- 1Heilongjiang Province Key Laboratory of Research on Anesthesiology and Critical Care Medicine, Harbin, China
- 2The Key Laboratory of Myocardial Ischemia Organization, Chinese Ministry of Education, Harbin, China
- 3Department of Anesthesiology, The Second Affiliated Hospital of Harbin Medical University, Harbin, China
- 4Department of Anesthesiology, The First Affiliated Hospital of Harbin Medical University, Harbin, China
Background: Ferroptosis has a vital role in sepsis, but the mechanism is not known. Understanding the mechanism of ferroptosis during sepsis will aid in developing improved therapeutic strategies.
Methods: We used the Gene Expression Omnibus database and FerrDb database to obtain ferroptosis-related differentially expressed genes (DEGs) between sepsis patients and healthy volunteers (HVs). Analyses of PPI networks, functional enrichment, as well as use of the MCODE algorithm were used to identify key ferroptosis-related DEGs. Expression of key ferroptosis-related DEGs was verified using: GSE57065 and GSE65682 datasets; rats in which ferroptosis was induced with erastin; sepsis-induced acute lung injury (siALI) rats. The effects of acupoint catgut embedding (ACE) on ferroptosis and expression of key ferroptosis-related DEGs in the lungs of siALI rats were also observed. A Cox proportional hazard model was used to verify the effect of key ferroptosis-related DEGs on the survival of sepsis patients. Cytoscape was used to construct ceRNA networks and gene–transcription factor networks.
Results: Between sepsis patients and HVs, we identified 33 ferroptosis-related DEGs. According to analyses of PPI networks and the MCODE algorithm, we obtained four modules, of which the most significant module contained nine ferroptosis-related DEGs. Functional-enrichment analyses showed that four of the nine DEGs were enriched in the MAPK signaling pathway: MAPK14, VEGFA, TGFBR1, and DUSP1. We verified expression of these four genes in GSE57065 and GSE65682 datasets and ferroptosis rats. In addition, expression of these four genes and that of the oxidative-stress indicators GSSG and MDA was upregulated, and glutathione peroxidase-4 (GPX4) expression was downregulated, in siALI rats, but ACE reversed these changes. The Cox proportional hazard model showed that survival of sepsis patients in the high-risk group was shorter than that in the low-risk group. We found that the XIST−hsa-let-7b-5p−TGFBR1/DUSP1 ceRNA network and transcription factor E2F1 may be important regulators of these four DEGs.
Conclusion: Our results suggest that MAPK14, VEGFA, TGFBR1, and DUSP1 may be key regulatory targets of ferroptosis in sepsis, and that ACE pretreatment may be antioxidant treatment for sepsis and alleviate ferroptosis. These findings provide a basis for further ferroptosis-related study in sepsis and provide new targets for its treatment.
Introduction
Sepsis has a global mortality rate of 20%, affects 49 million patients, and causes 11 million deaths per year: it is a significant healthcare problem worldwide (Rudd et al., 2020). However, how sepsis occurs is very complex, and has not been elucidated fully (Tian et al., 2019). Programmed cell death plays an essential part in sepsis and organ dysfunction, and is an essential target for its intervention, including ferroptosis, pyroptosis, and apoptosis (Li N. et al., 2020). Among them, ferroptosis is a new form of programmed cell death and is one of the leading causes of organ dysfunction due to sepsis (Li J. et al., 2021), which has attracted a great deal of interest among researchers.
Ferroptosis is one of the main pathogenic mechanisms of ischemia–reperfusion injury, degenerative diseases, and carcinogenesis (Chen et al., 2020). It is characterized by iron-dependent lipid peroxidation and, in addition, by a decrease in the expression of the antioxidant systems glutathione and glutathione peroxidase 4 (GPX4) (Imai et al., 2017). The decrease in GPX4 activity leads to oxidative cell death. In recent years, ferroptosis has been found to play an important role in the multi-organ dysfunction caused by sepsis, and inhibition of ferroptosis can alleviate acute injury to the kidney (Mishima et al., 2020), lung (Li J. et al., 2021), and liver (Wei et al., 2020). However, the specific molecular mechanisms of ferroptosis in sepsis and the resulting organ dysfunction are unknown.
Bioinformatics analysis (BA) has been used to explore the pathogenesis of diseases (Goh, 2018), however, there are no bioinformatics-based studies on the mechanism of ferroptosis occurrence in sepsis. By analyzing the dataset from the GEO database and intersecting it with the FerrDB database, we identified ferroptosis-related differentially expressed genes (DEGs) in sepsis patients. Since the new definition of sepsis places more emphasis on organ dysfunction caused by infection, and the lung is often the most susceptible organ to be involved in the early stage of sepsis (Heming et al., 2021), we used cecum ligation puncture (CLP) to construct sepsis-induced acute lung injury (siALI) animal models to verify our results obtained by bioinformatics analysis. Acupuncture is one of the most important therapeutic tools in treating various inflammatory diseases in traditional Chinese medicine (Oh and Kim, 2021). Acupoint catgut embedding (ACE) is an extension of acupuncture with a more sustained therapeutic effect, and studies have shown ACE to inhibit inflammation-based pain by inhibiting the MAPK signaling pathway (Du et al., 2017). Based on the above theory, this study explored the effect of ACE on ferroptosis in sepsis. In addition, we constructed a Cox proportional hazard model using target genes to observe its effect on the survival of sepsis patients. Finally, a gene−microRNA−long non-coding RNA (gene−miRNA−lncRNA) network was constructed and transcription factors (TFs) were predicted based on key ferroptosis-related DEGs. Our results could aid understanding of ferroptosis in sepsis and broaden treatment options for sepsis.
Materials and methods
Microarray data
We visited the Gene Expression Omnibus (GEO) database (www.ncbi.nlm.nih.gov/geo/) and obtained the datasets GSE95233, GSE57065, and GSE65682. Fifty-one patients with sepsis and 22 healthy volunteers (HVs) were in GSE95233. GSE57065 comprised 28 patients with sepsis and 25 HVs. GSE65682 comprised 760 patients with sepsis and 42 HVs. GSE95233 was the analysis dataset; GSE57065 and GSE65682 were the validation datasets. In addition, GSE65682 was the clinical-data source for sepsis patients in the present study for survival analyses.
Normalization of microarray data
After obtaining the data, the data were corrected and normalized using R software. The probes corresponding to multiple molecules were removed, and when probes corresponding to the same molecule were encountered, only the probe with the highest signal value was retained.
Identification of ferroptosis-related differentially expressed genes
We used GEO2R (www.ncbi.nlm.nih.gov/geo/geo2r/; 3 January 2022) to compare the gene-expression data between the sepsis patients and HVs of GSE95233 with the screening criteria of DEGs of |log2 (fold-change)| >1 and padjusted < 0.05. Ultimately, the ferroptosis-related genes obtained from FerrDb (www.zhounan.org/ferrdb/index.html/) were intersected with the DEGs of GSE95233 to identify ferroptosis-related DEGs.
Analyses of functional enrichment
The packages clusterProfiler (statistical analysis and visualization) and org. Hs.eg.db (ID conversion) were loaded using R in order to perform Genome-Ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of ferroptosis-related DEGs (significant as p < 0.05).
Protein–protein interaction networks
To obtain PPI networks for ferroptosis-related DEGs, we uploaded ferroptosis-related DEGs to the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database (www.string-db.org/) for prediction of PPI networks (interaction score >0.4). We used Cytoscape v.3.7.2 and the MCODE plug-in to undertake clustering analyses of ferroptosis-related DEGs.
Dataset validation of ferroptosis-related differentially expressed genes
First, we downloaded the GPL570 and GPL13667 gene-annotation files and used Perl scripts to annotate the raw data of GSE57065 and GSE65682 to obtain the gene-expression matrix of these two datasets. We analyzed expression of the key ferroptosis-related DEGs in GSE57065 and GSE65682 to verify the accuracy of differences in target-gene expression.
Animals and grouping
The animal study was reviewed and approved by the Animal Care and Use Committee of the Second Affiliated Hospital of Harbin Medical University (Approval No. SYDW 2021-087, Nangang, China). Male adult Sprague–Dawley rats (8–9 weeks) were purchased from the Experimentation Center of the Second Affiliated Hospital of Harbin Medical University. Rats were assigned randomly to three groups: sham, cecum ligation puncture (CLP), and CLP + ACE.
Surgical procedure for rats with sepsis-induced acute lung injury
We used CLP to construct a model of siALI to verify BA results. An siALI model in rats was constructed in accordance with previous studies (Rittirsch et al., 2009). In brief, isoflurane was used to anesthetize rats. Using a 5-0 surgical suture to ligate the cecum. An 18-G puncture needle was employed to penetrate the midpoint of the ligated cecum. We squeezed out an appropriate amount of intestinal contents to ensure the patency of the penetration site, and returned the cecum to the abdominal cavity. In the sham group, the cecum is not ligated and punctured and sent back to the abdominal cavity, and the remaining procedures were identical to those undertaken in the CLP group.
