- 1Department of Pharmacy, The Second Xiangya Hospital of Central South University, Changsha, China
- 2Menzies Institute for Medical Research, University of Tasmania, Hobart, TAS, Australia
- 3Department of Nuclear Medicine/PET Image Center, The Second Xiangya Hospital of Central South University, Changsha, China
Objective To compare the cost-effectiveness of the combination of pembrolizumab and chemotherapy (Pembro+Chemo) versus pembrolizumab monotherapy (Pembro) as the first-line treatment for metastatic non-squamous and squamous non-small-cell lung cancer (NSCLC) with PD-L1expression ≥50%, respectively, from a US health care perspective.
Material and Methods A comprehensive Makrov model were designed to compare the health costs and outcomes associated with first-line Pembro+Chemo and first-line Pembro over a 20-years time horizon. Health states consisted of three main states: progression-free survival (PFS), progressive disease (PD) and death, among which the PFS health state was divided into two substates: PFS while receiving first-line therapy and PFS with discontinued first-line therapy. Two scenario analyses were performed to explore satisfactory long-term survival modeling.
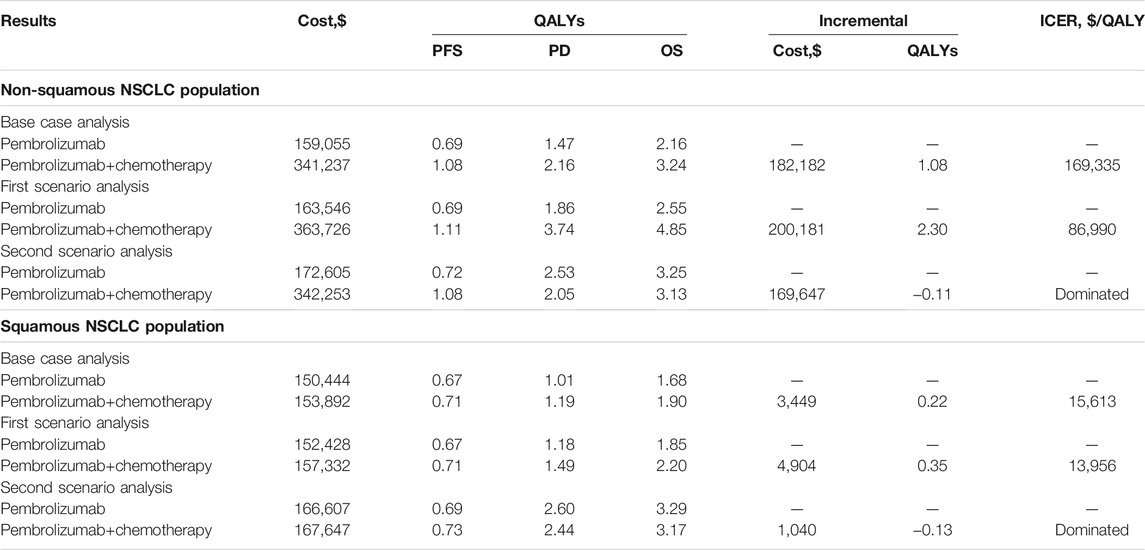
Results In base case analysis, for non-squamous NSCLC patients, Pembro+Chemo was associated with a significantly longer life expectancy [3.24 vs 2.16 quality-adjusted life-years (QALYs)] and a substantially greater healthcare cost ($341,237 vs $159,055) compared with Pembro, resulting in an ICER of $169,335/QALY; for squamous NSCLC patients, Pembro+Chemo was associated with a slightly extended life expectancy of 0.22 QALYs and a marginal incremental cost of $3,449 compared with Pembro, resulting in an ICER of $15,613/QALY. Our results were particularly sensitive to parameters that determine QALYs. The first scenario analysis yielded lower ICERs than our base case results. The second scenario analysis founded Pembro+Chemo was dominated by Pembro.
Conclusion For metastatic non-squamous NSCLC patients with PD-L1 expression ≥50%, first-line Pembro+Chemo was not cost-effective when compared with first-line Pembro. In contrast, for the squamous NSCLC patient population, our results supported the first-line Pembro+Chemo as a cost-effective treatment. Although there are multiple approaches that are used for extrapolating long-term survival, the optimal method has yet to be determined.
Introduction
Lung cancer is the leading cause of cancer-related deaths in the United States and globally, contributing to roughly 25% of cancer-related deaths. Most lung cancers (∼84%) are non-small-cell lung cancer (NSCLC). During the past decade, gene therapies targeting the oncogenic drivers such as sensitizing epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) translocations, has showed a great efficacy on the management of NSCLC (Brahmer et al., 2015; Borghaei et al., 2015; Garon et al., 2015; Herbst et al., 2016; Kazandjian et al., 2016; Reck et al., 2016; Rittmeyer et al., 2017; Barlesi et al., 2018; Gandhi et al., 2018; Paz-Ares et al., 2018; Antonia et al., 2019; Mok et al., 2019; Reck et al., 2021). However, these therapies do not take effect in patients with metastatic NSCLC without driver molecular alterations, who constitutes approximately 80% of the NSCLC cases (Aisner and Marshall, 2012). This has led to a revolution in the treatment paradigm for metastatic NSCLC patients with negative targetable driver alteration. Immune-checkpoint inhibitors (ICIs), either as a monotherapy or in combination with chemotherapies, have become the backbone of the standard of care for this disease (National Comprehensive Cancer Network, 2021). Tumor cell programmed death-ligand 1 (PD-L1), as the most robust predictor of the clinical response to ICIs, is recommended to be tested to guide the selection of treatment strategies for metastatic driver-negative NSCLC (Di Federico et al., 2021; Grant et al., 2021; Pathak et al., 2021). The latest National Comprehensive Cancer Network (NCCN) guidelines for NSCLC recommend replacing traditional chemotherapies with ICIs-containing regimens as the preferred first-line therapies for NSCLC when PD-L1 expresses in at least 50% of tumor cells (National Comprehensive Cancer Network, 2021).
