Feasibilty of Transcutaneous pCO2 Monitoring During Immediate Transition After Birth—A Prospective Observational Study
- 1Research Unit for Neonatal Micro- and Macrocirculation, Department of Pediatrics, Medical University of Graz, Graz, Austria
- 2Division of Neonatology, Department of Pediatrics, Medical University of Graz, Graz, Austria
- 3NICU “V. Buzzi” Children's Hospital, ASST-FBF-Sacco, Milan, Italy
- 4Institute for Medical Informatics, Statistics and Documentation, Medical University of Graz, Graz, Austria
Background: According to recommendations, non-invasive monitoring during neonatal resuscitation after birth includes heart rate (HR) and oxygen saturation (SpO2). Continuous transcutaneous monitoring of carbon dioxide partial pressure (tcpCO2) may further offer quantitative information on neonatal respiratory status.
Objective: We aimed to investigate feasibility of tcpCO2 measurements in the delivery room during immediate neonatal transition and to compare the course of tcpCO2 between stable term and preterm infants.
Methods: Neonates without need for cardio-respiratory intervention during immediate transition after birth were enrolled in a prospective observational study. In these term and preterm neonates, we measured HR and SpO2 by pulse oximetry on the right wrist and tcpCO2 with the sensor applied on the left hemithorax during the first 15 min after birth. Courses of tcpCO2 were analyzed in term and preterm neonates and groups were compared.
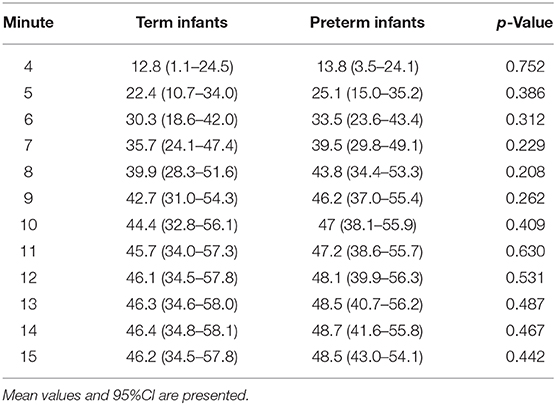
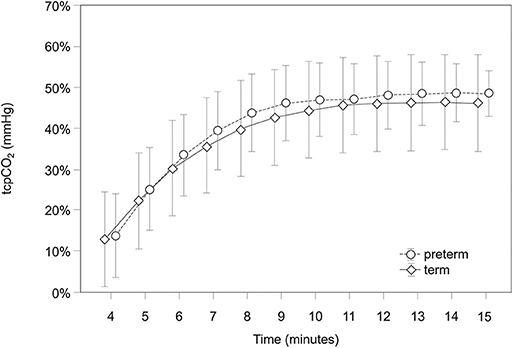
Results: Fifty-three term (gestational age: 38.8 ± 0.9 weeks) and 13 preterm neonates (gestational age: 34.1 ± 1.5 weeks) were included. First tcpCO2 values were achieved in both groups at minute 4 after birth, which reached a stable plateau after the equilibration phase at minute 9. Mean tcpCO2 values 15 min after birth were 46.2 (95% CI 34.5–57.8) mmHg in term neonates and 48.5 (95%CI 43.0–54.1) mmHg in preterm neonates. Preterm and term infants did not show significant differences in the tcpCO2 values at any time point.
Conclusion: This study demonstrates that tcpCO2 measurement is feasible during immediate neonatal transition after birth and that tcpCO2 values were comparable in stable term and preterm neonates.
Introduction
Neonatologist are confronted with the complexity of monitoring transition from intra- to extra-uterine life. It is a multi-step event, characterized by rapid physiological changes in respiratory, cardio-circulatory and metabolic status.
The decrease in partial pressure of oxygen and the increase in that of carbon dioxide (pCO2) followed by respiratory acidosis is one of the main triggers for initiation of breathing (1). After proper and effective establishment of respiratory function, the exchange of CO2 and O2 should reach a steady state.