Acupoint catgut embedding
In reference to the Rat Acupoint Atlas, we selected the bilateral Feishu acupoints (BL13: 3-mm adjacent to the third thoracic vertebra on the back) combined with the bilateral Zusanli acupoints (ST36; 5-mm adjacent to the anterior tibial tuberosity) as treatment acupoints. ACE was undertaken 24-h before CLP. Rats in the sham group and CLP group received the same level of anesthesia and puncture of the same site, but without catgut embedding.
Erastin administration
To revalidate the correlation between key ferroptosis-related DEGs and ferroptosis, an animal model of ferroptosis was constructed using the classical ferroptosis inducer erastin with reference to previous studies (Zhao et al., 2021). This was done by randomly dividing the rats into two groups: Solvent group (n = 6) and erastin group (n = 6). The rats were injected intraperitoneally with 25 mg/kg of Erastin (MCE) or solvent (5% DMSO + 40% PEG400 + 5% Tween-80 + 50% saline) at 12-h intervals for 2 days.
ELISA analysis
Blood was collected from rats via the abdominal aorta 24 h after CLP, and samples were stored at −20°C until use (n = 6 per group). Serum levels of TNF-α and IL-6 were determined using the respective ELISA kits (Jingkang, Beijing, China).
Wet/dry weight ratios
The exudate from the superficial layer of the lower lobe of the right lung of rats were blotted out. We weighed them to obtain the wet weight (W), followed by baking in an oven at 70°C for 48 h. Then, they were weighed to obtain the dry weight (D). Next, the W/D ratio was calculated, which we used to evaluate the degree of pulmonary edema (n = 6 per group).
Histology
The right middle lobe of the rat lung was isolated and immersed in paraformaldehyde for 24 h. Subsequently, we stained lung tissue using hematoxylin & eosin (H&E). Light microscopy was employed to observe histopathological changes in the lungs by two researchers blinded to the study protocol. Lung tissue damage was scored with reference to the scoring method of the previous study (Matute-Bello et al., 2011). (A) neutrophil aggregation in alveolar lumina; (B) neutrophil infiltration in the interstitial lung; (C) formation of hyaline membranes; (D) formation of protein fragments in alveolar lumina; (E) thickening of the alveolar wall. The final score of the pathological section was calculated according to the formula
Western blotting
Rats were executed 24 h after CLP. The right lobe of the lung was taken for testing (n = 6 per group). Protein was extracted using a mixture of RIPA lysis buffer. Primary antibodies against GPX4 (catalog number: ab125066; Abcam, Cambridge, UK), β-actin (AC026; ABclonal, Wuhan, China) and anti-rabbit secondary antibody (Ab6721; Abcam) were used.
Malondialdehyde detection
A lipid peroxidation malondialdehyde assay kit (Beyotime, Shanghai, China) was used to measure malondialdehyde content in lungs following manufacturer instructions. Absorbance measurements were taken at 532 nm (n = 5 per group).
Detection of glutathione disulfide
Oxidized GSSG is a common marker for measuring levels of oxidative stress. The GSSG level in lungs was quantified using a detection kit (S0053; Beyotime Institute of Biotechnology). Absorbance measurements were taken at 412 nm (n = 5 per group).
qRT-PCR
TRIzol™ Reagent (Invitrogen, United States) was used to extract RNA from the lung tissues (n = 5 per group). Complementary-DNA was synthesized from 1 μg of total RNA (Toyobo, Osaka, Japan) on an S1000 system (Bio-Rad Laboratories). Quantitative RT-PCR was performed using AceQ Universal SYBR qPCR Master Mix (Q511-02/03, Vazyme, Nanjing, China). The messenger (m) RNA level was standardized to that of endogenous glyceraldehyde 3-phosphate dehydrogenase. Expression of target genes was analyzed by the 2−△△ct method. The primer sequences for rat samples were:
Mapk14-F:5′- GATGCCAAGCCATGAGGCAA.
Mapk14-R:5′- GGGTCGTGGTACTGAGCAAA.
Vegfa-F:5′- ACTCATCAGCCAGGGAGTCT.
Vegfa-R:5′- GGGAGTGAAGGAGCAACCTC.
Tgfbr1-F:5′- ACTCCCAACTACAGAAAAGCA.
Tgfbr1-R:5′- AAGGGCGATCTAGTGAGGGA.
Dusp1-F:5′- ATATCGTGCCGAACACCGAA.
Dusp1-R:5′- ACGCTTCATATCCTCCTTGG.
Gapdh-F:5′- GTCGGTGTGAACGGATTT.
Gapdh-R:5′- ACTCCACGACGTACTCAGC.
Construction and validation of cox proportional hazard model
Considering the importance of the key ferroptosis-related DEGs, the Cox proportional hazard model was constructed. The GSE65682 dataset was first screened to remove samples with missing prognostic data, and combined with normalized gene expression data, 479 samples were finally obtained for subsequent analysis. The 479 samples were randomly grouped in a 1:1 ratio into the training group (n = 240) and the test group (n = 239). The “survival” package and “survminer” package were loaded in the R software, and the Cox proportional hazard model was constructed in the training group to score the risk of sepsis patients.
Survival analyses
Patients were classified into high-risk and low-risk groups based on the median risk score. Kaplan-Meier analysis was used to explore the prognostic significance of the ferroptosis-related gene signature on sepsis patients. The log-rank test was employed to analyze the difference in survival probability between the two groups.
Construction of the ceRNA network and the target gene-transcription factor network
We utilized miRDB, miRmap, miRWalk, miRTarBase, RNA22, and TargetScan databases to predict miRNAs that could regulate key ferroptosis-related DEGs. Prediction of a miRNA for each target referred to the prediction results of ≥4 databases. After obtaining the predicted miRNAs, prediction of lncRNAs was done on the selected miRNAs using starBase (https://starbase.sysu.edu.cn/; mammalian, human h19 genome, strict stringency (≥5) of CLIP-Data and with/without degradome data). We used NetworkAnalyst (www.networkanalyst.ca/), referring to the JASPAR database, to predict TFs that could regulate key ferroptosis-related DEGs.
Statistical analyses
Statistical analyses were done using SPSS 25.0 (IBM, Armonk, NY, United States), Prism 6.01 (GraphPad, San Diego, CA, United States), and R. Data were displayed as mean ± SD. The Student’s t-test was used for comparison between two groups. One-way ANOVA was employed for comparison between multiple groups, and the post hoc multiple comparisons least significant difference and Student–Newman–Keuls model were selected for analyses, p < 0.05 was considered significant.
