Cardio-Oncology: A Myriad of Relationships Between Cardiovascular Disease and Cancer
- 1Department of Cardiovascular Centre, The First Hospital of Jilin University, Changchun, China
- 2Department of Cardiology, Nepal APF Hospital, Kathmandu, Nepal
- 3The Second Hospital of Jilin University, Changchun, China
- 4Department of Cardiology, Union Hospital, Fujian Medical University, Fuzhou, China
Cardiovascular disease (CVD) and cancer are the leading causes of death worldwide. With an increasing number of the elderly population, and early cancer screening and treatment, the number of cancers cases are rising, while the mortality rate is decreasing. However, the number of cancer survivors is increasing yearly. With the prolonged life span of cancer patients, the adverse effects of anti-tumor therapy, especially CVD, have gained enormous attention. The incidence of cardiovascular events such as cardiac injury or cardiovascular toxicity is higher than malignant tumors' recurrence rate. Numerous clinical studies have also shifted their focus from the study of a single disease to the interdisciplinary study of oncology and cardiology. Previous studies have confirmed that anti-tumor therapy can cause CVD. Additionally, the treatment of CVD is also related to the tumors incidence. It is well established that the increased incidence of CVD in cancer patients is probably due to an unmodified unhealthy lifestyle among cancer survivors or cardiotoxicity caused by anti-cancer therapy. Nevertheless, some patients with CVD have a relatively increased cancer risk because CVD and malignant tumors are highly overlapping risk factors, including gender, age, hypertension, diabetes, hyperlipidemia, inflammation, and obesity. With advancements in the diagnosis and treatment, many patients simultaneously suffer from CVD and cancer, and most of them have a poor prognosis. Therefore, clinicians should understand the relationship between CVD and tumors, effectively identify the primary and secondary prevention for these diseases, and follow proper treatment methods.
Introduction
Currently, cardiovascular disease (CVD) and cancer have the highest morbidity and mortality worldwide. They are closely related in terms of many factors, including risk factors, pathogenesis, and iatrogenic side effects. The lifetime risk of CVD in people >30 years old is close to 50%, causing ~17.3 million deaths worldwide each year (1). In 2020, ~19.3 million people were diagnosed with cancer globally, resulting in approximately 10 million deaths. It is worth noting that the first year of cancer is the period with the highest mortality from cardiovascular complications (2). Therefore, as a severe global public health threat, the relationship between CVD and cancer is actively being studied and updated. Various exogenous factors and genes have contributed to the onset of these diseases. Anti-cancer treatments have led to an increase in the incidence of CVD. Similarly, antihypertensive drugs (angiotensin converting enzyme inhibitors; ACEI) and aspirin affect the occurrence of different types of cancer. We summarized relevant studies and proposed that CVD and cancer are predisposing factors for each other. Starting from the pathogenesis, this article systematically summarizes the epidemiological status, points out the common occurrence and pathogenic mechanisms of various CVD and cancer, lists anti-cancer drugs, and discusses several cardiovascular side effects induced by anti-cancer therapy. Finally, we provide the latest strategies for the clinical management of such patients.
Relationship Between Cancer and Cardiovascular Disease
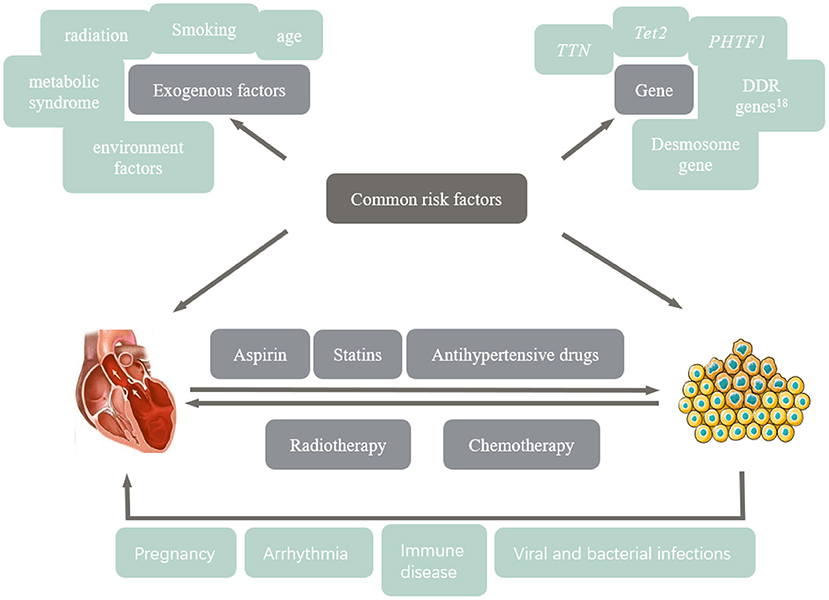
Common Risk Factors
CVD and cancer are multifactorial, with highly overlapping risk factors, including smoking, metabolic syndrome, radiation, age, air pollution, and environmental toxins (3). Recent studies have pointed out that CVD and tumors have direct mutual effects in addition to the above risk factors. CVD can increase the overall incidence of cancer. In patients with early-stage breast cancer, 59% of post-treatment recurrence and 60% of cancer-specific deaths are related to cardiovascular events (4, 5). Heart failure may be a risk factor for tumors by releasing particular heart failure-associated proteins, such as SERPINA3, into the bloodstream, leading to tumor development and growth, while elevated cardiac and inflammatory markers may indicate new cancers, according to an experimental study (6). Hypertension has a similar mechanism to tumors. Hypertension affects the arterial wall through oxidative stress and is related to cell canceration (7). Patients with hypertension have 2-fold higher risk cancer of normal people.
With normal blood pressure, and the incidence of malignant tumors increases with the increase of blood pressure. Active and effective antihypertensive treatment can prevent cardiovascular complications and improve the quality of life in cancer patients. Grossman et al. (8) further showed that high blood pressure increased the risk of cancer death by 23%. Myocardial infarction, as an acute rational stressor that accelerates breast cancer progression, accelerates tumor growth by activating systemic host response; meanwhile, bone marrow cells present an immunosuppressive state, accelerating the division of monocytes, and promoting tumor proliferation (8, 9). In addition, tumors promote the development of CVD. Tumors consume glucose in the body in a form other than insulin, leading to systemic insulin loss, and cardiac atrophy and heart failure, and possibly accelerating tumor progression (10). Moreover, many drugs and radiotherapy in cancer treatment are cardiotoxic, which aggravate the occurrence and development of heart failure, arrhythmia, coronary artery disease, hypertension and other CVD.
Genetic Susceptibility
TET2 is the most common mutated gene associated with increased incidence and mortality due to CVD, which is also the first gene identified as an acquired mutation in individuals without hematological malignancies. Preclinical studies have shown that TET2-deficient cells can accelerate atherosclerosis in mice, promoting the release of IL-1β from macrophages; consequently, perpetuating vascular inflammation and inducing monocyte aggregation at the lesion site to aggravate inflammation (11). Hereditary/familial cardiomyopathy (CMY) is an autosomal dominant monogenic disease with no clear cardiac abnormality (12). Hypertrophic cardiomyopathy (HCM) has the highest incidence among CMY, followed by dilated cardiomyopathy (DCM), arrhythmogenic cardiomyopathy (ACM), and restrictive cardiomyopathy (13). In 2012, Truncations of titin (TTN) was first proposed as a DCM related gene, which encodes myotin in sarcomere (14). Truncating variants in the TTN gene (TTNtv), i.e., TTN trunk-frame-frame-mutation was detected in 25% of familial cardiomyopathy, 18% of sporadic cardiomyopathy, 10% of perinatal cardiomyopathy, and 25% of alcoholic cardiomyopathy. Patients with TTNtv had worse cardiac function (15). TTNtv carriers are likely to have cancer treatment-induced cardiomyopathy (CCM). Genetic susceptibility to DCM increases susceptibility to CCM (16). 90% of patients with CCM received anthracyclines. An animal study showed that mice with TTNtv showed left ventricular cardiomyocyte elongation and dysfunction after treatment with anthracycline (13). In addition, desmosomes, as the main structure of the connections between cells, inhibit cell motor ability. Mutations in desmosome genes have been detected in various cancers and ACM patients (13). In a study of cardiomyopathy induced by cancer treatment in children, the risk of CCM in Africa is higher than in Europe, possibly due to abnormal expression of putative homeodomain transcription factor 1 (PHTF1) and associated with long-term response to adriamycin therapy (17). Therefore, genetic screening provides guidance to identify patients at high risk for CCM and helps evaluate drugs for prevention and treatment and optimize the treatment of cancer and CVD.
Clonal Hematopoiesis
Clonal hematopoiesis (CH) is defined as the clonal expansion of blood cells in the presence of somatic mutations and is an age-related biological state (18). The concept of clonal hematopoiesis of indeterminate potential (CHIP) was first introduced in 2015 as the presence of somatic mutations associated with hematologic malignancies in the blood or bone marrow of individuals with non-malignant hematologic diseases (19), involved in tumor development and CVD (20). CHIP is a biological state associated with aging that is virtually absent in children and its expression increases with age, mainly in the form of hematopoietic stem cell mutations. CHIP produces clonal white blood cells to populate peripheral blood, and individuals with 2–3 of these somatic mutations in a row are at increased risk of developing leukemia (21). Therefore, CHIP is considered a preclinical state for malignant blood disorders. Notably, CHIP alters innate immune cells to promote lymphoid malignancy and accelerates solid cancer progression by disrupting acquired immune cell homeostasis (22). In addition, chemotherapy promotes clonal expansion of specific mutations, leading to poor outcomes (23).
CHIP is an independent risk factor for CVD. CHIP increases the risk of atherogenesis and accelerates atherosclerosis and chronic cardiac insufficiency, leading to a poor prognosis for such patients (20). In 2017 Jaiswal et al. (24) enrolled 4,726 participants with coronary heart disease and 3,529 controls and confirmed that the presence of CHIP in peripheral blood cells can lead to a doubling of the risk of developing coronary artery disease. The study also found that the degree of coronary artery calcification and the incidence of coronary events were positively associated with CHIP (24). Atherosclerosis due to CHIP-associated mutations are primarily mediated by inflammation. The presence of CHIP-associated mutations in macrophages stimulates inflammation and changes the levels of inflammatory factors. Moreover, persistent chronic inflammatory state positively feeds back into somatic mutations leading to increased CHIP-associated mutations, promoting the development and progression of atherosclerosis (25–28). This finding also explains the role of CHIP in patients with valvular lesions, where the presence of CHIP has been shown to accelerate valve sclerosis in patients with aortic stenosis and often leads to a poor prognosis, and CHIP increases mortality even after aortic valve replacement (29, 30). Besides, 2021 Pascual et al. (31) observed that CHIP was common in patients with reduced left ventricular ejection fraction (LVEF) and was highly associated with accelerated heart failure progression regardless of etiology.
Multiple Strike Theory
Multiple strike theory (Figure 1) is critical in the pathogenesis of cancer and CVD; the more the risk factors, the higher the incidence of disease. Children and adolescents with cancer have a good prognosis after antitumor therapy, while poor prognosis with cardiovascular side effects when the heart is stressed by pregnancy, hypertension, diabetes and hyperthyroidism (3).
First, tumors are associated with the pathogenesis of CVD, including the induction of exogenous factors, such as adverse lifestyle and endogenous factors, namely certain gene mutations, such as TTNtv, affecting the occurrence of DCM and CCM. Secondly, both anti-tumor therapy and treatment of CVD may further deteriorate cardiovascular function, cause tumor, and affect the prognosis. These common risk factors, genetic predisposition, therapeutic interventions, and the progressive involvement of certain diseases that stress the heart may contribute to the gradual development of cardiotoxicity during antitumor therapy.
Commonly Used Drugs for Patients With Cardiovascular Disease May Affect the Occurrence of Cancer
Aspirin
Aspirin is widely used in the primary and secondary prevention of CVD due to its effects on platelet aggregation, atherosclerotic thrombosis, and embolization. Platelet activation prevents tumor cells from elimination by an immune response and promotes their retention in endothelial cells and growth of metastatic cells, as well as contributes to angiogenesis, thereby promoting metastasis (32). Therefore, aspirin can slow the metastatic spread of cancer cells by inhibiting platelet aggregation (33, 34).
