- 1Department of Hepatic Surgery, Third Affiliated Hospital of Second Military Medical University, Shanghai, China
- 2Department of Hepatic Surgery, The First Hospital of Putian, Putian, China
- 3Tumor Immunology and Gene Therapy Center, Third Affiliated Hospital of Second Military Medical University, Shanghai, China
Purpose: To compare the efficacy and safety of the combination of transcatheter arterial chemoembolization (TACE), Lenvatinib, and programmed cell death protein-1 (PD-1) inhibitors (combination group) with TACE (TACE group) in the treatment of patients with unresectable hepatocellular carcinoma (uHCC).
Methods: We consecutively enrolled 110 patients with uHCC in this prospective cohort study, with 56 patients receiving combination treatment and 54 patients receiving TACE from November 2017 to September 2020. The differences in tumor response, survival benefit, and adverse events (AEs) were compared between the two groups. Factors affecting survival were identified via Cox regression analysis.
Results: Compared with the TACE group, the combination group had a higher objective response rate (ORR) (67.9% vs. 29.6%, p < 0.001), longer median progression-free survival (mPFS) (11.9 vs. 6.9 months, P = 0.003) and overall survival (mOS) (23.9 vs. 15.3 months, p < 0.001). Multivariate analysis showed that the neutrophil-to-lymphocyte ratio (NLR) and the treatment option were independent factors associated with the PFS and OS. Further subgroup analysis showed that patients with low NLR (≤median 3.11) receiving combination therapy had better mPFS (20.1 vs. 6.2 months, P < 0.001) and mOS (28.9 vs. 15.2 months, P < 0.001) than those receiving TACE, while no obvious difference in PFS or OS was observed between the two groups in patients with high NLR (> 3.11). There were no unexpected toxicities in the combination group.
Conclusion: Compared with TACE, the combination treatment demonstrated an improved clinical efficacy and manageable safety profile in patients with uHCC. Combination treatment showed better therapeutic efficacy in patients with low NLR; therefore, this ratio could be used to identify patients who will benefit from this treatment.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the second leading cause of cancer-related death worldwide (1). Approximately 70%–80% of patients with HCC are diagnosed at the intermediate-advanced stage (2), and only candidates for palliative treatments such as chemotherapy, transcatheter arterial chemoembolization (TACE), radiotherapy or sorafenib, resulting in a dismal prognosis. Recently, little improvements in overall survival (OS) with monotherapy have been noted despite the rapid evolution of systemic agents in patients with uHCC (3, 4). Therefore, there is currently an unmet need to explore more effective treatment modalities with improved antitumoral efficacy in patients with uHCC.
TACE, which is the first therapeutic modality to provide survival benefits in patients with uHCC (5), is the standard treatment for BCLC stage B HCC in Western countries (6) and stage B and C in China (7). In the real-world BRIDGE study that included 18,031 patients from 14 countries, TACE was identified as the first-line treatment for approximately 50% of patients at the BCLC C stage of HCC (2). Also, some previous studies have demonstrated that TACE therapy alone could be of more benefit to patients at the BCLC C stage of HCC than the best supportive care (8, 9). Some studies even demonstrated that TACE could achieve a comparable survival outcome to sorafenib in BCLC stage C HCC patients (10, 11). TACE was also found to promote the immunogenic cell death of cancer cells (12) and induce the release of the tumor-associated antigen (13), enabling more antitumor activity of immune checkpoint inhibitors (ICIs). Furthermore, The upregulated expression of Vascular Endothelial Growth Factor (VEGF) and Fibroblast Growth Factor (FGF) (14) by TACE could be effectively inhibited by tyrosine kinase inhibitors(TKIs) in a preclinical study (15), leading to better clinical outcomes combined with TKIs (16, 17). Thus, TACE may hold a greater promise in the context of rapid advances in systemic therapy.
Several recent studies have demonstrated that the combination of ICIs and antiangiogenic agents exhibits superior antitumor activity and a significantly improved survival compared to monotherapies. In the IMbrave150 trial (18), the combination of atezolizumab and bevacizumab showed better tumor response and survival than sorafenib, leading to accelerated Food and Drug Administration approval for advanced HCC. Similarly promising efficacy was also reported in the KEYNOTE 524 trial (19) that was based on pembrolizumab plus Lenvatinib and demonstrated a mOS of 22 months, a mPFS of 9.3 months, and an objective response rate (ORR) of 46% per modified Response Evaluation Criteria in Solid Tumors (mRECIST). However, it should be noted that clinical benefits from such combinations with systemic therapies remain limited to a subset of patients because of the high proportion of primary drug resistance.
Considering the potential synergistic effect of the combination of TACE, Lenvatinib, and PD-1 inhibitors, we conducted this prospective cohort study to compare the safety and efficacy of the triple combination treatment with those of TACE alone for patients with uHCC.
Patients and Methods
Patients
This prospective cohort study was conducted at the Department of Hepatic Surgery, Third Affiliated Hospital of the Second Military Medical University, Shanghai, China, from November 2017 to September 2020.The inclusion criteria were as follows: patients were diagnosed with HCC by histopathological biopsy or clinical features according to the American Association for the Study of Liver Diseases guidelines; lesions not amenable to curative resection evaluated by surgeons due to an insufficient future liver remnant, extensive or multifocal bi-lobar tumors, extrahepatic metastasis, and major vascular invasion (20); Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0 or 1; Child-Pugh class A or B7; accept heart, kidney and bone marrow function; at least one measurable target lesion by mRECIST; no prior systemic therapy for liver cancer. Patients were excluded if they had any of the following conditions: life expectancy was less than 3 months; patients simultaneously received other forms of therapy such as radiotherapy and ablation; uncontrollable ascites; decompensated liver cirrhosis; concomitant with other primary malignancies. The study protocol was approved by the ethical committee of the Third Affiliated Hospital of the Second Military Medical University. Written informed consent was obtained from all patients.
TACE Procedure
The vascular catheter was inserted through a femoral artery using the Seldinger technique to the hepatic artery, then hepatic angiography was performed. The catheter tip was inserted selectively into the tumor-feeding artery and the pirarubicin manually emulsified with iodized oil was injected into these vessels, followed by embolization with absorbable gelatin sponge particles. TACE was conducted repeatedly on demand according to investigators’ consideration, mainly based on the proportion of viable tumors and status of hepatic function.
Medication Treatment
All patients were discussed and evaluated by multidisciplinary teams. The TACE-Lenvatinib-PD-1 inhibitor treatment strategy was recommended by physicians if the patient was suitable for the combination therapy. Also, the patient was fully informed about the efficacy and cost of the drugs and the potential adverse effects (AEs). If the patient agreed to the physician’s recommendation, Lenvatinib and PD-1 inhibitors were administered, otherwise, the patient received TACE treatment alone.
Lenvatinib was administered one week prior to the first TACE procedure. Patients received Lenvatinib at a dose of 12 mg/d if they weighed ≥60 kg and 8 mg/d if they weighed <60 kg. Lenvatinib was interrupted for two days before and after each TACE session if no obvious symptoms occurred after TACE. Dose modifications and discontinuation were permitted according to the label.
Patients received 200 mg pembrolizumab or 240 mg toripalimab intravenously every three weeks based on their labels. PD-1 inhibitors were administered one day prior to the first TACE. Discontinuation was allowed if patients experienced severe AEs. Each cycle of combination therapy was defined as four doses of PD-1 inhibitors and 1–2 episodes of TACE.
Patients discontinued the combination treatment in the case of unacceptable toxicity or disease progression.
All patients with hepatitis B virus (HBV) infection received antiviral treatment initially and continued antiviral treatment throughout the treatment period.
Efficacy Assessment
The primary endpoints were the OS. The OS was defined as the time from treatment initiation to death of any cause. The secondary endpoints were the PFS, the ORR and safety profiles. The PFS was defined as the time from treatment initiation to disease progression according to mRECIST or death of any cause. The mRECIST assessment is a method of evaluating the therapeutic response based on the viable tumor by an enhanced scan in HCC (21). The objective response rate (ORR) was calculated as the sum of the complete response (CR) and partial response (PR). The disease control rate (DCR) was the sum of the CR, PR, and stable disease (SD). Early tumor shrinkage (ETS) was defined as tumor shrinkage at the first radiologic evaluation (approximately eight weeks after treatment initiation) in the sum of target lesions’ longest diameters from baseline according to the Response Evaluation Criteria in Solid Tumors (RECIST) (22).
Safety Assessment
Safety was evaluated via vital signs, clinical laboratory testing, and an assessment of the incidence and severity of AEs according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0.
Follow-up
All patients were monitored regularly. Followed-up assessments were conducted every 6–8 weeks. Each session consisted of tumor response assessment by enhanced computed tomography (CT) or magnetic resonance imaging (MRI) and laboratory tests including blood and urine routine, liver and kidney function tests, thyroid function tests, HBV-DNA, and tumor markers, including alpha-fetoprotein (AFP) and des-gamma-carboxy prothrombin (DCP) in the two groups. An additional myocardial enzymology examination was necessary in the combination group. In addition, chest X-ray was also regular test. 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PETCT) examination was performed if necessary.