Results
Ferroptosis-related differentially expressed genes
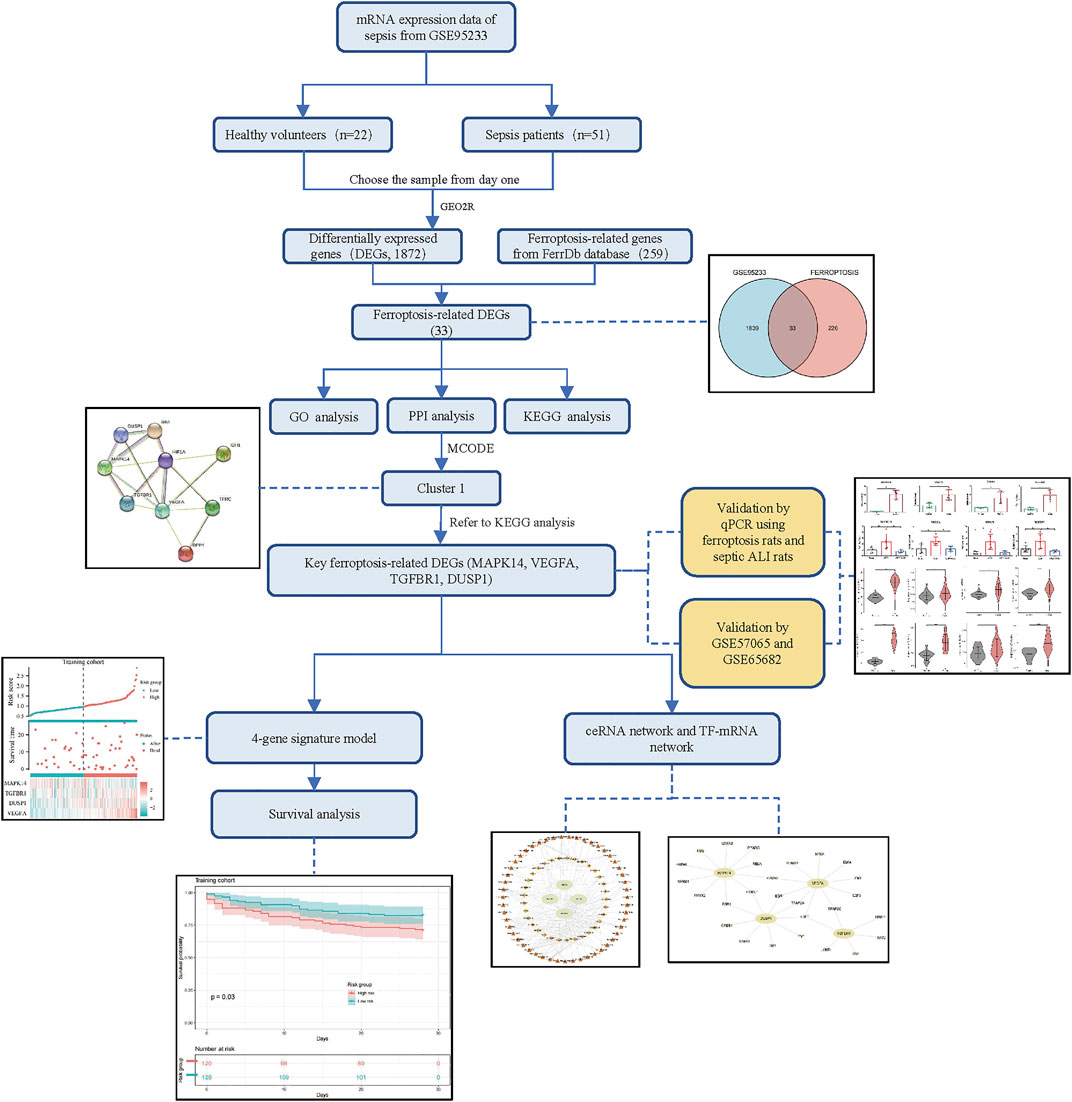
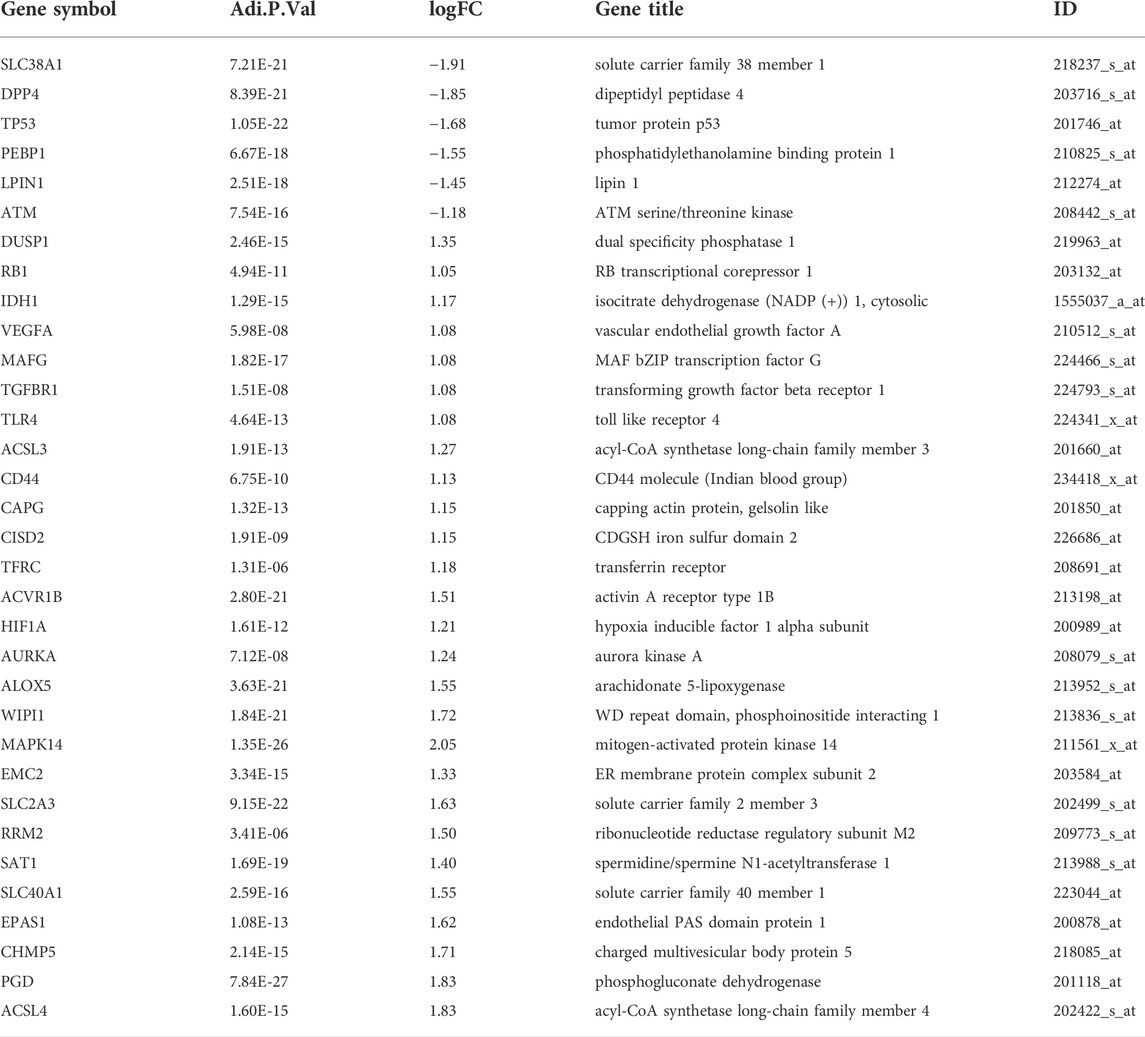
The flowchart of our study is displayed as Figure 1. By analyzing GSE95233 using GEO2R, we obtained 1872 DEGs: expression of 1070 genes was upregulated and expression of 802 genes was downregulated (Figures 2A,B). We identified 259 ferroptosis-related genes from FerrDb, which intersected with 1872 DEGs and, finally, we obtained 33 ferroptosis-related DEGs (Figure 2C): 27 showed upregulated expression and 6 showed downregulated expression (Table 1).
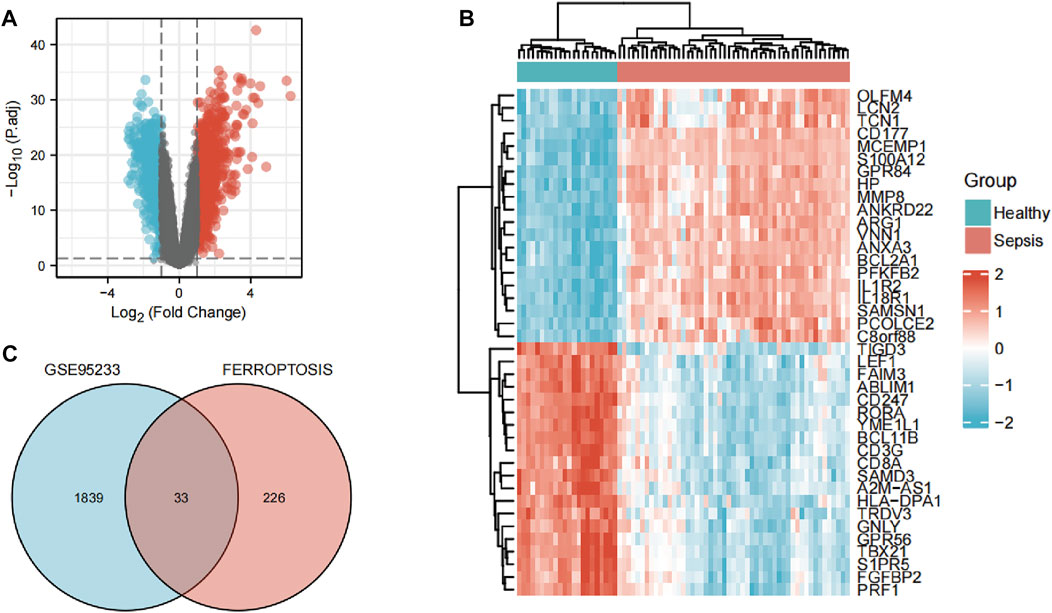
FIGURE 2. Identification of ferroptosis-related differentially expressed genes (DEGs). (A) Volcano plots of DEGs. (B) Heatmap of DEGs identified in GSE95233. (C) Venn diagram of ferroptosis-related DEGs.
