- 1Department of Ultrasound, Southern University of Science and Technology Hospital, Shenzhen, China
- 2Department of Ultrasound, The First Affiliated Hospital of Shenzhen University, Shenzhen Second People’s Hospital, Shenzhen, China
- 3Department of Ultrasound, First Affiliated Hospital of Southern University of Science and Technology, Second Clinical College of Jinan University, Shenzhen Medical Ultrasound Engineering Center, Shenzhen People’s Hospital, Shenzhen, China
Purpose: For men suspected of having prostate cancer (PCa), the transrectal ultrasound (TRUS)-guided systematic biopsy (SB) was performed. MRI/TRUS fusion guided-targeted biopsy (MRI-TB) could enhance PCa detection, allowing sampling of sites at higher risk which were not obvious with TRUS alone. The aim of this systematic review and meta-analysis was to compare the detection rates of prostate cancer by MRI-TB or MRI-TB plus SB versus SB, mainly for diagnosis of high-risk PCa.
Methods: A literature Search was performed on PubMed, Cochrane Library, and Embase databases. We searched from inception of the databases up to January 2021.
Results: A total of 5831 patients from 26 studies were included in the present meta-analysis. Compared to traditional TRUS-guided biopsy, MRI-TB had a significantly higher detection rate of clinically significant PCa (RR=1.27; 95%CI 1.15-1.40; p<0.001) and high-risk PCa (RR=1.41; 95% CI 1.22-1.64; p<0.001), while the detection rate of clinically insignificant PCa was lower (RR=0.65; 95%CI 0.55-0.77; p<0.001). MRI-TB and SB did not significantly differ in the detection of overall prostate cancer (RR=1.04; 95%CI 0.95-1.12; p=0.41). Compared with SB alone, we found that MRI-TB plus SB diagnosed more cases of overall, clinically significant and high-risk PCa (p<0.001).
Conclusion: Compared with systematic protocols, MRI-TB detects more clinically significant and high-risk PCa cases, and fewer clinically insignificant PCa cases. MRI-TB combined with SB enhances PCa detection in contrast with either alone but did not reduce the diagnosis rate of clinically insignificant PCa.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/#searchadvanced, CRD42021218475.
1 Introduction
Prostate cancer (PCa) is the most common type of cancer in men (1). Conventional methods for diagnosis of prostate cancer include prostate-specific antigen (PSA) blood examinations and digital rectal examination (DRE). Diagnosis using these two methods is confirmed through transrectal ultrasound(TRUS)-guided systematic prostate biopsy (SB) (2). However, low sensitivity and specificity of conventional TRUS-guided biopsy limits its application (3). In addition, TRUS-guided biopsy does not detect approximately 20% of significant PCa (csPCa) cases (4). Moreover, TRUS-guided biopsy over detects clinically insignificant PCa (cisPCa), increasing overtreatment and associated side events like erectile dysfunction and urinary incontinence (5).
Multiple methods have been developed based on MpMRI (multiparametric magnetic resonance imaging) to obtaining prostate biopsy cores. Currently, MRI-guided in-bore biopsies (within the MRI machine) are available, but they are costly and time consuming, particularly given the requirement of non-ferromagnetic biopsy instruments in the MRI setting (6). Cognitive fusion is a process where the examining specialist assesses MRI images before ultrasound (US)-guided assessment, reduces costs as well as the spatial correlation capacity (7). Therefore, platforms which allow instant mpMRI and US image fusion biopsy have several advantages, such as implementation outside the MRI setting, good spatial correlation and are not costly (8). MRI-TRUS fusion guided-targeted biopsy (MRI-TB) combines mpMRI’s high accuracy in lesion detection and characterization and cost-efficiency with the simple-to use TRUS-guided platforms for urologists (9, 10). A previous study reports that targeted biopsy using mpMRI-TRUS fusion enhances PCa detection, allowing sampling of sites at higher risk which could not be detected with TRUS alone (11). In addition, it reduces over detection of insignificant tumors, thus avoiding unwarranted radical treatment (11).
Several meta-analyses have been published on diagnostic approaches of prostate cancer (12, 13). However, they focused on evaluating diagnosis of clinically significant and insignificant PCa, excluding high-risk PCa and did not explore benefits of MRI-TB in combination with SB. High-risk prostate cancer is an aggressive disease characterized by relapse after definitive treatment (14). Therefore, the aim of this systematic review was to compare the detection rates of prostate cancer by MRI-TB or MRI-TB plus SB versus SB in men with high serum PSA levels and/or abnormal digital rectal examination, mainly for diagnosis of high-risk PCa.
2 Methods
2.1 Literature Search
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (15) were followed in this systematic review and meta-analysis. The study was registered with the international prospective register of systematic reviews (PROSPERO, ID CRD42021218475). Related studies were searched in PubMed, Cochrane Library, and Embase databases without any restriction on publication language. The search key words included prostate cancer (prostate neoplasm), MRI, ultrasonography, image-guided biopsy. Related studies were retrieved from the inception of the database to January 2021.
2.2 Inclusion and Exclusion Criteria
Paired retrospective and prospective articles were included in this study. Patients with suspected PCa showed increased serum PSA (prostate-specific antigen) contents, uncertain results from digital rectal assessment, and were biopsy naïve or reported previous negative biopsy. Studies included in this review explored MRI-TB and transrectal SB, to determine the effectiveness of MRI-TB in detection of PCa compared with traditional SB (SB relative to MRI-TB, and/or MRI-TB+SB relative to SB). Moreover, only studies with SB standard of 12 or close to 12 (12 ± 2) cores and those guided by the MRI/TRUS fusion prostate model or TRUS were included. Studies not published in English, studies involving proven PCa cases on active surveillance, studies involving transperineal biopsy, studies not comparing MRI-TB vs SB, studies that did not report outcomes, non-primary studies, literature reviews, and meta- analyses were excluded from this study. No standard definition of clinically significant, and clinically insignificant cancers exists, therefore, definitions of such cases in respective articles were allowed. In the absence of a clear definition, where applicable Gleason grade ≥3+4 cancer was regarded clinically significant, whereas Gleason grade 3 + 3 was regarded clinically insignificant (16). Similarly, it is also hard to define the high risk PCa.The American Urological Association (AUA) defines “high-risk” as a clinical T stage ≥cT2c, a Gleason score≥8, or a PSA >20 ng/mL. The National Comprehensive Cancer Network (NCCN) defines “high-risk” as T3a, Gleason ≥8, or PSA ≥20, and “very high risk” as T3b or T4 disease.High risk in theThe Radiation Therapy Oncology Group (RTOG) classification includes 1) Gleason ≥8, or 2) Gleason =7 plus either ≥cT3 or node-positive. Included articles that met any of the above definitions could be considered high-risk prostate cancer (17).
2.3 Study Selection and Extraction of Data
Data retrieval was performed by 2 independent reviewers and discrepancies between them were solved through discussions or consulting a third reviewer. Titles and abstracts were examined for relevance before full-text reviews of articles. Information on the article, participant, MRI, as well as biopsy features were recorded in a standard form as follows: 1) article: origin (authors, publication year), definition of csPCa, definition of high risk PCa; 2) study participant: clinical setting (prior negative biopsy or biopsy naïve), number of participants (overall), MRI sequence for defining target, age, mean prostate volume, and mean PSA; 3) MRI: magnet strength, MRI sequences, and number of patients with positive MRI scan; 4) biopsy: core numbers and lesion numbers for MRI-TB/SB, previous biopsy status, rates of detection of csPCa, overall PCa, cisPCa, as well as high risk PCa.
2.4 Quality Assessment of Included Studies
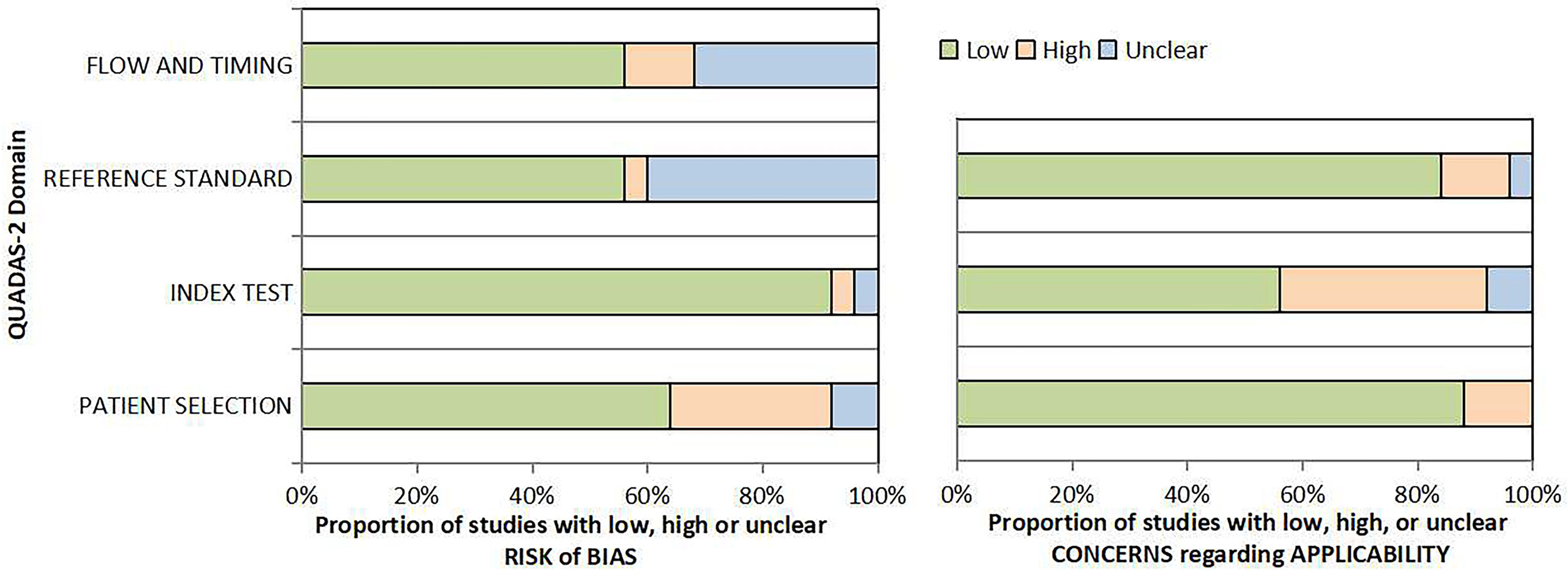
Two reviewers independently assessed the risk of bias and applicability concern in a single study using a revised tool for Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) checklist. RCTs were assessed using Cochrane risk of bias 2.0 tool to evaluate the risk of bias.
