Machine Learning for Assessment of Coronary Artery Disease in Cardiac CT: A Survey
- 1Department of Biomedical Engineering and Physics, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, Netherlands
- 2Amsterdam Cardiovascular Sciences, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, Netherlands
- 3Image Sciences Institute, University Medical Center Utrecht, Utrecht, Netherlands
- 4Department of Radiology, University Medical Center Utrecht, Utrecht, Netherlands
- 5Department of Radiology and Nuclear Medicine, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, Netherlands
Cardiac computed tomography (CT) allows rapid visualization of the heart and coronary arteries with high spatial resolution. However, analysis of cardiac CT scans for manifestation of coronary artery disease is time-consuming and challenging. Machine learning (ML) approaches have the potential to address these challenges with high accuracy and consistent performance. In this mini review, we present a survey of the literature on ML-based analysis of coronary artery disease in cardiac CT. We summarize ML methods for detection and characterization of atherosclerotic plaque as well as anatomically and functionally significant coronary artery stenosis.
1. Introduction
Diagnosis and monitoring of coronary artery disease (CAD) is increasingly based on non-invasive imaging with computed tomography (CT), allowing excellent visualization of the coronary arteries with high spatial resolution. Cardiac CT exams consist of hundreds of slices and the number of cardiac CT studies has been steadily increasing (1). This has led to an increased workload for medical professionals, which in combination with shortages of trained cardiac imagers (2) might lead to cardiac CT underuse in the clinic. Machine learning (ML) could offer a way to address these challenges and facilitate automatic cardiac CT analysis with consistent and accurate results. Furthermore, ML algorithms might enable an increased range of secondary diagnoses.
This survey provides an overview of ML algorithms for detection, characterization, and quantification of CAD in cardiac CT. We searched PubMed for articles related to ML-based assessment of CAD in cardiac CT published within the last 10 years (search strategy in Supplementary Materials) which led to inclusion of 59 studies. The structure of this survey is as follows. We provide a brief primer on ML in section 2. Applications of ML for automatic detection and characterization of atherosclerotic plaque are summarized in section 3. Studies focusing on ML for anatomical and functional evaluation of luminal stenosis are summarized in section 4. Finally, section 5 provides a discussion of outstanding challenges for transfer of ML algorithms into the clinic.
2. Machine Learning
Machine learning comes in many flavors, but most applications in cardiac CT use supervised learning. In supervised learning, a model is optimized to provide the correct labels as defined by the reference standard during training, and predict a label to new and unseen samples during testing.
Each sample can be described based on characteristics or features. Among the simplest ML algorithms are k-nearest neighbor (kNN) classifiers, which look for training samples with similar feature values to a test sample, and assign the test sample to the majority class among these training samples. Linear classifier (LC) models like support vector machines (SVM) aim to find a linear combination of features to separate samples in different classes. Alternatively, samples can be separated by thresholding feature values along a single axis. This is unlikely to lead to highly accurate classifiers, but by consecutively applying thresholds, a decision tree model can be built for more accurate classification.
ML performance can often be improved by combining predictions of multiple models. Ensembles (E) combine predictions of multiple simultaneously executed models, e.g., by averaging predictions of decision trees in a random forest (RF). In boosting (BO), models are applied consecutively and each model is trained to correct errors of its predecessors. Finally, artificial neural networks (ANNs) transform samples into targets through layers of trainable neurons, which are loosely based on biological neurons. While ANNs have been around since the 1950s, it has recently become possible to train networks that have many layers, i.e., deep learning. The success of deep learning in medical image analysis has been to a large extent due to the inclusion of trainable image filters in so-called convolutional neural networks (CNNs), which can be trained to extract valuable features from raw image data (3). For a more in-depth introduction to ML and deep learning, please refer to Jordan and Mitchell (4).
3. Atherosclerotic Plaque Detection, Characterization, and Quantification
CT offers a non-invasive alternative to e.g., catheter-guided X-ray angiography, optical coherence tomography, and intravascular ultrasound (IVUS) for atherosclerotic plaque visualization. Characterization and quantification of plaque in CT provide insight in different stages of CAD (5). In this section, we survey analysis of methods for calcified plaque (section 3.1) and non-calcified and mixed plaque (section 3.2). Reviewed papers are listed in Table 1.
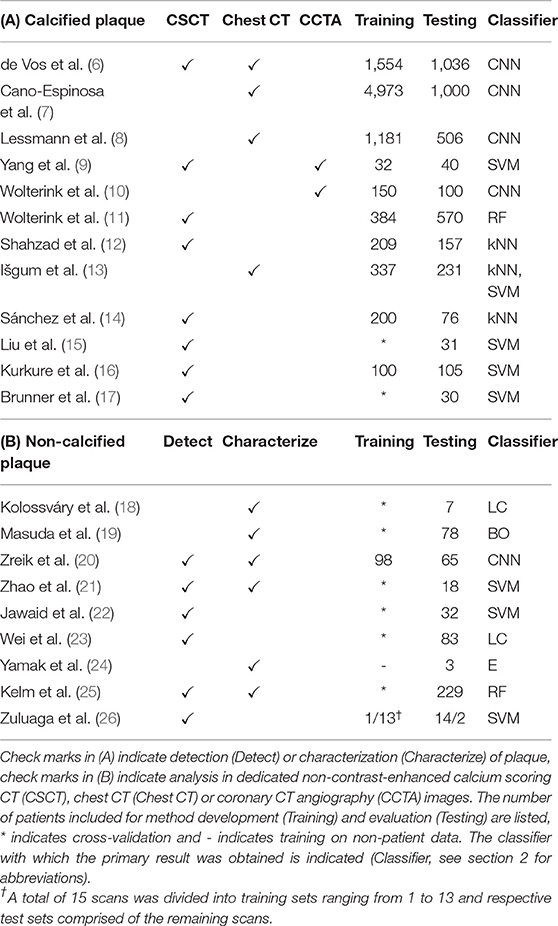
Table 1. Publications related to analysis of (A) calcified and (B) non-calcified and mixed atherosclerotic plaque.
