- 1Department of Endocrinology, Key Laboratory of Endocrinology, Ministry of Health, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, China
- 2Department of Nutrition, Peking Union Medical College Hospital, Beijing, China
- 3Department of Endocrinology, Fuxing Hospital, the Eighth Clinical Medical College, Capital Medical University, Beijing, China
- 4State Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 5Diabetes Research Center of Chinese Academy of Medical Sciences, Beijing, China
Background: The data on the relationship between normal-ranged serum uric acid (SUA), β-cell function, and non-alcoholic fatty liver disease (NAFLD) are complicated and insufficient. Moreover, uric acid is excreted by kidney, and SUA levels may be affected by renal function. Thus, we introduced a renal function-normalized index [serum uric acid to creatinine ratio (SUA/Cr)] into the study and explored the association between SUA/Cr, C‐peptide and NAFLD in a Chinese population with normal SUA levels by a cross-sectional analysis.
Materials and Methods: A total of 282 individuals with normal SUA levels and different glucose tolerance status from a diabetes project were included in the study (mean age = 53.7± 10.5 years; women = 64.50%). NAFLD was diagnosed by abdominal ultrasonography (NAFLD, n=86; without NAFLD, n=196). Trapezoid formula was used to calculate area under the curve of C‐peptide (AUCCP) from 4 points (including 0, 30,60, and 120min) during 2-h oral glucose tolerance test. Spearman correlation analysis was used to explore the correlation between SUA/Cr, AUCCP and NAFLD risk factors. Multiple logistic regression analysis was used to explore the association between SUA/Cr or AUCCP and NAFLD. Mediation analysis was used to explore whether AUCCP mediated the association between SUA/Cr and NAFLD.
Results: Individuals with NAFLD had significantly higher SUA/Cr and AUCCP than those without NAFLD(P<0.05). Spearman correlation analysis showed that both SUA/Cr and AUCCP were significantly associated with many NAFLD risk factors, and SUA/Cr was positively correlated with AUCCP (P<0.05). Multiple logistic regression analysis indicated that SUA/Cr and AUCCP were positively associated with NAFLD incidence (P<0.05). Medication analysis indicated that SUA/Cr had a significant direct effect on NAFLD (β =0.5854, 95% CI: 0.3232–0.8966), and AUCCP partly mediated the indirect effect of SUA/Cr on NAFLD incidence (β =0.1311, 95% CI: 0.0168–0.4663).
Conclusions: SUA/Cr was positively associated with NAFLD incidence, and AUCCP partly mediated the association in a Chinese population with normal SUA levels. Thus, we should pay more attention to high-normal SUA and C-peptide levels due to their predictive power in NAFLD incidence.
Introduction
Non-alcoholic fatty liver disease (NAFLD), characterized by lipid accumulation in liver with no significant alcohol intake, is one of the most common chronic liver diseases in the world. NAFLD can develop into cirrhosis, and even hepatocellular carcinoma and liver failure (1).NAFLD is also closely associated with cardiovascular disease (2), diabetes (3), and obesity (4).Moreover, patients with NAFLD exhibited relative high mortality compared to the general population (5). Hence, finding risk factors and the mechanism of NAFLD is warranted to prevent it.
Serum uric acid (SUA), major product of purine metabolism, is independently associated with NAFLD incidence (6, 7). Moreover, the significant association between SUA and NAFLD incidence was also established even in some individuals with normal SUA levels (8, 9). SUA is excreted via kidney, and the clearance of SUA is often affected by renal function, while none of the previous studies (6–9) considered the effects from kidney. SUA to creatinine ratio (SUA/Cr) is an index of renal function-normalized SUA, reflecting endogenous UA levels more precisely than SUA. Moreover, SUA/Cr is associated with β-cell function, metabolic syndrome and incident chronic kidney disease (10–12).However, there has been no study focused on the association between SUA/Cr and NAFLD yet.
In addition to NAFLD, SUA was also associated with islet β-cell function and β-cell secretion in patients with type 2 diabetes (T2DM) and prediabetes (13, 14). Moreover, SUA, within normal range, was related to β-cell function in overweight/obesity or male T2DM patients (15). Although the causal relationship between SUA and β-cell function was not conclusive, one study indicated that elevated SUA was the precursor of T2DM (16).There were also some studies reporting the significant association between β-cell secretion and NAFLD. An American study indicated that fasting C-peptide (FCP) was associated with NAFLD (17). A Chinese study based on obese children also found that FCP is a significant indicator of NAFLD (18). C-peptide and the area under the curve of C‐peptide (AUCCP) could reflect the secretion ability of islet β-cell, while the latter is a better indicator of overall and residual islet β-cell secretion compared to FCP. However, the studies involving the association between AUCCP and NAFLD are limited.
Several clinical studies have indicated that increased SUA, within the normal range, is closely associated with many diseases (19, 20), and high-normal SUA(elevated SUA within normal range) has already hold our attention. The intrinsic relationship between SUA, β-cell function and NAFLD is complicated, and related studies involving normal-ranged SUA are insufficient. Moreover, renal function may be also a potential confounding factor of the association between SUA and NAFLD. Thus, we performed a cross-sectional study based on a Chinese population with normal SUA levels and introduced an index of renal function-normalized SUA into the study, namely, SUA/Cr, and explored the association between SUA/Cr, AUCCP, and NAFLD.
Materials and Methods
Study Population
A total of 599 individuals from a T2DM project were recruited between 2014 and 2015 (21), and 333 of them completed liver ultrasound. Participants with other liver diseases, estimated glomerular filtration rate <60 ml/min/1.73 m2, alcohol consumption >20 g/day, hyperuricemia (SUA ≥ 416 µmol/L for male or SUA ≥ 357 umol/L for female or uric acid-lowering drugs treatment) or missing data were also excluded (n=51). A total of 282 individuals with normal SUA levels and with different glucose tolerance status were included in the study (Figure 1).
