- 1Department of Inflammation-Immunopathology-Biotherapy (I2B), Clinical Investigation Center for Biotherapies (CIC-BTi), Hôpital Pitié-Salpêtrière, Assistance Publique Hôpitaux de Paris (AP-HP), Paris, France
- 2UMRS 959, Immunology-Immunopathology-Immunotherapy (I3), Sorbonne Université, Université Pierre-et-Marie-Curie, Institut national de la santé et de la recherche médicale (INSERM), Paris, France
- 3FRE 3632, Immunology-Immunopathology-Immunotherapy (I3), Centre national de la recherche scientifique (CNRS), Paris, France
In addition to CD4+ regulatory T cells (Tregs), CD8+ suppressor T cells are emerging as an important subset of regulatory T cells. Diverse populations of CD8+ T cells with suppressive activities have been described. Among them, a small population of CD8+CD25+FOXP3+ T cells is found both in mice and humans. In contrast to thymic-derived CD4+CD25+FOXP3+ Tregs, their origin and their role in the pathophysiology of autoimmune diseases (AIDs) are less understood. We report here the number, phenotype, and function of CD8+ Tregs cells in mice and humans, at the steady state and in response to low-dose interleukin-2 (IL-2). CD8+ Tregs represent approximately 0.4 and 0.1% of peripheral blood T cells in healthy humans and mice, respectively. In mice, their frequencies are quite similar in lymph nodes (LNs) and the spleen, but two to threefold higher in Peyer patches and mesenteric LNs. CD8+ Tregs express low levels of CD127. CD8+ Tregs express more activation or proliferation markers such as CTLA-4, ICOS, and Ki-67 than other CD8+ T cells. In vitro, they suppress effector T cell proliferation as well as or even better than CD4+ Tregs. Owing to constitutive expression of CD25, CD8+ Tregs are 20- to 40-fold more sensitive to in vitro IL-2 stimulation than CD8+ effector T cells, but 2–4 times less than CD4+ Tregs. Nevertheless, low-dose IL-2 dramatically expands and activates CD8+ Tregs even more than CD4+ Tregs, in mice and humans. Further studies are warranted to fully appreciate the clinical relevance of CD8+ Tregs in AIDs and the efficacy of IL-2 treatment.
Introduction
T cell development in the thymus comprises the positive selection of functional T cells capable of supporting adaptive immunity and the elimination of highly self-reactive T cells. The latter process is leaky and some autoreactive effector T cells (Teffs) escape into the periphery where they are regulated by peripheral tolerance mechanisms. In healthy individuals, multiple cell subsets with immunoregulatory properties, among CD4+ and CD8+ T cell or B cell populations, control these potentially harmful Teffs (1–3).
The best characterized of such regulatory cell populations are the natural CD4+CD25+FOXP3+ thymic-derived regulatory T cells (CD4+ Tregs). Their major role in the maintenance of immunological self-tolerance and immune homeostasis (4, 5) is illustrated by the rapid development of autoimmune diseases (AIDs) in normal mice upon their depletion (4) and also by the occurrence of severe AID, allergy, and immunopathology in humans with a mutated FOXP3 gene (6). CD4+ Treg biology has dominated research on regulatory cells in AIDs. More recently, other regulatory cell populations have received attention, and notably CD8+ suppressor cells (CD8+ Tsups) for which evidence that they are involved in AIDs is growing (7–9). However, CD8+ Tsups are less well characterized than CD4+ Tregs.
CD8+ Tsups are functionally defined populations of CD8+ T cells endowed with immunosuppressive functions (10–14). Several subpopulations of CD8+ Tsups have been described based on the expression of CD8αα, CD25, CD38, CD45RA, CD45RO, CD56, FOXP3, CXCR3, LAG-3, CD103, CD122, and/or HLA-G, as well as the absence of CD28 and CD127 (2, 7, 8, 15). The different CD8+ Tsup subsets are multiply involved in the pathophysiology of different AIDs (16). Their suppressive activity in AIDs was first demonstrated in CD8-depleted mice, which were more susceptible to a second induction of experimental autoimmune encephalomyelitis (17). CD8-deficient mice were also more susceptible to relapse of autoimmune arthritis after immunization with self-antigens (18). In non-obese diabetic mice, antigen-specific CD8+ Tsups were able to not only prevent but also reverse type 1 diabetes (15). Notably, in patients with systemic lupus erythematosus (19–21), inflammatory bowel disease (22), or multiple sclerosis (23–26) defective functions and/or reduced numbers of CD8+ Tsups have been reported. CD8+ Tsup biology is also less well known than that of CD4+ Tregs, due in part to their small numbers, which render functional studies difficult.