To date, there are different methods and devices to assess oxygenation and cardio-circulatory status during neonatal transition. The most widespread monitoring is pulse oximetry, a non-invasive mode to detect heart rate (HR) and peripheral oxygen saturation (SpO2). It is recommended as standard practice in the delivery room (DR) by current international guidelines (2). Reference values for both parameters during immediate transition are available (3, 4).
Although gas exchange problems may be frequent events during immediate post-natal transition, there is currently no evidence on the role of CO2 and continuous pCO2 monitoring in the DR. Moreover, uncertainty remains about its feasibility and reliability in this setting.
In this study we aimed to evaluate the feasibility of transcutaneous pCO2 (tcpCO2) monitoring in stable neonates during the first 15 min after birth. Moreover, we compared tcpCO2 values between term and preterm neonates without need for any intervention immediately after birth. We hypothesized that tcpCO2 monitoring during immediate transition after birth is feasible and that term and preterm neonates differ in tcpCO2 during this phase.
Methods
A prospective observational study was conducted from October 2015 to June 2018 at the Division of Neonatology, Department of Pediatrics and Adolescent Medicine, Medical University of Graz, Austria. Institutional ethical approval (EC number: 27-465 ex 14/15) and written parental consent were obtained before inclusion. Term and preterm neonates born by cesarean section without any intervention immediately after birth were included in the study. Exclusion criteria for this analysis were congenital malformations and/or need for cardio-respiratory support.
Monitoring was performed during the first 15 min after birth. As soon as the neonate was placed on the resuscitation table in supine position, a pulse oximeter probe was applied on the right wrist and HR and SpO2 were measured using the IntelliVue MP50 monitor (Philips, The Netherlands). The tcpCO2 sensor (TC Sensor 84, Radiometer RSCH GmbH, Thalwil, Switzerland) was applied on the infant's left hemithorax and values were displayed on a separate monitor (IntelliVue MP70, Philips, The Netherlands). In accordance with the manufacturer's guidelines, the sensor's temperature was set at 39°C, the lowest level for tcpCO2 measurements. The fixation of the tcpCO2 sensor was observed continuously and data were only recorded when the sensor was in place and remained attached to the skin.
Statistical Analysis
Demographic variables are presented as absolute counts, mean and standard deviation (SD) or median and interquartile range (IQR), as appropriate. Comparisons of categorical baseline characteristics between preterm and term neonates were made using chi-square test, t-test or Mann Whitney U-test, as appropriate. tcpCO2, SpO2, and HR data are presented as mean and 95% confidence interval (95% CI). We investigated tcpCO2, SpO2, and HR across time within the first 15 min using a linear mixed model with fixed effects for time and gestational age (preterm vs. term neonates). A first order autoregressive covariance structure was used. The autoregressive covariance structure assumes a systematically decreasing correlation with increasing distance between time points. Therefore, adjacent time points have the highest correlations. Post-hoc analyses for differences between groups at each minute were performed for the comparison of gestational age groups (pre-term vs. term neonates). A p-value < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS Statistics 24.0.0 (IBM, USA).
Results
Out of 197 measured neonates in the DR, this study included 53 term and 13 preterm neonates without any cardio-respiratory intervention immediately after birth (Table 1).
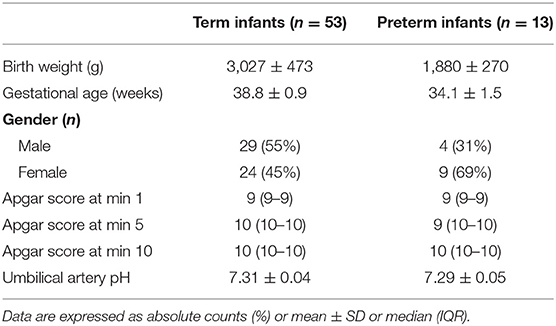
Table 1. Demographic data of term and preterm neonates with tcpCO2 monitoring immediately after birth.