2.5 Statistical Analysis
All statistical analyses were performed with Stata V.15 software. Relative risk (RR) and 95% CI were implemented to characterize the dichotomous variables comprising overall PCa, clinically insignificant PCa, clinically significant PCa, and high risk PCa. Q and I² statistics were employed to assess study heterogeneity. The random-effect model was implemented where P<0.1 or I²>50%, whereas fixed-effect model was used when this threshold was not met. Sensitivity analyses were performed by eliminating one article consecutively to validate the stability of final results. Subgroup analyses were performed based on available information. Egger’s and Begg’s tests were used to determine publication bias. p<0.05 represented statistical significance, and all tests were two-sided.Subgroup assessments were carried out based on prior biopsy status due to potential heterogeneity. Studies were then divided into 3 sub-groups including a previous prostate cancer biopsy that was negative, biopsy naïve, and mixed prostate cancer biopsy.
3 Results
3.1 Summary of Studies
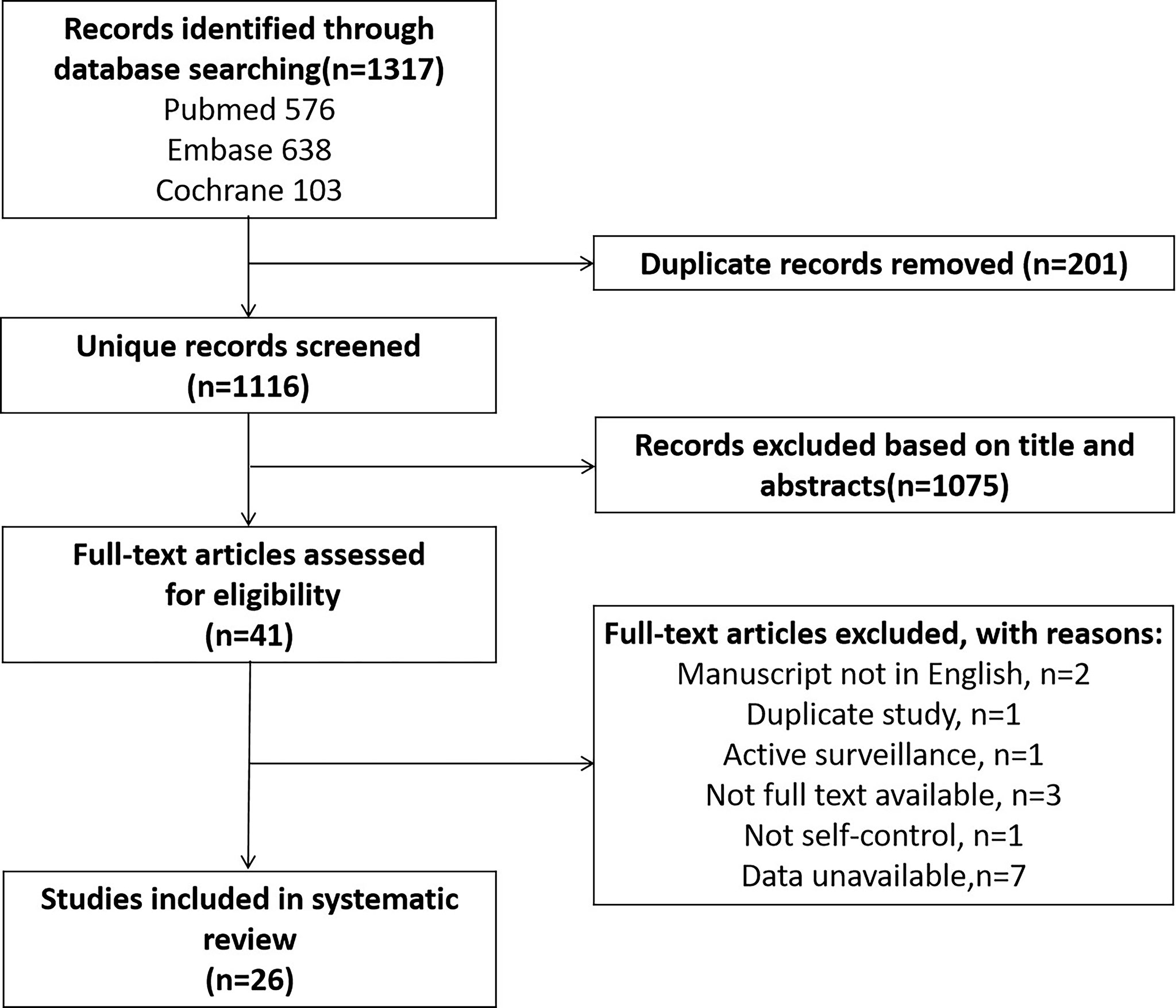
A total of 1317 articles were retrieved. After elimination of duplicates, and review of titles and abstracts, and full-text review, a total of 26 articles including 5831 PCa cases were included in this study (Figure 1).
3.2 Characteristics of Enrolled Studies
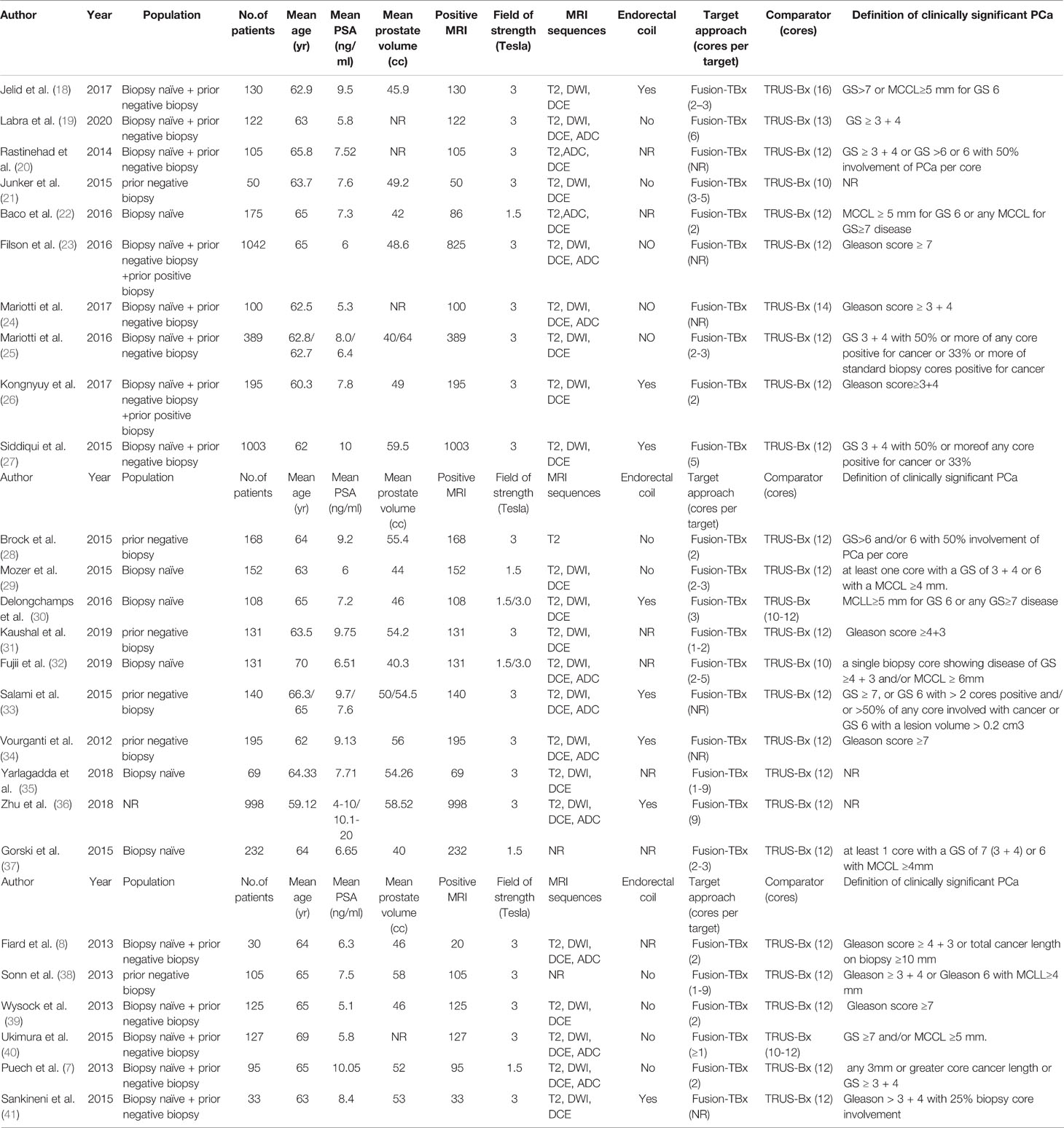
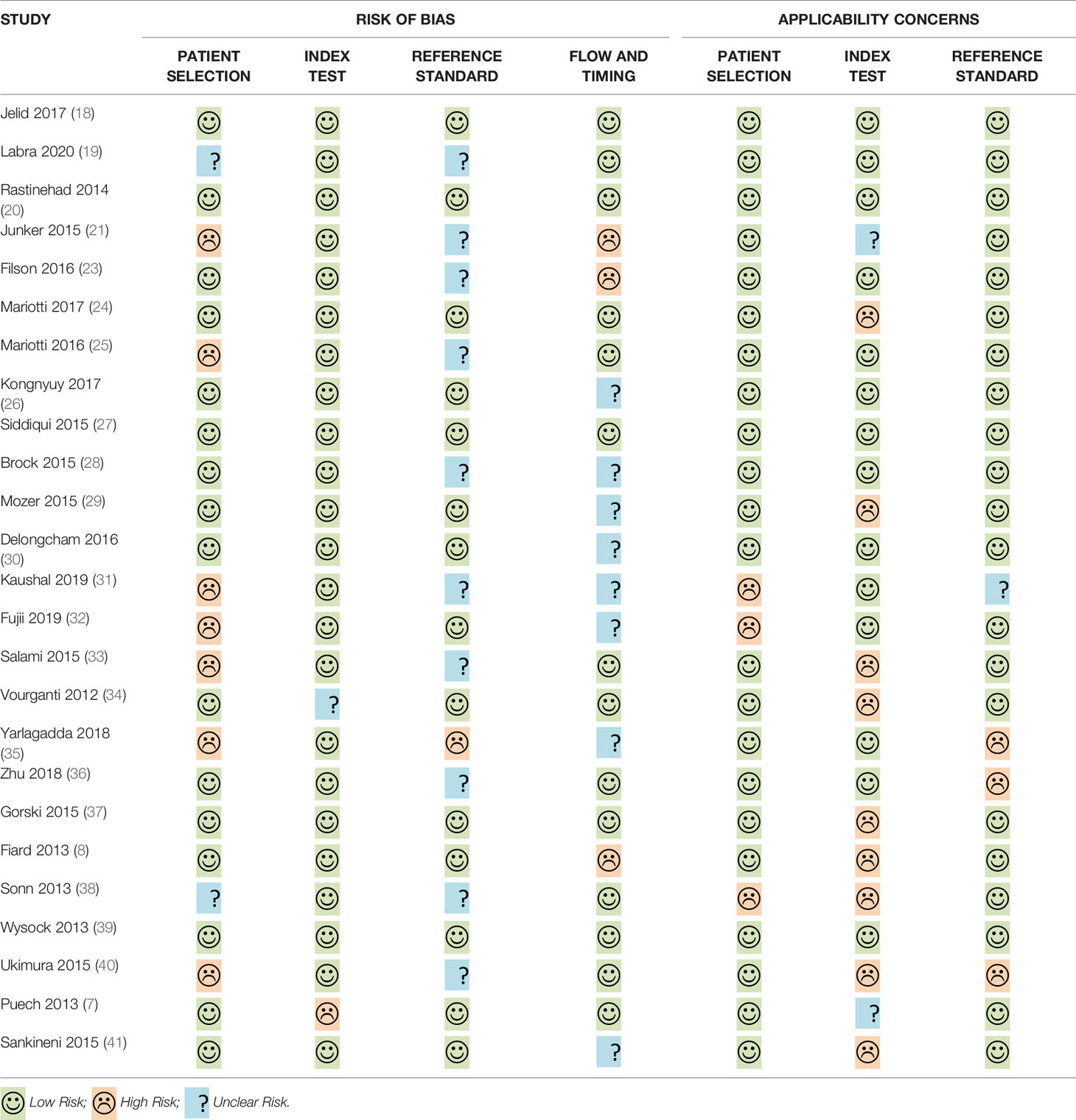
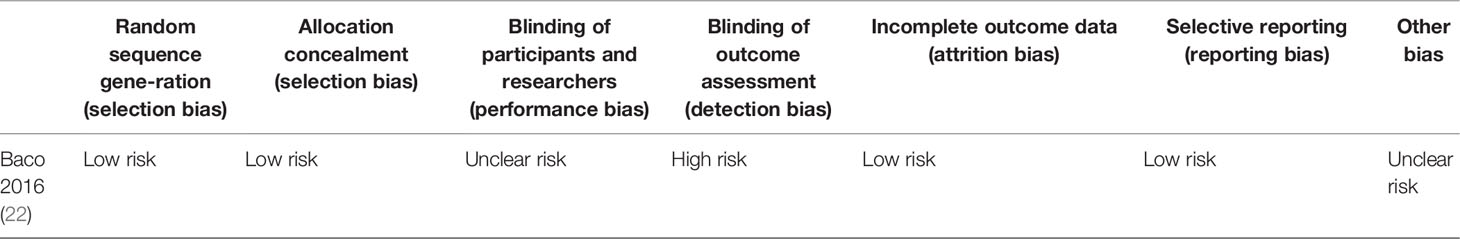
Out of the 26 enrolled studies, 25 used paired designs and 1 was a RCT. MRI-TB was used in all MRI navigation methods. Six studies included biopsy naïve patients, whereas six studies enrolled participants with prior negative biopsy results. The other studies used mixed biopsy, however, one study did not specify the biopsy type. 23 of the included studies provided clinically significant PCa’s definition and reported its detection rate. Some articles defined clinically significant PCa based on Gleason score ≥7 or >2 positive cores. Some studies used Gleason score with other criteria based on core information (maximum cancer core length ≥4 mm). The sample size in each article ranged between 33 and 1042. Patient age ranged from 59 to 70 years. 