Statistical Analysis
Statistical analyses were performed using STATA version 15 for Windows. Categorical variables were compared using the χ2 test or Fisher’s exact test. Continuous variables were compared using either the t-test for normally distributed variables or the Mann–Whitney U test for variables with a non-normal distribution. The median NLR value was chosen as the cutoff to divide the data into high and low subgroups. The survival analysis was carried out using the Kaplan-Meier method and compared using the log-rank test. All variables with p < 0.1 during univariate analyses were included in multivariate analyses, which included a Cox regression analysis to identify factors independently associated with PFS and OS. P < 0.05 was considered statistically significant.
Results
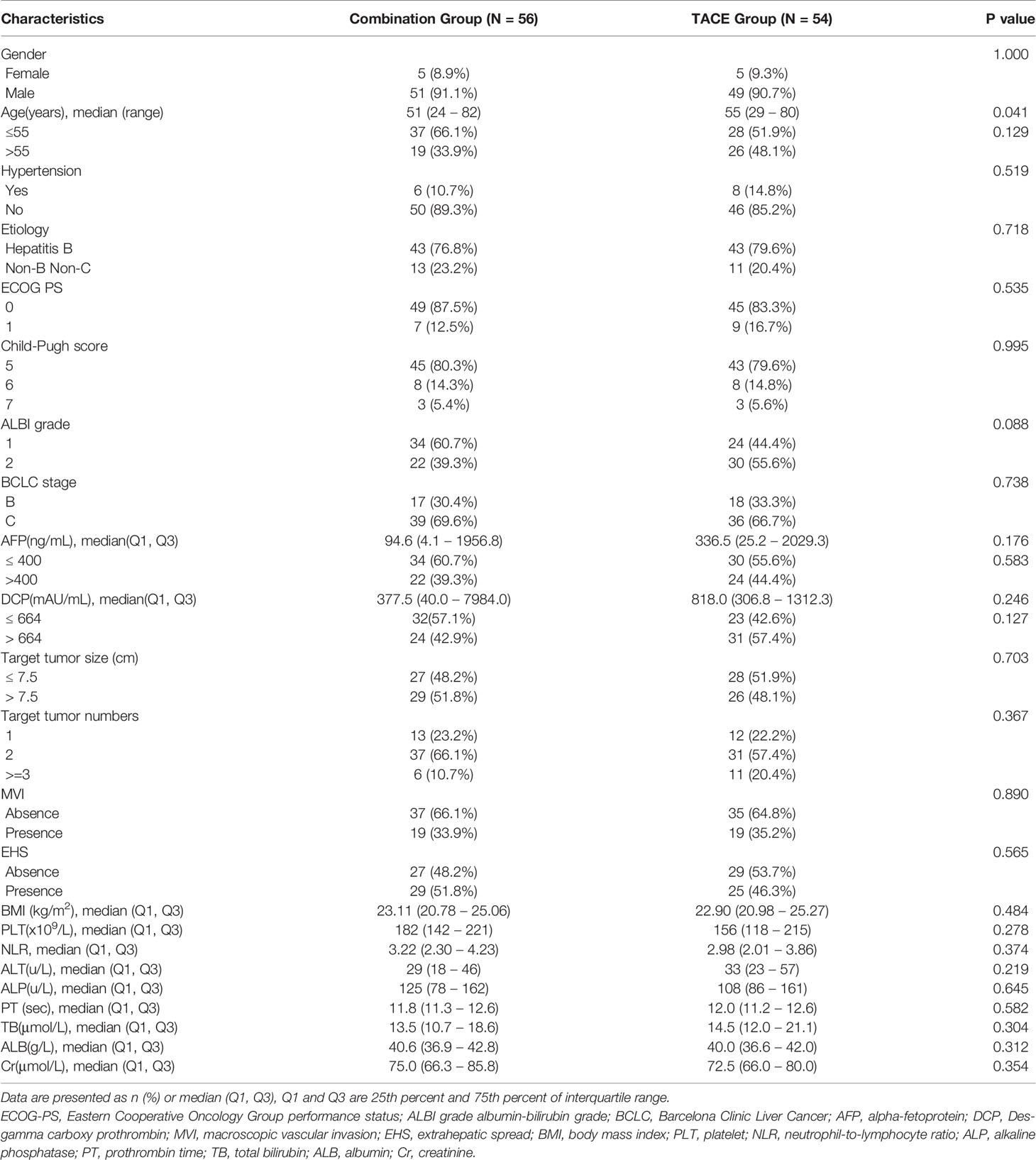
Baseline Characteristics
From November 2017 to September 2020, 326 patients with uHCC were recruited and 216 patients were excluded. Finally, 110 patients were enrolled in this study and were observed either until death or the last follow-up date (Mar 31, 2021) for living patients. Fifty-six patients received combination therapy and 54 patients received TACE alone (Figure 1). Nine patients received pembrolizumab while the other 47 patients received toripalimab in the combination group. There was no statistically significant difference between the baseline characteristics of the patients in both groups, except for the age that was slightly younger in the combination group (Table 1). At the time of data cutoff, the median duration of follow-up was 21.3 months (range, 9.3-38.5) in the combination group and 12.4 months (range, 3.5-19.5) in the TACE group. The median Lenvatinib treatment duration was 10.7 months (range, 2.4-35.0) and the median number of PD-1 inhibitor administrations was 11.0(range, 2-32). One hundred and thirty-six TACE courses were performed in the combination group (a mean of 2.4 procedures, range: 1–4) and 160 TACE courses were performed in the TACE group (a mean of 3.0 courses, range, 2–4). Patients with TACE alone received more TACE courses compared with those in the combination group (P = 0.001).

Figure 1 Flowchart of the patient selection process. HCC, hepatocellular carcinoma; ECOG-PS, Eastern Cooperative Oncology Group performance status; BCLC, Barcelona Clinic Liver Cancer; TACE, transcatheter arterial chemoembolization; PD-1, programmed cell death-1.
Tumor Response
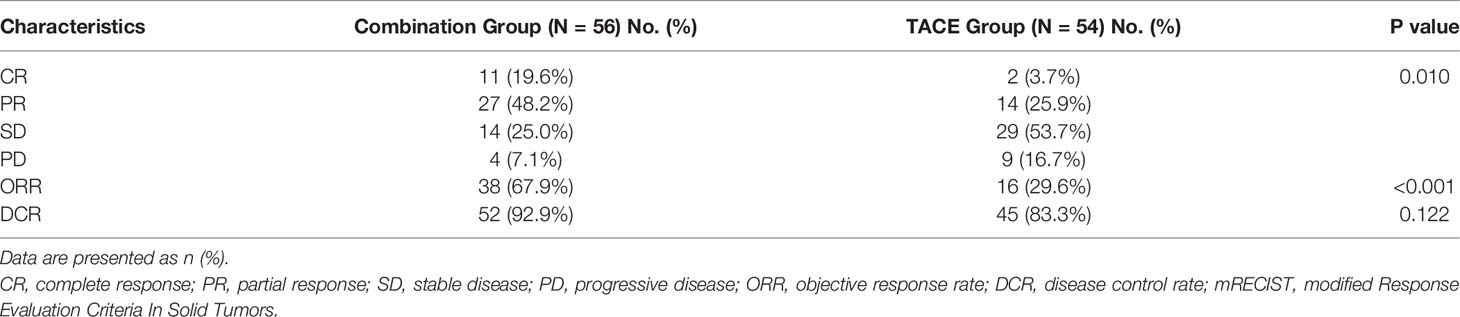
The best tumor response rates are shown in Table 2. The response rates of patients, according to mRECIST, differed significantly between the combination group and the TACE group. The ORR in the combination group was significantly higher than that in the TACE groups (67.9% vs. 29.6%, p < 0.001). The DCR in the combination group was numerically higher than that in the TACE group; however, the difference was not statistically significant (92.9% vs. 83.3%, p = 0.122). Among the 54 patients treated with TACE alone, ETS ≥ 10% was achieved in 11 (20.4%) patients while in the combination group, the higher rate of ETS ≥ 10% was achieved in 29 (51.8%) patients.
Survival Assessment
At the time of last follow-up date (Mar 31,2021), 42 patients were found disease progression and 30 patients had died in the combination group, while the disease was found to have progressed in 41 patients and 33 patients had died in the TACE group. Both PFS and OS of patients in the combination group were better than those of patients in the TACE group. The mPFS was 11.9 months for patients in the combination group as compared to 6.9 months for patients in the TACE group (p = 0.003, HR = 0.51, 95% CI 0.32–0.80; Figure 2A). Similarly, the mOS was significantly higher for patients in the combination group than that in the TACE group (23.9 vs. 15.3 months; p < 0.001, HR = 0.23, 95% CI 0.12–0.42; Figure 2B).
