- 1Department of Otolaryngology-Head and Neck Surgery, Jinling Hospital, Medical School of Nanjing University, Nanjing, China
- 2The First Clinical Medical College, Nanjing Medical University, Nanjing, China
Background: Trace metals have side-effect on human health. The association between trace metals exposure and hearing loss remains unclear.
Methods: A total of 8,128 participants were exacted for analysis of association between trace metals and hearing loss from the database of the National Health and Nutrition Examination Survey (NHANES) (2013–2018). Multivariable logistic regression and restricted cubic spline models were used to examine the association between trace metals and hearing loss.
Results: Participants with hearing loss had a higher level of lead, cadmium, molybdenum, tin, thallium, and tungsten (all p < 0.05). After adjusting for confounders, compared with the reference of the lowest quartile, the ORs with 95%CIs for hearing loss across quartiles were 1.14 (0.86, 1.51), 1.49 (1.12, 1.98), 1.32 (0.97, 1.80) for cobalt, and 1.35 (0.98, 1.87), 1.58 (1.15, 2.16), 1.75 (1.28, 2.40) for tin. Individuals with the level of cobalt at third quartile had 49% higher risks of hearing loss than those at lowest quartile. And participants with highest quartile of tin had 1.75-folds risks of hearing loss than those with lowest quartile of tin. There were increasing trends in risks of hearing loss with a raised level of thallium (p for trend <0.05). Restricted cubic spline regression analysis indicated that there was a nonlinear association between hearing loss and the levels of tin (p for nonlinearity = 0.021). Subgroup analysis showed that individuals of female, without hypertension and diabetes, and with a higher level of low-density lipoprotein cholesterol had modified effects on the associations between hearing loss and exposure to tin.
Conclusions: Our study indicated that exposure to cobalt and tin were significantly associated with hearing loss.
Introduction
It is widely known that metals perform a critical function in the biochemical process. Essential trace elements (e.g., cobalt, iron, zinc, or copper) play a crucial role in enzyme synthesis, which regulate cellular homeostasis and energy production (1). Other elements such as lead, cadmium, tin, or mercury have an unknown impact on human body and are even toxic at certain levels (1, 2). Exposure to trace metals mainly occurred from our daily diet, inhalation, and dermal contact (1, 3). Source of lead can be released from batteries, pipes, pottery, and roofing materials, and cadmium can be touched by burning fossil fuels, cigarette smoke, and contaminated foods (4). Chronic or acute exposure to trace metals, even though the accumulation of essential elements at a high level, has a serious adverse effect on health (2, 5, 6). Excessive concentration of cobalt may lead to peripheral neuropathy, vision loss, sensorineural hearing loss, and cognitive decline (7). It was reported that early childhood exposure to lead may be a vital risk factor for hearing loss (8). Moreover, an epidemiologic study revealed that low-level exposure to cadmium was associated with hearing loss (9). Due to regeneration disabilities of the sensory receptor cells in the inner ear, the hearing loss induced by such toxicants is permanent (4).
Hearing loss is the fourth leading contributor to years lived with a disability worldwide (10). Various factors may contribute to hearing loss, including congenital, infectious, noise exposure, and aging. In clinical practice, hearing loss is defined as a pure-tone average exceeding 25dB (11). Several studies revealed that exposure to lead and cadmium could cause auditory dysfunction using 25dB as the threshold to identify hearing loss (9, 12, 13). But the grade of hearing conditions varied broadly, mild hearing loss may be reversible after correcting incentives or self-healing. It might be inappropriate to recklessly include all grades of hearing status to determine the association between hearing loss and heavy metals. Therefore, we extracted the data of hearing conditions with serious hearing difficulty from the National Health and Nutrition Examination Survey (NHANES), aiming to explore the relationship between severe hearing loss and urine trace metals.
Methods
Study population
The NHANES survey is a cross-sectional program recruiting a representative sample of participants from the United States population based on a complex, multistage probability design. The program collected data from demographic, clinical, and laboratory tests for surveyed individuals. The NHANES database can be acquired publicly from the Centers for Disease Control and Prevention National Center for Health Statistics (https://www.cdc.gov/nchs/nhanes/index.htm). Potential participants were exacted from the dataset of 2013–2018 (N = 29,400). Excluding those with unclear hearing conditions (n = 1,159), and missing data on urine trace metals (n = 20,113). A total of 8,128 participants were enrolled for further analysis. The survey protocol was approved by the NCHS research ethics review board, and all participants provided written informed consent. This study complied with the Declaration of Helsinki.
