- 1Northwest Pituitary Center, Oregon Health & Science University, Portland, OR, United States
- 2Centre Hospitalier Universitaire de Sherbrooke, Fleurimont, QC, Canada
- 3Singapore General Hospital, Singapore, Singapore
Background: Hypercortisolism has been implicated in the development of venous thromboembolic events (VTE). We aimed to characterize VTE risk in endogenous Cushing's syndrome (CS) patients, compare that risk to other pathologies, and determine if there are any associated coagulation factor changes.
Methods: Medline and Scopus search for “hypercortisolism” and “thromboembolic disease” from January 1980 to April 2017 to include studies that reported VTE rates and/or coagulation profile of CS patients. A systematic review and meta-analysis were performed.
Results: Forty-eight studies met inclusion criteria. There were 7,142 CS patients, average age was 42 years and 77.7% female. Odds ratio of spontaneous VTE in CS is 17.82 (95%CI 15.24–20.85, p < 0.00001) when comparing to a healthy population. For CS patients undergoing surgery, the odds ratio (both with / without anticoagulation) of spontaneous VTE is 0.26 (95%CI 0.07–0.11, p < 0.00001)/0.34 (0.19–0.36, p < 0.00001) when compared to patients undergoing hip fracture surgery who were not treated with anticoagulants. Coagulation profiles in patients with CS showed statistically significant differences compared to controls, as reflected by increases in von Willebrand factor (180.11 vs. 112.53 IU/dL, p < 0.01), as well as decreases in activated partial thromboplastin time (aPTT; 26.91 vs. 30.65, p < 0.001) and increases in factor VIII (169 vs. 137 IU/dL, p < 0.05).
Conclusion: CS is associated with significantly increased VTE odds vs. general population, but lower than in patients undergoing major orthopedic surgery. Although exact timing, type, and dose of anticoagulation medication remains to be established, clinicians might consider monitoring vWF, PTT, and factor VIII when evaluating CS patients and balance advantages of thromboprophylaxis with risk of bleeding.
Introduction
Endogenous Cushing's syndrome (CS) results in multiple systemic manifestations related to excess cortisol, including catabolic effects, central fat redistribution and psychiatric manifestations (1). Endogenous CS can be stratified into two subtypes; adrenocorticotropic hormone (ACTH)-dependent CS, where excess ACTH leads to overproduction of cortisol, or ACTH-independent disease where cortisol is produced regardless of input from ACTH. The former is attributed to an ACTH-secreting pituitary adenoma (Cushing's disease; CD), or ectopic ACTH-secretion while autonomous adrenal hypersecretion of cortisol results in ACTH-independent disease (2–4). The most common is ACTH-dependent CS, where an estimated 70% of cases are due to a pituitary adenoma; CD (5). Though probably underdiagnosed, reported incidence of CD is between 6.2 and 7.6 per million patient years, while the reported incidence of adrenal CS is approximately 0.6 per million per year (3, 4). First-line treatment in most cases is surgical resection of the culprit tumor; some patients may further require medical therapy, bilateral adrenalectomy, and/or radiation (4, 6).
An association between hypercortisolism and hypercoagulability has been previously documented (7–11). A state of hypercoagulability reflects German pathologist Rudolph Virchow's postulation that venous thrombosis and/or arterial thrombosis are preceded by identifiable factors (12). Risk of postoperative venous thromboembolism events (VTE) was significantly higher in patients with CD compared to patients undergoing pituitary surgery for a non-functioning pituitary adenoma, suggesting that the high risk for postoperative VTE in CS is cortisol mediated (13). Importantly, other disease states, such as patients undergoing orthopedic surgery for hip replacement, are routinely treated with thromboprophylaxis (14). However, there is no consensus about the exact risk of thrombosis in patients with endogenous CS. Quantifying this risk is important to balance the advantages and complications associated with anticoagulation treatment. The Endocrine Society Guidelines for Treatment of Cushing's syndrome suggests clinicians be aware of the altered coagulation profile of CS patients for up to 1-year following surgical cure, and consider anticoagulation on an individual basis (15). The guidelines, however, do not offer screening metrics for venous thromboembolism (VTE) assessment risk in CS patients. Further, few observational studies and case-series have demonstrated changes in von Willebrand factor (vWF), factor-VIII (fVIII), or homocysteine in CS patients (9, 16–19). Other coagulation markers could also play a role (20–22).
Here, we performed a meta-analysis to study the association between endogenous hypercortisolism and hypercoagulability (venous thrombosis events and alteration of coagulation profile). We hypothesized that patients with hypercortisolism due to CS have a significantly increased risk of VTE compared to the general population. We also reviewed available evidence of coagulation profiles for CS patients and compared to the general population. Lastly, we quantified the odds of VTE in CS and compared it with the odds of patients undergoing major orthopedic surgery.
Materials and Methods
Study Identification
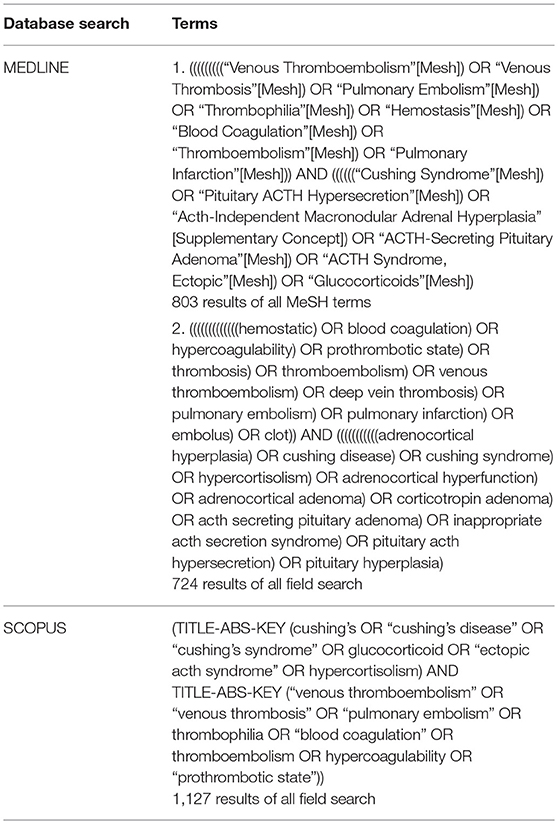
Studies were identified using search terms in both MEDLINE and SCOPUS databases up to April 2017. The query in PubMed, the search engine to access the MEDLINE database, included MeSH terms and non-MeSH. Terms were related to both endogenous hypercortisolism and hypercoagulability, more specifically detailed in Table 1.
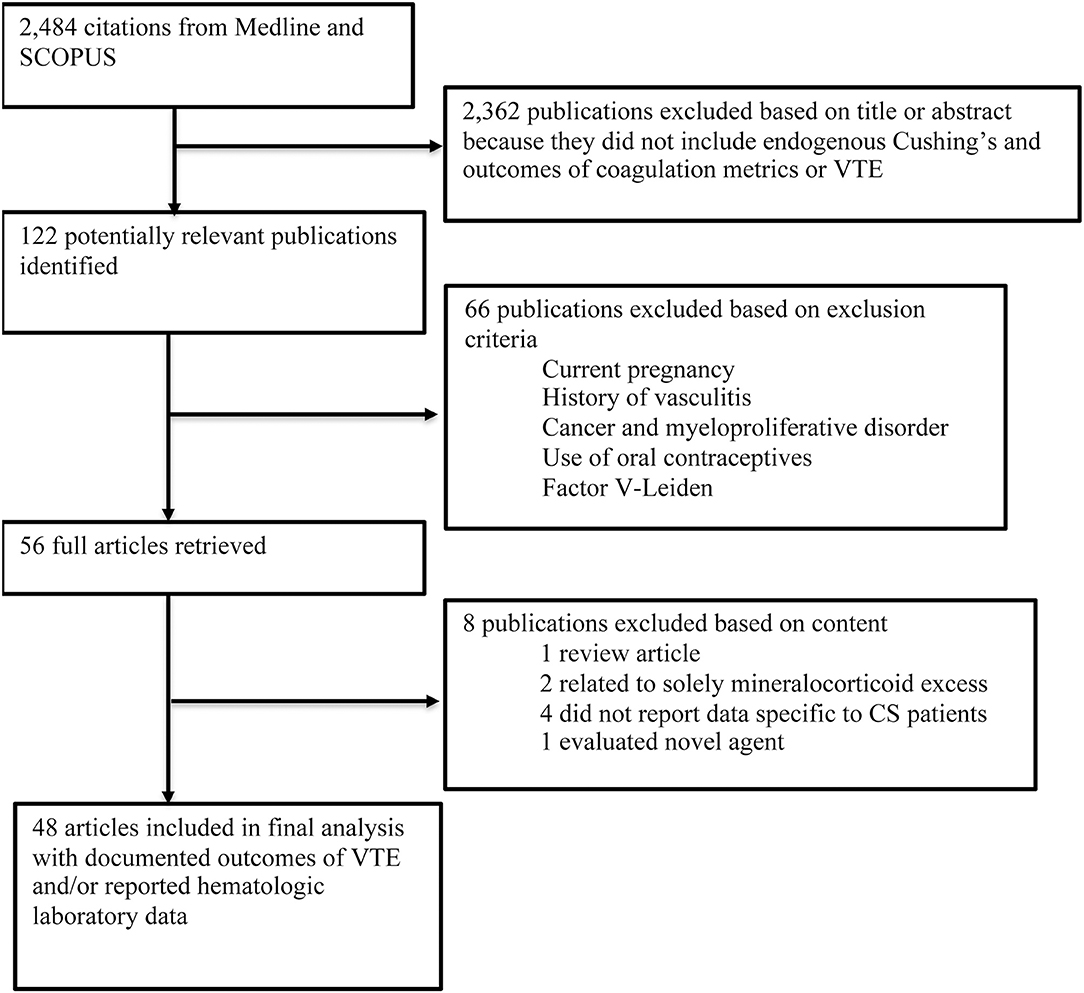
In MEDLINE, a cumulative search generated 803 articles using MeSH terms and 724 articles using non-MeSH terms. The same search terms were used in SCOPUS, which identified 1,127 articles. References from included articles were also searched for additional articles. Articles from both databases queries were combined to compromise comprehensive review of the literature. Duplicate studies identified in both databases were reduced so each article was only accounted for once in the cumulative dataset with all duplicates removed, resulting in 2,484 articles (Figure 1).