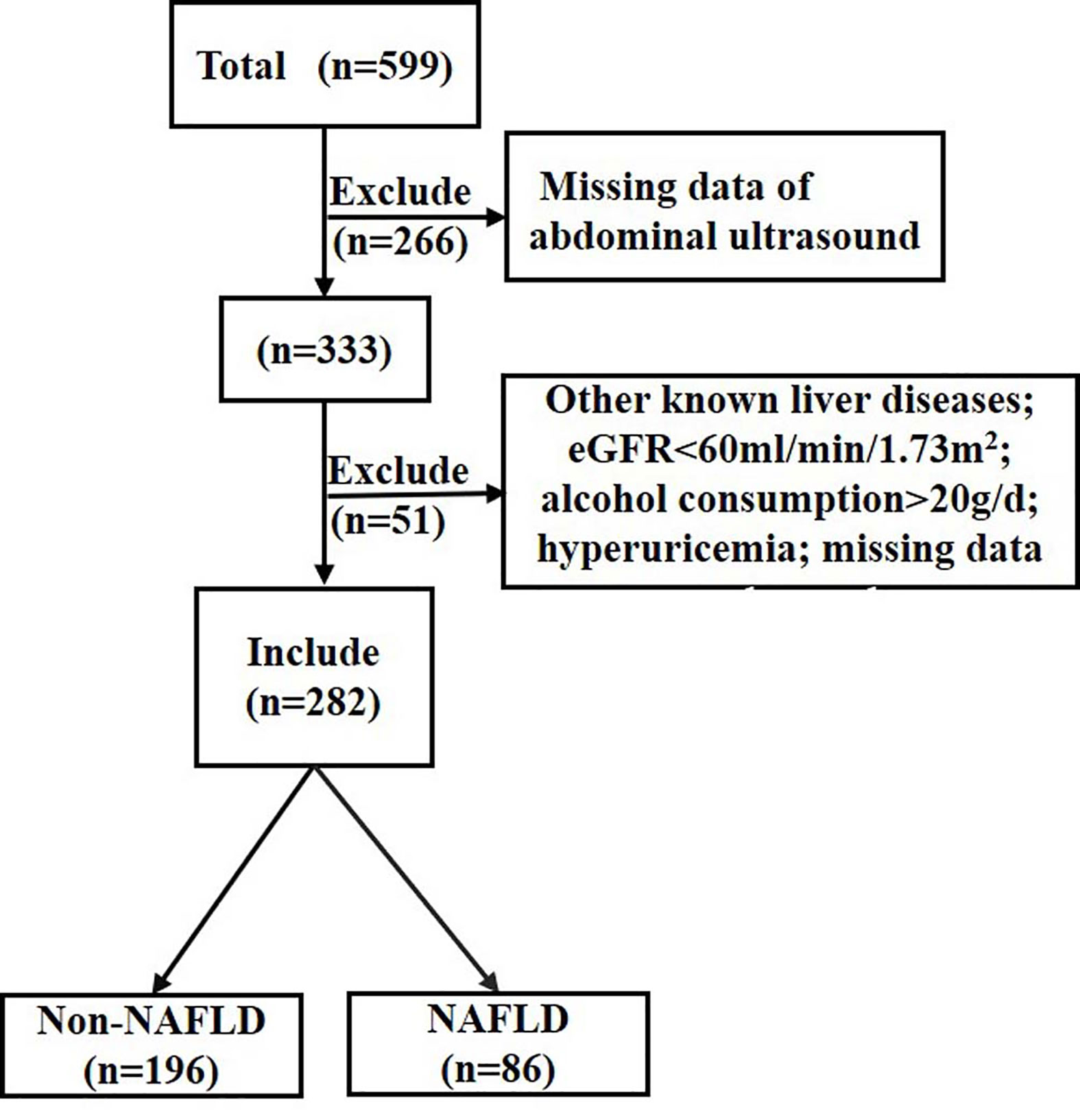
Figure 1 Flow chart of the population inclusion. NAFLD, non-alcoholic fatty liver disease; eGFR, estimated glomerular filtration rate. eGFR was calculated with the chronic kidney disease epidemiology collaboration equation.
Anthropometric and Biochemical Measurements
All participants were asked to complete a questionnaire, including gender, age, and medical history. Blood pressure, waist circumference (WC) and body mass index (BMI) were measured using standard methods. Blood samples for glucose and C-peptide were obtained at 0, 30, 60, and 120 min after a 75-g oral glucose load. Serum C peptide levels were measured by chemiluminescence immunoassay using a Siemens ADIVA Centaur XP analyzer (Siemens Healthcare Diagnostics Inc., Tarrytown, NY, USA), while serum glucose concentrations were assayed using a glucose oxidase assay. Hemoglobin A1c (HbA1c) analysis was analyzed in whole blood using high-performance liquid chromatography. The diagnosis of diabetes and prediabetes were defined based on the1999 World Health Organization criteria after the oral glucose tolerance test (OGTT) (22). Fasting serum alanine transaminase (ALT), aspartate transaminase (AST), SUA, creatinine, and lipids were measured by an automated analyzer. Trapezoid formula was used to calculate AUC for glucose and C-peptide. Insulin resistance and insulin sensitivity was estimated using homeostasis model assessment of insulin resistance (HOMA-IR) (23).
Liver Ultrasonography Evaluation
NAFLD was diagnosed by abdominal ultrasonography. The ultrasound results were assessed by physicians who were blind to the subjects’ biochemical results. Except for individuals with significant alcohol consumption, subjects were diagnosed with NAFLD by ultrasonography if at least two of the following three ultrasonic characteristics were positive: bright liver, liver echo greater than kidney, vascular blurring, and deep attenuation of ultrasound signal (24).