Only some CD8+ Tsups express FOXP3, the master regulator of CD4+ Treg differentiation and function, as well as CD25, and will be referred to here as CD8+ Tregs. Many groups are now suggesting that FOXP3 expression might represent a good indicator of a bona fide suppressive function (7, 27–30). Notably, it was demonstrated in lupus-prone mice that silencing FOXP3 with siRNA abrogates the ability of CD8+ Tregs to suppress anti-DNA antibodies (27, 29).
As CD8+ Tregs express CD25, the question of their sensitivity to interleukin-2 (IL-2) is relevant. Stimulation with IL-2 is indeed crucial for CD4+ Tregs. Mice deficient in IL-2 or in IL-2 receptor develop systemic AIDs that have been related to impaired development, survival, and function of CD4+ Treg cells (1, 31). However, the lack of IL-2 signaling effects on CD8+ Tregs has not been specifically evaluated in these models. Nevertheless, it is already known that, like CD4+ Tregs, CD8+ Tregs expand in response to IL-2 treatment, as we previously showed in a clinical trial using low-dose IL-2 in hepatitis C virus-induced vasculitis (32) and type 1 diabetes (33).
We sought to characterize CD8+CD25+FOXP3+ Tregs in mice and humans, phenotypically and functionally, at steady state and under IL-2 stimulation in vitro and in vivo. We show here that CD8+ Tregs are highly suppressive and responsive to IL-2. Our results warrant further study of these bona fide CD8+ Tregs in AID pathophysiology and therapy.
Materials and Methods
Human Blood Samples
Blood samples from healthy volunteers were obtained from the Etablissement français du sang (EFS) at the Pitié-Salpêtrière Hospital in Paris, France. Informed consent was obtained from each volunteer.
Blood samples from type 1 diabetes patients were obtained from the DF-IL2 trial (clinicaltrials.gov identifier NCT01353833). Patients were treated at the Centre d’Investigation Clinique (CIC)-Paris Est of la Pitié-Salpêtrière Hospital in Paris, France. Details about the clinical trial and the patients’ clinical characteristics are described in Hartemann et al. (34). Written informed consent was obtained from all participants before enrolment in the study. The study was approved by the institutional review board of the Pitié-Salpêtrière Hospital, and was done in accordance with the Declaration of Helsinki and good clinical practice guidelines.
Mice
BALB/cJRj and C57Bl/6 mice were from Janvier. Transgenic C57Bl/6 FOXP3-GFP mice that express green fluorescent protein (GFP) in FOXP3+ cells were kindly provided by Dr. Malissen of the Centre d’immunologie de Marseille Luminy (France). CD3 KO mice were from CDTA of Orléans (France). Animals were maintained in our animal facility under specific pathogen-free conditions in agreement with current European legislation on animal care, housing, and scientific experimentation. All procedures were approved by the Regional Ethics Committee on Animal Experimentation No. 5 of the Ile-de-France region (Ce5/2012/031).
Preparation of tissue-infiltrating lymphocytes in mice
Spleen, lymph nodes (LNs) (mesenteric LN, MLN; cervical LN, CLN; pancreatic LN, PaLN), and Peyer’s patches (PP) were isolated and dissociated in PBS 3% fetal calf serum (FCS). Pancreas was digested with collagenase/DNase solution in RPMI medium, and filtered as described (35). A Ficoll (Sigma-Aldrich) gradient was used to isolate tissue-infiltrating lymphocytes.
Interleukin-2 treatment in mice
Eight-week-old female BALB/c mice received intraperitoneal injections of 50,000 or 100,000 IU of recombinant human IL-2 (Proleukin, Novartis) or PBS daily for 5 days. Twenty-four hours after the last injection, blood was collected and analyzed by flow cytometry.