The titles and abstracts were reviewed by two of three independent reviewers. Inclusion criteria included both diagnosis of hypercortisolism due to endogenous CS and either reporting of VTE events or coagulation laboratory data. The search was also limited to human studies published after 1980 in English-language literature, and reports of more than 1 patient. Studies that included patients undergoing treatment with systemic supraphysiological glucocorticoids were excluded. Similarly, malignant causes of CS, mostly ectopic ACTH-syndromes were also excluded. Notable exceptions were made for studies that pooled total of 81 CS patients with ectopic ACTH in their analyses where authors did not stratify patients by underlying diagnosis. Those studies were not excluded since included the majority of patients had hypercortisolism of pituitary or adrenal origin.
Exclusion criteria also included studies that were unrelated to CS population or any of the following: diagnosis of current pregnancy, vasculitis, cancer or myeloproliferative disease, systemic lupus erythematosus or other inflammatory condition, heparin induced thrombocytopenia, antithrombin-III /protein-C/protein-S deficiencies, factor-V-Leiden, prothrombin mutation, paroxysmal nocturnal hemoglobinuria, dysfibrinogenemia, and use of oral contraceptives, or received fibrinolysis within the past 30 days were excluded.
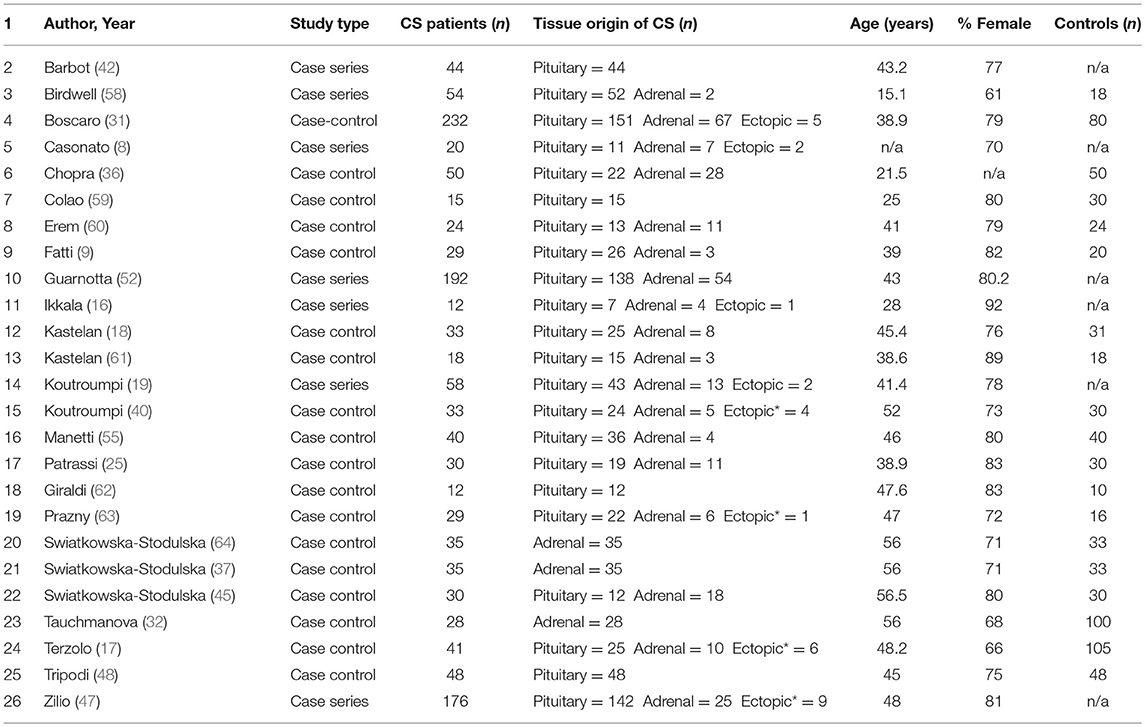
Following review of literature title and abstracts, 122 manuscripts were further reviewed to determine if methods and data reported ultimately met inclusion criteria for data extraction. Manuscripts were excluded due to a variety of criteria, which included: pilot studies characterizing a non-validated test, methodology data, results did not stratify for CS, or article type was not original research such as a review article. Following final review, 48 studies were included for use in the analysis (7, 9, 10, 13, 16, 18, 19, 23–48).
Data Extraction
Data were extracted by three independent reviewers (JW, FL, DL). Inclusion and exclusion criteria were predefined (as above) and validated through discussion with another author (MF). Studies were included if there was agreement between two independent reviewers. Discrepancies were resolved by discussion among all authors of this report on a study-by-study basis.
Data extraction was stratified into three subgroups: background, laboratory, and VTE event data. The following data were extracted for both CS and controls (if used in study) patients: number of patients, mean (± standard deviation; SD) age, gender distribution, and mean (± SD) BMI. Ethnicity was not reported in most studies. For CS patients, additional data included: source of hypercortisolism (ACTH-dependent or independent) and mean 24-h urinary free cortisol (UFC; μg/24-h) if available. Preoperative laboratory data was used for patients with a confirmed diagnosis of CS. VTE event data included the number of patients with the following outcomes for both CS and control patients: development of VTE, postoperative VTE, pulmonary embolism (PE), and VTE on prophylactic anticoagulation.
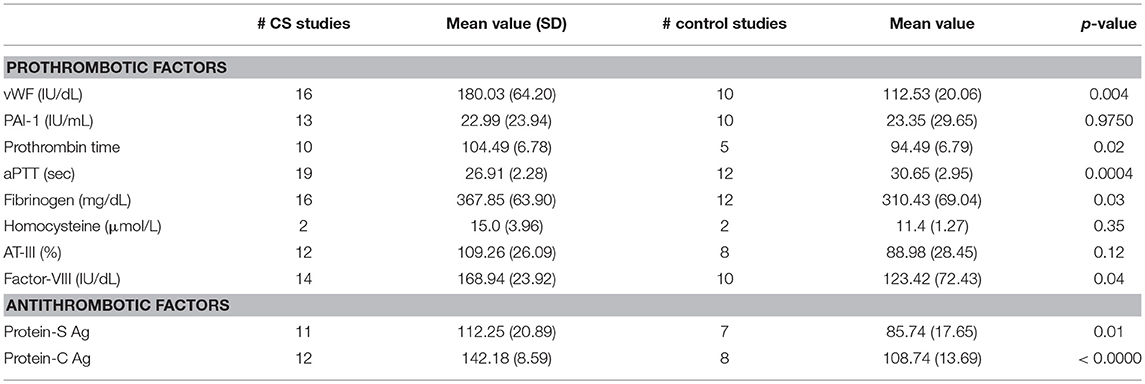
The following laboratory data was retrospectively collected: protein-S antigen, protein-C antigen, von Willebrand factor (IU/dL), plasminogen activator inhibitor-1 (IU/mL), % prothrombin time, activated partial thromboplastin time (seconds), fibrinogen (mg/dL), homocysteine (μmoL/L), % antithrombin-III, and factor VIII (IU/dL).
Study Controls
For a control group to be acceptable given the heterogeneity of different criteria among included studies, studies were at minimum required to specify if they enrolled healthy or patients with other adrenal or pituitary diseases, and age-matched controls. However, no controls from included studies developed VTE. Therefore, we referenced the literature to determine the baseline risk of VTE in the general population to act as the control group in our analysis. A large review (49) among people of European ancestry estimated annual incidence of VTE from 104 to 183 per 100,000 person years. Using this population data as a control, we calculated the odds ratio (OR) using the higher estimate of 183 cases of VTE per 100,000 person years in the general population.