3.1. Calcified Plaque
Coronary artery calcification (CAC) quantification or scoring is typically performed in dedicated non-contrast-enhanced, ECG-triggered, calcium scoring CT images (CSCT). Using dedicated software, an expert identifies voxels with a density over 130 Hounsfield units (HU) in the coronary arteries. Identified CAC is then quantified according to its volume, density, or a combination of both (27). CAC cannot only be quantified in CSCT, but also in other kinds of CT images visualizing the heart, such as cardiac CT angiography (CCTA) and non-gated chest CT. Calcium scoring is not considered a difficult task for trained clinicians, but it is time-consuming when performed in large numbers of images. Hence, automatic ML-based methods have been proposed.
ML-based calcium scoring methods proposed prior to the advent of deep learning have focused on identification of CAC lesions among a large set of samples, i.e., groups of connected voxels above 130 HU. Samples are described with features such as size, shape, appearance and location to distinguish CAC from other candidate lesions such as calcifications in the aorta. Location features are of particular importance, as recognized by Liu et al. (15), Kurkure et al. (16), and Brunner et al. (17) who proposed a heart coordinate system. Similarly, Sánchez et al. (14) described candidate locations relative to anatomical landmarks. Išgum et al. (13) used multi-atlas registration to estimate the location of the coronary artery tree, while Shahzad et al. (12) and Wolterink et al. (11) estimated the location of three major coronary arteries for per-vessel calcium scoring. Yang et al. (9) extracted coronary artery centerlines in CCTA images and propagated these to CSCT images of the same patients to provide location features.
Deep learning-based methods have typically classified individual voxels instead of candidate lesions. Due to the extreme imbalance between numbers of CAC and background voxels in CT images, Wolterink et al. (10) proposed to use two CNNs, where one CNN identified candidate voxels in CCTA and the second CNN further discriminated among identified candidates. Similarly, Lessmann et al. (8) used two CNNs to identify calcified voxels in chest CT. Cano-Espinosa et al. (7) and de Vos et al. (6) avoid voxel-based classification altogether by directly regressing calcium scores in chest CT, enabling automatic scoring in less than a second.
Automatic CAC scoring methods have been validated in large data sets (28) and in other types of CT scans in which the heart is routinely visualized, such as attenuation correction images for PET-CT (29) and CT images acquired for radiotherapy treatment planning (30–32). Wolterink et al. presented a public data set with reference standard for standardized evaluation of CAC scoring in CSCT (33).
3.2. Non-calcified Plaque
Non-calcified plaque is typically lipid-rich and vulnerable to rupture, causing acute coronary syndrome (34). ML-based analysis methods in CCTA have been developed for detection or localization of non-calcified plaque, as well as characterization of lipid and fibrous plaque components.
Coronary artery localization by means of centerline extraction is a typical preprocessing step for ML-based plaque analysis. Traditionally, many automatic centerline extraction methods have been based on minimum cost paths between proximal and distal artery points (35, 36). ML has been used to verify automatic centerline extraction results with an RF (25) or CNN (37). Alternatively, centerlines can be iteratively extracted based on a single seed point. Wolterink et al. (38) showed how such a tracker can be guided by a 3D CNN that locally detects the artery orientation.
Coronary artery centerlines can be used to reconstruct CCTA volumes into images that allow better plaque visualization and identification. Zhao et al. (21), Jawaid et al. (22), Wei et al. (23), and Zuluaga et al. (26) used cross-sectional images along the coronary artery centerline to extract features describing the vessel wall shape and texture. In Jawaid et al. (22) and Wei et al. (23), these features were used in an SVM or linear classifier to determine whether the image contained non-calcified plaque. Similarly, Zuluaga et al. (26) used such features to train an SVM classifying lesion segments as either healthy or diseased, i.e., containing non-calcified or calcified plaque. Zhao et al. (21) trained an SVM to classify cross-sectional images as healthy or containing non-calcified, calcified, or mixed plaque. For the same task, Zreik et al. (20) trained a recurrent CNN that did not depend on hand-crafted feature extraction. Kelm et al. (25) used an RF classifier to classify whether non-calcified or calcified plaque was present along a coronary artery centerline segment.
Characterization of individual components in non-calcified plaque is a challenging task due to low-contrast boundaries between plaque components (39). Yamak et al. (24) exploited additional attenuation data provided by dual-energy CT to characterize plaque in manually determined regions of interest in axial slices. To validate their model in patient scans, manual CCTA annotations by an expert were used. However, obtaining reliable manual reference annotations for non-calcified plaque in CCTA is challenging. Kolossváry et al. (18) determined the reference standard in CCTA through registration of histology images to ex-vivo CCTA scans. Features were extracted for each cross-sectional image and lesions were classified into advanced or early stage atherosclerosis using a linear classifier. Alternatively, Masuda et al. (19) used an in-vivo IVUS-based reference standard to train a boosting classifier with histogram-based features distinguishing fibrous from lipid plaque in CCTA.
4. Coronary Stenosis Detection and Characterization
Non-invasive assessment of CAD-induced stenotic lesions in CT prior to invasive treatment may prevent unnecessary costs and complications (40). Therefore, CT images have long been used to assess the anatomical significance of lesions by a local measurement of luminal narrowing. However, determination of the functional significance of a lesion by taking physiology into account can better stratify patients in need of treatment (41). In this section, we review ML algorithms for the detection and quantification of anatomically (section 4.1) and functionally (section 4.2) significant stenosis. Reviewed papers are listed in Table 2.
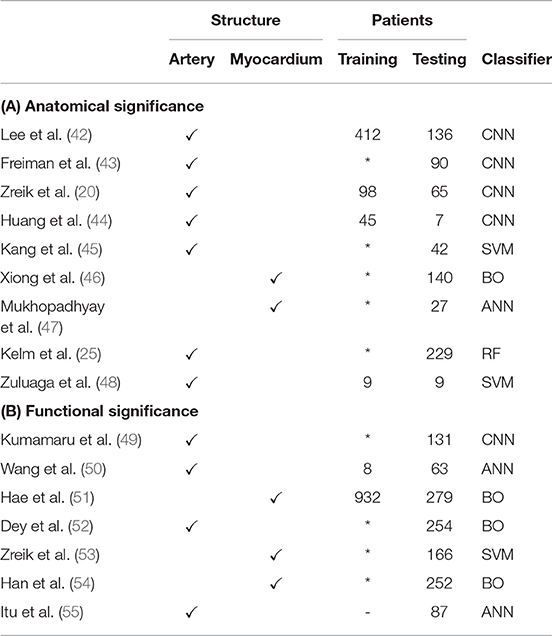
Table 2. Publications related to (A) anatomically and (B) functionally significant stenosis detection.