Pembrolizumab, used as the first-line treatment for metastatic driver-negative NSCLC with PD-L1expression ≥50%, is viewed as an important milestone in the era of immunotherapy. Precipitated by the favorable net benefits of pembrolizumab reported in the KEYNOTE-024 trial, and later, the KEYNOTE-042 trial (Reck et al., 2016; Mok et al., 2019; Reck et al., 2021), it becomes the first ICI approved by the U.S. Food and Drug Administration (FDA) used as the first-line therapy for this subset of NSCLC patients (US Food and Drug Administration, 2021). Two years later, results from the KEYNOTE-189 and KEYNOTE-407 trials found that pembrolizumab in combination with chemotherapy resulted in the higher response rate and longer survival than platinum-based chemotherapy among metastatic non-squamous NSCLC patients as well as squamous NSCLC patients, regardless of the level of PD-L1 expression. Based on this evidence, pembrolizumab +chemotherapy is recommended as a standard first-line treatment for the PD-L1-high patient population (Gandhi et al., 2018; Paz-Ares et al., 2018). However, it remains unclear whether the combination therapy is superior to the pembrolizumab monotherapy due to the lack of a decent clinical trial with head-to-head comparisons. This has posed a challenge for oncologists when making treatment decisions.
The American Cancer Society estimates that, there will be about 118, 800 new cases of metastatic NSCLC in the United States (US) in 2021 (American Cancer Society, 2021), and approximately 25–35% of these cases are expected to have high levels of tumor cell PD- L1expression (≥50%) (Sezer et al., 2021), corresponding to nearly 35,640 potential patients. ICIs-containing regimens thus represent as one of the most pressing needs in the oncology therapeutics market. Whether their excellent efficacy outweighs the financial burden they impose is the key to determine the appropriateness for their widespread use, and this emphasizes the need for economic analysis for these approved therapies. Although several US-based studies have evaluated the cost-effectiveness of pembrolizumab or pembrolizumab plus chemotherapy against platinum-based chemotherapy in the first-line treatment for this disease (Huang et al., 2017; She et al., 2019), their studies were not able to answer the comparative cost-effectiveness of using pembrolizumab alone versus using in combination with chemotherapy, and this led to an evidence-practice gap in the real-life practice. To assist in clinical decision-making, the aim of this study was to compare the cost-effectiveness of the combination of pembrolizumab and chemotherapy versus pembrolizumab monotherapy as the first-line treatment for metastatic non-squamous and squamous NSCLC with PD-L1expression ≥50% from a US health care perspective.
Materials and Methods
Overview
Through mathematical modeling using TreeAge Pro software (version 2021, https://www.treeage.com/) and network meta-analysis (NMA) implemented in R software (version 4.0.4, http://www.r-project.org), we compared the cost-effectiveness between first-line pembrolizumab combined chemotherapy (Pembro+Chemo) and pembrolizumab monotherapy (Pembro) indirectly among patients with metastatic non-squamous and squamous NSCLC with PD-L1 of at least 50% from a US health care perspective. This study is exempted from the institutional review board approval because it used only existing data to inform the model. Our study followed the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guideline.
Simulation Model
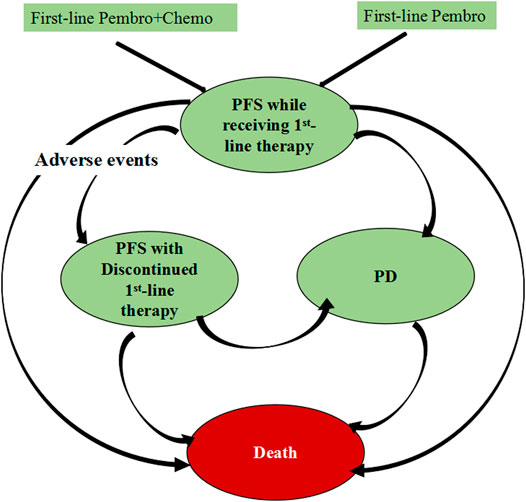
For this economic evaluation, we built a Markov model composed of three main health states: progression-free survival (PFS), progressive disease (PD) and death, in which PFS health state was divided into two sub-health states: progression-free survival (PFS) while receiving first-line therapy and PFS with discontinued first-line therapy (Figure 1). All patients began in the health state of PFS while receiving first-line therapy and were randomized to 2 first-line treatment strategies. Individuals who experienced intolerable toxicity during first-line treatment but did not develop disease progression could enter the health state of PFS with discontinued first-line therapy. Individuals with disease progression would enter the PD health state and receive subsequent anticancer therapy if there is sustained survival benefit, otherwise they will receive best supportive care (BSC). To better reflect the real-world practice, patients were proceeded to palliative care before death. The first-line and subsequent treatment regimens was detailed in Supplementary Table S1.
With a 1-month Markov cycle and a 20-years time horizon, the Markov model was used to project the cumulative costs and quality-adjusted life-years (QALYs) corresponding to each regimen. The incremental cost-effectiveness ratio (ICER) between two competitive regimens was then generated and compared with a willingness-to-pay (WTP) threshold of $100,000 per QALY to determine the cost-effective regimen (Neumann et al., 2014). Costs were reported in 2021 US dollars and an annual discount rate of 3% was applied for both costs and QALYs (Sanders et al., 2016).