Enrichment analyses
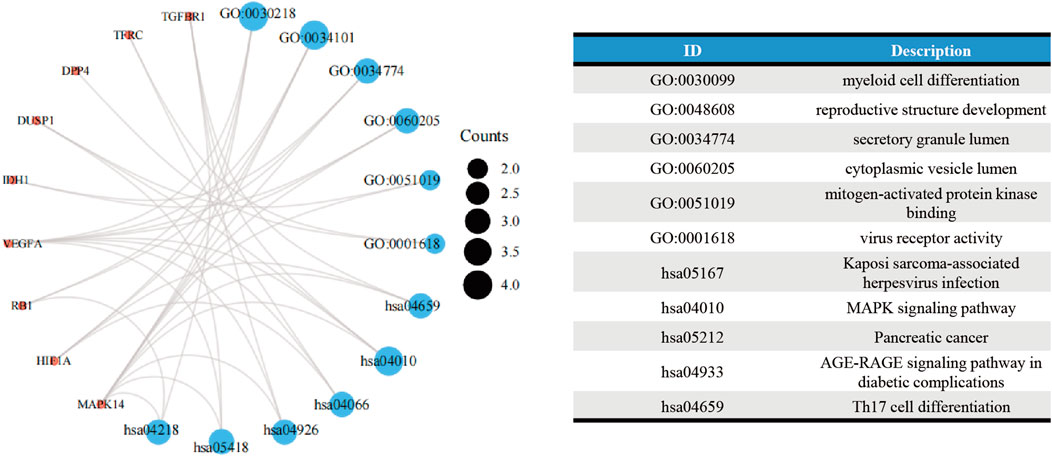
The GO analysis showed the most significant enrichment terms to be “myeloid cell differentiation”, spindle and “protein serine/threonine kinase activity” individually for biological processes, cell component, and molecular function (Figure 3A). The KEGG analysis showed that “MAPK signaling pathway” was one of the major enrichment pathways for ferroptosis-related DEGs (Figure 3B).
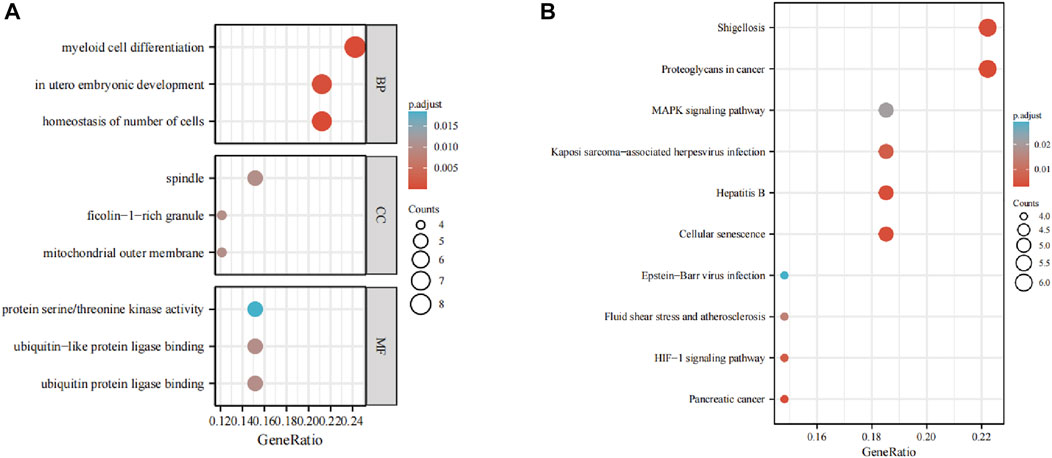
FIGURE 3. Enrichment analyses of (A) function of differentially expressed genes (DEGs) using the Gene Ontology database and (B) signaling pathways of DEGs using the Kyoto Encyclopedia of Genes and Genomes database.
Analyses of PPI networks for ferroptosis-related differentially expressed genes
According to the STRING database and Cytoscape, we obtained a PPI network comprising 33 nodes and 71 edges (Figure 4A). We used MCODE to identify key modules. We obtained four modules in this PPI network, in which the highest-scoring gene clusters were eight genes with upregulated expression (MAPK14, TGFBR1, VEGFA, DUSP1, RB1, HIF1A, TFRC, IDH1) and one gene with downregulated expression (DPP4) (Figure 4B). Based on functional-enrichment analysis of the cluster1 gene, we found four genes to be enriched in the MAPK signaling pathway. Considering the importance of this pathway for sepsis and ferroptosis, we chose these key ferroptosis-related DEGs for subsequent analyses: MAPK14, VEGFA, TGFBR1, and DUSP1 (Figure 5).
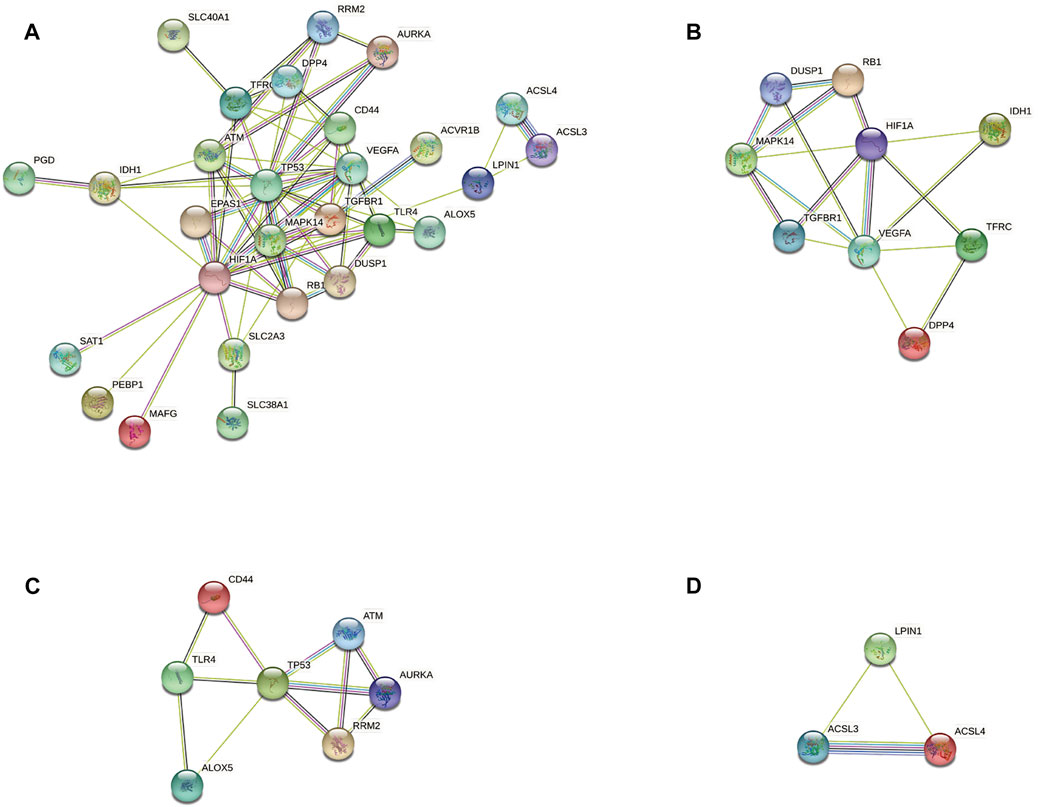
FIGURE 4. Protein–protein interaction network analyses of ferroptosis-related differentially expressed genes (DEGs). (A) PPI network of ferroptosis-related DEGs. (B) Cluster 1. (C) Cluster 2. (D) Cluster 3.
Verification of the key ferroptosis-related differentially expressed genes
To verify key ferroptosis-related DEGs, we selected GSE57065 and GSE65682 as validation datasets. mRNA expression of the four key ferroptosis-related DEGs was significantly higher in sepsis patients than that in HVs in GSE57065 and GSE65682 (Figure 6). Meanwhile, qRT-PCR results in erastin-induced ferroptosis animal models showed that these four key ferroptosis-related DEGs were highly expressed in the Erastin group compared to the Solvent group (Supplementary Figure S1).
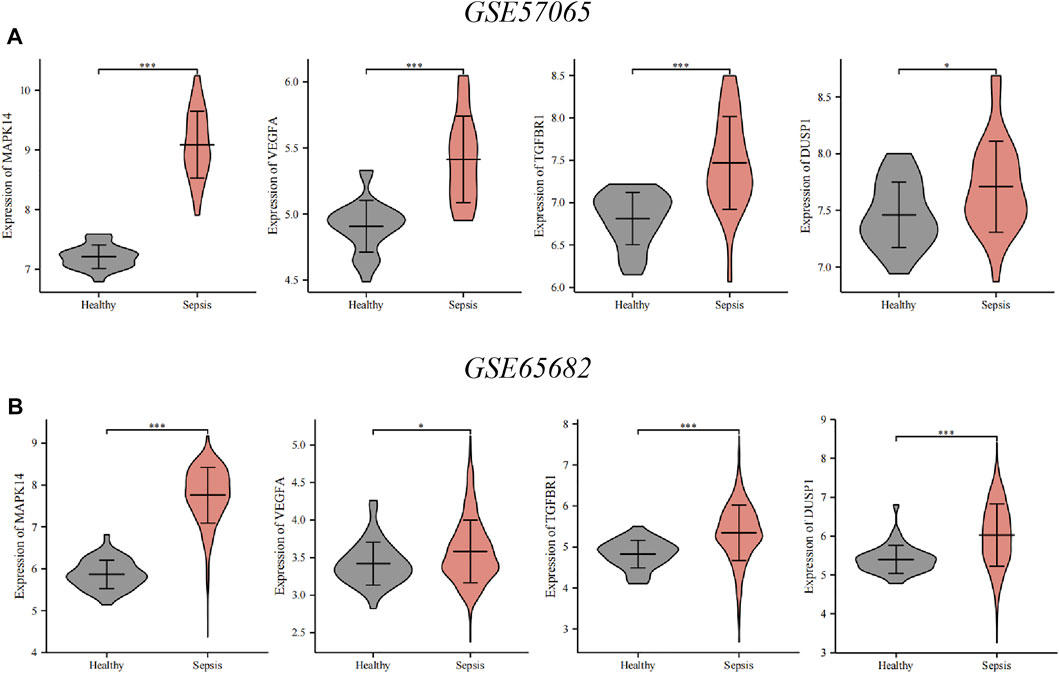
FIGURE 6. Verification of the key ferroptosis-related differentially expressed genes (DEGs) using datasets from the GEO database. (A) Verification by the GSE57065 dataset. Compared with healthy volunteers, expression of all key ferroptosis-related DEGs was upregulated in sepsis patients. (B) Verification by the GSE65682 dataset. Compared with healthy volunteers, expression of all key ferroptosis-related DEGs was upregulated in sepsis patients. ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05, ns, no significant difference.
