- Department of Urology, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, China
Purpose: To investigate the relationship between the Oxidative Balance Score (OBS) and kidney stone risk using NHANES 2007-2018 data, and to explore potential mechanisms and population-specific effects.
Materials and methods: Data from the NHANES 2007-2018 were analyzed. OBS was calculated based on 16 dietary components and 4 lifestyle components. Multivariate logistic regression was employed to investigate the relationship between OBS and kidney stone. Further stratified analyses were conducted to examine the associations across different subgroups.
Results: A total of 19,799 participants were included in the study. There was a consistent inverse association between OBS and the risk of kidney stones (OR = 0.97; 95% CI: 0.96–0.99). After dividing the participants into quartiles based on OBS, compared to the lowest quartile of OBS, the risk of kidney stones in the highest quartile of OBS was reduced by 33% (95% CI 0.50–0.89; p = 0.002). This association was consistent across both dietary and lifestyle OBS scores. The protective effect of OBS was notably pronounced among Non-Hispanic white and Other race groups, and among individuals with a higher level of education. However, the association was not significant among individuals with diabetes.
Conclusion: A higher OBS, indicating a balance skewed towards antioxidants, is associated with a reduced risk of kidney stones, especially among specific population subgroups. These findings underscore the potential role of oxidative balance in kidney stone pathogenesis and highlight the importance of considering individual and population-specific factors in future research and preventive strategies.
1 Introduction
The prevalence and incidence of kidney stones worldwide have been increasing over the past few decades. The burden of kidney stones on healthcare systems is substantial, with annual healthcare expenditures exceeding $2 billion in the United States alone (Thongprayoon et al., 2020). Kidney stone disease is a systemic disorder associated with chronic kidney disease, bone loss and fractures, increased risk of coronary artery disease, hypertension, type 2 diabetes, and metabolic syndrome (Sakhaee et al., 2012). The formation of kidney stones is a complex process regulated by metabolic changes in various substances, including oxalate, reactive oxygen species (ROS), hormones, and others. Among these, oxidative stress (OS) induced by ROS plays a key regulatory role in the formation of kidney stones (Xu et al., 2023). Animal and in vitro studies have confirmed that oxalate and calcium oxalate crystals can induce OS in renal epithelial cells, which is a critical factor leading to the formation of kidney stones (Umekawa et al., 2004; Zhu et al., 2019; Khan et al., 2021; Khan, 2014; W et al., 2021).
OS refers to a condition where the production of oxidants exceeds the body’s antioxidant defenses. Dietary intake is an important source of antioxidants and oxidants. However, the relationship between individual nutrient components and kidney stones remains contradictory due to conflicting study results and the complexity of the human diet. In vitro studies have confirmed that antioxidants such as vitamins C and E can protect against oxalate-induced OS and kidney damage (Thamilselvan et al., 2014; Jaturakan et al., 2017). However, excessive intake of vitamin C may increase urinary oxalate excretion and increase the risk of kidney stones (Ferraro et al., 2016; Jaturakan et al., 2017). Increasing fiber intake and maintaining balanced calcium intake can reduce the risk of kidney stone formation (Nirumand et al., 2018; Ferraro et al., 2020). Studies on the relationship between vitamin D and kidney stones are also inconsistent (Letavernier et al., 2016; Malihi et al., 2016; Ferraro et al., 2017; Bouderlique et al., 2019; Malihi et al., 2019; Taheri et al., 2019). Another study found that increasing the six major dietary sources of antioxidants (including vitamins A, beta-carotene, B6, C, E, and lycopene) did not significantly reduce the risk of kidney stone disease (Jian et al., 2021). In addition, lifestyle factors are also a major source of OS. It has been established that obesity is a risk factor for kidney stones (Milicevic et al., 2013; Sasaki et al., 2015; Lee et al., 2022a). Alcohol can cause OS damage to kidney tissues, leading to the formation of kidney stones (Jones et al., 2021). Whether smoking (including active and passive smoking) (Tamadon et al., 2013; Marić et al., 2019; Chen et al., 2021) and physical activity (Sorensen et al., 2014; Ferraro et al., 2015; Aune et al., 2018; Zhuo et al., 2019) significantly increase the risk of kidney stones is also inconclusive.
The Oxidative Balance Score (OBS) is a comprehensive measure that reflects both pro-oxidant and antioxidant exposures, mainly composed of dietary and lifestyle components (Hernández-Ruiz Á et al., 2022). A higher OBS indicates a higher antioxidant and lower pro-oxidant exposure, suggesting a lower level of OS. Previous studies have confirmed that a higher OBS can reduce the risk of some diseases, such as better blood sugar control (Golmohammadi et al., 2021), lower incidence of hypertension (Lee et al., 202b), as well as reducing depression (Liu et al., 2023), improving sleep quality (Lei et al., 2023), etc. However, the relationship between OBS and the risk of kidney stones has not been studied. Therefore, we used the NHANES 2007-2018 data to conduct a cross-sectional study on the relationship between OBS and kidney stones. This study could lead to a better understanding of the role of OS in kidney stone formation and potentially lead to new prevention strategies.
2 Materials and methods
2.1 Study population
The National Health and Nutrition Examination Survey (NHANES), conducted every 2 years, is a program designed to assess the health and nutritional status of adults and children in the United States. All participants have provided informed consent. For our study, we incorporated data from five NHANES cycles spanning 2007–2018, initially involving 59,842 participants. We applied several exclusion criteria: 1) Individuals under 20 years of age (n = 25072); 2) pregnant individuals (n = 274); 3) individuals with missing OBS component data (n = 13276); 4) individuals with missing kidney stone questionnaire data (n = 46); 5) individuals with unreliable energy intake (for males: <800 kcal/d or >4,200 kcal/d, for females: <500 kcal/d or >3,500 kcal/d) (n = 1375) (Zhang et al., 2022). After applying these criteria, our final study population comprised 19,799 participants (Figure 1).
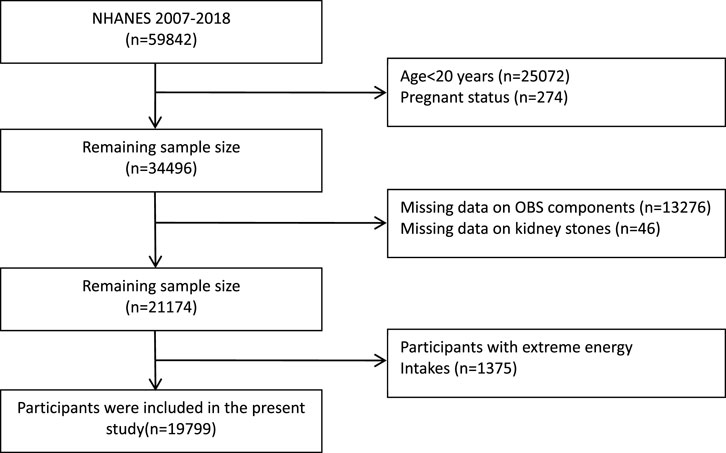
FIGURE 1. Flowchart of the study population. NHANES, National Health and Nutrition Examination Survey; OBS, oxidative balance score.