4.1. Anatomical Significance
Identification of anatomically significant stenotic lesions in CCTA, i.e., those lesions causing a luminal narrowing of at least 50%, allows a first assessment of the severity of stenosis in patients with symptoms of CAD. While this assessment is often based on visual estimation by a clinician, this is a difficult task (56) with substantial inter-observer variability (57). ML-based automatic approaches could reduce this variability.
Stenosis detection typically requires a local measurement of the lumen diameter and an estimation of the healthy lumen diameter. These estimates can be based on automatically extracted centerlines (section 3.2). Many centerline extraction methods also estimate the luminal radius at each centerline point, assuming a circular coronary artery profile (25, 38). However, circular artery profiles are not a realistic assumption for diseased vessel segments. Automatically extracted centerlines can also be used as an initialization for more detailed lumen segmentation. Huang et al. (44) used centerlines to obtain a reformatted image in which the lumen was segmented using a 3D CNN. Lee et al. (42) use centerlines to obtain a tube-shaped prior that is deformed to segment the coronary lumen.
Lumen segmentation is often considered a preprocessing step for stenosis detection, but it has been shown that stenosis degree can also be directly determined based on image data. Zuluaga et al. (48) detected stenosis and artery bifurcations with an SVM based on features obtained from concentric circles in cross-sectional images. Similarly, Kang et al. (45) used geometrical and plaque features in an SVM to detect obstructive lesions (> 50% narrowing) and non-obstructive lesions (25–50% narrowing). Zreik et al. (20) used a recurrent CNN to detect anatomically significant stenosis along the centerline. Freiman et al. (43) detected stenosis of at least intermediate severity (> 40% narrowing) using deep sparse autoencoders, a variation on CNNs.
Coronary stenoses are located in the arteries, but may restrict blood flow to myocardial segments. Mukhopadhyay et al. (47) used an ML approach to identify myocardial segments (58) affected by coronary stenosis. Hand-crafted feature vectors describing the endocardial surface shape were combined using a bag-of-words approach and classified with an ANN to identify affected segments. Xiong et al. (46) performed analysis of the full myocardium to detect existence of at least one anatomically significant stenosis. Instead of the shape of the endocardial surface, features in this approach described the attenuation and wall thickness of myocardial segments.
4.2. Functional Significance
The sensitivity of CCTA-based anatomical stenosis evaluation for detection of functionally significant stenosis is high when evaluated visually, but its specificity is moderate (41). The current reference standard for determination of functional significance of a stenosis is given by its fractional flow reserve (FFR), i.e., the ratio of flow distal of the stenosis to the flow proximal of the stenosis. FFR is measured invasively by inserting a special catheter in the coronary artery under hyperemic conditions. FFR below 0.80 indicates need for intervention (59). Treatment based on invasive FFR measurements can improve patient outcomes (59), but measurement of FFR is still relatively uncommon, which is due to associated cost and risk, as well as lack of vasodilator drugs (60).
FFR estimation based on CCTA scans (FFRCT) could provide reproducible physical measurement without the drawbacks of invasive procedures. FFRCT has traditionally been based on computational fluid dynamics (CFD) (61, 62), i.e., numerical simulation of blood flow in a coronary tree model extracted from CCTA using lumen segmentation methods (section 4.1). These methods are accurate (63) but computationally expensive due to their iterative nature. This precludes their deployment on local workstations, and instead CFD simulations are typically performed on off-site dedicated systems. ML could be used to significantly speed up estimation of FFRCT.
Itu et al. (55) proposed an ANN model to predict an FFR value for each segment in the coronary artery tree, given local features based on the segment's geometry and global features based on the most severe stenoses. To train this model, a large data set of 12,000 synthetic coronary artery trees was generated and a reference standard was obtained through conventional CFD simulation. By only performing CFD simulations once in a training phase, the time required to perform FFRCT was reduced by two orders of magnitude. The diagnostic value of this method has been demonstrated thoroughly (64–76). Yu et al. (77) further demonstrated additional prognostic value of CT morphological index for the method proposed by Itu et al. (55). Wang et al. (50) proposed to use a recurrent ANN that can model long-range dependencies between segments.
Both conventional CFD-based FFRCT and the methods proposed in Wang et al. (50) and Itu et al. (55) are based only on the geometry of the coronary artery tree model, and are thus susceptible to errors by the segmentation method used to obtain this model (78). Instead, Dey et al. (52) proposed to combine geometric features with semi-automatically obtained plaque and attenuation gradient measurements to identify arteries with functionally significant stenosis. Other methods skip explicit coronary artery centerline extraction and lumen segmentation altogether. Kumamaru et al. (49) trained a CNN to extract a map showing the contrast-enhanced territories in CCTA and used this map in a classifier to predict the minimum FFR value in a patient. Alternatively, analysis can be moved from the cause—the coronary arteries—to the effect, i.e., the myocardium. Han et al. (54) subdivided the separated endocardium and epicardium into the American Heart Association (AHA) 17 segments (58), with 3 features per segment characterizing perfusion and wall thickness. However, the trained boosting classifier showed only moderate accuracy for patientwise prediction of abnormal FFR values. For the same purpose, Zreik et al. (53) trained an SVM based on features from myocardial regions extracted from CCTA. Clinical evaluation of this method yielded improved diagnostic accuracy of FFRCT over visual evaluation of stenosis (79). Hae et al. (51) increased accuracy of FFR-prediction by including the tissue volume subtended to a stenotic lesion in analysis. However, determination of lesion position required additional analysis including artery tree segmentation.
5. Discussion
We have presented a survey of applications of ML for detection, characterization and quantification of atherosclerotic plaque and stenosis in cardiac CT. We found that while ML has been a mainstay of cardiac image analysis for years, the recent emergence of deep learning has accelerated progress in the field. Machine learning has the potential to unburden clinicians from time-consuming tasks and change diagnostic procedures, thereby reducing healthcare costs. Moreover, low-cost ML-based analysis could be added to screening studies as a secondary goal. In this survey, we have focused on ML for CAD analysis. For a broader scope the reader is referred to Al'Aref et al. (80), Litjens et al. (81), Nicol et al. (82), Petersen et al. (83), and Singh et al. (84).