Flow Cytometry
Analysis of cell surface and intracellular markers and FOXP3 expression in mice
Fresh total cells from the respective tissues were directly stained with the following monoclonal antibodies (mAbs) at predetermined optimal dilutions for 20 min at 4°C: CD3-PE, CD8-Alexa700, CD4-HorizonV500, CD127-FITC, and CD25-PeCy7 (eBioscience). Intracellular detection of FOXP3 (FOXP3-E450, eBioscience), CTLA-4 (CTLA-4-APC, eBioscience), Ki-67 (Ki-67-FITC, BD Pharmingen), and Bcl-2 (Bcl-2-PE, BD Pharmingen) was performed on fixed and permeabilized cells using appropriate buffer (eBioscience) (incubation 30 min at 4°C). Cells were acquired on an LSR II flow cytometer (Becton Dickinson) and analyzed using FlowJo software (Tree Star, Inc.). Dead cells were excluded by forward/side scatter gating.
Analysis of cell surface markers and FOXP3 expression in humans
Direct ex vivo immunostaining was performed on 50 μL of lithium heparinized fresh whole blood from healthy donors using the PerFix-nc kit (Beckman Coulter) according to the manufacturer’s instructions. Briefly, 5 μL of fixative reagent was added to the blood for 15 min at room temperature in the dark, before adding antibodies diluted in 300 μL of permeabilizing reagent for 1 h at room temperature in the dark. Staining was performed with FOXP3− AF647 (clone 259D), CD25-PE, CD127-PE-Cy7, CD8-Chrome Orange, CD4-Pacific Blue, CD3-FITC, CD103-APC, ICOS-FITC, CD122-FITC, and CCR7-PE mAbs, all from Beckman Coulter. CTLA-4-PeCy7 mAb was from Biolegend. Samples were acquired on a Navios cytometer (Beckman Coulter) and analyses were performed using Kaluza software (Beckman Coulter). Matched mouse isotype control antibodies were used.
Instrument settings (gain, compensation, and threshold) were set with machine software (Navios Software; Beckman Coulter) in conjunction with calibration beads (Flow-set beads, Cytocomp kit, and CYTO-TROL Control Cells). Machine reproducibility was verified with standardized beads (Flow-check).
In mice and in humans, CD4+ Tregs were defined as CD25+ FOXP3+ cells among CD4+ T cells, and effector CD4+ T cells as FOXP3− cells among CD4+ T cells. CD8+ Tregs were defined as CD25+FOXP3+ cells among CD8+ T cells, and effector CD8+ T cells as FOXP3− cells among CD8+ T cells.
Absolute numbers of CD8+ Tregs and CD4+ Tregs in peripheral blood
Briefly, PBMC subsets (CD4+ Tregs, CD8+ Tregs) counts (cells/μL) were established from fresh blood samples using Flowcount fluorescents beads (Beckman Coulter) as internal standard (33).
pSTAT5 staining procedure
The pSTAT5 staining was assessed using PerFix EXPOSE reagents from Beckman Coulter as previously described (36). Briefly, fresh lithium heparinized whole blood was stained using anti-CD4, anti-CD25, anti-FOXP3, and PE-conjugated anti-phosphorylated STAT5 (Beckman Coulter) antibodies. Blood samples were stimulated with increasing hIL-2 (proleukin, Novartis) concentrations at 37°C for 10 min. Cell surface staining was then performed. Whole blood was incubated for 5 min (37°C, incubator). Samples were fixed for 10 min at room temperature in the dark, using 50 μL of fixative reagent (PerFix EXPOSE). Aliquots were permeabilized using 1 mL of permeabilizing reagent (PerFix EXPOSE), and incubated for 5 min at 37°C. Samples were centrifuged at 300 × g for 5 min, and the supernatant was completely discarded by aspiration. Then, intracellular staining with a mixture of 100 μL of staining reagent including PE-anti-pSTAT5 and AF647-anti-FOXP3 antibodies was performed for 30 min (room temperature) and cells were washed with 3 mL of washing buffer (PerFix EXPOSE). The supernatant was completely discarded by aspiration and 300 μL of PBS was added.
In vitro Suppression Assay
Four C57Bl/6 FOXP3-GFP mice were sacrificed, and spleen and LN were collected and dissociated in PBS 3% FCS. Cells were stained with CD3, CD4, CD8, and CD25 (as described above) and then sorted using a FACS ARIA cell sorter (Becton Dickinson). CD4+GFP− (CD4+ Teffs), CD4+CD25+GFP+ (CD4+ Tregs), and CD8+CD25+GFP+ (CD8+ Tregs) were collected. The purity of cell preparations exceeded 97%. CD4+ Teffs were cultured in RPMI 1640 medium supplemented with 5% FCS, 2 mmol/L l-glutamine, 100 U/mg/mL penicillin/streptomycin at 5 × 104 cells/well, and variable numbers of regulatory cells were added in the presence of 7.5 × 104 total splenocytes from CD3 KO mice and anti-CD3 (final concentration 0.1 μg/mL, BioXell).
