Pulmonary Embolism and Pregnancy—Challenges in Diagnostic and Therapeutic Decisions in High-Risk Patients
- 1Department of Cardiology, University Medical Center of the Johannes Gutenberg-University, Mainz, Germany
- 2Center for Thrombosis and Hemostasis (CTH), University Medical Center of the Johannes Gutenberg University, Mainz, Germany
- 3German Center for Cardiovascular Research (DZHK), Partner Site Rhine Main, Mainz, Germany
- 4Department of Cardiology, Democritus University of Thrace, Komotini, Greece
- 5Medical Clinic VII, University Hospital Heidelberg, Heidelberg, Germany
Diagnosis of acute PE in pregnant women with haemodynamic instability is following the general integrated risk-adapted diagnostic algorithm and starts with bedside echocardiography to assess RV function. If RV dysfunction is identified, a prompt and immediate reperfusion without further imaging should be initiated. Although pregnancy is listed as a relative contraindication of systemic thrombolysis, in pregnant women with acute PE and haemodynamic instability thrombolysis must be considered. In those cases, other treatment strategies as surgical embolectomy or catheter-directed low-dose thromboylysis or percutaneous thrombectomy should be taken into consideration as well. A multidisciplinary team with experience of PE management in pregnancy should be consulted to reach consensus on the best treatment approach.
Introduction
Venous thromboembolism (VTE) is considered globally as the third most frequent acute cardiovascular syndrome and is an umbrella term for the clinical entities of acute pulmonary embolism (PE) and deep vein thrombosis (DVT) (1). For PE, annual incidence rates range from 39 to 115 per 100,000 population; for DVT, annual incidence rates between 53 and 162 per 100,000 population were reported (2, 3).
Although an overall decreasing trend in PE-related mortality over the past two decades was observed in a recent analysis of vital registration data in Europe, more than 1% of all deaths in women aged 15–50 years are caused by PE (3, 4). VTE occurs and complicates one of 500–3,000 pregnancies and acute PE is still one of the leading causes of maternal death, also in high-income countries with highly developed medical health services (5, 6). Data from the UK and Ireland demonstrated that thrombosis and thromboembolism were the most common causes of direct maternal death in the years 2013–2015 resulting in 1.13 deaths per 100,000 maternities (7). Additionally, based on current epidemiological data from Germany, PE-related deaths in hospitalized women accounted for almost 14% of all maternal deaths (8).
The management of acute PE during pregnancy is challenging since:
• symptoms of PE (particularly dyspnoea) as well as DVT (especially leg swelling) in pregnant women can in part be difficult to distinguish from “physiological” symptoms of pregnancy,
• lower threshold of PE suspicion,
• fewer publications on validation of PE diagnostic algorithms,
• potential concerns regarding the harm of radiations or iodine contrast exposure regarding PE diagnostics and
• lack of direct evidence from interventional trials regarding PE reperfusion treatment, notably systemic thrombolysis, surgical embolectomy or catheter-directed treatment options (9–11).
Initial risk stratification is based on assessment of the patient's vital/haemodynamic parameters. In haemodynamically stable patients, significant progress has been made in the validation of clinical and biochemical criteria, which are generally considered to apply to pregnant patients as well (7). In contrast, haemodynamic instability in acute PE indicates a high risk of early death and, therefore, rapid reperfusion treatment is recommended, which can however be challenging due to a high risk of bleeding complications in pregnant women.
Aim of this review is to provide a framework for the management of pregnancy- associated PE, especially focusing on critically ill patients.
Diagnostic Strategies in All patients Vs. Pregnant Women With Suspected PE in the 2019 ESC Guidelines
The diagnostic management of PE in pregnancy is particularly challenging due to the fact that pregnant women often have clinical symptoms, such as shortness of breath or tachycardia, which could point to the suspicion of PE, but can also be present as physiological changes during pregnancy (12). Moreover, overlooking and missing a PE diagnosis could have fatal consequences for mother and child (8), while, on the other hand, thoughtless use of imaging tests could lead to harmful radiation to both mother and fetus (13).
All patients with suspected PE and signs of haemodynamic compromise have a high-risk of death during the first hours and days (14). Thus, initiation of heparin anticoagulation is recommended without delay in patients with high or intermediate clinical probability of PE, while diagnostic workup is in progress (7). The recent published European Society of Cardiology (ESC) guidelines for the diagnosis and management of acute PE underline the importance of a bedside transthoracic echocardiography (TTE) examination in patients with haemodynamic instability. Acute right ventricular (RV) dysfunction can rapidly be detected by TTE if acute PE is the cause of patient's haemodynamic deterioration. If no signs of RV dysfunction exist, other causes of haemodynamic deterioration such as cardiac tamponade, acute coronary syndrome, aortic dissection, acute valvular dysfunction and/or hypovolaemia could be assessed by TTE as well. Additionally, bedside compression ultrasound (CUS) can be used as a further radiation-free diagnostic approach to detect or exclude proximal DVT. If PE is (in)directly confirmed, in all PE patients with haemodynamic instability a rescue thrombolytic treatment is recommended, if no absolute contraindications for systemic thrombolysis are present (7). If these do exist, alternative treatment strategies such as (percutaneous) thrombectomy should be considered. However, there are occasions as haemodynamic collapse with concomitant cardiac arrest and the necessity of cardio-pulmonary resuscitation (CPR) given very limited treatment options. Even if pregnancy is listed as a relative contraindication for systemic thrombolysis, guidelines recommend to consider thrombolysis or surgical embolectomy as the first reperfusion option in these patient group (7, 15). Recent data demonstrated that one third of haemodynamically unstable pregnant women with PE received systemic thrombolytic treatment (8).