2.2 Exposure and outcome definitions
Since 2007, NHANES has incorporated kidney stone-related information into its questionnaire. Participants who responded “yes” to the question “Have you/Has sample person (SP) ever had kidney stones?” were identified as having a history of kidney stones.
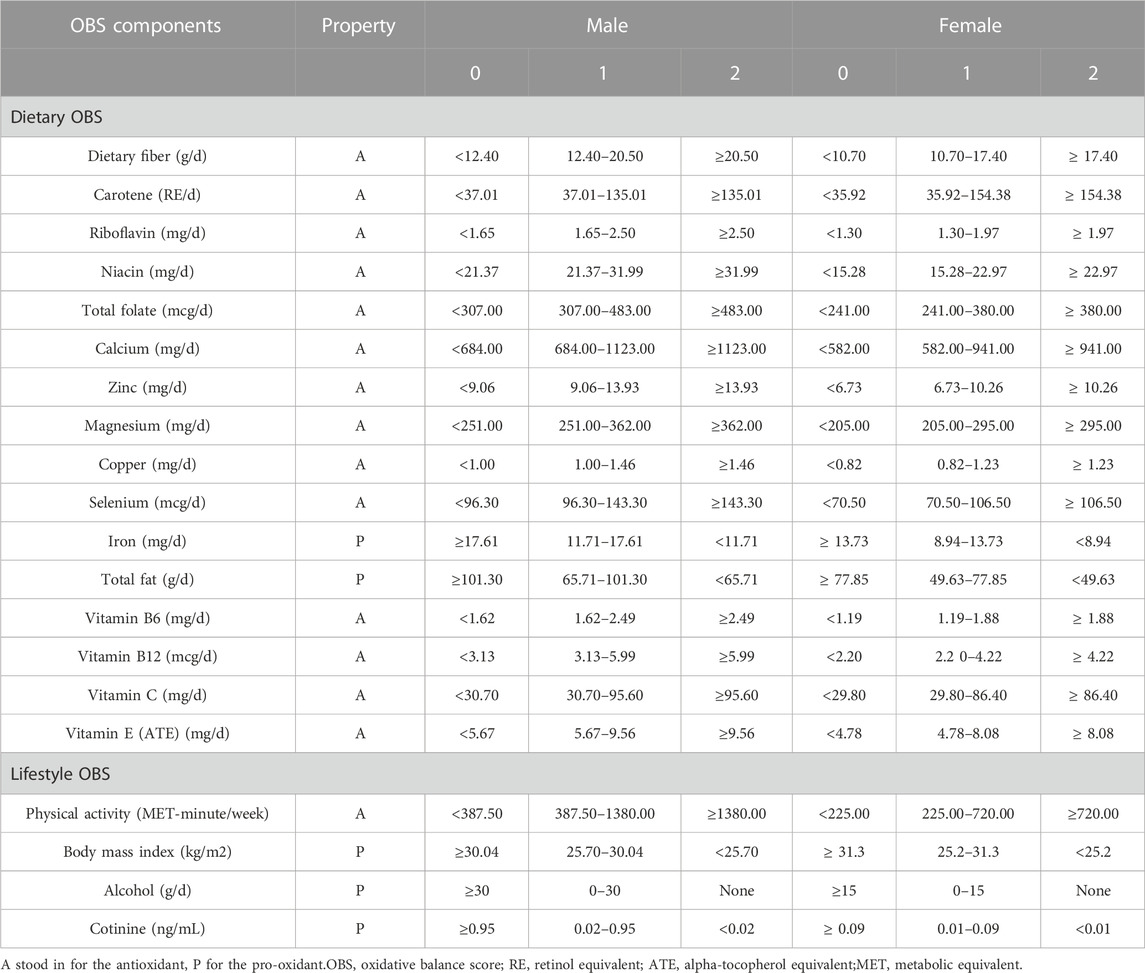
We adopted a scoring method from previous research (Zhang et al., 2022) for the Oxidative Balance Score (OBS), which comprises 16 dietary and 4 lifestyle components. Among these, 15 are classified as antioxidants and 5 as pro-oxidants. The dietary data was derived from NHANES’ 24-h dietary recall. Lifestyle factors included physical activity, alcohol consumption, body mass index, and cotinine levels. Physical activity was quantified as a metabolic equivalent (MET) score. Alcohol consumption was categorized into three groups: nondrinkers, non-heavy drinkers, and heavy drinkers. Serum cotinine, a metabolite of nicotine, was used as an indicator of exposure to tobacco smoke, encompassing both active and passive smoking. All factors, except for alcohol, were divided into gender-specific tertiles. Antioxidant factors were scored as 0, 1, and 2 from the lowest to highest tertiles, while pro-oxidant factors were scored inversely, from 2 to 0, across the same tertiles. The total OBS score, the sum of all factor scores, indicates a better oxidative balance when higher. Table 1 presents the classification and assigned scores for each OBS component.
2.3 Covariates
We included the following variables in our research: Age, Race (categorized as Non-Hispanic White, Non-Hispanic Black, Mexican American and Other Race), Marital Status (classified as Married or Living with Partner, and Single), Poverty Income Ratio (PIR) (grouped as<=1.3, 1.3–3.5, >3.5), Education Level (divided into Less than High School, High School Grad/GED or Equivalent, and More than High School), Total Energy Intake, and the presence or absence of Hyperlipidemia, Hypertension, Diabetes, and Gout.
2.4 Statistical analysis
In line with the NHANES weighting selection guidelines, we utilized the weights derived from the 24-h dietary recall interview for our study sample. Baseline characteristics of the study population were stratified according to quartiles of the Oxidative Balance Score (OBS). Continuous variables were expressed as weighted means (standard errors), and categorical variables were presented as frequencies (weighted percentages). Differences in baseline characteristics across OBS quartiles were calculated using chi-square tests for categorical variables and t-tests or one-way analysis of variance for continuous variables.
We constructed three weighted multivariate logistic regression models to evaluate the relationship between OBS and the kidney stones. The P for trend value was calculated when OBS was categorized into quartiles. Model 1 was unadjusted; Model 2 adjusted for age, gender, and race; and Model 3 further adjusted for marital status, poverty income ratio (PIR), education level, energy intake, diabetes mellitus, hypertension, hyperlipidemia, and gout.
Separate analyses were conducted to calculate the associations between dietary OBS scores, lifestyle OBS scores, and the risk of kidney stones. Subgroup analyses were performed based on age, gender, race, marital status, PIR, education level, diabetes, hypertension, hyperlipidemia, and gout to explore potential variations in the relationship between OBS and kidney stones across different subgroups.