Subsequently, to calculate the odds of VTE for CS patients in the perioperative setting, we selected the control group consisting of orthopedic repair for hip or knee fractures in patients who received prophylactic anticoagulation with low molecular weight heparin. This patient population currently is indicated for perioperative anticoagulation due to a well-documented risk of VTE. Comparing the odds in both patient populations head-to-head allowed us to compare the odds of known high-risk population (orthopedic fracture) to that of an unknown population (CS). Since a minority, 5.89% (385/6,537) of the CS patients included in our analysis received anticoagulation in the perioperative period, we opted for a control group also treated with anticoagulation (incidence of VTE 1.5%; 36/2,420) (50). Conversely, we used the risk of pulmonary embolism in patients undergoing surgery for femur fracture without thromboprophylaxis in order to calculate the OR for the remaining 94.11% percent of our CS cohort undergoing surgery without anticoagulation: the estimated risk of VTE in the control arm was 14% (21/150) (51).
Statistical Analysis
Laboratory results from all studies were pooled for analysis. Descriptive statistics using mean ± SD, and 95% confidence intervals were calculated for the pooled data. Analysis of pooled data was conducted via two-sided t-tests with assumed equal variance, comparing diagnosis of CS vs. control patients. We performed simple linear regression for the statistically significant laboratory values and percentage of CS patients who developed VTE.
We calculated the OR of VTE in CS patients compared to controls using outcomes data from the included studies. For patients undergoing surgery, data on the time of VTE diagnosis relative to operation was collected and post-operative period was defined as within 30 days after surgery.
Heterogeneity among studies was assessed by calculating an I2 statistic, as well as assessing the visual variability between studies by funnel plot. All analyses were conducted with STATA 15.1 statistical software (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC).
Results
Background Analysis
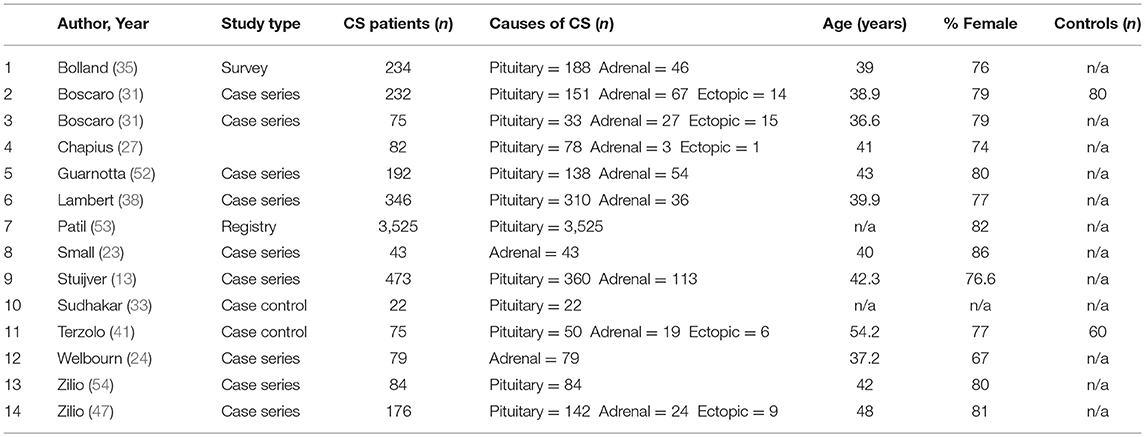
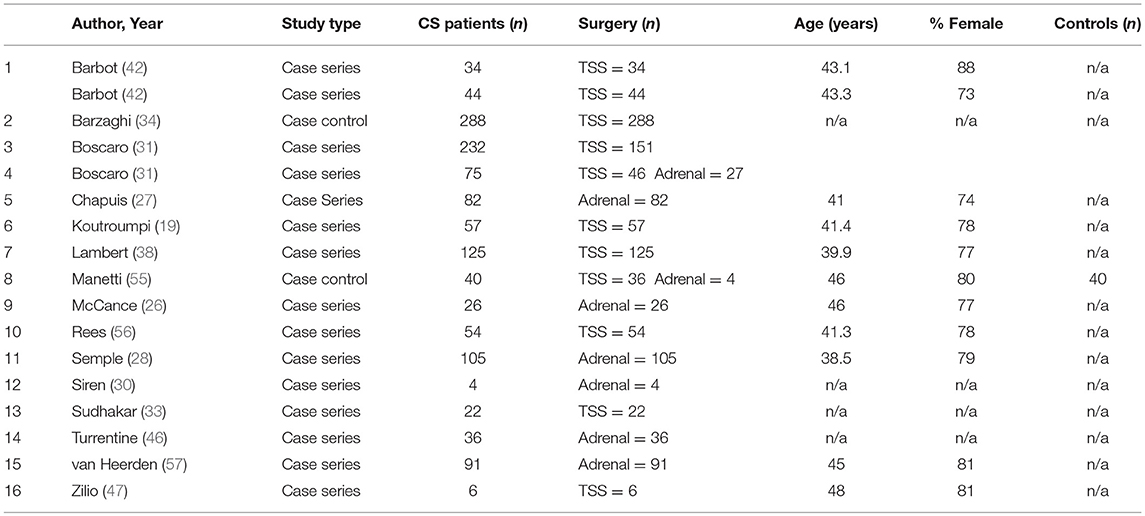
Characteristics of the 48 included studies are shown in Tables 2–4. Eleven studies contributed data to both outcome of VTE analysis and outcome laboratory data analysis. Thirty-five studies were retrospective in design, compared to 14 prospective studies. Twenty-one studies were cross-sectional analyses and 25 were case series. Of the remaining 2 studies, one was a survey, and one a cohort study.
In total, pooled studies represented 7,142 patients with endogenous hypercortisolism with mean follow-up of 38.89 ± 25.49 months. The underlying cause of hypercortisolism was CD in 6,174, adrenal origin in 888, ectopic CS in 80. Mean age of patients was 42.1 years of age (range 15.1–56.5 years), and patients were more likely to be female (76.2% ± 8.7), with mean BMI of 29.3 ± 1.7 kg/m2. Baseline UFC for patients with CS was 1061.9 ± 499.2 nmol/24 h which corresponds to 7 to 8 times upper limit of normal (ULN).
Laboratory Analysis
Laboratory coagulation metrics are shown in Table 5. The pooled mean values for CS patients were compared to values for controls. Of those evaluated, vWF, protein-C antigen, protein-S antigen, activated partial thromboplastin time (aPTT), fibrinogen, and factor VIII had statistically significant differences between CS and control patients. Some prothrombotic parameters were noted: mean vWF in CS was higher: 180.03 ± 64.20 IU/dL (95% CI 145.81–214.24) vs. 112.53 ± 20.06 IU/dL (95% CI 98.18–126.88) in controls (p < 0.01), mean fibrinogen was also elevated in CS 367.85 ± 63.90 (95% CI 333.80–402.00) vs. 310.43 ± 69.04 (95% CI 266.57–354.30) in controls (p < 0.05), mean factor VIII in CS was higher: 168.94 ± 6.39 (95% CI 155.13–182.75) vs. 123.42 ± 72.43 (95% CI 71.61–175.24) in controls (p < 0.05) and mean aPTT in CS was lower: 26.91 ± 0.56 (95% CI 25.81–28.0) vs. 30.65 ± 2.95 (95% CI 28.78–32.52) in controls (p < 0.001). On the other hand, other antithrombotic parameters were observed in CS: Mean protein-C antigen in CS was increased: 142.18 ± 8.59 (95% CI 136.72–147.63) vs. 108.74 ± 13.69 (95%CI 97.29–120.18) in controls (p < 0.00001). Mean protein-S antigen in CS was also elevated: 112.25 ± 20.90 (95% CI 98.21–126.28) vs. 85.74 ± 17.65 (95% CI 69.42–102.06) in controls (p < 0.05).
Simple linear regression revealed no linear relationship between laboratory metrics and number of thrombotic events (Table 6). We additionally performed regression analysis that demonstrated no coagulation related labs were linearly associated with the severity of CS as evidenced by UFC elevation.
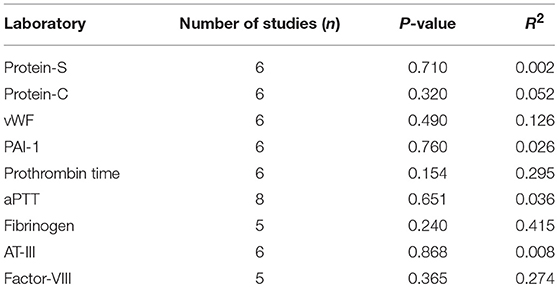
Table 6. Laboratory variables tested in associated with development of VTE following simple linear regression.
Odds of VTE in Patients With CS Compared to General Population
In pooled analysis, 3.21% (210/6,537) of CS patients experienced VTE. 62 of these events were due to pulmonary embolism and 48 deaths were attributed to VTE. The determined odds ratio is 17.8 when unadjusted for perioperative risk (95%CI 15.24–20.85, p < 0.00001; Figure 2). Confidence intervals were wide and displayed non-uniform overlap. Calculated I-squared statistic was highly elevated at 91%, suggesting a large degree of heterogeneity among reported odds of VTE in patients with CS. Follow-up funnel plot in assessment of bias demonstrated the cumulative literature reporting cases of VTE in patients with underlying CS is likely subject to bias and is additionally influenced by overestimation of risk (Figure 3). Other inherent risk factors for VTE were included in pooled analysis to evaluate for prognostic association for the following parameters: age, sex, active smoker, undergoing surgery (transsphenoidal surgery-TSS or bilateral adrenalectomy), and history of diabetes mellitus, when available. Simple linear regression did not indicate a significant association between any of these and the outcome of VTE (Table 7).