Transition Probabilities
The transition probabilities between three main health states were estimated by published extrapolation techniques and standard NMA techniques (Diaby et al., 2014; Guyot et al., 2012). For non-squamous and squamous NSCLC patients treated with first-line Pembro+Chemo, we ascertained overall survival (OS) and PFS rates from the Kaplan-Meier (KM) curves reported in the KEYNOTE-189 and KEYNOTE-407 trials, respectively (Gandhi et al., 2018; Paz-Ares et al., 2018). Then, the log-logistic distributions were selected to fit these recreated individual patient-level data because they yielded the lowest AIC and BIC statistics (Supplementary Table S2; Figure 1). In calculating the transition probabilities for patients receiving first-line Pembro, the hazard ratios (HRs) of first-line Pembro vs Pembro+Chemo generated by implementing NMA for non-squamous and squamous patient populations, respectively, were applied (Reck et al., 2016; Mok et al., 2019; Reck et al., 2021). Transition probabilities between the two PFS sub-health states were calculated using the clinical data regarding the discontinuation of first-line therapy owing to adverse events (AEs) (Supplementary Table S3) (Reck et al., 2016; Reck et al., 2021; Mok et al., 2019; Gandhi et al., 2018; Paz-Ares et al., 2018). Data in KYNOTE-024 trial were preferentially selected for estimating this model parameter due to its longer follow-up period (5 years) compared with the KYNOTE-042 trial (Mok et al., 2019; Reck et al., 2021). Table 1 summarizes parameters used for transition probabilities estimation.
To explore satisfactory survival modeling, our base case analysis elected to use trial-based parametric distributions to project survival for the first 5 years, followed by the survival data from the Surveillance, Epidemiology, and End Results (SEER) database for non-squamous and squamous NSCLC patients (Supplementary Table S4) (National Cancer Institute, 2021). We also performed two scenario analyses based on other alternative methods that were used in previous cost-effectiveness studies (Wan et al., 2019; Watson et al., 2020; Patel et al., 2021). In our first scenario analysis, we applied the parametric extrapolation approach to project long-term survival for both Pembro+Chemo and Pembro arms. In our second scenario analysis, differed from estimating transition probabilities for death based on parametric distributions for the first 5 years and the SEER-observed survivals afterwards in our base case model, we combined an age-matched background mortality rate from US life tables (Supplementary Table S5) with the data regarding fatal treatment-related AEs from each clinical trial (Supplementary Table S3) for such calculations (Arias et al., 2019).
Costs and Health Utility
Regimen related cost, AEs management costs and general treatment costs (including routine follow-up, BSC, and death-associated costs) were considered in our study and collected from a US health care perspective. The prices of first-line drugs were sourced from October 2021 Average Sales Price Drug Pricing Files available at the Centers for Medicare & Medicaid Services (CMS) (Centers for Medicare and Medicaid Services, 2021a). Acquisition of drug administration costs depended on the infusion price retrieved through the CMS Physician Fee Schedule Look-up Tool and the infusion duration requirements for each administration (Centers for Medicare and Medicaid Services, 2021b). For dosage calculation, we modeled the base case patients as having a body surface of 1.79 m2 and a creatinine clearance rate of 70 ml/min (Criss et al., 2019; Zhang et al., 2020), and then rounded to an integral multiple of single-use vial size to account for drug waste (Lien et al., 2016).
Costs for treating grade 3 + AEs with an incidence of at least 1% were considered in the model and were calculated as frequency-weighted averages according to the safety data reported in clinical trials (Reck et al., 2016; Gandhi et al., 2018; Paz-Ares et al., 2018). Each AE was matched to a Clinical Classification Software Refined (CCSR) diagnosis to obtain a corresponding management cost per event from the Healthcare Cost and Utilization Project (HCUP) (Supplementary Table S6) (Agency for Healthcare Research and Quality, 2021). We modeled routine follow-up as a monthly physician visit and a quarterly imaging examination and retrieved these costs from the CMS Physician Fee Schedule (Centers for Medicare and Medicaid Services, 2021b). Costs of subsequent anticancer therapy, BSC, and palliative care were obtained from the literature (Insinga et al., 2018; Criss et al., 2019; Insinga et al., 2019). All costs are outlined in Table 1.
Treatment effectiveness was measured in QALY, which was calculated as a health utilities-weighted life expectancy (overall survival). Considering that cancer patients’ health utilities varied by tumor histology, the health-related quality of life data were collected from the KEYNOTE-189 and KEYNOTE-407 trials for patients with metastatic non-squamous and squamous NSCLC, respectively (Garassino et al., 2020; Mazieres et al., 2020). A time-to-death approach described in previous studies was used to reflect the decline in quality of life as patients approach death (Insinga et al., 2018; Insinga et al., 2019). Utility decrements due to grade III/IV AEs were also considered in our model (Nafees et al., 2017). Details regarding health utilities used in the model are available in the Supplementary Table S6.
Statistical Analysis
This Cost-effectiveness analysis was performed for patients with non-squamous and squamous NSCLC, respectively. We performed one-way deterministic sensitivity analyses (DSA) by varying individual parameters within the plausible ranges to ascertain their role in the ICER. Ranges for each parameter were modeled as the reported 95% confidence intervals (CIs), or within ±50% of the baseline value provided that its 95% CIs was not available. To further evaluate the robustness of model results, we performed a probabilistic sensitivity analysis (PSA) using Monte Carlo simulations with 10,000 iterations to determine the impact of variation in multiple parameters on the ICER. During each Monte Carlo simulation, relevant parameters were random sampled from an appropriate distribution to generate a cost and a QALY estimate. The DAS ranges and parameter distributions used in the PSA were detailed in Table 1.
Results
Incremental Cost-Effectiveness Ratios
The summary results of the base case analysis and scenario analyses are shown in Table 2. In our base case analysis, first-line therapy of Pembro+Chemo in metastatic non-squamous NSCLC patients was associated with a significantly longer life expectancy (3.24 vs 2.16 QALYs) and a substantially greater healthcare cost ($341,237 vs $159,055) compared with first-line Pembro, producing an ICER of $169,335 per QALY above the WTP threshold of $100,000 per QALY. For metastatic squamous NSCLC patients, first-line therapy of Pembro+Chemo was associated with a slightly extended life expectancy of 0.22 QALYs and a marginal incremental cost of $3,449 compared with first-line Pembro, generating an ICER of $15,613 per QALY below the WTP threshold used in the model.