A sensitivity analysis was conducted by sequentially excluding each component of the OBS to assess the robustness of our findings (Table 2). All statistical analyses were performed using R version 4.2.2, and statistical significance was determined at a two-sided p-value of 0.05.
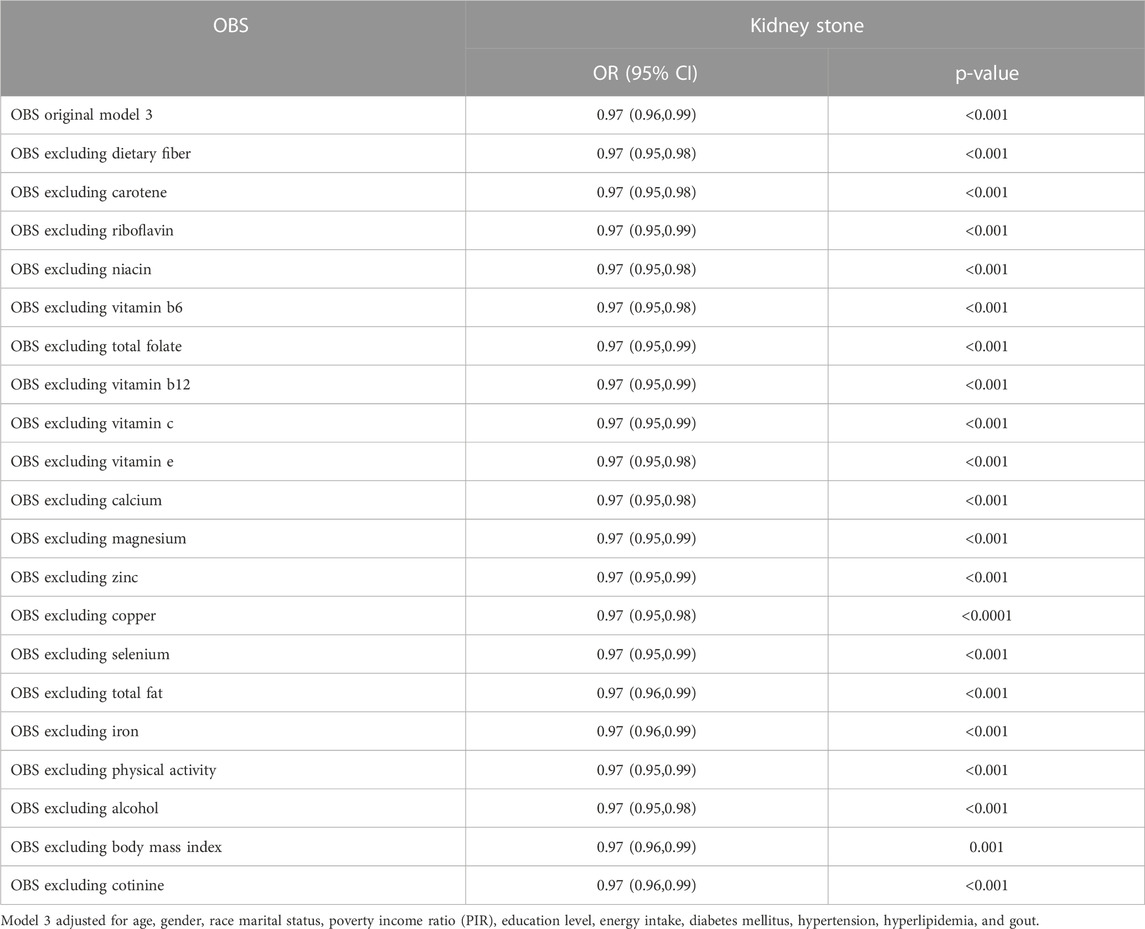
TABLE 2. Sensitivity analyses to assess the effects of individual OBS components on the kidney stone.
3 Results
3.1 Baseline characteristics
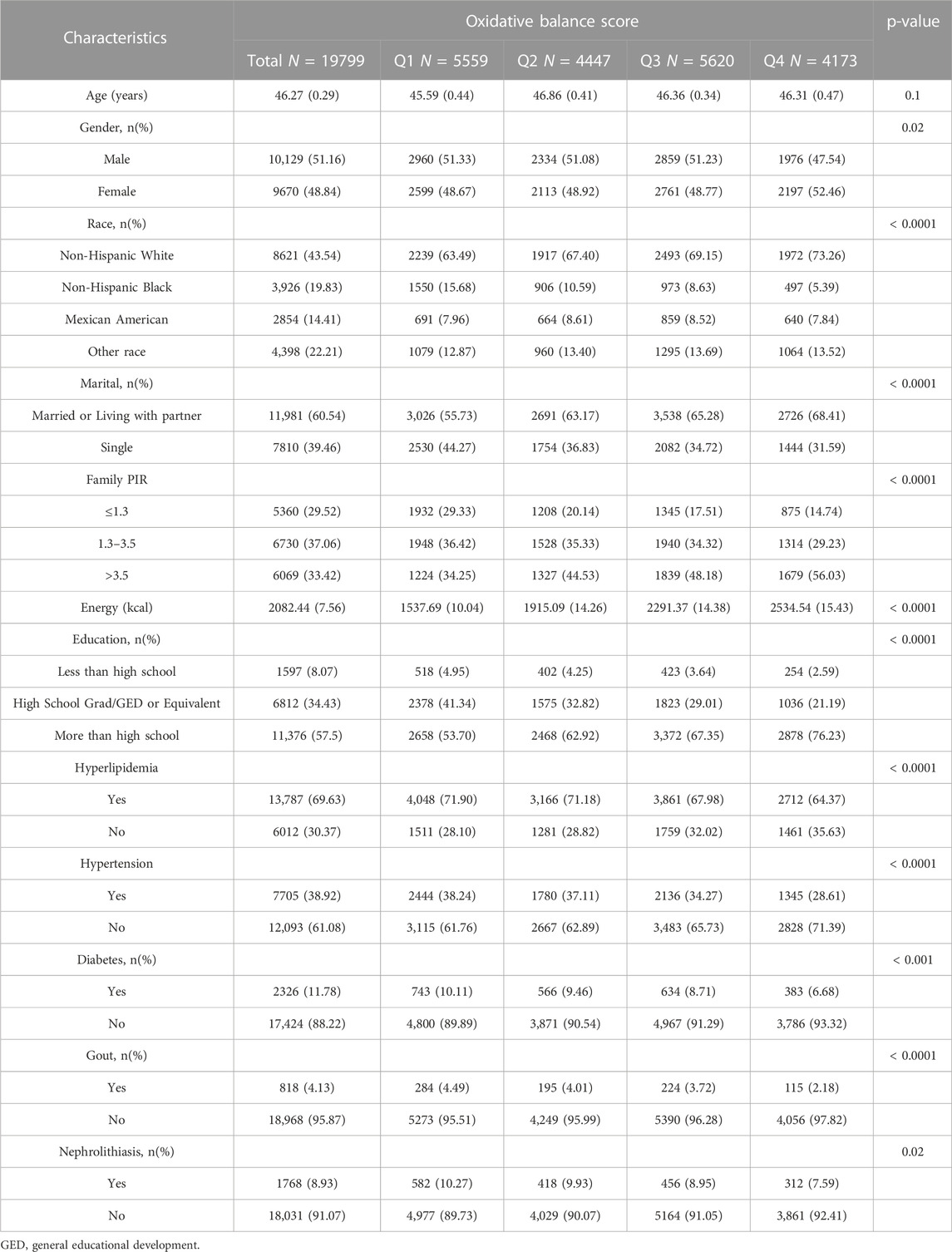
In this study, a total of 19,799 participants from the NHANES (2007–2018) were included, with a weighted mean age of 46.3 (0.29) years, and 51.16% being male. The basic demographic characteristics and covariates of the population, stratified by OBS score quartiles, are presented in Table 3. Notably, the highest quartile of OBS had a significantly higher proportion of female participants compared to the other three quartiles. Participants with higher OBS scores were more likely to be Non-Hispanic White, more educated, had a higher PIR, and a higher energy intake. Interestingly, individuals who were single tended to have lower OBS scores. As OBS scores increased (from Q1 to Q4), there was a decrease in the prevalence of hypertension, diabetes, hyperlipidemia, and gout in the population, paralleled by a decrease in the prevalence of kidney stones (7.59% in Q4 vs. 10.27% in Q1, p = 0.02).