3H-thymidine (1 μCi/well) incorporation was evaluated during the final 16 h of the 3-day culture.
Statistical Analyses
Statistical significance was evaluated using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA, http://www.graphpad.com) and calculated using the paired t-test, Mann–Whitney test (comparison of means, unpaired test, non-parametric test, two-tail p value), or one-way ANOVA test, with p < 0.05 (*) taken as statistical significance (**p < 0.01, ***p < 0.001, NS, non-significant).
Results
Characterization and Phenotype of CD8+CD25+FOXP3+ Tregs
We measured the percentages and absolute numbers of CD8+ Tregs and CD4+ Tregs in human blood and in blood and lymphoid organs from C57Bl/6 and BALB/c mice. A representative gating strategy for CD8+CD25+FOXP3+ Treg immunophenotyping by flow cytometry in humans (Figure 1A) and in BALB/c mice (Figure 1B) is shown in Figure 1. In mice, CD4+ Tregs and CD8+ Tregs were solely defined by the co-expression of FOXP3 and CD25. In human and to a lesser extent in mouse, CD4+ Tregs and CD8+ Tregs were also characterized by low levels of CD127 compared to Teffs (Figure S1 in Supplementary Material).
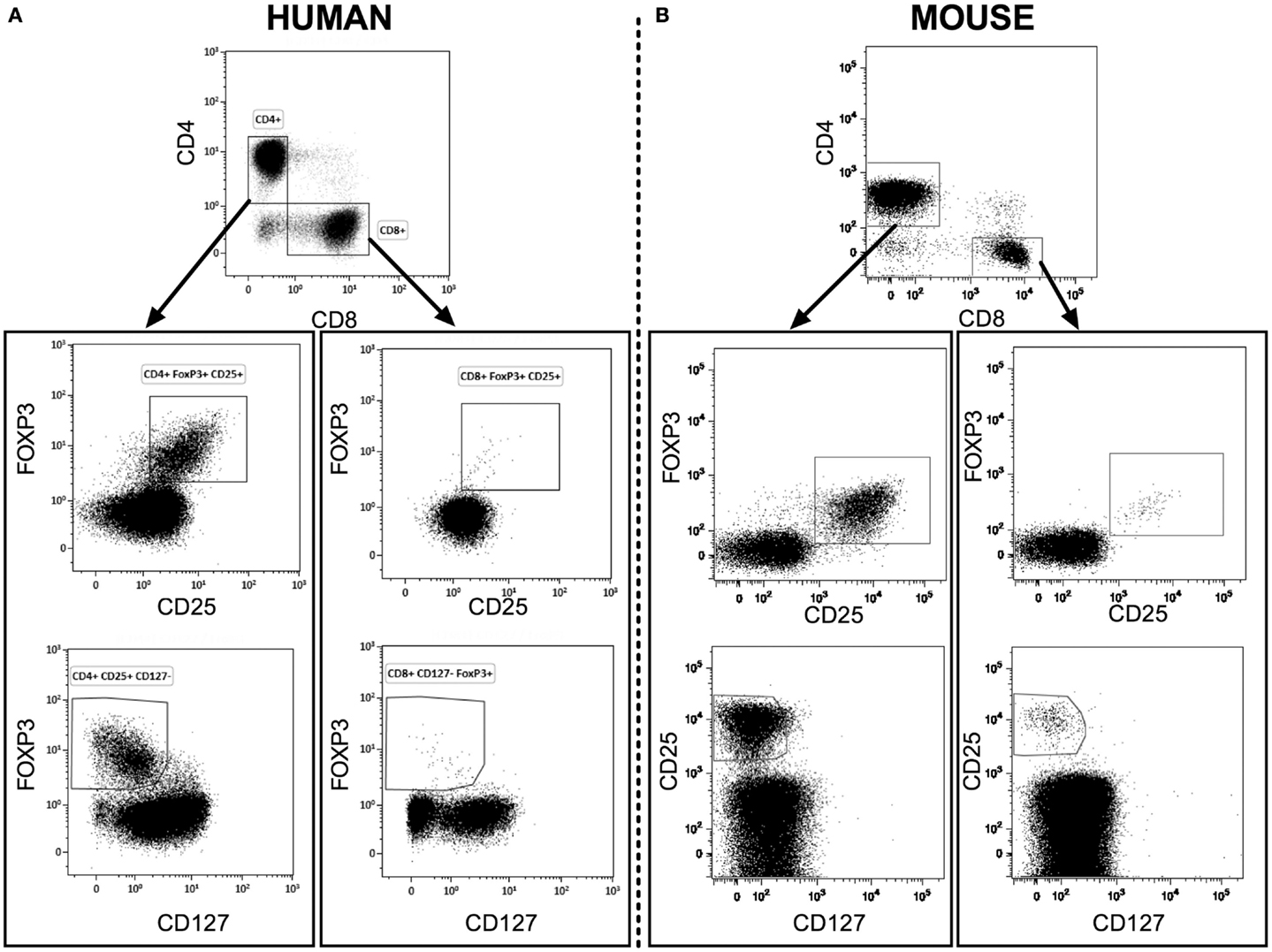
Figure 1. Representative flow cytometry analysis of CD4+ Tregs and CD8+ Tregs from fresh heparinized peripheral blood of human healthy donors [human (A)] and of naive BALB/c mice [mouse (B)]. CD4+ and CD8+ T cells were gated from CD3+ lymphocytes.