Statistical Analysis
Normally distributed continuous variables were recorded as mean ± standard deviation. Non-normal distribution parameters were transformed or presented as the median (25th–75th percentile). Categorical data were presented as number and percentages (n, %). Differences between groups were compared by Student’s t test or Chi-squared test or Mann–Whitney’s U-test. Spearman correlation analysis was used to explore the association between SUA/Cr or AUCCP and potential NAFLD risk factors. Multiple logistic regression analysis was used to evaluate the association between SUA/Cr or AUCCP and NAFLD. Mediation models were established to explore whether AUCCP mediated the association between SUA/Cr and NAFLD.
Analyses were conducted using the SPSS (version 22.0). P < 0.05 (2-tailed) was considered statistically significant.
Results
Clinical Characteristics of Individuals in NAFLD and Non-NAFLD Groups
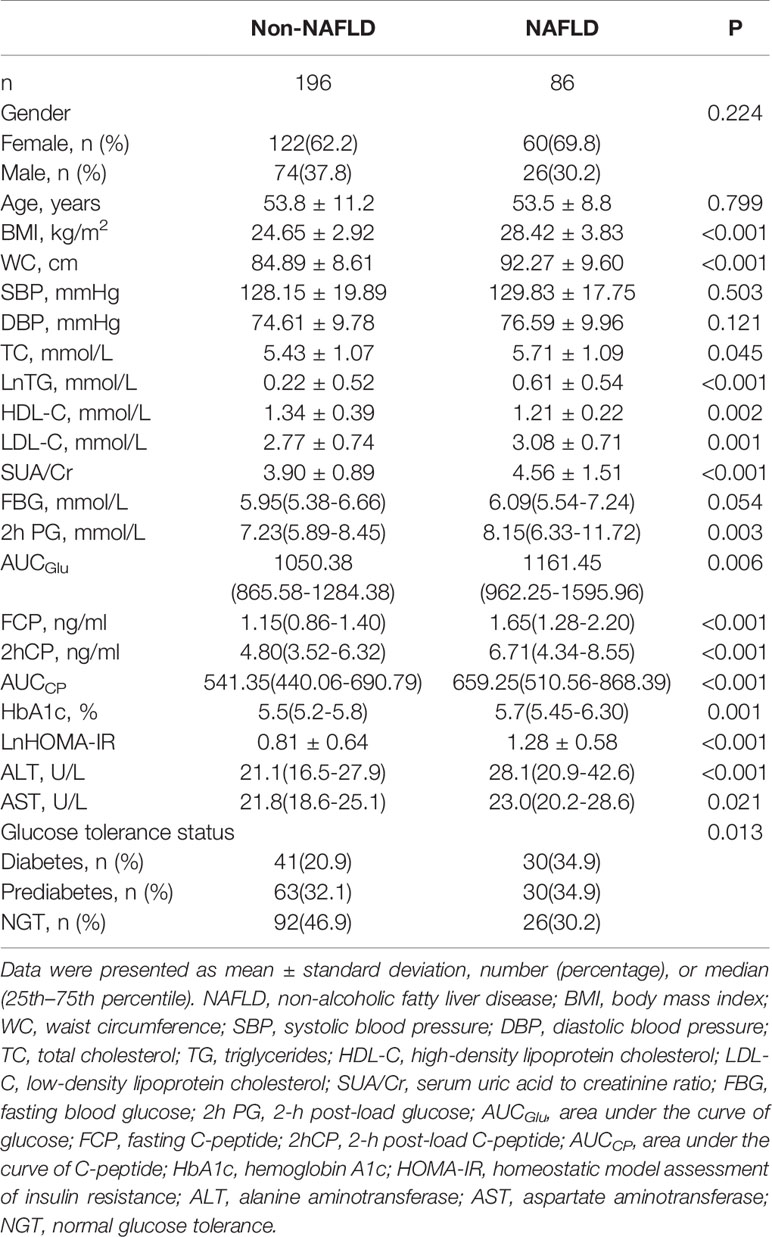
According to the liver ultrasonography, individuals were divided into a NAFLD group (n=86) and a non-NAFLD group (n=196). As recorded in Table 1, individuals with NAFLD had higher BMI, WC, total cholesterol (TC), triglycerides (TG), low- density lipoprotein cholesterol (LDL-C), HbA1c, 2-h post-load glucose(2hPG), area under the curve of glucose (AUCGlu), FCP, 2-h post-load C-peptide (2hCP), AUCCP, HOMA-IR, ALT, AST, and SUA/Cr but lower high-density lipoprotein cholesterol (HDL-C) compared to those without NAFLD(P<0.05). In addition, individuals with NAFLD were more likely to have higher prevalence of diabetes than those without NAFLD (Table 1).
Spearman Correlation of SUA/Cr or AUCCP With Potential NAFLD Risk Factors
As shown in Table 2, Spearman correlation analysis indicated that SUA/Cr was positively correlated with BMI (r=0.208, P<0.001),WC (r=0.217, P<0.001), TG (r=0.285, P<0.001), LDL-C (r=0.151, P=0.011), HbA1c(r=0.120, P=0.045), HOMA-IR(r=0.198, P=0.001), ALT(r=0.190, P=0.001), AST(r=0.183, P=0.002) and AUCGlu (r=0.124, P=0.037) but negatively correlated with HDL-C (r=-0.176, P=0.003). Similarly, AUCCP was positively correlated with BMI (r=0.256, P<0.001),WC (r=0.197, P=0.001), TG (r=0.295, P<0.001), LDL-C (r=0.137, P=0.022), HOMA-IR(r=0.455, P<0.001), ALT(r=0.159, P=0.007), and AST(r=0.117, P=0.049) but negatively correlated with HDL-C(r=-0.251, P<0.001).There were also significant correlations between SUA/Cr and C-peptide related markers [FCP(r=0.246, P<0.001), 2hCP(r=0.190, P=0.001), AUCCP (r=0.208, P<0.001)].