We have reviewed plaque and stenosis analysis methods in separate sections, but formation of plaque and stenosis is naturally related and many papers have proposed simultaneous analysis [e.g., (48, 53)]. Moreover, (semi-)automatic identification of plaque or stenosis is often only an intermediate step for prediction of cardiovascular events. Motwani et al. (85) used stenosis scores and plaque characteristics to develop a model for 5 years all-cause mortality prediction. Similarly, Johnson et al. (86) showed that an ML model taking into account per segment coronary artery characteristics can outperform hand-crafted models for prediction of adverse cardiac events. Van Rosendael et al. (87) developed a model for all-cause mortality prediction in combination with future myocardial infarction based only on hand-crafted features derived from CCTA scans. Furthermore, some methods directly predict presence of CAD from medical images, i.e., chest CT (88) or non-contrast-enhanced cardiac CT (89). While these approaches only require one label per patient and large data sets are thus not expensive to obtain, the interpretability of predictions may be limited. Interpretability might constitute an opportunity, not only to improve reliability but also as it might increase medical knowledge by quantifying the diagnostic relevance of underlying phenomena.
The readiness of automatic analysis methods for clinical implementation depends on the complexity of the task, but also on other factors. ML algorithms require large training sets, and tasks with abundant data may be easier to automate. For example, obtaining a ground truth for e.g., non-calcified plaque characterization is very challenging. Therefore, data sets are generally small and ML algorithms remain at an early developmental stage. In contrast, large data sets are available for the development of ML-based CAC scoring methods, which has led to highly accurate results in both dedicated cardiac CT images (11) and other CT images visualizing the heart (8, 32). Similarly, ML-based FFRCT development is aided by the availability of large data sets with CFD-derived reference values. An important remaining step toward clinical application of FFRCT lies in performance evaluation specifically for subjects around the FFR threshold of 0.8, which were shown to be most challenging (90). Furthermore, a recent study showed that not all CCTA exams are suitable for FFRCT analysis (78).
Many challenges in the adoption of machine learning methods in the clinic are not exclusive to CAD detection in cardiac CT. For example, ML algorithms could show unexpected behavior, motivating research into ML interpretability and explainability (91). Furthermore, it is important to point out that ML algorithms are often trained and evaluated on single center studies with high risk for selective biases, and under exclusion of low quality scans.
Despite these challenges, current rapid development allows for justifiable hope that the importance of ML algorithms in cardiac CT will not cease to increase in near future, with benefits for clinicians and patients alike.
Author Contributions
NH and SV drafted the manuscript, which was critically revised and edited by JW, TL, and II. Authors agree to be accountable for all aspects of the work.
Funding
This study has been financially supported by Pie Medical Imaging BV.
Conflict of Interest
II and TL received institutional research projects by Dutch Technology Foundation cofunded by Pie Medical Imaging and Philips Healthcare (P15-26), the Netherlands Organisation for Health Research and Development with participation of Pie Medical Imaging (104003009). II received institutional research project by Dutch Technology Foundation cofunded by Pie Medical Imaging (12726). II and TL are cofounders and shareholders of Quantib-U BV.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2019.00172/full#supplementary-material
References
1. Levin DC, Parker L, Halpern EJ, Rao VM. Coronary CT angiography: reversal of earlier utilization trends. J Am Coll Radiol. (2019) 16:147–55. doi: 10.1016/j.jacr.2018.07.022
2. Dreisbach JG, Nicol ED, Roobottom CA, Padley S, Roditi G. Challenges in delivering computed tomography coronary angiography as the first-line test for stable chest pain. Heart. (2018) 104:921–7. doi: 10.1136/heartjnl-2017-311846
3. Litjens G, Kooi T, Bejnordi BE, Setio AAA, Ciompi F, Ghafoorian M, et al. A survey on deep learning in medical image analysis. Med Image Anal. (2017) 42:60–88. doi: 10.1016/j.media.2017.07.005
4. Jordan MI, Mitchell TM. Machine learning: trends, perspectives, and prospects. Science. (2015) 349:255–60. doi: 10.1126/science.aaa8415
5. Insull W. The pathology of atherosclerosis: plaque development and plaque responses to medical treatment. Am J Med. (2009) 122(1, Suppl.):S3–14. doi: 10.1016/j.amjmed.2008.10.013
6. de Vos BD, Wolterink JM, Leiner T, Jong PAd, Lessmann N, Išgum I. Direct automatic coronary calcium scoring in cardiac and chest CT. IEEE Trans. Med. Imaging. (2019) 38:2127–38. doi: 10.1109/TMI.2019.2899534
7. Cano-Espinosa C, González G, Washko GR, Cazorla M, Estépar RSJ. Automated agatston score computation in non-ECG gated CT scans using deep learning. In: Proceedings of SPIE–the International Society for Optical Engineering. vol. 10574 (2018). Available online at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6095680/
8. Lessmann N, Ginneken Bv, Zreik M, Jong PAd, Vos BDd, Viergever MA, et al. Automatic calcium scoring in low-dose chest CT using deep neural networks with dilated convolutions. IEEE Trans Med Imaging. (2018) 37:615–25. doi: 10.1109/TMI.2017.2769839
9. Yang G, Chen Y, Ning X, Sun Q, Shu H, Coatrieux JL. Automatic coronary calcium scoring using noncontrast and contrast CT images. Med Phys. (2016) 43:2174. doi: 10.1118/1.4945045
10. Wolterink JM, Leiner T, de Vos BD, van Hamersvelt RW, Viergever MA, Išgum I. Automatic coronary artery calcium scoring in cardiac CT angiography using paired convolutional neural networks. Med Image Anal. (2016) 34:123–36. doi: 10.1016/j.media.2016.04.004
11. Wolterink JM, Leiner T, Takx RAP, Viergever MA, Išgum I. Automatic coronary calcium scoring in non-contrast-enhanced ECG-triggered cardiac CT with ambiguity detection. IEEE Trans Med Imaging. (2015) 34:1867–78. doi: 10.1109/TMI.2015.2412651
12. Shahzad R, van Walsum T, Schaap M, Rossi A, Klein S, Weustink AC, et al. Vessel specific coronary artery calcium scoring: an automatic system. Acad Radiol. (2013) 20:1–9. doi: 10.1016/j.acra.2012.07.018
13. Išgum I, Prokop M, Niemeijer M, Viergever MA, van Ginneken B. Automatic coronary calcium scoring in low-dose chest computed tomography. IEEE Trans Med Imaging. (2012) 31:2322–34. doi: 10.1109/TMI.2012.2216889
14. Sánchez CI, Niemeijer M, Išgum I, Dumitrescu A, Suttorp-Schulten MSA, Abràmoff MD, et al. Contextual computer-aided detection: improving bright lesion detection in retinal images and coronary calcification identification in CT scans. Med Image Anal. (2012) 16:50–62. doi: 10.1016/j.media.2011.05.004
15. Liu Q, Qian Z, Marvasty I, Rinehart S, Voros S, Metaxas DN. Lesion-specific coronary artery calcium quantification for predicting cardiac event with multiple instance support vector machines. In: Medical Image Computing and Computer-Assisted Intervention: MICCAI, Vol. 13. Beijing (2010). p. 484–92.