Transcutaneous pCO2 values were analyzed from minute 4 after birth, which we considered the first proper detection time. Earlier values were detected in just a few patients; thus, they were not included in the analysis. Values reached a stable plateau after the equilibration phase at minute 9. Mean tcpCO2 values 15 min after birth were 46.2 (95% CI 34.5–57.8) mmHg in term neonates and 48.5 (43.0–54.1) mmHg in preterm infants. They both showed comparable courses of tcpCO2 without significant differences at any time point (p = 0.812) (Table 2 and Figure 1).
SpO2 and HR values were within targets (2), although SpO2 values in preterm neonates were significantly higher at minutes 2, 4, and 5 (in preterm infants at min 2: 75.8, 95% CI: 55.7–84.9, at min 4: 82.7, 95% CI: 73.9–91.5; at min 5: 87.3 95%CI: 78.6–96.0; in term infants at min 2: 68.7, 95% CI: 60.4–77.0, at min 4: 78.3, 95% CI: 70.0–86.5, at min 5: 87.3 95% CI: 82.5–90.8) and HR values were significantly lower at minute 2 (in preterm infants: 106 bpm, 95% CI: 71.5–141.9; in term infants: 138.3 bpm, 95% CI: 107.4–169.2).
Discussion
To our knowledge, this is the first description of tcpCO2 measurements in term and preterm neonates in the DR during immediate post-natal transition (5). In this study, we demonstrated that the use of tcpCO2 monitoring is feasible and safe.
First values could be obtained rather quickly at minute 4 after birth. We observed a progressive increase of values over the first minutes, until a plateau was reached at minute 9 after birth. This initial trend of tcpCO2 is attributed to the equilibration phase of the skin sensor which is necessary to obtain reliable values.
As we only enrolled neonates who did not require cardio-respiratory intervention and completed post-natal transition in a physiological way, it is reasonable to assume that the observed tcpCO2 values reflect uncompromised neonatal transition.
The importance of pCO2 levels in neonates is well-known. In fact, evidence clearly shows that pCO2 strongly influences cerebral perfusion in newborn infants, especially in those of low gestational age (6), and its uncontrolled increase may lead to brain injury. Moreover, the fact that blood gas values change quite rapidly has raised interest for continuous monitoring of pCO2 (7). Therefore, invasive and non-invasive pCO2 monitoring is widely used in neonates admitted to neonatal intensive care units (NICU). Two non-invasive methods for pCO2 monitoring are available: the transcutaneous measurement and the exhaled or end-tidal CO2 (etCO2) measurement (8, 9). They both provide continuous estimation of pCO2 and they significantly reduce blood samples, which is particularly important in neonates. However, they also have some limitations mainly due to technical reasons.
EtCO2 monitoring during immediate transition has shown valuable results in neonates needing respiratory support (10) but its use is limited to ventilated patients. It reflects the efficacy of lung aeration and the establishment of effective V/Q match in the first minutes after birth and it seems to be a promising instrument in the DR more to confirm correct endotracheal tube placement or to verify the efficacy of mask ventilation (5). However, it is not that accurate in providing information on arterial pCO2 levels when neonates experience respiratory distress. This condition, peculiar of preterm infants, leads to poor pulmonary perfusion and compromises gas exchange. Therefore, the etCO2 device may underestimate high arterial pCO2 levels. Transcutaneous pCO2 might overcome this limitation and, if combined with etCO2, could contribute to a more reliable estimation of respiratory gas imbalances also in ventilated patients.
Of note, reference ranges for etCO2 in neonates during post-natal transition without resuscitation have been proposed recently (11).
Transcutaneous pCO2 monitoring is used in neonates during NICU care and relies on the fact that CO2 propagates through tissue and can be measured by a skin sensor. However, the correlation between tcpCO2 and blood pCO2 is related to the perfusion of the capillary bed, so heating of the skin sensor is required. However, this may cause skin burns. On the other side, the use of low sensor temperature has been correlated to an overestimation of the pCO2 (12).