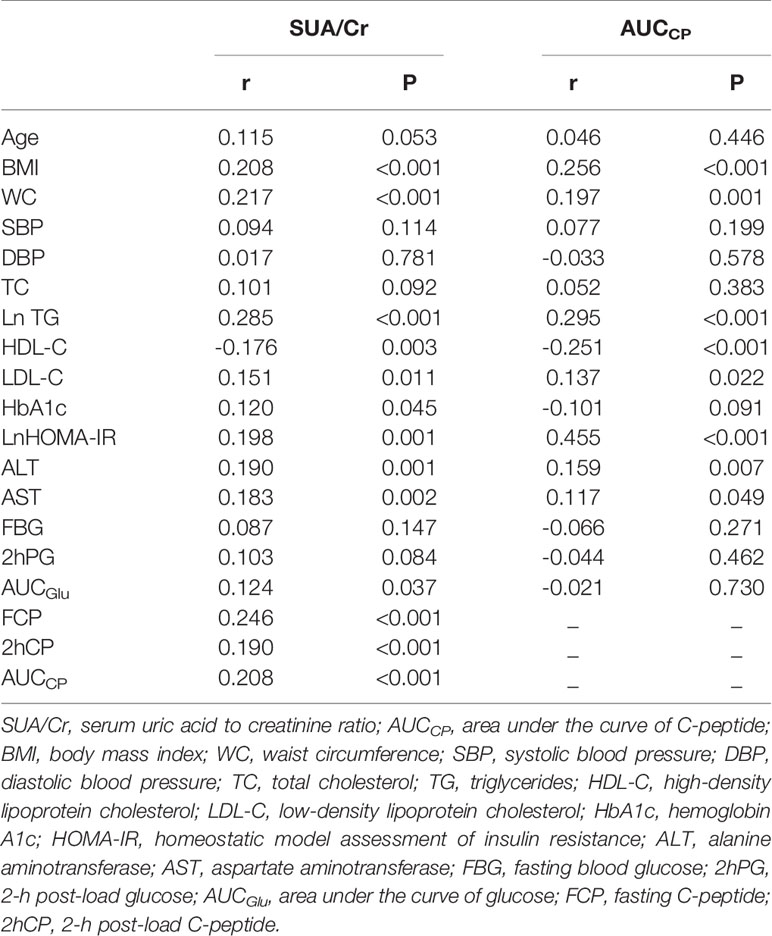
Table 2 Spearman’s correlation of SUA/Cr or AUCCP with potential risk factors of non-alcoholic fatty liver disease.
Association Between SUA/Cr and the Prevalence of NAFLD
As shown in Table 3, multiple logistic analysis indicated SUA/Cr was positively associated with the prevalence of NAFLD [Odds ratio (OR): 2.288, 95% confidence intervals (CI): 1.592–3.288, P<0.001] after adjustment for age and gender (Model 1). After further adjustment for BMI, WC, systolic blood pressure (SBP), TG, HDL-C, LDL-C, HOMA-IR, and glucose tolerance status, the association between SUA/Cr and NAFLD incidence remained significant (OR: 1.529, 95% CI: 1.011-2.310, P=0.044, Model 2). In addition, SUA/Cr still showed a significant association with NAFLD incidence after additional adjustment for ALT and AST (OR: 1.548, 95% CI: 1.018–2.352, P=0.041, Model 3).
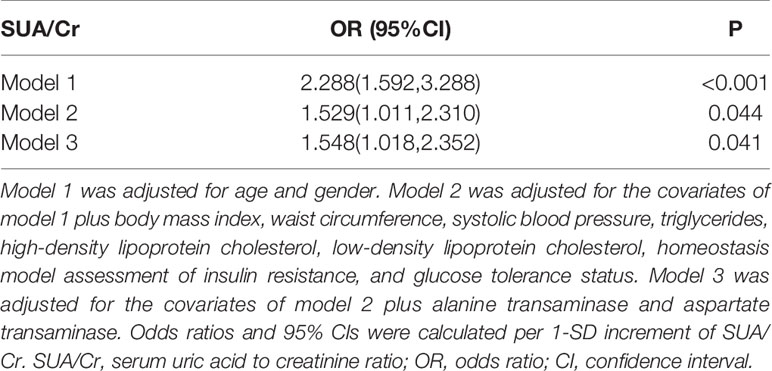
Table 3 Logistic regression analysis for association of SUA/Cr with non-alcoholic fatty liver disease.
Association Between AUCCP and the Prevalence of NAFLD
As shown in Table 4, multiple logistic analysis indicated a positive association between AUCCP and the prevalence of NAFLD (OR: 5.649 95% CI: 2.666-11.971, P<0.001) after adjustment for age and gender (Model 1). After further adjustment for BMI, WC, SBP, TG, HDL-C, LDL-C, HOMA-IR, and glucose tolerance status, the association between AUCCP and NAFLD incidence remained significant (OR: 3.074, 95% CI: 1.166–8.105, P=0.023, Model 2). SUA/Cr still showed a significant association with NAFLD after additional adjustment for ALT and AST (OR: 2.763, 95% CI: 1.012–7.544, P=0.047, Model 3).
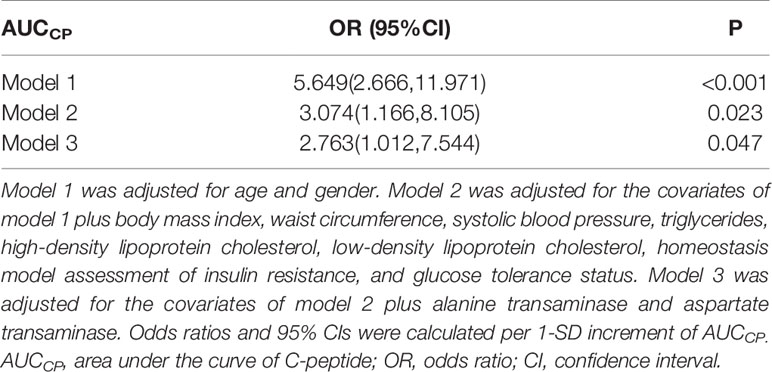
Table 4 Logistic regression analysis for association of AUCCP with non-alcoholic fatty liver disease.