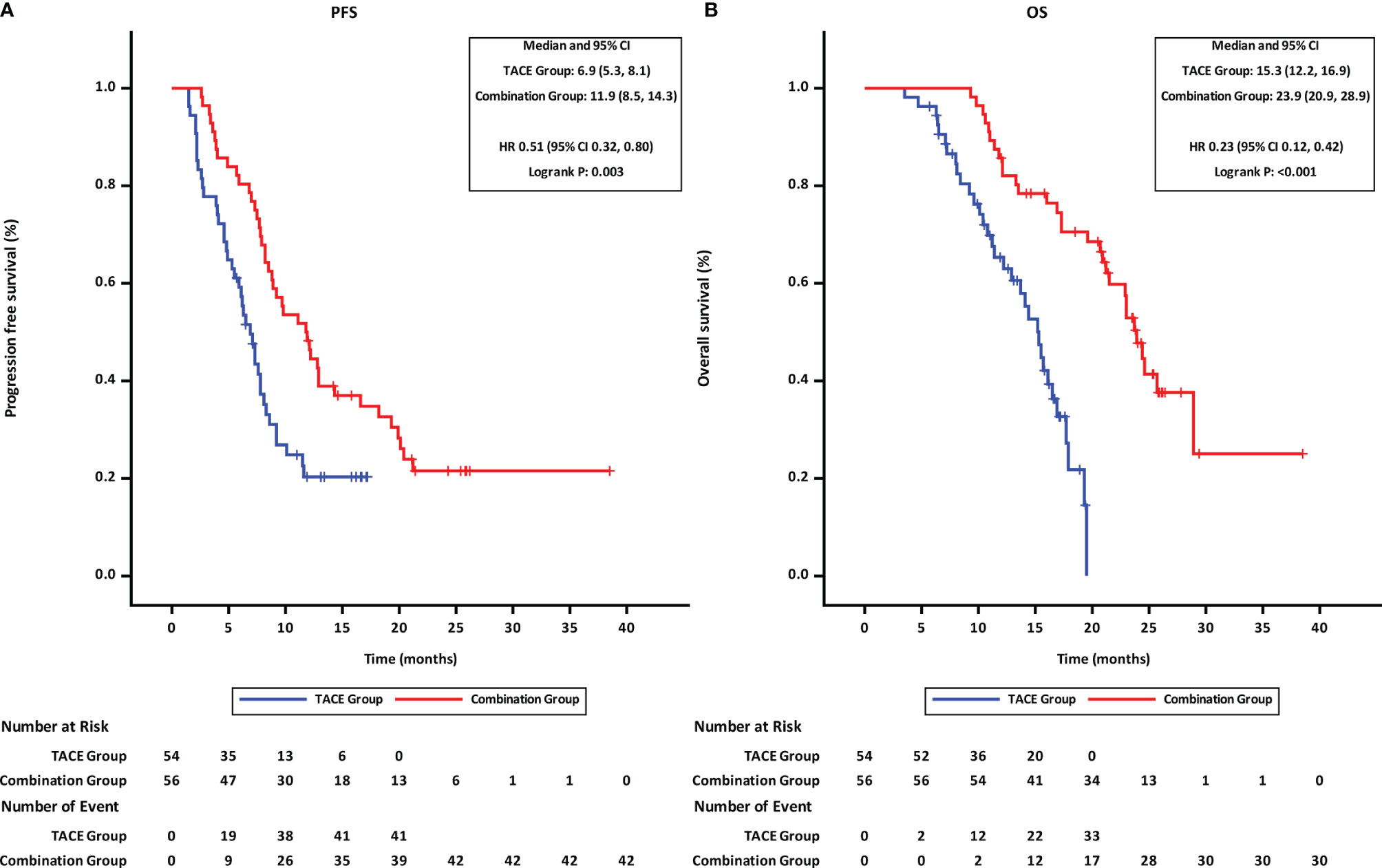
Figure 2 Progression-free and overall survival of patients receiving the different treatments. (A) progression-free survival. (B) overall survival. TACE, transcatheter arterial chemoembolization; PD-1, programmed cell death-1; HR, hazard ratio; CI, confidence interval.
Prognostic Factors for PFS and OS
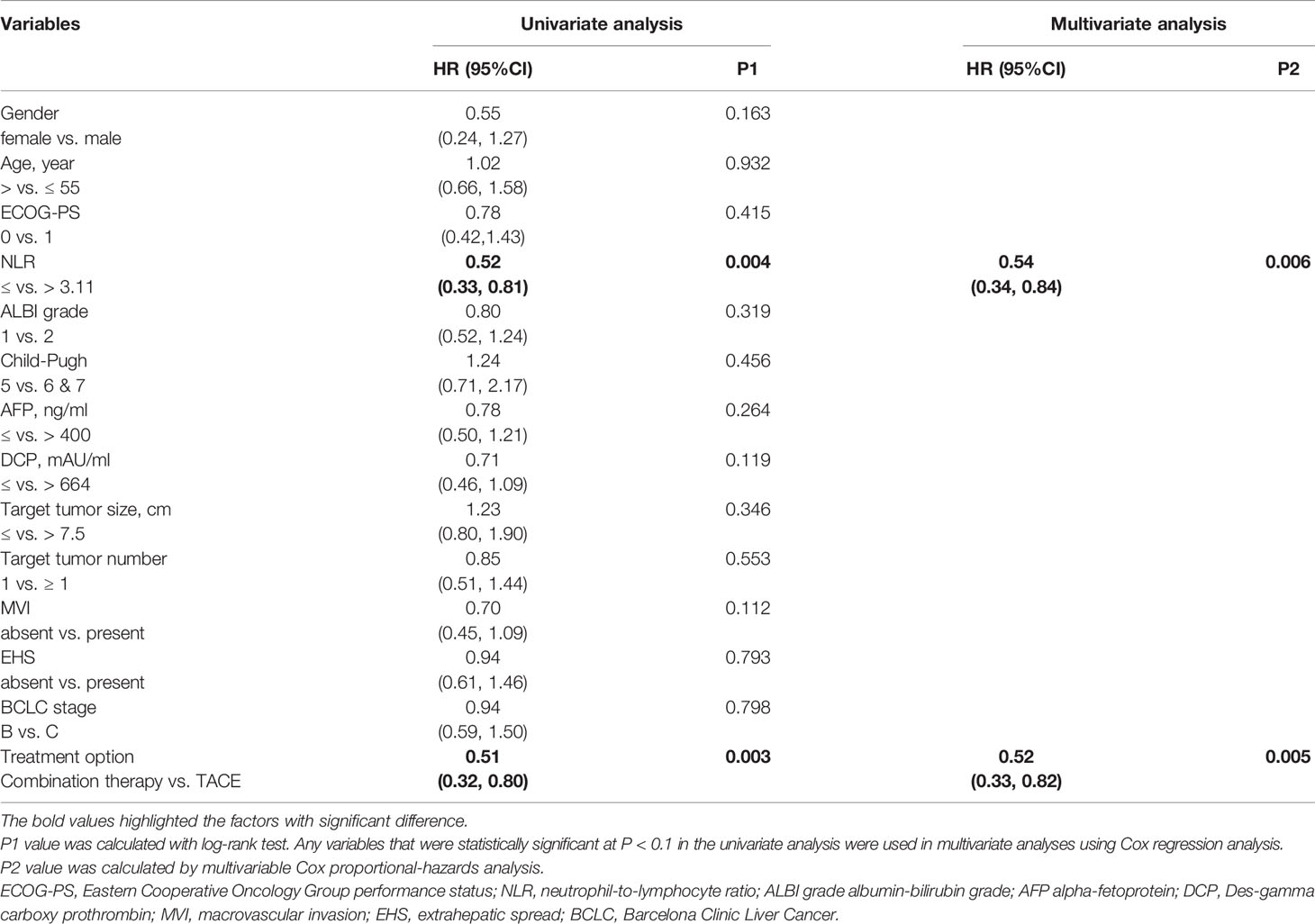
The factors influencing PFS by univariate and multivariate analysis are shown in Table 3. Univariate analysis showed that PFS was associated with NLR (≤ vs. > 3.11) and the treatment option (Combination therapy vs. TACE). On the multivariate analysis, NLR (≤ vs. > 3.11) and treatment option (Combination therapy vs. TACE) were also identified as independent prognostic factors of PFS.