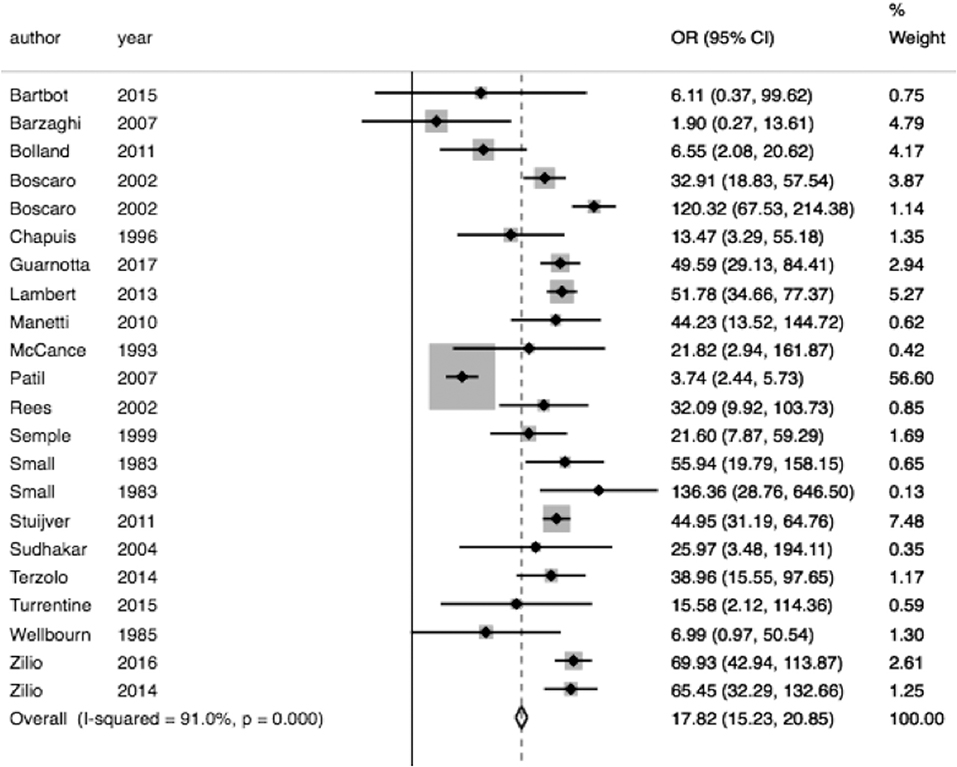
Figure 2. Increase odds of VTE in CS. Total OR 17.82 (95% CI 15.24–20.85) of VTE in CS compared to general population when unadjusting for VTE within 30 days of an operation. However, the interpretability of this analysis is limited by the degree of heterogeneity of odds ratios between studies included, as indicated by the I2 statistic of 91.0%.
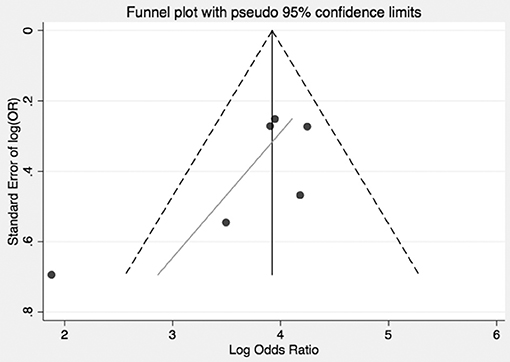
Figure 3. Bias and overestimation of risk impact interpretation of risk of VTE in CS from literature. Funnel plot visually representing the heterogeneity among trials reporting odds of VTE in CS. As shown, included studies are asymmetrically distributed relative to the point estimate. This suggests that pooled results are influenced both by bias and overestimate of risk in the CS population.
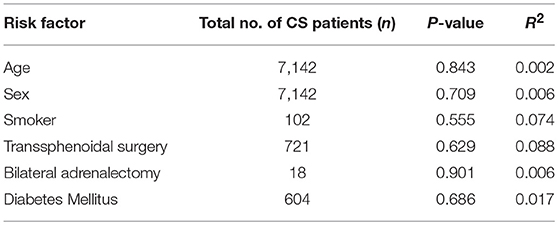
Table 7. Additional Risk factors tested in association with outcome of VTE in pooled CS cohort following simple linear regression.
Odds of Perioperative VTE in Patients With CS Compared to Patients Undergoing Orthopedic Surgery
Of the 6,537 cases included, a total of 2,083 operative cases were patients undergoing surgery, either transphenoidal surgery (TSS) or adrenalectomy for CS was undertaken. A total of 1,476 TSS cases were included compared to 607 adrenalectomies for from 16 studies. 4.75% (99/2,083) of patients had a VTE event within 30 days of surgery. The determined odds ratio for perioperative VTE in CS vs. hip fracture repair without anticoagulation is 0.26 (95% CI 0.19–0.136, p < 0.00001; Figure 4). Confidence intervals were wide and displayed non-uniform overlap. Calculated I-squared statistic of 63.4% indicates the greater body of literature reporting VTE events in CS patients related to surgery has less heterogeneity when accounting for the CS population as a whole, but sizable heterogeneity remains. The determined odds ratio for perioperative VTE in CS vs. hip fracture repair with anticoagulation is 0.34 (95% CI 0.20–0.61, p < 0.00001; Figure 5). Confidence intervals were wide and displayed non-uniform overlap. Calculated I-squared statistic of 58.7% indicates the greater body of literature reporting VTE events in CS patients related to surgery has less heterogeneity when accounting for the CS population as a whole, but sizable heterogeneity remains. In assessing for the potential influence of bias, funnel plot demonstrates moderate asymmetrical distribution of included studies (Figure 3). This suggests our pooled analysis comparing the odds of VTE in CS patients vs. the control data from trials reporting the risk of VTE in hip surgery is less subject to bias and may accurately represent a reasonable estimation of the perioperative risk of VTE in CS patients.
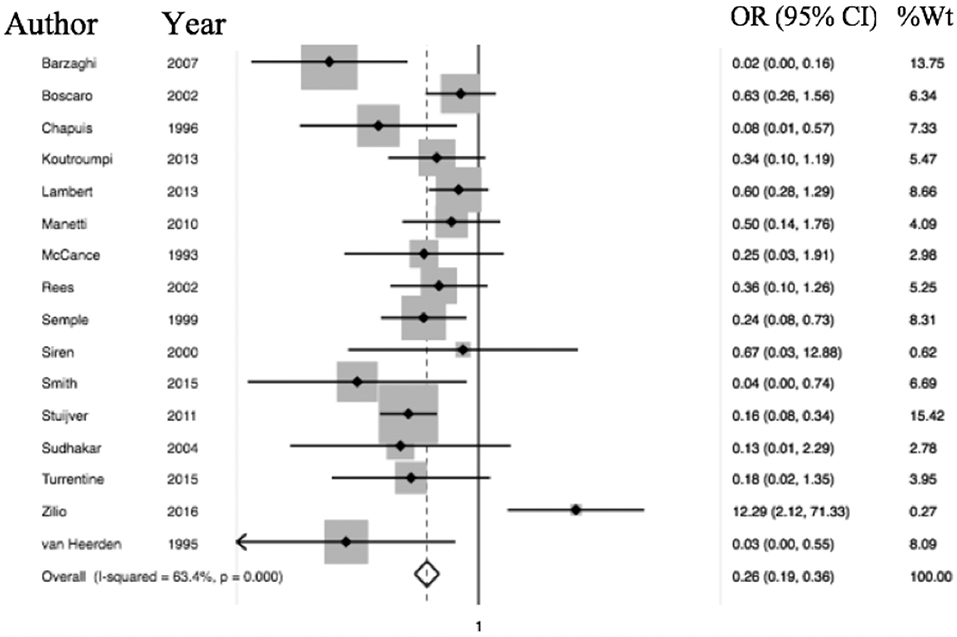
Figure 4. Odds of VTE in CS without anticoagulation significantly less than hip fracture surgery without anticoagulation. Total OR 0.26 (95% CI 0.19–0.36) of postop (within 30 days of surgery) VTE in CS without anticoagulation compared to odds of postoperative VTE in cases of hip fracture repair without anticoagulation. This suggests the odds of VTE in CS patients in the perioperative period for those undergoing adrenalectomy or TSS are 84% less than that of patients undergoing surgery for hip fracture.