Effect of acupoint catgut embedding on sepsis-induced acute lung injury rats
As illustrated in Figures 7A,B, ACE enhanced survival within 5 days in siALI rats. The TNF-α and IL-6 were decreased under ACE treatment (Figures 7C,D). We calculated the W/D ratio and observed the pathological changes in lungs. The W/D ratio in the CLP group was significantly increased, whereas that in the ACE group was decreased (Figure 7E). The lung tissues of rats in the sham group had a normal structure. The lung tissues of rats in the CLP group were severely damaged with many inflammatory cells infiltrating the alveolar septum (and widening it), and alveolar and septal hemorrhage and higher-lung injury scores were observed. The above-mentioned manifestations were reduced significantly in ACE-group rats (Figures 7F,G). These results suggested that ACE could improve the severity of lung injury in siALI rats.
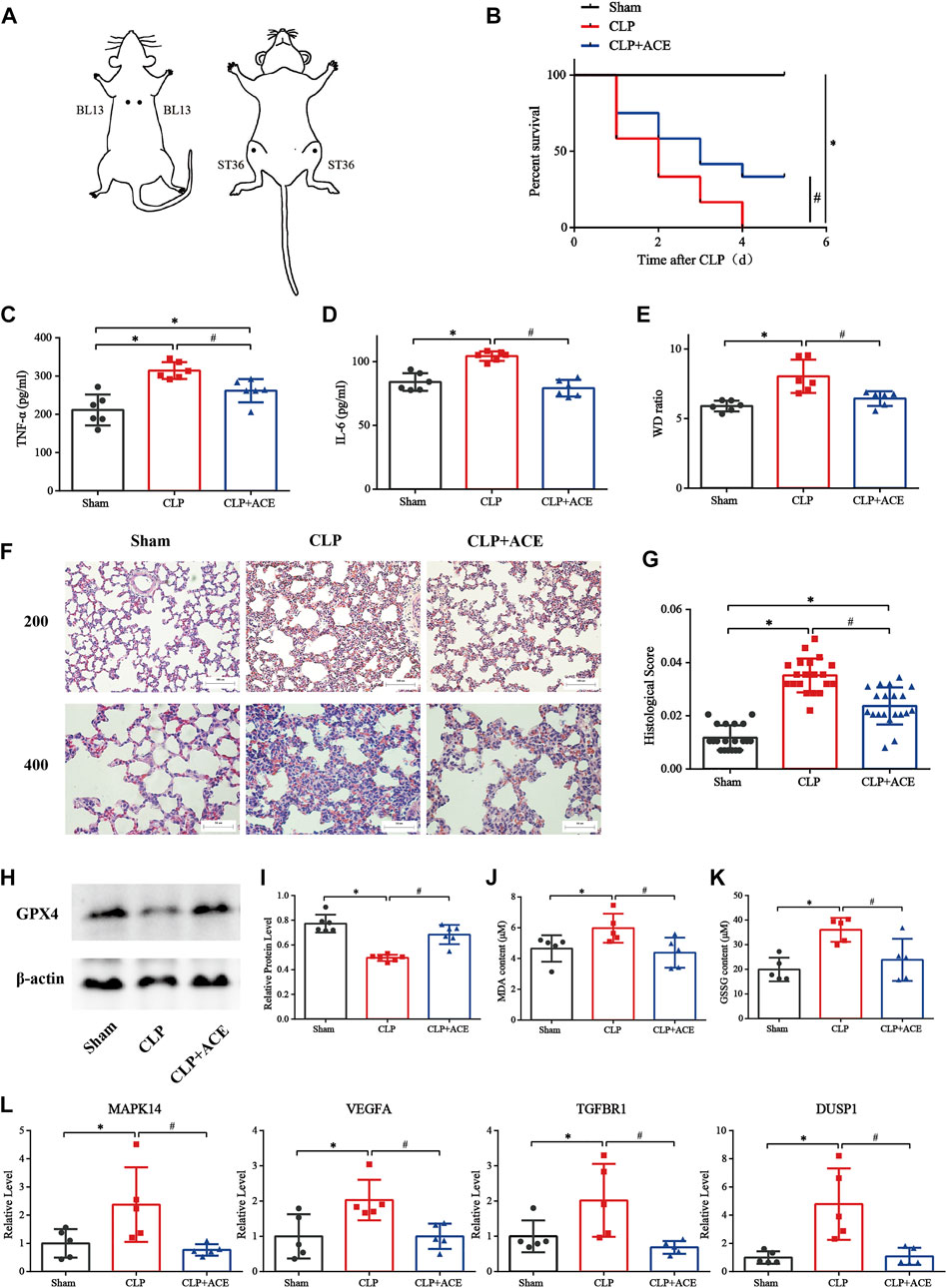
FIGURE 7. Effect of acupoint catgut embedding (ACE) on sepsis-induced acute lung injury (siALI) rats and ferroptosis. (A) Acupoint location (schematic). (B) ACE increased the 5-day survival of siALI rats (n = 12 per group). (C) TNF-α level in serum. (D) IL-6 level in serum. (E) W/D ratio in lungs. (F) H&E staining of pulmonary sections: scale bar is 50 µm or 100 µm. (G) Lung-injury scores. (H,I) Western blotting shows that ACE upregulated of GPX4 expression in the lungs of siALI rats. (J,K) Levels of GSSG and MDA were measured using assay kits. (L) qRT-PCR shows that expression of MAPK14, VEGFA, TGFBR1, and DUSP1 was higher in siALI rats than that in the sham group. After ACE, expression of these four genes was downregulated. Data are the mean ± SD (n = 5 or 6 per group). ∗p < 0.05 vs. sham group, #p < 0.05 vs. CLP group.
Effect of acupoint catgut embedding on ferroptosis
The expression of GPX4 in the lung tissues of siALI rats was reduced significantly, whereas it was increased in the ACE group compared with CLP group. (Figures 7H,I). The levels of MDA and GSSG were increased in siALI rats and decreased in the ACE group (Figures 7J,K). In addition, the mRNA expression of MAPK14, VEGFA, TGFBR1, and DUSP1 was significantly increased in the lungs of rats in the CLP group, while the expression of these genes was downregulated in the ACE group (Figure 7L). These results were consistent with BA trends. Taken together, these results suggested that the administration of ACE could alleviate ferroptosis in siALI rats, and validated the accuracy and importance of BA results.
Construction of prognostic signature and survival analysis
We wished to ascertain if the four key ferroptosis-related DEGs could influence the prognosis of sepsis patients. We constructed a Cox proportional hazard model for risk scoring using these four key ferroptosis-related DEGs with data from training group. Then, we divided patients into high-risk and low-risk groups (Figures 8A,C) and observed the survival rate both in training group and test group. The survival rate of sepsis patients in the high-risk group was reduced within 28 days compared with that of sepsis patients in the low-risk group (Figures 8B,D). This result suggested that these four key ferroptosis-related DEGs may influence the prognosis of sepsis patients.