16. Kurkure U, Chittajallu DR, Brunner G, Le YH, Kakadiaris IA. A supervised classification-based method for coronary calcium detection in non-contrast CT. Int J Cardiovasc Imaging. (2010) 26:817–28. doi: 10.1007/s10554-010-9607-2
17. Brunner G, Chittajallu DR, Kurkure U, Kakadiaris IA. Toward the automatic detection of coronary artery calcification in non-contrast computed tomography data. Int J Cardiovasc Imaging. (2010) 26:829–38. doi: 10.1007/s10554-010-9608-1
18. Kolossváry M, Karády J, Kikuchi Y, Ivanov A, Schlett CL, Lu MT, et al. Radiomics versus visual and histogram-based assessment to identify atheromatous lesions at coronary CT angiography: an ex vivo study. Radiology. (2019) 293:190407. doi: 10.1148/radiol.2019190407
19. Masuda T, Nakaura T, Funama Y, Okimoto T, Sato T, Higaki T, et al. Machine-learning integration of CT histogram analysis to evaluate the composition of atherosclerotic plaques: validation with IB-IVUS. J Cardiovasc Comput Tomogr. (2019) 13:163–9. doi: 10.1016/j.jcct.2018.10.018
20. Zreik M, van Hamersvelt RW, Wolterink JM, Leiner T, Viergever MA, Išgum I. A recurrent CNN for automatic detection and classification of coronary artery plaque and stenosis in coronary CT angiography. IEEE Trans Med Imaging. (2019) 38:1588–98. doi: 10.1109/TMI.2018.2883807
21. Zhao F, Wu B, Chen F, Cao X, Yi H, Hou Y, et al. An automatic multi-class coronary atherosclerosis plaque detection and classification framework. Med Biol Eng Comput. (2019) 57:245–57. doi: 10.1007/s11517-018-1880-6
22. Jawaid MM, Riaz A, Rajani R, Reyes-Aldasoro CC, Slabaugh G. Framework for detection and localization of coronary non-calcified plaques in cardiac CTA using mean radial profiles. Comput Biol Med. (2017) 89:84–95. doi: 10.1016/j.compbiomed.2017.07.021
23. Wei J, Zhou C, Chan HP, Chughtai A, Agarwal P, Kuriakose J, et al. Computerized detection of noncalcified plaques in coronary CT angiography: evaluation of topological soft gradient prescreening method and luminal analysis. Med Phys. (2014) 41:081901. doi: 10.1118/1.4885958
24. Yamak D, Panse P, Pavlicek W, Boltz T, Akay M. Non-calcified coronary atherosclerotic plaque characterization by dual energy computed tomography. IEEE J Biomed Health Informat. (2014) 18:939–45. doi: 10.1109/JBHI.2013.2295534
25. Kelm BM, Mittal S, Zheng Y, Tsymbal A, Bernhardt D, Vega-Higuera F, et al. Detection, grading and classification of coronary stenoses in computed tomography angiography. In: Medical Image Computing and Computer-Assisted Intervention: MICCAI, Vol. 14. Toronto, ON (2011). p. 25–32.
26. Zuluaga MA, Hush D, Delgado Leyton EJF, Hernández Hoyos M, Orkisz M. Learning from only positive and unlabeled data to detect lesions in vascular CT images. In: Medical Image Computing and Computer-Assisted Intervention: MICCAI, Vol. 14. Toronto, ON (2011). p. 9–16.
27. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. (1990) 15:827–32. doi: 10.1016/0735-1097(90)90282-T
28. Takx RAP, de Jong PA, Leiner T, Oudkerk M, de Koning HJ, Mol CP, et al. Automated coronary artery calcification scoring in non-gated chest CT: agreement and reliability. PLoS ONE. (2014) 9:e91239. doi: 10.1371/journal.pone.0091239
29. Išgum I, de Vos BD, Wolterink JM, Dey D, Berman DS, Rubeaux M, et al. Automatic determination of cardiovascular risk by CT attenuation correction maps in Rb-82 PET/CT. J Nucl Cardiol. (2018) 25:2133–42. doi: 10.1007/s12350-017-0866-3
30. Emaus MJ, Išgum I, van Velzen SGM, van den Bongard HJGD, Gernaat SAM, Lessmann N, et al. Bragatston study protocol: a multicentre cohort study on automated quantification of cardiovascular calcifications on radiotherapy planning CT scans for cardiovascular risk prediction in patients with breast cancer. BMJ Open. (2019) 9:e028752. doi: 10.1136/bmjopen-2018-028752
31. Gernaat SAM, Išgum I, de Vos BD, Takx RAP, Young-Afat DA, Rijnberg N, et al. Automatic coronary artery calcium scoring on radiotherapy planning CT scans of breast cancer patients: reproducibility and association with traditional cardiovascular risk factors. PLoS ONE. (2016) 11:e0167925. doi: 10.1371/journal.pone.0167925
32. Gernaat SAM, van Velzen SGM, Koh V, Emaus MJ, Išgum I, Lessmann N, et al. Automatic quantification of calcifications in the coronary arteries and thoracic aorta on radiotherapy planning CT scans of Western and Asian breast cancer patients. Radiother Oncol. (2018) 127:487–92. doi: 10.1016/j.radonc.2018.04.011
33. Wolterink JM, Leiner T, de Vos BD, Coatrieux JL, Kelm BM, Kondo S, et al. An evaluation of automatic coronary artery calcium scoring methods with cardiac CT using the orCaScore framework. Med Phys. (2016) 43:2361. doi: 10.1118/1.4945696
34. Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. (2006) 47(Suppl. 8):C13–18. doi: 10.1016/j.jacc.2005.10.065
35. Lesage D, Angelini ED, Bloch I, Funka-Lea G. A review of 3D vessel lumen segmentation techniques: models, features and extraction schemes. Med Image Anal. (2009) 13:819–45. doi: 10.1016/j.media.2009.07.011
36. Schaap M, Metz CT, van Walsum T, van der Giessen AG, Weustink AC, Mollet NR, et al. Standardized evaluation methodology and reference database for evaluating coronary artery centerline extraction algorithms. Med Image Anal. (2009) 13:701–14. doi: 10.1016/j.media.2009.06.003
37. Gülsün MA, Funka-Lea G, Sharma P, Rapaka S, Zheng Y. Coronary centerline extraction via optimal flow paths and CNN path pruning. In: Ourselin S, Joskowicz L, Sabuncu MR, Unal G, Wells W, editors. Medical Image Computing and Computer-Assisted Intervention: MICCAI. Lecture Notes in Computer Science. Athens: Springer International Publishing (2016). p. 317–25.