Assessment of trace metals
Urine samples were measured for trace level element analysis by inductively coupled plasma mass spectrometry (ICP-MS) coupled with dynamic reaction cell technology (DRC). Liquid samples were introduced into the ICP through a nebulizer and spray chamber carried by a flowing argon stream, which ionizes the atoms. The ions are finally counted in rapid sequence at the detector allowing individual isotopes of an element to be determined. More detailed descriptions can be available at https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/labmethods/UTAS_UTASS_UM_UMS_I_MET.PDF. The current study included 10 trace metals elements (lead, barium, cadmium, cobalt, cesium, molybdenum, antimony, tin, thallium, and tungsten).
Definition of hearing loss
Hearing loss in this study was defined as severe difficulty hearing. A household questionnaire on disability was provided to the surveyed participants by trained interviewers. Hearing loss was recorded if the person answered “yes” to the following question: are you deaf or do you have serious difficulty hearing.
Covariates
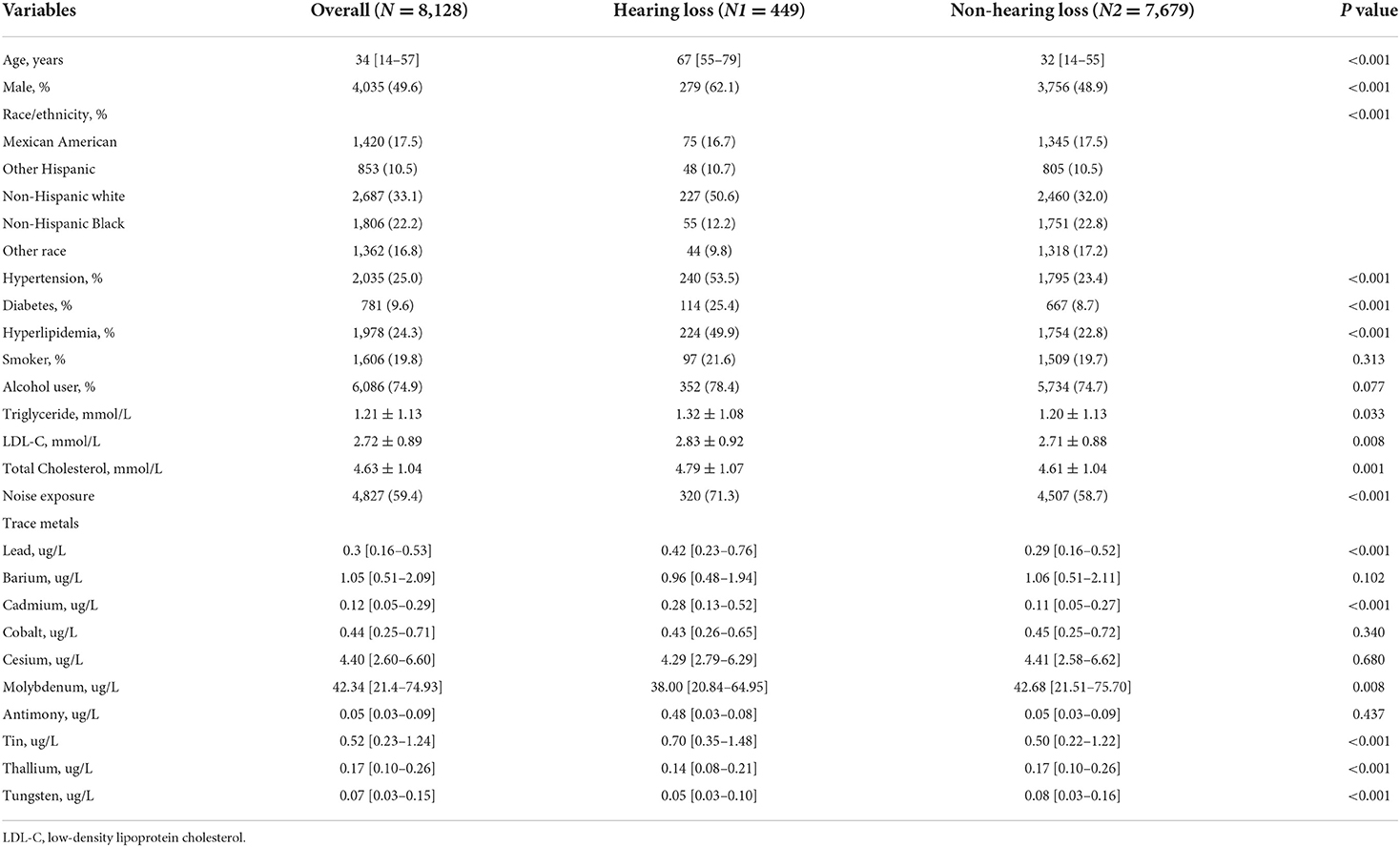
Demographic data including age, gender, and race/ethnicity (Mexican American, other Hispanic, non-Hispanic white, non-Hispanic black, and other race) were collected from self-reports. As atherogenic risk factors are involved in the pathogenesis of hearing loss, covariates related to atherosclerosis were selected and controlled. Self-reported diabetes history, hypertension history, or hyperlipidemia history (yes/no), smoker (smoked at least 100 cigarettes in life), and alcohol user (at least 12 alcoholic drinks per year or not) were documented from questionnaire data. Noise exposure was defined as “ever exposed to very loud noise at work.” The level of triglyceride, total cholesterol, low-density lipoprotein cholesterol, and urine trace metals were obtained from laboratory data.
