ER, Sherman SI, Clayman GL. In Papillary Thyroid Cancer, Preoperative Central Neck Ultrasound Detects Only Macroscopic Surgical Disease, But Negative Findings Predict Excellent Long-Term Regional Control and Survival. Thyroid (2012) 22(4):347–55. doi: 10.1089/thy.2011.0121
13. Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Clinical Significance of Metastasis to the Central Compartment From Papillary Microcarcinoma of the Thyroid. World J Surg (2006) 30(1):91–9. doi: 10.1007/s00268-005-0113-y
14. Liu FH, Kuo SF, Hsueh C, Chao TC, Lin JD. Postoperative Recurrence of Papillary Thyroid Carcinoma With Lymph Node Metastasis. J Surg Oncol (2015) 112(2):149–54. doi: 10.1002/jso.23967
15. Wada N, Duh Q-Y, Sugino K, Iwasaki H, Kameyama K, Mimura T, et al. Lymph Node Metastasis From 259 Papillary Thyroid Microcarcinomas: Frequency, Pattern of Occurrence and Recurrence, and Optimal Strategy for Neck Dissection. Ann Surg (2003) 237(3):399. doi: 10.1097/01.SLA.0000055273.58908.19
16. Shuai Y, Yue K, Duan Y, Zhou M, Fang Y, Liu J, et al. Surgical Extent of Central Lymph Node Dissection for Papillary Thyroid Carcinoma Located in the Isthmus: A Propensity Scoring Matched Study. Front Endocrinol (2021) 12:220. doi: 10.3389/fendo.2021.620147
17. Medas F, Canu GL, Cappellacci F, Anedda G, Conzo G, Erdas E, et al. Prophylactic Central Lymph Node Dissection Improves Disease-Free Survival in Patients With Intermediate and High Risk Differentiated Thyroid Carcinoma: A Retrospective Analysis on 399 Patients. Cancers (2020) 12(6):1658. doi: 10.3390/cancers12061658
18. Çolakoğlu B, Sağlam B, Sezer H, Kapran Y, Aydın Ö, Demirkol MO, et al. Effect of Prophylactic Central Neck Dissection on the Surgical Outcomes in Papillary Thyroid Cancer: Experience in a Single Center. Eur Arch Oto-Rhino-Laryngol (2020) 277(5):1491–7. doi: 10.1007/s00405-020-05830-1
19. Chen L, Wu Y-H, Lee C-H, Chen H-A, Loh E-W, Tam K-W. Prophylactic Central Neck Dissection for Papillary Thyroid Carcinoma With Clinically Uninvolved Central Neck Lymph Nodes: A Systematic Review and Meta-Analysis. World J Surg (2018) 42(9):2846–57. doi: 10.1007/s00268-018-4547-4
20. Gyorki DE, Untch B, Tuttle RM, Shaha AR. Prophylactic Central Neck Dissection in Differentiated Thyroid Cancer: An Assessment of the Evidence. Ann Surg Oncol (2013) 20(7):2285–9. doi: 10.1245/s10434-013-2897-6
21. Conzo G, Calò PG, Sinisi AA, De Bellis A, Pasquali D, Iorio S, et al. Impact of Prophylactic Central Compartment Neck Dissection on Locoregional Recurrence of Differentiated Thyroid Cancer in Clinically Node-Negative Patients: A Retrospective Study of a Large Clinical Series. Surgery (2014) 155(6):998–1005. doi: 10.1016/j.surg.2014.02.010
22. Scherl S, Mehra S, Clain J, Dos Reis LL, Persky M, Turk A, et al. The Effect of Surgeon Experience on the Detection of Metastatic Lymph Nodes in the Central Compartment and the Pathologic Features of Clinically Unapparent Metastatic Lymph Nodes: What are We Missing When We Don’t Perform a Prophylactic Dissection of Central Compartment Lymph Nodes in Papillary Thyroid Cancer? Thyroid (2014) 24(8):1282–8. doi: 10.1089/thy.2013.0600
23. Calò PG, Pisano G, Medas F, Marcialis J, Gordini L, Erdas E, et al. Total Thyroidectomy Without Prophylactic Central Neck Dissection in Clinically Node-Negative Papillary Thyroid Cancer: Is it an Adequate Treatment? World J Surg Oncol (2014) 12(1):1–8. doi: 10.1186/1477-7819-12-152
24. Sippel RS, Robbins SE, Poehls JL, Pitt SC, Chen H, Leverson G, et al. A Randomized Controlled Clinical Trial: No Clear Benefit to Prophylactic Central Neck Dissection in Patients With Clinically Node Negative Papillary Thyroid Cancer. Ann Surg (2020) 272(3):496–503. doi: 10.1097/SLA.0000000000004345
25. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
26. Siraj AK, Parvathareddy SK, Pratheeshkumar P, Divya SP, Al-Sobhi SS, Al-Dayel F, et al. PD-L1 Is an Independent Prognostic Marker in Middle Eastern PTC and Its Expression Is Upregulated by BRAFV600E Mutation. Cancers (2021) 13(3):555. doi: 10.3390/cancers13030555
27. Bu R, Siraj AK, Divya SP, Kong Y, Parvathareddy SK, Al-Rasheed M, et al. Telomerase Reverse Transcriptase Mutations are Independent Predictor of Disease-Free Survival in M Iddle E Astern Papillary Thyroid Cancer. Int J Cancer (2018) 142(10):2028–39. doi: 10.1002/ijc.31225
28. Doubi A, Al-Qannass A, Al-Angari SS, Al-Qahtani KH, Alessa M, Al-Dhahri S. Trends in Thyroid Carcinoma Among Thyroidectomy Patients: A 12-Year Multicenter Study. Ann Saudi Med (2019) 39(5):345–9. doi: 10.5144/0256-4947.2019.345
29. Safavi A, Azizi F, Jafari R, Chaibakhsh S, Safavi AA. Thyroid Cancer Epidemiology in Iran: A Time Trend Study. Asian Pacific J Cancer Prev (2016) 17(1):407–12. doi: 10.7314/APJCP.2016.17.1.407
30. Doubleday A, Sippel RS. Surgical Options for Thyroid Cancer and Post-Surgical Management. Expert Rev Endocrinol Metab (2018) 13(3):137–48. doi: 10.1080/17446651.2018.1464910
31. Patel KN, Yip L, Lubitz CC, Grubbs EG, Miller BS, Shen W, et al. The American Association of Endocrine Surgeons Guidelines for the Definitive Surgical Management of Thyroid Disease in Adults. Ann Surg (2020) 271(3):e21–93. doi: 10.1097/SLA.0000000000003580
32. Sippel RS, Chen H. Controversies in the Surgical Management of Newly Diagnosed and Recurrent/Residual Thyroid Cancer. Thyroid (2009) 19(12):1373–80. doi: 10.1089/thy.2009.1606
33. White ML, Gauger PG, Doherty GM. Central Lymph Node Dissection in Differentiated Thyroid Cancer. World J Surg (2007) 31(5):895–904. doi: 10.1007/s00268-006-0907-6
34. Pereira JA, Jimeno J, Miquel J, Iglesias M, Munné A, Sancho JJ, et al. Nodal Yield, Morbidity, and Recurrence After Central Neck Dissection for Papillary Thyroid Carcinoma. Surgery (2005) 138(6):1095–101. doi: 10.1016/j.surg.2005.09.013
35. Calò PG, Lombardi CP, Podda F, Sessa L, Santini L, Conzo G. Role of Prophylactic Central Neck Dissection in Clinically Node-Negative Differentiated Thyroid Cancer: Assessment of the Risk of Regional Recurrence. Updates Surg (2017) 69(2):241–8. doi: 10.1007/s13304-017-0438-8
36. Raef H, Alfadhli E, Al-Hajjaj A, Malabu UH, Al-Sobhi S, Rifai A, et al. High Rate of Persistent/Recurrent Disease Among Patients With Differentiated Thyroid Cancer in Saudi Arabia: Factors Affecting Non-Remission. Ann Saudi Med (2008) 28(4):277–81. doi: 10.5144/0256-4947.2008.277
37. Arianpoor A, Asadi M, Amini E, Ziaeemehr A, Ahmadi Simab S, Zakavi SR. Investigating the Prevalence of Risk Factors of Papillary Thyroid Carcinoma Recurrence and Disease-Free Survival After Thyroidectomy and Central Neck Dissection in Iranian Patients. Acta Chirurgica Belgica (2020) 120(3):173–8. doi: 10.1080/00015458.2019.1576447
38. Siraj AK, Parvathareddy SK, Qadri Z, Siddiqui K, Al-Sobhi SS, Al-Dayel F, et al. Annual Hazard Rate of Recurrence in Middle Eastern Papillary Thyroid Cancer Over a Long-Term Follow-Up. Cancers (2020) 12(12):3624. doi: 10.3390/cancers12123624
39. Siddiqui S, White MG, Antic T, Grogan RH, Angelos P, Kaplan EL, et al. Clinical and Pathologic Predictors of Lymph Node Metastasis and Recurrence in Papillary Thyroid Microcarcinoma. Thyroid (2016) 26(6):807–15. doi: 10.1089/thy.2015.0429
40. Xue S, Zhang L, Pang R, Wang P, Jin M, Guo L, et al. Predictive Factors of Central-Compartment Lymph Node Metastasis for Clinical N0 Papillary Thyroid Carcinoma With Strap Muscle Invasion. Front Endocrinol (2020) 11. doi: 10.3389/fendo.2020.00511