38. Wolterink JM, van Hamersvelt RW, Viergever MA, Leiner T, Išgum I. Coronary artery centerline extraction in cardiac CT angiography using a CNN-based orientation classifier. Med Image Anal. (2019) 51:46–60. doi: 10.1016/j.media.2018.10.005
39. Kristanto W, van Ooijen PMA, Jansen-van der Weide MC, Vliegenthart R, Oudkerk M. A meta analysis and hierarchical classification of HU-based atherosclerotic plaque characterization criteria. PLoS ONE. (2013) 8:e73460. doi: 10.1371/journal.pone.0073460
40. Pijls NHJ, Tanaka N, Fearon WF. Functional assessment of coronary stenoses: can we live without it? Eur Heart J. (2013) 34:1335–44. doi: 10.1093/eurheartj/ehs436
41. Meijboom WB, Van Mieghem CAG, van Pelt N, Weustink A, Pugliese F, Mollet NR, et al. Comprehensive assessment of coronary artery stenoses: computed tomography coronary angiography versus conventional coronary angiography and correlation with fractional flow reserve in patients with stable angina. J Am Coll Cardiol. (2008) 52:636–43. doi: 10.1016/j.jacc.2008.05.024
42. Lee MCH, Petersen K, Pawlowski N, Glocker B, Schaap M. TETRIS: template transformer networks for image segmentation with shape priors. IEEE Trans Med Imaging. (2019) 38:2596–606. doi: 10.1109/TMI.2019.2905990
43. Freiman M, Manjeshwar R, Goshen L. Unsupervised abnormality detection through mixed structure regularization (MSR) in deep sparse autoencoders. Med Phys. (2019) 46:2223–31. doi: 10.1002/mp.13464
44. Huang W, Huang L, Lin Z, Huang S, Chi Y, Zhou J, et al. Coronary artery segmentation by deep learning neural networks on computed tomographic coronary angiographic images. In: Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference, Vol. 2018. Honolulu, HI (2018). p. 608–11.
45. Kang D, Dey D, Slomka PJ, Arsanjani R, Nakazato R, Ko H, et al. Structured learning algorithm for detection of nonobstructive and obstructive coronary plaque lesions from computed tomography angiography. J Med Imaging. (2015) 2:014003. doi: 10.1117/1.JMI.2.1.014003
46. Xiong G, Kola D, Heo R, Elmore K, Cho I, Min JK. Myocardial perfusion analysis in cardiac computed tomography angiographic images at rest. Med Image Anal. (2015) 24:77–89. doi: 10.1016/j.media.2015.05.010
47. Mukhopadhyay A, Qian Z, Bhandarkar SM, Liu T, Rinehart S, Voros S. Morphological analysis of the left ventricular endocardial surface and its clinical implications. In: Medical Image Computing and Computer-Assisted Intervention: MICCAI, Vol. 15. Nice (2012). p. 502–10.
48. Zuluaga MA, Magnin IE, Hernández Hoyos M, Delgado Leyton EJF, Lozano F, Orkisz M. Automatic detection of abnormal vascular cross-sections based on density level detection and support vector machines. Int J Comput Assist Radiol Surg. (2011) 6:163–74. doi: 10.1007/s11548-010-0494-8
49. Kumamaru KK, Fujimoto S, Otsuka Y, Kawasaki T, Kawaguchi Y, Kato E, et al. Diagnostic accuracy of 3D deep-learning-based fully automated estimation of patient-level minimum fractional flow reserve from coronary computed tomography angiography. Eur Heart J Cardiovasc Imaging. (2019). doi: 10.1093/ehjci/jez160. [Epub ahead of print].