Mediated Effect of AUCCP on the Association Between SUA/Cr and NAFLD
Both SUA/Cr and AUCCP were positively associated with NAFLD incidence, while SUA/Cr was positively correlated with AUCCP, suggesting a mechanistic link between SUA/Cr and NAFLD, possibly explained by AUCCP. To explore the internal relationships between AUCCP, SUA/Cr and NAFLD, we conducted mediation analysis to explore whether AUCCP mediated the association between SUA/Cr and NAFLD incidence.
As shown in Figure 2, mediation analysis indicated that SUA/Cr had a significant direct effect on NAFLD incidence (β =0.5854, 95% CI: 0.3232–0.8966), and AUCCP partly mediated the indirect effect of SUA/Cr on NAFLD incidence (β =0.1311, 95% CI: 0.0168–0.4663).
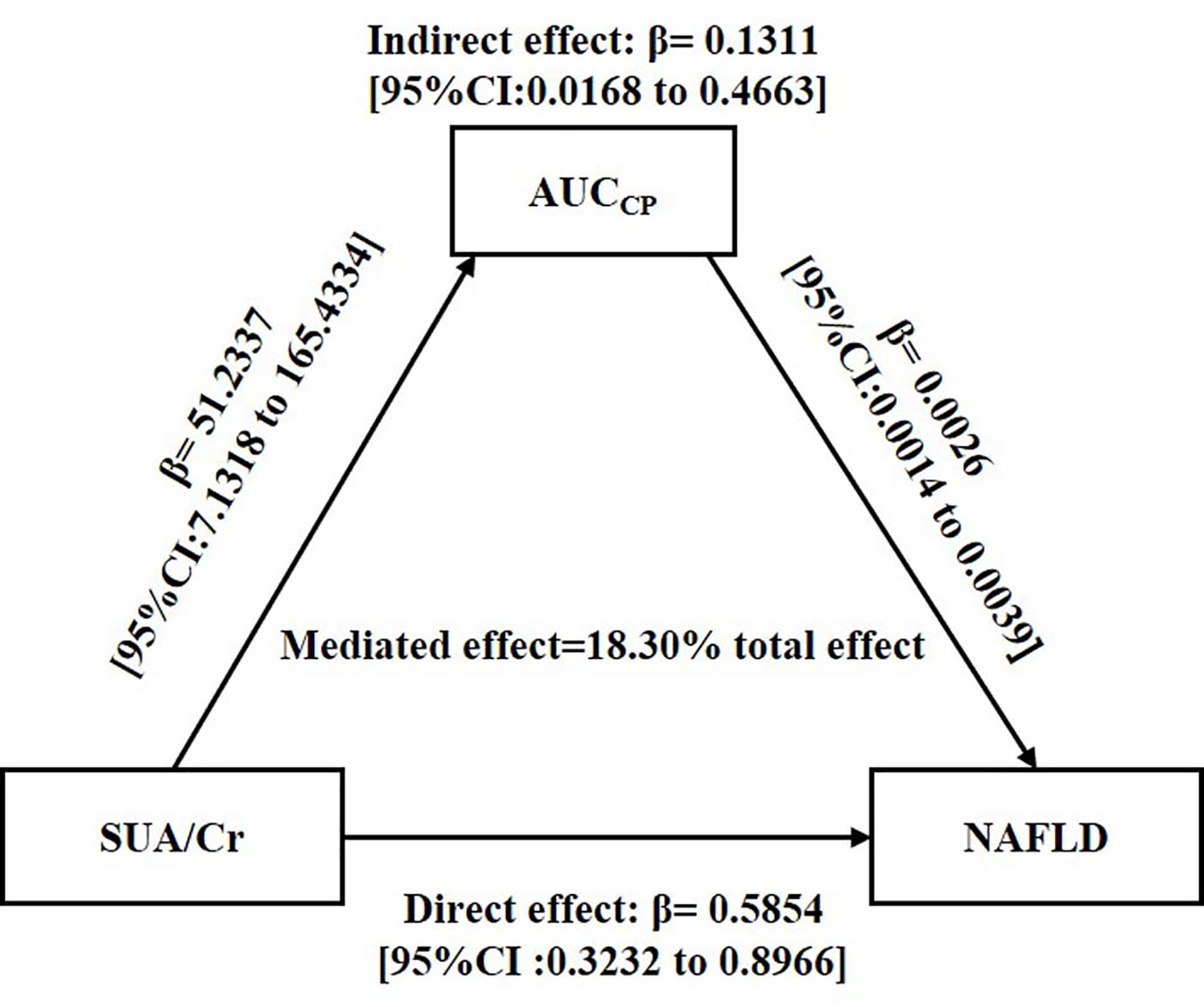
Figure 2 Mediation of AUCCP on the association between SUA/Cr and NAFLD. Zero was not included in 95% confidence intervals representing statistical significance. SUA/Cr, serum uric acid to creatinine ratio; AUCCP, area under the curve of C-peptide; NAFLD, non-alcoholic fatty liver disease.
Discussion
This study, based on a Chinese population with normal SUA levels, indicated that individuals with NAFLD had higher SUA/Cr and AUCCP than those without NAFLD. Both SUA/Cr and AUCCP were significantly correlated with many conventional risk factors of NAFLD, and the correlation between SUA/Cr and AUCCP was positive. In addition, SUA/Cr was positively associated with NAFLD incidence, and AUCCP partly mediated the indirect effect of SUA/Cr on NAFLD incidence in a population with normal SUA levels.