During the past 3 decades, studies have shown that regular, low doses (75 to 300 mg) of aspirin reduce the risk of cancer in the general population, with a significant benefit (35). Since 1988, many studies have demonstrated the positive effect of aspirin on colorectal cancer (36, 37). Recent studies have shown that the risk of colorectal cancer is significantly reduced after using aspirin continuously for 5 years and that the protective effect persists at 20 years of follow-up, and a longer duration of aspirin use is related to higher protection (38, 39). Similarly, aspirin may reduce deaths from prostate, biliary, and liver cancers (40–42). However, studies have also suggested that aspirin may accelerate cancer progression in people over 70 years because of its bleeding risk (32, 43). Therefore, aspirin should be used carefully in population with high risk of bleeding, while long-term use is recommended for those at high risk of CVD.
Statins
Statins can reduce blood cholesterol levels by inhibiting the rate-limiting enzyme of the MVA metabolic pathway, namely HMG-CoA reductase (HMGCR), which can significantly reduce morbidity and mortality due to CVD. Meanwhile, statins also inhibit the transport of receptors on the surface of cell membranes, thereby reducing cancer cell growth, survival, migration, metastasis, inflammation, angiogenesis, promoting apoptosis, and having a protective effect on tumors. Statins may also have an anti-cancer effect by depleting cholesterol in certain situations. Lipophilic statins are more effective in inhibiting viral replication, enhancing therapeutic effectiveness, and passively entering the cell membrane, providing a more sustained and effective cholesterol-dependent anti-HCC effect (44, 45).
Previous studies have shown that statins use is associated with a 13–40% reduction in the incidence of cholangiocarcinoma (46, 47). In a large clinical case analysis, statins are associated with a 25% reduction in the risk of extrahepatic cholangiocarcinoma and improved survival for patients with distal cholangiocarcinoma. Moreover, the risk decreases with the duration of statin use (48). A previous study has suggested that statins may prevent cholangiocarcinoma, but due to the low incidence of cholangiocarcinoma, the association between statins and cholangiocarcinoma still needs to be further verified (49). A recent Swedish viral hepatitis cohort study reported a dose-dependent and time-dependent reduction in the risk of liver cancer, all-cause mortality and liver-related mortality in patients with viral hepatitis treated with lipophilic statins (50). Many studies have shown that statins are associated with a lower incidence of colon cancer. Statins also reduce the risk of progression from non-advanced adenomas to colon cancer, especially proximal lesions, and prevent colorectal cancer recurrence after treatment (51). Additionally, inhibition of the HMG-CoA reductase gene is associated with a lower incidence of epithelial ovarian cancer. However, the effect of statins on ovarian cancer has not been determined and needs to be further investigated (52). Furthermore, retrospective studies have shown that breast cancer patients receiving both statins and anthracyclines have a lower risk of heart failure than those who do not receive statins; however, the difference is not significant in breast cancer patients receiving trastuzumab (53).
Antihypertensive Drugs
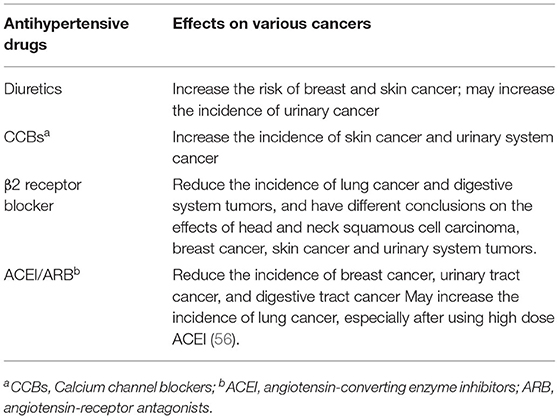
According to statistics, about 37% of cancer patients have hypertension, and the active and effective control of blood pressure by antihypertensive drugs can prevent the occurrence of cardiovascular complications and improve the quality of life in cancer patients (54). At present, the widely using antihypertensive drugs include ACEIs, angiotensin-receptor antagonists (ARBs), β-blockers, calcium antagonists, diuretics, α-blockers and central sympatholytic drugs. The relationship between antihypertensive drugs and cancer has received widespread attention recently, and the interaction between several antihypertensive drugs and malignant tumors is still unclear. A 1,998 article published in The Lancet studied the patients who used blood pressure drugs for more than 3 years. Patients who used ACE inhibitors had the lowest relative risk of developing cancer, while patients who used calcium channel blockers (CCBs), diuretics, and beta-blockers, had no significant effect on cancer risk (55). In recent years, studies have shown that various antihypertensive drugs may be associated with cancer, while renin-angiotensin system inhibitors (RAS inhibitors) may have a more comprehensive protective effect (Table 1).
Diuretics
Certain diuretics (such as thiazide diuretics), are considered photosensitive drugs and can increase the risk of skin cancer associated with ultraviolet (UV) light by damaging DNA (57). Thiazide diuretics are associated with insulin resistance, a recognized risk factor for breast cancer (58).
Two large studies from Denmark and Iceland have shown that hydrochlorothiazide is significantly associated with an increased risk of skin cancer, possibly in a dose-dependent relationship (59, 60). However, other studies have shown no significant relationship between hydrochlorothiazide and the risk of skin cancer, which may be due to individual sensitivity to UV light, which led to different results (61). Since 1980, many studies have shown that diuretics are positively correlated with the risk of breast cancer. The incidence of breast cancer increases by 16% in using diuretics for more than 10 years, and the use of diuretics is highly correlated with the poor prognosis of breast cancer patients (62). A case-control study of hypertensive and non-hypertensive patients on antihypertensive drugs showed that methotrexate, thiazide, and loop diuretics increased the risk of renal cell carcinoma by 40%, with women at a higher risk than men (63, 64). However, high blood pressure itself can cause kidney damage, so more clinical studies are needed to confirm this statement.
Calcium Channel Blockers
To date, available data suggest that CCBs increase the tumors incidence by inhibiting apoptosis or interfering with cell differentiation through calcium triggering signals. Moreover, CCBs reduce intracellular calcium levels and impair the process of programmed cell death. The body is prevented from destroying damaged cells to prevent malignancy, leaving them to replicate when desired (65, 66).
A meta-analysis combining 11 related studies showed that long-term (>9 years) treatment with CCBs increased the incidence of malignancy (67). Rothschild's large population-based study and meta-analysis showed a slightly increased risk of lung and prostate cancer in calcium antagonist users, both in a time-dependent manner (68–70). CCBs were associated with a 1.6-fold increased risk of breast cancer in those who used CCBs for more than 2 years, especially invasive ductal carcinoma and invasive lobular carcinoma of the breast thant hose who had never used antihypertensive drugs (71, 72).
Beta-Receptor Blockers
In recent years, several studies have found that selective beta 2 blockers (β2 blockers) may reduce the recurrence and metastasis of cancer and thus increase overall survival in cancer patients. Selective beta 1 blockers have been shown to have no beneficial effect on cancer (73). Epinephrine and norepinephrine can induce tumor cell invasion and migration, thereby affecting lymph node invasion and metastasis, and this effect is mediated by the β-adrenergic pathway, especially the β2 receptors (74–77). Therefore, beta-blockers compete with epinephrine and norepinephrine for effective beta-adrenergic receptors to reduce the migratory activity of cancer cells and can also alter tumor growth, invasion, apoptosis, and angiogenesis to prevent tumor metastasis (78). Furthermore, the selective β2 receptor blocker propranolol can reduce the expression of the proliferative antigen Ki-67 and increase the phosphorylation of the tumor suppressor gene P53 in early breast cancer, thus slowing down cell proliferation and inducing cell apoptosis, which has a positive effect on breast cancer pateints (79).
Barron suggested that women who took propranolol in the years before breast cancer diagnosis were significantly less likely to develop T4 tumors in a large population study, with positive lymph nodes (N2/N3), or metastases and significantly lower mortality rate than women who did not take propranolol. Besides, prolonged use of propranolol may reduce T4 tumorigenicity (73). Moreover, propranolol has a protective effect on head and neck cancer, stomach, colon, and prostate cancer, especially when used for more than 1,000 days (46). Inhibition of angiogenesis reduces bacterial translocation; thus, non-selective beta-blockers have been shown to reduce the incidence of liver cancer in patients with cirrhosis (80).
ACEI/ARB
RAS is well known for its control over the body's internal environment stability. Recently, there have been many studies reported RAS involvement in the complex carcinogenic mechanism. It is associated with proliferation signaling, resistance to cell death, induction of angiogenesis, energy metabolism reprogramming, inflammation, cell migration, invasion, and metastasis, thereby promoting vascular endothelial growth factor-mediated angiogenesis in malignant tumors and increasing proliferation of malignant tumor cells (81, 82). Therefore, using drugs that inhibit the RAS (mainly ACEI and ARB) can slow the rate of tumor growth. Furthermore, the risk of most cancers also decreases by using for long time (83).
A recent retrospective study of 73,170 patients with breast cancer patients using ARB improved patient's survival rate and reduced mortality. Patients who used other antihypertensive drugs also had reduced mortality, but cannot rule out it is due to blood pressure control or have positive effects on cancer (84). Similarly, compared with other antihypertensive drugs, patients on ACEI have a lower incidence of prostate cancer; hence, ACEI may improve their survival rate (85, 86). RAS inhibitors slow the progression of gastrointestinal cancer. Using ACEI over 3 years was associated with a 29% reduction in the risk of esophageal adenocarcinoma, and high daily doses were associated with a 45% risk reduction (87). RAS inhibitors have been linked to a protective effect against pancreatic cancer, with a 39% risk reduction after 1–3 years of use, but no significant effect on long-term use (88). A case-control study of patients with hypertension suggested that RAS inhibitors reduced the incidence of colon cancer, with long-term use decreasing the risk by 16 and a 25% reduction after 5 years of use. The greater the dose, the more significant the positive effect (89). The dose of ACEI is inversely proportional to the size of adenomatous polyps.
However, ACEI increases the incidence of lung cancer in patients with hypertension. The existing research points out that bradykinin (BK, a 9-peptide substance with cardioprotective effects) was found in lung cancer tissue and substance P. Many tumor cells expressed higher levels of BK and the related receptors that directly release vascular endothelial growth factor that stimulate the growth of cancer cells and angiogenesis, leading to increased risk of lung cancer. Additionally, ACEI promotes the buildup of these two chemicals in the lungs (90, 91).
Hicks et al. (92) in 2018 conducted a study, which included more than 90,000 patients with hypertension, was followed for 13 years to compare ACEI use and lung cancer incidence. This study confirmed that ACEIs could lead to an increased incidence of lung cancer. Lin et al. (93) further compared lung cancer incidence in hypertensive patients using ACEIs vs. ARBs and found that lung cancer incidence was significantly higher in patients using ACEIs than in those using ARBs. Moreover, they found that the higher the dose and longer the duration of ACEI use, the higher the incidence of lung cancer. Kumar et al. (94) and Hsu et al. (95) subsequent studies support this view. There are different conclusions, a 2021 study by Lee et al. (96) concluded that there was no significant difference in the effect of ACEI and ARB on lung cancer. Similarly, a meta-analysis, enrolled 13 observational studies with 458,686 ACEI users, conducted by Batais et al. (97) suggested that ACEIs were not associated with an increased risk of lung cancer. Although most studies reported a negative effect of ACEIs on lung cancer, more prospective studies are needed to confirm the effect of antihypertensive drugs on cancer incidence and progression.
Different cancer stages or different types of cancer have different responses to RAS blockers. As the only antihypertensive drugs that have definite effects on cancer, RAS blockers should be used carefully in clinical practice to achieve treatment optimization.
Other Drugs
A retrospective study suggested that in patients with atrial fibrillation (AF), oral anticoagulants, gastrointestinal bleeding, urogenital bleeding, and bronchopulmonary bleeding often increased the risk of cancer, and there is a strong correlation with the severity of bleeding. It is worth mentioning that this new cancer is often detected within 6 months after bleeding, but this may be related to more frequent follow-ups. In any case, patients with AF receiving oral anticoagulants should be alert to the occurrence of cancer once they have bleeding in the above-mentioned organs (98).
Early-Onset and High-Incidence Cardiovascular Risks of Cancer Patients and Their Management Strategies
Cancer Treatment Is Prone to Cardiovascular Disease
Cardiotoxicity due to antineoplastic therapy is induced by multiple factors, mainly including oxidative stress (99) [OS; including mitochondrial functional impairment (100), myocardial apoptosis (101, 102)], microtubule dysfunction (103, 104), and disruption of myocardial immune homeostasis (105, 106).