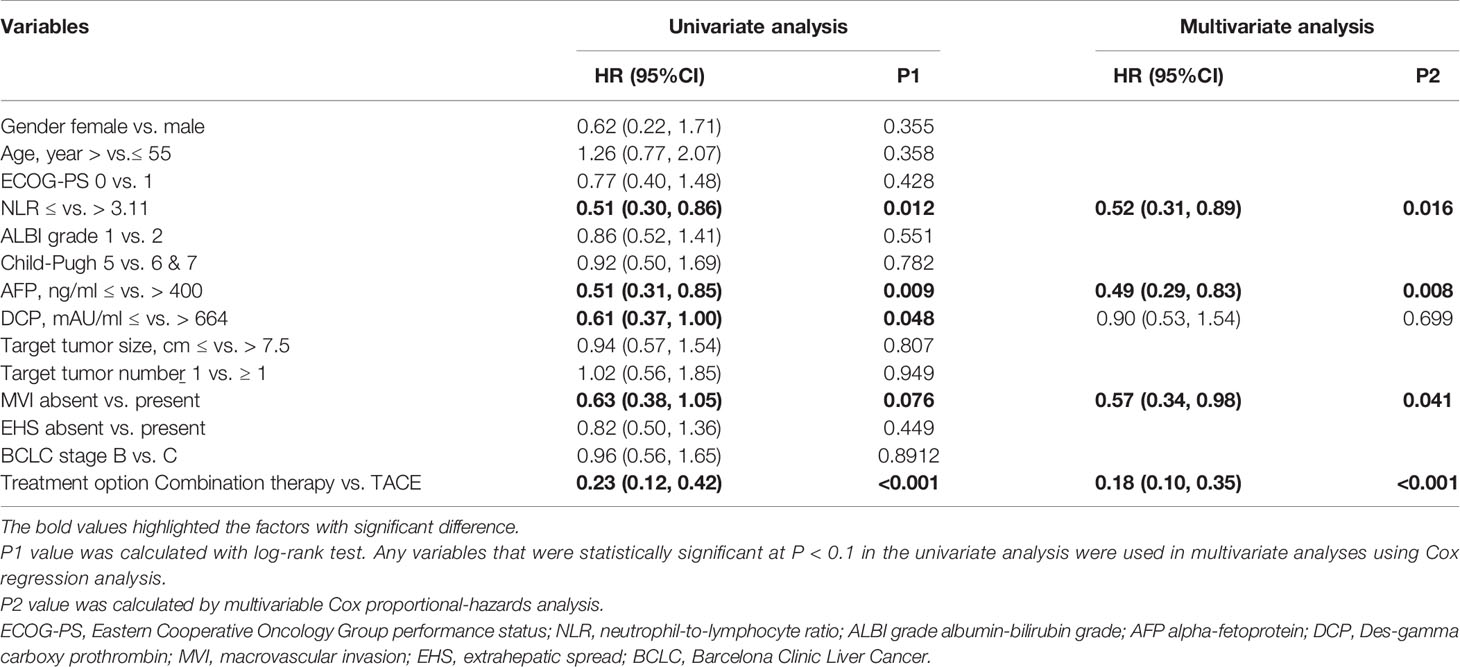
The factors influencing the OS identified via univariate and multivariate analyses of the factors are shown in Table 4. Univariate analyses revealed that the OS was significantly associated with the NLR (≤ vs. > 3.11), AFP (≤ vs.> 400 ng/ml), DCP (≤ vs.> 664 mAU/ml), macroscopic vascular invasion (MVI) (absent vs. present) and treatment option (combination therapy vs. TACE). Upon multivariate analysis, NLR (≤ vs.> 3.11), AFP (≤ vs.> 400 ng/ml), MVI (absent vs. present), and treatment option (combination therapy vs. TACE) were identified as independent prognostic factors of OS.
Subgroup Analysis of Factors Associated With Patients’ Survival
Patients were stratified into two groups by the NLR value (≤3.11 and >3.11; with 3.11 being the median). Subgroup analyses revealed that in patients with low NLR (≤3.11), the PFS (Figure 3A) and OS (Figure 4A) in the combination group were significantly prolonged compared with those in the TACE group, while no statistically significant differences in PFS (Figure 3B) and OS (Figure 4B) were found in patients with high NLR (>3.11) between the two groups.
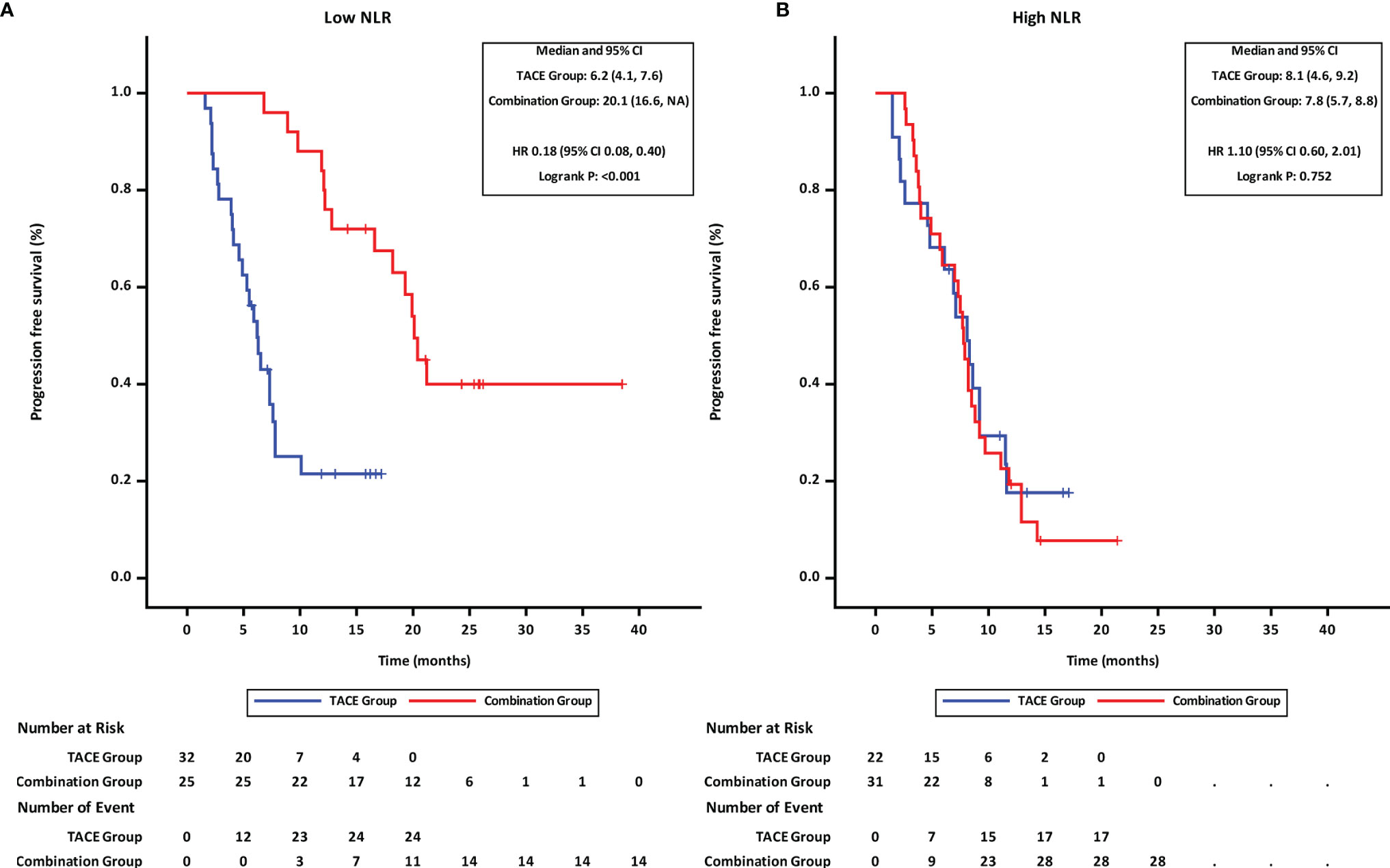
Figure 3 Progression-free survival of groups of patients with different NLR statuses. (A) low NLR. (B) high NLR. TACE, transcatheter arterial chemoembolization; PD-1 programmed cell death-1; NLR, neutrophil-to-lymphocyte ratio; HR, hazard ratio; CI, confidence interval.