1.5-T or 3.0-T scanner was used for mp-MRI examination in all included studies. Each patient presented with at least one disputable lesion as shown by MRI results, and each lesion was obtained from at least one targeted core. Systematic biopsies from the same session were carried out using a median of 10 - 13 cores through the transrectal route. Main features of included studies and PCa cases are presented in Table 1. The quality assessment of these enrolled studies is shown in Tables 2, 3 and Figure 2.
3.3 MRI-TB Compared With SB for Prostate Cancer Detection
3.3.1 Performance of MRI-TB Compared With SB in Clinically Significant and Insignificant PCa Diagnosis
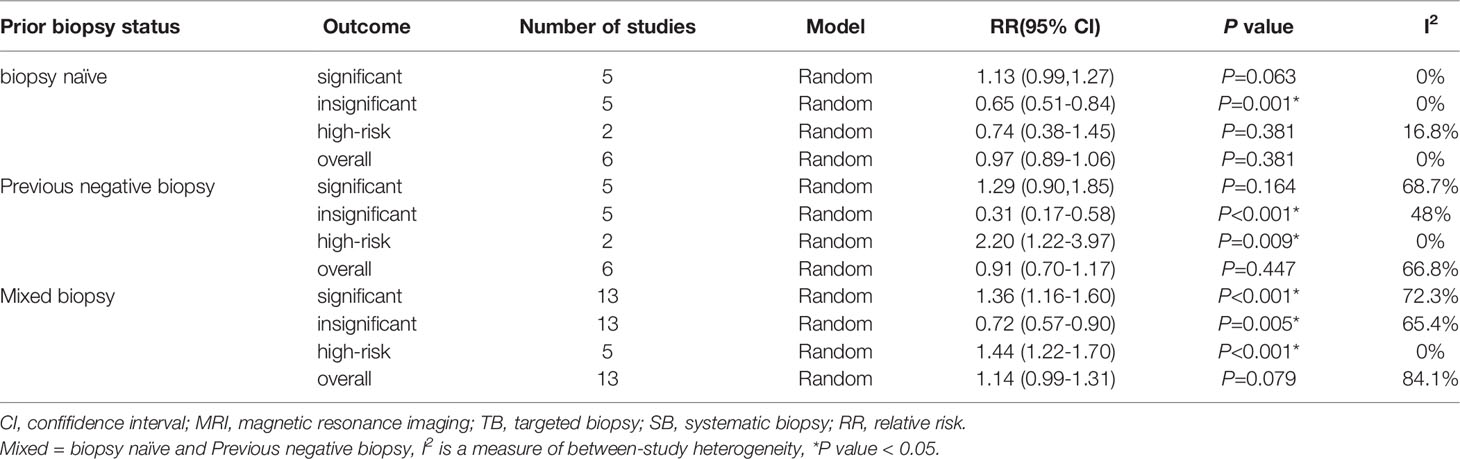
A total of 24 study cohorts, including 5712 participants were included in the analysis. The rate of diagnosis of clinically significant PCa using MRI-TB was significantly higher compared with the rate for TRUS (RR=1.27, 95% CI =1.15-1.4, p<0.001, I2 = 61.6%, Figure 3A). MRI-TB diagnosed fewer cases of clinically insignificant PCa compared with TRUS biopsy (RR=0.65, 95% CI=0.55-0.77, p<0.001, I2 = 61.4%, Figure 3B). Subgroup analysis showed that in the biopsy naïve population, the rate of detection of clinically significant PCa was not significantly different between MRI-TB and SB (RR=1.13, 95% CI =0.99-1.27, p=0.063; I2 = 0%). On the other hand, clinically insignificant PCa cases diagnosed through MRI-TB diagnosed were significantly fewer compared with those detected through SB (RR=0.65. 95% CI=0.51-0.84, p=0.001; I2 = 0%). In the previous negative biopsy group, no significant differences were reported in clinical significance between the groups (RR=1.29, 95% CI =0.90-1.85, p=0.164, I2 = 68.7%). Similarly, MRI-TB showed lower rates of clinically insignificant PCa compared with SB (RR=0.31, 95% CI=0.17-0.58, p<0.001, I2 = 48%). In the mixed biopsy population, significant differences were observed in diagnosis of clinically significant PCa (RR=1.36, 95%CI=1.16-1.60, p<0.001, I2 = 72.3%) and clinically insignificant PCa using the two methods (RR=0.72, 95% CI=0.57-0.9, p=0.005, I2 = 65.4%, Table 4).