OS refers to an imbalance in the body's oxidative and antioxidant systems that tend toward oxidation, causing abnormalities in the body's biochemical and physiological processes and damaging endothelial tissue (107). Oxidants of oxidative stress refer to reactive oxygen species (ROS) or nitrogen substances (RNS) as well as free radicals. The direct effects of both inflammation and ROS are mediated by the activation of macrophages in the arterial wall (108). Neutrophils and monocytes/macrophages are the main sources of ROS, and oxidative stress increases the production of chemokines (MCP-1, CSF-1) and adhesion molecules (ICAM-1), tending to shift the redox balance toward a peroxidized state by promoting the aggregation of these cells (109). Since the heart has a weak antioxidant capacity (102), high concentrations of ROS predispose cardiomyocytes to mitochondrial damage and lipid peroxidation, affecting myocardial function. High oxidative status in elderly patients could explain the high incidence of cancer in elderly patients; therefore, chronic inflammation and oxidative stress should also be considered risk factors for cancer in the elderly (110). Drugs that mediate cardiotoxicity through oxidative stress are mainly anthracyclines and anti-epidermal growth factor receptor 2 (ErbB2) drugs, which increase the production of ROS and RNS, inhibit oxidative phosphorylation (111), lead to mitochondrial damage in cardiomyocytes (110), and ultimately result in irreversible myocardial damage. Mitochondria are important target tissues, and patients receiving tyrosine kinase inhibitors (TKI) can also suffer from cardiac complications due to impaired mitochondrial function that lead to cell death (106). Paclitaxel alters the process of cell division by affecting microtubule function, and it also affects the level of histamine in the body and stimulates the development of cardiotoxicity (106). Notably, immune checkpoint inhibitors (e.g., PD-1) mainly affect immune regulation, i.e., they influence T-cell effector function by inhibiting T-cell downstream signaling and hinder the immune organ from fighting against cancer cells (112, 113). Therefore, immune checkpoint inhibitors have been used in solid and hematological cancers to enhance the immune system's potential to fight cancer cells (114). Although existing studies suggest that immune checkpoint inhibitors are less likely to be cardiotoxic, immunotherapy may cause life-threatening events in patients (cardiac arrest, fulminant myocarditis, shock) by disrupting immune homeostasis in the myocardium (115, 116).
Cardiac Dysfunction
In patients with antineoplastic therapy-induced congestive heart failure, a reduction in LVEF of more than 10% and <50% is diagnostic of cancer therapy-induced cardiac insufficiency (cancer therapy drug-associated cardiac insufficiency/CTRCD). CTRCD usually appears months to years after treatment and is reversible in 75% of patients after withdrawal; however, it may affect long-term prognosis in 25% of patients, especially in patients with left bundle branch block. Most of these patients have no obvious symptoms, left ventricular dysfunction was diagnosed in some patients, while only a small number of patients develop symptomatic heart failure (117).
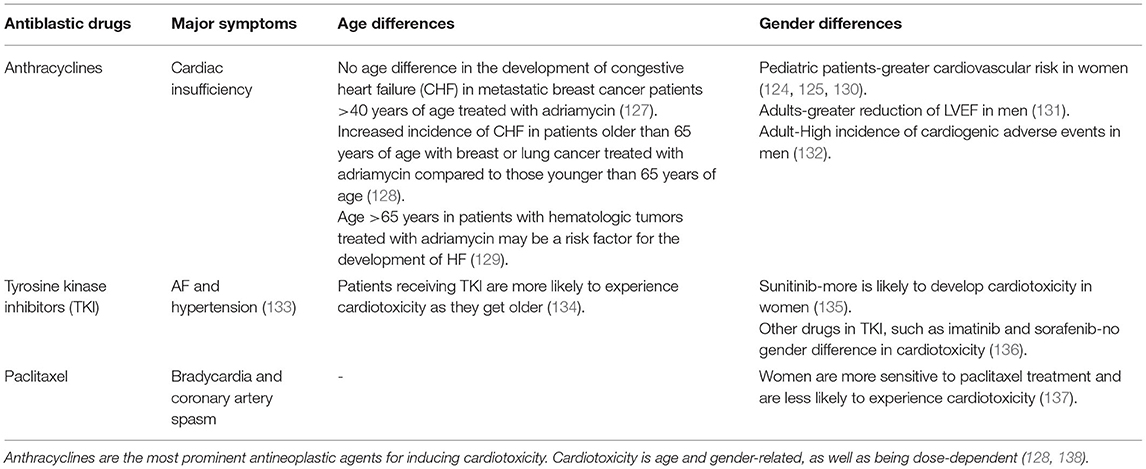
The most common anti-cancer drug that causes heart failure is anthracycline, the main treatment drug for many lymphomas, soft tissue sarcomas, and breast cancer, causing about 43% of those at risk (118). The main mechanism is the irreversible damage of cardiomyocytes with a high density of mitochondria, which induces myocardial remodeling and leads to cardiomyopathy (119). This is followed by TKI and immunotherapy, but the damage to the heart muscle is temporary and reversible. Previous studies suggested that the targeted drug trastuzumab may also increase the risk of cardiomyopathy by four times, and that when combined with anthracyclines, the risk increases by seven times (120, 121).
Women receiving anthracyclines are less likely than men to have cardiac insufficiency secondary to chemotherapy (122). The mechanism of cardiac insufficiency caused by anthracyclines is mainly due to oxidative stress-mediated oxidative damage in cardiomyocytes and abnormal mitochondrial function (123), which is less reported in female individuals (122). Notably, the occurrence of cardiac insufficiency complications in pediatric cancer patients does not appear to be significantly correlated with gender, as pediatric and postmenopausal females are more likely to develop cardiac insufficiency from antineoplastic therapy, suggesting that estrogen modulates abnormal oxidative stress in cancer patients receiving anthracyclines (124, 125). Estrogen enhances myocardial resistance to ischemia/reperfusion injury, i.e., attenuates abnormal oxidative stress and apoptosis (126). Thus, estrogens are cardioprotective. Sex differences in cardiotoxicity are not only present in anthracycline treatment but also cardiotoxicity caused by paclitaxel and tyrosine kinase inhibitors. The cardiotoxicity produced by different drugs differed concerning age and gender (Table 2). Sex-related genes are also involved in regulating the development of chemotherapy-related cardiac insufficiency (139). However, there are few studies related to the effect of gender on the efficacy and complications of antitumor therapy, and more gender- and age-specific studies are needed in the future to clarify the effectiveness of antitumor therapy and provide more theoretical support for clinical use.
Coronary Artery Disease (Acute Vasospasm and Atherosclerosis)
With the increasing number of cancer survivors, vascular toxicity has become the second most widely concerned disease after cardiac toxicity. In addition to the high incidence of venous thromboembolism, arterial toxicity has also attracted attention due to the increase of cancer patients, the prolonged life span, and the continuous progress of cancer treatment. A case study of children's study points out that had not been treated for cancer patients with systemic inflammation and increased risk of atherosclerosis, and a diagnosis of age is smaller, the higher their risk of dying from heart disease, 2 years after another child study, points out that accepting radiotherapy or chemotherapy and survival over the age of 35 patients compared with a normal person five times the risk of myocardial infarction (AMI) (140, 141). However, the overall incidence of myocardial infarction is not high. Arterial thromboembolism is also common in pancreatic, gastric, and lung cancer patients, like venous thromboembolism. Among all patients with vascular complications, lung cancer patients have the highest mortality rate and colon cancer patients have the highest bleeding risk (142, 143). Patients with metastatic cancer complicated with acute myocardial infarction have a poor prognosis, and the cause of death is usually associated with hemorrhage and reinfarction (144).
Ischemic heart disease is critical cardiotoxicity in patients treated with 5-fluorouracil during antitumor therapy. Vascular endothelial growth factor inhibitors can cause coronary spasms and even acute myocardial infarction due to endotheliotropic and erosion of monolayer vascular endothelial cells (145, 146). Paclitaxel can cause acute coronary spasms and even myocardial infarction (147). In such patients, the ST segment elevation rapidly reduces after immediate administration of nitrates. Due to its endothelial toxicity, which is not easy to eliminate, cisplatin can also cause coronary artery diseases, and the toxicity increases with the increase of its dose (148). Existing findings show that radiation therapy can change vascular reactivity and vascular spasm and possible acute severe endothelial injury and acute myocardial infarction (MI). The prolonged radiation exposure time is proportional to the risk of myocardial infarction. Due to the cancer patients own existence of cardiovascular risk factors and the role of anti-cancer treatment, prolonged endothelial cells to rebuild (149, 150). Protecting the heart from radiation may significantly reduce the risk of coronary atherosclerotic heart disease (151).
Arrhythmias
Various arrhythmias may occur in cancer patients during anti-cancer treatment. Inflammatory infiltration of the heart may result in pericarditis or cardiomyopathy that involves the cardiac conduction system and results in the atrioventricular block, prolonged QT interval, and AF. Radiotherapy causes radiation injury and promotes myocardial fibrosis, resulting in an atrioventricular block and AF, but rarely causes ventricular arrhythmias (152), so it may become an alternative therapy for invasive ventricular ablation of ventricular arrhythmias. The arrhythmias induced by antitumor therapy may also be related to drug interactions, drug accumulation, and electrolyte disturbance. The common arrhythmias in cancer patients mainly include AF, prolonged QT interval, ventricular arrhythmias, and cardiac arrest (152).
In 1993, the first arrhythmia caused by antineoplastic therapy was proposed, about 30% of patients who used chemotherapy drug paclitaxel developed asymptomatic bradycardia (104). Later studies suggested that thalidomide, palazonib, sunitinib, and crizotinib may also cause bradycardia. About 72% of patients treated with the chemothermic arsenic trioxide extended their QT interval from baseline by more than 30 ms, with half of those exceeding 60 ms (153). Many targeted drugs cause prolongation of the QT interval (154, 155). Generally, the prolonged QT interval resolves gradually as the drugs are metabolized. However, if the upper limit is exceeded, antitumor drugs should be discontinued to avoid torsade de points ventricular tachycardia. AF is closely linked to cancer, and they share the same risk factors: obesity and inflammation. A study showed a 20% increase in the incidence of cancer within 1 year of AF onset. AF occurred as a cardiotoxic complication of antitumor therapy with anthracycline, ibrutinib, melphalan, and paclitaxel (156, 157).
Thrombotic Disease and Peripheral Vascular Disease
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), is second only to disease progression as the leading cause of death in cancer patients. Pulmonary embolism occurred in half of untreated DVT patients. About one-third of untreated pulmonary embolism patients die, most of whom have recurrent thromboembolism (158, 159). Compared with normal people, the incidence of VTE in cancer patients is at least 4 times higher, and a higher risk of VTE often indicates a poor prognosis of the cancer patients. A previous study showed that cancer of the pancreas, bile duct, and liver is associated with a higher risk of VTE (160).
Cancer patients release pro-inflammatory factors and pro-coagulation active substances, thereby promoting the adhesion between blood cells and blood vessels, resulting in a high coagulation state. The main factors of cancer patients that predispose to VTE include the type of cancer, central venous catheter chemotherapy, radiotherapy, surgical treatment, and related drug side effects (161).
Others: Hypertension, Valvular Heart Disease, Pericardial Disease
Hypertension is closely related to the occurrence of tumors. High blood pressure and cancer have some common risk factors (age, active or passive smoking, diabetes, dyslipidemia, overweight or obesity, low physical activity, unhealthy diet) (162). Vascular endothelial growth factor (VEGF) possibly plays an important role in the pathogenesis of hypertension and tumor by stimulating angiogenesis (163, 164). Forty-one years after Judah Folkman (165) proposed that tumor growth depends on the formation of new blood vessels by secreting factors, hyperactive angiogenesis is now becoming a therapeutic target for cancer (166). However, given the common biological characteristics of tumors and hypertension, some anti-tumor drugs can increase the incidence of hypertension. At present, it is believed that the anti-tumor drugs that can cause hypertension mainly refer to VEGF signal inhibitors, with a 19–47% incidence of hypertension (167). VEGF signal inhibitors may lead to an imbalance between vasodilators and vasoconstrictors, loss of capillary microcirculation, and altered glomerular function, all of which contribute to hypertension (168, 169). Some scholars believe that hypertension may be related to the effectiveness of anti-cancer treatment. Tanaka et al. (170) found that the development of hypertension in the early stage of treatment is related to the anti-tumor effect and maybe a predictor of treatment effect.