50. Wang ZQ, Zhou YJ, Zhao YX, Shi DM, Liu YY, Liu W, et al. Diagnostic accuracy of a deep learning approach to calculate FFR from coronary CT angiography. J Geriatr Cardiol. (2019) 16:42–8. doi: 10.11909/j.issn.1671-5411.2019.01.010
51. Hae H, Kang SJ, Kim WJ, Choi SY, Lee JG, Bae Y, et al. Machine learning assessment of myocardial ischemia using angiography: development and retrospective validation. PLoS Med. (2018) 15:e1002693. doi: 10.1371/journal.pmed.1002693
52. Dey D, Gaur S, Ovrehus KA, Slomka PJ, Betancur J, Goeller M, et al. Integrated prediction of lesion-specific ischemia from quantitative coronary CT Angiography using machine learning: a multicenter study. Eur Radiol. (2018) 28:2655–64. doi: 10.1007/s00330-017-5223-z
53. Zreik M, Lessmann N, van Hamersvelt RW, Wolterink JM, Voskuil M, Viergever MA, et al. Deep learning analysis of the myocardium in coronary CT angiography for identification of patients with functionally significant coronary artery stenosis. Med Image Anal. (2018) 44:72–85. doi: 10.1016/j.media.2017.11.008
54. Han D, Lee JH, Rizvi A, Gransar H, Baskaran L, Schulman-Marcus J, et al. Incremental role of resting myocardial computed tomography perfusion for predicting physiologically significant coronary artery disease: a machine learning approach. J Nucl Cardiol. (2018) 25:223–33. doi: 10.1007/s12350-017-0834-y
55. Itu L, Rapaka S, Passerini T, Georgescu B, Schwemmer C, Schoebinger M, et al. A machine-learning approach for computation of fractional flow reserve from coronary computed tomography. J Appl Physiol. (2016) 121:42–52. doi: 10.1152/japplphysiol.00752.2015
56. Pugliese F, Hunink MGM, Gruszczynska K, Alberghina F, Malagó R, van Pelt N, et al. Learning curve for coronary CT angiography: what constitutes sufficient training? Radiology. (2009) 251:359–68. doi: 10.1148/radiol.2512080384
57. Arbab-Zadeh A, Hoe J. Quantification of coronary arterial stenoses by multidetector CT angiography in comparison with conventional angiography methods, caveats, and implications. JACC Cardiovasc Imaging. (2011) 4:191–202. doi: 10.1016/j.jcmg.2010.10.011
58. Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. (2002) 105:539–42. doi: 10.1161/hc0402.102975
59. Tonino PAL, De Bruyne B, Pijls NHJ, Siebert U, Ikeno F, van' t Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. (2009) 360:213–24. doi: 10.1056/NEJMoa0807611
60. Petraco R, Park JJ, Sen S, Nijjer SS, Malik IS, Echavarría-Pinto M, et al. Hybrid iFR-FFR decision-making strategy: implications for enhancing universal adoption of physiology-guided coronary revascularisation. EuroIntervention. (2013) 8:1157–65. doi: 10.4244/EIJV8I10A179
61. Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol. (2013) 61:2233–41. doi: 10.1016/j.jacc.2012.11.083
62. Tesche C, Vliegenthart R, Duguay TM, De Cecco CN, Albrecht MH, De Santis D, et al. Coronary computed tomographic angiography-derived fractional flow reserve for therapeutic decision making. Am J Cardiol. (2017) 120:2121–7. doi: 10.1016/j.amjcard.2017.08.034
63. Nørgaard BL, Leipsic J, Gaur S, Seneviratne S, Ko BS, Ito H, et al. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: next steps). J Am Coll Cardiol. (2014) 63:1145–55. doi: 10.1016/j.jacc.2013.11.043
64. Baumann S, Renker M, Schoepf UJ, De Cecco CN, Coenen A, De Geer J, et al. Gender differences in the diagnostic performance of machine learning coronary CT angiography-derived fractional flow reserve -results from the MACHINE registry. Eur J Radiol. (2019) 119:108657. doi: 10.1016/j.ejrad.2019.108657
65. Coenen A, Kim YH, Kruk M, Tesche C, De Geer J, Kurata A, et al. Diagnostic accuracy of a machine-learning approach to coronary computed tomographic angiography-based fractional flow reserve: result from the MACHINE consortium. Circulat Cardiovasc Imaging. (2018) 11:e007217. doi: 10.1161/CIRCIMAGING.117.007217
66. Duguay TM, Tesche C, Vliegenthart R, De Cecco CN, Lin H, Albrecht MH, et al. Coronary computed tomographic angiography-derived fractional flow reserve based on machine learning for risk stratification of non-culprit coronary narrowings in patients with acute coronary syndrome. Am J Cardiol. (2017) 120:1260–6. doi: 10.1016/j.amjcard.2017.07.008
67. Hu X, Yang M, Han L, Du Y. Diagnostic performance of machine-learning-based computed fractional flow reserve (FFR) derived from coronary computed tomography angiography for the assessment of myocardial ischemia verified by invasive FFR. Int J Cardiovasc Imaging. (2018) 34:1987–996. doi: 10.1007/s10554-018-1419-9
68. Kurata A, Fukuyama N, Hirai K, Kawaguchi N, Tanabe Y, Okayama H, et al. On-site computed tomography-derived fractional flow reserve using a machine-learning algorithm - clinical effectiveness in a retrospective multicenter cohort. Circulat J. (2019) 83:1563–71. doi: 10.1253/circj.CJ-19-0163
69. Nous FMA, Coenen A, Boersma E, Kim YH, Kruk MBP, Tesche C, et al. Comparison of the diagnostic performance of coronary computed tomography angiography-derived fractional flow reserve in patients with versus without diabetes mellitus (from the MACHINE consortium). Am J Cardiol. (2019) 123:537–43. doi: 10.1016/j.amjcard.2018.11.024
70. Tang CX, Wang YN, Zhou F, Schoepf UJ, Assen Mv, Stroud RE, et al. Diagnostic performance of fractional flow reserve derived from coronary CT angiography for detection of lesion-specific ischemia: a multi-center study and meta-analysis. Eur J Radiol. (2019) 116:90–7. doi: 10.1016/j.ejrad.2019.04.011
71. Tesche C, De Cecco CN, Albrecht MH, Duguay TM, Bayer RR, Litwin SE, et al. Coronary CT angiography-derived fractional flow reserve. Radiology. (2017) 285:17–33. doi: 10.1148/radiol.2017162641
72. Tesche C, Otani K, De Cecco CN, Coenen A, De Geer J, Kruk M, et al. Influence of coronary calcium on diagnostic performance of machine learning CT-FFR: results from MACHINE registry. JACC Cardiovasc Imaging. (2019). doi: 10.1016/j.jcmg.2019.06.027