3.2 Relationship between OBS and kidney stone
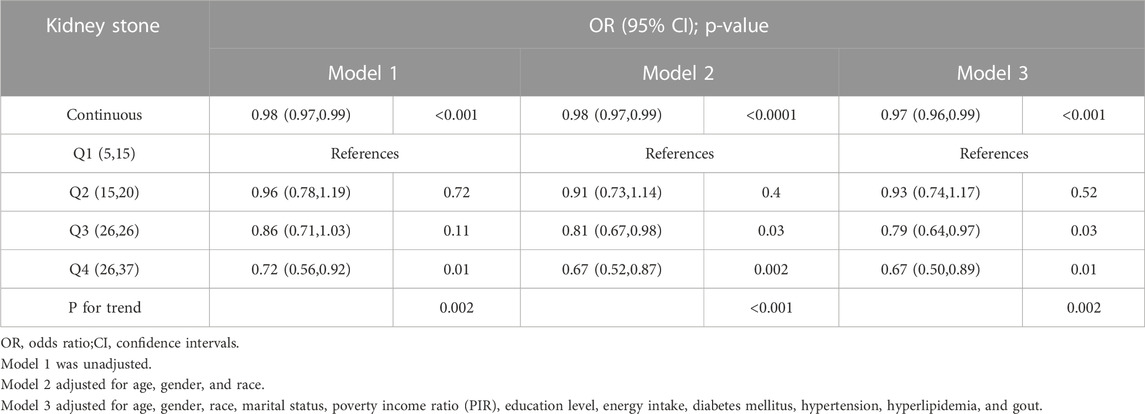
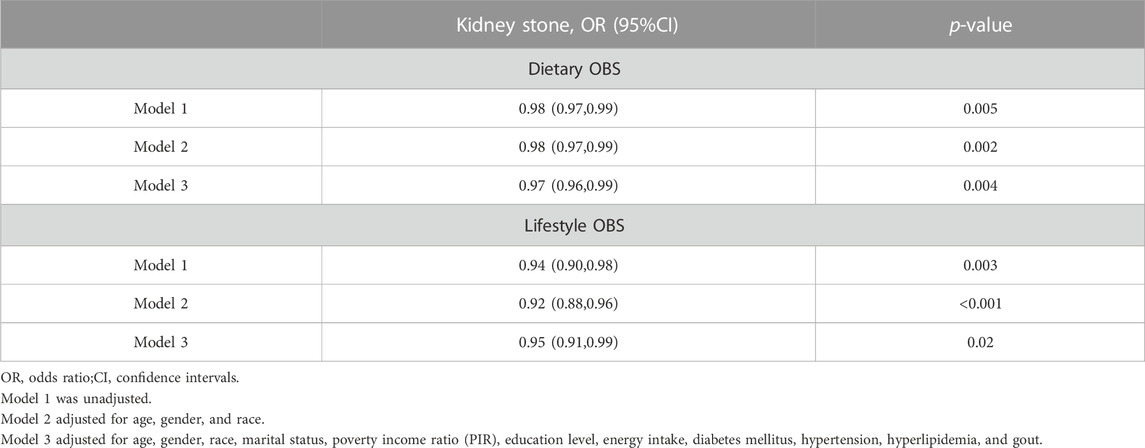
We employed three weighted logistic regression models to investigate the association between OBS and the risk of kidney stones, as presented in Table 4. Across all three models, we observed a protective influence of higher OBS scores on the risk of kidney stones (p < 0.05). In Model 3, which accounted for a variety of confounding factors, the findings indicated that with each unit increase in OBS, the risk of kidney stones diminished by 3% (95% CI 0.96–0.99; p < 0.001). This negative correlation persisted even after categorizing OBS, with the P for trend in Models 1, 2, and 3 being 0.002, <0.001, and 0.002, respectively. In Model 3, when using the lowest quartile OBS group as a reference, the risk of kidney stones in the highest quartile group was reduced by 33% (95% CI 0.50–0.89; p = 0.002).Additionally, we investigated the associations of both dietary OBS and lifestyle OBS with the risk of kidney stones (Table 5). A persistent negative correlation between both dietary and lifestyle OBS and the risk of kidney stones was consistently observed across all three models (All p-values <0.05).