Anti-tumor therapy-related valvular heart disease is mainly caused by radiotherapy, especially involving the left heart valve. The pathological manifestations were valve tip and leaflet thickening, calcification, and retraction. Similarly, radiotherapy over 2 years can lead to pericarditis in up to 20% of tumor patients, so it is recommended that radiotherapy doses of <10 Gy should be limited to patients without prior cardiac disease during radiotherapy for thoracic tumors (171).
Cardiac metabolic syndrome is a condition caused by various metabolic disorders that affect about one in four adults. Saxena et al. (172) proposed that cardiac metabolic syndrome is associated with various cancers, especially pancreatic and rectal cancer in females and prostate cancer in males.
Cancer and Cardiovascular Disease Prevention
Poor lifestyle is a common risk factor for CVD and cancer, while a low-risk lifestyle reduces the incidence of cancer, CVD and diabetes, as well as the mortality due to related diseases, and prolongs the life expectancy of healthy people. A low-risk lifestyle includes non-smoking, a BMI of 18.5–24.9 kg/m2, moderate daily exercise (≥30 min/day), moderate alcohol consumption (5–15g/day for women, 5–30g/day for men), and a high-quality diet (173). Moderate drinking may prevent CVD, but cancer risk is relatively increasing, so non-alcoholic drinkers are not recommended to start drinking to prevent CVD (173). Dietary supplements of omega-3 fatty acids or vitamin D to prevent cancer and CVD are also not recommended for the general population, as they may cause problems with existing health conditions (174).
Prior to cancer treatment, especially before using treatment measures known to have cardiovascular toxicity, patients should be screened for the risk of underlying CVD, diabetes, and other related diseases through Electrocardiogram (ECG), echocardiography, biomarkers, and other tests. Heart rate variability (HRV) may predict cardiovascular complications in breast cancer (175). More elaborate screening tests for underlying diseases and comorbid conditions should be undertaken for patients with pre-existing CVD. For patients with tumors at high cardiovascular risk, close monitoring of relevant indicators is recommended and antitumor therapies with clear cardiovascular toxicity should be avoided.
Recommended Diagnostic Methods
Blood Pressure Monitoring and Electrocardiogram
The diagnostic criteria for hypertension in cancer patients are the same as those in the general population.
The diagnosis of arrhythmia and acute ST-segment elevation myocardial infarction in cancer survivors can be confirmed by paying close attention to the dynamic changes of ECG. For paroxysmal arrhythmia, a dynamic ECG is feasible to make a clear diagnosis. Aggressive electrophysiological tests can be used to diagnose suspected arrhythmias, but the necessity of these tests depends on the patient's general state and life expectancy (176).
Imaging Examination
Echocardiography is the first choice for cancer patients to monitor cardiac function (i.e., LVEF) and diagnose valvular heart disease. 3D echocardiography is recommended as the first choice so that the endocardial boundary can be seen more clearly (177). Cardiac magnetic resonance imaging (CMR) has become the clinical gold standard for measuring left ventricular volume and ejection fraction, followed by radionuclide ventricular angiography (RVG)/ multigate cardiac pool imaging (multigate acquisition, MUGA). An endomyocardial biopsy can determine the extent of myocardial injury in cancer patients, but due to its invasive nature, the diagnosis is usually confirmed by the patient's symptoms and imaging examination (178, 179).
Arterial and venous ultrasound of bilateral lower extremities is preferred for VTE diagnosis. Computed tomography pulmonary angiography (CTPA) is recommended to confirm PE after DVT is diagnosed. CTPA is the preferred imaging modality for diagnosing PE. When patients have symptoms highly suspicion of PE, such as dyspnea, chest pain, hemoptysis, and cough, accompanied by hypoxemia, along with DVT, as suggested by arterial and venous ultrasound of lower limbs, the CTPA should be performed timely to make a confirmed diagnosis. CTPA is contraindicated in patients with contrast hypersensitivity, renal insufficiency, hypotension, advanced heart failure, or unable to perform CT scanning due to complex comorbid conditions or difficulty lying flat (159).
Biomarker
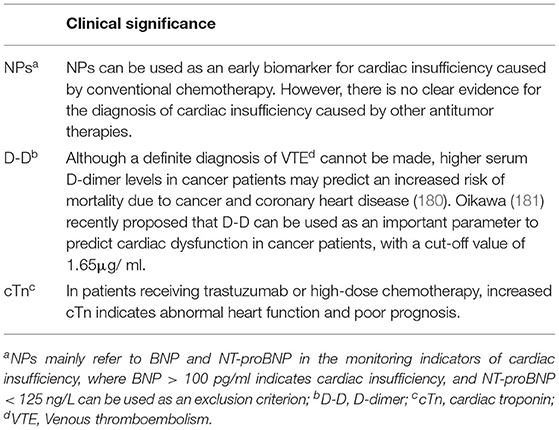
Biomarkers play an important role in the prevention and diagnosis of CVD (Table 3). However, a single biomarker has certain limitations, and many factors can lead to abnormal results, so a definite diagnosis generally requires imaging findings and lab tests.
Natriuretic Peptide
Nearly half of the patients with cardiac dysfunction are not accompanied by reduced LVEF, so when patients have overt dyspnea, but no history of myocardial infarction and signs of pulmonary edema, it is recommended to measure natriuretic peptide (NP): B-type natriuretic peptide (BNP) or N-terminal precursor B-type brain natriuretic peptide (NT-proBNP) level (182).
D-Dimer
D-dimer (D-D) is the first choice for VTE diagnosis in a normal population. However, given the higher pathophysiological coagulation tendency in cancer patients, increased plasma D-D level is generally found in cancer patients. Therefore, arterial and venous color Doppler of the bilateral lower extremity is considered the first choice for VTE diagnosis in cancer patients (183).
Cardiac Troponin
The diagnostic approach of coronary heart disease in cancer patients is the same as that in normal people. Cardiac injury markers include creatine kinase MB (CK-MB), myoglobin, and cardiac troponin (cTn); among them, cTn is considered more important. For early reinfarction in patients with MI, CK-MB is relatively more significant (184).
Coronary Angiography
In addition to the patient's symptoms and vital signs, coronary angiography is the gold standard for diagnosing coronary artery disease (CAD) and evaluating vascular status. Diagnosis of CAD can be challenging in cancer patients, and some of the anti-tumor drugs mentioned earlier can cause transient coronary spasms that mimic the symptoms of a heart attack.
For patients at low risk for CAD, CCTA can be considered. Indications for coronary angiography are that the patient is suitable for coronary revascularization and that acute coronary syndrome or angina is not adequately controlled by optimal medication (185).
Early Detection and Treatment of Cardiovascular Diseases in Cancer Patients
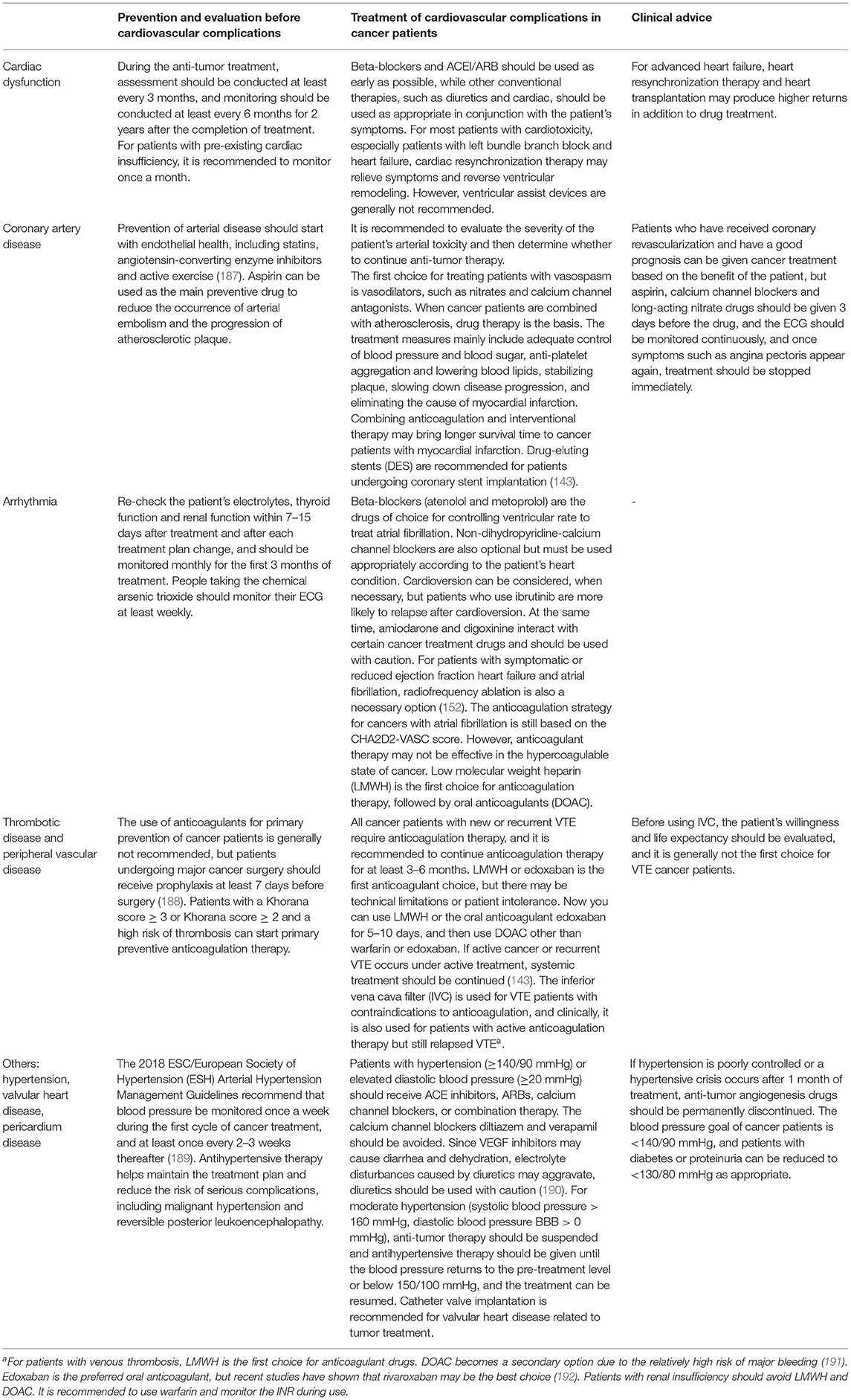
When the detection time and treatment time for complications of cardiac insufficiency in cancer patients are doubled, the chance of LVEF returning to normal will be reduced by 25% (186). Therefore, cardiovascular complications in cancer patients should be strengthened (Table 4). First, it should be clear whether the patient is a patient with a high risk of CVD such as hypertension, diabetes, smoking, alcohol consumption, hyperlipidemia, obesity, poor lifestyle and presence of a family history of heart disease, and a 12-lead ECG should be routinely checked before anti-tumor treatment. Blood pressure and myocardial enzymes and other indicators should be recorded. Given that the electrolyte imbalance, abnormal thyroid function, or renal function may cause arrhythmia (193), patients should be routinely checked before cardiovascular toxicity occurs, and changes in the patient's ECG and myocardial enzymes should be monitored during anti-tumor treatment. Some drugs for treating tumor comorbidities, such as antiemetics and psychotropic drugs, can prolong the DT interval. It should be clear whether the arrhythmia caused by antitumor therapy or the treatment of comorbid drugs is caused. In high-risk situations, it is recommended to switch to other drugs to treat comorbidities (186).
Conclusion
Cancer survivors face several risks, including cancer recurrence and cardiovascular events. Cardio-Oncology provides a multidisciplinary approach to the entire treatment process and guides effective treatment of cancer patients with cardiovascular complications. However, the relevant studies are in their preliminary stages with several limitations. The positive results obtained from many studies may vary with age, gender, race, marital status, stage of cancer, time of diagnosis and surgical intervention. Therefore, large-scale study results are needed for further confirmation. Secondly, there is still much room to explore the interaction between cardiovascular drugs and anti-tumor drugs as well the effect of genes on the mechanism of these drugs. Clinical studies are required to decide the proper timing of discontinuation and restart of antitumor therapy due to cardiovascular complications and provide alternative therapies if needed.