73. von Knebel Doeberitz PL, De Cecco CN, Schoepf UJ, Duguay TM, Albrecht MH, van Assen M, et al. Coronary CT angiography-derived plaque quantification with artificial intelligence CT fractional flow reserve for the identification of lesion-specific ischemia. Eur Radiol. (2019) 29:2378–87. doi: 10.1007/s00330-018-5834-z
74. Wardziak L, Kruk M, Pleban W, Demkow M, Ruzyllo W, Dzielinska Z, et al. Coronary CTA enhanced with CTA based FFR analysis provides higher diagnostic value than invasive coronary angiography in patients with intermediate coronary stenosis. J Cardiovasc Comput Tomogr. (2019) 13:62–7. doi: 10.1016/j.jcct.2018.10.004
75. Yu M, Lu Z, Shen C, Yan J, Wang Y, Lu B, et al. The best predictor of ischemic coronary stenosis: subtended myocardial volume, machine learning-based FFRCT, or high-risk plaque features? Eur. Radiol. (2019) 29:3647–57. doi: 10.1007/s00330-019-06139-2
76. Zhou F, Tang CX, Schoepf UJ, Tesche C, Rollins JD, Liu H, et al. Machine learning using CT-FFR predicts proximal atherosclerotic plaque formation associated with LAD myocardial bridging. JACC Cardiovasc Imaging. (2019) 12:1591–3. doi: 10.1016/j.jcmg.2019.01.018
77. Yu M, Lu Z, Li W, Wei M, Yan J, Zhang J. CT morphological index provides incremental value to machine learning based CT-FFR for predicting hemodynamically significant coronary stenosis. Int J Cardiol. (2018) 265:256–61. doi: 10.1016/j.ijcard.2018.01.075
78. Pontone G, Weir-McCall JR, Baggiano A, Del Torto A, Fusini L, Guglielmo M, et al. Determinants of rejection rate for coronary CT angiography fractional flow reserve analysis. Radiology. (2019) 292:597–605. doi: 10.1148/radiol.2019182673
79. van Hamersvelt RW, Zreik M, Voskuil M, Viergever MA, Išgum I, Leiner T. Deep learning analysis of left ventricular myocardium in CT angiographic intermediate-degree coronary stenosis improves the diagnostic accuracy for identification of functionally significant stenosis. Eur Radiol. (2019) 29:2350–9. doi: 10.1007/s00330-018-5822-3
80. Al'Aref SJ, Anchouche K, Singh G, Slomka PJ, Kolli KK, Kumar A, et al. Clinical applications of machine learning in cardiovascular disease and its relevance to cardiac imaging. Eur Heart J. (2019) 40:1975–86. doi: 10.1093/eurheartj/ehy404
81. Litjens G, Ciompi F, Wolterink JM, de Vos BD, Leiner T, Teuwen J, et al. State-of-the-art deep learning in cardiovascular image analysis. JACC Cardiovasc Imaging. (2019) 12(8 Pt 1):1549–65. doi: 10.1016/j.jcmg.2019.06.009
82. Nicol ED, Norgaard BL, Blanke P, Ahmadi A, Weir-McCall J, Horvat PM, et al. The future of cardiovascular computed tomography: advanced analytics and clinical insights. JACC. (2019) 12:1058–72. doi: 10.1016/j.jcmg.2018.11.037
83. Petersen SE, Abdulkareem M, Leiner T. Artificial intelligence will transform cardiac imaging-opportunities and challenges. Front Cardiovasc Med. (2019) 6:133. doi: 10.3389/fcvm.2019.00133
84. Singh G, Al'Aref SJ, Van Assen M, Kim TS, van Rosendael A, Kolli KK, et al. Machine learning in cardiac CT: basic concepts and contemporary data. J Cardiovasc Comput Tomogr. (2018) 12:192–201. doi: 10.1016/j.jcct.2018.04.010
85. Motwani M, Dey D, Berman DS, Germano G, Achenbach S, Al-Mallah MH, et al. Machine learning for prediction of all-cause mortality in patients with suspected coronary artery disease: a 5-year multicentre prospective registry analysis. Eur Heart J. (2017) 38:500–7. doi: 10.1093/eurheartj/ehw188
86. Johnson KM, Johnson HE, Zhao Y, Dowe DA, Staib LH. Scoring of coronary artery disease characteristics on coronary CT angiograms by using machine learning. Radiology. (2019) 292:354–62. doi: 10.1148/radiol.2019182061
87. Van Rosendael AR, Maliakal G, Kolli KK, Beecy A, Al'Aref SJ, Dwivedi A, et al. Maximization of the usage of coronary CTA derived plaque information using a machine learning based algorithm to improve risk stratification; insights from the CONFIRM registry. J Cardiovasc Comput Tomogr. (2018) 12:204–9. doi: 10.1016/j.jcct.2018.04.011
88. Dormer JD, Halicek M, Ma L, Reilly CM, Schreibmann E, Fei B. Convolutional neural networks for the detection of diseased hearts using CT images and left atrium patches. In: Proceedings of SPIE–the International Society for Optical Engineering, Vol. 10575. San Diego, CA (2018). doi: 10.1117/12.2293548
89. Mannil M, von Spiczak J, Manka R, Alkadhi H. Texture analysis and machine learning for detecting myocardial infarction in noncontrast low-dose computed tomography: unveiling the invisible. Investigat Radiol. (2018) 53:338–43. doi: 10.1097/RLI.0000000000000448
90. Cook CM, Petraco R, Shun-Shin MJ, Ahmad Y, Nijjer S, Al-Lamee R, et al. Diagnostic accuracy of computed tomography-derived fractional flow reserve: a systematic review. JAMA Cardiol. (2017) 2:803–10. doi: 10.1001/jamacardio.2017.1314
91. Samek W, Montavon G, Vedaldi A, Hansen LK, Muller KR, editors. Explainable AI: interpreting, explaining and visualizing deep learning. In: Lecture Notes in Artificial Intelligence, Lecture Notes ComputerState-of-the-Art Surveys. Springer International Publishing (2019). Available online at: https://www.springer.com/gp/book/9783030289539
Keywords: machine learning, coronary artery disease, atherosclerotic plaque, coronary artery stenosis, cardiac CT
Citation: Hampe N, Wolterink JM, van Velzen SGM, Leiner T and Išgum I (2019) Machine Learning for Assessment of Coronary Artery Disease in Cardiac CT: A Survey. Front. Cardiovasc. Med. 6:172. doi: 10.3389/fcvm.2019.00172
Received: 30 September 2019; Accepted: 12 November 2019;
Published: 26 November 2019.
Edited by:
Fabrizio Ricci, G. d'Annunzio University of Chieti and Pescara, ItalyCopyright © 2019 Hampe, Wolterink, van Velzen, Leiner and Išgum. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nils Hampe, n.hampe@amsterdamumc.nl
 Nils Hampe
Nils Hampe Jelmer M. Wolterink
Jelmer M. Wolterink Sanne G. M. van Velzen
Sanne G. M. van Velzen Tim Leiner
Tim Leiner Ivana Išgum
Ivana Išgum