In contrast to pregnant women with haemodynamic instability, the diagnostic algorithm for normotensive pregnant women may occasionally vary from that used for patients without pregnancy. A pre-test clinical probability assessment along with high-sensitivity D-dimer testing as well as bilateral lower limb CUS are in the center of the diagnostic algorithm for normotensive pregnant women with suspected PE. If there is a high or intermediate pre-test probability, empirical heparin anticoagulation should be administered before diagnostic imaging is initiated (Figure 1). If there are signs/symptoms of DVT, CUS should be performed. If CUS identifies DVT, the diagnosis of PE is—per definition—confirmed indirectly. If no proximal DVT is present or the CUS is inconclusive, chest X-ray followed (in the absence of parenchymal pulmonary changes) by ventilation/perfusion scintigraphy (V/Q scan), or computed tomography pulmonary angiography (CTPA), should be considered to rule out suspected PE (Figure 1).
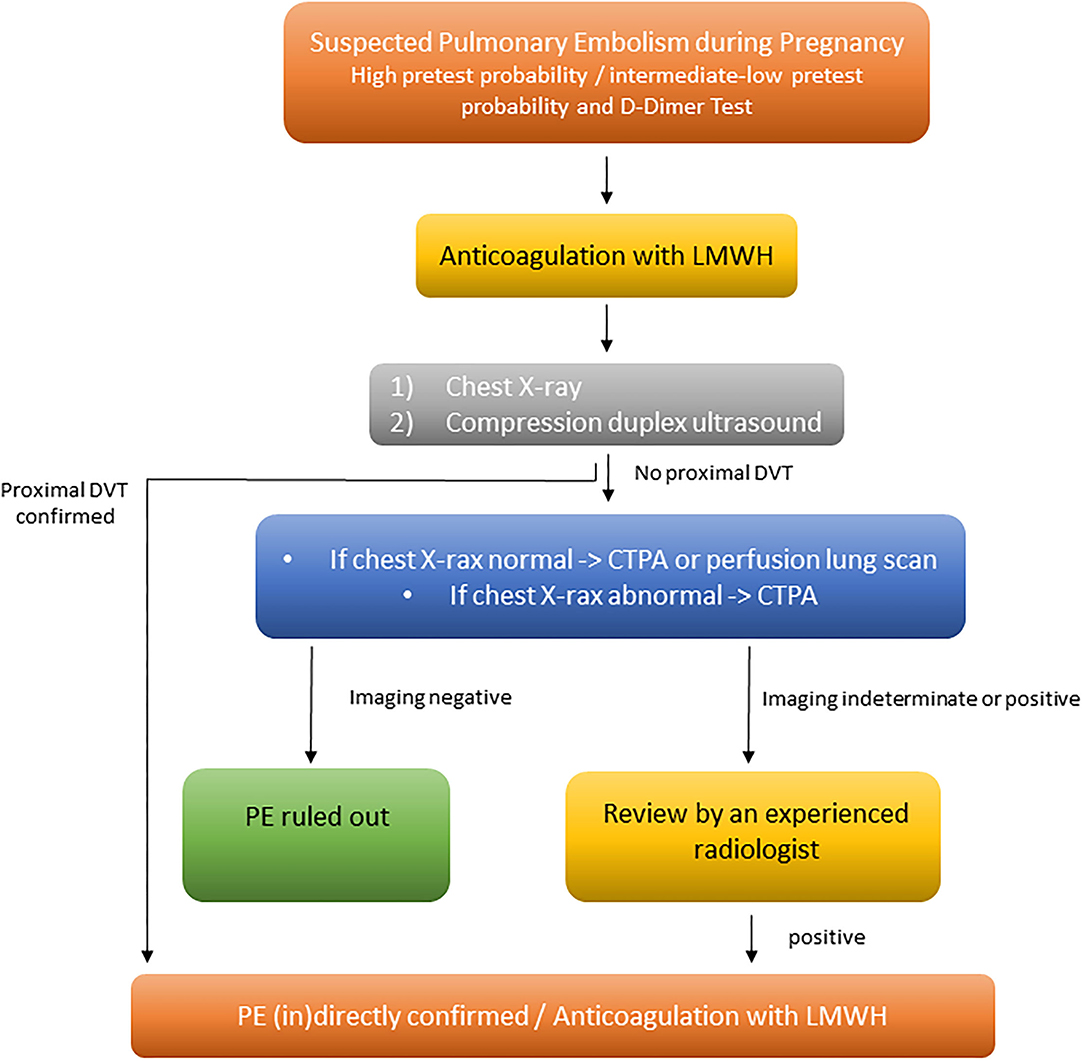
Figure 1. Diagnosis and management of women with suspected acute PE, modified from Konstantinides et al. (7). CTPA, computed tomography pulmonary angiography; DVT, deep vein thrombosis; LMWH, low-molecular-weight heparin; PE, pulmonary embolism.