In our first scenario analysis, the increases in QALYs associated with first-line Pembro+Chemo were more significant than the increase in cost, resulting in relatively lower ICERs than our base case results ($86,990/QALY vs $169,335/QALY for non-squamous NSCLC patient and $13,956/QALY vs $15,613/QALY for squamous NSCLC patient, respectively). In our second scenario analysis, first-line Pembro+Chemo was associated with lower QALYs and higher costs when compared with first-line Pembro, resulting in first-line Pembro+Chemo being dominated by first-line Pembro.
Sensitivity Analysis
DSA results of our base case analysis for non-squamous NSCLC patient population showed that only the fluctuations in the OS HR for first-line Pembro+Chemo relative to Pembro, and pemetrexed price/mg had the potential to make first-line Pembro+Chemo cost-effective compared with first-line Pembro. Meanwhile, the lower limit of PFS HR of Pembro+Chemo vs Pembro led to the ICER approaching the WTP threshold of $100,000 per QALY ($102,964 per QALY). Other parameters had minimal effects on our model results (the ICERs ranged between $154,536/QALY and $179,304/QALY). When DSA was performed in squamous NSCLC patient population, the ICERs of first-line Pembro+Chemo vs Pembro remained below the WTP threshold at the lower or upper limits of any tested parameter except for PFS HR for Pembro+Chemo vs Pembro. The tornado diagram in Figure 2 shows the DSA results.
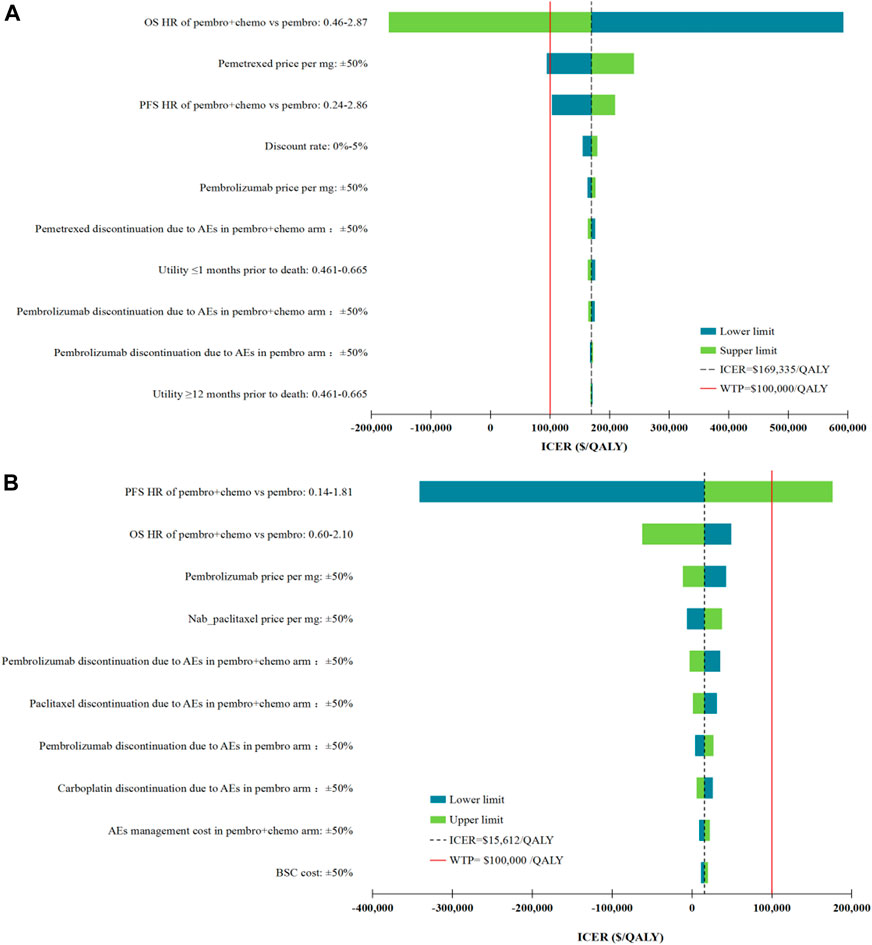
FIGURE 2. Deterministic sensitivity analysis results. (A), the top 10 parameters with the greatest influence on the ICER of first-line Pembro+Chemo vs Pembro in non-squamous NSCLC patient population; (B), the top 10 parameters with the greatest influence on the ICER of first-line Pembro+Chemo vs Pembro in squamous NSCLC patient population. ICER, incremental cost-effectiveness ratios; QALY, quality-adjusted life-years; AEs, adverse events; OS, overall survival; PFS, progression-free survival; HR, hazard ratios; BSC, best supportive care.
The PSA results of the base case revealed that, at a WTP threshold of $100,000 per QALY, the probability of first-line Pembro+Chemo being cost-effective in non-squamous and squamous NSCLC patient populations were 3.6 and 87.4%, respectively (Supplementary Figure S2). When we increased the WTP threshold, an expected increase in the cost-effectiveness probability of first-line Pembro+Chemo was observed.
Discussion
Through mathematical modeling and NMA, we evaluated the cost-effectiveness of first-line Pembro+Chemo relative to first-line Pembro among metastatic NSCLC patients with PD-L1 expression ≥50% from the US health care sector perspective. In our base case analysis, we found that in non-squamous NSCLC patient population, first-line Pembro+Chemo was superior to first-line Pembro in survival, but was associated with an overwhelming healthcare cost. Since the reported ICER ($169,335 per QALY) exceeded the WTP threshold of $100,000 per QALY used in the current study, first-line Pembro+Chemo was not cost-effective compared with first-line Pembro. In contrast, for the squamous NSCLC patient population, our results support the first-line use of Pembro+Chemo as a cost-effective treatment by showing that first-line Pembro+Chemo added a 0.22 QALYs at a marginal incremental cost of $3,449 and the generated ICER ($15,613 per QALY) was far below the WTP threshold.