Statistical analysis
Data distribution was determined by the Kolmogorov-Smirnov test. Continuous data with normal distribution were presented as mean ± deviation and were analyzed with independent sample student's t-test. Nonnormal distributed continuous data were reported as median with interquartile range (IQR) and compared by nonparametric Mann–Whitney U-test. Categorical variables were calculated with counts and percentages and were assessed by the Chi-square test. Log2-transform of trace metals was performed to facilitate the interpretation due to its skewed distribution.
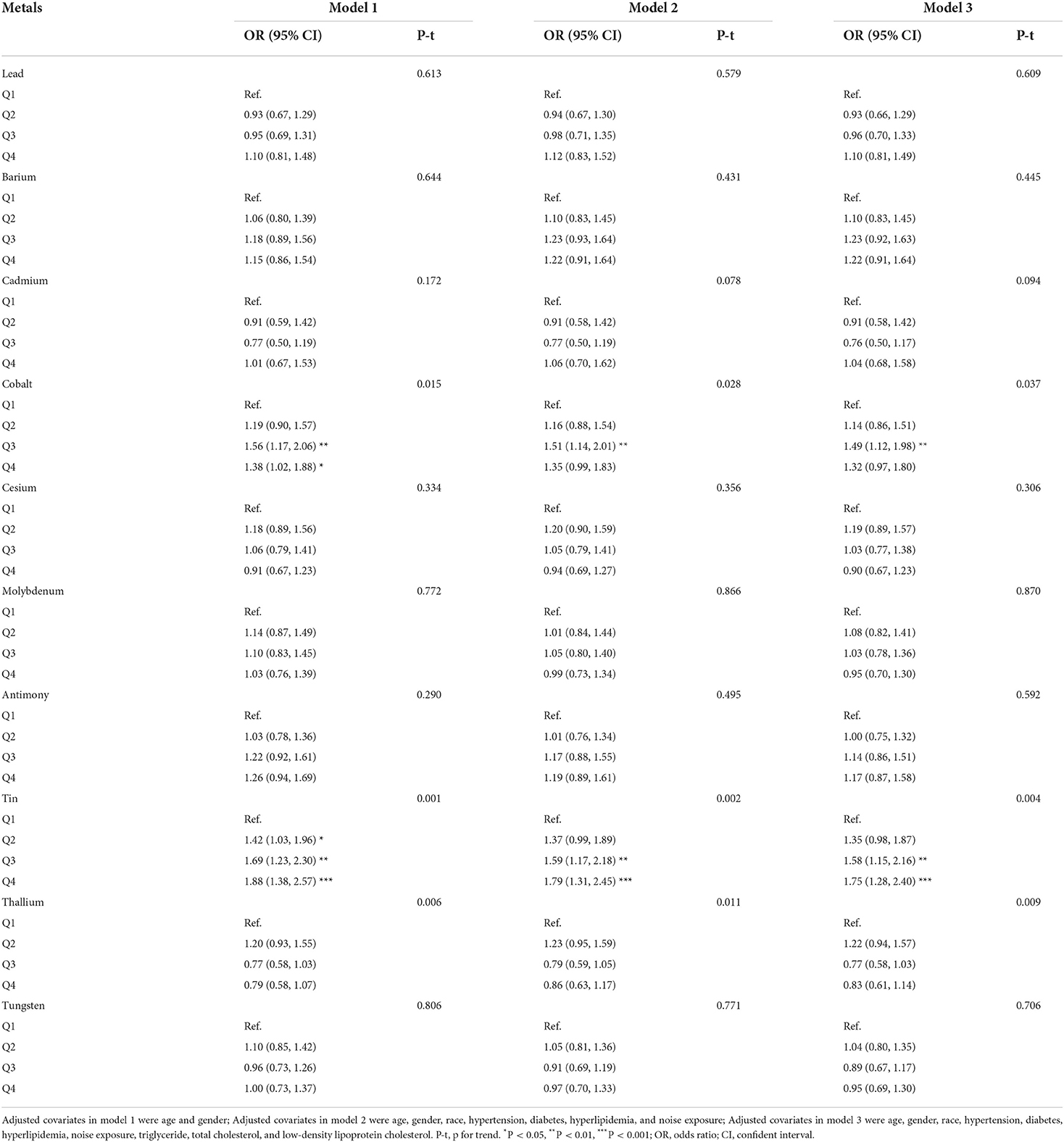
Multivariate logistic regression analysis was used to assess the association of trace metals quartiles with hearing loss. Model 1 was adjusted for age and gender. Model 2 was adjusted for age, gender, race, hypertension, diabetes, hyperlipidemia, and noise exposure. And model 3 was adjusted for age, gender, race, hypertension, diabetes, hyperlipidemia, noise exposure, triglyceride, total cholesterol, and low-density lipoprotein cholesterol. In order to explore the effect of multiple-metal on hearing