The overall prevalence of confirmed PE among women is low (2 to 7%) and underlines the diagnostic challenges (16–18). Because of this, and due to the weak level of evidence, current guidelines vary in their approach to diagnosing PE in pregnancy (19). However, recently, two prospective studies have investigated a diagnostic algorithm in women with suspected PE during pregnancy (9, 10). A multicentre prospective diagnostic management study validated the combination of pre-test clinical probability assessment based on the Geneva score, high-sensitivity D-dimer testing, CUS and CTPA in a diagnostic strategy for pregnant women with suspected PE (10). With a low or intermediate pre-test clinical probability and a negative D-dimer result, PE was excluded. All other patients underwent lower limb CUS and, if results were negative, CTPA was performed. In total, 395 women were included and among these, PE was diagnosed in 28 (7.1%) and excluded in 367 (92.9%). The rate of symptomatic venous thromboembolic events was 0.0% (95% CI, 0.0 to 1.0%) among untreated pregnant women after exclusion of PE on the basis of negative results on the diagnostic work-up. Therefore, this diagnostic algorithm involving sequential assessment of pre-test clinical probability based on the Geneva score, D-dimer measurement, lower limb CUS and CTPA or V/Q scan is able to safely rule out PE in pregnancy (10). Another prospective study involving pregnant women with suspected PE assessed three criteria from the so-called YEARS algorithm (clinical signs of DVT, haemoptysis, and PE as the most likely diagnosis), also taking the D-dimer levels into account. A total of 498 women were included in this study and of these, PE was diagnosed in 20 (4.0%) of the examined patients and excluded in 478 (96%) women.
The current ESC guidelines recommend to perform an X-ray in pregnant women with suspected PE. If the X-ray is normal, V/Q scan should be performed, due to the fact, that V/Q scan is associated with low fetal and maternal radiation exposure. If the X-ray is abnormal, showing, for example, pulmonary infiltrates, then CTPA should be performed directly (7, 17) (Figure 1).
Diagnostic Strategies Across Guidelines and Societies in Pregnant Women With Suspected High-Risk PE
International medical society guidelines address new evidence of diagnostic strategies in pregnant women with suspected PE (7, 20–25). In line, to the aforementioned 2019 ESC guidelines, the American Thoracic and Radiology Society (ATS-STR), Society of Thrombosis and Haemostasis (GTH) and Royal College of Obstetricians and Gynaecologists (RCOG) guidelines begin with administering empirical therapeutic anticoagulation, if haemodynamic instability is present, even before any diagnostic work-up is started. The RCOG (24) and ESC (7) guidelines recommend early treatment for all patients suspected of PE with high- or intermediate clinical probability, while diagnostic workup is in progress. GTH (23) and ATS-STR (21) guidelines recommend empirical treatment in patients with a high clinical probability of having PE only (26). The remaining guidelines of Australasian Society of Thrombosis and Haemostasis and the Society of Obstetric Medicine of Australia and New Zealand (ASTH-SOMANZ), European Association of Nuclear Medicine (EANM), and Society of Obstetricians and Gynaecologists of Canada (SOGC) do not mention any empirical treatment (20, 22, 25). The ESC guidelines, as the only one, recommend the use of echocardiography as a first risk assessment strategy in all patients with haemodynamic instability (7).
Treatment of Acute Pulmonary Embolism in Pregnant Women—High-Risk vs. Not High-Risk
Especially high-risk PE in pregnancy can be a devastating event with a high case-fatality rate up to 37% (8). In patients with haemodynamic instability, unfractionated heparin (UFH) is used as a first-line medication. If the haemodynamic status aggravates, thrombolytic agents may be necessary to administer. Immediate thrombolytic treatment is recommended unless absolute contraindications for systemic thrombolysis are present (7). Besides thrombolysis, other treatment options of high-risk PE as surgical or percutaneous thrombectomy in should be taken into account. If necessary also extra-corporeal membrane oxygenation (ECMO) can be considered for depressurize the right ventricle and pulmonary circulation (27). Although pregnancy is reported as a relative contraindication of thrombolysis, haemodynamic collapse with concomitant cardiac arrest and the necessity of CPR leave the clinician with limited alternative treatment options (7). Recent data demonstrated that one third of unstable women with PE receive systemic thrombolytic treatment (8). Thrombolysis might be associated with a favorable outcome (94 and 88% of maternal and fetal survival, respectively) (27). However, other data of retrospective nature provide a more ominous prognostic depiction of thrombolysis in the context of high-risk PE. A mortality rate of 42.6% were reported among 67 pregnant women who received thrombolysis (8). Furthermore, in the same study, thrombolysis was sparsely used and regarded as a last resort option; even in the presence of haemodynamic collapse, only 37.8% of patients received thrombolysis.
Bleeding complications are reported as a common adverse event after thrombolytic treatment in 18 to 58% cases during pregnancy and in the post-partum period, respectively (27). Maternal major bleeding was reported in 3 out of 10 cases. Most of them were vaginal or abdominal C-section associated occurring in the early post-partum period. Especially the peripartum phase as well as spinal or epidural anesthesia are associated with high risk of bleeding (7). Therefore, thrombolytic therapy should be used peripartum in a life-threatening context only. The risk for the fetus is low, because a transplacental crossing of fibrinolytic drugs is very unlikely due to the fact that their components are larger than 1,000 Dalton (28, 29). However, the lack of prospectively designed controlled studies precludes conclusions regarding the efficacy and safety profile of thrombolysis in high-risk pregnancy-associated PE. Thus, causalities of fatal maternal and fetal outcomes cannot be deduced to the administration of the thrombolytic agent only.