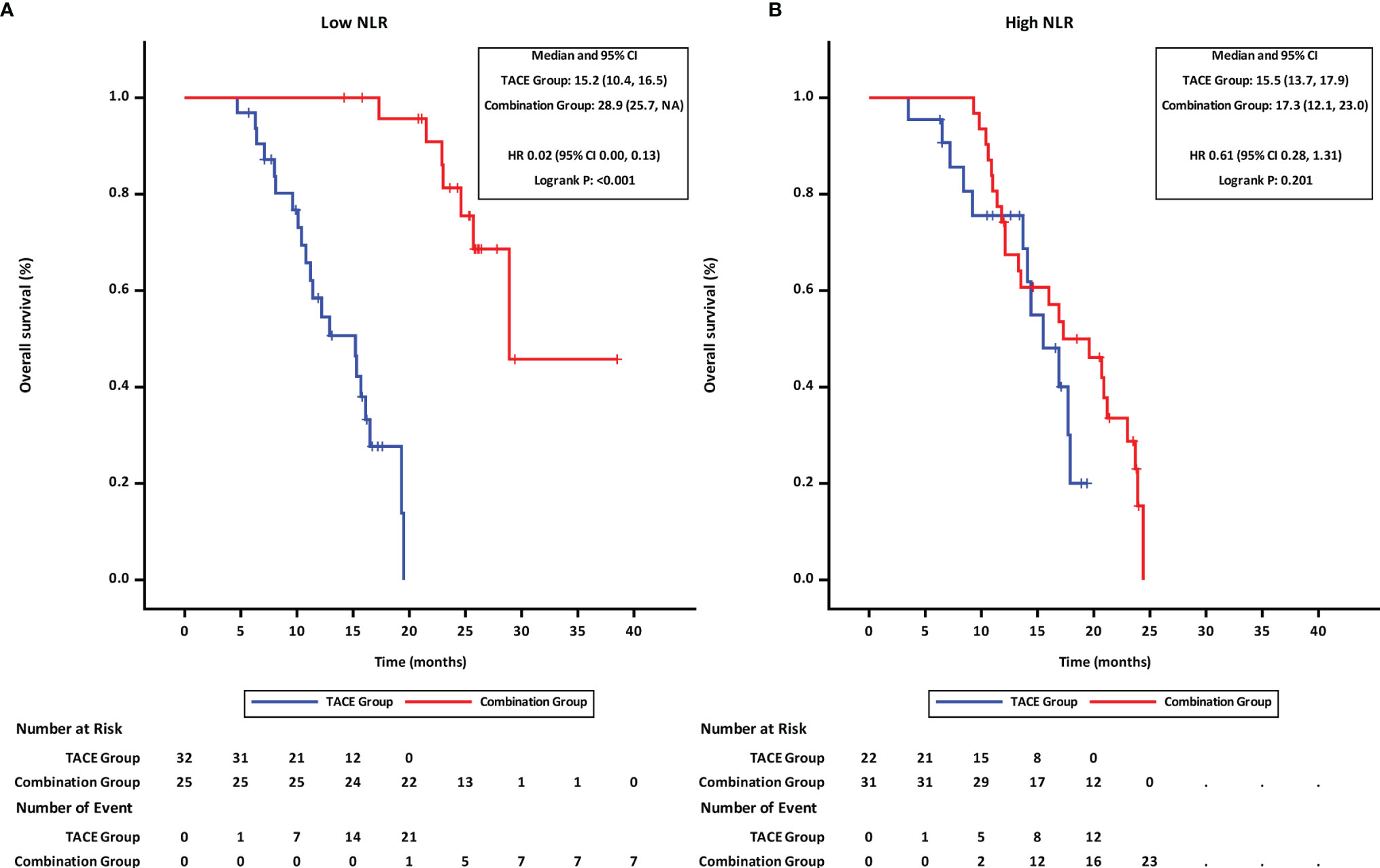
Figure 4 Overall survival of groups of patients with different NLR statuses. (A) low NLR. (B) high NLR. TACE, transcatheter arterial chemoembolization; PD-1, programmed cell death-1; NLR, neutrophil-to-lymphocyte ratio; HR, hazard ratio; CI, confidence interval.
Further, the effects of treatment options and tumor-related factors on survival were analyzed. The OS in the combination group was longer than that in TACE group irrespective of the tumor stage (mOS of BCLC stage B: 25.7 months in the combination group vs. 16.9 months in the TACE group, HR = 0.29,95%CI 0.10–0.87, p = 0.019 (Figure 5A); mOS of BCLC stage C: 23.7 months in the combination group vs. 14.1 months in the TACE group, HR = 0.20,95%CI 0.09–0.43, p < 0.001 (Figure 5B).
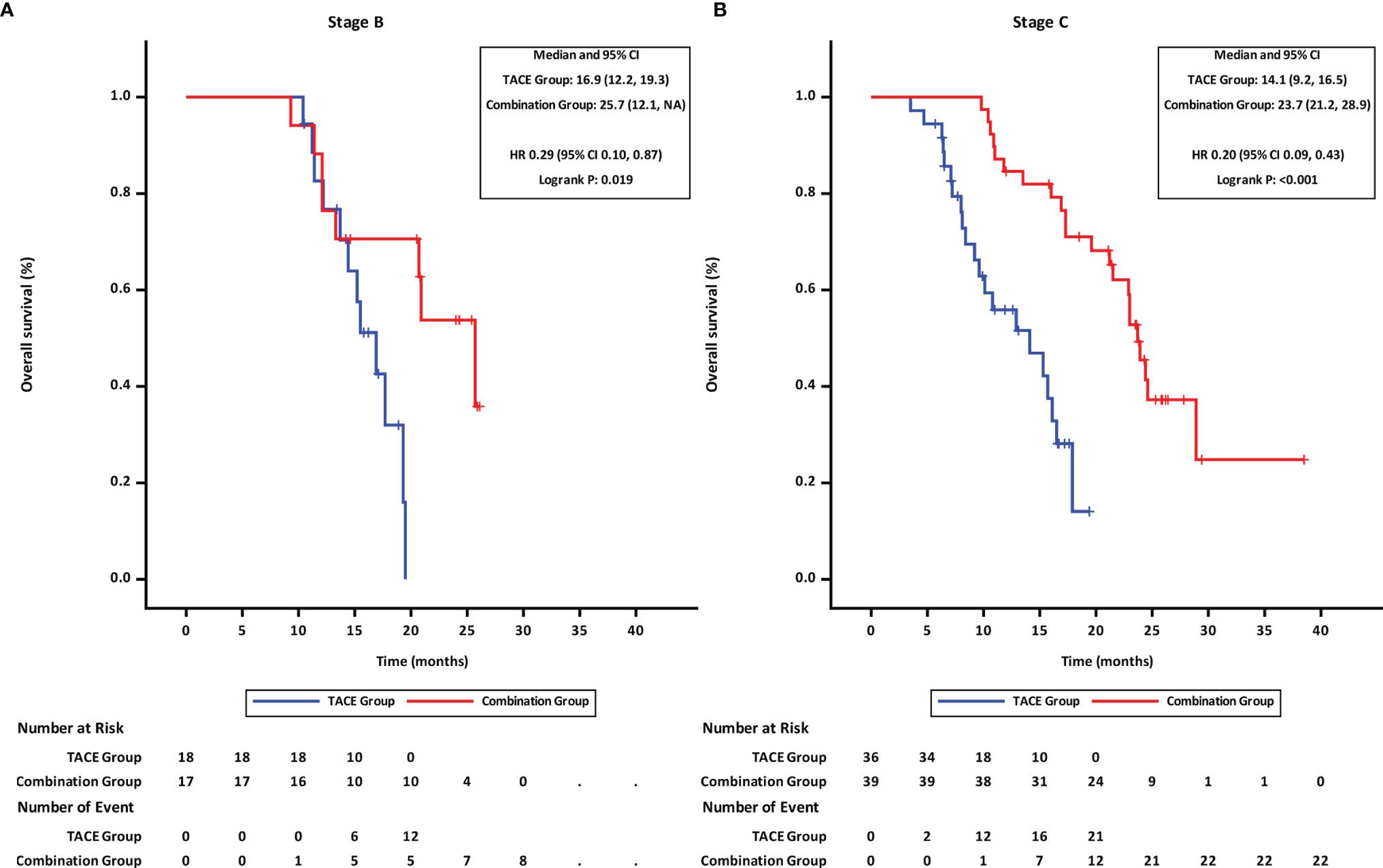
Figure 5 Overall survival of groups of patients at different BCLC stages. (A) BCLC stage B, (B) BCLC stage C TACE, transcatheter arterial chemoembolization; PD-1, programmed cell death-1; BCLC, Barcelona Clinic Liver Cancer; HR, hazard ratio; CI, confidence interval.
Similar results were also found in the analysis of AFP and MVI, as the OS in the combination group was longer than that in the TACE group irrespective of the stratification of AFP or MVI (Figures S1, 2).