Among the top 10 most sensitive parameters, the parameters that determine QALY were superior in numbers, including HRs of first-line Pembro+Chemo vs Pembro, first-line drug discontinuation due to AEs and health utilities (Figure 2). Of note, these parameters, as quantitative indicators reflecting the efficacy and safety of cancer treatment, were difficult to be changed through clinical or policy interventions. Apart from these QALY drivers, drug prices also had considerable influences on our cost-effectiveness results, raising concerns regarding the role of drug cost in determining the preferred regimen. Combining this findings with the findings in our previous cost-effectiveness studies (Liu et al., 2020a; Liu et al., 2021), we found that the cost variance between two competing treatments has the most potential to reverse the results of cost-effectiveness analysis. DSA confirmed that among all drugs, the result of non-squamous NSCLC patient population was most affected by the pemetrexed price/mg, because pemetrexed was a supplement to first-line Pembro+Chemo compared with first-line Pembro, with maintenance treatment costs of $ 6,700 per 3-weeks. For squamous NSCLC patient population, the pembrolizumab price/mg ranked the first in the DSA among all drugs, mainly due to the difference in the probabilities of first-line pembrolizumab discontinuation due to AEs.
Since cost-effectiveness evaluation focuses on whether a new treatment can prolong life expectancy (overall survival) at an affordable cost (Ferguson et al., 2000), we pay more attention to the estimation of overall survival in the current study. From the existing literature, parameter fitting method alone or combined with SEER-observed survivals, as well as background mortality rate application technology, are the three mainstream methods to estimate overall survivals in cost-effectiveness research (Criss et al., 2019; Wan et al., 2019; Watson et al., 2020; Zhang et al., 2020; Patel et al., 2021). To better understand the applicability of these methods, two scenario analyses were conducted in the present study. In the first scenario analysis, we found that the best fitting parametric projection increased discounted life expectancy relative to SEER population data. Our previous studies have pointed out that extrapolating long-term survival from the trial-based parameter distribution inevitably suffers from uncertainty (Wan et al., 2019; Liu et al., 2020b). SEER-observed survival data may be more applicable as the data reflect the real-world performance. In the second scenario analysis, when we combined an age-matched background mortality rate with the clinical data of fatal treatment-related AEs to calculate the transition probabilities for death, the results showed that first-line Pembro+Chemo gained lower QALYs than first-line Pembro. However, first-line Pembro+Chemo is generally known to be superior to first-line Pembro in survival (Di Federico et al., 2021; Pathak et al., 2021), so this result is likely to be untenable. Therefore, background mortality rate application technology should be used with caution because it lacks the ability to directly map clinical effects of treatments.
To our knowledge, there are two existing studies from the same authors (Insinga et al.) on the cost-effectiveness of first-line Pembro+Chemo vs first-line Pembro for the US non-squamous and squamous NSCLC patient population, respectively (Insinga et al., 2018; Insinga et al., 2019). The study of non-squamous NSCLC with PD-L1 expression≥50% reported a slightly lower ICER for first-line Pembro+Chemo vs first-line Pembro ($147,365 per QALY vs $169,335 QALY, respectively) (Insinga et al., 2018). The authors cited a WTP threshold of 3 times per capita gross domestic product (GDP) ($180,000/QALY) and concluded that the first-line Pembro+Chemo was cost-effective among this patient population. Another cost-effectiveness study found that first-line Pembro+Chemo was associated with reduced net cost and improved QALYs, and therefore a cost-saving strategy (Insinga et al., 2019). Some inconsistency between our findings and the findings in the above two studies can be explained by the facts that the current model considered the first-line treatment discontinuations due to AEs and the utility decrements due to AEs, and the current study applied NMA to perform the indirect cost-effectiveness comparison between first-line Pembro+Chemo and first-line Pembro while the above studies used Bucher method.
This study has several notable strengths. First, we utilized the most detailed clinical efficacy and safety data regarding first-line Pembro+Chemo and Pembro to describe the cost-effectiveness of these two widely used and controversial treatments for non-squamous and squamous NSCLC patients with PD-L1 expression ≥50%, respectively. Therefore, our analysis has provided valuable evidence for clinicians to make relevant decisions on treatments. Second, we employed three mainstream survival modeling methods to build the cost-effectiveness model and analyzed the corresponding results to judge their applicability to the current research. This study is the first to examine the applicability of the three methods and therefore have implications for survival modeling in future economic evaluation. Third, our model comprehensively considers the impact of AEs, including the first-line treatment discontinuations due to AEs, as well as the incidences, costs and disutility associated with grade III/IV AEs.
This study also has several limitations. First, there is inherent uncertainty in the costs used to populate the model. However, a series of sensitivity analyses found that our results are not particularly sensitive to cost parameters, indicating that including more accurate estimates is unlikely to change our results. Second, we modeled proportions of patients receiving subsequent anticancer therapy based on clinical trial data, which may not reflect the prevalence of subsequent anticancer therapy used in real-world practice. Third, our model did not include traditional chemotherapy because ICI-containing regimens have replaced traditional chemotherapy as the standard first-line therapy for metastatic NSCLC with PD-L1 expression≥50%. Fourth, the treatments analyzed in this trial-based economic assessment may not fully reflect the real-world performance. More evidence from real-life scenarios is needed to be collected to verify our results.
Conclusion
This economic evaluation found that for metastatic non-squamous NSCLC patients with PD-L1expression ≥50%, first-line Pembro+Chemo was not cost-effective when compared with first-line Pembro. In contrast, for the squamous NSCLC patient population, our results support the first-line Pembro+Chemo as a cost-effective treatment. Although there are multiple approaches that are used for extrapolating long-term survival, the optimal method has yet to be determined.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
XZ and CT contributed to the conception, design of the primarily model and interpreted the results. QL and ZZ developed the economic model, performed the analyses and drafted the manuscript. XL and LY collected and reviewed data. QL, LP and XW provided clinical input, validated the model assumptions. All authors read and approved the final manuscript.