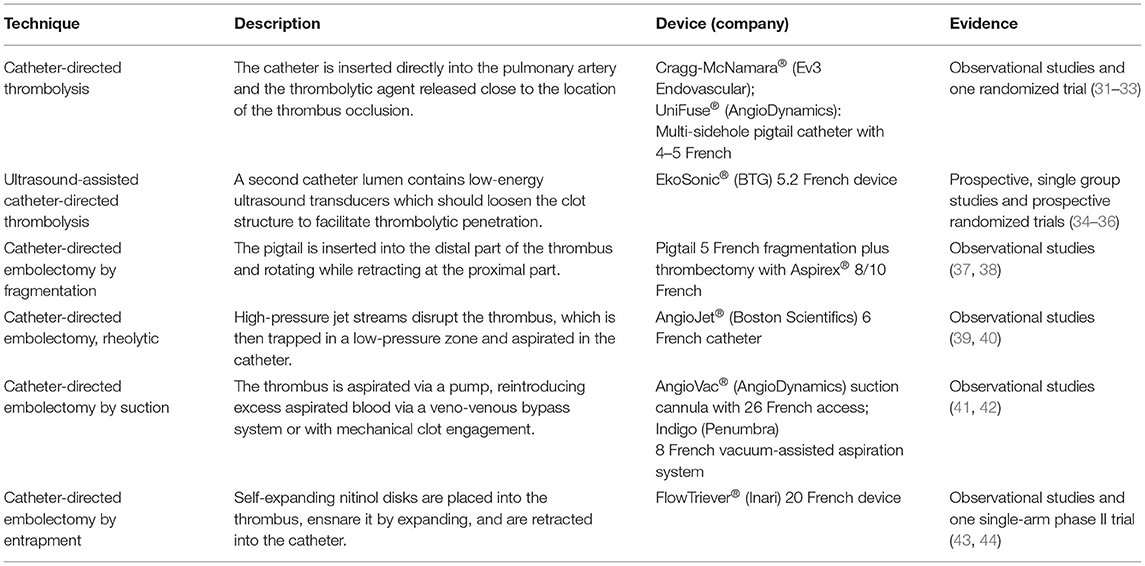
In the case of absolute contraindications, alternative treatment strategies such as surgical embolectomy or percutaneous low-dose thrombolysis (CDT) or thrombectomy should be considered (30) (Table 1). Results of several studies confirm that CDT, a novel treatment modality for high- and intermediate high-risk PE, is associated with a favorable outcome regarding bleeding complications in comparison to systemic thrombolysis in patients with PE (31). However, randomized studies using standardized clinical outcomes such as mortality and recurrent VTE are missing. In order to close this gap, CDT is currently being evaluated in a phase III clinical trial (NCT04790370). However, pregnancy constitutes an exclusion criterion of the trial and only few cases of pregnant women treated with CDT have been published in literature yet (27, 45, 46). Surgical embolectomy or percutaneous thrombectomy are reasonable treatment options, when needed in the immediate postpartum period, to avoid the bleeding risks of thrombolysis. However, these methods are limited in their availability and are used as last life-saving therapy option only (27). However, if reperfusion treatment is not effective or not available in the setting of haemodynamic instability, data indicate that the temporary use of mechanical circulatory support via ECMO as a bridging therapy might improve outcomes until pharmacological or mechanical thrombolysis or embolectomy is applied (47). In patients with acute PE and pregnancy ECMO has not been widely used. In a systematic review of 21 pregnant women with PE and ECMO support, the maternal survival rate was 76%, while the fetal survival rate was 63% (48).
An additional treatment option for pregnant women with absolute contraindications for anticoagulation could be the placement of an inferior vena cava (IVC) filter (7). Data on this preventive approach is limited. A systematic review including 124 pregnant women with DVT, in whom an IVC filter was inserted, were analyzed. No fatal PE occurred after filter placement and retrieval complication rates appeared comparable to those in the general population (49). However, even if the authors concluded that IVC filters can be used effectively in pregnancy to prevent PE, there is currently not enough evidence to suggest that IVC filters should be used routinely (50–52). In exceptional cases with absolute contraindications for anticoagulation, or if recurrent PE is present despite adequate therapeutic anticoagulation, IVC should be taken into consideration (7). Overall, the evidence for advanced treatment options in high-risk PE during pregnancy is poor. A prospective international registry investigating the effectiveness and safety of advanced methods in massive pregnancy-related PE is currently underway (MAPP registry endorsed by the International Society on Thrombosis and Haemostasis) (53). Due to the diagnostic and treatment complexity, a multidisciplinary team (with experience in PE management in pregnancy) should be consulted to evaluate the best and treatment approach (7).