loss, we conducted a collinearity diagnostic to determine whether there are collinearities among these metals. Variance inflation factors (VIF) and tolerance are used to identify a collinearity (Tolerance<0.1 or VIF>10 is considered to be collinear). We include ten trace metals into covariates when they are non-collinear, as well as the covariates in the model 3 to build a multiple-metal based model. The odds ratio (OR) with 95% confidence intervals (CI) was calculated.
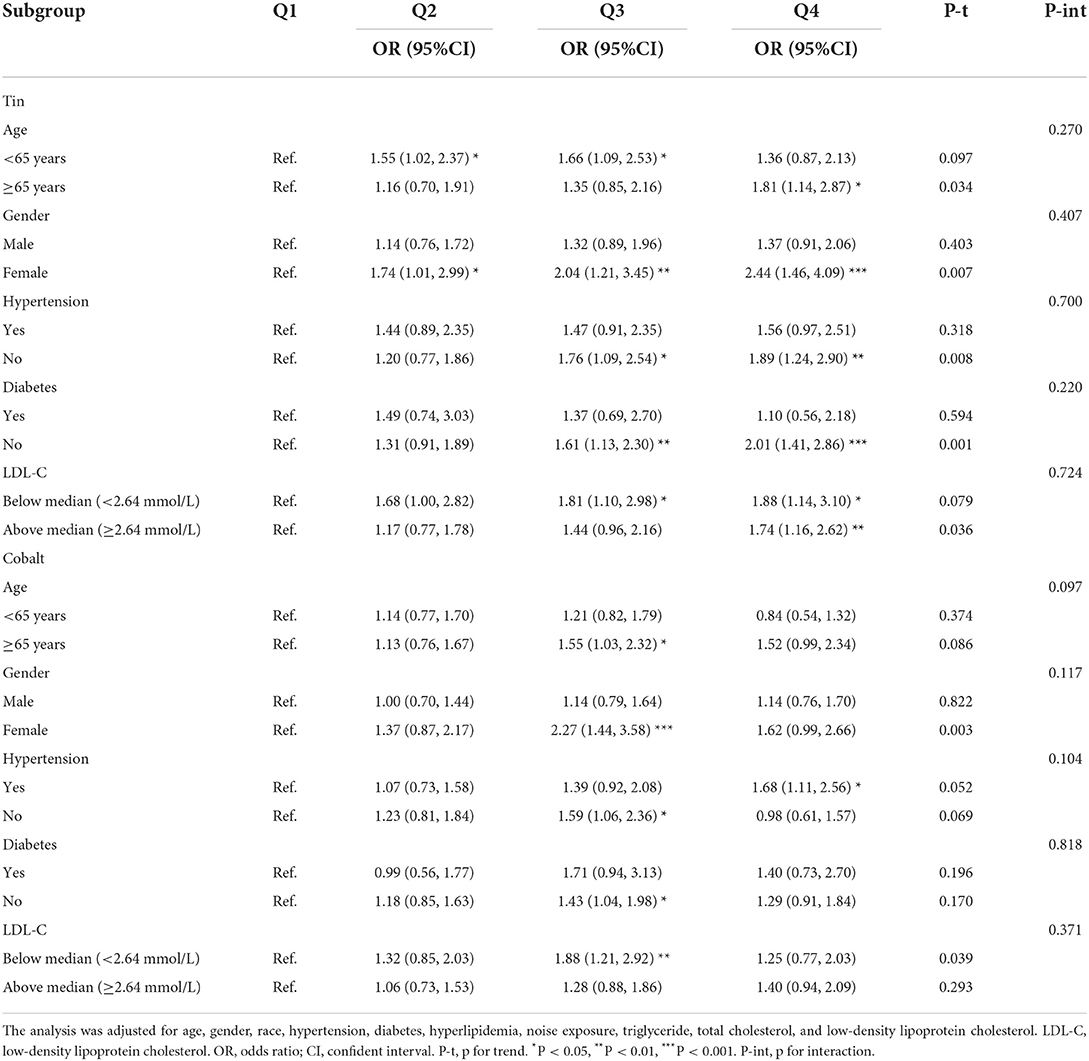
A restricted cubic spline regression model was applied to explore any nonlinear relationship between trace metals and hearing loss [with 3 knots located at 10th, 50th, and 90th percentiles confirmed according to the Akaike information criterion (14)]. Subgroup analyses stratified by age, gender, hypertension, diabetes, and level of low-density lipoprotein cholesterol were conducted to determine the associations between trace metals and hearing loss. All statistical analyses were performed by R software (version 4.0.5) and SPSS software (version 25). Two-sided p < 0.05 was considered statistically significant.