When patients with both CVD and cancer are encountered clinically, the proper treatment strategies should be followed by clinicians. Moreover, oncologists should be informed of cardiovascular complications of antitumor therapy and their prevention, diagnosis and treatment. Furthermore, cardiologists should be alert to the high incidence of tumors caused by certain cardiovascular drugs in high-risk patients. Similarly, it calls on society to enhance the awareness and attention of cancer and CVD and hopes that doctors from all departments can cooperate to promote the continuous development of this discipline.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author Contributions
YiW: reviewed the literature and drafted this review. YoW, XH, and CL: reviewed the literature, gave critical comments, and revised the manuscript. JS, BA, and JZ: gave critical comments and revised the manuscript. XM and ZC: reviewed the literature, gave critical comments, and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by the National Natural Science Foundation of China [YoW, Grant Number: 82170362], [CL, Grant Number: 82000347], and [JS, Grant Number: 81770374]. This study was also supported by Jilin Province Science and technology development plan project [YoW, Grant Number: 20190701066GH], [XM, Grant Number: 20210101246JC], China Postdoctoral Science Foundation [YoW, 2021M691209], and Jilin Medical and Health Talents Special [YoW, JLSWSRCZX2021-061].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S. Blood pressure and incidence of twelve cardiovascular diseases: lifetime risks, healthy life-years lost, and age-specific associations in 125 million people. Lancet. (2014) 383:1899–911. doi: 10.1016/S0140-6736(14)60685-1
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
3. Pfeffer TJ, Pietzsch S, Hilfiker-Kleiner D. Common genetic predisposition for heart failure and cancer. Herz. (2020) 45:632–6. doi: 10.1007/s00059-020-04953-9
4. Guha A, Fradley MG, Dent SF, Weintraub NL, Lustberg MB, Alonso A, et al. Incidence, risk factors, and mortality of atrial fibrillation in breast cancer: a SEER-medicare analysis. Eur Heart J. (2021) 43:300–12. doi: 10.1093/eurheartj/ehab745
5. Chen D, Kelly C, Haw TJ, Lombard JM, Nordman IIC, Croft AJ, et al. Heart Failure in breast cancer survivors: focus on early detection and novel biomarkers. Curr Heart Fail Rep. (2021) 18:362–77. doi: 10.1007/s11897-021-00535-w
6. Meijers WC, Maglione M, Bakker SJL, Oberhuber R, Kieneker LM, de Jong S, et al. Heart failure stimulates tumor growth by circulating factors. Circulation. (2018) 138:678–91. doi: 10.1161/CIRCULATIONAHA.117.030816
7. Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. (2016) 133:1104–14. doi: 10.1161/CIRCULATIONAHA.115.020406
8. Grossman E, Messerli FH, Boyko V, Goldbourt U. Is there an association between hypertension and cancer mortality? Am J Med. (2002) 112:479–86. doi: 10.1016/S0002-9343(02)01049-5
9. Koelwyn GJ, Newman AAC, Afonso MS, van Solingen C, Corr EM, Brown EJ, Moore KJ. Myocardial infarction accelerates breast cancer via innate immune reprogramming. Nat Med. (2020) 26:1452–8. doi: 10.1038/s41591-020-0964-7
10. Thackeray JT, Pietzsch S, Stapel B, Ricke-Hoch M, Lee CW, Bankstahl JP, et al. Insulin supplementation attenuates cancer-induced cardiomyopathy and slows tumor disease progression. JCI Insight. (2017) 2. doi: 10.1172/jci.insight.93098
11. Evans MA, Sano S, Walsh K. Cardiovascular disease, aging, and clonal hematopoiesis. Annu Rev Pathol. (2020) 15:419–38. doi: 10.1146/annurev-pathmechdis-012419-032544
12. Huang YC, Wang CY. Telomere attrition and clonal hematopoiesis of indeterminate potential in cardiovascular disease. Int J Mol Sci. (2021) 22:9867. doi: 10.3390/ijms22189867
13. Sabater-Molina M, Navarro-Penalver M, Munoz-Esparza C, Esteban-Gil A, Santos-Mateo JJ, Gimeno JR. Genetic factors involved in cardiomyopathies and in cancer. J Clin Med. (2020) 9:1702. doi: 10.3390/jcm9061702
14. Herman DS, Lam L, Taylor MR, Wang L, Teekakirikul P, Christodoulou D, et al. Truncations of titin causing dilated cardiomyopathy. N Engl J Med. (2012) 366:619–28. doi: 10.1056/NEJMoa1110186
15. Garcia-Pavia P, Kim Y, Restrepo-Cordoba MA, Lunde IG, Wakimoto H, Smith AM, et al. Genetic variants associated with cancer therapy-induced cardiomyopathy. Circulation. (2019) 140:31–41. doi: 10.1161/CIRCULATIONAHA.118.037934
16. Linschoten M, Teske AJ, Baas AF, Vink A, Dooijes D, Baars HF, et al. Truncating titin (TTN) variants in chemotherapy-induced cardiomyopathy. J Card Fail. (2017) 23:476–9. doi: 10.1016/j.cardfail.2017.03.003
17. Sapkota Y, Qin N, Ehrhardt MJ, Wang Z, Chen Y, Wilson CL, et al. Genetic variants associated with therapy-related cardiomyopathy among childhood cancer survivors of African ancestry. Cancer Res. (2021) 81:2556–65. doi: 10.1158/0008-5472.CAN-20-2675
18. Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. (2014) 371:2488–98. doi: 10.1056/NEJMoa1408617
19. Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. (2015) 126:9–16. doi: 10.1182/blood-2015-03-631747
20. Jaiswal S, Ebert BL. Clonal hematopoiesis in human aging and disease. Science. (2019) 366:eaan4673. doi: 10.1126/science.aan4673
21. Silver AJ, Jaiswal S. Clonal hematopoiesis: pre-cancer PLUS. Adv Cancer Res. (2019) 141:85–128. doi: 10.1016/bs.acr.2018.12.003
22. Asada S, Kitamura T. Clonal hematopoiesis and associated diseases: a review of recent findings. Cancer Sci. (2021) 112:3962–71. doi: 10.1111/cas.15094
23. Severson EA, Riedlinger GM, Connelly CF, Vergilio JA, Goldfinger M, Ramkissoon S, et al. Detection of clonal hematopoiesis of indeterminate potential in clinical sequencing of solid tumor specimens. Blood. (2018) 131:2501–5. doi: 10.1182/blood-2018-03-840629
24. Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. (2017) 377:111–21. doi: 10.1056/NEJMoa1701719
25. Mann M, Mehta A, de Boer CG, Kowalczyk MS, Lee K, Haldeman P, et al. Heterogeneous responses of hematopoietic stem cells to inflammatory stimuli are altered with age. Cell Rep. (2018) 25:2992–3005. doi: 10.1016/j.celrep.2018.11.056
26. Bolton KL, Koh Y, Foote MB, Im H, Jee J, Sun CH, et al. Clonal hematopoiesis is associated with risk of severe Covid-19. Nat Commun. (2021) 12:5975. doi: 10.1038/s41467-021-26138-6
27. Zuriaga MA, Fuster JJ. Emerging role of acquired mutations and clonal hematopoiesis in atherosclerosis- beyond conventional cardiovascular risk factors. Circ J. (2021). doi: 10.1253/circj.CJ-21-0505
28. Mozzini C, Pagani M. Clonal hematopoiesis and cardiovascular diseases: the connection. Curr Probl Cardiol. (2021) 100962. doi: 10.1016/j.cpcardiol.2021.100962 Available online at: https://www.sciencedirect.com/science/article/pii/S0146280621001778?via%3Dihub
29. Galli D, Manuguerra R, Monaco R, Manotti L, Goldoni M, Becchi G, et al. Understanding the structural features of symptomatic calcific aortic valve stenosis: a broad-spectrum clinico-pathologic study in 236 consecutive surgical cases. Int J Cardiol. (2017) 228:364–74. doi: 10.1016/j.ijcard.2016.11.180
30. Mas-Peiro S, Hoffmann J, Fichtlscherer S, Dorsheimer L, Rieger MA, et al. Clonal haematopoiesis in patients with degenerative aortic valve stenosis undergoing transcatheter aortic valve implantation. Eur Heart J. (2020) 41:933–9. doi: 10.1093/eurheartj/ehz591
31. Pascual-Figal DA, Bayes-Genis A, Diez-Diez M, Hernandez-Vicente A, Vazquez-Andres D, de la Barrera J, et al. Clonal hematopoiesis and risk of progression of heart failure with reduced left ventricular ejection fraction. J Am Coll Cardiol. (2021) 77:1747–59. doi: 10.1016/j.jacc.2021.02.028
32. Agarwal A, Holbrook AM. In healthy older adults, daily aspirin increased cancer mortality. Ann Intern Med. (2021) 174:JC74. doi: 10.7326/ACPJ202107200-074
33. Gasic GJ. Role of plasma, platelets, and endothelial cells in tumor metastasis. Cancer Metastasis Rev. (1984) 3:99–114. doi: 10.1007/BF00047657
34. Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. (2011) 11:123–34. doi: 10.1038/nrc3004
35. Cao Y, Nishihara R, Wu K, Wang M, Ogino S, Willett WC, et al. Population-wide impact of long-term use of aspirin and the risk for cancer. JAMA Oncol. (2016) 2:762–9. doi: 10.1001/jamaoncol.2015.6396
36. Bosetti C, Santucci C, Gallus S, Martinetti M, La Vecchia C. Aspirin and the risk of colorectal and other digestive tract cancers: an updated meta-analysis through 2019. Ann Oncol. (2020) 31:558–68. doi: 10.1016/j.annonc.2020.02.012
37. Steering Committee of the Physicians' Health Study Research Group. Preliminary report: findings from the aspirin component of the ongoing physicians' health study. N Engl J Med. (1988) 318:262–4. doi: 10.1056/NEJM198801283180431
38. Burn J, Sheth H, Elliott F, Reed L, Macrae F, Mecklin JP, et al. Cancer prevention with aspirin in hereditary colorectal cancer (Lynch syndrome), 10-year follow-up and registry-based 20-year data in the CAPP2 study: a double-blind, randomised, placebo-controlled trial. Lancet. (2020) 395:1855–63. doi: 10.1016/S0140-6736(20)30366-4
39. Katona BW, Weiss JM. Chemoprevention of colorectal cancer. Gastroenterology. (2020) 158:368–88. doi: 10.1053/j.gastro.2019.06.047
40. Simon TG, Duberg AS, Aleman S, Chung RT, Chan AT, Ludvigsson JF. Association of aspirin with hepatocellular carcinoma and liver-related mortality. N Engl J Med. (2020) 382:1018–28. doi: 10.1056/NEJMoa1912035
41. Skriver C, Dehlendorff C, Borre M, Brasso K, Larsen SB, Dalton SO, et al. Use of low-dose aspirin and mortality after prostate cancer diagnosis: a nationwide cohort study. Ann Intern Med. (2019) 170:443–52. doi: 10.7326/M17-3085
42. Jackson SS, Pfeiffer RM, Liu Z, Anderson LA, Tsai HT, Gadalla SM, et al. Association between aspirin use and biliary tract cancer survival. JAMA Oncol. (2019) 5:1802–4. doi: 10.1001/jamaoncol.2019.4328