Anticoagulation remains the mainstay of treatment in pregnancy and must be administered to all patients with high-risk suspicion of PE and confirmed PE (7). Since heparins do not pass the placenta and are not associated with teratogen effects on the fetus, they can be safely administered in pregnant women. Low molecular weight heparins (LMWH) are the agents of choice, because they have a predictable pharmacodynamic profile (54). In contrast, vitamin K antagonists (VKAs) can cause teratogenicity and fetal bleeding during the first and the third trimester and should therefore not used during those periods (55). Due to the insufficient safety data, direct oral anticoagulants (DOACs) are also contraindicated during pregnancy (56, 57). UFH may be associated with heparin-induced thrombocytopenia, resulting in restriction of recommendation regarding their use. However, in pregnant women heparin-induced thrombocytopenia is extremely rare (<0.1%) (58). UFH is used predominantly for patients with severe renal impairment, extreme body weight, high-risk PE, and PE occurring very close to delivery (59). Dosing strategies of LMWH generally follow these of the non-pregnant population, as there is a lack of specific randomized data (60). Although evidence suggest that most anticoagulated patients lie in a sub-therapeutic range, anti-Xa level monitoring has not be shown to be beneficial. LMWH use is currently recommend only for patients with severe renal impairment and extremes of body weight (61–63). However, therapeutic use of LMWH or UFH has a 3 and 2% incidence risk for antepartum and postpartum hemorrhagic complications, respectively (64). Approaching delivery, LMWH is usually converted to a continuous UFH infusion ≥36 h prior to delivery, especially if neuraxial anesthesia is planned. Finally, UFH should be paused 4–6 h prior to delivery. The timeframe of the post-partum re-initiation of LMWH should be decided by a multidisciplinary team and depends on the mode of delivery as well as the thrombotic and bleeding risk profile of the patient. Importantly, re-initiation of LMWH should not start 4 h after the epidural catheter has been removed (7). If there is an allergy or adverse response to LMWH, Fondaparinux is given as an alternative drug, although solid data are lacking and minor transplacental passage has been demonstrated (65).
Conclusion
Diagnosis of acute PE in pregnant women with haemodynamic instability
• is following the general integrated risk-adapted diagnostic PE algorithm PE and
• starts with bedside echocardiography to assess RV function. If RV dysfunction is identified, a prompt and immediate reperfusion without further imaging should be initiated.
Although pregnancy is listed as a relative contraindication of systemic thrombolysis, in pregnant women with acute PE and haemodynamic instability
• systemic thrombolysis must be considered and
• other treatment strategies as surgical embolectomy or catheter-directed low-dose thromboylysis or percutaneous thrombectomy should be taken into consideration as well.
A multidisciplinary team with experience of PE management in pregnancy should be consulted to reach consensus on the best treatment approach.
Author Contributions
All authors: conception and design of the study, data collection, analysis of the data, interpretation of data, drafting of the manuscript and revising of the manuscript critically for important intellectual content, and final approval of the manuscript submitted.
Conflict of Interest
LH reports lecture/consultant fees from MSD and Janssen, outside the submitted work. SK reports institutional grants and personal lecture/consultant fees from Bayer AG, Daiichi-Sankyo, and Boston Scientific; institutional grants from Inari Medical; and personal lecture/consultant fees from Pfizer- Bristol-Myers Squibb and MSD, all outside the submitted work.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wendelboe AM, Raskob GE. Global burden of thrombosis: epidemiologic aspects. Circ Res. (2016) 118:1340–7. doi: 10.1161/CIRCRESAHA.115.306841
2. Keller K, Hobohm L, Ebner M, Kresoja KP, Munzel T, Konstantinides SV, et al. Trends in thrombolytic treatment and outcomes of acute pulmonary embolism in Germany. Eur Heart J. (2020) 41:522–9. doi: 10.1093/eurheartj/ehz236
3. Barco S, Mahmoudpour SH, Valerio L, Klok FA, Munzel T, Middeldorp S, et al. Trends in mortality related to pulmonary embolism in the European Region, 2000-15: analysis of vital registration data from the WHO Mortality Database. Lancet Respir Med. (2020) 8:277–87. doi: 10.1016/S2213-2600(19)30354-6
4. Hobohm L, Sebastian T, Valerio L, Mahmoudpour SH, Vatsakis G, Johner F, et al. [Trends in mortality related to pulmonary embolism in the DACH countries]. Med Klin Intensivmed Notfmed. (2021). doi: 10.1007/s00063-021-00854-9. [Epub ahead of print].
5. Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstetr Gynecol. (2010) 116:1302–9. doi: 10.1097/AOG.0b013e3181fdfb11