Results
Baseline characteristics
A total of 8,128 participants were included in this study, with a prevalence of hearing loss of approximately 5.5%. There were significant differences between the hearing group and non-hearing group in age, gender, race/ethnicity, hypertension, diabetes, hyperlipidemia, noise exposure, levels of triglyceride, total cholesterol, and low-density lipoprotein cholesterol (All p < 0.05). As for the concentration of urine trace metals, the hearing loss group had a higher level of lead, cadmium, molybdenum, tin, thallium, and tungsten (All p < 0.05). The general presentation of baseline characteristics was shown in Table 1.
Association between trace metals and hearing loss
Table 2 presented the results of logistic regression analyses for the association between trace metals and hearing loss. When fully adjusted for potential confounders, compared with the reference of lowest quartile, the ORs with 95%CIs for hearing loss across highest quartile was 1.10 (0.81, 1.49) for lead, 1.22 (0.91, 1.64) for barium, 1.04 (0.68, 1.58) for cadmium, 1.32 (0.97, 1.80) for cobalt, 0.90 (0.67, 1.23) for cesium, 0.95 (0.70, 1.30) for molybdenum, 1.17 (0.87, 1.58) for antimony, 1.75 (1.28, 2.40) for tin, 0.83 (0.61, 1.14) for thallium, and 0.95 (0.69, 1.30) for tungsten, respectively. Cobalt, and tin were significantly associated with hearing loss (p < 0.05). Individuals with the level of cobalt at third quartile had 49% higher risks of hearing loss than those at lowest quartile. And participants with highest quartile of tin had 1.75-folds risks of hearing loss than those with lowest quartile of tin. Restricted cubic spline indicated that there was a nonlinear association between hearing loss and the levels of tin (p for nonlinearity = 0.021, Figure 1A). However, the association between the risk of hearing loss and levels of cobalt was not nonlinear (p for nonlinearity > 0.05, Figure 1B). We also conducted multiple-metal based Cox-regression analyses including 10 trace metals as covariates (Table S1). It showed that higher level of lead, cadmium, tin, thallium, and tungsten were statistically associated with hearing loss in an unadjusted model. After adjusting for all confounders, higher tin concentration retained higher risk of hearing loss. The highest quartile of tin had 2.09-folds risks for hearing loss compared with the lowest quartile. And cobalt was also closely related to the occurrence of hearing loss in the full adjusted model.