41. Falvo L, Catania A, D’Andrea V, Marzullo A, Giustiniani MC, De Antoni E. Prognostic Importance of Histologic Vascular Invasion in Papillary Thyroid Carcinoma. Ann Surg (2005) 241(4):640. doi: 10.1097/01.sla.0000157317.60536.08
42. Tang T, Li J, Zheng L, Zhang L, Shi J. Risk Factors of Central Lymph Node Metastasis in Papillary Thyroid Carcinoma: A Retrospective Cohort Study. Int J Surg (2018) 54:129–32. doi: 10.1016/j.ijsu.2018.04.046
43. Li X, Zhang H, Zhou Y, Cheng R. Risk Factors for Central Lymph Node Metastasis in the Cervical Region in Papillary Thyroid Carcinoma: A Retrospective Study. World J Surg Oncol (2021) 19(1):1–9. doi: 10.1186/s12957-021-02247-w
44. Prescott JD, Parangi S. Bilaterality in Papillary Thyroid Carcinoma: Does It Influence Prognosis? Ann Surg Oncol (2012) 19(1):1–2. doi: 10.1245/s10434-011-2098-0
45. Moore SE, Kasaian K, Jones S, Melck A, Wiseman SM. Clinical Importance of Bilateral Disease in Patients With Papillary Thyroid Cancer. Can J Surg (2016) 59(3):213. doi: 10.1503/cjs.014615
46. So YK, Kim MW, Son Y-I. Multifocality and Bilaterality of Papillary Thyroid Microcarcinoma. Clin Exp Otorhinolaryngol (2015) 8(2):174. doi: 10.3342/ceo.2015.8.2.174
47. Rodríguez-Cuevas S, Labastida-Almendaro S, Cortés-Arroyo H, López-Garza J, Barroso-Bravo S. Multifactorial Analysis of Survival and Recurrences in Differentiated Thyroid Cancer. Comparative Evaluation of Usefulness of AGES, MACIS, and Risk Group Scores in Mexican Population. J Exp Clin Cancer Res: CR (2002) 21(1):79–86.
48. Siraj AK, Masoodi T, Bu R, Beg S, Al-Sobhi SS, Al-Dayel F, et al. Genomic Profiling of Thyroid Cancer Reveals a Role for Thyroglobulin in Metastasis. Am J Hum Genet (2016) 98(6):1170–80. doi: 10.1016/j.ajhg.2016.04.014
49. Li J, Zhang S, Zheng S, Zhang D, Qiu X. The BRAF V600E Mutation Predicts Poor Survival Outcome in Patients With Papillary Thyroid Carcinoma: A Meta Analysis. Int J Clin Exp Med (2015) 8(12):22246.
50. Yang J, Gong Y, Yan S, Chen H, Qin S, Gong R. Association Between TERT Promoter Mutations and Clinical Behaviors in Differentiated Thyroid Carcinoma: A Systematic Review and Meta-Analysis. Endocrine (2020) 67(1):44–57. doi: 10.1007/s12020-019-02117-2
51. Zhao W-J, Luo H, Zhou Y-M, Dai W-Y, Zhu J-Q. Evaluating the Effectiveness of Prophylactic Central Neck Dissection With Total Thyroidectomy for Cn0 Papillary Thyroid Carcinoma: An Updated Meta-Analysis. Eur J Surg Oncol (2017) 43(11):1989–2000. doi: 10.1016/j.ejso.2017.07.008
Keywords: head and neck cancer, papillary thyroid carcinoma, prophylactic central lymph node dissection, central lymph node metastasis, risk factors
Citation: Parvathareddy SK, Siraj AK, Ahmed SO, DeVera F, Al-Sobhi SS, Al-Dayel F and Al-Kuraya KS (2022) Risk Factors for Central Lymph Node Metastases and Benefit of Prophylactic Central Lymph Node Dissection in Middle Eastern Patients With cN0 Papillary Thyroid Carcinoma. Front. Oncol. 11:819824. doi: 10.3389/fonc.2021.819824
Received: 22 November 2021; Accepted: 27 December 2021;
Published: 17 January 2022.
Edited by:
Giuseppe Mercante, Humanitas University, ItalyReviewed by:
Roberto Valcavi, Endocrine & Thyroid Clinic, ItalyGerardo Petruzzi, Hospital Physiotherapy Institutes (IRCCS), Italy
Copyright © 2022 Parvathareddy, Siraj, Ahmed, DeVera, Al-Sobhi, Al-Dayel and Al-Kuraya. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khawla S. Al-Kuraya, kkuraya@kfshrc.edu.sa
†These authors have contributed equally to this work
 Sandeep Kumar Parvathareddy1†
Sandeep Kumar Parvathareddy1† Abdul K. Siraj
Abdul K. Siraj Khawla S. Al-Kuraya
Khawla S. Al-Kuraya