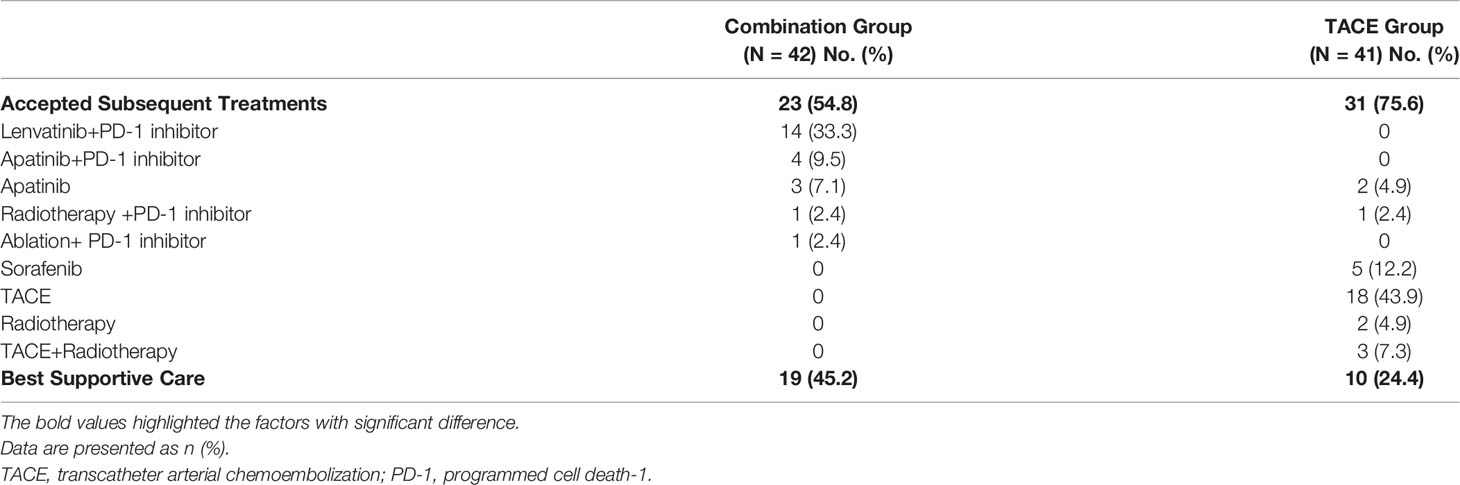
Subsequent Therapy After Progression
As shown in Table 5, 23 patients (54.8%) received subsequent treatments in the combination group while 31 patients (75.6%) did in the TACE group. In the TACE group, many patients (n = 18, 43.9%) still chose TACE while only 8 patients (19.5%) received systemic therapy. In the combination group, 23 (54.8%) patients opted for systemic-therapy-based treatment, with 14 of them continuing Lenvatinib combined with PD-1 inhibitor due to the limited treatment options after progression.
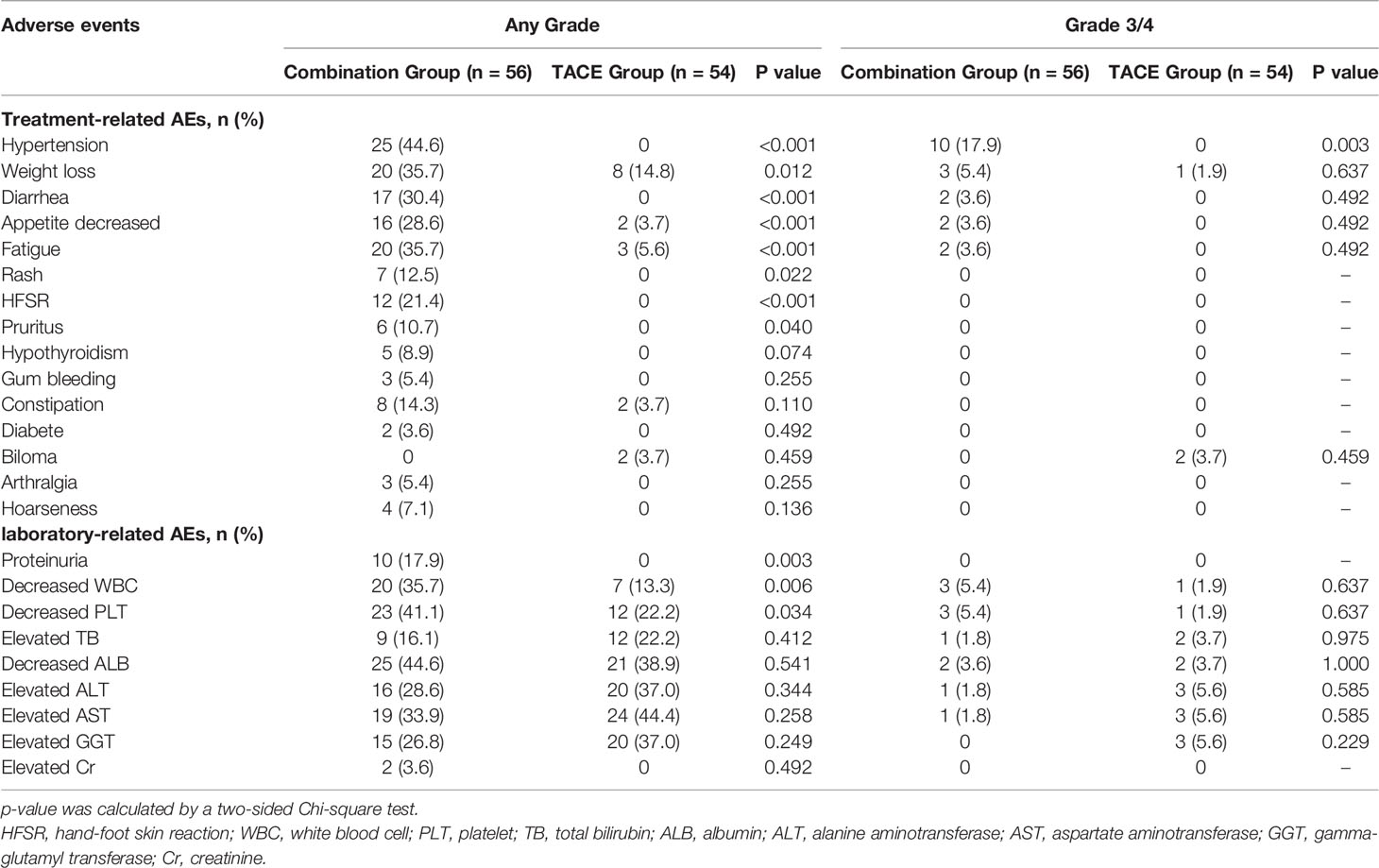
Safety Assessment
All reported AEs were evaluated and found to be mild and tolerable, and no toxicity-related deaths occurred in this study. More Grade 1–2 AEs were found in the combination group, and only hypertension (Grade 3–4 AEs) was more frequent in the combination group than that in the TACE group (17.9% vs. 0,χ2 = 8.556, p = 0.003). The details of AEs are summarized in Table 6. Immune-related AEs included diabetes with Grade 1 in 2 patients, decreased PLT counts with Grade 3 in 1 patient, and hepatitis with Grade 2 in 1 patient. The decreased PLT count and hepatitis were resolved after treatment with oral corticosteroids at a dose of 1 mg/kg/day.
Discussion
In this study, we found that TACE combined with Lenvatinib plus a PD-1 inhibitor achieved more favorable results than TACE alone in patients with uHCC. Although there was a high percentage of patients with advanced stage disease (BCLC C 69.6%), MVI (33.9%), and extrahepatic metastasis (EHS) (51.8%) among those treated with the combination therapy, they had a higher rate of ORR and better survival benefits than those treated with the control therapy (ORR 67.9 vs. 29.6%; mPFS 11.9 vs. 6.9 months; mOS 23.9 vs. 15.3 months). Some of these patients even were found to have an early and quick response after only 1–2 cycles of combination therapy, leading to the normalization of tumor markers, shrinkage and fusion necrosis of tumors, disappearance of tumor daughter nodules, and hypertrophy of the future liver remnant, which may increase the possibility of a conversion hepatectomy in patients initially diagnosed with uHCC. Therefore, the triple combination treatment of TACE-Lenvatinib-PD-1 inhibitors might be an effective and promising treatment strategy for uHCC patients.
Studies on TKIs in combination with ICIs in patients with uHCC proved the potential synergetic effect. TKI and ICI monotherapies were shown to have limited efficacy in clinical studies, with a limited ORR of 9.2% in Sorafenib (3), 17% in PD-1 inhibitors (23), and 24.1% in Lenvatinib (3); however, the combination therapy of TKIs and ICIs were found to improve the survival significantly. An ORR of 33.2% was reported in the IMbrave 150 study with the combination of atezolizumab plus bevacizumab (18) and a higher ORR of 46% was reported in the Keynote 524 study with pembrolizumab plus Lenvatinib (19). These indicated that the combination treatment may have synergistic antitumor effects. The predominant effects not only mainly depend on the role of the PD-1/PD-L1 interaction but are also significantly affected by the multiple roles of anti-angiogenesis drugs. The Anti-VEGF functions were found to be involved in several steps of T-cell activation, including the restoration of antigen presentation, the priming and activation of T-cell responses, and the modulation of the tumor immune microenvironment (24, 25). Furthermore, Lenvatinib was also found to have other pathways in the modulation of antitumor immunity, including the reduction of tumor PD-L1 expression levels and Treg differentiation by blocking FGFR4 (26) and reducing the Treg proportion through TGF-β pathway inhibition (27).