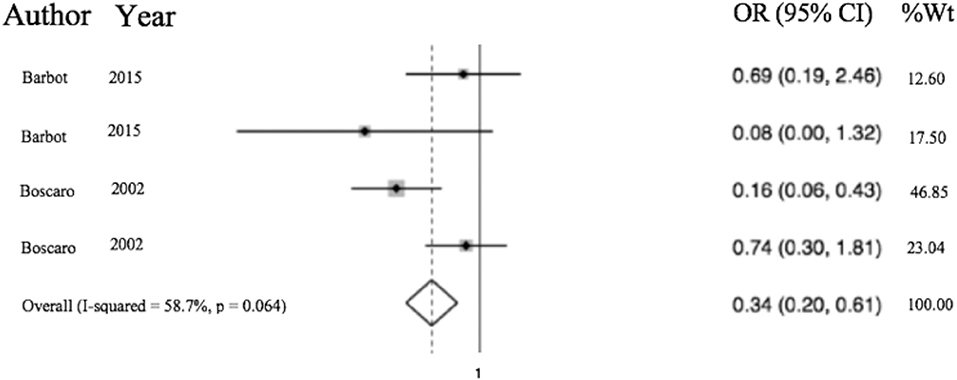
Figure 5. Odds of VTE in CS on anticoagulation significantly less than hip fracture surgery with anticoagulation. Total OR 0.34 (95% CI 0.20–0.61) of postop (within 30 days of surgery) VTE in CS with anticoagulation compared to odds of postoperative VTE in cases of hip fracture repair with anticoagulation. This suggests the odds of VTE in CS patients in the perioperative period for those undergoing adrenalectomy or TSS are 66% less than that of patients undergoing surgery for hip fracture.
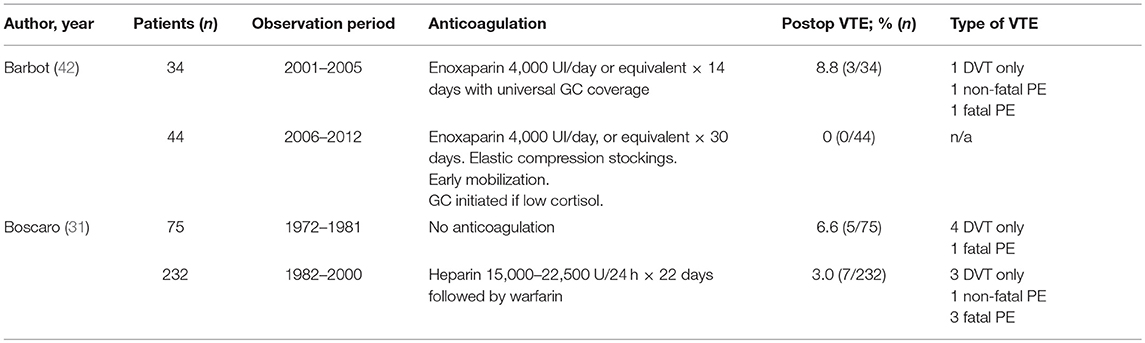
Only two studies reported use of thromboprophylaxis in CS and their results are presented in Table 8. However, this data is insufficient to support universal use of anticoagulation in CS undergoing intervention.
Discussion
An association between CS and a hypercoagulable state has been previously suggested (11, 13, 65), however, prevalence of VTE in patients with endogenous CS remains unclear. Furthermore, the role of anticoagulation is unknown. To shed light on this we undertook a systematic review of published studies to conduct a meta-analysis regarding the reported odds of VTE in CS patients compared to the general population. Subsequently, in attempt to clarify the potential role of perioperative anticoagulation to avert this risk, we compared the odds of VTE in CS patients undergoing surgery to those of patients undergoing hip replacement. This was done in order to identify whether the odds is reasonably similar between the two by comparing them head-to-head, where similar odds would argue a greater likelihood CS patients would benefit from anticoagulation, since thromboprophylaxis indication is well established in hip surgery patients.
We found that the odds of VTE is significantly increased in CS patients compared to the general population. The calculated odds of VTE in CS patients in this study was 17.82 vs. general population. Patients with CS in our meta-analysis were most often females with a mean age of 42 years, while expected rate of VTE in young women is much lower than in general population, 27 per 100,000 person-years (66), which would have resulted in much higher OR of VTE comparing to the specific CS subpopulation. No linear relationship was found correlating the severity of endogenous hypercortisolism with the number of thrombotic events or laboratory metrics coagulation. Other studies have confirmed that clinical manifestations of CS do not always correlate with the severity of hypercortisolism (52) thus we can assume the same rationale may be true for hypercoagulation and VTE.
The non-operative VTE risk in hospitalized patients is highly variable and depends on many individual factors including age >70 years malignancy, acute infection, etc. Pooled risks of inpatient-population average 0.8% of DVT and 0.4% of pulmonary embolism, but in a high- risk population, risk may increase up to 11% (67). To date, no risk assessment scales nor models have included Cushing/hypercortisolism (68–71). A key question for clinicians is whether this risk should be mitigated by treatment with anticoagulation. On the other hand, risks to anticoagulation include major bleeding, which has been reported to be between 2.8 and 6 per 100 person years (72). The risk-benefit analysis in deciding to treat with anticoagulation however necessitates indicated patients be at substantial risk of VTE in order to justify acceptable risk of hemorrhage.
In the perioperative setting, we show that the odds of VTE in patients undergoing TSS or adrenalectomy, is substantially lower than patients undergoing orthopedic surgery for hip fracture repair. CS carries a lower risk than a procedure for which prophylactic anticoagulation is currently the standard of care. Though this analysis prevents a definitive comment on risk, we can assume that CS-associated VTE risk is intermediate between general population and orthopedic surgery. Decision of prophylactic anticoagulation in CS patients undergoing surgery must then be individualized. Furthermore, bleeding risk should also be balanced in this population, especially in patient undergoing neurological surgery.
Interestingly, multiple prothrombotic laboratory metrics (vWF, fibrinogen, factor-VIII) were increased, while others were decreased (aPTT) in CS patients and anti-thrombotic markers (protein-C and protein-S) appear paradoxically to be elevated. To our knowledge, this is the first systematic review to characterize the association of increases of both protein-C and protein-S with CS. The increase in antithrombotic protein-C and protein-S has been described and is thought to be a compensatory physiological response to higher levels of clotting factors, such as FVIII, in order to promote degradation of factor excess (40, 60, 64, 73). This compensatory response could possibly vary from patient to patient and may explain why some individuals are at higher VTE risk. The underlying mechanism for this finding is likely related to a significant increase in prothrombotic metrics such as vWF, fibrinogen, and factor-VIII. All of these factors play a role in the activation of the coagulation cascade through either the intrinsic or extrinsic pathways, see Figure 2 in Isidori et al. (22). For example, vWF mediates platelet adhesion at site of endothelial damage and stabilizes FVIII, and FVIII has been described as a risk factor for VTE formation (22, 74). A corresponding decrease in aPTT, which reflects increased tendency to form clots, further supports that enhanced coagulation cascade activation occurs in these patients. The physiological explanation to link hypercortisolism and hypercoagulability is not fully understood. In one study, level of factor VIII positively correlated with UFC levels (11, 58), (not observed in our meta-analysis). Obesity also confers a higher risk of VTE, but the risk in CS seems higher than in otherwise healthy obese patients who are at low risk (14). Inflammation may induce a hypercoagulable state, but this is counterintuitive in cases of endogenous CS, since high levels of cortisol should rather have an anti-inflammatory effect (75). Many VTE events occur postoperatively and previous studies have suggested that an abrupt decrease in cortisol after surgery can increase VTE risk (2, 39, 42). A rapid drop in cortisol level could lead to a paradoxical increase in coagulation factors by withdrawal of the anti-inflammatory effect of cortisol. However, in our meta-analysis, there was insufficient data concerning remission of CS post-surgery, and adrenal insufficiency by itself does not confer an increased risk of VTE (76). Moreover, an increase in anti-coagulation parameters has been observed post-surgery, in pediatric and adult patients (11, 13, 77). Further, VTE risk decreased, but persisted even in patients who underwent anticoagulation therapy (31).
Karamouzis et al. reported data on increase platelet aggregation measured by thromboxane B2 levels and increased oxidative stress in patient with CS (not measured in our meta-analysis) (76). This may result in both arterial and venous increased risk of thrombosis. A systematic review in 2009 found an increased risk of VTE in CS patients, both postoperatively (0–0.6%) and unprovoked by surgery (1.9–2.5%) (65). A difference in methods between our analyses was inclusion of case series, due to limited availability of prospective studies; we also calculated an OR as these case series studies lack the rigor to properly define risk. Thus, outcome of VTE events from our analysis is relatable, though not identical in nature. Our analysis also included 33 articles additional articles beyond the 2009 (2010–2017), which represents a significant body of literature and warrants revisiting this topic (65).
Our meta- analysis further confirms an association between CS and VTE, and changes in coagulation parameters. Clinicians and patients should be aware of this association, and CS management tailored on an individual basis. A recent Pituitary Society survey showed awareness regarding hypercoagulability in CD quadrupled in 2 years (78); routine VTE prophylaxis increased from 50 to 75% peri-operatively and doubled in patients who underwent bilateral inferior petrosal sinus sampling (BIPSS). However, prophylactic treatment for hypercoagulability was not universally administered. For centers using anticoagulation, low-molecular heparin was the preferred agent for VTE prophylaxis and most of the responders used it for approximately 2 weeks post-operatively. It is the authors' opinion that more rigorous trials are needed to identify additional VTE risk factors and possible mitigating factors.