3.3 Subgroup analysis
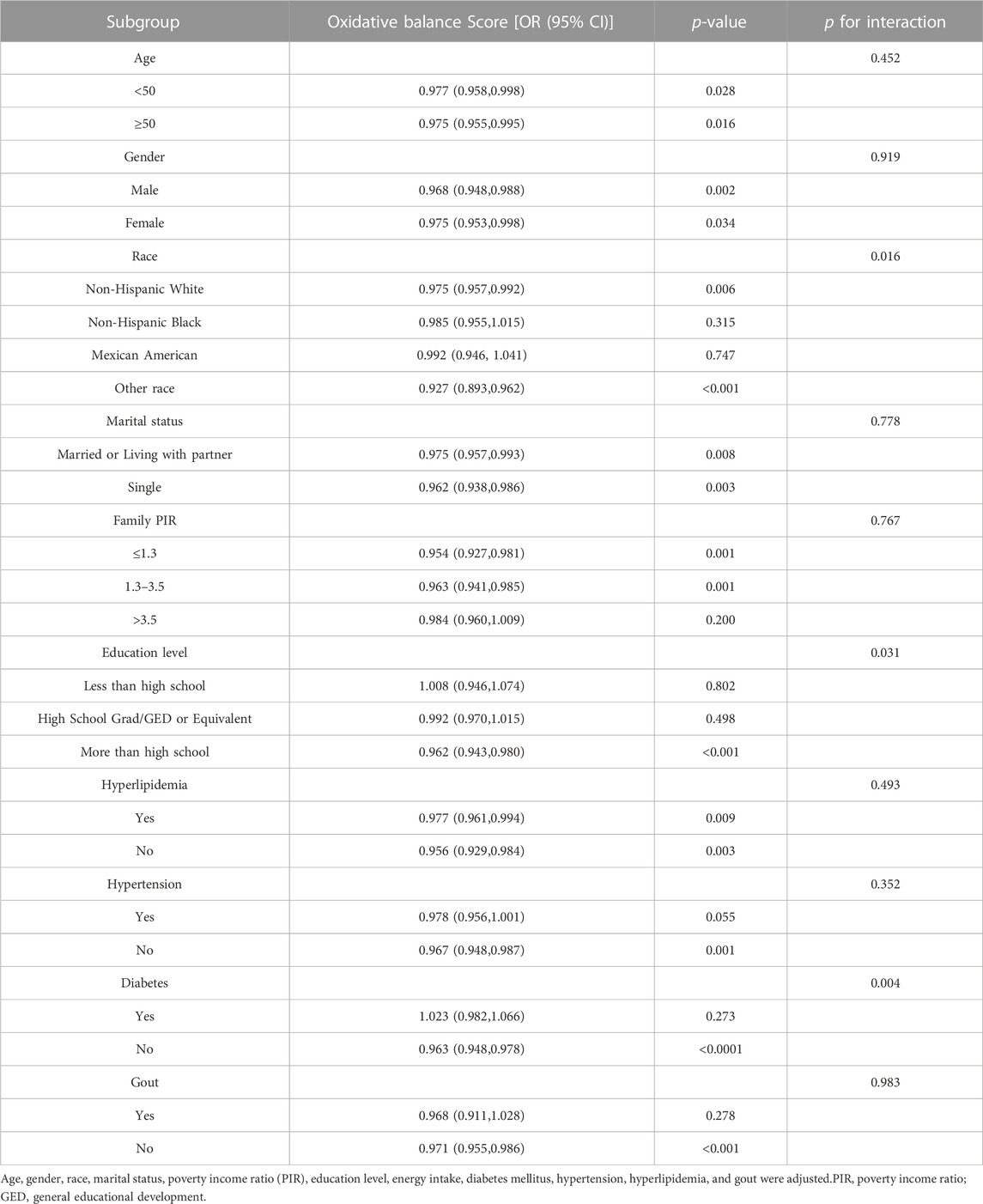
As depicted in Table 6, further stratified analysis revealed that among Non-Hispanic White and Other race groups, a higher OBS was associated with a significant reduction in the risk of kidney stones (P for interaction = 0.016). Another variable exhibiting an interaction effect was the level of education (P for interaction = 0.031). Among individuals with a higher level of education (more than high school), the relationship between OBS and kidney stones was more pronounced (OR 0.962, 95% CI 0.943, 0.980, p < 0.001). In the population without diabetes, it was observed that for each unit increase in OBS, the risk of kidney stones decreased by 3.7% (p < 0.0001). However, the association was not significant in the population with diabetes.
4 Discussion
Using data from the NHANES 2007–2018, our study provides new insights into the relationship between the Oxidative Balance Score (OBS) and the risk of kidney stones. We consistently found an inverse association between OBS and the risk of kidney stones, suggesting that a higher antioxidant and lower pro-oxidant exposure, as indicated by a higher OBS, could potentially reduce the risk of kidney stone formation. This association remained significant even after adjusting for potential confounders and was consistent across both dietary and lifestyle OBS.
The pathogenesis of kidney stones is multifactorial and intricate, involving a blend of genetic, environmental, and dietary factors (Howles and Thakker, 2020; Siener and Hesse, 2021). Oxidative stress (OS), defined by an imbalance between the production of oxidants and the body’s antioxidant defenses, has been implicated in the formation of kidney stones (Xu et al., 2023). Several studies have highlighted the elevated oxidative stress levels in renal stone patients. For instance, Williams et al. (2016) found that mitochondrial function in monocytes from kidney stone patients was compromised, leading to increased levels of inflammation and oxidative stress. Additionally, research by Ma et al. (2014) indicated that, compared to a healthy control group, erythrocytes from stone patients showed diminished antioxidant protein levels and activity. Furthermore, animal experiments (Huang et al., 2009; Huang and Ma, 2015) have revealed that the increase in intrarenal oxidative stress in rats with kidney stones is due to the enhanced excretion of ROS in the urine. Oxalate and calcium oxalate crystals, common constituents of kidney stones, can induce OS in renal epithelial cells, contributing to stone formation (Umekawa et al., 2004; Zhu et al., 2019; Khan et al., 2021; Khan, 2014; W et al., 2021). Our findings, demonstrating a protective effect of higher OBS against kidney stone formation, align with the current understanding of the role of OS in kidney stone pathogenesis.
While several methods exist to calculate OBS, we adopted the approach described by Zhang, dividing OBS into 16 dietary components (14 antioxidants and 2 pro-oxidants) and 4 lifestyle components (1 antioxidant and 3 pro-oxidants). Numerous studies have identified that some of these dietary antioxidants can inhibit the formation of kidney stones, such as dietary fiber (Ferraro et al., 2020), riboflavin (Williams et al., 2016), zinc (Ma et al., 2014), magnesium (Huang and Ma, 2015), copper (Huang et al., 2009), and selenium (Di et al., 2023). However, the relationship between other antioxidants and kidney stones remains heterogeneous in various studies (Ferraro et al., 2016; Letavernier et al., 2016; Malihi et al., 2016; Ferraro et al., 2017; Jaturakan et al., 2017; Bouderlique et al., 2019; Malihi et al., 2019; Taheri et al., 2019). Regarding lifestyle factors, it is well-established that a higher BMI correlates with an increased risk of kidney stones (Tasian et al., 2017; Aune et al., 2018). Some studies have found that physical activity can, to some extent, reduce the risk of kidney stones (Aune et al., 2018), while others found no such association (Ferraro et al., 2015). Surprisingly, alcohol consumption might lower kidney stone risk, possibly because it increases fluid intake (Wu et al., 2020; Zhu et al., 2022). However, considering the other health risks of alcohol, we do not recommend it as a preventive strategy.