43. McNeil JJ, Gibbs P, Orchard SG, Lockery JE, Bernstein WB, Cao Y, et al. Effect of aspirin on cancer incidence and mortality in older adults. J Natl Cancer Inst. (2021) 113:258–65. doi: 10.1093/jnci/djaa114
44. Ren QW, Yu SY, Teng TK, Li X, Cheung KS, Wu MZ, et al. Statin associated lower cancer risk and related mortality in patients with heart failure. Eur Heart J. (2021) 42:3049–59. doi: 10.1093/eurheartj/ehab325
45. Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. (2005) 5:930–42. doi: 10.1038/nrc1751
46. Chang PY, Huang WY, Lin CL, Huang TC, Wu YY, Chen JH, et al. Propranolol reduces cancer risk: a population-based cohort study. Medicine. (2015) 94:e1097. doi: 10.1097/MD.0000000000001097
47. Wijarnpreecha K, Aby ES, Ghoz H, Cheungpasitporn W, Lukens FJ, Harnois DM, et al. Statins and risk of cholangiocarcinoma: a systematic review and meta-analysis. J Gastrointestin Liver Dis. (2020) 29:629–35. doi: 10.15403/jgld-2990
48. Lavu S, Therneau TM, Harmsen WS, Mara KC, Wongjarupong N, Hassan M, et al. Effect of statins on the risk of extrahepatic cholangiocarcinoma. Hepatology. (2020) 72:1298–309. doi: 10.1002/hep.31146
49. Liu Z, Alsaggaf R, McGlynn KA, Anderson LA, Tsai HT, Zhu B, et al. Statin use and reduced risk of biliary tract cancers in the UK clinical practice research datalink. Gut. (2019) 68:1458–64. doi: 10.1136/gutjnl-2018-317504
50. Simon TG, Duberg AS, Aleman S, Hagstrom H, Nguyen LH, Khalili H, et al. Lipophilic statins and risk for hepatocellular carcinoma and death in patients with chronic viral hepatitis: results from a nationwide Swedish population. Ann Intern Med. (2019) 171:318–27. doi: 10.7326/M18-2753
51. Cheung KS, Chen L, Chan EW, Seto WK, Wong ICK, Leung WK. Statins reduce the progression of non-advanced adenomas to colorectal cancer: a postcolonoscopy study in 187 897 patients. Gut. (2019) 68:1979–85. doi: 10.1136/gutjnl-2018-317714
52. Yarmolinsky J, Bull CJ, Vincent EE, Robinson J, Walther A, Smith GD, et al. Association between genetically proxied inhibition of hmg-coa reductase and epithelial ovarian cancer. JAMA. (2020) 323:646–55. doi: 10.1001/jama.2020.0150
53. Abdel-Qadir H, Bobrowski D, Zhou L, Austin PC, Calvillo-Arguelles O, Amir E, et al. Statin exposure and risk of heart failure after anthracycline- or trastuzumab-based chemotherapy for early breast cancer: a propensity scorematched cohort study. J Am Heart Assoc. (2021) 10:e018393. doi: 10.1161/JAHA.119.018393
54. Malyszko J, Malyszko M, Kozlowski L, Kozlowska K, Malyszko J. Hypertension in malignancy-an underappreciated problem. Oncotarget. (2018) 9:20855–71. doi: 10.18632/oncotarget.25024
55. Lever AF, Hole DJ, Gillis CR, McCallum IR, McInnes GT, MacKinnon PL, et al. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet. (1998) 352:179–84. doi: 10.1016/S0140-6736(98)03228-0
56. Kristensen KB, Hicks B, Azoulay L, Pottegard A. Use of ACE (angiotensin-converting enzyme) inhibitors and risk of lung cancer: a nationwide nested case-control study. Circ Cardiovasc Qual Outcomes. (2021) 14:e006687. doi: 10.1161/CIRCOUTCOMES.120.006687
57. Kunisada M, Masaki T, Ono R, Morinaga H, Nakano E, Yogianti F, et al. Hydrochlorothiazide enhances UVA-induced DNA damage. Photochem Photobiol. (2013) 89:649–54. doi: 10.1111/php.12048
58. Li CI, Malone KE, Weiss NS, Boudreau DM, Cushing-Haugen KL, Daling JR. Relation between use of antihypertensive medications and risk of breast carcinoma among women ages 65-79 years. Cancer. (2003) 98:1504–13. doi: 10.1002/cncr.11663
59. Adalsteinsson JA, Muzumdar S, Waldman R, Hu C, Wu R, Ratner D, et al. Association between hydrochlorothiazide and the risk of in situ and invasive squamous cell skin carcinoma and basal cell carcinoma: a population-based case-control study. J Am Acad Dermatol. (2021) 84:669–75. doi: 10.1016/j.jaad.2020.08.025
60. Pedersen SA, Gaist D, Schmidt SAJ, Holmich LR, Friis S, Pottegard A. Hydrochlorothiazide use and risk of nonmelanoma skin cancer: a nationwide case-control study from Denmark. J Am Acad Dermatol. (2018) 78:673–81. doi: 10.1016/j.jaad.2017.11.042
61. Gandini S, Palli D, Spadola G, Bendinelli B, Cocorocchio E, Stanganelli I, et al. Anti-hypertensive drugs and skin cancer risk: a review of the literature and meta-analysis. Crit Rev Oncol Hematol. (2018) 122:1–9. doi: 10.1016/j.critrevonc.2017.12.003
62. Rizos CV, Elisaf MS. Antihypertensive drugs and glucose metabolism. World J Cardiol. (2014) 6:517–30. doi: 10.4330/wjc.v6.i7.517
63. Colt JS, Hofmann JN, Schwartz K, Chow WH, Graubard BI, Davis F, et al. Antihypertensive medication use and risk of renal cell carcinoma. Cancer Causes Control. (2017) 28:289–97. doi: 10.1007/s10552-017-0857-3
64. Grossman E, Messerli FH, Goldbourt U. Does diuretic therapy increase the risk of renal cell carcinoma? Am J Cardiol. (1999) 83:1090–3.
65. Daling JR. Calcium channel blockers and cancer: is an association biologically plausible? Am J Hypertens. (1996) 9:713–4.
66. Pahor M, Guralnik JM, Ferrucci L, Corti MC, Salive ME, Cerhan, et al. Calcium-channel blockade and incidence of cancer in aged populations. Lancet. (1996) 348:493–7. doi: 10.1016/S0140-6736(96)04277-8
67. Thakur AA, Wang X, Garcia-Betancourt MM, Forse RA. Calcium channel blockers and the incidence of breast and prostate cancer: a meta-analysis. J Clin Pharm Ther. (2018) 43:519–29. doi: 10.1111/jcpt.12673
68. Rotshild V, Azoulay L, Feldhamer I, Perlman A, Glazer M, Muszkat M, et al. Calcium channel blockers and the risk for lung cancer: a population-based nested case-control study. Ann Pharmacother. (2019) 53:445–52. doi: 10.1177/1060028018814684
69. Rotshild V, Azoulay L, Zarifeh M, Masarwa R, Hirsh-Raccah B, Perlman A, et al. The risk for lung cancer incidence with calcium channel blockers: a systematic review and meta-analysis of observational studies. Drug Saf. (2018) 41:555–64. doi: 10.1007/s40264-018-0644-4
70. Rotshild V, Azoulay L, Feldhamer I, Perlman A, Muszkat M, Matok I. Calcium channel blocker use and the risk for prostate cancer: a population-based nested case-control study. Pharmacotherapy. (2019) 39:690–6. doi: 10.1002/phar.2266
71. Saltzman BS, Weiss NS, Sieh W, Fitzpatrick AL, McTiernan A, Daling JR, et al. Use of antihypertensive medications and breast cancer risk. Cancer Causes Control. (2013) 24:365–71. doi: 10.1007/s10552-012-0122-8
72. Li CI, Daling JR, Tang MT, Haugen KL, Porter PL, Malone KE. Use of antihypertensive medications and breast cancer risk among women aged 55 to 74 years. JAMA Intern Med. (2013) 173:1629–37. doi: 10.1001/jamainternmed.2013.9071
73. Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population- based study. J Clin Oncol. (2011) 29:2635–44. doi: 10.1200/JCO.2010.33.5422
74. Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. (2015) 15:563–72. doi: 10.1038/nrc3978
75. Masur K, Niggemann B, Zanker KS, Entschladen F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. (2001) 61:2866–9. Available online at: https://aacrjournals.org/cancerres/article/61/7/2866/508514/Norepinephrine-induced-Migration-of-SW-480-Colon
76. Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. (2006) 12:369–75. doi: 10.1158/1078-0432.CCR-05-1698
77. Palm D, Lang K, Niggemann B, Drell TL, Masur K, Zaenker KS, et al. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. Int J Cancer. (2006) 118:2744–9. doi: 10.1002/ijc.21723
78. Pon CK, Lane JR, Sloan EK, Halls ML. The beta2-adrenoceptor activates a positive cAMP-calcium feedforward loop to drive breast cancer cell invasion. FASEB J. (2016) 30:1144–54. doi: 10.1096/fj.15-277798
79. Montoya A, Amaya CN, Belmont A, Diab N, Trevino R, Villanueva G, et al. Use of non-selective beta-blockers is associated with decreased tumor proliferative indices in early stage breast cancer. Oncotarget. (2017) 8:6446–60. doi: 10.18632/oncotarget.14119
80. Thiele M, Albillos A, Abazi R, Wiest R, Gluud LL, Krag A. Non-selective beta-blockers may reduce risk of hepatocellular carcinoma: a meta-analysis of randomized trials. Liver Int. (2015) 35:2009–16. doi: 10.1111/liv.12782
81. George AJ, Thomas WG, Hannan RD. The renin-angiotensin system and cancer: old dog, new tricks. Nat Rev Cancer. (2010) 10:745–59. doi: 10.1038/nrc2945
82. Wegman-Ostrosky T, Soto-Reyes E, Vidal-Millan S, Sanchez-Corona J. The renin-angiotensin system meets the hallmarks of cancer. J Renin Angiotensin Aldosterone Syst. (2015) 16:227–33. doi: 10.1177/1470320313496858
83. Sanidas E, Velliou M, Papadopoulos D, Fotsali A, Iliopoulos D, Mantzourani M, et al. Antihypertensive drugs and risk of cancer: between scylla and charybdis. Am J Hypertens. (2020) 33:1049–58. doi: 10.1093/ajh/hpaa098
84. Santala EEE, Murto MO, Artama M, Pukkala E, Visvanathan K, Murtola TJ. Angiotensin receptor blockers associated with improved breast cancer survival-a nationwide cohort study from finland. Cancer Epidemiol Biomarkers Prev. (2020) 29:2376–82. doi: 10.1158/1055-9965.EPI-20-0711
85. Ronquist G, Rodriguez LA, Ruigomez A, Johansson S, Wallander MA, Frithz G, et al. Association between captopril, other antihypertensive drugs and risk of prostate cancer. Prostate. (2004) 58:50–6. doi: 10.1002/pros.10294
86. Siltari A, Murtola TJ, Talala K, Taari K, Tammela TLJ, Auvinen A. Antihypertensive drug use and prostate cancer-specific mortality in Finnish men. PLoS ONE. (2020) 15:e0234269. doi: 10.1371/journal.pone.0234269
87. Sjoberg T, Garcia Rodriguez LA, Lindblad M. Angiotensin-converting enzyme inhibitors and risk of esophageal and gastric cancer: a nested case-control study. Clin Gastroenterol Hepatol. (2007) 5:1160–6. doi: 10.1016/j.cgh.2007.08.005
88. Mandilaras V, Bouganim N, Yin H, Asselah J, Azoulay L. The use of drugs acting on the renin-angiotensin system and the incidence of pancreatic cancer. Br J Cancer. (2017) 116:103–8. doi: 10.1038/bjc.2016.375
89. Makar GA, Holmes JH, Yang YX. Angiotensin-converting enzyme inhibitor therapy and colorectal cancer risk. J Natl Cancer Inst. (2014) 106:djt374. doi: 10.1093/jnci/djt374