Steps such as screening coagulation metrics in CS patients or even considering anticoagulation may be appropriate, especially in perioperative setting or around procedures such a BIPSS. However, this cannot be definitely inferred from the data we present. Such recommendations require well designed, prospective analyses. Nevertheless, screening CS patients for inherited coagulation pathologies and other additional risk factors would help determine the highest “at risk” subgroup of patients (22). Regarding timing of anticoagulation, in patients with gliomas (albeit with much higher risk of VTE), recent guidelines suggest VTE prophylaxis with low-molecular-weight heparin in brain tumor patients to be started postoperatively within 24 h to decrease risk of hemorrhage (79). A similar pattern can be potentially applicable to selected high risk VTE cases in patients with CS, but further studies are needed.
Data on medical therapy directed to lower cortisol values on VTE risk is sparse (80). In a study of 17 patients with CD on short-term triple combination medical therapy (pasireotide, cabergoline and ketoconazole), both increased production of procoagulant factors and impaired fibrinolysis, have not been shown to reverse despite biochemical remission after successful medical therapy (81). The authors found a slight decrease in antithrombin levels after 3 months of successful medical therapy (levels were normal at baseline), but no changes in fibrinogen, D-dimer levels, factor VIII activity, and protein C and S activity. Another prospective study in 21 patients treated with short-acting pasireotide confirmed the lack of effect of medical therapy of CS on changing either clotting or anticoagulant factors during therapy; no patients developed thrombotic complications during treatment (81).
Limitations
There are inherent study limitations. Inclusion of case series and lower quality studies rules out a determination of risk per se in the meta-analysis, as presented. To address this we computed an OR. The OR calculations were, however, limited by the lack of controls who developed VTE, which was addressed by referencing epidemiologic data from a population of white-European descent, the majority of patients in the CS studies. Additionally, the inclusion and exclusion criteria varied between the included studies. We attempted to address this via verification that authors at a minimum specified that controls were health- and age-matched in an attempt to limit variation a priori. However, when determining the OR of VTE in CS, the degree of heterogeneity indicates the included studies differed greatly (Figures 4, 5). Unfortunately, factors required to evaluate this concern, such as severity of illness, methods of diagnosis for both CS and VTE were not available. The diagnosis of VTE was clinical for most studies or studies did not objectively report how VTE was diagnosed. For example, confirmatory testing for deep vein thrombosis with doppler ultrasound and venography with rule in and rule out patients under different operative characteristics of that given test (i.e., sensitivity and specificity). Since no standardized diagnosis method was used to make the diagnosis of VTE, we are left to assume each patient who had a reported VTE event per study did indeed have the appropriate diagnosis regardless of workup and did not involve systematic search for VTE, some events may have been missed and VTE prevalence underestimated. Concomitant interventions such as elastic stockings and early mobilization may not have been reported or may not have been consistent in studies over a long observation period. As previously mentioned, trial designs reviewed and included are of lower evidence quality, which lends the pooled results to the biases of such designs. However, the degree of heterogeneity is, impart, and accounted for by these biases as evidenced in the funnel plot where smaller studies are distributed asymmetrically (Figure 3). Lastly, due to the lack of prospective data on this topic, we utilized the literature to provide data on the risk of VTE in all of our control calculation. The risk data in the control group was used in order to calculate odds, resulting in data from multiple trial designs being synthesized into a single calculation in order to perform the meta-analysis.
Conclusions
Endogenous Cushing's syndrome is associated with significantly high odds of developing VTE. Our meta-analysis, which includes case-series in addition to prospective and retrospective studies, shows that risk of VTE (both non-operative and post-operative cases) in CS is significantly increased compared with general population. Rates of VTE in CS seem lower than in patients with hip fracture postsurgery, a disease well- known to carry high risk of VTE and in which most patients require prophylactic anticoagulation. Nonetheless, a definitive determination of risk and benefits of anticoagulation in CS is needed through large, prospective controlled trials. Exact timing (at diagnosis or before and after surgery), type, doses and duration of prophylaxis medications remains to be established. It is essential that endocrinologists bear in mind the close association of CS with VTE when caring for patients and consider prophylactic intervention and/or monitoring on an individualized basis.
Author Contributions
JW, FL, DL, and MF contributed to study conception and design, acquisition of data, and analysis and interpretation of data. JW, FL, SM, and MF drafted the manuscript. JW, FL, DL, SM, and MF critically revised and reviewed the submitted version of the manuscript. JW and FL contributed to statistical analysis. SM and MF provided administrative and technical support. MF provided overall study supervision.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Nieman LK. Diagnosis of Cushing's syndrome in the Modern Era. Endocrinol Metab Clin North Am. (2018) 47:259–73. doi: 10.1016/j.ecl.2018.02.001
2. Etxabe J, Vazquez JA. Morbidity and mortality in Cushing's disease: an epidemiological approach. Clin Endocrinol. (1994) 40:479–84. doi: 10.1111/j.1365-2265.1994.tb02486.x
3. Lindholm J, Juul S, Jørgensen JO, Astrup J, Bjerre P, Feldt-Rasmussen U, et al. Incidence and late prognosis of cushing's syndrome: a population-based study. J Clin Endocrinol Metab. (2001) 86:117–23. doi: 10.1210/jc.86.1.117
4. Broder MS, Neary MP, Chang E, Cherepanov D, Ludlam WH. Incidence of Cushing's syndrome and Cushing's disease in commercially-insured patients < 65 years old in the United States. Pituitary (2015) 18:283–9. doi: 10.1007/s11102-014-0569-6
5. Newell-Price J, Bertagna X, Grossman AB, Nieman LK. Cushing's syndrome. Lancet (2006) 367:1605–17. doi: 10.1016/S0140-6736(06)68699-6
6. Fleseriu M, Petersenn S. Medical management of Cushing's disease: what is the future? Pituitary (2012) 15:330–41. doi: 10.1007/s11102-012-0397-5
7. Zanon RDB, Fornasiero L, Boscaro M, Cappellato G, Fabris F, Girolami A. Increased factor VIII associated activities in Cushing's syndrome: a probable hypercoagulable state. Thromb Haemost. (1982) 47:116–7. doi: 10.1055/s-0038-1657142
8. Casonato A, Pontara E, Boscaro M, Sonino N, Sartorello F, Ferasin S, et al. Abnormalities of von Willebrand factor are also part of the prothrombotic state of Cushing's syndrome. Blood Coagul Fibrinolysis (1999) 10:145–51. doi: 10.1097/00001721-199904000-00006
9. Fatti LM, Bottasso B, Invitti C, Coppola R, Cavagnini F, Mannucci PM. Markers of activation of coagulation and fibrinolysis in patients with Cushing's syndrome. J Endocrinol Invest. (2000) 23:145–50. doi: 10.1007/BF03343697
10. Dekkers OM, Horváth-Puhó E, Jørgensen JOL, Cannegieter SC, Ehrenstein V, Vandenbroucke JP, et al. Multisystem morbidity and mortality in Cushing's syndrome: a cohort study. J Clin Endocrinol Metab. (2013) 98:2277–84. doi: 10.1210/jc.2012-3582
11. van der Pas R, Leebeek FWG, Hofland LJ, de Herder WW, Feelders RA. Hypercoagulability in Cushing's syndrome: prevalence, pathogenesis and treatment. Clin Endocrinol (2013) 78:481–8. doi: 10.1111/cen.12094
12. Kitchens CS. Concept of hypercoagulability: a review of its development, clinical application, and recent progress. Semin Thromb Hemost. (1985) 11:293–315. doi: 10.1055/s-2007-1004383
13. Stuijver DJF, van Zaane B, Feelders RA, Debeij J, Cannegieter SC, Hermus AR, et al. Incidence of venous thromboembolism in patients with Cushing's syndrome: a multicenter cohort study. J Clin Endocrinol Metab. (2011) 96:3525–32. doi: 10.1210/jc.2011-1661
14. Kearon C, Akl EA, Ornelas J, Blaivas A, Jimenez D, Bounameaux H, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest (2016) 149:315–52. doi: 10.1016/j.chest.2015.11.026
15. Nieman LK, Biller BMK, Findling JW, Murad MH, Newell-Price J, Savage MO, et al. Treatment of Cushing's syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2015) 100:2807–31. doi: 10.1210/jc.2015-1818
16. Ikkala E, Myllylä G, Pelkonen R, Rasi V, Viinikka L, Ylikorkala O. Haemostatic parameters in Cushing's syndrome. Acta Med. Scand. (1985) 217:507–11. doi: 10.1111/j.0954-6820.1985.tb03254.x
17. Terzolo M, Allasino B, Bosio S, Brusa E, Daffara F, Ventura M, et al. Hyperhomocysteinemia in patients with Cushing's syndrome. J Clin Endocrinol Metab. (2004) 89:3745–51. doi: 10.1210/jc.2004-0079
18. Kastelan D, Dusek T, Kraljevic I, Polasek O, Giljevic Z, Solak M, et al. Hypercoagulability in Cushing's syndrome: the role of specific haemostatic and fibrinolytic markers. Endocrine (2009) 36:70–4. doi: 10.1007/s12020-009-9186-y
19. Koutroumpi S, Daidone V, Sartori MT, Cattini MG, Albiger NM, Occhi G, et al. Venous thromboembolism in patients with Cushing's syndrome: need of a careful investigation of the prothrombotic risk profile. Pituitary (2013) 16:175–81. doi: 10.1007/s11102-012-0398-4
20. Trementino L, Arnaldi G, Appolloni G, Daidone V, Scaroni C, Casonato A, et al. Coagulopathy in Cushing's syndrome. Neuroendocrinology (2010) 92(Suppl. 1):55–9. doi: 10.1159/000314349
21. van Zaane B, Stuijver DJF, Squizzato A, Gerdes VEA. Arterial and venous thrombosis in endocrine diseases. Semin Thromb Hemost. (2013) 39:489–95. doi: 10.1055/s-0033-1343889
22. Isidori AM, Minnetti M, Sbardella E, Graziadio C, Grossman AB. Mechanisms in endocrinology: ‘the spectrum of haemostatic abnormalities in glucocorticoid excess and defect. Eur J Endocrinol. (2015) 173:R101–13. doi: 10.1530/EJE-15-0308
23. Small M, Lowe GD, Forbes CD, Thomson JA. Thromboembolic complications in Cushing's syndrome. Clin Endocrinol (1983) 19:503–11. doi: 10.1111/j.1365-2265.1983.tb00025.x