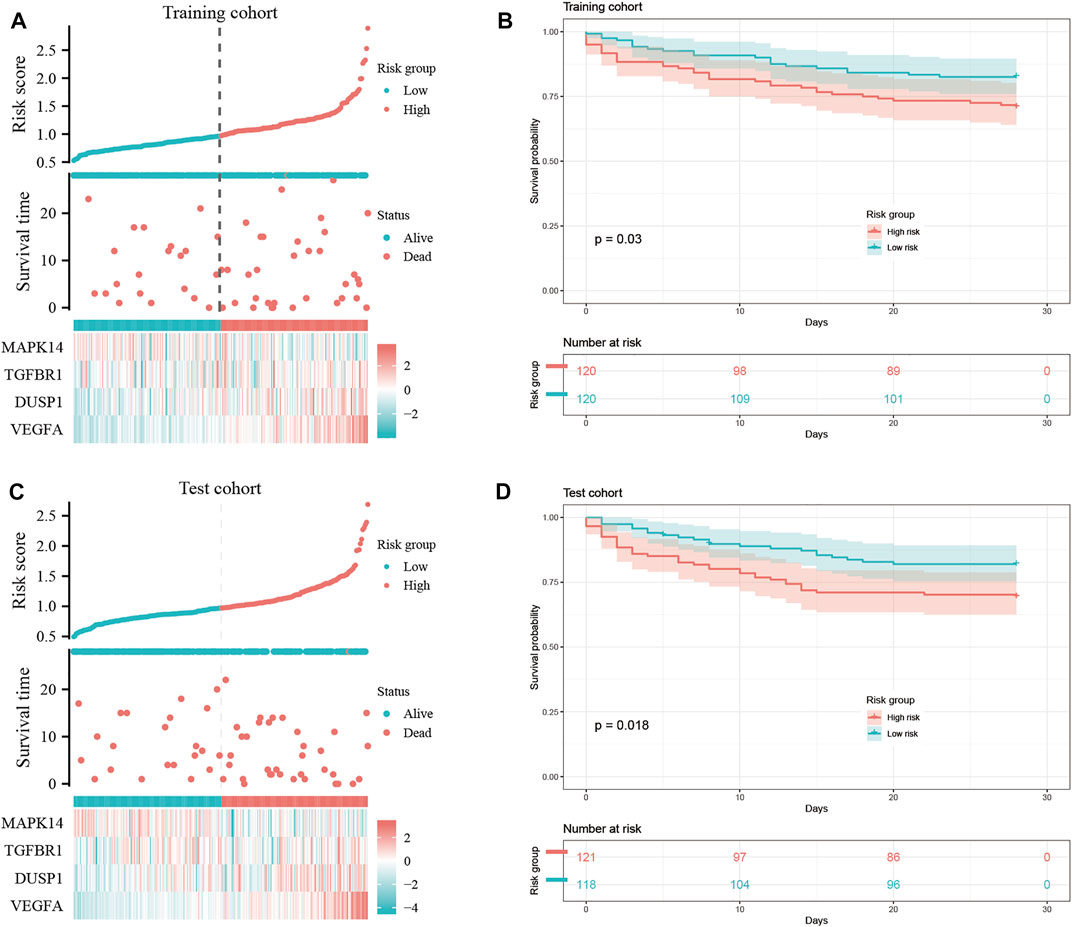
FIGURE 8. Construction of a “prognostic signature” and survival analyses. Risk scores (A,C) and survival outcome (B,D) of each case are shown.
Construction of a ceRNA network and transcription factor-gene network
We aimed to predict the ceRNA networks that may be involved in regulating key ferroptosis-related DEGs. The results showed that we identified 51 target miRNAs and 114 mRNA−miRNAs. Six of the miRNAs could regulate more than one target gene (Table 2). Finally, we got the 34 target lncRNAs based on these miRNAs (Figure 9A). Among these six miRNAs, hsa-let-7b-5p could match to the most lncRNAs (AC124045.1, SNHG4, LINC00265, AP000766.1, LINC02381, AC007228.2, MIRLET7BHG, XIST). We also used NetworkAnalyst to predict the TFs that could regulate key ferroptosis-related DEGs: 27 target TFs that may be involved in regulating key ferroptosis-related DEGs were identified. Among these TFs, E2F transcription factor 1 (E2F1) could match the most target genes (Figure 9B).
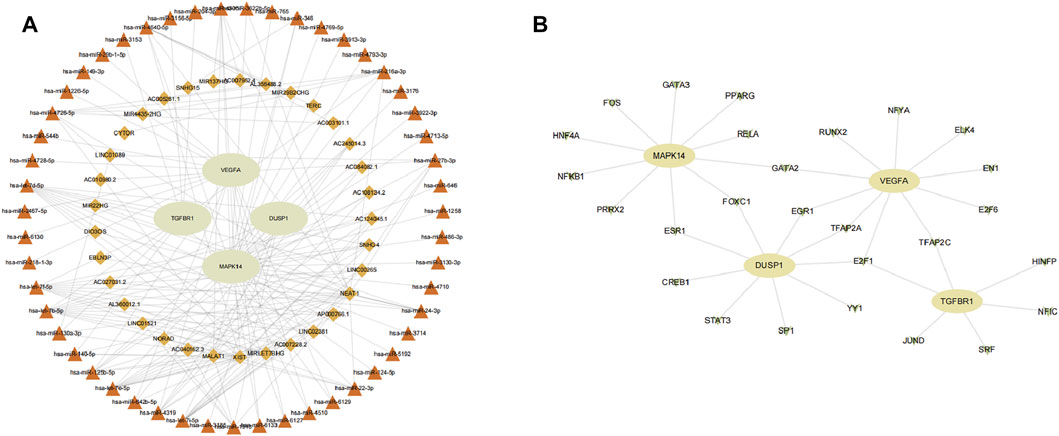
FIGURE 9. Network construction. (A) ceRNA network of key ferroptosis-related differentially expressed genes (DEGs). (B) TF–gene network of key ferroptosis-related DEGs. Ellipse nodes are genes, diamond nodes are lncRNAs, triangle nodes are miRNAs, and V nodes are TFs.
Discussion
Recent researches have revealed that ferroptosis plays an essential part in organ dysfunction due to sepsis. Inhibition of ferroptosis is considered an effective treatment for sepsis (Li N. et al., 2020). However, the specific molecular mechanisms underlying ferroptosis during sepsis are unknown.
In the current study, we identified key genes involved in the regulation of ferroptosis in sepsis. We identified 33 ferroptosis-related DEGs: 27 had upregulated expression and 6 had downregulated expression. The MAPK signaling pathway was one of the major pathways to be enriched. We chose siALI rats for experimental validation. Based on BA results, we selected ACE as a treatment method to observe its effect on ferroptosis and key ferroptosis-related DEGs. The results showed that ACE could alleviate ferroptosis in siALI rats and reverse the upregulation of key ferroptosis-related DEGs. In addition, we constructed a Cox proportional hazard model using the key ferroptosis-related DEGs to score the risk of sepsis patients: sepsis patients in the high-risk group had higher mortality within 28 days. Among the key ferroptosis-related DEGs we identified, several genes have not been mentioned before in the context of sepsis or ferroptosis. Our data provide a reference for study of the pathogenesis of sepsis and the resulting organ dysfunction.
Iron is important in various biochemical processes. If cells are stressed, iron accumulates within them (“iron overload”). This process leads to redox imbalance, production of lipid ROS, one of the characteristics of ferroptosis (Zhou et al., 2018) and, ultimately, to cell death. Activation of MAPK signaling pathway has been shown to lead to increased levels of oxidative stress, which triggers ferroptosis (Lv et al., 2020). Lipid ROS can also activate the MAPK signaling pathway (Nakamura et al., 2019). BA showed that the MAPK signaling pathway was enriched in ferroptosis-related DEGs, and most genes enriched in this pathway had upregulated expression, which is consistent with other researches (Nakamura et al., 2019). Hence, we hypothesized that the MAPK signaling pathway was activated by ferroptosis in sepsis, which aggravated the level of oxidative stress in the organism and increased ROS production, thus making the organism enter a “vicious cycle”.
Based on the above theory, we suggest that the MAPK pathway-related genes MAPK14, VEGFA, TGFBR1 and DUSP1 may be important regulatory targets of ferroptosis in sepsis. Mitogen-Activated Protein Kinase 14 (MAPK14) is a serine-threonine protein kinase that belongs to the p38-MAPK subfamily (Luo et al., 2016). It has been shown that MAPK14 plays an important regulatory role in diseases (e.g., pneumonia, acute lung injury, diabetes mellitus) and that inhibition of this pathway is an effective measure to alleviate the level of the inflammatory response and oxidative stress in disease states (Jackson et al., 1998). In addition, the MAPK signaling pathway has been found to be involved in the regulation of ferroptosis in a variety of diseases, including tumors, cold stress, and osteoporosis (Lin et al., 2022; Song et al., 2022). Vascular Endothelial Growth Factor A (VEGFA) shows abundant expression in lungs (Leung et al., 1989; Mura et al., 2006). VEGFA may also be involved in the inflammatory response by inducing expression of intracellular adhesion molecules in endothelial cells, including E-selectin, intercellular adhesion molecule 1, and vascular cell adhesion molecule 1, which promote leukocyte adhesion (Kim et al., 2001). Studies have shown that anti-VEGF antibody alleviates the inflammatory response in mice suffering from sepsis in vivo and reduces vascular leak in lungs (Jeong et al., 2013). Transforming Growth Factor Beta Receptor 1 (TGFBR1) is a key component in transmission of extracellular stimuli to the downstream TGF-β signaling pathway, and has vital roles in the growth and development of the organism and homeostasis maintenance (Vander Ark et al., 2018). It was found that when a specific inhibitor of TGFBR1 was used, the inflammatory response caused by LPS-stimulated macrophages was reduced (Chen et al., 2008). However, the current studies on TGFBR1 in animal models of sepsis are scarce and deserve further exploration. Dual Specificity Phosphatase 1 (DUSP1) can regulate cell proliferation and division, and is also thought to be a central mediator of the inflammatory response (Hoppstädter and Ammit, 2019). When DUSP1 is knocked out in septic mice, it leads to excessive release of inflammatory factors in vivo and a further increase in mortality (Hammer et al., 2010). Those results suggest that DUSP1 may have a positive regulatory effect on the inflammatory response: this is contradictory to our results showing ACE to downregulate mRNA expression of DUSP1. This contradiction may be related to the post-transcriptional modification of DUSP1 or post-translational modification at the protein level. No studies have shown that MAPK14, VEGFA, TGFBR1 and DUSP1 regulate ferroptosis in sepsis. Therefore, we hypothesize that these genes might influence ferroptosis in sepsis and the resulting organ dysfunction.