The association between SUA and NAFLD has been explored for a long time. A Chinese study contained 21,798 subjects revealed that SUA was significantly associated with NAFLD incidence (25). A prospective observational study demonstrated that high SUA independently predicted 3-year’s incidence of NAFLD (26). Moreover, even in individuals with normal SUA levels, increased SUA was independently associated with NAFLD (8, 9). UA is the end product of human purine metabolism and is excreted by the kidney. SUA level will increase due to its impaired clearance in individuals with impaired renal function (27).However, many studies ignored the effect of kidney on SUA, while SUA/Cr is a renal function-normalized index and may be a more precise indicator than SUA. A Chinese study based on 713 diabetics revealed that SUA/Cr was significantly associated with β-cell function (11). Another study suggested that SUA/Cr in T2DM patients was closely linked to metabolic syndrome and its components (10). Furthermore, a longitudinal study indicated that SUA/Cr had stronger associations with chronic kidney disease than SUA alone (12). Similarly, our present study firstly demonstrated that SUA/Cr was an independent risk factor of NAFLD in individuals with normal SUA levels, and mediation analysis indicated SUA/Cr had direct effect on NAFLD. Although the detailed mechanism of NAFLD remains uncertain, many studies have indicated that there is a close association between SUA and NAFLD. SUA may function as a pro-oxidant and react with oxidants, inducing the production of free radicals and oxidative stress (28), which are critical factors in the development of NAFLD (29).Thus, SUA may have direct effect on NAFLD as a pro-oxidant.
We also found the effect of SUA/Cr on NAFLD partly via AUCCP. Several studies have indicated that FCP was independently associated with NAFLD (17, 18),while we used a more accurate index (AUCCP), which could reflect overall β-cell secretion. Equimolar amount of C-peptide is produced when insulin is secreted. However, insulin, not C-peptide, is partly cleared in liver with first-pass hepatic extraction (30). Thus, serum C-peptide was a well-established marker of the endogenous insulin secretion. We found that individuals with NAFLD had higher AUCCP and HOMA-IR, and AUCCP was significantly correlated with HOMA-IR. We also found AUCCP partly mediated the association between SUA/Cr and NAFLD. Although the causal relationship between UA and insulin resistance was not conclusive, elevated SUA may aggravate insulin resistance to some extent. A clinical study indicated that elevated SUA was the precursor of T2DM (16). SUA could induce endothelial dysfunction and inhibit nitric oxide bioavailability, which is involved in insulin resistance (31). Thus, higher AUCCP may represent higher endogenous insulin secretion and may be a compensatory of insulin resistance due to higher SUA, namely, higher AUCCP is a sign of insulin resistance, which plays important role in the progress of NAFLD (32). In other hand, many metabolic regulators such as follistatin and fibroblast growth factor (FGF21) were regulated by islet hormone (33, 34). Moreover, fold changes of C-peptide during an OGTT were inversely associated with those of FGF21 in individuals with normal glucose tolerance (35), and FGF21 related signal pathways played important roles in the progression of NAFLD (36). In addition, previous studies have indicated that C-peptide may be also a predictive marker of the severity of the cardiovascular disease (37) and mortality (38), which suggested C-peptide was a bioactive peptide with other potential physiological functions. Thus, AUCCP may partly mediate the association between SUA/Cr and NAFLD via insulin resistance and other potential physiological function.
Besides AUCCP, other mechanism may be also involved in the association between SUA and NAFLD. A previous study indicated that SUA, within normal range, was positively associated with inflammation markers (39), which may be the important mediator in the development of NAFLD (40). Basic studies showed that SUA also caused hepatic steatosis and liver fat accumulation via endoplasmic reticulum stress (41) and mitochondrial oxidative stress (42).In addition, SUA may generate from fructose metabolism, which could induce hepatic steatosis (43). Overall, high-normal SUA was positively associated with NAFLD incidence via its direct pro-oxidant effect, C-peptide, and other signal pathways. Thus, high-normal SUA and C-peptide levels are important factors in the pathological process of NAFLD, and we should pay enough attention to these indicators.
Our present study had some advantages. First, present study introduced SUA/Cr as a newer index into the study and revealed that there was a significant association between SUA/Cr and NAFLD incidence, which brought a more accurate predictor of NAFLD. Second, we explored the internal relationship between SUA/Cr, AUCCP, and NAFLD by different statistical methods, which strengthens our understanding of their internal relationship. Third, our present study based on a population with normal SUA levels and found its strong predictive ability for NAFLD, which suggested that high-normal SUA should cause our attention. Our resent study also had some limitations. First, liver biopsy has been established as the gold diagnosis standard of NAFLD, while NAFLD was determined by ultrasonography with no histologic confirmation in our present study. Nevertheless, ultrasonography is the widely-used methodology to detect NAFLD because of safety, availability, and economy. Second, the nature of the cross-sectional study and the relatively small sample size were also the limitations of the present study, and therefore larger scale and longitude studies are warranted in the future.
Conclusions
SUA/Cr was positively associated with NAFLD incidence, and AUCCP partly mediated the association between SUA/Cr and NAFLD incidence in a Chinese population with normal SUA levels. This finding indicates that we should pay more attention to high-normal SUA and C-peptide levels due to their predictive power in NAFLD incidence.
Data Availability Statement
All datasets presented in this study are included in the article/supplementary material.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Peking Union Medical College Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CFM conducted the research, performed the statistical analysis, and wrote the first draft of the manuscript. YiL, SH, JZ, PL, CXM, FP, HZ, LX, and WL contributed to the discussion, conducted the research, and collected the data. YuL designed the study and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This project was supported by the CAMS Innovation Fund for Medical Sciences (CIFMS) (CIFMS2016-I2M-4-001) and the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (No. 2017PT32020, No. 2018PT32001, and No. 2019PT320007).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgment
We thank all participants in this study.
Abbreviations
ALT, alanine transaminase; AST, aspartate transaminase; AUCCP, area under the curve of C‐peptide; AUCGlu, area under the curve of glucose; BMI, body mass index; CI, confidence interval; FCP, fasting C-peptide; 2hCP, 2-h post-load C-peptide; FGF21, fibroblast growth factor 21; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance; 2hPG, 2-h post-load glucose; LDL-C, low-density lipoprotein cholesterol, NAFLD, non-alcoholic fatty liver disease; OR, odds ratio; SUA, serum uric acid; SUA/Cr, serum uric acid to creatinine ratio; TC, total cholesterol; T2DM, type 2 diabetes; TG, triglycerides; WC, waist circumference.