24. Welbourn RB. Survival and causes of death after adrenalectomy for Cushing's disease. Surgery (1985) 97:16–20.
25. Patrassi GM, Sartori MT, Viero ML, Scarano L, Boscaro M, Girolami A. The fibrinolytic potential in patients with Cushing's disease: a clue to their hypercoagulable state. Blood Coagul Fibrinolysis (1992) 3:789–93. doi: 10.1097/00001721-199212000-00013
26. McCance DR, Russell CF, Kennedy TL, Hadden DR, Kennedy L, Atkinson AB. Bilateral adrenalectomy: low mortality and morbidity in Cushing's disease. Clin Endocrinol. (1993) 39:315–21. doi: 10.1111/j.1365-2265.1993.tb02371.x
27. Chapuis Y, Pitre J, Conti F, Abboud B, Pras-Jude N, Luton JP. Role and operative risk of bilateral adrenalectomy in hypercortisolism. World J Surg. (1996) 20:775–9. discussion: 779–80. doi: 10.1007/s002689900118
28. Semple PL, Laws ER Jr. Complications in a contemporary series of patients who underwent transsphenoidal surgery for Cushing's disease. J Neurosurg. (1999) 91:175–9. doi: 10.3171/jns.1999.91.2.0175
29. Henry JF, Defechereux T, Raffaelli M, Lubrano D, Gramatica L. Complications of laparoscopic adrenalectomy: results of 169 consecutive procedures. World J Surg. (2000) 24:1342–6. doi: 10.1007/s002680010222
30. Sirén J, Haglund C, Haapiainen R. An institutional experience with 40 first lateral transperitoneal laparoscopic adrenalectomies. Surg Laparosc Endosc Percutan Tech. (2000) 10:382–6. doi: 10.1097/00129689-200012000-00009
31. Boscaro M, Sonino N, Scarda A, Barzon L, Fallo F, Sartori MT, et al. Anticoagulant prophylaxis markedly reduces thromboembolic complications in Cushing's syndrome. J Clin Endocrinol Metab. (2002) 87:3662–6. doi: 10.1210/jcem.87.8.8703
32. Tauchmanov L, Rossi R, Biondi B, Pulcrano M, Nuzzo V, Palmieri E-A, et al. Patients with subclinical Cushing's syndrome due to adrenal adenoma have increased cardiovascular risk. J Clin Endocrinol Metab. (2002) 87:4872–8. doi: 10.1210/jc.2001-011766
33. Sudhakar N, Ray A, Vafidis JA. Complications after trans-sphenoidal surgery: our experience and a review of the literature. Br J Neurosurg. (2004) 18:507–12. doi: 10.1080/02688690400012459a
34. Barzaghi LR, Losa M, Giovanelli M, Mortini P. Complications of transsphenoidal surgery in patients with pituitary adenoma: experience at a single centre. Acta Neurochir. (2007) 149:877–85. discussion: 885–76. doi: 10.1007/s00701-007-1244-8
35. Bolland MJ, Holdaway IM, Berkeley JE, Lim S, Dransfield WJ, Conaglen JV, et al. Mortality and morbidity in Cushing's syndrome in New Zealand. Clin Endocrinol. (2011) 75:436–42. doi: 10.1111/j.1365-2265.2011.04124.x
36. Chopra A, Kumar R, Kishore K, Tandon N, Yusuf T, Kumar S, et al. Effect of glucocorticoids on von Willebrand factor levels and its correlation with von Willebrand factor gene promoter polymorphism. Blood Coagul Fibrinolysis (2012) 23:514–9. doi: 10.1097/MBC.0b013e3283548dfc
37. Swiatkowska-Stodulska R, Kaniuka-Jakubowska S, Wiśniewski P, Skibowska-Bielinska A, Babinska A, Sowinska-Przepiera E, et al. Homocysteine and alpha-1 antitrypsin concentration in patients with subclinical hypercortisolemia. Adv Med Sci. (2012) 57:302–7. doi: 10.2478/v10039-012-0032-8
38. Lambert JK, Goldberg L, Fayngold S, Kostadinov J, Post KD, Geer EB. Predictors of mortality and long-term outcomes in treated Cushing's disease: a study of 346 patients. J Clin Endocrinol Metab. (2013) 98:1022–30. doi: 10.1210/jc.2012-2893
39. Coelho MCA, Vieira Neto L, Kasuki L, Wildemberg LE, Santos CV, Castro G, et al. Rotation thromboelastometry and the hypercoagulable state in Cushing's syndrome. Clin Endocrinol (2014) 81:657–64. doi: 10.1111/cen.12491
40. Koutroumpi S, Spiezia L, Albiger N, Barbot M, Bon M, Maggiolo S, et al. Thrombin generation in Cushing's Syndrome: do the conventional clotting indices tell the whole truth? Pituitary (2014) 17:68–75. doi: 10.1007/s11102-013-0467-3
41. Terzolo M, Allasino B, Pia A, Peraga G, Daffara F, Laino F, et al. Surgical remission of Cushing's syndrome reduces cardiovascular risk. Eur J Endocrinol. (2014) 171:127–36. doi: 10.1530/EJE-13-0555
42. Barbot M, Daidone V, Zilio M, Albiger N, Mazzai L, Sartori MT, et al. Perioperative thromboprophylaxis in Cushing's disease: what we did and what we are doing? Pituitary (2015) 18:487–93. doi: 10.1007/s11102-014-0600-y
43. Smith TR, Hulou MM, Huang KT, Nery B, de Moura SM, Cote DJ, et al. Complications after transsphenoidal surgery for patients with Cushing's disease and silent corticotroph adenomas. Neurosurg Focus (2015) 38:E12. doi: 10.3171/2014.10.FOCUS14705
44. Swiatkowska-Stodulska R, Mital A, Wiśniewski P, Babinska A, Skibowska-Bielinska A, Sworczak K. Assessment of platelet function in endogenous hypercortisolism. Endokrynol Pol. (2015) 66:207–13. doi: 10.5603/EP.2015.0014
45. Swiatkowska-Stodulska R, Skibowska-Bielinska A, Wiśniewski P, Sworczak K. Activity of selected coagulation factors in overt and subclinical hypercortisolism. Endocr J. (2015) 62:687–94. doi: 10.1507/endocrj.EJ14-0539
46. Turrentine FE, Stukenborg GJ, Hanks JB, Smith PW. Elective laparoscopic adrenalectomy outcomes in 1099 ACS NSQIP patients: identifying candidates for early discharge. Am Surg. (2015) 81:507–14.
47. Zilio M, Mazzai L, Sartori MT, Barbot M, Ceccato F, Daidone V, et al. A venous thromboembolism risk assessment model for patients with Cushing's syndrome. Endocrine (2016) 52:322–32. doi: 10.1007/s12020-015-0665-z
48. Tripodi A, Ammollo CT, Semeraro F, Colucci M, Malchiodi E, Verrua E, et al. Hypercoagulability in patients with Cushing disease detected by thrombin generation assay is associated with increased levels of neutrophil extracellular trap-related factors. Endocrine (2017) 56:298–307. doi: 10.1007/s12020-016-1027-1
49. Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol. (2015) 12:464–74. doi: 10.1038/nrcardio.2015.83
50. Bjørnarå BT, Gudmundsen TE, Dahl OE. Frequency and timing of clinical venous thromboembolism after major joint surgery. J Bone Joint Surg Br. (2006) 88:386–91. doi: 10.1302/0301-620X.88B3.17207
51. Sevitt S, Gallagher NG. Prevention of venous thrombosis and pulmonary embolism in injured patients. A trial of anticoagulant prophylaxis with phenindione in middle-aged and elderly patients with fractured necks of femur. Lancet (1959) 2:981–9. doi: 10.1016/S0140-6736(59)91464-3
52. Guarnotta V, Amato MC, Pivonello R, Arnaldi G, Ciresi A, Trementino L, et al. The degree of urinary hypercortisolism is not correlated with the severity of cushing's syndrome. Endocrine (2017) 55:564–72. doi: 10.1007/s12020-016-0914-9
53. Patil CG, Lad SP, Harsh GR, Laws ER Jr, Boakye M. National trends, complications, and outcomes following transsphenoidal surgery for Cushing's disease from 1993 to 2002. Neurosurg Focus (2007) 23:E7.