In peripheral blood mononuclear cells (PBMCs) from human healthy donors, the percentage of CD8+ Tregs among CD8+ T cells was variable and below 1% (0.38 ± 0.26, mean ± SD, n = 37; Figure 2A). It is noteworthy that a wide range of values was observed (0.1–1%) with a quite heterogeneous distribution, since few individuals had markedly higher values (Figure 2A). In comparison, the percentage of CD4+ Tregs among CD4+ T cells in human blood was higher and less variable (8.3 ± 1.6) (Figure 2C).
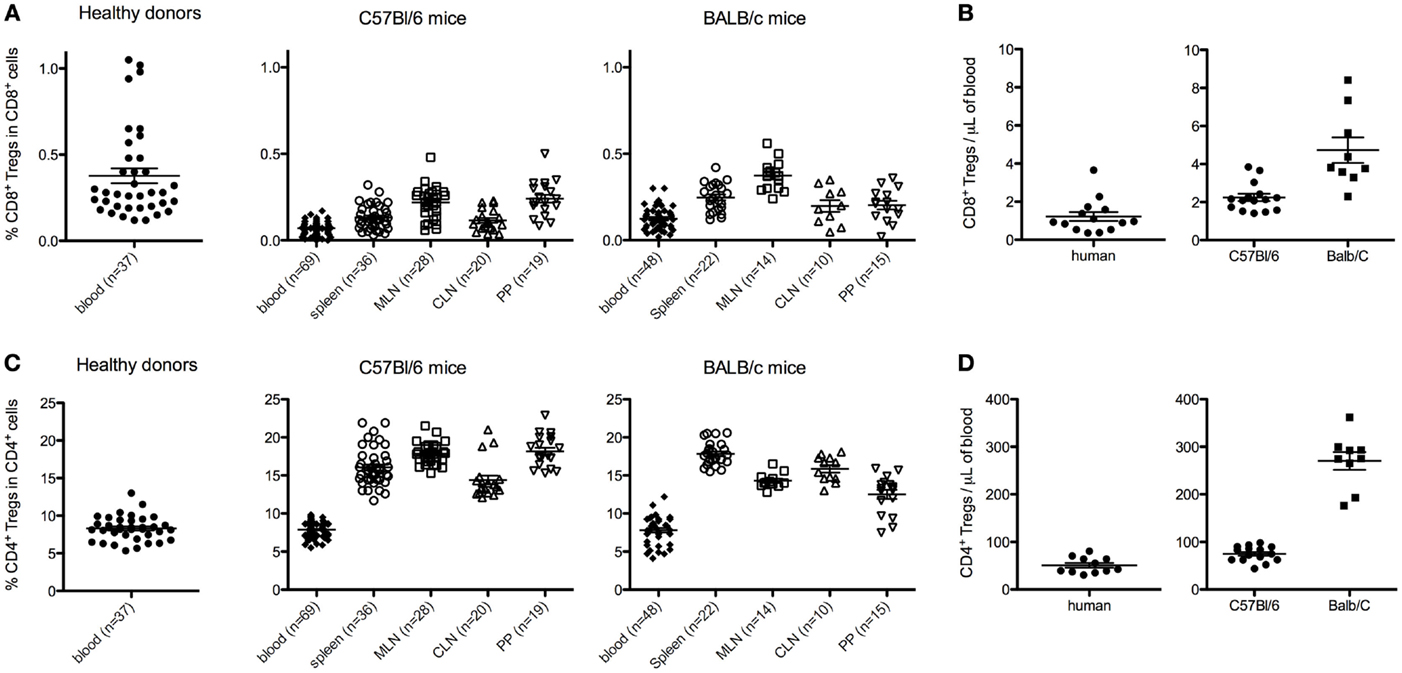
Figure 2. Percentages and absolute counts of CD8+ Tregs among CD8+ T cells [respectively (A,B)], and of CD4+ Tregs among CD4+ T cells [respectively (C,D)] in human healthy donors and C57Bl/6 or BALB/c naive mice. Human data are from 10 to 37 healthy blood donors. Mouse data are from a pool of at least three different experiments analyzing 8- to 12-week-old female mice. Individual values and mean ± SEM are shown. MLN, mesenteric lymph nodes; CLN, cervical lymph nodes; PP, Peyer’s patches.
In mice, the percentages of CD8+ Tregs among CD8+ T cells were 0.07 ± 0.04 and 0.12 ± 0.07% in peripheral blood of C57Bl/6 and BALB/c (Figure 2A). Similar or slightly higher values were observed in spleen and CLN in both mouse