Funding
This work was supported by the Hunan Provincial Natural Science Foundation (grant numbers 2019JJ50864); Hunan Provincial Natural Science Foundation (grant numbers 2021JJ80080).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.803626/full#supplementary-material
Supplementary Figure S1 | Model Validation for first-line Pembro+Chemo. NSCLC, non-small-cell lung cancer; OS, overall survival; PFS, progression-free survival.
Supplementary Figure S2 | The probability of first-line Pembro+Chemo being cost-effective against first-line Pembro under different WTP thresholds in the non-squamous and squamous NSCLC patient populations. QALY, quality-adjusted life-year; NSCLC, non-small-cell lung cancer.
References
Agency for Healthcare Research and QualityUS Dept of Health & Human Services (2021). Healthcare Cost and Utilization Project. Available at: https://hcupnet.ahrq.gov (Accessed January 2, 2021).
Aisner, D. L., and Marshall, C. B. (2012). Molecular Pathology of Non-small Cell Lung Cancer: a Practical Guide. Am. J. Clin. Pathol. 138 (3), 332–346. doi:10.1309/AJCPFR12WJKCEEZZ
American Cancer Society (2021). Key Statistics for Lung Cancer. Available at: https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html (Accessed May 06, 2021).
Antonia, S. J., Borghaei, H., Ramalingam, S. S., Horn, L., De Castro Carpeño, J., Pluzanski, A., et al. (2019). Four-year Survival with Nivolumab in Patients with Previously Treated Advanced Non-small-cell Lung Cancer: a Pooled Analysis. Lancet Oncol. 20 (10), 1395–1408. doi:10.1016/S1470-2045(19)30407-3
Arias, E., Rostron, B. L., and Tejada-Vera, B. (2019). United States Life Tables, 2005. Natl. Vital Stat. Rep. 58 (7), 1–132.
Barlesi, F., Vansteenkiste, J., Spigel, D., Ishii, H., Garassino, M., de Marinis, F., et al. (2018). Avelumab versus Docetaxel in Patients with Platinum-Treated Advanced Non-small-cell Lung Cancer (JAVELIN Lung 200): an Open-Label, Randomised, Phase 3 Study. Lancet Oncol. 19 (11), 1468–1479. doi:10.1016/S1470-2045(18)30673-9
Borghaei, H., Paz-Ares, L., Horn, L., Spigel, D. R., Steins, M., Ready, N. E., et al. (2015). Nivolumab versus Docetaxel in Advanced Nonsquamous Non-small-cell Lung Cancer. N. Engl. J. Med. 373 (17), 1627–1639. doi:10.1056/NEJMoa1507643
Brahmer, J., Reckamp, K. L., Baas, P., Crinò, L., Eberhardt, W. E., Poddubskaya, E., et al. (2015). Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-small-cell Lung Cancer. N. Engl. J. Med. 373 (2), 123–135. doi:10.1056/NEJMoa1504627
Centers for Medicare and Medicaid Services (2021a). 2021 ASP Drug Pricing Files. Available at: https://www.cms.gov/medicare/medicare-part-b-drug-average-sales-price/2021-asp-drug-pricing-files (Accessed January 1, 2021).
Centers for Medicare and Medicaid Services (2021b). Physician Fee Schedule Search. Available at: https://www.cms.gov/medicare/physician-fee-schedule/search (Accessed January 8, 2021).
Criss, S. D., Mooradian, M. J., Watson, T. R., Gainor, J. F., Reynolds, K. L., and Kong, C. Y. (2019). Cost-effectiveness of Atezolizumab Combination Therapy for First-Line Treatment of Metastatic Nonsquamous Non-small Cell Lung Cancer in the united states. JAMA Netw. Open 2 (92), e1911952. doi:10.1001/jamanetworkopen.2019.11952
Di Federico, A., De Giglio, A., Parisi, C., Gelsomino, F., and Ardizzoni, A. (2021). PD-1/PD-L1 Inhibitor Monotherapy or in Combination with Chemotherapy as Upfront Treatment for Advanced NSCLC with PD-L1 Expression ≥ 50%: Selecting the Best Strategy. Crit. Rev. Oncol. Hematol. 160, 103302. doi:10.1016/j.critrevonc.2021.103302
Diaby, V., Adunlin, G., and Montero, A. J. (2014). Survival Modeling for the Estimation of Transition Probabilities in Model-Based Economic Evaluations in the Absence of Individual Patient Data: A Tutorial. Pharmacoeconomics 32 (2), 101–108. doi:10.1007/s40273-013-0123-9
Ferguson, J. S., Summerhayes, M., Masters, S., Schey, S., and Smith, I. E. (2000). New Treatments for Advanced Cancer: an Approach to Prioritization. Br. J. Cancer 83 (10), 1268–1273. doi:10.1054/bjoc.2000.1406
Gandhi, L., Rodríguez-Abreu, D., Gadgeel, S., Esteban, E., Felip, E., De Angelis, F., et al. (2018). Pembrolizumab Plus Chemotherapy in Metastatic Non-small-cell Lung Cancer. N. Engl. J. Med. 378 (22), 2078–2092. doi:10.1056/NEJMoa1801005
Garassino, M. C., Gadgeel, S., Esteban, E., Felip, E., Speranza, G., Domine, M., et al. (2020). Patient-reported Outcomes Following Pembrolizumab or Placebo Plus Pemetrexed and Platinum in Patients with Previously Untreated, Metastatic, Non-squamous Non-small-cell Lung Cancer (KEYNOTE-189): a Multicentre, Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Oncol. 21 (3), 387–397. doi:10.1016/S1470-2045(19)30801-0