54. Zilio M, Barbot M, Ceccato F, Camozzi V, Bilora F, Casonato A, et al. Diagnosis and complications of Cushing's disease: gender-related differences. Clin Endocrinol. (2014) 80:403–10. doi: 10.1111/cen.12299
55. Manetti L, Bogazzi F, Giovannetti C, Raffaelli V, Genovesi M, Pellegrini G, et al. Changes in coagulation indexes and occurrence of venous thromboembolism in patients with Cushing's syndrome: results from a prospective study before and after surgery. Eur J Endocrinol. (2010) 163:783–91. doi: 10.1530/EJE-10-0583
56. Rees DA, Hanna FW, Davies JS, Mills RG, Vafidis J, Scanlon MF. Long-term follow-up results of transsphenoidal surgery for Cushing's disease in a single centre using strict criteria for remission. Clin Endocrinol. (2002) 56:541–51. doi: 10.1046/j.1365-2265.2002.01511.x
57. van Heerden JA, Young WF Jr, Grant CS, Carpenter PC. Adrenal surgery for hypercortisolism–surgical aspects. Surgery (1995) 117:466–72.
58. Birdwell L, Lodish M, Tirosh A, Chittiboina P, Keil M, Lyssikatos C, et al. Coagulation profile dynamics in pediatric patients with Cushing syndrome: a prospective, observational comparative study. J Pediatr. (2016) 177:227–31. doi: 10.1016/j.jpeds.2016.06.087
59. Colao A, Pivonello R, Spiezia S, Faggiano A, Ferone D, Filippella M, et al. Persistence of increased cardiovascular risk in patients with Cushing's disease after five years of successful cure. J Clin Endocrinol Metab. (1999) 84:2664–72.
60. Erem C, Nuhoglu I, Yilmaz M, Kocak M, Demirel A, Ucuncu O, et al. Blood coagulation and fibrinolysis in patients with Cushing's syndrome: increased plasminogen activator inhibitor-1, decreased tissue factor pathway inhibitor, and unchanged thrombin-activatable fibrinolysis inhibitor levels. J Endocrinol Invest. (2009) 32:169–74. doi: 10.1007/BF03345709
61. Kastelan D, Dusek T, Kraljevic I, Aganovic I. Hypercoagulable state in Cushing's syndrome is reversible following remission. Clin Endocrinol. (2013) 78:102–6. doi: 10.1111/j.1365-2265.2012.04479.x
62. Giraldi PF, Ambrogio AG, Fatti LM, Rubini V, Cozzi G, Scacchi M, et al. Von Willebrand factor and fibrinolytic parameters during the desmopressin test in patients with Cushing's disease. Br J Clin Pharmacol. (2011) 71:132–6. doi: 10.1111/j.1365-2125.2010.03812.x
63. Prazny M, Jezkova J, Horova E, Lazarova V, Hana V, Kvasnicka J, et al. Impaired microvascular reactivity and endothelial function in patients with Cushing's syndrome: influence of arterial hypertension. Physiol Res. (2008) 57:13–22.
64. Swiatkowska-Stodulska R, Kaniuka-Jakubowska S, Wisniewski P, Skibowska-Bielinska A, Sworczak K. The estimation of selected endogenous anticoagulation system parameters in patients with subclinical Cushing's syndrome. Eur J Endocrinol. (2011) 165:865–71. doi: 10.1530/EJE-11-0535
65. Van Zaane B, Nur E, Squizzato A, Dekkers OM, Twickler MB, Fliers E, et al. Hypercoagulable state in Cushing's syndrome: a systematic review. J Clin Endocrinol Metab. (2009) 94:2743–50. doi: 10.1210/jc.2009-0290
66. Næss IA, Christiansen SC, Romundstad P, Cannegieter SC, Rosendaal FR, Hammerstrøm J. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost. (2007) 5:692–9. doi: 10.1111/j.1538-7836.2007.02450.x
67. Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest (2012) 141:e419S−96S. doi: 10.1378/chest.11-2301
68. Caprini JA, Arcelus JI, Hasty JH, Tamhane AC, Fabrega F. Clinical assessment of venous thromboembolic risk in surgical patients. Semin Thromb Hemost. (1991) 17(Suppl. 3):304–12.
69. Wells PS, Anderson DR, Rodger M, Stiell I, Dreyer JF, Barnes D, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. (2001) 135:98–107. doi: 10.7326/0003-4819-135-2-200107170-00010
70. Wells PS, Anderson DR, Rodger M, Forgie M, Kearon C, Dreyer J, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. (2003) 349:1227–35. doi: 10.1056/NEJMoa023153
71. Barbar S, Noventa F, Rossetto V, Ferrari A, Brandolin B, Perlati M, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. (2010) 8:2450–7. doi: 10.1111/j.1538-7836.2010.04044.x
72. Adeboyeje G, Sylwestrzak G, Barron JJ, White J, Rosenberg A, Abarca J, et al. Major bleeding risk during anticoagulation with warfarin, dabigatran, apixaban, or rivaroxaban in patients with nonvalvular atrial fibrillation. J Manag Care Spec Pharm. (2017) 23:968–78. doi: 10.18553/jmcp.2017.23.9.968
73. Dahlbäck B. Blood coagulation and its regulation by anticoagulant pathways: genetic pathogenesis of bleeding and thrombotic diseases. J Intern Med. (2005) 257:209–23. doi: 10.1111/j.1365-2796.2004.01444.x
74. Jenkins PV, Rawley O, Smith OP, O'Donnell JS. Elevated factor VIII levels and risk of venous thrombosis. Br J Haematol. (2012) 157:653–63. doi: 10.1111/j.1365-2141.2012.09134.x
75. Greenhill C. Pituitary disease: inflammation in patients with Cushing disease. Nat Rev Endocrinol. (2016) 12:687. doi: 10.1038/nrendo.2016.170
76. Karamouzis I, Berardelli R, D'Angelo V, Fussotto B, Zichi C, Giordano R, et al. Enhanced oxidative stress and platelet activation in patients with Cushing's syndrome. Clin Endocrinol. (2015) 82:517–24. doi: 10.1111/cen.12524
77. Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet (2014) 383:2152–67. doi: 10.1016/S0140-6736(13)61684-0
78. Fleseriu M, Biller BMK, Swearingen AGB, Melmed S. P7 Hypercoagulability in Cushing's disease: a risk awareness and prophylaxis survey on behalf of the Pituitary Society (2017). p. 35. Available online at: https://www.pituitarysociety.org/sites/all/pdfs/15_Pituitary_Congress_program.pdf
79. Pace A, Dirven L, Koekkoek JAF, Golla H, Fleming J, Rudà R, et al. European Association for Neuro-Oncology (EANO) guidelines for palliative care in adults with glioma. Lancet Oncol. (2017) 18:e330–40. doi: 10.1016/S1470-2045(17)30345-5
80. van der Pas R, de Bruin C, Leebeek FWG, de Maat MPM, Rijken DC, Pereira AM, et al. The hypercoagulable state in Cushing's disease is associated with increased levels of procoagulant factors and impaired fibrinolysis, but is not reversible after short-term biochemical remission induced by medical therapy. J Clin Endocrinol Metab. (2012) 97:1303–10. doi: 10.1210/jc.2011-2753
Keywords: venous thromboembolism, Cushing disease, Cushing syndrome, hypercoagulability, anticoagulation
Citation: Wagner J, Langlois F, Lim DST, McCartney S and Fleseriu M (2019) Hypercoagulability and Risk of Venous Thromboembolic Events in Endogenous Cushing's Syndrome: A Systematic Meta-Analysis. Front. Endocrinol. 9:805. doi: 10.3389/fendo.2018.00805
Received: 20 September 2018; Accepted: 21 December 2018;
Published: 28 January 2019.
Edited by:
Nienke Biermasz, Leiden University, NetherlandsReviewed by:
Leila Warszawski, Instituto Estadual de Diabetes e Endocrinologia Luiz Capriglione, BrazilLucio Vilar, Federal University of Pernambuco, Brazil
Copyright © 2019 Wagner, Langlois, Lim, McCartney and Fleseriu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Fleseriu, fleseriu@ohsu.edu
†These authors have contributed equally to this work
 Jeffrey Wagner
Jeffrey Wagner Fabienne Langlois
Fabienne Langlois Dawn Shao Ting Lim
Dawn Shao Ting Lim Shirley McCartney
Shirley McCartney Maria Fleseriu
Maria Fleseriu