In our subgroup analysis, we found a stronger protective effect of OBS among Non-Hispanic White and Other race groups, as well as among individuals with a higher level of education. These findings suggest that the impact of OBS on kidney stone risk may vary across different population subgroups, possibly due to differences in genetic susceptibility, dietary habits, or other lifestyle factors. A review (Wang et al., 2023) examined health disparities in kidney stone disease concerning race, ethnicity, socioeconomic status, and place of residence, and found that white males had the highest risk of kidney stones. A study based on the NHANES database (Feng et al., 2021) found that males, non-Hispanic white people, and obese individuals had a higher prevalence of kidney stones, and the prevalence in females showed a catching-up trend. Research has found that education level and lifestyle play a significant role in the formation of kidney stones (Barghouthy et al., 2021). A higher level of education predicts a relatively higher income level and may be associated with healthier dietary and lifestyle habits. However, the underlying mechanisms for these differences warrant further investigation.
Additionally, we observed no significant link between OBS and kidney stones in diabetic individuals. This could be due to the complex interplay between diabetes, OS, and kidney stone formation. Oxidative stress (OS) plays a pivotal role in the onset and progression of diabetes (Littlejohns et al., 2020; Crivelli et al., 2021). OS can interfere with the metabolic processes of glucose, such as glycolysis, leading to hyperglycemia (Pietropaolo et al., 2017; Abufaraj et al., 2021), which could potentially overshadow the impact of OBS on kidney stone risk in this population. Alternatively, the metabolic abnormalities associated with diabetes, such as insulin resistance and hyperglycemia, could influence kidney stone formation through mechanisms independent of OS (Tangvarasittichai, 2015).
One of our study’s strengths is the use of a large, nationally representative sample, bolstering the applicability of our findings. Furthermore, the comprehensive measure of OBS, incorporating both dietary and lifestyle components, provides a more holistic assessment of antioxidant and pro-oxidant exposures. However, our study has several limitations. The cross-sectional design precludes causal inferences. The NHANES database lacks specific data on the type of kidney stones, and while calcium oxalate stones are predominant, the absence of this specificity is a limitation. The method used to determine stone recurrence in NHANES might not be entirely accurate. Reliance on self-reported data and the 24-h dietary recall could introduce recall bias, and the study’s conclusions might not be generalizable to all kidney stone patients. Future studies should consider these factors and potentially incorporate oxidative stress biomarkers for more accurate results. Future longitudinal studies are needed to confirm our findings and elucidate the potential mechanisms underlying the observed associations.
5 Conclusion
Our analysis of the NHANES 2007-2018 data revealed a significant inverse association between the Oxidative Balance Score (OBS) and risk of kidney stone. This relationship suggests that higher antioxidant and lower pro-oxidant exposures may reduce kidney stone risk. Notably, the protective effect of OBS varied across different population subgroups, with pronounced benefits observed among Non-Hispanic White and Other race groups, and those with higher education levels. However, the association was not significant among diabetic individuals. Further longitudinal studies are essential to validate these findings and explore the underlying mechanisms.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: www.cdc.gov/nchs/nhanes/.
Ethics statement
The studies involving humans were approved by the National Center for Health Statistics (NCHS) Ethics Review Board, Centers for Disease Control and Prevention (CDC). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
RK: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Software, Writing–original draft, Writing–review and editing. YH: Project administration, Resources, Supervision, Validation, Writing–review and editing. CC: Project administration, Resources, Supervision, Validation, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abufaraj M., Xu T., Cao C., Waldhoer T., Seitz C., D'Andrea D., et al. (2021). Prevalence and trends in kidney stone among adults in the USA: analyses of national health and nutrition examination survey 2007-2018 data. Eur. Urol. Focus 7 (6), 1468–1475. doi:10.1016/j.euf.2020.08.011
Aune D., Mahamat-Saleh Y., Norat T., Riboli E. (2018). Body fatness, diabetes, physical activity and risk of kidney stones: a systematic review and meta-analysis of cohort studies. Eur. J. Epidemiol. 33 (11), 1033–1047. doi:10.1007/s10654-018-0426-4
Barghouthy Y., Corrales M., Doizi S., Somani B. K., Traxer O. (2021). Tea and coffee consumption and pathophysiology related to kidney stone formation: a systematic review. World J. Urol. 39 (7), 2417–2426. doi:10.1007/s00345-020-03466-8
Bouderlique E., Tang E., Perez J., Coudert A., Bazin D., Verpont M. C., et al. (2019). Vitamin D and calcium supplementation accelerates randall's plaque formation in a murine model. Am. J. Pathol. 189 (11), 2171–2180. doi:10.1016/j.ajpath.2019.07.013
Chen C. H., Lee J. I., Jhan J. H., Lee Y. C., Geng J. H., Chen S. C., et al. (2021). Secondhand smoke increases the risk of developing kidney stone disease. Sci. Rep. 11 (1), 17694. doi:10.1038/s41598-021-97254-y
Crivelli J. J., Maalouf N. M., Paiste H. J., Wood K. D., Hughes A. E., Oates G. R., et al. (2021). Disparities in kidney stone disease: a scoping review. J. Urol. 206 (3), 517–525. doi:10.1097/JU.0000000000001846
Cutiongco M. F. A., Chua B. M. X., Neo D. J. H., Rizwan M., Yim E. K. F. (2018). Functional differences between healthy and diabetic endothelial cells on topographical cues. Biomaterials 153, 70–84. doi:10.1016/j.biomaterials.2017.10.037