Garon, E. B., Rizvi, N. A., Hui, R., Leighl, N., Balmanoukian, A. S., Eder, J. P., et al. (2015). Pembrolizumab for the Treatment of Non-small-cell Lung Cancer. N. Engl. J. Med. 372 (21), 2018–2028. doi:10.1056/NEJMoa1501824
Grant, M. J., Herbst, R. S., and Goldberg, S. B. (2021). Selecting the Optimal Immunotherapy Regimen in Driver-Negative Metastatic NSCLC. Nat. Rev. Clin. Oncol. 18 (10), 625–644. doi:10.1038/s41571-021-00520-1
Guyot, P., Ades, A. E., Ouwens, M. J., and Welton, N. J. (2012). Enhanced Secondary Analysis of Survival Data: Reconstructing the Data from Published Kaplan-Meier Survival Curves. BMC Med. Res. Methodol. 12, 9. doi:10.1186/1471-2288-12-9
Herbst, R. S., Baas, P., Kim, D. W., Felip, E., Pérez-Gracia, J. L., Han, J. Y., et al. (2016). Pembrolizumab versus Docetaxel for Previously Treated, PD-L1-Positive, Advanced Non-small-cell Lung Cancer (KEYNOTE-010): a Randomised Controlled Trial. Lancet 387 (10027), 1540–1550. doi:10.1016/S0140-6736(15)01281-7
Huang, M., Lou, Y., Pellissier, J., Burke, T., Liu, F. X., Xu, R., et al. (2017). Cost Effectiveness of Pembrolizumab vs. Standard-Of-Care Chemotherapy as First-Line Treatment for Metastatic NSCLC that Expresses High Levels of PD-L1 in the United States. Pharmacoeconomics 35 (8), 831–844. doi:10.1007/s40273-017-0527-z
Insinga, R. P., Vanness, D. J., Feliciano, J. L., Vandormael, K., Traore, S., and Burke, T. (2018). Cost-effectiveness of Pembrolizumab in Combination with Chemotherapy in the 1st Line Treatment of Non-squamous NSCLC in the US. J. Med. Econ. 21, 1191–1205. doi:10.1080/13696998.2018.1521416
Insinga, R. P., Vanness, D. J., Feliciano, J. L., Vandormael, K., Traore, S., Ejzykowicz, F., et al. (2019). Cost-effectiveness of Pembrolizumab in Combination with Chemotherapy versus Chemotherapy and Pembrolizumab Monotherapy in the First-Line Treatment of Squamous Non-small-cell Lung Cancer in the US. Curr. Med. Res. Opin. 35 (7), 1241–1256. doi:10.1080/03007995.2019.1571297
Kazandjian, D., Suzman, D. L., Blumenthal, G., Mushti, S., He, K., Libeg, M., et al. (2016). FDA Approval Summary: Nivolumab for the Treatment of Metastatic Non-small Cell Lung Cancer with Progression on or after Platinum-Based Chemotherapy. Oncologist 21 (5), 634–642. doi:10.1634/theoncologist.2015-0507
Lien, K., Cheung, M. C., and Chan, K. K. (2016). Adjusting for Drug Wastage in Economic Evaluations of New Therapies for Hematologic Malignancies: a Systematic Review. J. Oncol. Pract. 12 (4), e369–79. doi:10.1200/JOP.2015.005876
Liu, Q., Luo, X., Peng, L., Yi, L., Wan, X., Zeng, X., et al. (2020). Cost-effectiveness Analysis of Adding Ramucirumab to the First-Line Erlotinib Treatment for Untreated EGFR-Mutated Metastatic Non-small Cell Lung Cancer in China. BMJ Open 10 (11), e040691. doi:10.1136/bmjopen-2020-040691
Liu, Q., Luo, X., Peng, L., Yi, L., Wan, X., Zeng, X., et al. (2020). Nivolumab versus Docetaxel for Previously Treated Advanced Non-small Cell Lung Cancer in China: A Cost-Effectiveness Analysis. Clin. Drug Investig. 40 (2), 129–137. doi:10.1007/s40261-019-00869-3
Liu, Q., Luo, X., Yi, L., Zeng, X., and Tan, C. (2021). First-Line Chemo-Immunotherapy for Extensive-Stage Small-Cell Lung Cancer: A United States-Based Cost-Effectiveness Analysis. Front. Oncol. 11, 699781. doi:10.3389/fonc.2021.699781
Mazieres, J., Kowalski, D., Luft, A., Vicente, D., Tafreshi, A., Gümüş, M., et al. (2020). Health-Related Quality of Life with Carboplatin-Paclitaxel or Nab-Paclitaxel with or without Pembrolizumab in Patients with Metastatic Squamous Non-small-cell Lung Cancer. J. Clin. Oncol. 38 (3), 271–280. doi:10.1200/JCO.19.01348
Mok, T. S. K., Wu, Y. L., Kudaba, I., Kowalski, D. M., Cho, B. C., Turna, H. Z., et al. (2019). Pembrolizumab versus Chemotherapy for Previously Untreated, PD-L1-Expressing, Locally Advanced or Metastatic Non-small-cell Lung Cancer (KEYNOTE-042): a Randomised, Open-Label, Controlled, Phase 3 Trial. Lancet 393 (10183), 1819–1830. doi:10.1016/S0140-6736(18)32409-7
Nafees, B., Lloyd, A. J., Dewilde, S., Rajan, N., and Lorenzo, M. (2017). Health State Utilities in Non-small Cell Lung Cancer: An International Study. Asia Pac. J. Clin. Oncol. 13 (5), e195–e203. doi:10.1111/ajco.12477
National Cancer Institute (2021). Surveillance, Epidemiology, and End Results Program. SEER*Stat Software Version 8.3.9. Available at: https://seer.cancer.gov/seerstat/software/ (Accessed June 11, 2021).
National Comprehensive Cancer Network (2021). NCCN Clinical Practice Guidelines in Oncology: Non-small Cell Lung Cancer, Version 5. 2021. Available at: https://www.nccn.org/professionals/physician_gls/default.aspx (Accessed June 25, 2021).