strains. When MLN and PP were analyzed, two to threefold higher values were observed as compared with blood values, except for PP in BALB/c mice. In comparison, in these different lymphoid tissues, the percentages of CD4+ Tregs among CD4+ T cells were around 50-fold higher in both C57Bl/6 and BALB/c mice (Figure 2C).
These 50-fold differences in percentages of CD4+ Tregs and CD8+ Tregs were also observed when looking at absolute numbers (Figures 2B,D) in both human and mouse.
We compared the phenotype of mouse CD8+ Tregs with that of other CD8+ T cells and of CD4+ T cells, focusing mainly on proteins associated with the regulatory function of CD4+ Tregs (Figure 3). Most CD8+ Tregs expressed CD103 (85.2 ± 11.8%) and some were CD122+ (11.9 ± 9.6%) with significantly higher levels compared with CD8+ Teffs only for CD103 expression (p < 0.0001). Approximately 36% of them were ICOS+ and only 9% expressed more CTLA-4 than CD8+ Teffs (p < 0.0001 and p = 0.0025, respectively). CD8+ Tregs had a proliferation rate measured by expression of Ki-67 (19.8 ± 11.7%) that was more than three times higher than that of CD8+ Teffs (6.6 ± 2.0%, p < 0.0001), whereas anti-apoptotic Bcl-2 marker expression was the same in CD8+ Tregs and CD8+ Teffs. This phenotype was similar in CD4+ Tregs, except for a lower expression of CD103, CD122, and ICOS (Figure 3).