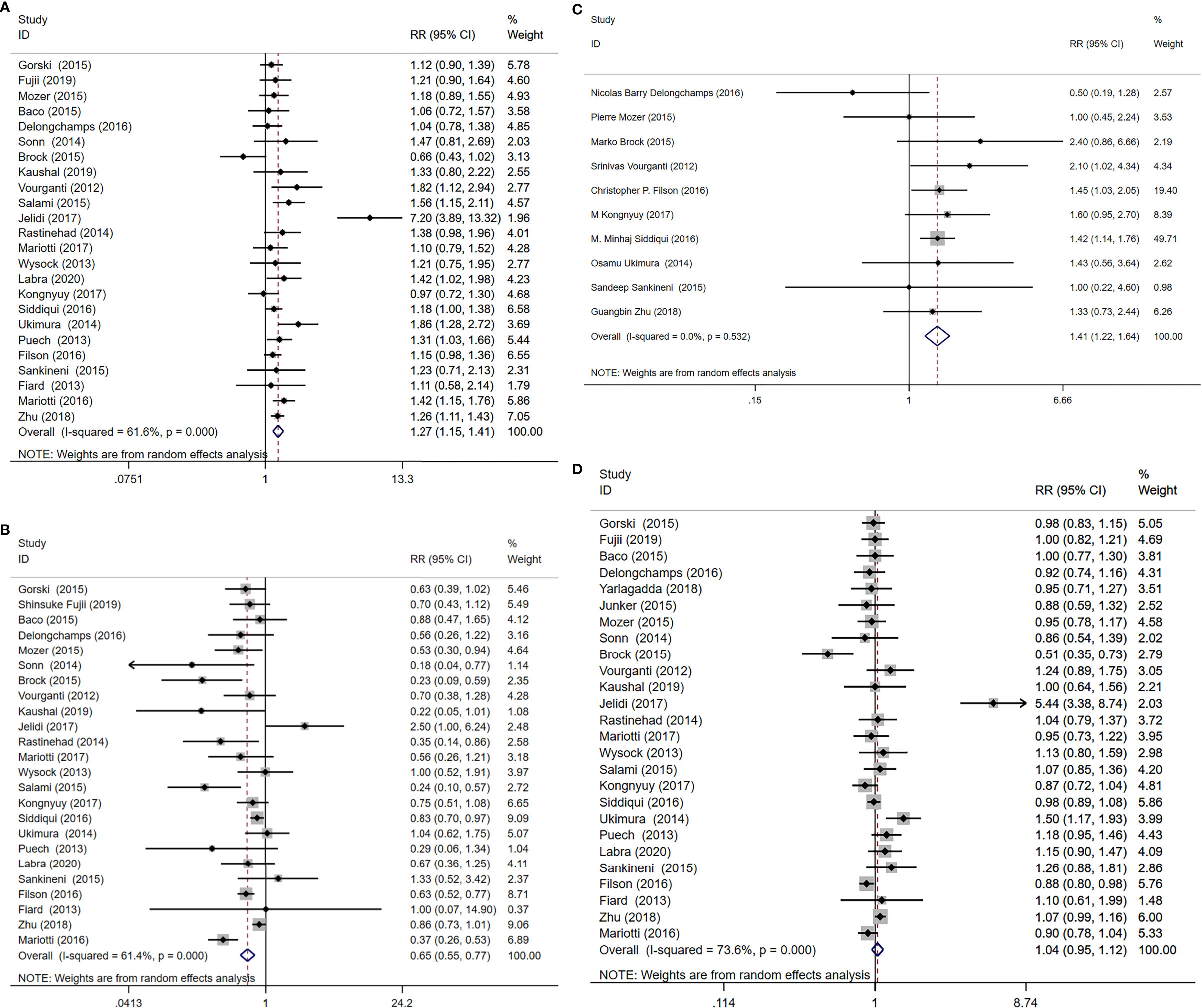
Figure 3 MRI/Transrectal Ultrasound (TRUS) fusion guided-targeted biopsy (MRI-TB) versus transrectal ultrasound-guided biopsy (SB) for the detection of (A) clinically significant prostate cancer, (B) clinically insignificant prostate cancer, (C) high-risk prostate cancer, (D) overall prostate cancer.
3.3.2 Performance of MRI-TB Compared With SB in High-Risk PCa Diagnosis
Ten studies including 3804 patients reported high-risk PCa, with MRI-TB detecting more cases of high-risk PCa compared with systematic biopsy (RR=1.41, 95% CI=1.22-1.64, p<0.001, I2 = 0%, Figure 3C).
3.3.3 Performance of MRI-TB Compared With SB in Overall PCa Diagnosis
The included studies involving 26 cohorts, reported overall PCa detection in 5831 cases. Overall PCa cases detected using MRI-TB method were slightly more compared with cases detected through SB method (RR=1.04, 95% CI=0.95-1.12, I2 = 73.6%), at 48.6% (2832/5826) vs 47.9% (2793/5831). However, the detection rates for the two groups were not significantly different (p =0.41, Figure 3D).
3.4 Performance of MRI-TB Integrated With SB Compared With SB Alone in PCa Diagnosis
3.4.1 Performance of MRI-TB+SB vs SB in Diagnosis of Clinically Significant and Insignificant PCa
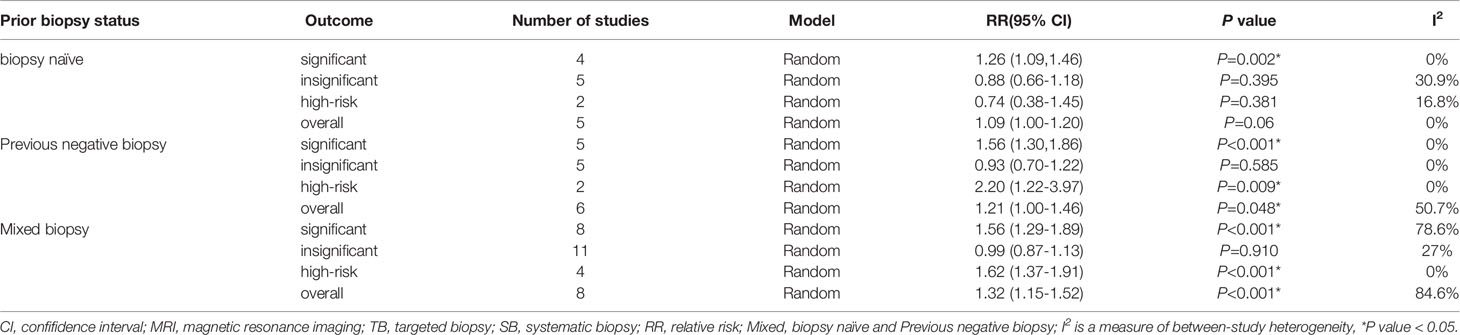
A total of 18 study cohorts with 5083 participants were included in the analysis. Diagnosis rate of clinically significant PCa using MRI-TB+SB method was significantly higher compared with use of SB alone (RR=1.44, 95% CI=1.30-1.59, p<0.001, I2 = 60.8%, Figure 4A). However, detection rates of clinically insignificant cases showed no significant differences for MRI-TB+SB and SB methods (RR=0.99, 95% CI=0.91-1.07, p=0.72, I2 = 7.9%, Figure 4B). Three subgroup analyses based on detection of clinically significant prostate cancer showed statistical differences. Notably, MRI-TB+SB showed a higher detection rate in biopsy naïve cohorts compared with use of SB alone (RR=1.26, 95% CI=1.09-1.46, p=0.002, I2 = 0%). Similar results were obtained in individuals with previous negative biopsy (RR=1.56, 95% CI=1.30-1.86. p<0.001, I2 = 0%) and mixed biopsy (RR=1.56, 95% CI=1.29-1.89, p<0.001, I2 = 78.6%). The two methods did not show significant differences between the three sub-group analysis in detection rates for diagnosis of insignificant PCa (Table 5).