In this study, no skin burns or other adverse reactions were observed, mainly due to the short measurement period in the DR, reducing the exposure of neonatal skin to high sensor temperatures.
This study also demonstrated that there was no difference between stable term and preterm neonates in regard to tcpCO2 courses and values during the first 15 min. The inclusion of neonates without need for cardio-respiratory support regardless of gestational age could in part explain this observation, even if these two populations are known to differ significantly in HR and SpO2 values during immediate transition, as also shown by our findings (3).
The present study has some limitations. First, we attributed the initial increase to the equilibration phase of the sensor. The current data set does not allow to differentiate exactly between equilibration of temperature, microcirculation and pCO2 of the tissue at the sensor site and true changes in arterial blood of the neonate. However, according the manufacturer's equilibration time and the observed increase of tcpCO2 to a stable plateau at minute 9 it can be assumed that thereafter tcpCO2 corresponds to pCO2 in the blood. Furthermore, even if this delay in acquisition of data cannot currently be avoided, gathering reliable data within 10 min after birth may still be helpful to guide medical DR interventions. Second, we did not compare tcpCO2 values and those measured from blood samples in the present analyses of neonates without any intervention. Since there is controversial evidence in this regard (13–15), this aspect warrants further investigation.
Conclusion
Non-invasive measurement of tcpCO2 seems to be feasible during immediate neonatal transition, even if measurements in the very first minutes after birth remain challenging. Further research is desirable to confirm our results and to investigate any clinical impact of additional tcpCO2 measurements on DR interventions in neonates with adverse conditions during the transitional period.
Data Availability Statement
The datasets analyzed in this article are not publicly available. Requests to access the datasets should be directed to Gerhard Pichler, gerhard.pichler@medunigraz.at.
Ethics Statement
This study was carried out in accordance with the recommendations of Regional Committee on Biomedical Research Ethics with written informed consent from all subjects. All parents gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Ethikkommission, Medizinische Universität Graz, Austria (EC number: 27-465 ex 14/15).
Author Contributions
IB, GP, and BU: conception and design. IB, MB, CM, NB-S, BS, LM, and GP: collection and assembly of data. IB, MB, CM, NB-S, BS, LM, AA, BU, and GP: analyses and interpretation of data, critical revision, editing, and final approval of the article. IB, AA, and GP: drafting of the article.
Funding
The study was partly funded by the Culture-Department/Science of the City Graz, Austria, which had no influence on design, collection, analyses, interpretation, writing, and submission of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
HR, heart rate; NICU, neonatal intensive care unit; SpO2, arterial oxygen saturation; tcpCO2, transcutaneous monitoring of carbon dioxide partial pressure; DR, delivery room.
References
1. Lista G, Maturana A, Moya FR. Achieving and maintaining lung volume in the preterm infant: from the first breath to the NICU. Eur J Pediatr. (2017) 176:1287–93. doi: 10.1007/s00431-017-2984-y
2. Wyllie J, Bruinenberg J, Roehr CC, Rudiger M, Trevisanuto D, Urlesberger B. European resuscitation council guidelines for resuscitation 2015: section 7. Resuscitation and support of transition of babies at birth. Resuscitation. (2015) 95:249–63. doi: 10.1016/j.resuscitation.2015.07.029