Lungs are the most susceptible organs to pathological changes during sepsis (Shankar-Hari et al., 2016). It is well established that patients with siALI have a higher mortality rate (Kumar, 2020). We employed siALI rats to validate the BA results and found that the expression of MAPK14, VEGFA, TGFBR1, and DUSP1 was upregulated in the lungs of siALI rats. These four genes are enriched mainly in the MAPK signaling pathway, and acupoint stimulation can exert anti-inflammatory effects by inhibiting the MAPK signaling pathway (Zhang et al., 2021). Hence, we chose ACE to observe the effects of ACE on siALI rats. ACE has been shown to contribute to the control of inflammation in organisms and to promote recovery of organ function in disease states (Li D. et al., 2020; Zhou et al., 2021). Recent studies have shown that electroacupuncture stimulation (another form of acupoint stimulation) of ST36 can alleviate lipopolysaccharide-induced ferroptosis in mice with sepsis-induced ARDS by modulating the alpha7 nicotinic acetylcholine receptor (Zhang et al., 2022). Consistently, our study showed that ACE alleviated ferroptosis in the lungs of siALI rats. Interestingly, ACE also decreased mRNA expression of MAPK14, VEGFA, TGFBR1, and DUSP1, which further confirmed the results of our BA and suggested that regulation of ferroptosis in siALI rats by ACE might be related to these four genes. In addition, we constructed a Cox proportional hazard model using MAPK14, VEGFA, TGFBR1, and DUSP1: they influenced survival within 28 days in sepsis patients, which emphasizes their importance in sepsis pathogenesis.
To explore the factors that can influence the expression of key ferroptosis-related DEGs, we used the bioinformatics website to predict the miRNAs, lncRNAs, and TFs that could regulate the expression of key ferroptosis-related DEGs. It was shown that in sepsis, hsa-let-7b-5p was able to regulate neutrophil function and reduce organismal inflammation, and when hsa-let-7b-5p agonist was used, it reduced neutrophil recruitment in sepsis mice and alleviated organ dysfunction (Chen et al., 2021). Among the upstream lncRNAs of hsa-let-7b-5p, only XIST has been found to be involved in sepsis regulation. Studies have shown that silencing or downregulation of XIST expression protects against sepsis-induced multiorgan dysfunction (Wang et al., 2021; Wang and Cao, 2022). Findings from those studies are consistent with our prediction that XIST−hsa-let-7b-5p−TGFBR1/DUSP1 may be a vital ceRNA regulatory network in sepsis. In addition, the TF that could match the most target genes was E2F1. Studies have shown that downregulation of E2F1 activates the NFκB signaling pathway, which leads to sepsis progression (Li B. et al., 2021). However, the above prediction has not yet been investigated and deserves further exploration.
We identified four ferroptosis-related genes that may play an important part in ferroptosis development in sepsis, and validated them from other datasets as well as animal models. A therapeutic approach that alleviates ferroptosis development in sepsis was also identified, which complements studies on the mechanism of action of ferroptosis in sepsis. However, our study had three main limitations. First, we could not validate our experimental results in human tissues due to the difficulty of obtaining samples. Second, we focused on four important genes among 33 ferroptosis-related DEGs but neglected to explore other genes: more comprehensive and in-depth studies are needed in the future. Third, we validated only the key ferroptosis-related gene signature in an internal dataset due to a lack of other relatively well-developed prognostic data from sepsis patients. Validation in an external dataset is needed. The results of our study need further confirmation from laboratory tests and clinical data.
Conclusion
We identified four ferroptosis-related genes (MAPK14, VEGFA, TGFBR1, DUSP1) that may influence the pathological progression of sepsis and the resulting organ dysfunction. ACE pretreatment alleviated pulmonary ferroptosis in siALI rats, and maybe novel adjuvant therapy for sepsis patients. We predicted that the XIST−hsa-let-7b-5p−TGFBR1/DUSP1 ceRNA network and transcription factor E2F1 might be important regulators of sepsis. Our results may extend knowledge of sepsis and provide novel ideas for treating it.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by the Animal Care and Use Committee of the Second Affiliated Hospital of Harbin Medical University.
Author contributions
ZL designed the study, performed experiments, analyzed data and wrote the manuscript. YY performed experiments and analyzed data. CL performed experiments and analyzed data. GC performed experiments. WG performed experiments. JL performed experiments. ZY approved the implementation of the study, refined this study, reviewed the manuscript and was responsible for financial support.
Acknowledgments
We would like to thank all members for their kind comments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.940261/full#supplementary-material
References
Chen, B., Han, J., Chen, S., Xie, R., Yang, J., Zhou, T., et al. (2021). MicroLet-7b regulates neutrophil function and dampens neutrophilic inflammation by suppressing the canonical TLR4/NF-κB pathway. Front. Immunol. 12, 653344. doi:10.3389/fimmu.2021.653344
Chen, P. H., Wu, J., Ding, C. C., Lin, C. C., Pan, S., Bossa, N., et al. (2020). Kinome screen of ferroptosis reveals a novel role of ATM in regulating iron metabolism. Cell Death Differ. 27 (3), 1008–1022. doi:10.1038/s41418-019-0393-7
Chen, Y., Kam, C. S., Liu, F. Q., Liu, Y., Lui, V. C., Lamb, J. R., et al. (2008). LPS-induced up-regulation of TGF-beta receptor 1 is associated with TNF-alpha expression in human monocyte-derived macrophages. J. Leukoc. Biol. 83 (5), 1165–1173. doi:10.1189/jlb.0807521
Du, K., Wang, X., Chi, L., and Li, W. (2017). Role of sigma-1 receptor/p38 MAPK inhibition in acupoint catgut embedding-mediated analgesic effects in complete freund's adjuvant-induced inflammatory pain. Anesth. Analg. 125 (2), 662–669. doi:10.1213/ane.0000000000001857
Goh, H. H. (2018). Integrative multi-omics through bioinformatics. Adv. Exp. Med. Biol. 1102, 69–80. doi:10.1007/978-3-319-98758-3_5
Hammer, M., Echtenachter, B., Weighardt, H., Jozefowski, K., Rose-John, S., Männel, D. N., et al. (2010). Increased inflammation and lethality of Dusp1-/- mice in polymicrobial peritonitis models. Immunology 131 (3), 395–404. doi:10.1111/j.1365-2567.2010.03313.x
Heming, N., Azabou, E., Cazaumayou, X., Moine, P., and Annane, D. (2021). Sepsis in the critically ill patient: Current and emerging management strategies. Expert Rev. anti. Infect. Ther. 19 (5), 635–647. doi:10.1080/14787210.2021.1846522
Hoppstädter, J., and Ammit, A. J. (2019). Role of dual-specificity Phosphatase 1 in glucocorticoid-driven anti-inflammatory responses. Front. Immunol. 10, 1446. doi:10.3389/fimmu.2019.01446
Imai, H., Matsuoka, M., Kumagai, T., Sakamoto, T., and Koumura, T. (2017). Lipid peroxidation-dependent cell death regulated by GPx4 and ferroptosis. Curr. Top. Microbiol. Immunol. 403, 143–170. doi:10.1007/82_2016_508
Jackson, J. R., Bolognese, B., Hillegass, L., Kassis, S., Adams, J., Griswold, D. E., et al. (1998). Pharmacological effects of SB 220025, a selective inhibitor of P38 mitogen-activated protein kinase, in angiogenesis and chronic inflammatory disease models. J. Pharmacol. Exp. Ther. 284 (2), 687–692.