6. Heit JA, Kobbervig CE, James AH, Petterson TM, Bailey KR, Melton LJ III. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med. (2005) 143:697–706. doi: 10.7326/0003-4819-143-10-200511150-00006
7. Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. (2020) 41:543–603. doi: 10.1093/eurheartj/ehz405
8. Hobohm L, Keller K, Valerio L, Ni Ainle F, Klok FA, Munzel T, et al. Fatality rates and use of systemic thrombolysis in pregnant women with pulmonary embolism. ESC Heart Fail. (2020) 7:2365–72. doi: 10.1002/ehf2.12775
9. van der Pol LM, Klok FA, Huisman MV, Artemis Study I. Diagnosis of suspected pulmonary embolism in pregnancy. N Engl J Med. (2019) 380:e49. doi: 10.1056/NEJMc1905283
10. Righini M, Robert-Ebadi H, Elias A, Sanchez O, Le Moigne E, Schmidt J, et al. Diagnosis of pulmonary embolism during pregnancy: a multicenter prospective management outcome study. Ann Intern Med. (2018) 169:766–73. doi: 10.7326/M18-1670
11. Barco S, Nijkeuter M, Middeldorp S. Pregnancy and venous thromboembolism. Semin Thromb Hemost. (2013) 39:549–58. doi: 10.1055/s-0033-1343893
12. Fukuda W, Chiyoya M, Taniguchi S, Daitoku K, Fukuda I. Management of deep vein thrombosis and pulmonary embolism (venous thromboembolism) during pregnancy. Gen Thorac Cardiovasc Surg. (2016) 64:309–14. doi: 10.1007/s11748-016-0635-2
13. Perisinakis K, Seimenis I, Tzedakis A, Damilakis J. Perfusion scintigraphy versus 256-slice CT angiography in pregnant patients suspected of pulmonary embolism: comparison of radiation risks. J Nucl Med. (2014) 55:1273–80. doi: 10.2967/jnumed.114.137968
14. Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. (2002) 121:877–905. doi: 10.1378/chest.121.3.877
15. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. (2016) 149:315–52. doi: 10.1016/j.chest.2015.11.026
16. Sheen JJ, Haramati LB, Natenzon A, Ma H, Tropper P, Bader AS, et al. Performance of low-dose perfusion scintigraphy and CT pulmonary angiography for pulmonary embolism in pregnancy. Chest. (2018) 153:152–60. doi: 10.1016/j.chest.2017.08.005
17. van Mens TE, Scheres LJ, de Jong PG, Leeflang MM, Nijkeuter M, Middeldorp S. Imaging for the exclusion of pulmonary embolism in pregnancy. Cochrane Database Syst Rev. (2017) 1:CD011053. doi: 10.1002/14651858.CD011053.pub2
18. Hamilton EJ, Green AQ, Cook JA, Nash H. Investigating for pulmonary embolism in pregnancy: five year retrospective review of referrals to the acute medical unit of a large teaching hospital. Acute Med. (2016) 15:58–62. doi: 10.52964/AMJA.0607
19. Wan T, Skeith L, Karovitch A, Rodger M, Le Gal G. Guidance for the diagnosis of pulmonary embolism during pregnancy: consensus and controversies. Thromb Res. (2017) 157:23–8. doi: 10.1016/j.thromres.2017.06.025
20. McLintock C, Brighton T, Chunilal S, Dekker G, McDonnell N, McRae S, et al. Recommendations for the diagnosis and treatment of deep venous thrombosis and pulmonary embolism in pregnancy and the postpartum period. Aust N Z J Obstet Gynaecol. (2012) 52:14–22. doi: 10.1111/j.1479-828X.2011.01361.x
21. Leung AN, Bull TM, Jaeschke R, Lockwood CJ, Boiselle PM, Hurwitz LM, et al. American thoracic society documents: an official American Thoracic Society/Society of Thoracic Radiology Clinical Practice guideline–evaluation of suspected pulmonary embolism in pregnancy. Radiology. (2012) 262:635–46. doi: 10.1148/radiol.11114045
22. Bajc M, Neilly JB, Miniati M, Schuemichen C, Meignan M, Jonson B. EANM guidelines for ventilation/perfusion scintigraphy: part 2. Algorithms and clinical considerations for diagnosis of pulmonary emboli with V/P(SPECT) and MDCT. Eur J Nucl Med Mol Imaging. (2009) 36:1528–38. doi: 10.1007/s00259-009-1169-y
23. Linnemann B, Bauersachs R, Rott H, Halimeh S, Zotz R, Gerhardt A, et al. Diagnosis of pregnancy-associated venous thromboembolism - position paper of the Working Group in Women's Health of the Society of Thrombosis and Haemostasis (GTH). Vasa. (2016) 45:87–101. doi: 10.1024/0301-1526/a000503
24. Royal College of Obstetricians and Gynaecologists. Thromboembolic Disease in Pregnancy and the Puerperium: Acute Management. Green-top Guideline. No. 37b (2015).
25. Chan WS, Rey E, Kent NE, Group VTEiPGW, Chan WS, Kent NE, et al. Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can. (2014) 36:527–53. doi: 10.1016/S1701-2163(15)30569-7
26. Cohen SL, Feizullayeva C, McCandlish JA, Sanelli PC, McGinn T, Brenner B, et al. Comparison of international societal guidelines for the diagnosis of suspected pulmonary embolism during pregnancy. Lancet Haematol. (2020) 7:e247–58. doi: 10.1016/S2352-3026(19)30250-9
27. Martillotti G, Boehlen F, Robert-Ebadi H, Jastrow N, Righini M, Blondon M. Treatment options for severe pulmonary embolism during pregnancy and the postpartum period: a systematic review. J Thromb Haemost. (2017) 15:1942–50. doi: 10.1111/jth.13802