Neumann, P. J., Cohen, J. T., and Weinstein, M. C. (2014). Updating Cost-Effectiveness-Tthe Curious Resilience of the $50,000-Per-QALY Threshold. N. Engl. J. Med. 371 (9), 796–797. doi:10.1056/NEJMp1405158
Patel, K. K., Giri, S., Parker, T. L., Bar, N., Neparidze, N., and Huntington, S. F. (2021). Cost-Effectiveness of First-Line versus Second-Line Use of Daratumumab in Older, Transplant-Ineligible Patients with Multiple Myeloma. Jco 39 (10), 1119–1128. doi:10.1200/jco.20.01849
Pathak, R., De Lima Lopes, G., Yu, H., Aryal, M. R., Ji, W., Frumento, K. S., et al. (2021). Comparative Efficacy of Chemoimmunotherapy versus Immunotherapy for Advanced Non-small Cell Lung Cancer: A Network Meta-Analysis of Randomized Trials. Cancer 127 (5), 709–719. doi:10.1002/cncr.3326910.1002/cncr.33269
Paz-Ares, L., Luft, A., Vicente, D., Tafreshi, A., Gümüş, M., Mazières, J., et al. (2018). Pembrolizumab Plus Chemotherapy for Squamous Non-small-cell Lung Cancer. N. Engl. J. Med. 379 (21), 2040–2051. doi:10.1056/NEJMoa181086510.1056/nejmoa1810865
Reck, M., Rodríguez-Abreu, D., Robinson, A. G., Hui, R., Csőszi, T., Fülöp, A., et al. (2021). Five-Year Outcomes with Pembrolizumab versus Chemotherapy for Metastatic Non-small-cell Lung Cancer with PD-L1 Tumor Proportion Score ≥ 50. J. Clin. Oncol. 39 (21), 2339–2349. doi:10.1200/JCO.21.00174
Reck, M., Rodríguez-Abreu, D., Robinson, A. G., Hui, R., Csőszi, T., Fülöp, A., et al. (2016). Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-small-cell Lung Cancer. N. Engl. J. Med. 375 (19), 1823–1833. doi:10.1056/NEJMoa1606774
Rittmeyer, A., Barlesi, F., Waterkamp, D., Park, K., Ciardiello, F., von Pawel, J., et al. (2017). Atezolizumab versus Docetaxel in Patients with Previously Treated Non-small-cell Lung Cancer (OAK): a Phase 3, Open-Label, Multicentre Randomised Controlled Trial. Lancet 389 (10066), 255–265. doi:10.1016/S0140-6736(16)32517-X
Sanders, G. D., Neumann, P. J., Basu, A., Brock, D. W., Feeny, D., Krahn, M., et al. (2016). Recommendations for Conduct, Methodological Practices, and Reporting of Cost-Effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA 316 (10), 1093–1103. doi:10.1001/jama.2016.12195
Sezer, A., Kilickap, S., Gümüş, M., Bondarenko, I., Özgüroğlu, M., Gogishvili, M., et al. (2021). Cemiplimab Monotherapy for First-Line Treatment of Advanced Non-small-cell Lung Cancer with PD-L1 of at Least 50%: a Multicentre, Open-Label, Global, Phase 3, Randomised, Controlled Trial. Lancet 397 (10274), 592–604. doi:10.1016/S0140-6736(21)00228-2
She, L., Hu, H., Liao, M., Xia, X., Shi, Y., Yao, L., et al. (2019). Cost-effectiveness Analysis of Pembrolizumab versus Chemotherapy as First-Line Treatment in Locally Advanced or Metastatic Non-small Cell Lung Cancer with PD-L1 Tumor Proportion Score 1% or Greater. Lung Cancer 138, 88–94. doi:10.1016/j.lungcan.2019.10.017
US Food and Drug Administration (2021). Pembrolizumab (KEYTRUDA) Checkpoint Inhibitor. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/pembrolizumab-keytruda-checkpoint-inhibitor (Accessed June 26, 2021).
Wan, X., Zhang, Y., Tan, C., Zeng, X., and Peng, L. (2019). First-line Nivolumab Plus Ipilimumab vs Sunitinib for Metastatic Renal Cell Carcinoma: A Cost-Effectiveness Analysis. JAMA Oncol. 5 (4), 491–496. doi:10.1001/jamaoncol.2018.7086
Watson, T. R., Gao, X., Reynolds, K. L., and Kong, C. Y. (2020). Cost-effectiveness of Pembrolizumab Plus Axitinib vs Nivolumab Plus Ipilimumab as First-Line Treatment of Advanced Renal Cell Carcinoma in the US. JAMA Netw. Open 3 (10), e2016144. doi:10.1001/jamanetworkopen.2020.16144
Keywords: NSCLC, PD-L1, cost-effectiveness, pembrolizumab, squamous, non-squamous
Citation: Liu Q, Zhou Z, Luo X, Yi L, Peng L, Wan X, Tan C and Zeng X (2022) Cost-Effectiveness of Pembrolizumab Plus Chemotherapy Versus Pembrolizumab Monotherapy in Metastatic Non-Squamous and Squamous NSCLC Patients With PD-L1 Expression ≥ 50%. Front. Pharmacol. 12:803626. doi: 10.3389/fphar.2021.803626
Received: 28 October 2021; Accepted: 13 December 2021;
Published: 10 January 2022.
Edited by:
Jean Paul Deslypere, Aesculape CRO, BelgiumReviewed by:
Brian Godman, University of Strathclyde, United KingdomAntonio Molino, University of Naples Federico II, Italy
Copyright © 2022 Liu, Zhou, Luo, Yi, Peng, Wan, Tan and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xia Luo, luoxia@csu.edu.cn; Xiaohui Zeng, zengxiaohui2008@csu.edu.cn
 Qiao Liu
Qiao Liu Zhen Zhou
Zhen Zhou Xia Luo
Xia Luo Lidan Yi
Lidan Yi Liubao Peng1
Liubao Peng1 Xiaomin Wan
Xiaomin Wan Chongqing Tan
Chongqing Tan Xiaohui Zeng
Xiaohui Zeng