90. Trifilieff A, Da Silva A, Gies JP. Kinins and respiratory tract diseases. Eur Respir J. (1993) 6:576–87.
91. Singh JSS, Burrell LM, Cherif M, Squire IB, Clark AL, Lang CC. Sacubitril/valsartan: beyond natriuretic peptides. Heart. (2017) 103:1569–77. doi: 10.1136/heartjnl-2017-311295
92. Hicks BM, Filion KB, Yin H, Sakr L, Udell JA, Azoulay L. Angiotensin converting enzyme inhibitors and risk of lung cancer: population based cohort study. BMJ. (2018) 363:k4209. doi: 10.1136/bmj.k4209
93. Lin SY, Lin CL, Lin CC, Hsu WH, Lin CD, Wang IK, et al. Association between angiotensin-converting enzyme inhibitors and lung cancer-a nationwide, population-based, propensity score-matched cohort study. Cancers. (2020) 12:747. doi: 10.3390/cancers12030747
94. Kumar P, Kumar V, Murlidhar F, Fatima A, Jahangir M, Khalid D, et al. Comparison between angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for incidence of lung cancer: a retrospective study. Cureus. (2021) 13:e14788. doi: 10.7759/cureus.14788
95. Hsu HL, Lee CH, Chen CH, Zhan JF, Wu SY. Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers might be associated with lung adenocarcinoma risk: a nationwide population-based nested case-control study. Am J Transl Res. (2020) 12:6615–25. doi: 10.2139/ssrn.3564398
96. Lee SH, Chun KJ, Park J, Kim J, Sung JD, Park RW, et al. Angiotensin converting enzyme inhibitors and incidence of lung cancer in a population based cohort of common data model in Korea. Sci Rep. (2021) 11:18576. doi: 10.1038/s41598-021-97989-8
97. Batais M, Almigbal T, Alotaibi K, Alodhayani A, Alkhushail A, Altheaby A, et al. Angiotensin converting enzyme inhibitors and risk of lung cancer: a systematic review and meta-analysis. Medicine. (2021) 100:e25714. doi: 10.1097/MD.0000000000025714
98. Raposeiras Roubin S, Abu Assi E, Barreiro Pardal C, Cespon Fernandez M, Munoz Pousa I, Cobas Paz R, et al. New cancer diagnosis after bleeding in anticoagulated patients with atrial fibrillation. J Am Heart Assoc. (2020) 9:e016836. doi: 10.1161/JAHA.120.016836
99. Mercurio V, Cuomo A, Cadeddu Dessalvi C, Deidda M, Di Lisi D, Novo G, et al. Redox imbalances in ageing and metabolic alterations: implications in cancer and cardiac diseases. an overview from the working group of cardiotoxicity and cardioprotection of the Italian society of cardiology (SIC). Antioxidants. (2020) 9:641. doi: 10.3390/antiox9070641
100. Hoshino A, Mita Y, Okawa Y, Ariyoshi M, Iwai-Kanai E, Ueyama T, et al. Cytosolic p53 inhibits Parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat Commun. (2013) 4:2308. doi: 10.1038/ncomms3308
101. Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. (2004) 56:185–229. doi: 10.1124/pr.56.2.6
102. Minotti G, Salvatorelli E, Menna P. Pharmacological foundations of cardio-oncology. J Pharmacol Exp Ther. (2010) 334:2–8. doi: 10.1124/jpet.110.165860
103. Al-Mahayri ZN, AlAhmad MM, Ali BR. Current opinion on the pharmacogenomics of paclitaxel-induced toxicity. Expert Opin Drug Metab Toxicol. (2021) 17:785–801. doi: 10.1080/17425255.2021.1943358
104. Rowinsky EK, Eisenhauer EA, Chaudhry V, Arbuck SG, Donehower RC. Clinical toxicities encountered with paclitaxel (Taxol). Semin Oncol. (1993) 20:1–15.
105. Michel L, Helfrich I, Hendgen-Cotta UB, Mincu RI, Korste S, Mrotzek SM, et al. Targeting early stages of cardiotoxicity from anti-PD1 immune checkpoint inhibitor therapy. Eur Heart J. (2021) 43:316–29. doi: 10.1093/eurheartj/ehab430
106. Tocchetti CG, Cadeddu C, Di Lisi D, Femmino S, Madonna R, Mele D, et al. From molecular mechanisms to clinical management of antineoplastic drug-induced cardiovascular toxicity: a translational overview. Antioxid Redox Signal. (2019) 30:2110–53. doi: 10.1089/ars.2016.6930
107. Podkowinska A, Formanowicz D. Chronic kidney disease as oxidative stress- and inflammatory-mediated cardiovascular disease. Antioxidants. (2020) 9:752. doi: 10.3390/antiox9080752
108. Yang XB, Hou FF, Wu Q, Zhou H, Liu ZR. [Increased levels of advanced oxidation protein products are associated with atherosclerosis in chronic kidney disease]. Zhonghua Nei Ke Za Zhi. (2005) 44:342–6. Available online at: http://rs.yiigle.com/CN112138200505/394459.htm
109. Vaziri ND, Dicus M, Ho ND, Boroujerdi-Rad L, Sindhu RK. Oxidative stress and dysregulation of superoxide dismutase and NADPH oxidase in renal insufficiency. Kidney Int. (2003) 63:179–85. doi: 10.1046/j.1523-1755.2003.00702.x
110. Varricchi G, Ameri P, Cadeddu C, Ghigo A, Madonna R, Marone G, et al. Antineoplastic drug-induced cardiotoxicity: a redox perspective. Front Physiol. (2018) 9:167. doi: 10.3389/fphys.2018.00167
111. Pereira GC, Pereira SP, Tavares LC, Carvalho FS, Magalhaes-Novais S, Barbosa IA, et al. Cardiac cytochrome c and cardiolipin depletion during anthracycline-induced chronic depression of mitochondrial function. Mitochondrion. (2016) 30:95–104. doi: 10.1016/j.mito.2016.07.005
112. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. (2012) 366:2443–54. doi: 10.1056/NEJMoa1200690
113. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. (2015) 27:450–61. doi: 10.1016/j.ccell.2015.03.001
114. Heinzerling L, Ott PA, Hodi FS, Husain AN, Tajmir-Riahi A, Tawbi H, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer. (2016) 4:50. doi: 10.1186/s40425-016-0152-y
115. Zarifa A, Lopez-Mattei J, Palaskas N, Iliescu C, Durand JB, Kim PY. Immune checkpoint inhibitors (ICIs)-related cardiotoxicity. Adv Exp Med Biol. (2020) 1244:277–85. doi: 10.1007/978-3-030-41008-7_15
116. Zaborowska-Szmit M, Krzakowski M, Kowalski DM, Szmit S. Cardiovascular complications of systemic therapy in non-small-cell lung cancer. J Clin Med. (2020) 9:1268. doi: 10.3390/jcm9051268
117. Chang HM, Moudgil R, Scarabelli T, Okwuosa TM, Yeh ETH. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 1. J Am Coll Cardiol. (2017) 70:2536–51. doi: 10.1016/j.jacc.2017.09.1096
118. Vasbinder A, Cheng R, Ray R, Langford D, Barac A, Anderson G, et al. Abstract 13885: phenotyping risk profiles for heart failure with preserved and reduced ejection fraction among breast cancer survivors. Circulation. (2020) 142:A13885. doi: 10.1161/circ.142.suppl_3.13885
119. Bhatia S. Genetics of anthracycline cardiomyopathy in cancer survivors: JACC: cardiooncology state-of-the-art review. JACC CardioOncol. (2020) 2:539–52. doi: 10.1016/j.jaccao.2020.09.006
120. Unitt C, Montazeri K, Tolaney S, Moslehi J. Cardiology patient page. breast cancer chemotherapy and your heart. Circulation. (2014) 129:e680–682. doi: 10.1161/CIRCULATIONAHA.113.007181
121. Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. (2002) 20:1215–21. doi: 10.1200/JCO.2002.20.5.1215
122. Cadeddu Dessalvi C, Pepe A, Penna C, Gimelli A, Madonna R, Mele D, et al. Sex differences in anthracycline-induced cardiotoxicity: the benefits of estrogens. Heart Fail Rev. (2019) 24:915–25. doi: 10.1007/s10741-019-09820-2
123. Menna P, Salvatorelli E, Minotti G. Anthracycline degradation in cardiomyocytes: a journey to oxidative survival. Chem Res Toxicol. (2010) 23:6–10. doi: 10.1021/tx9003424
124. Krischer JP, Epstein S, Cuthbertson DD, Goorin AM, Epstein ML, Lipshultz SE. Clinical cardiotoxicity following anthracycline treatment for childhood cancer: the pediatric oncology group experience. J Clin Oncol. (1997) 15:1544–52. doi: 10.1200/JCO.1997.15.4.1544
125. Lipshultz SE, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, et al. Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. (1995) 332:1738–43. doi: 10.1056/NEJM199506293322602
126. Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. (1999) 340:1801–11. doi: 10.1056/NEJM199906103402306
127. Ibrahim NK, Hortobagyi GN, Ewer M, Ali MK, Asmar L, Theriault RL, et al. Doxorubicin-induced congestive heart failure in elderly patients with metastatic breast cancer, with long-term follow-up: the MD anderson experience. Cancer Chemother Pharmacol. (1999) 43:471–8. doi: 10.1007/s002800050926
128. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. (2003) 97:2869–79. doi: 10.1002/cncr.11407
129. Kim YA, Cho H, Lee N, Jung SY, Sim SH, Park IH, et al. Doxorubicin-induced heart failure in cancer patients: a cohort study based on the Korean national health insurance database. Cancer Med. (2018) 7:6084–92. doi: 10.1002/cam4.1886
130. Silber JH, Jakacki RI, Larsen RL, Goldwein JW, Barber G. Increased risk of cardiac dysfunction after anthracyclines in girls. Med Pediatr Oncol. (1993) 21:477–9. doi: 10.1002/mpo.2950210704
131. Clements IP, Davis BJ, Wiseman GA. Systolic and diastolic cardiac dysfunction early after the initiation of doxorubicin therapy: significance of gender and concurrent mediastinal radiation. Nucl Med Commun. (2002) 23:521–7. doi: 10.1097/00006231-200206000-00003
132. Wang L, Tan TC, Halpern EF, Neilan TG, Francis SA, Picard MH, et al. Major cardiac events and the value of echocardiographic evaluation in patients receiving anthracycline-based chemotherapy. Am J Cardiol. (2015) 116:442–6. doi: 10.1016/j.amjcard.2015.04.064
133. Lee DH, Hawk F, Seok K, Gliksman M, Emole J, Rhea IB, et al. Association between ibrutinib treatment and hypertension. Heart. (2022) 108:445–50. doi: 10.1136/heartjnl-2021-319110
134. Cortes JE, Jean Khoury H, Kantarjian H, Brummendorf TH, Mauro MJ, Matczak E, et al. Long-term evaluation of cardiac and vascular toxicity in patients with Philadelphia chromosome-positive leukemias treated with bosutinib. Am J Hematol. (2016) 91:606–16. doi: 10.1002/ajh.24360
135. van der Veldt AA, Boven E, Helgason HH, van Wouwe M, Berkhof J, de Gast G, et al. Predictive factors for severe toxicity of sunitinib in unselected patients with advanced renal cell cancer. Br J Cancer. (2008) 99:259–65. doi: 10.1038/sj.bjc.6604456
136. Wang S, Cowley LA, Liu XS. Sex differences in cancer immunotherapy efficacy, biomarkers, and therapeutic strategy. Molecules. (2019) 24:3214. doi: 10.3390/molecules24183214
137. Schmetzer O, Florcken A. Sex differences in the drug therapy for oncologic diseases. Handb Exp Pharmacol. (2012) 214:411–42. doi: 10.1007/978-3-642-30726-3_19
138. Von Hoff DD, Layard MW, Basa P, Davis HL, Von Hoff AL, Rozencweig M, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. (1979) 91:710–7. doi: 10.7326/0003-4819-91-5-710
139. D'Amario D, Camilli M, Migliaro S, Canonico F, Galli M, Arcudi A, et al. Sex-related differences in dilated cardiomyopathy with a focus on cardiac dysfunction in oncology. Curr Cardiol Rep. (2020) 22:102. doi: 10.1007/s11886-020-01377-z
140. Lipshultz SE, Landy DC, Lopez-Mitnik G, Lipsitz SR, Hinkle AS, Constine LS, et al. Cardiovascular status of childhood cancer survivors exposed and unexposed to cardiotoxic therapy. J Clin Oncol. (2012) 30:1050–7. doi: 10.1200/JCO.2010.33.7907
141. Armstrong GT, Kawashima T, Leisenring W, Stratton K, Stovall M, Hudson MM, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. (2014) 32:1218–27. doi: 10.1200/JCO.2013.51.1055
142. Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. (2017) 70:926–38. doi: 10.1016/j.jacc.2017.06.047
143. Herrmann J. Vascular toxic effects of cancer therapies. Nat Rev Cardiol. (2020) 17:503–22. doi: 10.1038/s41569-020-0347-2
144. Bharadwaj A, Potts J, Mohamed MO, Parwani P, Swamy P, Lopez-Mattei JC, et al. Acute myocardial infarction treatments and outcomes in 65 million patients with a current or historical diagnosis of cancer in the USA. Eur Heart J. (2020) 41:2183–93. doi: 10.1093/eurheartj/ehz851
145. Lu JI, Carhart RL, Graziano SL, Gajra A. Acute coronary syndrome secondary to fluorouracil infusion. J Clin Oncol. (2006) 24:2959–60. doi: 10.1200/JCO.2005.04.0766
146. Jensen SA, Hasbak P, Mortensen J, Sorensen JB. Fluorouracil induces myocardial ischemia with increases of plasma brain natriuretic peptide and lactic acid but without dysfunction of left ventricle. J Clin Oncol. (2010) 28:5280–6. doi: 10.1200/JCO.2009.27.3953
147. Santoro C, Esposito R, Lembo M, Sorrentino R, De Santo I, Luciano F, et al. Strain-oriented strategy for guiding cardioprotection initiation of breast cancer patients experiencing cardiac dysfunction. Eur Heart J Cardiovasc Imaging. (2019) 20:1345–52. doi: 10.1093/ehjci/jez194
148. Haugnes HS, Wethal T, Aass N, Dahl O, Klepp O, Langberg CW, et al. Cardiovascular risk factors and morbidity in long-term survivors of testicular cancer: a 20-year follow-up study. J Clin Oncol. (2010) 28:4649–57. doi: 10.1200/JCO.2010.29.9362
149. Taylor C, McGale P, Bronnum D, Correa C, Cutter D, Duane FK, et al. Cardiac structure injury after radiotherapy for breast cancer: cross-sectional study with individual patient data. J Clin Oncol. (2018) 36:2288–96. doi: 10.1200/JCO.2017.77.6351
150. Atkins KM, Chaunzwa TL, Lamba N, Bitterman DS, Rawal B, Bredfeldt J, et al. Association of left anterior descending coronary artery radiation dose with major adverse cardiac events and mortality in patients with non-small cell lung cancer. JAMA Oncol. (2021) 7:206–19. doi: 10.1001/jamaoncol.2020.6332
151. Nepali PR, Gabriels K, Mathieu M, Russell J, te Poele JA, Kitz S, et al. Abstract 15364: radiation exposure of the base of the heart accelerates coronary atherosclerosis. Circulation. (2020) 142:A15364. doi: 10.1161/circ.142.suppl_3.15364
152. Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol. (2020) 17:474–502. doi: 10.1038/s41569-020-0348-1
153. Roboz GJ, Ritchie EK, Carlin RF, Samuel M, Gale L, Provenzano-Gober JL, et al. Prevalence, management, and clinical consequences of QT interval prolongation during treatment with arsenic trioxide. J Clin Oncol. (2014) 32:3723–8. doi: 10.1200/JCO.2013.51.2913
154. Cuni R, Parrini I, Asteggiano R, Conte MR. Targeted cancer therapies and QT interval prolongation: unveiling the mechanisms underlying arrhythmic complications and the need for risk stratification strategies. Clin Drug Investig. (2017) 37:121–34. doi: 10.1007/s40261-016-0460-5
155. Coppola C, Rienzo A, Piscopo G, Barbieri A, Arra C, Maurea N. Management of QT prolongation induced by anti-cancer drugs: target therapy and old agents. different algorithms for different drugs. Cancer Treat Rev. (2018) 63:135–43. doi: 10.1016/j.ctrv.2017.11.009
156. Arbuck SG, Strauss H, Rowinsky E, Christian M, Suffness M, Adams J, et al. A reassessment of cardiac toxicity associated with Taxol. J Natl Cancer Inst Monogr. (1993) 15:117–30.