Dariya B., Nagaraju G. P. (2020). Advanced glycation end products in diabetes, cancer and phytochemical therapy. Drug Discov. Today 25 (9), 1614–1623. doi:10.1016/j.drudis.2020.07.003
Di X. P., Gao X. S., Xiang L. Y., Wei X. (2023). The association of dietary intake of riboflavin and thiamine with kidney stone: a cross-sectional survey of NHANES 2007-2018. BMC Public Health 23 (1), 964. doi:10.1186/s12889-023-15817-2
Feng X., Wu W., Zhao F., Xu F., Han D., Guo X., et al. (2021). Relationship between body mass index and kidney stones based on dose-response analyses using restricted cubic splines applied to NHANES 2011-2016 data. J. Ren. Nutr. 31 (3), 263–269. doi:10.1053/j.jrn.2020.05.003
Ferraro P. M., Bargagli M., Trinchieri A., Gambaro G. (2020). Risk of kidney stones: influence of dietary factors, dietary patterns, and vegetarian-vegan diets. Nutrients 12 (3), 779. doi:10.3390/nu12030779
Ferraro P. M., Curhan G. C., Gambaro G., Taylor E. N. (2016). Total, dietary, and supplemental vitamin C intake and risk of incident kidney stones. Am. J. Kidney Dis. 67 (3), 400–407. doi:10.1053/j.ajkd.2015.09.005
Ferraro P. M., Curhan G. C., Sorensen M. D., Gambaro G., Taylor E. N. (2015). Physical activity, energy intake and the risk of incident kidney stones. J. Urol. 193 (3), 864–868. doi:10.1016/j.juro.2014.09.010
Ferraro P. M., Taylor E. N., Gambaro G., Curhan G. C. (2017). Vitamin D intake and the risk of incident kidney stones. J. Urol. 197 (2), 405–410. doi:10.1016/j.juro.2016.08.084
Golmohammadi M., Ayremlou P., Zarrin R. (2021). Higher oxidative balance score is associated with better glycemic control among Iranian adults with type-2 diabetes. Int. J. Vitam. Nutr. Res. 91 (1-2), 31–39. doi:10.1024/0300-9831/a000596
Hernández-Ruiz Á., García-Villanova B., Guerra-Hernández E. J., Carrión-García C. J., Amiano P., Sánchez M. J., et al. (2022). Oxidative balance scores (OBSs) integrating nutrient, food and lifestyle dimensions: development of the NutrientL-OBS and FoodL-OBS. Antioxidants (Basel) 11 (2), 300. doi:10.3390/antiox11020300
Howles S. A., Thakker R. V. (2020). Genetics of kidney stone disease. Nat. Rev. Urol. 17 (7), 407–421. doi:10.1038/s41585-020-0332-x
Huang H. S., Ma M. C. (2015). High sodium-induced oxidative stress and poor anticrystallization defense aggravate calcium oxalate crystal formation in rat hyperoxaluric kidneys. PLoS One 10 (8), e0134764. doi:10.1371/journal.pone.0134764
Huang H. S., Ma M. C., Chen J. (2009). Low-vitamin E diet exacerbates calcium oxalate crystal formation via enhanced oxidative stress in rat hyperoxaluric kidney. Am. J. Physiol. Ren. Physiol. 296 (1), F34–F45. doi:10.1152/ajprenal.90309.2008
Jaturakan O., Dissayabutra T., Chaiyabutr N., Kijtawornrat A., Tosukhowong P., Rungsipipat A., et al. (2017). Combination of vitamin E and vitamin C alleviates renal function in hyperoxaluric rats via antioxidant activity. J. Vet. Med. Sci. 79 (5), 896–903. doi:10.1292/jvms.17-0083
Jian Z., Wang M., Jin X., Li H., Wang K. (2021). Diet-derived antioxidants and risk of kidney stone disease: results from the NHANES 2007-2018 and mendelian randomization study. Front. Nutr. 8, 738302. doi:10.3389/fnut.2021.738302
Jones P., Karim Sulaiman S., Gamage K. N., Tokas T., Jamnadass E., Somani B. K. (2021). Do lifestyle factors including smoking, alcohol, and exercise impact your risk of developing kidney stone disease? Outcomes of a systematic review. J. Endourol. 35 (1), 1–7. doi:10.1089/end.2020.0378
Khan S. R. (2014). Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis. Transl. Androl. Urol. 3 (3), 256–276. doi:10.3978/j.issn.2223-4683.2014.06.04
Khan S. R., Canales B. K., Dominguez-Gutierrez P. R. (2021). Randall's plaque and calcium oxalate stone formation: role for immunity and inflammation. Nat. Rev. Nephrol. 17 (6), 417–433. doi:10.1038/s41581-020-00392-1
Khan S. R., Pearle M. S., Robertson W. G., Gambaro G., Canales B. K., Doizi S., et al. (2016). Kidney stones. Nat. Rev. Dis. Prim. 2, 16008. doi:10.1038/nrdp.2016.8
Lee J. H., Son D. H., Kwon Y. J. (2022b). Association between oxidative balance score and new-onset hypertension in adults: a community-based prospective cohort study. Front. Nutr. 9, 1066159. doi:10.3389/fnut.2022.1066159
Lee M. R., Ke H. L., Huang J. C., Huang S. P., Geng J. H. (2022a). Obesity-related indices and its association with kidney stone disease: a cross-sectional and longitudinal cohort study. Urolithiasis 50 (1), 55–63. doi:10.1007/s00240-021-01288-w
Lei X., Xu Z., Chen W. (2023). Association of oxidative balance score with sleep quality: NHANES 2007-2014. J. Affect Disord. 339, 435–442. doi:10.1016/j.jad.2023.07.040
Letavernier E., Verrier C., Goussard F., Perez J., Huguet L., Haymann J. P., et al. (2016). Calcium and vitamin D have a synergistic role in a rat model of kidney stone disease. Kidney Int. 90 (4), 809–817. doi:10.1016/j.kint.2016.05.027
Littlejohns T. J., Neal N. L., Bradbury K. E., Heers H., Allen N. E., Turney B. W. (2020). Fluid intake and dietary factors and the risk of incident kidney stones in UK biobank: a population-based prospective cohort study. Eur. Urol. Focus 6 (4), 752–761. doi:10.1016/j.euf.2019.05.002
Liu X., Liu X., Wang Y., Zeng B., Zhu B., Dai F. (2023). Association between depression and oxidative balance score: national health and nutrition examination survey (NHANES) 2005-2018. J. Affect Disord. 337, 57–65. doi:10.1016/j.jad.2023.05.071
Ma M. C., Chen Y. S., Huang H. S. (2014). Erythrocyte oxidative stress in patients with calcium oxalate stones correlates with stone size and renal tubular damage. Urology 83 (2), 510.e9–17. doi:10.1016/j.urology.2013.09.050
Malihi Z., Lawes C. M. M., Wu Z., Huang Y., Waayer D., Toop L., et al. (2019). Monthly high-dose vitamin D supplementation does not increase kidney stone risk or serum calcium: results from a randomized controlled trial. Am. J. Clin. Nutr. 109 (6), 1578–1587. doi:10.1093/ajcn/nqy378
Malihi Z., Wu Z., Stewart A. W., Lawes C. M., Scragg R. (2016). Hypercalcemia, hypercalciuria, and kidney stones in long-term studies of vitamin D supplementation: a systematic review and meta-analysis. Am. J. Clin. Nutr. 104 (4), 1039–1051. doi:10.3945/ajcn.116.134981