In this triple combination group, patients achieved a 67.9% ORR according to the mRECIST criteria, which significantly higher than that of dual combination, with an ORR of 33.2-46% (18, 19, 28) in PD-1/PD-L1 inhibitors combined with antiangiogenics, and 27.8-53.1% (29, 30) in TACE combined with Lenvatinib. It has been found that the application of TACE alongside TKI and ICI resulted in more synergistic antitumor effects. In addition to the conventional role of tumor necrosis leading by TACE (31), TACE was found to promote T-cell activation via abscopal effects. The tumor necrosis caused by TACE increased the release of tumor-associated antigens (32), which has been proven to recruit DCs (33), increase AFP-specific CD4+T-cell response (13), synergize with ICIs to increase cytotoxic T lymphocytes, and decrease tumor-infiltrating Treg cells (34). TKI (Lenvatinib) was also found to effectively inhibit the angiogenic growth factors triggered by the extensive ischemic necrosis in preclinical (15) or clinical studies (16, 17), which was also found to be an important factor associated with T-cell activation. These synergistic mechanisms might contribute to the favorable clinical outcomes. Recent studies have also demonstrated the encouraging clinical data of TKI and PD-1 inhibitor in combination with transarterial therapy with an ORR of 75.7-84.2% and a DCR of 86.5-94.7% (35, 36). The results of our study are comparable and might offer more evidence of this triple combination treatment approach for uHCC patients.
Upon multivariate analysis, NLR was found to be an independent prognostic factors associated with both OS and PFS. Several studied found NLR in peripheral blood significantly affected the survival in patients with uHCC treated with systemic therapy (37, 38). NLR reflects a potential balance between neutrophil-associated protumor inflammation and lymphocyte-dependent antitumor immune response. Based on the cutoff value of generally 3.0–4.5 (39), patients were divided into two groups with different responses to ICI (40). The exploration results in our study also demonstrated these findings. Patients with low NLR had a significant response to combination therapy and longer survival than those in the TACE group (mOS 28.9 vs. 15.2 months, p < 0.001). On the other hand, in those with high NLR, survival did not differ significantly between the combination group and the TACE group (mOS 17.3 vs. 15.5 months, p = 0.2013). Thus, NLR might be a noninvasive and predictive indicator to identify patients who may benefit from combination therapy.
A tolerable safety profile was observed with combination therapy in this study. There were no unexceptional toxicities in triple treatment group. The frequent AEs with any-grade AEs included hypertension, decreased ALB, and decreased PLT, in combination therapy. Moreover, the most common Grade 3 or Grade 4 AE in combination group was hypertension, which could be manageable by dose reduction or antihypertension drugs without treatment discontinuation. The tolerable safety profiles guaranteed the long-term medication to achieve survival benefits.
However, this study had several limitations. Firstly, it was a prospective, observational, cohort study. The patients selected the treatment strategy after being fully informed of the efficacy, potential AEs, and cost of each drug. The various selection biases and potential differences in baseline characteristics would affect the treatment results. Secondly, the sample size of our study was small. The enrolled patients with uHCC were heterogeneous, including patients at BCLC B and C stages, even though combination therapy could bring potential benefits for either B-stage or C-stage HCC patients; thus, the results of this study require further confirmation. Thirdly, the control group of our study enrolled patients who took only TACE. It was the recommended treatment for BCLC C-stage HCC patients in Chinese guidelines and could offer some survival benefits from the control of local lesions. However, it might have some bias on the results. Thus, the results should be interpreted cautiously. More large-scale, randomized, controlled clinical trials are needed to confirm the efficacy of triple combination therapy.
In conclusion, our study demonstrated that compared with TACE alone, combination therapy with TACE and Lenvatinib plus a PD-1 inhibitor might be associated with better survival benefits with manageable toxicity profiles and the magnitude of benefit was significantly more intense in patients with low NLR who received combination therapy.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The ethical committee of the Third Affiliated Hospital of the Second Military Medical University. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
SQ, XZ, LW, and DW were involved in the conception and design of the study. DW, LH, JZ, and RW were involved in the provision of study materials and the recruitment of patients. SQ, XZ, YW, YM, HP, and QF were involved in data acquisition and assembly. SQ, LH, JZ, RW, LW, and DW were involved in data analysis and interpretation. SQ, XZ, LW, and DW were involved in manuscript writing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant from the State Key Project on Infectious Diseases of China (2018ZX10723204), the National Natural Science Foundation of China (Grant NO. 81630070), the Program of Science and Technology Commission of the Shanghai Municipality (18411969200).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor JY declared a shared parent affiliation with the authors SQ, XZ, YM, HP, QF, LH, JZ, RW, LW, and DW at the time of review.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the patients and their families for their participation in this study. We equally thank Ms. Cheng Dantong and Wang Yalin from MSD medical affairs for their contribution in terms of material support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.874473/full#supplementary-material
References
1. Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, Allen C, et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results From the Global Burden of Disease Study 2015. JAMA Oncol (2017) 3(12):1683–91. doi: 10.1001/jamaoncol.2017.3055
2. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global Patterns of Hepatocellular Carcinoma Management From Diagnosis to Death: The BRIDGE Study. Liver Int (2015) 35(9):2155–66. doi: 10.1111/liv.12818
3. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib Versus Sorafenib in First-Line Treatment of Patients With Unresectable Hepatocellular Carcinoma: A Randomised Phase 3 Non-Inferiority Trial. Lancet (2018) 391(10126):1163–73. doi: 10.1016/s0140-6736(18)30207-1
4. Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, et al. Nivolumab Versus Sorafenib in Advanced Hepatocellular Carcinoma (CheckMate 459): A Randomised, Multicentre, Open-Label, Phase 3 Trial. Lancet Oncol (2022) 23(1):77–90. doi: 10.1016/s1470-2045(21)00604-5
5. Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial Embolisation or Chemoembolisation Versus Symptomatic Treatment in Patients With Unresectable Hepatocellular Carcinoma: A Randomised Controlled Trial. Lancet (2002) 359(9319):1734–9. doi: 10.1016/s0140-6736(02)08649-x
6. Forner A, Reig M, Bruix J. Hepatocellular Carcinoma. Lancet (2018) 391(10127):1301–14. doi: 10.1016/s0140-6736(18)30010-2
7. Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, et al. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer (2020) 9(6):682–720. doi: 10.1159/000509424
8. Yoo DJ, Kim KM, Jin YJ, Shim JH, Ko GY, Yoon HK, et al. Clinical Outcome of 251 Patients With Extrahepatic Metastasis at Initial Diagnosis of Hepatocellular Carcinoma: Does Transarterial Chemoembolization Improve Survival in These Patients? J Gastroenterol Hepatol (2011) 26(1):145–54. doi: 10.1111/j.1440-1746.2010.06341.x
9. Xiang X, Lau WY, Wu ZY, Zhao C, Ma YL, Xiang BD, et al. Transarterial Chemoembolization Versus Best Supportive Care for Patients With Hepatocellular Carcinoma With Portal Vein Tumor Thrombus:a Multicenter Study. Eur J Surg Oncol (2019) 45(8):1460–7. doi: 10.1016/j.ejso.2019.03.042
10. Koch C, Göller M, Schott E, Waidmann O, Op den Winkel M, Paprottka P, et al. Combination of Sorafenib and Transarterial Chemoembolization in Selected Patients With Advanced-Stage Hepatocellular Carcinoma: A Retrospective Cohort Study at Three German Liver Centers. Cancers (Basel) (2021) 13(9). doi: 10.3390/cancers13092121