References
1. Serfaty L, Lemoine M. Definition and natural history of metabolic steatosis: clinical aspects of NAFLD, NASH and cirrhosis. Diabetes Metab (2008) 34(6 Pt 2):634–7. doi: 10.1016/S1262-3636(08)74597-X
2. Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med (2010) 363(14):1341–50. doi: 10.1056/NEJMra0912063
3. Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol (2017) 14(1):32–42. doi: 10.1038/nrgastro.2016.147
4. Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol (2017) 67(4):862–73. doi: 10.1016/j.jhep.2017.06.003
5. Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology (2015) 61(5):1547–54. doi: 10.1002/hep.27368
6. Li Y, Xu C, Yu C, Xu L, Miao M. Association of serum uric acid level with non-alcoholic fatty liver disease: a cross-sectional study. J Hepatol (2009) 50(5):1029–34. doi: 10.1016/j.jhep.2008.11.021
7. Lee JW, Cho YK, Ryan M, Kim H, Lee SW, Chang E, et al. Serum uric Acid as a predictor for the development of nonalcoholic Fatty liver disease in apparently healthy subjects: a 5-year retrospective cohort study. Gut Liver (2010) 4(3):378–83. doi: 10.5009/gnl.2010.4.3.378
8. Hwang IC, Suh SY, Suh AR, Ahn HY. The relationship between normal serum uric acid and nonalcoholic fatty liver disease. J Korean Med Sci (2011) 26(3):386–91. doi: 10.3346/jkms.2011.26.3.386
9. Moon SS. Relationship between serum uric acid level and nonalcoholic fatty liver disease in pre- and postmenopausal women. Ann Nutr Metab (2013) 62(2):158–63. doi: 10.1159/000346202
10. Al-Daghri NM, Al-Attas OS, Wani K, Sabico S, Alokail MS. Serum Uric Acid to Creatinine Ratio and Risk of Metabolic Syndrome in Saudi Type 2 Diabetic Patients. Sci Rep (2017) 7(1):12104. doi: 10.1038/s41598-017-12085-0
11. Li M, Gu L, Yang J, Lou Q. Serum uric acid to creatinine ratio correlates with beta-cell function in type 2 diabetes. Diabetes Metab Res Rev (2018) 34(5):e3001. doi: 10.1002/dmrr.3001
12. Gu L, Huang L, Wu H, Lou Q, Bian R. Serum uric acid to creatinine ratio: A predictor of incident chronic kidney disease in type 2 diabetes mellitus patients with preserved kidney function. Diabetes Vasc Dis Res (2017) 14(3):221–5. doi: 10.1177/1479164116680318
13. Tang W, Fu Q, Zhang Q, Sun M, Gao Y, Liu X, et al. The association between serum uric acid and residual beta -cell function in type 2 diabetes. J Diabetes Res (2014) 2014:709691. doi: 10.1155/2014/709691
14. Wu Y, He H, Yu K, Zhang M, An Z, Huang H. The Association between Serum Uric Acid Levels and Insulin Resistance and Secretion in Prediabetes Mellitus: A Cross-Sectional Study. Ann Clin Lab Sci (2019) 49(2):218–23.
15. Zhong X, Zhang D, Yang L, Du Y, Pan T. The relationship between serum uric acid within the normal range and beta-cell function in Chinese patients with type 2 diabetes: differences by body mass index and gender. PeerJ (2019) 7:e6666. doi: 10.7717/peerj.6666
16. Juraschek SP, McAdams-Demarco M, Miller ER, Gelber AC, Maynard JW, Pankow JS, et al. Temporal relationship between uric acid concentration and risk of diabetes in a community-based study population. Am J Epidemiol (2014) 179(6):684–91. doi: 10.1093/aje/kwt320
17. Atsawarungruangkit A, Chenbhanich J, Dickstein G. C-peptide as a key risk factor for non-alcoholic fatty liver disease in the United States population. World J Gastroenterol (2018) 24(32):3663–70. doi: 10.3748/wjg.v24.i32.3663
18. Han X, Xu P, Zhou J, Liu Y, Xu H. Fasting C-peptide is a significant indicator of nonalcoholic fatty liver disease in obese children. Diabetes Res Clin Pract (2020) 160:108027. doi: 10.1016/j.diabres.2020.108027
19. Ray L, Mohandas K, Shamraj M, Akila B eds. Association of high-normal serum uric acid levels with abnormal lipid profile and high atherogenic index of plasma in apparently healthy South Indian males. Tirupati, India: Ambicon (2014).
20. Jung DH, Lee YJ, Lee HR, Lee JH, Shim JY. Association of renal manifestations with serum uric acid in Korean adults with normal uric acid levels. J Korean Med Sci (2010) 25(12):1766–70. doi: 10.3346/jkms.2010.25.12.1766
21. Zhou MC, Zhu L, Cui X, Feng L, Zhao X, He S, et al. Reduced peripheral blood mtDNA content is associated with impaired glucose-stimulated islet beta cell function in a Chinese population with different degrees of glucose tolerance. Diabetes Metab Res Rev (2016) 32(7):768–74. doi: 10.1002/dmrr.2814
22. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med (1998) 15: (7):539. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S
23. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia (1985) 28(7):412–9. doi: 10.1007/bf00280883
24. Fan JG, Jia JD, Li YM, Wang BY, Lu LG, Shi JP, et al. Guidelines for the diagnosis and management of nonalcoholic fatty liver disease: update 2010. J Dig Dis (2011) 12(1):38–44. doi: 10.1111/j.1751-2980.2010.00476.x. published in Chinese on Chinese Journal of Hepatology 2010; 18:163–166.
25. Liang J, Pei Y, Gong Y, Liu XK, Dou LJ, Zou CY, et al. Serum uric acid and non-alcoholic fatty liver disease in non-hypertensive Chinese adults: the Cardiometabolic Risk in Chinese (CRC) study. Eur Rev Med Pharmacol Sci (2015) 19(2):305–11.