28. Heavner MS, Zhang M, Bast CE, Parker L, Eyler RF. Thrombolysis for massive pulmonary embolism in pregnancy. Pharmacotherapy. (2017) 37:1449–57. doi: 10.1002/phar.2025
29. Leonhardt G, Gaul C, Nietsch HH, Buerke M, Schleussner E. Thrombolytic therapy in pregnancy. J Thromb Thrombol. (2006) 21:271–6. doi: 10.1007/s11239-006-5709-z
30. Hobohm L, Keller K, Munzel T, Gori T, Konstantinides SV. EkoSonic(R) endovascular system and other catheter-directed treatment reperfusion strategies for acute pulmonary embolism: overview of efficacy and safety outcomes. Expert Rev Med Devices. (2020) 17:739–49. doi: 10.1080/17434440.2020.1796632
31. Hobohm L, Schmidt FP, Gori T, Schmidtmann I, Barco S, Munzel T, et al. In-hospital outcomes of catheter-directed thrombolysis in patients with pulmonary embolism. Eur Heart J Acute Cardiovasc Care. (2021) 10:258–64. doi: 10.1093/ehjacc/zuaa026
32. Kuo WT, Banerjee A, Kim PS, DeMarco FJ Jr., Levy JR, et al. Pulmonary Embolism Response to Fragmentation, Embolectomy, and Catheter Thrombolysis (PERFECT): initial results from a prospective multicenter registry. Chest. (2015) 148:667–73. doi: 10.1378/chest.15-0119
33. Kaymaz C, Akbal OY, Hakgor A, Tokgoz HC, Karagoz A, Tanboga IH, et al. A five-year, single-centre experience on ultrasound-assisted, catheter-directed thrombolysis in patients with pulmonary embolism at high risk and intermediate to high risk. EuroIntervention. (2018) 14:1136–43. doi: 10.4244/EIJ-D-18-00371
34. Kucher N, Boekstegers P, Muller OJ, Kupatt C, Beyer-Westendorf J, Heitzer T, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. (2014) 129:479–86. doi: 10.1161/CIRCULATIONAHA.113.005544
35. Tapson VF, Sterling K, Jones N, Elder M, Tripathy U, Brower J, et al. A randomized trial of the optimum duration of acoustic pulse thrombolysis procedure in acute intermediate-risk pulmonary embolism: the OPTALYSE PE trial. JACC Cardiovasc Interv. (2018) 11:1401–10. doi: 10.1016/j.jcin.2018.04.008
36. Ozcinar E, Cakici M, Dikmen Yaman N, Baran C, Aliyev A, Inan B, et al. Thrombus resolution and right ventricular functional recovery using ultrasound-accelerated thrombolysis in acute massive and submassive pulmonary embolism. Int Angiol. (2017) 36:428–37. doi: 10.23736/S0392-9590.17.03775-0
37. Bayiz H, Dumantepe M, Teymen B, Uyar I. Percutaneous aspiration thrombectomy in treatment of massive pulmonary embolism. Heart Lung Circ. (2015) 24:46–54. doi: 10.1016/j.hlc.2014.06.014
38. Lichtenberg M, Stahlhoff WF, Ozkapi A, de Graaf R, Breuckmann F. Safety, procedural success and outcome of the Aspirex((R))S endovascular thrombectomy system in the treatment of iliofemoral deep vein thrombosis - data from the Arnsberg Aspirex registry. Vasa. (2019) 48:341–6. doi: 10.1024/0301-1526/a000779
39. Bonvini RF, Roffi M, Bounameaux H, Noble S, Muller H, Keller PF, et al. AngioJet rheolytic thrombectomy in patients presenting with high-risk pulmonary embolism and cardiogenic shock: a feasibility pilot study. EuroIntervention. (2013) 8:1419–27. doi: 10.4244/EIJV8I12A215
40. Li K, Cui M, Zhang K, Liang K, Liu H, Zhai S. Treatment of acute pulmonary embolism using rheolytic thrombectomy. EuroIntervention. (2021) 17:e158–66. doi: 10.4244/EIJ-D-20-00259
41. Al-Hakim R, Park J, Bansal A, Genshaft S, Moriarty JM. Early experience with angiovac aspiration in the pulmonary arteries. J Vasc Interv Radiol. (2016) 27:730–4. doi: 10.1016/j.jvir.2016.01.012
42. D'Ayala M, Worku B, Gulkarov I, Sista A, Horowitz J, Salemi A. Factors associated with successful thrombus extraction with the AngioVac device: an institutional experience. Ann Vasc Surg. (2017) 38:242–7. doi: 10.1016/j.avsg.2016.04.015
43. Wible BC, Buckley JR, Cho KH, Bunte MC, Saucier NA, Borsa JJ. Safety and efficacy of acute pulmonary embolism treated via large-bore aspiration mechanical thrombectomy using the inari flowtriever device. J Vasc Interv Radiol. (2019) 30:1370–5. doi: 10.1016/j.jvir.2019.05.024
44. Tu T, Toma C, Tapson VF, Adams C, Jaber WA, Silver M, et al. A prospective, single-arm, multicenter trial of catheter-directed mechanical thrombectomy for intermediate-risk acute pulmonary embolism: the FLARE study. JACC Cardiovasc Interv. (2019) 12:859–69. doi: 10.1016/j.jcin.2018.12.022
45. Gowda N, Nwabuobi CK, Louis JM. Catheter-directed thrombolytic therapy in the management of massive pulmonary embolism in pregnancy. Obstet Gynecol. (2019) 134:1002–4. doi: 10.1097/AOG.0000000000003532
46. Compadre AJ, Kohi M, Lokken RP, Blissett S, Harris IS, Lucero J, et al. Catheter-directed thrombolysis for submassive pulmonary embolism in the third trimester of pregnancy. JACC Case Rep. (2020) 2:1899–904. doi: 10.1016/j.jaccas.2020.08.022
47. Hobohm L, Sagoschen I, Habertheuer A, Barco S, Valerio L, Wild J, et al. Clinical use and outcome of extracorporeal membrane oxygenation in patients with pulmonary embolism. Resuscitation. (2021) 170:285–92. doi: 10.1016/j.resuscitation.2021.10.007