11. Pinter M, Hucke F, Graziadei I, Vogel W, Maieron A, Königsberg R, et al. Advanced-Stage Hepatocellular Carcinoma: Transarterial Chemoembolization Versus Sorafenib. Radiology (2012) 263(2):590–9. doi: 10.1148/radiol.12111550
12. Kohles N, Nagel D, Jüngst D, Stieber P, Holdenrieder S. Predictive Value of Immunogenic Cell Death Biomarkers HMGB1, sRAGE, and DNase in Liver Cancer Patients Receiving Transarterial Chemoembolization Therapy. Tumour Biol (2012) 33(6):2401–9. doi: 10.1007/s13277-012-0504-2
13. Ayaru L, Pereira SP, Alisa A, Pathan AA, Williams R, Davidson B, et al. Unmasking of Alpha-Fetoprotein-Specific CD4(+) T Cell Responses in Hepatocellular Carcinoma Patients Undergoing Embolization. J Immunol (2007) 178(3):1914–22. doi: 10.4049/jimmunol.178.3.1914
14. Sergio A, Cristofori C, Cardin R, Pivetta G, Ragazzi R, Baldan A, et al. Transcatheter Arterial Chemoembolization (TACE) in Hepatocellular Carcinoma (HCC): The Role of Angiogenesis and Invasiveness. Am J Gastroenterol (2008) 103(4):914–21. doi: 10.1111/j.1572-0241.2007.01712.x
15. Ogasawara S, Mihara Y, Kondo R, Kusano H, Akiba J, Yano H. Antiproliferative Effect of Lenvatinib on Human Liver Cancer Cell Lines In Vitro and In Vivo. Anticancer Res (2019) 39(11):5973–82. doi: 10.21873/anticanres.13802
16. Fu Z, Li X, Zhong J, Chen X, Cao K, Ding N, et al. Lenvatinib in Combination With Transarterial Chemoembolization for Treatment of Unresectable Hepatocellular Carcinoma (uHCC): A Retrospective Controlled Study. Hepatol Int (2021) 15(3):663–75. doi: 10.1007/s12072-021-10184-9
17. Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, et al. Randomised, Multicentre Prospective Trial of Transarterial Chemoembolisation (TACE) Plus Sorafenib as Compared With TACE Alone in Patients With Hepatocellular Carcinoma: TACTICS Trial. Gut (2020) 69(8):1492–501. doi: 10.1136/gutjnl-2019-318934
18. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab Plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
19. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol (2020) 38(26):2960–70. doi: 10.1200/jco.20.00808
20. Lau WY, Lai EC. Salvage Surgery Following Downstaging of Unresectable Hepatocellular Carcinoma–A Strategy to Increase Resectability. Ann Surg Oncol (2007) 14(12):3301–9. doi: 10.1245/s10434-007-9549-7
21. Lencioni R, Llovet JM. Modified RECIST (mRECIST) Assessment for Hepatocellular Carcinoma. Semin Liver Dis (2010) 30(1):52–60. doi: 10.1055/s-0030-1247132
22. Cremolini C, Loupakis F, Antoniotti C, Lonardi S, Masi G, Salvatore L, et al. Early Tumor Shrinkage and Depth of Response Predict Long-Term Outcome in Metastatic Colorectal Cancer Patients Treated With First-Line Chemotherapy Plus Bevacizumab: Results From Phase III TRIBE Trial by the Gruppo Oncologico Del Nord Ovest. Ann Oncol (2015) 26(6):1188–94. doi: 10.1093/annonc/mdv112
23. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib (KEYNOTE-224): A Non-Randomised, Open-Label Phase 2 Trial. Lancet Oncol (2018) 19(7):940–52. doi: 10.1016/s1470-2045(18)30351-6
24. Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular Normalization as an Emerging Strategy to Enhance Cancer Immunotherapy. Cancer Res (2013) 73(10):2943–8. doi: 10.1158/0008-5472.can-12-4354
25. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing Cancer Immunotherapy Using Antiangiogenics: Opportunities and Challenges. Nat Rev Clin Oncol (2018) 15(5):325–40. doi: 10.1038/nrclinonc.2018.29
26. Yi C, Chen L, Lin Z, Liu L, Shao W, Zhang R, et al. Lenvatinib Targets FGF Receptor 4 to Enhance Antitumor Immune Response of Anti-Programmed Cell Death-1 in HCC. Hepatology (2021) 74(5):2544–60. doi: 10.1002/hep.31921
27. Torrens L, Montironi C, Puigvehí M, Mesropian A, Leslie J, Haber PK, et al. Immunomodulatory Effects of Lenvatinib Plus Anti-Programmed Cell Death Protein 1 in Mice and Rationale for Patient Enrichment in Hepatocellular Carcinoma. Hepatology (2021) 74(5):2652–69. doi: 10.1002/hep.32023
28. Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in Combination With Apatinib in Patients With Advanced Hepatocellular Carcinoma (RESCUE): A Nonrandomized, Open-Label, Phase II Trial. Clin Cancer Res (2021) 27(4):1003–11. doi: 10.1158/1078-0432.Ccr-20-2571
29. Chen S, Wu Z, Shi F, Mai Q, Wang L, Wang F, et al. Lenvatinib Plus TACE With or Without Pembrolizumab for the Treatment of Initially Unresectable Hepatocellular Carcinoma Harbouring PD-L1 Expression: A Retrospective Study. J Cancer Res Clin Oncol (2021). doi: 10.1007/s00432-021-03767-4
30. Ding X, Sun W, Li W, Shen Y, Guo X, Teng Y, et al. Transarterial Chemoembolization Plus Lenvatinib Versus Transarterial Chemoembolization Plus Sorafenib as First-Line Treatment for Hepatocellular Carcinoma With Portal Vein Tumor Thrombus: A Prospective Randomized Study. Cancer (2021) 127(20):3782–93. doi: 10.1002/cncr.33677
31. Lencioni R. Loco-Regional Treatment of Hepatocellular Carcinoma. Hepatology (2010) 52(2):762–73. doi: 10.1002/hep.23725
32. den Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Frielink C, Toonen LW, et al. Efficient Loading of Dendritic Cells Following Cryo and Radiofrequency Ablation in Combination With Immune Modulation Induces Anti-Tumour Immunity. Br J Cancer (2006) 95(7):896–905. doi: 10.1038/sj.bjc.6603341
33. Apetoh L, Mignot G, Panaretakis T, Kroemer G, Zitvogel L. Immunogenicity of Anthracyclines: Moving Towards More Personalized Medicine. Trends Mol Med (2008) 14(4):141–51. doi: 10.1016/j.molmed.2008.02.002
34. Rios-Doria J, Durham N, Wetzel L, Rothstein R, Chesebrough J, Holoweckyj N, et al. Doxil Synergizes With Cancer Immunotherapies to Enhance Antitumor Responses in Syngeneic Mouse Models. Neoplasia (2015) 17(8):661–70. doi: 10.1016/j.neo.2015.08.004
35. Song T, Lang M, Lu W, Zhang T, Li H, Wu Q, et al. Conversion of Initially Unresectable Hepatocellular Carcinoma (HCC) With Triple-Combination Therapy (Lenvatinib, Anti-PD-1 Antibodies, and Transarterial Therapy): A Retrospective Analysis. J Clin Oncol (2022) 40(4_suppl):413. doi: 10.1200/JCO.2022.40.4_suppl.413
36. Zhang X, Zhu X, Liu C, Lu W, Li Q, Chen W, et al. The Safety and Efficacy of Transarterial Chemoembolization (TACE) + Lenvatinib + Programmed Cell Death Protein 1 (PD-1) Antibody of Advanced Unresectable Hepatocellular Carcinoma. J Clin Oncol (2022) 40(4_suppl):453. doi: 10.1200/JCO.2022.40.4_suppl.453
37. Valero C, Lee M, Hoen D, Weiss K, Kelly DW, Adusumilli PS, et al. Pretreatment Neutrophil-to-Lymphocyte Ratio and Mutational Burden as Biomarkers of Tumor Response to Immune Checkpoint Inhibitors. Nat Commun (2021) 12(1):729. doi: 10.1038/s41467-021-20935-9
38. Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic Factors and Predictors of Sorafenib Benefit in Patients With Hepatocellular Carcinoma: Analysis of Two Phase III Studies. J Hepatol (2017) 67(5):999–1008. doi: 10.1016/j.jhep.2017.06.026
39. Tada T, Kumada T, Hiraoka A, Michitaka K, Atsukawa M, Hirooka M, et al. Neutrophil-To-Lymphocyte Ratio Is Associated With Survival in Patients With Unresectable Hepatocellular Carcinoma Treated With Lenvatinib. Liver Int (2020) 40(4):968–76. doi: 10.1111/liv.14405
Keywords: hepatocellular carcinoma, transcatheter arterial chemoembolization, lenvatinib, programmed cell death-1 inhibitor, neutrophil-to-lymphocyte ratio
Citation: Qu S, Zhang X, Wu Y, Meng Y, Pan H, Fang Q, Hu L, Zhang J, Wang R, Wei L and Wu D (2022) Efficacy and Safety of TACE Combined With Lenvatinib Plus PD-1 Inhibitors Compared With TACE Alone for Unresectable Hepatocellular Carcinoma Patients: A Prospective Cohort Study. Front. Oncol. 12:874473. doi: 10.3389/fonc.2022.874473
Received: 12 February 2022; Accepted: 29 March 2022;
Published: 21 April 2022.
Edited by:
Jianhua Yin, Second Military Medical University, ChinaReviewed by:
Hong Zhixian, Fifth Medical Center of the PLA General Hospital, ChinaHaiguang Xin, Shanghai Jiao Tong University, China
Minggen Hu, Institute of Hepatobiliary Surgery of Chinese PLA, China
Copyright © 2022 Qu, Zhang, Wu, Meng, Pan, Fang, Hu, Zhang, Wang, Wei and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lixin Wei, weilixin_smmu@163.com; Dong Wu, wudong1005@sina.com.cn
†These authors have contributed equally to this work and share first authorship
 Shuping Qu
Shuping Qu Xiaobing Zhang
Xiaobing Zhang Yutian Wu
Yutian Wu Yan Meng
Yan Meng Hongyu Pan
Hongyu Pan Qiang Fang
Qiang Fang Lei Hu
Lei Hu Jin Zhang
Jin Zhang Ruoyu Wang
Ruoyu Wang Lixin Wei
Lixin Wei Dong Wu
Dong Wu