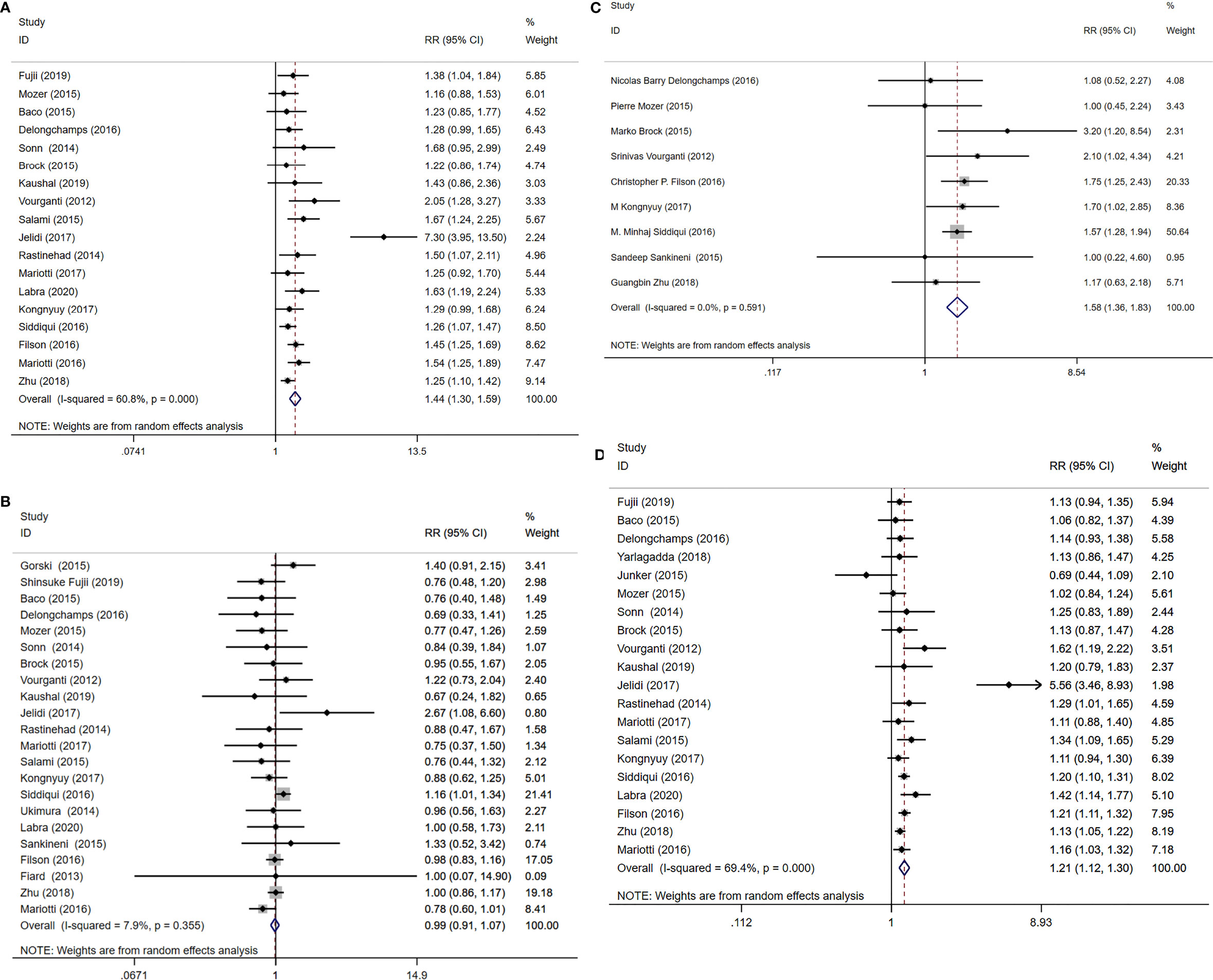
Figure 4 MRI/Transrectal Ultrasound (TRUS) fusion guided-targeted biopsy (MRI-TB) Combined with systematic biopsy(SB) versus SB for the detection of (A) clinically significant prostate cancer, (B) clinically insignificant prostate cancer, (C) high-risk prostate cancer, (D) overall prostate cancer.
3.4.2 Performance of MRI-TB+SB Compared With SB in High-Risk PCa Detection
Nine studies including 3677 cases reported high-risk PCa detection rates. Detection rates of high-risk PCa cases using MRI-TB+SB were significantlyu higher compared with use of SB alone (RR=1.58, 95% CI=1.36-1.83, p<0.001, I2 = 0%, Figure 4C).
3.4.3 MRI-TB+SB vs SB in Overall PCa Detection
A total of 20 studies that reported 5220 PCa cases were included in this meta-analysis. MRI-TB+SB exhibited significantly higher overall PCa detection rate compared with SB alone (RR=1.21, 95% CI=1.12-1.30, p<0.001, I2 = 69.4%, Figure 4D). Subgroup analysis did not show statistically significant differences in detection of biopsy naïve patients using the two methods (p=0.06). However, in previous negative biopsy group (RR=1.21, 95% CI=1.00-1.46, p=0.048, I2 = 50.7%) and mixed biopsy patients (RR=1.32, 95% CI=1.15-1.52, p<0.001, I2 = 84.6%), the detection rate of MRI-TB+SB for overall PCa cases was significantly higher compared with use of SB alone (Table 5).
3.5 Sensitivity Analysis
In the analysis of MRI-TB vs SB to detect prostate cancer, heterogeneity was observed in diagnosis of clinically significant PCa and insignificant PCa. Subgroup analysis showed high heterogeneity in previous negative biopsy and mixed biopsy subgroups and low heterogeneity in the biopsy naïve subgroup, implying that prior biopsy status may not be the source of heterogeneity. Therefore, we performed a sensitivity analysis for clinically significant PCa, and insignificant PCa. Sensitivity analysis did not show any effect on the results when we excluded the studies one by one (Figure 5), implying that heterogeneity was caused by differences in other variables. Although subgroup analyses were performed, several variables were not available for correlation analysis.
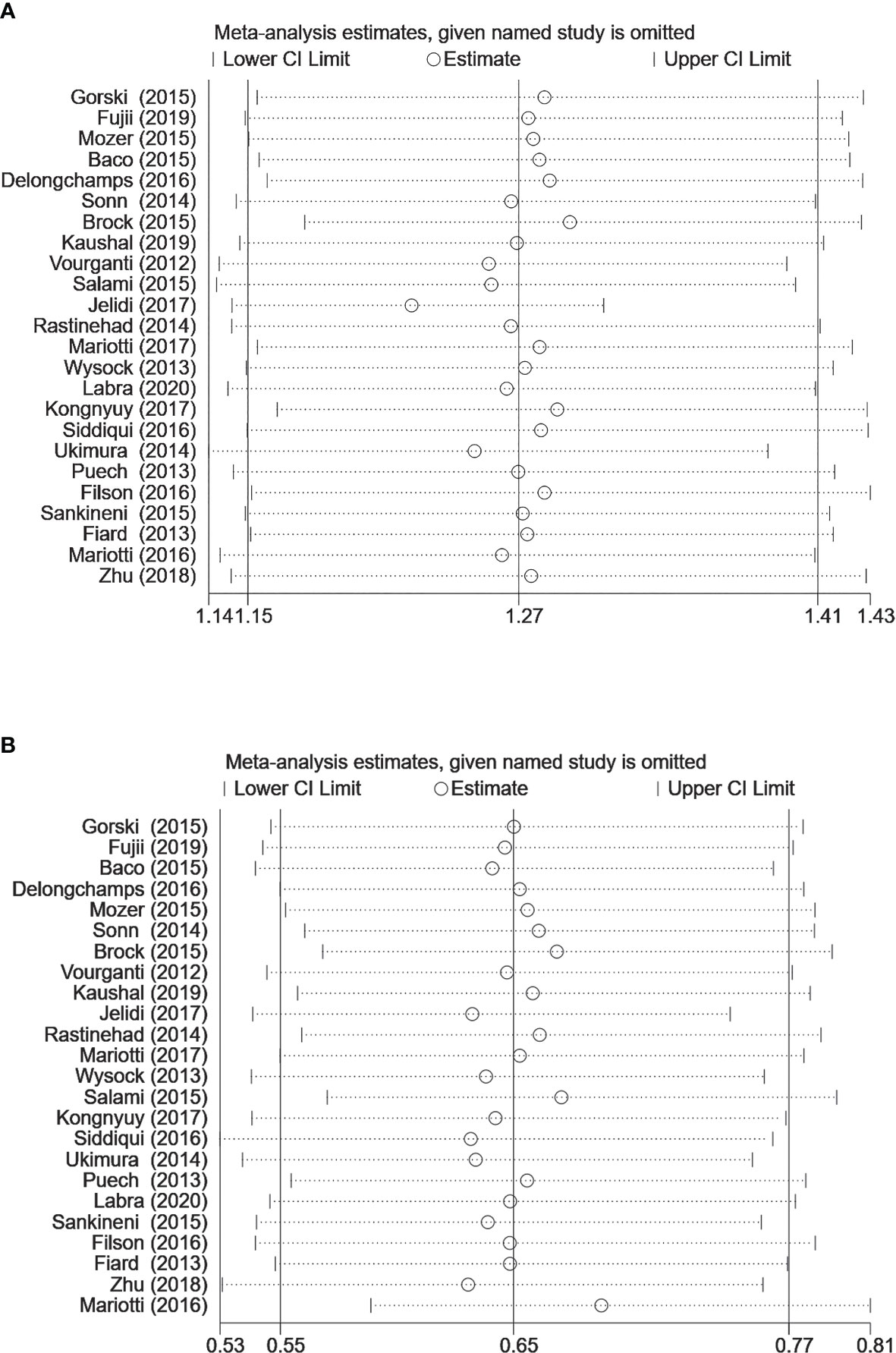
Figure 5 Sensitivity analysis for (A) clinically significant prostate cancer detection (B) clinically insignificant prostate cancer detection (MRI-TB vs. systematic biopsy). The circles represent the RR estimate and the horizontal lines represent the 95% CI. CI, confidence interval; RR, relative risk.
3.6 Publication Bias
In MRI-TB vs SB analysis of PCa detection, no publication bias was observed using Begg’s test or Egger’s test. In MRI-TB+SB vs SB analysis of PCa detection no publication bias was detected except for clinically significant PCa cases (Egger’s test, P=0.036. Begg’s test, P=0.081) (Figure 6).