48. Blondon M, Martinez de Tejada B, Glauser F, Righini M, Robert-Ebadi H. Management of high-risk pulmonary embolism in pregnancy. Thromb Res. (2021) 204:57–65. doi: 10.1016/j.thromres.2021.05.019
49. Harris SA, Velineni R, Davies AH. Inferior vena cava filters in pregnancy: a systematic review. J Vasc Interv Radiol. (2016) 27:354–60 e8. doi: 10.1016/j.jvir.2015.11.024
50. Decousus H, Leizorovicz A, Parent F, Page Y, Tardy B, Girard P, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prevention du Risque d'Embolie Pulmonaire par Interruption Cave Study Group. N Engl J Med. (1998) 338:409–15. doi: 10.1056/NEJM199802123380701
51. Bikdeli B, Chatterjee S, Desai NR, Kirtane AJ, Desai MM, Bracken MB, et al. Inferior vena cava filters to prevent pulmonary embolism: systematic review and meta-analysis. J Am Coll Cardiol. (2017) 70:1587–97. doi: 10.1016/j.jacc.2017.07.775
52. Jia Z, Wu A, Tam M, Spain J, McKinney JM, Wang W. Caval penetration by inferior vena cava filters: a systematic literature review of clinical significance and management. Circulation. (2015) 132:944–52. doi: 10.1161/CIRCULATIONAHA.115.016468
53. Othman M, Santamaria Ortiz A, Cerda M, Erez O, Minford A, Obeng-Tuudah D, et al. Thrombosis and hemostasis health in pregnancy: registries from the International Society on Thrombosis and Haemostasis. Res Pract Thromb Haemost. (2019) 3:607–14. doi: 10.1002/rth2.12243
54. Bates SM, Rajasekhar A, Middeldorp S, McLintock C, Rodger MA, James AH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: venous thromboembolism in the context of pregnancy. Blood Adv. (2018) 2:3317–59. doi: 10.1182/bloodadvances.2018024802
55. Vitale N, De Feo M, De Santo LS, Pollice A, Tedesco N, Cotrufo M. Dose-dependent fetal complications of warfarin in pregnant women with mechanical heart valves. J Am Coll Cardiol. (1999) 33:1637–41. doi: 10.1016/S0735-1097(99)00044-3
56. Cohen H, Arachchillage DR, Middeldorp S, Beyer-Westendorf J, Abdul-Kadir R. Management of direct oral anticoagulants in women of childbearing potential: guidance from the SSC of the ISTH. J Thromb Haemost. (2016) 14:1673–6. doi: 10.1111/jth.13366
57. Hoeltzenbein M, Beck E, Meixner K, Schaefer C, Kreutz R. Pregnancy outcome after exposure to the novel oral anticoagulant rivaroxaban in women at suspected risk for thromboembolic events: a case series from the German Embryotox Pharmacovigilance Centre. Clin Res Cardiol. (2016) 105:117–26. doi: 10.1007/s00392-015-0893-5
58. Fausett MB, Vogtlander M, Lee RM, Esplin MS, Branch DW, Rodgers GM, et al. Heparin-induced thrombocytopenia is rare in pregnancy. Am J Obstetr Gynecol. (2001) 185:148–52. doi: 10.1067/mob.2001.114690
59. Martel N, Lee J, Wells PS. Risk for heparin-induced thrombocytopenia with unfractionated and low-molecular-weight heparin thromboprophylaxis: a meta-analysis. Blood. (2005) 106:2710–5. doi: 10.1182/blood-2005-04-1546
60. Voke J, Keidan J, Pavord S, Spencer NH, Hunt BJ, British Society for Haematology Obstetric Haematology G. The management of antenatal venous thromboembolism in the UK and Ireland: a prospective multicentre observational survey. Br J Haematol. (2007) 139:545–58. doi: 10.1111/j.1365-2141.2007.06826.x
61. Friedrich E, Hameed AB. Fluctuations in anti-factor Xa levels with therapeutic enoxaparin anticoagulation in pregnancy. J Perinatol. (2010) 30:253–7. doi: 10.1038/jp.2009.164
62. McDonnell BP, Glennon K, McTiernan A, O'Connor HD, Kirkham C, Kevane B, et al. Adjustment of therapeutic LMWH to achieve specific target anti-FXa activity does not affect outcomes in pregnant patients with venous thromboembolism. J Thromb Thrombol. (2017) 43:105–11. doi: 10.1007/s11239-016-1409-5
63. Boban A, Paulus S, Lambert C, Hermans C. The value and impact of anti-Xa activity monitoring for prophylactic dose adjustment of low-molecular-weight heparin during pregnancy: a retrospective study. Blood Coagul Fibrinol. (2017) 28:199–204. doi: 10.1097/MBC.0000000000000573
64. Romualdi E, Dentali F, Rancan E, Squizzato A, Steidl L, Middeldorp S, et al. Anticoagulant therapy for venous thromboembolism during pregnancy: a systematic review and a meta-analysis of the literature. J Thromb Haemost. (2013) 11:270–81. doi: 10.1111/jth.12085
Keywords: pulmonary embolism, pregnancy, thrombolysis, outcome, venous thromboembolism
Citation: Hobohm L, Farmakis IT, Münzel T, Konstantinides S and Keller K (2022) Pulmonary Embolism and Pregnancy—Challenges in Diagnostic and Therapeutic Decisions in High-Risk Patients. Front. Cardiovasc. Med. 9:856594. doi: 10.3389/fcvm.2022.856594
Received: 17 January 2022; Accepted: 10 February 2022;
Published: 08 March 2022.
Edited by:
Bernard Tardy, Inserm CIC1408, FranceReviewed by:
Håkan Wallen, Karolinska Institutet (KI), SwedenCopyright © 2022 Hobohm, Farmakis, Münzel, Konstantinides and Keller. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lukas Hobohm, lukas.hobohm@unimedizin-mainz.de
 Lukas Hobohm
Lukas Hobohm Ioannis T. Farmakis2
Ioannis T. Farmakis2  Thomas Münzel
Thomas Münzel