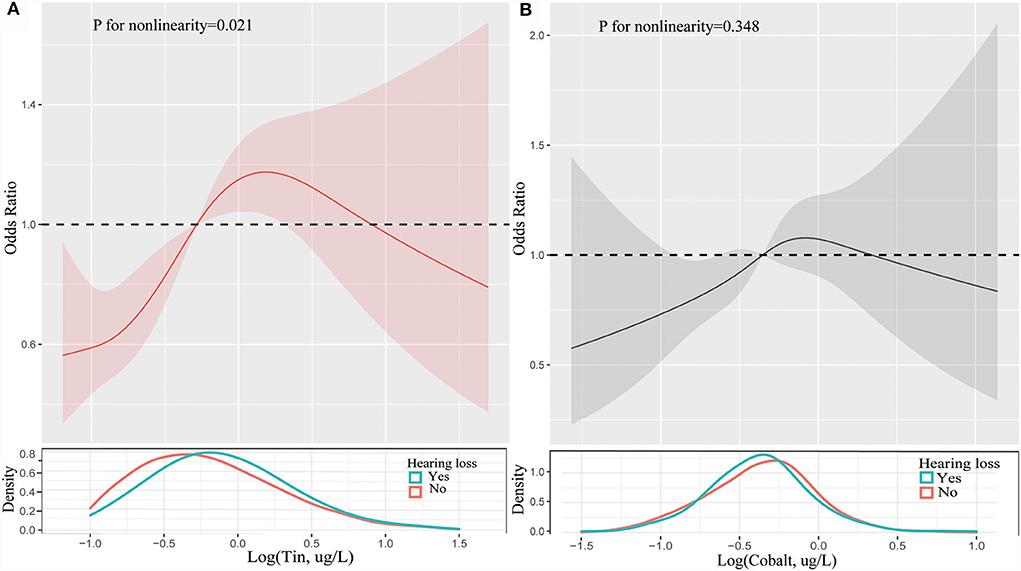
Figure 1. Restricted cubic spline of association between the levels of tin (A) and cobalt (B) and the risks of hearing loss. Analyses were adjusted for age, gender, race, hypertension, diabetes, hyperlipidemia, noise exposure, triglyceride, total cholesterol and low-density lipoprotein cholesterol. The solid line and dashed line represent the log-transformed odds ratios and corresponding 95% confidence intervals.
Subgroup analysis
When stratified by age, gender, hypertension, diabetes, and the level of low-density lipoprotein cholesterol, we determined the effect size of these factors on hearing loss. As shown in Table 3, individuals of female, without hypertension and diabetes, and with a higher level of LDL-C had modified effects on the association between hearing loss and exposure to tin. Similarly, compared with reference of lowest quartile, participants of female still had higher risks of hearing loss when exposed to higher level of cobalt (OR 2.27, 95% CI 1.44–3.58). Interestingly, participants with a lower level of LDL-C were more likely to have hearing loss in the third quartile of cobalt (OR 1.88, 95%CI 1.21–2.92).
Discussion
This is the first study that implicated the association between trace metals and severe hearing loss. There are three main findings of the current study. Firstly, we highlighted the role of atherogenic risk factors (aging, hypertension, diabetes, hyperlipidemia, et. al) in the prevalence of hearing loss. Secondly, tin was firstly found to be significantly associated with hearing loss in a large sample cross-sectional study. Lastly, the trends of association between hearing loss and the level of tin were nonlinear.
Hearing loss affects individuals worldwide with nearly 6%-8% of the world population (10). In our study, we reported the prevalence of hearing loss of 5.5% based on a representative sample, which was closed to the above literature. Traditional risk factors for hearing loss were infectious, noise exposure, and aging (10). It was demonstrated that atherosclerosis may influence hearing loss (15), so we compared the difference in atherogenic risk factors between the population with hearing loss and without hearing loss. Consistently, the proportions of hypertension, diabetes, hyperlipidemia, and the level of blood lipid profile were significantly higher in the population with hearing loss. The above comorbidities were well confirmed in the pathophysiological process of atherosclerosis and were independent risk factors for coronary artery disease and stroke. These comorbidities might contribute to sclerosis of the blood vessels that supply hearing organs, leading to limited blood flow and declining hearing function.
A series of case reports showed that systemic cobalt toxicity was closed related to peripheral neuropathy, vision loss, sensorineural hearing loss, and cognitive decline (6, 7, 12). Rizzetti et al. reported a case of hearing and vision loss after hip-arthroplasty, which contained cobalt, with increased concentration of cobalt in urine and blood samples (7). Cobalt can produce various toxicological effects including local respiratory toxicity, neurological toxicity, and immunological toxicity (7). Cobalt can induce a hypoxia-like effect, possibly targeting mitochondria (7) and impairing nerve function, with patients presenting hearing loss. An experimental study using rabbits to explore its pathological alterations after repeated intravenous injection of cobalt indicated clinical signs indicative of auditory and optic system toxicity (16). In addition, histopathological examination showed severe retinal and cochlear ganglion cell depletion along with optic nerve damage and loss of sensory cochlear hair cells in these experimental rabbits (16). This evidence could further confirm the neurological toxic effect of cobalt. It was reported that higher level of tin in individuals with hearing frustration (17). And we also determined that cobalt is closely associated with hearing loss from the perspective of epidemiological study. Continuous cobalt exposure mainly concerning workers exposed in occupational setting is deemed to be detrimental to health.