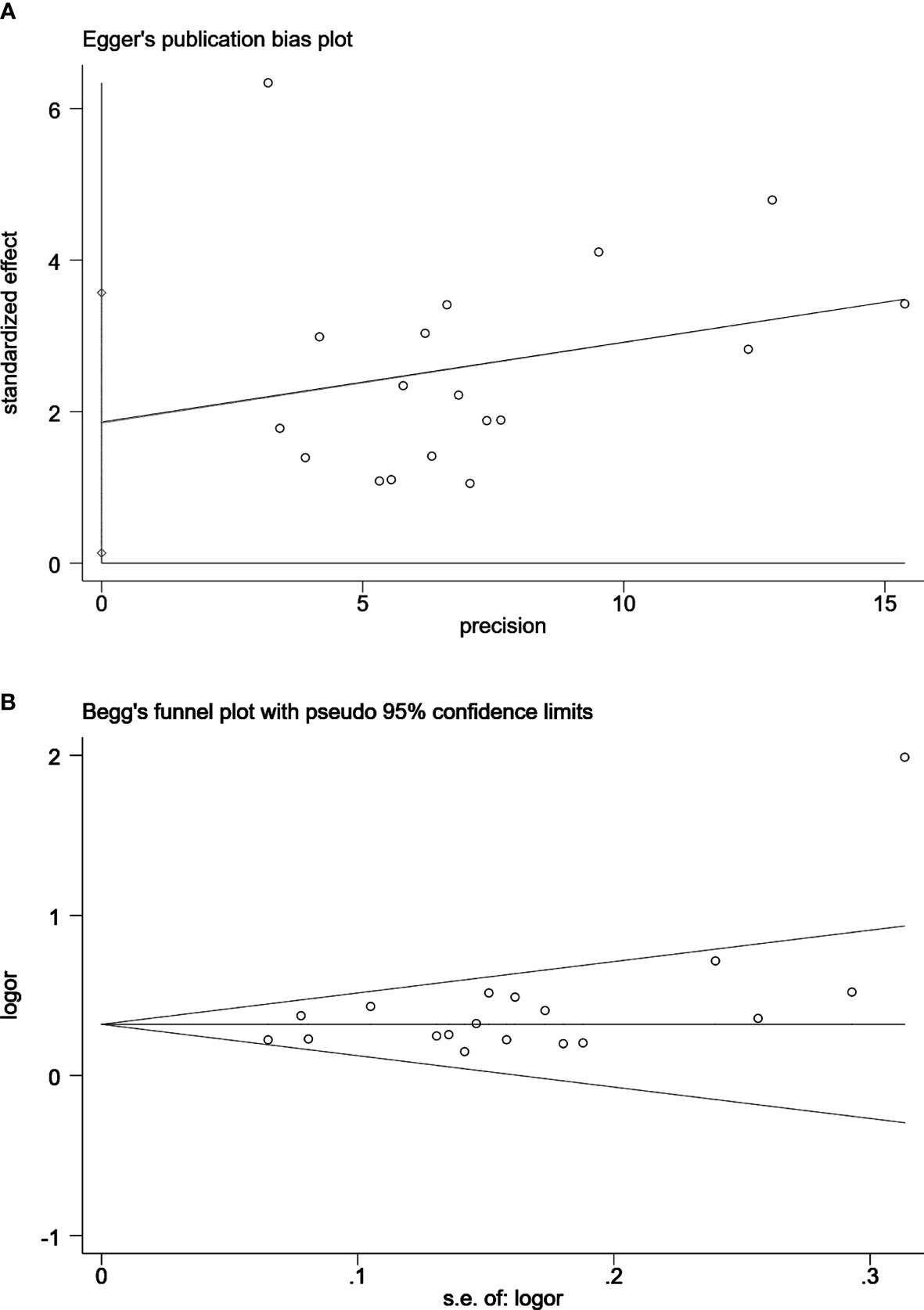
Figure 6 Egger’s publication bias plot (A) and Begg’s funnel plot (B) for the assessment of publication bias for clinically significant prostate cancer detection by MRI/Transrectal Ultrasound (TRUS) fusion guided-targeted biopsy (MRI-TB) Combined with systematic biopsy(SB) versus SB. Egger’s test, P=0.036. Begg’s test, P=0.081.
4 Discussion
Although TRUS-guided prostate biopsy is widely used for diagnosis of prostate cancer, it is not effective in determining tumor location (17). Furthermore, incidence of clinically relevant tumor false-negative biopsy using TRUS is approximately 47%, which can delay treatment of tumors with high Gleason scores (42). Multiparametric MRI comprises contrast-enhanced dynamic imaging, T2-weighted, and diffusion-weighted strategies, and is considered the most sensitive imaging approach for PCa detection (11). Prostate gland mp-MRI is a valuable imaging technique for patients that require targeted prostate biopsy as it effectively identifies higher grades and PCa volume compared with systematic 12-core prostate biopsy. Therefore, mp-MRI increases accuracy of prostate biopsy analysis by guiding clinicians to suspicious areas during biopsy rather than random sampling (19). The findings of this systematic review show that MRI-TRUS fusion biopsy diagnoses more clinically significant PCa and high-risk PCa compared with systematic biopsy, with lower rate of detection of clinically insignificant PCa, thus avoiding consequent overtreatment.
Analysis showed that MRI-TB has a higher detection rate of clinically significant PCa and high-risk PCa compared with traditional TRUS-guided biopsy. On the other hand, the rate of detection of clinically insignificant PCa using MRI-TB was lower compared with the rate of detection using traditional TRUS-guided biopsy. MRI/TRUS fusion biopsy did not show significant differences in overall PCa diagnosis compared with systematic biopsy. These results are consistent with previous findings that MRI-TB diagnoses more clinically significant PCa and fewer clinically insignificant PCa cases compared with standard biopsy (12, 13). Similarly, in the study carried out by Pepe et al., they included a total of 1032 patients suspicion of cancer and found that MRI targeted fusion biopsy had a lower detection rate of clinically insignificant prostate cancer than transperineal saturation biopsy (43). However, the previous systematic reviews did not explore the detection rate of MRI-TB for high-risk PCa. Currently, very few systematic reviews and meta-analyses have explored detection rate of high-risk PCa using MRI-TB. Similar findings were reported by Tang et al. on high-risk PCa detection, however, meta-analysis mainly evaluated MRI/TRUS fusion 3D model-guided targeted biopsy for PCa (44). Patients with high-risk prostate cancer are at an increased risk of biochemical recurrence, metastatic progression and cancer-related death following primary treatment compared with patients with low-risk or intermediate-risk disease (45). Individuals with high-risk PCa have high risk of systemic or local relapse and are more likely to experience symptoms and/or higher risk of death (17). Therefore, we explored high-risk PCa detection in the present meta-analysis. Our findings show that MRI-TB may help clinicians to effectively screen high-risk PCa, thus improving treatment decisions, which are consistent with previous findings (27).
Analysis of the 3 PCa diagnosis methods (including MRI-TB combined with SB) showed that MRI-TB+SB was superior in detection of overall, clinically significant, and high-risk PCa, which was consistent with reports from previous studies (23, 27–30). Rapisarda et al. compared the coincidence rate in the detection of clinically significant PCa cancers (GS≥7) between combined biopsy (fusion plus standard) and final histological report to 73.6%. They suggest that targeted biopsy combined with systematic biopsy can further improve the detection rate of clinically significant prostate cancer and reduce the missed diagnosis rate with targeted biopsy alone (46). This observation coincides with our findings.However, MRI-TB+SB did not exhibit significantly different diagnostic rate for clinically insignificant PCa compared with SB method. Therefore, MRI-TB+SB did not reduce detection of clinically insignificant PCa compared with SB.
This review had a few limitations. Firstly, most of the included studies had paired designs, therefore, the analysis introduces bias because conclusions from such data are limited to MRI patients with suspicious findings using MRI-TB, and systematic biopsy. Secondly, MRI image quality and definitions of clinically significant and high-risk PCa were different across centers, causing heterogeneity. Finally, integrating MRI-TB with SB improves rate of detection of overall clinically significant cases, and high-risk PCa case compared with other biopsy methods. However, MRI-TB+SB needs more biopsy cores. Therefore, further studies should be performed to determine MRI-TB+SB results in more complications compared with systematic biopsy.
Finally, we note some new directions in PCa research and give an outlook to the future research. Recently, radiomics and genomics have increasingly become a prospective topic in the field of prostate cancer research. A combination of these fields, radiogenomics, is an emerging fifield that studies the correlation between image phenotypes and genomics inside a tumor. Prostate cancer has been extensively investigated using radiogenomics, with experiments between quantitative image features and single gene expression, which have yielded promising results (47). Some studies showed that radiomic features correlation with Gleason score and PIRADS sum scores (48–50). In the detection of clinically significant PCa, Combining radiological and clinical radiomic models was indeed effective in predicting clinically significant PCa in patients with a PIRADS score of three or more. It is possible to further improve the radiomic potential of this issue by developing different models automatically, using machine learning and artificial intelligence techniques, and by creating nomograms (51). However, At present, radiogenomics holds great promise, but it is a new area of research, the use of radiogenomics in clinical practice regarding prostate cancer does not have any utility or effectiveness.We need to fill many gaps to achieve clinical implementation of radiogenomics.