26. Xu C, Yu C, Xu L, Miao M, Li Y. High serum uric acid increases the risk for nonalcoholic Fatty liver disease: a prospective observational study. PloS One (2010) 5(7):e11578. doi: 10.1371/journal.pone.0011578
27. Johnson RJ, Kang DH, Feig D, Kivlighn S, Kanellis J, Watanabe S, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension (2003) 41(6):1183–90. doi: 10.1161/01.HYP.0000069700.62727.C5
28. Sautin YY, Imaram W, Kim KM, Angerhofer A, Henderson G, Johnson R. Uric Acid and Oxidative Stress. In: Miyata T, Eckardt K-U, Nangaku M, editors. Studies on Renal Disorders. Totowa, NJ: Humana Press (2011). p. 143–59.
29. Spahis S, Delvin E, Borys JM, Levy E. Oxidative Stress as a Critical Factor in Nonalcoholic Fatty Liver Disease Pathogenesis. Antioxid Redox Signal (2017) 26(10):519–41. doi: 10.1089/ars.2016.6776
30. Polonsky KS, Rubenstein AH. C-peptide as a measure of the secretion and hepatic extraction of insulin. Pitfalls and limitations. Diabetes (1984) 33(5):486–94. doi: 10.2337/diab.33.5.486
31. Li C, Hsieh MC, Chang SJ. Metabolic syndrome, diabetes, and hyperuricemia. Curr Opin Rheumatol (2013) 25(2):210–6. doi: 10.1097/BOR.0b013e32835d951e
32. Akhtar DH, Iqbal U, Vazquez-Montesino LM, Dennis BB, Ahmed A. Pathogenesis of Insulin Resistance and Atherogenic Dyslipidemia in Nonalcoholic Fatty Liver Disease. J Clin Transl Hepatol (2019) 7(4):362–70. doi: 10.14218/JCTH.2019.00028
33. Hansen JS, Rutti S, Arous C, Clemmesen JO, Secher NH, Drescher A, et al. Circulating Follistatin Is Liver-Derived and Regulated by the Glucagon-to-Insulin Ratio. J Clin Endocrinol Metab (2016) 101(2):550–60. doi: 10.1210/jc.2015-3668
34. Hansen JS, Clemmesen JO, Secher NH, Hoene M, Drescher A, Weigert C, et al. Glucagon-to-insulin ratio is pivotal for splanchnic regulation of FGF-21 in humans. Mol Metab (2015) 4(8):551–60. doi: 10.1016/j.molmet.2015.06.001
35. Lin Z, Gong Q, Wu C, Yu J, Lu T, Pan X, et al. Dynamic change of serum FGF21 levels in response to glucose challenge in human. J Clin Endocrinol Metab (2012) 97(7):E1224–8. doi: 10.1210/jc.2012-1132
36. Tucker B, Li H, Long X, Rye KA, Ong KL. Fibroblast growth factor 21 in non-alcoholic fatty liver disease. Metabolism (2019) 101:153994. doi: 10.1016/j.metabol.2019.153994
37. Harnishsingh B, Rama B. Is C-peptide a predictor of severity of coronary artery disease in metabolic syndrome? An observational study. Indian Heart J (2018) 70(Suppl 3):S105–S9. doi: 10.1016/j.ihj.2018.07.005
38. Min JY, Min KB. Serum C-peptide levels and risk of death among adults without diabetes mellitus. CMAJ (2013) 185(9):E402–8. doi: 10.1503/cmaj.121950
39. Ruggiero C, Cherubini A, Ble A, Bos AJ, Maggio M, Dixit VD, et al. Uric acid and inflammatory markers. Eur Heart J (2006) 27(10):1174–81. doi: 10.1093/eurheartj/ehi879
40. Stojsavljevic S, Gomercic Palcic M, Virovic Jukic L, Smircic Duvnjak L, Duvnjak M. Adipokines and proinflammatory cytokines, the key mediators in the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol (2014) 20(48):18070–91. doi: 10.3748/wjg.v20.i48.18070
41. Choi YJ, Shin HS, Choi HS, Park JW, Jo I, Oh ES, et al. Uric acid induces fat accumulation via generation of endoplasmic reticulum stress and SREBP-1c activation in hepatocytes. Lab Invest (2014) 94(10):1114–25. doi: 10.1038/labinvest.2014.98
42. Lanaspa MA, Sanchez-Lozada LG, Choi YJ, Cicerchi C, Kanbay M, Roncal-Jimenez CA, et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J Biol Chem (2012) 287(48):40732–44. doi: 10.1074/jbc.M112.399899
Keywords: C‐peptide (blood), mediated effect, non-alcoholic fatty liver disease, normal-ranged uric acid, serum uric acid to creatinine ratio
Citation: Ma C, Liu Y, He S, Zeng J, Li P, Ma C, Ping F, Zhang H, Xu L, Li W and Li Y (2020) C-Peptide: A Mediator of the Association Between Serum Uric Acid to Creatinine Ratio and Non-Alcoholic Fatty Liver Disease in a Chinese Population With Normal Serum Uric Acid Levels. Front. Endocrinol. 11:600472. doi: 10.3389/fendo.2020.600472
Received: 30 August 2020; Accepted: 22 October 2020;
Published: 19 November 2020.
Edited by:
Karine Clément, Sorbonne Universités, FranceReviewed by:
Ying Zhao, Zhejiang University, ChinaMagdalene K. Montgomery, The University of Melbourne, Australia
Copyright © 2020 Ma, Liu, He, Zeng, Li, Ma, Ping, Zhang, Xu, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuxiu Li, liyuxiu@medmail.com.cn
 Chifa Ma
Chifa Ma Yiwen Liu1
Yiwen Liu1 Lingling Xu
Lingling Xu Yuxiu Li
Yuxiu Li