Tin is a naturally existing element, which is found in inorganic and organic forms in the environment. Exposure to tin and tin compounds in the U.S. population is almost ubiquitous (9). Tin levels in the body increase with age in adult (9). World Health Organization classified the tin as potentially toxic (18). Studies investigated that indium-tin-oxide could cause pulmonary and systemic toxicity in rat models (19). It also demonstrated that elevated tin level was associated with increased fasting glucose level in coke oven workers in China (20). Shiue et al. reported that the level of tin was higher in hearing frustration population (17), which was consistent with our results. However, experimental investigations for the links of tin to auditory dysfunction are less conducted, mechanism of tin-induced hearing loss remained unclear.
Heavy metals are particular chemical class of elements and present in the environment. Most of them are harmful even at low concentrations. Several potential mechanisms are implicated in metals toxicity including production of oxygen reactive species (ROS), interaction with thiol groups of proteins, incorrect protein folding and mimicry of the essential elements for intracellular transport, and depletion of antioxidants enzymes (1). Cadmium is capable to bypass the blood-brain barrier (21). On the other hand, in human autopsies of individuals with diverse neurodegenerative diseases, significantly higher level of accumulation of Cd in locus ceruleus was observed (22). Similarly, tin as a heavy metal element, plays a critical role in neurological disease and autoimmune disease (23, 24). Researchers and in our study, tin was dramatically associated with hearing loss. We assumed that it impaired hearing through the destruction of neurons. Further cellular and animal research is prompted for verification.
There are several limitations of our study. Firstly, a cross-sectional study design can only infer the correlation, but causality. Secondly, despite the attempts to adjust hearing-related confounders, unmeasured or unknown covariates may influence the effectiveness of the conclusion. Additionally, the mechanism of the association between hearing loss and trace metals has not been fully addressed. Finally, the data of this study was derived from the United state, the generalizability of the conclusion to other regions or populations should be very cautious.
Conclusions
Our study indicated that exposure to cobalt and tin were significantly associated with hearing loss.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
The studies involving human participants were reviewed and the survey protocol was approved by the NCHS research ethics review board. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
PZ: conceptualization, methodology, and writing-original draft. ML: data curation, software, formal analysis, and writing-review and editing. WC: writing-original draft. JJ, FX, and LX: investigation. YC: validation. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Strengthening Health through Science and Education Program of Nanjing Commission of Health (SZDZK202002). The funding body played no role in the design of the study and collection, analysis, and interpretation of data, and in writing the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.973832/full#supplementary-material
References
1. Cannas D, Loi E, Serra M, Firinu D, Valera P, Zavattari P. Relevance of essential trace elements in nutrition and drinking water for human health and autoimmune disease risk. Nutrients. (2020) 12:2074. doi: 10.3390/nu12072074
2. Esplugas R, Mari M, Marquès M, Schuhmacher M, Domingo JL, Nadal M. Biomonitoring of trace elements in hair of schoolchildren living near a hazardous waste incinerator-A 20 years follow-up. Toxics. (2019) 7:52. doi: 10.3390/toxics7040052
3. Mamtani R, Stern P, Dawood I, Cheema S. Metals and disease: a global primary health care perspective. J Toxicol. (2011) 2011:319136. doi: 10.1155/2011/319136
4. Rosati R, Jamesdaniel S. Environmental exposures and hearing loss. Int J Environ Res Public Health. (2020) 17:4879. doi: 10.3390/ijerph17134879
5. Domingo JL, Marquès M. The effects of some essential and toxic metals/metalloids in COVID-19: a review. Food Chem Toxicol. (2021) 152:112161. doi: 10.1016/j.fct.2021.112161
6. Devlin JJ, Pomerleau AC, Brent J, Morgan BW, Deitchman S, Schwartz M. Clinical features, testing, and management of patients with suspected prosthetic hip-associated cobalt toxicity: a systematic review of cases. J Med Toxicol. (2013) 9:405–15. doi: 10.1007/s13181-013-0320-0