5 Conclusion
The findings of this study show that MRI-TB has higher detection rate for clinically significant and high-risk PCa cases, and fewer rate for clinically insignificant PCa cases compared with systematic protocols. Combination of MRI-TB with SB improves PCa detection in compared with use of either of the methods alone. However, MRI-TB+SB does not reduce the diagnosis rate of clinically insignificant PCa. The findings of this study provide information for clinicians and patients on the risks and benefits of using MRI-TB or systematic biopsy.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author Contributions
JX and CJ have contributed equally. JX, conception, manuscript preparation, data collection and analysis. CJ, conception, data analysis, manuscript editing and manuscript review. ML, Study design, manuscript editing and manuscript review. KS, study design and manuscript review. ZJ, data collection and analysis. ZD and XG, substantial contributions, manuscript editing and manuscript review. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from Shenzhen Science and Technology Innovation Committee (JCYJ20170413161913429), Shenzhen Key Medical Discipline Construction Fund (SZXK052) and Sanming Project of Medicine in Shenzhen (SZSM201612027).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank the members of their research group for useful discussions.
References
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2018. CA Cancer J Clin (2018) 68(1):7–30. doi: 10.3322/caac.21442
2. Descotes JL. Diagnosis of Prostate Cancer. Asian J Urol (2019) 6(2):129–36. doi: 10.1016/j.ajur.2018.11.007
3. Wang X, Bao J, Ping X, Hu C, Hou J, Dong F, et al. The Diagnostic Value of Pi-Rads V1 and V2 Using Multiparametric Mri in Transition Zone Prostate Clinical Cancer. Oncol Lett (2018) 16(3):3201–6. doi: 10.3892/ol.2018.9038
4. Fütterer JJ, Briganti A, De Visschere P, Emberton M, Giannarini G, Kirkham A, et al. Can Clinically Significant Prostate Cancer Be Detected With Multiparametric Magnetic Resonance Imaging? A Systematic Review of the Literature. Eur Urol (2015) 68(6):1045–53. doi: 10.1016/j.eururo.2015.01.013
5. Geiger-Gritsch S, Oberaigner W, Mühlberger N, Siebert U, Ladurner M, Klocker H, et al. Patient-Reported Urinary Incontinence and Erectile Dysfunction Following Radical Prostatectomy: Results From the European Prostate Centre Innsbruck. Urol Int (2015) 94(4):419–27. doi: 10.1159/000369475
6. Beyersdorff D, Winkel A, Hamm B, Lenk S, Loening SA, Taupitz M. Mr Imaging-Guided Prostate Biopsy With a Closed Mr Unit at 1.5 T: Initial Results. Radiology (2005) 234(2):576–81. doi: 10.1148/radiol.2342031887
7. Puech P, Rouvière O, Renard-Penna R, Villers A, Devos P, Colombel M, et al. Prostate Cancer Diagnosis: Multiparametric Mr-Targeted Biopsy With Cognitive and Transrectal Us-Mr Fusion Guidance Versus Systematic Biopsy–Prospective Multicenter Study. Radiology (2013) 268(2):461–9. doi: 10.1148/radiol.13121501
8. Fiard G, Hohn N, Descotes JL, Rambeaud JJ, Troccaz J, Long JA. Targeted Mri-Guided Prostate Biopsies for the Detection of Prostate Cancer: Initial Clinical Experience With Real-Time 3-Dimensional Transrectal Ultrasound Guidance and Magnetic Resonance/Transrectal Ultrasound Image Fusion. Urology (2013) 81(6):1372–8. doi: 10.1016/j.urology.2013.02.022
9. Kam J, Yuminaga Y, Kim R, Aluwihare K, Macneil F, Ouyang R, et al. Does Magnetic Resonance Imaging-Guided Biopsy Improve Prostate Cancer Detection? A Comparison of Systematic, Cognitive Fusion and Ultrasound Fusion Prostate Biopsy. Prostate Int (2018) 6(3):88–93. doi: 10.1016/j.prnil.2017.10.003
10. Costa DN, Pedrosa I, Donato F Jr., Roehrborn CG, Rofsky NM. Mr Imaging-Transrectal Us Fusion for Targeted Prostate Biopsies: Implications for Diagnosis and Clinical Management. Radiographics (2015) 35(3):696–708. doi: 10.1148/rg.2015140058
11. Siddiqui MM, Rais-Bahrami S, Truong H, Stamatakis L, Vourganti S, Nix J, et al. Magnetic Resonance Imaging/Ultrasound-Fusion Biopsy Significantly Upgrades Prostate Cancer Versus Systematic 12-Core Transrectal Ultrasound Biopsy. Eur Urol (2013) 64(5):713–9. doi: 10.1016/j.eururo.2013.05.059
12. Schoots IG, Roobol MJ, Nieboer D, Bangma CH, Steyerberg EW, Hunink MG. Magnetic Resonance Imaging-Targeted Biopsy May Enhance the Diagnostic Accuracy of Significant Prostate Cancer Detection Compared to Standard Transrectal Ultrasound-Guided Biopsy: A Systematic Review and Meta-Analysis. Eur Urol (2015) 68(3):438–50. doi: 10.1016/j.eururo.2014.11.037
13. Kasivisvanathan V, Stabile A, Neves JB, Giganti F, Valerio M, Shanmugabavan Y, et al. Magnetic Resonance Imaging-Targeted Biopsy Versus Systematic Biopsy in the Detection of Prostate Cancer: A Systematic Review and Meta-Analysis. Eur Urol (2019) 76(3):284–303. doi: 10.1016/j.eururo.2019.04.043
14. Spiotto MT, Hancock SL, King CR. Radiotherapy After Prostatectomy: Improved Biochemical Relapse-Free Survival With Whole Pelvic Compared With Prostate Bed Only for High-Risk Patients. Int J Radiat Oncol Biol Phys (2007) 69(1):54–61. doi: 10.1016/j.ijrobp.2007.02.035
15. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The Prisma Statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
16. Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. Mri-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N Engl J Med (2018) 378(19):1767–77. doi: 10.1056/NEJMoa1801993
17. Chang AJ, Autio KA, Roach M 3rd, Scher HI. High-Risk Prostate Cancer-Classification and Therapy. Nat Rev Clin Oncol (2014) 11(6):308–23. doi: 10.1038/nrclinonc.2014.68
18. Jelidi A, Ohana M, Labani A, Alemann G, Lang H, Roy C. Prostate Cancer Diagnosis: Efficacy of a Simple Electromagnetic Mri-Trus Fusion Method to Target Biopsies. Eur J Radiol (2017) 86:127–34. doi: 10.1016/j.ejrad.2016.11.016
19. Labra A, González F, Silva C, Franz G, Pinochet R, Gupta RT. Mri/Trus Fusion Vs. Systematic Biopsy: Intra-Patient Comparison of Diagnostic Accuracy for Prostate Cancer Using Pi-Rads V2. Abdominal Radiol (2020) 45(7):2235–43. doi: 10.1007/s00261-020-02481-y
20. Rastinehad AR, Turkbey B, Salami SS, Yaskiv O, George AK, Fakhoury M, et al. Improving Detection of Clinically Significant Prostate Cancer: Magnetic Resonance Imaging/Transrectal Ultrasound Fusion Guided Prostate Biopsy. J Urol (2014) 191(6):1749–54. doi: 10.1016/j.juro.2013.12.007
21. Junker D, Schäfer G, Heidegger I, Bektic J, Ladurner M, Jaschke W, et al. Multiparametric Magnetic Resonance Imaging/Transrectal Ultrasound Fusion Targeted Biopsy of the Prostate: Preliminary Results of a Prospective Single-Centre Study. Urol Int (2015) 94(3):313–8. doi: 10.1159/000365489
22. Baco E, Rud E, Eri LM, Moen G, Vlatkovic L, Svindland A, et al. A Randomized Controlled Trial to Assess and Compare the Outcomes of Two-Core Prostate Biopsy Guided by Fused Magnetic Resonance and Transrectal Ultrasound Images and Traditional 12-Core Systematic Biopsy. Eur Urol (2016) 69(1):149–56. doi: 10.1016/j.eururo.2015.03.041
23. Filson CP, Natarajan S, Margolis DJ, Huang J, Lieu P, Dorey FJ, et al. Prostate Cancer Detection With Magnetic Resonance-Ultrasound Fusion Biopsy: The Role of Systematic and Targeted Biopsies. Cancer (2016) 122(6):884–92. doi: 10.1002/cncr.29874
24. Mariotti GC, Falsarella PM, Garcia RG, Queiroz MRG, Lemos GC, Baroni RH. Incremental Diagnostic Value of Targeted Biopsy Using Mpmri-Trus Fusion Versus 14-Fragments Prostatic Biopsy: A Prospective Controlled Study. Eur Radiol (2017) 28(1):11–6. doi: 10.1007/s00330-017-4939-0
25. Mariotti GC, Costa DN, Pedrosa I, Falsarella PM, Martins T, Roehrborn CG, et al. Magnetic Resonance/Transrectal Ultrasound Fusion Biopsy of the Prostate Compared to Systematic 12-Core Biopsy for the Diagnosis and Characterization of Prostate Cancer: Multi-Institutional Retrospective Analysis of 389 Patients. Urol Oncol (2016) 34(9):416.e9–e14. doi: 10.1016/j.urolonc.2016.04.008