Marić I., Kizivat T., Smolić M., Smolić R., Opačak-Bernardi T., Šolić K., et al. (2019). LIFESTYLE RISK FACTORS AND BONE MASS IN RECURRENT STONE-FORMING PATIENTS: A CROSS-SECTIONAL STUDY IN 144 SUBJECTS. Acta Clin. Croat. 58 (3), 439–445. doi:10.20471/acc.2019.58.03.06
Milicevic S., Bijelic R., Krivokuca V., Bojic M., Popovic-Pejicic S., Bojanic N. (2013). Correlation of the body mass index and calcium nephrolithiasis in adult population. Med. Arch. 67 (6), 423–427. doi:10.5455/medarh.2013.67.423-427
Nirumand M. C., Hajialyani M., Rahimi R., Farzaei M. H., Zingue S., Nabavi S. M., et al. (2018). Dietary plants for the prevention and management of kidney stones: preclinical and clinical evidence and molecular mechanisms. Int. J. Mol. Sci. 19 (3), 765. doi:10.3390/ijms19030765
Pietropaolo A., Proietti S., Geraghty R., Skolarikos A., Papatsoris A., Liatsikos E., et al. (2017). Trends of 'urolithiasis: interventions, simulation, and laser technology' over the last 16 years (2000-2015) as published in the literature (PubMed): a systematic review from European section of Uro-technology (ESUT). World J. Urol. 35 (11), 1651–1658. doi:10.1007/s00345-017-2055-z
Rehman K., Akash M. S. H. (2017). Mechanism of generation of oxidative stress and pathophysiology of type 2 diabetes mellitus: how are they interlinked? J. Cell Biochem. 118 (11), 3577–3585. doi:10.1002/jcb.26097
Sakhaee K., Maalouf N. M., Sinnott B. (2012). Clinical review. Kidney stones 2012: pathogenesis, diagnosis, and management. J. Clin. Endocrinol. Metab. 97 (6), 1847–1860. doi:10.1210/jc.2011-3492
Sasaki Y., Kohjimoto Y., Iba A., Matsumura N., Hara I. (2015). Weight loss intervention reduces the risk of kidney stone formation in a rat model of metabolic syndrome. Int. J. Urol. 22 (4), 404–409. doi:10.1111/iju.12691
Siener R., Hesse A. (2021). Effect of Black tea consumption on urinary risk factors for kidney stone formation. Nutrients 13 (6), 4434. doi:10.3390/nu13124434
Sorensen M. D., Chi T., Shara N. M., Wang H., Hsi R. S., Orchard T., et al. (2014). Activity, energy intake, obesity, and the risk of incident kidney stones in postmenopausal women: a report from the Women's Health Initiative. J. Am. Soc. Nephrol. 25 (2), 362–369. doi:10.1681/ASN.2013050548
Taheri M., Tavasoli S., Shokrzadeh F., Amiri F. B., Basiri A. (2019). Effect of vitamin D supplementation on 24-hour urine calcium in patients with calcium Urolithiasis and vitamin D deficiency. Int. Braz J. Urol. 45 (2), 340–346. doi:10.1590/S1677-5538.IBJU.2018.0522
Tamadon M. R., Nassaji M., Ghorbani R. (2013). Cigarette smoking and nephrolitiasis in adult individuals. Nephrourol. Mon. 5 (1), 702–705. doi:10.5812/numonthly.5251
Tangvarasittichai S. (2015). Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 6 (3), 456–480. doi:10.4239/wjd.v6.i3.456
Tasian G. E., Ross M. E., Song L., Grundmeier R. W., Massey J., Denburg M. R., et al. (2017). Dietary zinc and incident calcium kidney stones in adolescence. J. Urol. 197 (5), 1342–1348. doi:10.1016/j.juro.2016.11.096
Thamilselvan V., Menon M., Thamilselvan S. (2014). Oxalate at physiological urine concentrations induces oxidative injury in renal epithelial cells: effect of α-tocopherol and ascorbic acid. BJU Int. 114 (1), 140–150. doi:10.1111/bju.12642
Thongprayoon C., Krambeck A. E., Rule A. D. (2020). Determining the true burden of kidney stone disease. Nat. Rev. Nephrol. 16 (12), 736–746. doi:10.1038/s41581-020-0320-7
Umekawa T., Hatanaka Y., Kurita T., Khan S. R. (2004). Effect of angiotensin II receptor blockage on osteopontin expression and calcium oxalate crystal deposition in rat kidneys. J. Am. Soc. Nephrol. 15 (3), 635–644. doi:10.1097/01.asn.0000113321.49771.2d
Wang A., Wang N., Zhang D., Wen J., Wang W. (2023). Relationship between serum selenium level and self-reported history of kidney stone. Nutrients 15 (11), 2549. doi:10.3390/nu15112549
Wang Z., Zhang Y., Zhang J., Deng Q., Liang H. (2021). Recent advances on the mechanisms of kidney stone formation (Review). Int. J. Mol. Med. 48 (2), 149. doi:10.3892/ijmm.2021.4982
Williams J., Holmes R. P., Assimos D. G., Mitchell T. (2016). Monocyte mitochondrial function in calcium oxalate stone formers. Urology 93, 224.e1–e6. doi:10.1016/j.urology.2016.03.004
Wu J., Yang Z., Wei J., Zeng C., Wang Y., Yang T. (2020). Association between serum magnesium and the prevalence of kidney stones: a cross-sectional study. Biol. Trace Elem. Res. 195 (1), 20–26. doi:10.1007/s12011-019-01830-3
Xu Z., Yao X., Duan C., Liu H., Xu H. (2023). Metabolic changes in kidney stone disease. Front. Immunol. 14, 1142207. doi:10.3389/fimmu.2023.1142207
Zhang W., Peng S. F., Chen L., Chen H. M., Cheng X. E., Tang Y. H. (2022). Association between the oxidative balance score and telomere length from the national health and nutrition examination survey 1999-2002. Oxid. Med. Cell Longev. 2022, 1345071. doi:10.1155/2022/1345071
Zhu J., Wang Q., Li C., Lu Y., Hu H., Qin B., et al. (2019). Inhibiting inflammation and modulating oxidative stress in oxalate-induced nephrolithiasis with the Nrf2 activator dimethyl fumarate. Free Radic. Biol. Med. 134, 9–22. doi:10.1016/j.freeradbiomed.2018.12.033
Zhu W., Wang C., Wu J., Chen S., Mao W., Chen Y., et al. (2022). Dietary copper intake and the prevalence of kidney stones among adult in the United States: a propensity score matching study. Front. Public Health 10, 973887. doi:10.3389/fpubh.2022.973887
Keywords: oxidative balance score, kidney stones, NHANES, antioxidants, pro-oxidants
Citation: Ke R, He Y and Chen C (2023) Association between oxidative balance score and kidney stone in United States adults: analysis from NHANES 2007-2018. Front. Physiol. 14:1275750. doi: 10.3389/fphys.2023.1275750
Received: 18 August 2023; Accepted: 17 October 2023;
Published: 03 November 2023.
Edited by:
Tomasz Kostka, Medical University of Lodz, PolandReviewed by:
Felix Grases, University of the Balearic Islands, SpainThasinas Dissayabutra, Chulalongkorn University, Thailand
Copyright © 2023 Ke, He and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chaohao Chen, krjtc123@126.com
 Runjiang Ke
Runjiang Ke Chaohao Chen
Chaohao Chen