7. Rizzetti MC, Liberini P, Zarattini G, Catalani S, Pazzaglia U, Apostoli P, et al. Loss of sight and sound Could it be the hip? Lancet. (2009) 373:1052. doi: 10.1016/S0140-6736(09)60490-6
8. Liu Y, Huo X, Xu L, Wei X, Wu W, Wu X, et al. Hearing loss in children with e-waste lead and cadmium exposure the science of the total environment. (2018) 624:621–7. doi: 10.1016/j.scitotenv.2017.12.091
9. Choi YH, Hu H, Mukherjee B, Miller J. Park SK. Environmental cadmium and lead exposures and hearing loss in US adults: the National Health and Nutrition Examination Survey, 1999 to 2004. Environ Health Perspect. (2012) 120:1544–50. doi: 10.1289/ehp.1104863
10. Brown CS, Emmett SD, Robler SK, Tucci DL. Global hearing loss prevention. Otolaryngol Clin North Am. (2018) 51:575–92. doi: 10.1016/j.otc.2018.01.006
11. Olusanya BO, Davis AC, Hoffman HJ. Hearing loss grades and the International classification of functioning, disability and health. Bull World Health Organ. (2019) 97:725–8. doi: 10.2471/BLT.19.230367
12. Ghiasvand M, Mohammadi S, Roth B, Ranjbar M. The relationship between occupational exposure to lead and hearing loss in a cross-sectional survey of Iranian workers. Front Public Health. (2016) 4:19. doi: 10.3389/fpubh.2016.00019
13. Shargorodsky J, Curhan SG, Henderson E, Eavey R, Curhan GC. Heavy metals exposure and hearing loss in US adolescents. Arch Otolaryngol Head Neck Surg. (2011) 137:1183–9. doi: 10.1001/archoto.2011.202
14. Portet S. A primer on model selection using the Akaike Information Criterion. Infect Dis Model. (2020) 5:111–28. doi: 10.1016/j.idm.2019.12.010
15. Frederiksen TW, Ramlau-Hansen CH, Stokholm ZA, Brødsgaard Grynderup M, Hansen ÅM, Lund SP, et al. Atherogenic risk factors and hearing thresholds. Audiol Neurootol. (2014) 19:310–8. doi: 10.1159/000365439
16. Apostoli P, Catalani S, Zaghini A, Mariotti A, Poliani PL, Vielmi V, et al. High doses of cobalt induce optic and auditory neuropathy. Exp Toxicol Pathol. (2013) 65:719–27. doi: 10.1016/j.etp.2012.09.006
17. Shiue I. Urinary heavy metals, phthalates, perchlorate, nitrate, thiocyanate, hydrocarbons, and polyfluorinated compounds are associated with adult hearing disturbance: USA NHANES, 2011–2012. Environ Sci Pollut Res Int. (2015) 22:20306–11. doi: 10.1007/s11356-015-5546-8
18. World Health Organization, International Atomic Energy Agency & Food Agriculture Organization of the United Nations. (1996). Trace Elements in Human Nutrition and Health. World Health Organization. Available online at: https://apps.who.int/iris/handle/10665/37931
19. Liu N, Guan Y, Zhou C, Wang Y, Ma Z, Yao S, et al. Pulmonary and systemic toxicity in a rat model of pulmonary alveolar proteinosis induced by indium-tin oxide nanoparticles. Int J Nanomed. (2022) 17:713–31. doi: 10.2147/IJN.S338955
20. Liu B, Feng W, Wang J, Li Y, Han X, Hu H, et al. Association of urinary metals levels with type 2 diabetes risk in coke oven workers. Environ Pollut. (2016) 210:1–8. doi: 10.1016/j.envpol.2015.11.046
21. Oggiano R, Pisano A, Sabalic A, Farace C, Fenu G, Lintas S, et al. An overview on amyotrophic lateral sclerosis and cadmium. Neurol Sci. (2021) 42:531–7. doi: 10.1007/s10072-020-04957-7
22. Pamphlett R, Bishop DP, Kum Jew S, Doble PA. Age-related accumulation of toxic metals in the human locus ceruleus. PLoS ONE. (2018) 13:e0203627. doi: 10.1371/journal.pone.0203627
23. Lehmler HJ, Gadogbe M, Liu B. Bao W. Environmental tin exposure in a nationally representative sample of US adults and children: the national health and nutrition examination survey 2011-2014. Environ Pollut. (2018) 240:599–606. doi: 10.1016/j.envpol.2018.05.019
Keywords: trace metals, exposure, health, hearing loss, association
Citation: Zou P, Li M, Chen W, Ji J, Xue F, Wang Z, Xu L and Cheng Y (2022) Association between trace metals exposure and hearing loss. Front. Public Health 10:973832. doi: 10.3389/fpubh.2022.973832
Received: 20 June 2022; Accepted: 01 August 2022;
Published: 17 August 2022.
Edited by:
Fabrizio Bianchi, National Research Council (CNR), ItalyReviewed by:
Yansen Bai, Guangzhou Medical University, ChinaAntonio Caputi, University of Bari Aldo Moro, Italy
Copyright © 2022 Zou, Li, Chen, Ji, Xue, Wang, Xu and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: You Cheng, dzchengyou@163.com
†These authors have contributed equally to this work
 Peixi Zou1†
Peixi Zou1† Menghuan Li
Menghuan Li