26. Kongnyuy M, Siddiqui MM, George AK, Muthigi A, Sidana A, Maruf M, et al. Multiparametric Mri/Ultrasound Fusion-Guided Biopsy Decreases Detection of Indolent Cancer in African-American Men. Prostate Cancer Prostatic Dis (2017) 20(3):348–51. doi: 10.1038/pcan.2017.21
27. Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of Mr/Ultrasound Fusion-Guided Biopsy With Ultrasound-Guided Biopsy for the Diagnosis of Prostate Cancer. Jama (2015) 313(4):390–7. doi: 10.1001/jama.2014.17942
28. Brock M, von Bodman C, Palisaar J, Becker W, Martin-Seidel P, Noldus J. Detecting Prostate Cancer. Dtsch Arztebl Int (2015) 112(37):605–11. doi: 10.3238/arztebl.2015.0605
29. Mozer P, Rouprêt M, Le Cossec C, Granger B, Comperat E, de Gorski A, et al. First Round of Targeted Biopsies Using Magnetic Resonance Imaging/Ultrasonography Fusion Compared With Conventional Transrectal Ultrasonography-Guided Biopsies for the Diagnosis of Localised Prostate Cancer. BJU Int (2015) 115(1):50–7. doi: 10.1111/bju.12690
30. Delongchamps NB, Portalez D, Bruguière E, Rouvière O, Malavaud B, Mozer P, et al. Are Magnetic Resonance Imaging-Transrectal Ultrasound Guided targeted Biopsies Noninferior to Transrectal Ultrasound Guided Systematic Biopsies for the Detection of Prostate Cancer? J Urol (2016) 196(4):1069–75. doi: 10.1016/j.juro.2016.04.003
31. Kaushal R, Das CJ, Singh P, Dogra PN, Kumar R. Multiparametric Magnetic Resonance Imaging-Transrectal Ultrasound Fusion Biopsies Increase the Rate of Cancer Detection in Populations With a Low Incidence of Prostate Cancer. Investig Clin Urol (2019) 60(3):156–61. doi: 10.4111/icu.2019.60.3.156
32. Fujii S, Hayashi T, Honda Y, Terada H, Akita R, Kitamura N, et al. Magnetic Resonance Imaging/Transrectal Ultrasonography Fusion Targeted Prostate Biopsy Finds More Significant Prostate Cancer in Biopsy-Naive Japanese Men Compared With the Standard Biopsy. Int J Urol (2020) 27(2):140–6. doi: 10.1111/iju.14149
33. Salami SS, Ben-Levi E, Yaskiv O, Ryniker L, Turkbey B, Kavoussi LR, et al. In Patients With a Previous Negative Prostate Biopsy and a Suspicious Lesion on Magnetic Resonance Imaging, Is a 12-Core Biopsy Still Necessary in Addition to a Targeted Biopsy? BJU Int (2015) 115(4):562–70. doi: 10.1111/bju.12938
34. Vourganti S, Rastinehad A, Yerram N, Nix J, Volkin D, Hoang A, et al. Multiparametric Magnetic Resonance Imaging and Ultrasound Fusion Biopsy Detect Prostate Cancer in Patients With Prior Negative Transrectal Ultrasound Biopsies. J Urol (2012) 188(6):2152–7. doi: 10.1016/j.juro.2012.08.025
35. Yarlagadda VK, Lai WS, Gordetsky JB, Porter KK, Nix JW, Thomas JV, et al. Mri/Us Fusion-Guided Prostate Biopsy Allows for Equivalent Cancer Detection With Significantly Fewer Needle Cores in Biopsy-Naive Men. Diagn Interv Radiol (2018) 24(3):115–20. doi: 10.5152/dir.2018.17422
36. Zhu G, Wang Q. Comparisons Between Magnetic Resonance/Ultrasound Fusion-Guided Biopsy and Standard Biopsy in the Diagnosis of Prostate Cancer: A Prospective Cohort Study. Med (Baltimore) (2018) 97(36):e11962. doi: 10.1097/MD.0000000000011962
37. de Gorski A, Roupret M, Peyronnet B, Le Cossec C, Granger B, Comperat E, et al. Accuracy of Magnetic Resonance Imaging/Ultrasound Fusion Targeted Biopsies to Diagnose Clinically Significant Prostate Cancer in Enlarged Compared to Smaller Prostates. J Urol (2015) 194(3):669–73. doi: 10.1016/j.juro.2015.03.025
38. Sonn GA, Chang E, Natarajan S, Margolis DJ, Macairan M, Lieu P, et al. Value of Targeted Prostate Biopsy Using Magnetic Resonance-Ultrasound Fusion in Men With Prior Negative Biopsy and Elevated Prostate-Specific Antigen. Eur Urol (2014) 65(4):809–15. doi: 10.1016/j.eururo.2013.03.025
39. Wysock JS, Rosenkrantz AB, Huang WC, Stifelman MD, Lepor H, Deng FM, et al. A Prospective, Blinded Comparison of Magnetic Resonance (Mr) Imaging-Ultrasound Fusion and Visual Estimation in the Performance of Mr-Targeted Prostate Biopsy: The Profus Trial. Eur Urol (2014) 66(2):343–51. doi: 10.1016/j.eururo.2013.10.048
40. Ukimura O, Marien A, Palmer S, Villers A, Aron M, de Castro Abreu AL, et al. Trans-Rectal Ultrasound Visibility of Prostate Lesions Identified by Magnetic Resonance Imaging Increases Accuracy of Image-Fusion Targeted Biopsies. World J Urol (2015) 33(11):1669–76. doi: 10.1007/s00345-015-1501-z
41. Sankineni S, George AK, Brown AM, Rais-Bahrami S, Wood BJ, Merino MJ, et al. Posterior Subcapsular Prostate Cancer: Identification With Mpmri and Mri/Trus Fusion-Guided Biopsy. Abdom Imaging (2015) 40(7):2557–65. doi: 10.1007/s00261-015-0426-8
42. Sonn GA, Natarajan S, Margolis DJ, MacAiran M, Lieu P, Huang J, et al. Targeted Biopsy in the Detection of Prostate Cancer Using an Office Based Magnetic Resonance Ultrasound Fusion Device. J Urol (2013) 189(1):86–91. doi: 10.1016/j.juro.2012.08.095
43. Pepe P, Garufi A, Priolo GD, Galia A, Fraggetta F, Pennisi M. Is It Time to Perform Only Magnetic Resonance Imaging Targeted Cores? Our Experience With 1,032 Men Who Underwent Prostate Biopsy. J Urol (2018) 200(4):774–8. doi: 10.1016/j.juro.2018.04.061
44. Tang Y, Liu Z, Tang L, Zhang R, Lu Y, Liang J, et al. Significance of Mri/Transrectal Ultrasound Fusion Three-Dimensional Model-Guided, Targeted Biopsy Based on Transrectal Ultrasound-Guided Systematic Biopsy in Prostate Cancer Detection: A Systematic Review and Meta-Analysis. Urol Int (2018) 100(1):57–65. doi: 10.1159/000484144
45. Devos G, Devlies W, De Meerleer G, Baldewijns M, Gevaert T, Moris L, et al. Neoadjuvant Hormonal Therapy Before Radical Prostatectomy in High-Risk Prostate Cancer. Nat Rev Urol (2021) 18(12):739–62. doi: 10.1038/s41585-021-00514-9
46. Rapisarda S, Bada M, Crocetto F, Barone B, Arcaniolo D, Polara A, et al. The Role of Multiparametric Resonance and Biopsy in Prostate Cancer Detection: Comparison With Definitive Histological Report After Laparoscopic/Robotic Radical Prostatectomy. Abdom Radiol (NY) (2020) 45(12):4178–84. doi: 10.1007/s00261-020-02798-8
47. Ferro M, de Cobelli O, Vartolomei MD, Lucarelli G, Crocetto F, Barone B, et al. Prostate Cancer Radiogenomics-From Imaging to Molecular Characterization. Int J Mol Sci (2021) 22(18):9971. doi: 10.3390/ijms22189971
48. Switlyk MD, Salberg UB, Geier OM, Vlatkovic L, Lilleby W, Lyng H, et al. Pten Expression in Prostate Cancer: Relationship With Clinicopathologic Features and Multiparametric Mri Findings. AJR Am J Roentgenol (2019) 212:1–9. doi: 10.2214/ajr.18.20743
49. Renard-Penna R, Cancel-Tassin G, Comperat E, Varinot J, Léon P, Roupret M, et al. Multiparametric Magnetic Resonance Imaging Predicts Postoperative Pathology But Misses Aggressive Prostate Cancers as Assessed by Cell Cycle Progression Score. J Urol (2015) 194(6):1617–23. doi: 10.1016/j.juro.2015.06.107
50. VanderWeele DJ, McCann S, Fan X, Antic T, Jiang Y, Oto A. Radiogenomics of Prostate Cancer: Association Between Qunatitative Multiparametric Mri Features and Pten. J Clin Oncol (2015) 33(7_suppl):126–. doi: 10.1200/jco.2015.33.7_suppl.126
Keywords: magnetic resonance imaging, transrectal ultrasound, prostate cancer, targeted biopsy, meta-analysis
Citation: Xie J, Jin C, Liu M, Sun K, Jin Z, Ding Z and Gong X (2022) MRI/Transrectal Ultrasound Fusion-Guided Targeted Biopsy and Transrectal Ultrasound-Guided Systematic Biopsy for Diagnosis of Prostate Cancer: A Systematic Review and Meta-analysis. Front. Oncol. 12:880336. doi: 10.3389/fonc.2022.880336
Received: 21 February 2022; Accepted: 11 April 2022;
Published: 23 May 2022.
Edited by:
Felix Preisser, Universitätsklinikum Frankfurt, GermanyReviewed by:
Pietro Pepe, Cannizzaro Hospital, ItalyFelice Crocetto, Federico II University Hospital, Italy
Copyright © 2022 Xie, Jin, Liu, Sun, Jin, Ding and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhimin Ding, jimding03@126.com; Xuehao Gong, fox_gxh@sina.com
†These authors have contributed equally to this work and share first authorship
 Jianfeng Xie
Jianfeng Xie Chunchun Jin
Chunchun Jin Mengmeng Liu
Mengmeng Liu Kun Sun1
Kun Sun1 Zhimin Ding
Zhimin Ding Xuehao Gong
Xuehao Gong