Jeong, S. J., Han, S. H., Kim, C. O., Choi, J. Y., and Kim, J. M. (2013). Anti-vascular endothelial growth factor antibody attenuates inflammation and decreases mortality in an experimental model of severe sepsis. Crit. Care 17 (3), R97. doi:10.1186/cc12742
Kim, I., Moon, S. O., Kim, S. H., Kim, H. J., Koh, Y. S., Koh, G. Y., et al. (2001). Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J. Biol. Chem. 276 (10), 7614–7620. doi:10.1074/jbc.M009705200
Kumar, V. (2020). Pulmonary innate immune response determines the outcome of inflammation during pneumonia and sepsis-associated acute lung injury. Front. Immunol. 11, 1722. doi:10.3389/fimmu.2020.01722
Leung, D. W., Cachianes, G., Kuang, W. J., Goeddel, D. V., and Ferrara, N. (1989). Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246 (4935), 1306–1309. doi:10.1126/science.2479986
Li, B., Zhang, L., Zhu, L., Cao, Y., Dou, Z., Yu, Q., et al. (2021a). HDAC5 promotes intestinal sepsis via the Ghrelin/E2F1/NF-κB axis. Faseb J. 35 (7), e21368. doi:10.1096/fj.202001584R
Li, D., Sun, T., Chi, L., Zhao, D., and Li, W. (2020a). Acupoint catgut embedding improves the lipopolysaccharide-induced acute respiratory distress syndrome in rats. Biomed. Res. Int. 2020, 2394734. doi:10.1155/2020/2394734
Li, J., Lu, K., Sun, F., Tan, S., Zhang, X., Sheng, W., et al. (2021b). Panaxydol attenuates ferroptosis against LPS-induced acute lung injury in mice by Keap1-Nrf2/HO-1 pathway. J. Transl. Med. 19 (1), 96. doi:10.1186/s12967-021-02745-1
Li, N., Wang, W., Zhou, H., Wu, Q., Duan, M., Liu, C., et al. (2020b). Ferritinophagy-mediated ferroptosis is involved in sepsis-induced cardiac injury. Free Radic. Biol. Med. 160, 303–318. doi:10.1016/j.freeradbiomed.2020.08.009
Lin, Y., Shen, X., Ke, Y., Lan, C., Chen, X., Liang, B., et al. (2022). Activation of osteoblast ferroptosis via the METTL3/ASK1-p38 signaling pathway in high glucose and high fat (HGHF)-induced diabetic bone loss. Faseb J. 36 (3), e22147. doi:10.1096/fj.202101610R
Luo, F., Shi, J., Shi, Q., Xu, X., Xia, Y., He, X., et al. (2016). Mitogen-activated protein kinases and hypoxic/ischemic nephropathy. Cell. Physiol. biochem. 39 (3), 1051–1067. doi:10.1159/000447812
Lv, H., Zhen, C., Liu, J., and Shang, P. (2020). β-Phenethyl isothiocyanate induces cell death in human osteosarcoma through altering iron metabolism, disturbing the redox balance, and activating the MAPK signaling pathway. Oxid. Med. Cell. Longev. 2020, 5021983. doi:10.1155/2020/5021983
Matute-Bello, G., Downey, G., Moore, B. B., Groshong, S. D., Matthay, M. A., Slutsky, A. S., et al. (2011). An official American thoracic society workshop report: Features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 44 (5), 725–738. doi:10.1165/rcmb.2009-0210ST
Mishima, E., Sato, E., Ito, J., Yamada, K. I., Suzuki, C., Oikawa, Y., et al. (2020). Drugs repurposed as antiferroptosis agents suppress organ damage, including AKI, by functioning as lipid peroxyl radical scavengers. J. Am. Soc. Nephrol. 31 (2), 280–296. doi:10.1681/asn.2019060570
Mura, M., Han, B., Andrade, C. F., Seth, R., Hwang, D., Waddell, T. K., et al. (2006). The early responses of VEGF and its receptors during acute lung injury: Implication of VEGF in alveolar epithelial cell survival. Crit. Care 10 (5), R130. doi:10.1186/cc5042
Nakamura, T., Naguro, I., and Ichijo, H. (2019). Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim. Biophys. Acta. Gen. Subj. 1863 (9), 1398–1409. doi:10.1016/j.bbagen.2019.06.010
Oh, J. E., and Kim, S. N. (2021). Anti-inflammatory effects of acupuncture at ST36 point: A literature review in animal studies. Front. Immunol. 12, 813748. doi:10.3389/fimmu.2021.813748
Rittirsch, D., Huber-Lang, M. S., Flierl, M. A., and Ward, P. A. (2009). Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 4 (1), 31–36. doi:10.1038/nprot.2008.214
Rudd, K. E., Johnson, S. C., Agesa, K. M., Shackelford, K. A., Tsoi, D., Kievlan, D. R., et al. (2020). Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the global burden of disease study. Lancet 395 (10219), 200–211. doi:10.1016/s0140-6736(19)32989-7
Shankar-Hari, M., Phillips, G. S., Levy, M. L., Seymour, C. W., Liu, V. X., Deutschman, C. S., et al. (2016). Developing a new definition and assessing new clinical criteria for septic shock: For the third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama 315 (8), 775–787. doi:10.1001/jama.2016.0289
Song, Q., Peng, S., Che, F., and Zhu, X. (2022). Artesunate induces ferroptosis via modulation of p38 and ERK signaling pathway in glioblastoma cells. J. Pharmacol. Sci. 148 (3), 300–306. doi:10.1016/j.jphs.2022.01.007
Tian, L., Zhu, J., Jin, J., Tong, C., Zeng, W., Deng, S., et al. (2019). Prognostic value of circulating lymphocyte B and plasma immunoglobulin M on septic shock and sepsis: A systematic review and meta-analysis. Am. J. Transl. Res. 11 (12), 7223–7232.
Vander Ark, A., Cao, J., and Li, X. (2018). TGF-β receptors: In and beyond TGF-β signaling. Cell. Signal. 52, 112–120. doi:10.1016/j.cellsig.2018.09.002
Wang, L., and Cao, Q. M. (2022). Long non-coding RNA XIST alleviates sepsis-induced acute kidney injury through inhibiting inflammation and cell apoptosis via regulating miR-155-5p/WWC1 axis. Kaohsiung J. Med. Sci. 38 (1), 6–17. doi:10.1002/kjm2.12442
Wang, X., Li, X. L., and Qin, L. J. (2021). The lncRNA XIST/miR-150-5p/c-Fos axis regulates sepsis-induced myocardial injury via TXNIP-modulated pyroptosis. Lab. Invest.. 101 (9), 1118–1129. doi:10.1038/s41374-021-00607-4
Wei, S., Bi, J., Yang, L., Zhang, J., Wan, Y., Chen, X., et al. (2020). Serum irisin levels are decreased in patients with sepsis, and exogenous irisin suppresses ferroptosis in the liver of septic mice. Clin. Transl. Med. 10 (5), e173. doi:10.1002/ctm2.173
Zhang, X. F., Xiang, S. Y., Lu, J., Li, Y., Zhao, S. J., Jiang, C. W., et al. (2021). Electroacupuncture inhibits IL-17/IL-17R and post-receptor MAPK signaling pathways in a rat model of chronic obstructive pulmonary disease. Acupunct. Med. 39 (6), 663–672. doi:10.1177/0964528421996720
Zhang, Y., Zheng, L., Deng, H., Feng, D., Hu, S., Zhu, L., et al. (2022). Electroacupuncture alleviates LPS-induced ARDS through α7 nicotinic acetylcholine receptor-mediated inhibition of ferroptosis. Front. Immunol. 13, 832432. doi:10.3389/fimmu.2022.832432
Zhao, J., Xu, B., Xiong, Q., Feng, Y., and Du, H. (2021). Erastin-induced ferroptosis causes physiological and pathological changes in healthy tissues of mice. Mol. Med. Rep. 24 (4), 713. doi:10.3892/mmr.2021.12352
Zhou, B., Zhang, J. Y., Liu, X. S., Chen, H. Z., Ai, Y. L., Cheng, K., et al. (2018). Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis. Cell Res. 28 (12), 1171–1185. doi:10.1038/s41422-018-0090-y
Keywords: ferroptosis, sepsis, acute lung injury, bioinformatics, MAPK signaling pathway, acupoint catgut embedding
Citation: Li Z, Yu Y, Liu C, Chen G, Gong W, Luo J and Yue Z (2022) Identification of the key ferroptosis-related genes involved in sepsis progression and experimental validation in vivo. Front. Pharmacol. 13:940261. doi: 10.3389/fphar.2022.940261
Received: 11 May 2022; Accepted: 12 July 2022;
Published: 11 August 2022.
Edited by:
Hailin Tang, Sun Yat-sen University Cancer Center (SYSUCC), ChinaReviewed by:
Song Wu, Sun Yat-sen University Cancer Center (SYSUCC), ChinaChuanxi Cai, The Ohio State University, United States
Copyright © 2022 Li, Yu, Liu, Chen, Gong, Luo and Yue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ziyong Yue, yueziyong@126.com
 Zhixi Li
Zhixi Li Yongjing Yu1,3
Yongjing Yu1,3 Chang Liu
Chang Liu