157. Jain P, Zhao S, Lee HJ, Hill HA, Ok CY, Kanagal-Shamanna R, et al. Ibrutinib with rituximab in first-line treatment of older patients with mantle cell lymphoma. J Clin Oncol. (2021) 40:202–12. doi: 10.1200/JCO.21.01797
158. McCormack T, Harrisingh MC, Horner D, Bewley S, Guideline C. Venous thromboembolism in adults: summary of updated NICE guidance on diagnosis, management, and thrombophilia testing. BMJ. (2020) 369:m1565. doi: 10.1136/bmj.m1565
159. Khan F, Tritschler T, Kahn SR, Rodger MA. Venous thromboembolism. Lancet. (2021) 398:64–77. doi: 10.1016/S0140-6736(20)32658-1
160. Yuichiro O, Kusunose K, Ise T, Tobiume T, Yamaguchi K, Yagi S et al. Abstract 13145: differences of cancer types in hospital mortality in patients with venous thromboembolism. Circulation. (2020) 142:A13145. doi: 10.1161/circ.142.suppl_3.13145
161. Farge D, Frere C, Connors JM, Ay C, Khorana AA, Munoz A, et al. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. (2019) 20:e566–81. doi: 10.1016/S1470-2045(19)30750-8
162. Souza VB, Silva EN, Ribeiro ML, Martins Wde A. Hypertension in patients with cancer. Arq Bras Cardiol. (2015) 104:246–52. doi: 10.5935/abc.20150011
163. Felmeden DC, Spencer CG, Belgore FM, Blann AD, Beevers DG, Lip GY. Endothelial damage and angiogenesis in hypertensive patients: relationship to cardiovascular risk factors and risk factor management. Am J Hypertens. (2003) 16:11–20. doi: 10.1016/S0895-7061(02)03149-7
164. Aguilar-Cazares D, Chavez-Dominguez R, Carlos-Reyes A, Lopez-Camarillo C, Hernadez de la Cruz ON, Lopez-Gonzalez JS. Contribution of angiogenesis to inflammation and cancer. Front Oncol. (2019) 9:1399. doi: 10.3389/fonc.2019.01399
165. Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. (1971) 285:1182–6. doi: 10.1056/NEJM197111182852108
167. Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med. (2016) 375:1457–67. doi: 10.1056/NEJMra1100265
168. Li W, Croce K, Steensma DP, McDermott DF, Ben-Yehuda O, Moslehi J. Vascular and metabolic implications of novel targeted cancer therapies: focus on kinase inhibitors. J Am Coll Cardiol. (2015) 66:1160–78. doi: 10.1016/j.jacc.2015.07.025
169. Lankhorst S, Saleh L, Danser AJ, van den Meiracker AH. Etiology of angiogenesis inhibition-related hypertension. Curr Opin Pharmacol. (2015) 21:7–13. doi: 10.1016/j.coph.2014.11.010
170. Tanaka H, Takahashi K, Yamaguchi K, Kontani K, Motoki T, Asakura M, et al. Hypertension and proteinuria as predictive factors of effects of bevacizumab on advanced breast cancer in Japan. Biol Pharm Bull. (2018) 41:644–8. doi: 10.1248/bpb.b17-00605
171. Fillon M. Lung cancer radiation may increase the risk of major adverse cardiac events. CA Cancer J Clin. (2019) 69:435–7. doi: 10.3322/caac.21581
172. Saxena A, Rubens M, Ramamoorthy V, Das S, Bhatt CB, Salami JA, et al. Abstract P339: association between cardiometabolic syndrome and cancer: systematic review. Circulation. (2020) 141:AP339. doi: 10.1161/circ.141.suppl_1.P339
173. Li Y, Schoufour J, Wang DD, Dhana K, Pan A, Liu X, et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: prospective cohort study. BMJ. (2020) 368:l6669. doi: 10.1136/bmj.l6669
174. Keaney JF, Rosen CJ. VITAL signs for dietary supplementation to prevent cancer and heart disease. N Engl J Med. (2019) 380:91–3. doi: 10.1056/NEJMe1814933
175. Branch M, Schaich CL, Beavers D, Soliman EZ, Reding K, Shadyab A, et al. Abstract 13500: Association between breast cancer and cardiac autonomic function as measured by heart rate variability in the women's health initiative. Circulation. (2020) 142:A13500. doi: 10.1161/circ.142.suppl_3.13500
176. Tini G, Sarocchi M, Tocci G, Arboscello E, Ghigliotti G, Novo G, et al. Arterial hypertension in cancer: the elephant in the room. Int J Cardiol. (2019) 281:133–9. doi: 10.1016/j.ijcard.2019.01.082
177. Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popovic ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. (2013) 61:77–84. doi: 10.1016/j.jacc.2012.09.035
178. Lambert J, Lamacie M, Thampinathan B, Altaha MA, Esmaeilzadeh M, Nolan M, et al. Variability in echocardiography and MRI for detection of cancer therapy cardiotoxicity. Heart. (2020) 106:817–23. doi: 10.1136/heartjnl-2019-316297
179. Armstrong GT, Plana JC, Zhang N, Srivastava D, Green DM, Ness KK, et al. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. (2012) 30:2876–84. doi: 10.1200/JCO.2011.40.3584
180. Simes J, Robledo KP, White HD, Espinoza D, Stewart RA, Sullivan DR, et al. D-Dimer predicts long-term cause-specific mortality, cardiovascular events, and cancer in patients with stable coronary heart disease: LIPID study. Circulation. (2018) 138:712–23. doi: 10.1161/CIRCULATIONAHA.117.029901
181. Oikawa M, Yaegashi D, Yokokawa T, Misaka T, Sato T, Kobayashi A, et al. Abstract 13441: D-dimmer is a predictor of cancer therapeutics-related cardiac dysfunction in patients treated with cardiotoxic chemotherapy. Circulation. (2020) 142:A13441. doi: 10.1161/circ.142.suppl_3.13441
182. Goetze JP, Bruneau BG, Ramos HR, Ogawa T, de Bold MK, de Bold AJ. Cardiac natriuretic peptides. Nat Rev Cardiol. (2020) 17:698–717. doi: 10.1038/s41569-020-0381-0
183. Renner E, Barnes GD. Antithrombotic management of venous thromboembolism: JACC focus seminar. J Am Coll Cardiol. (2020) 76:2142–54. doi: 10.1016/j.jacc.2020.07.070
184. Semeraro GC, Cipolla CM, Cardinale DM. Role of cardiac biomarkers in cancer patients. Cancers. (2021) 13:5426. doi: 10.3390/cancers13215426
185. Curigliano G, Cardinale D, Dent S, Criscitiello C, Aseyev O, Lenihan D, et al. Cardiotoxicity of anticancer treatments: epidemiology, detection, and management. CA Cancer J Clin. (2016) 66:309–25. doi: 10.3322/caac.21341
186. Bertero E, Canepa M, Maack C, Ameri P. Linking heart failure to cancer: background evidence and research perspectives. Circulation. (2018) 138:735–42. doi: 10.1161/CIRCULATIONAHA.118.033603
187. Touyz RM, Herrmann SMS, Herrmann J. Vascular toxicities with VEGF inhibitor therapies-focus on hypertension and arterial thrombotic events. J Am Soc Hypertens. (2018) 12:409–25. doi: 10.1016/j.jash.2018.03.008
188. Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AYY, Arcelus JI, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. (2020) 38:496–520. doi: 10.1200/JCO.19.01461
189. Williams B, Mancia G, Spiering W, Rosei EA, Azizi M, Burnier M, et al. [2018 ESC/ESH guidelines for the management of arterial hypertension]. Kardiol Pol. (2019) 77:71–159. doi: 10.5603/KP.2019.0018
190. Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines: the task force for cancer treatments and cardiovascular toxicity of the European society of cardiology (ESC). Eur Heart J. (2016) 37:2768–801. doi: 10.1093/eurheartj/ehw211
191. Mulder FI, Di Nisio M, Ay C, Carrier M, Bosch FTM, Segers A, et al. Clinical implications of incidental venous thromboembolism in cancer patients. Eur Respir J. (2020) 55:1901697. doi: 10.1183/13993003.01697-2019
192. Khorana AA, Soff GA, Kakkar AK, Vadhan-Raj S, Riess H, Wun T, et al. Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med. (2019) 380:720–8. doi: 10.1056/NEJMoa1814630
Keywords: cardiology, oncology, cardiovascular disease, cancer, cardiotoxicity
Citation: Wang Y, Wang Y, Han X, Sun J, Li C, Adhikari BK, Zhang J, Miao X and Chen Z (2022) Cardio-Oncology: A Myriad of Relationships Between Cardiovascular Disease and Cancer. Front. Cardiovasc. Med. 9:727487. doi: 10.3389/fcvm.2022.727487
Received: 18 June 2021; Accepted: 21 February 2022;
Published: 17 March 2022.
Edited by:
Nicola Maurea, G. Pascale National Cancer Institute Foundation (IRCCS), ItalyCopyright © 2022 Wang, Wang, Han, Sun, Li, Adhikari, Zhang, Miao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Miao, miaoxiao@jlu.edu.cn; Zhaoyang Chen, chenzhaoyang888@126.com
 Yinghui Wang
Yinghui Wang Yonggang Wang
Yonggang Wang Xiaorong Han1
Xiaorong Han1  Jian Sun
Jian Sun Binay Kumar Adhikari
Binay Kumar Adhikari Zhaoyang Chen
Zhaoyang Chen