3. Dawson JA, Kamlin CO, Vento M, Wong C, Cole TJ, Donath SM, et al. Defining the reference range for oxygen saturation for infants after birth. Pediatrics. (2010) 125:e1340–7. doi: 10.1542/peds.2009-1510
4. Dawson JA, Kamlin CO, Wong C, te Pas AB, Vento M, Cole TJ, et al. Changes in heart rate in the first minutes after birth. Arch Dis Child Fetal Neonatal Ed. (2010) 95:F177–81. doi: 10.1136/adc.2009.169102
5. Hawkes GA, Kelleher J, Ryan CA, Dempsey EM. A review of carbon dioxide monitoring in preterm newborns in the delivery room. Resuscitation. (2014) 85:1315–9. doi: 10.1016/j.resuscitation.2014.07.012
6. Pryds O, Greisen G, Skov LL, Friis-Hansen B. Carbon dioxide-related changes in cerebral blood volume and cerebral blood flow in mechanically ventilated preterm neonates: comparison of near infrared spectrophotometry and 133Xenon clearance. Pediatr Res. (1990) 27:445–9. doi: 10.1203/00006450-199005000-00006
7. Bruschettini M, Romantsik O, Zappettini S, Ramenghi LA, Calevo MG. Transcutaneous carbon dioxide monitoring for the prevention of neonatal morbidity and mortality. Cochrane Database Syst Rev. (2016) 2:CD011494. doi: 10.1002/14651858.CD011494.pub2
8. Eberhard P. The design, use, and results of transcutaneous carbon dioxide analysis: current and future directions. Anesth Analg. (2007) 105:S48–52. doi: 10.1213/01.ane.0000278642.16117.f8
9. Levene M. Minimising neonatal brain injury: how research in the past 5 years has changed my clinical practice. Arch Dis Child. (2007) 92:261–5. doi: 10.1136/adc.2005.086371
10. van Vonderen JJ, Lista G, Cavigioli F, Hooper SB, te Pas AB. Effectivity of ventilation by measuring expired CO2 and RIP during stabilisation of preterm infants at birth. Arch Dis Child Fetal Neonatal Ed. (2015) 100:F514–8. doi: 10.1136/archdischild-2014-307412
11. Blank DA, Gaertner VD, Kamlin COF, Nyland K, Eckard NO, Dawson JA, et al. Respiratory changes in term infants immediately after birth. Resuscitation. (2018) 130:105–10. doi: 10.1016/j.resuscitation.2018.07.008
12. Sørensen LC, Brage-Andersen L, Greisen G. Effects of the transcutaneous electrode temperature on the accuracy of transcutaneous carbon dioxide tension. Scand J Clin Lab Invest. (2011) 71:548–52. doi: 10.3109/00365513.2011.590601
13. Lambert LL, Baldwin MB, Gonzalez CV, Lowe GR, Willis JR. Accuracy of transcutaneous CO2 values compared with arterial and capillary blood gases. Respir Care. (2018) 63:907–12. doi: 10.4187/respcare.05936
14. Mukhopadhyay S, Maurer R, Puopolo KM. Neonatal transcutaneous carbon dioxide monitoring–effect on clinical management and outcomes. Respir Care. (2016) 61:90–7. doi: 10.4187/respcare.04212
Keywords: transcutaneous, carbon dioxide, neonate, transition, delivery room
Citation: Bresesti I, Bruckner M, Mattersberger C, Baik-Schneditz N, Schwaberger B, Mileder L, Avian A, Urlesberger B and Pichler G (2020) Feasibilty of Transcutaneous pCO2 Monitoring During Immediate Transition After Birth—A Prospective Observational Study. Front. Pediatr. 8:11. doi: 10.3389/fped.2020.00011
Received: 17 September 2019; Accepted: 10 January 2020;
Published: 29 January 2020.
Edited by:
Hans Fuchs, Freiburg University Medical Center, GermanyReviewed by:
Helmut Dietmar Hummler, University of Ulm, GermanyJesper Padkær Petersen, Aarhus University Hospital, Denmark
Copyright © 2020 Bresesti, Bruckner, Mattersberger, Baik-Schneditz, Schwaberger, Mileder, Avian, Urlesberger and Pichler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gerhard Pichler, gerhard.pichler@medunigraz.at
†ORCID: Gerhard Pichler orcid.org/0000-0003-2405-7143
 Ilia Bresesti
Ilia Bresesti Marlies Bruckner
Marlies Bruckner Christian Mattersberger
Christian Mattersberger Nariae Baik-Schneditz
Nariae Baik-Schneditz Bernhard Schwaberger
Bernhard Schwaberger Lukas Mileder
Lukas Mileder Alexander Avian
Alexander Avian Berndt Urlesberger
Berndt Urlesberger Gerhard Pichler
Gerhard Pichler