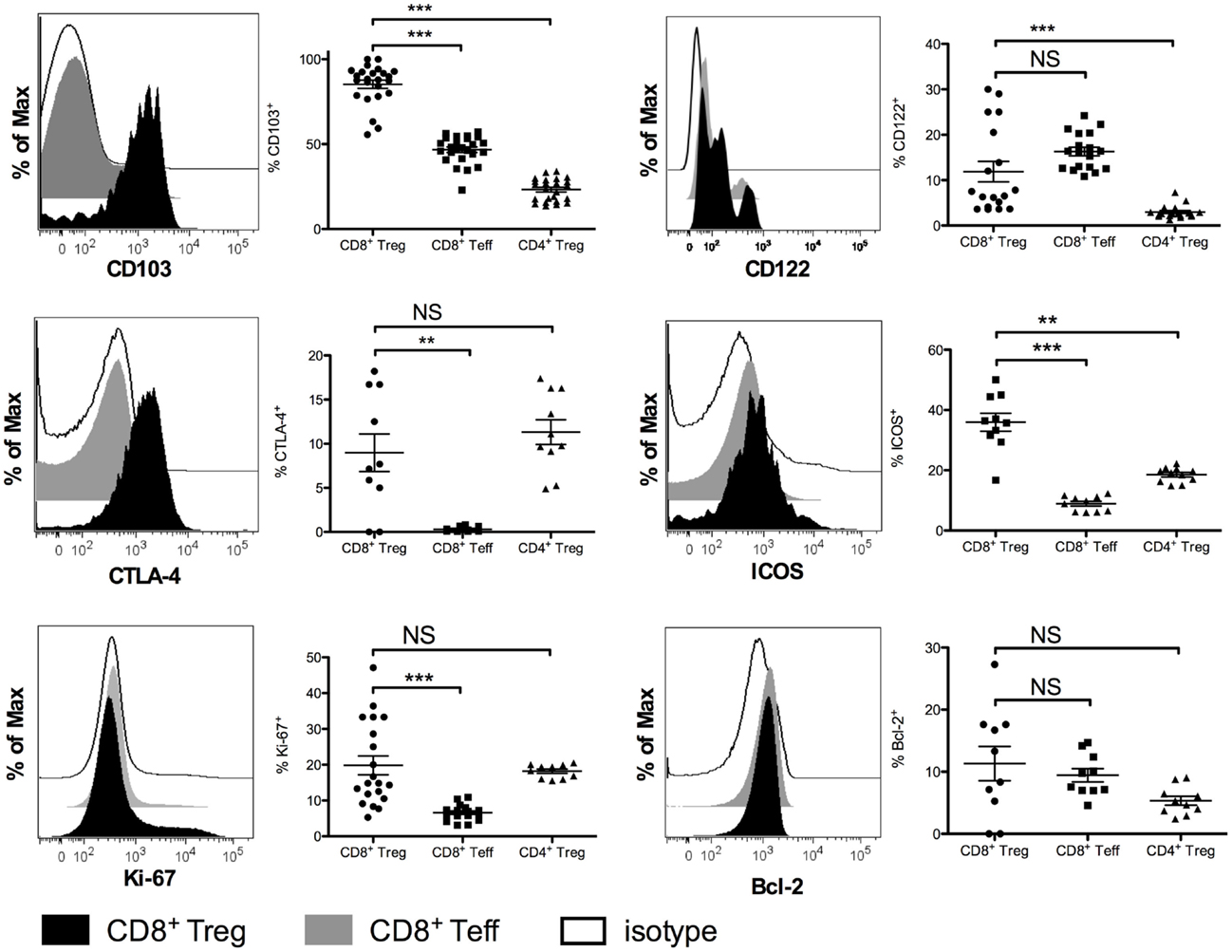
Figure 3. Phenotypic characterization of CD8+CD25+FOXP3+ Tregs (CD8+ Tregs) compared with CD8+CD25−FOXP3−effector CD8+ T cells (CD8+ Teffs) and with CD4+CD25+FOXP3+ Tregs (CD4+ Tregs) in female BALB/c mice. Representative histograms showing expression of CD103, CTLA-4, Ki-67, CD122, ICOS, and Bcl-2 in CD8+ Teffs (gray) and in CD8+ Tregs (black) compared to isotype control (white) are shown. Corresponding percentages in the overall population are shown. Individual values and mean ± SEM are shown.
A large fraction of human CD8+ Tregs express CTLA-4 (59 ± 19%) (Figure S2 in Supplementary Material) as do CD4+ Tregs. In contrast, CD8+ Tregs do not express any of the other markers we tested, i.e., CD103, CD122, ICOS, and CCR7 (data not shown).
Suppressive Activity of CD4+ Tregs and CD8+ Tregs
We next evaluated the suppressive capacity of CD8+ Tregs. For that purpose, we used C57Bl/6 FOXP3-GFP mice. In these knock-in mice, GFP is expressed only in FOXP3 positive cells and can thus be used as a marker for flow cytometry sorting of viable CD8+ Tregs. CD8+ Tregs (CD8+CD25+GFP+) and CD4+ Tregs (CD4+CD25+GFP+) were sorted and evaluated for their capacity to suppress CD4+ Teffs (CD4+GFP− cells). CD8+ Tregs appeared slightly more suppressive than CD4+ Tregs (Figure 4). At a 1:1 Treg:Teff ratio, 95% of CD4+ Teff suppression was observed with CD8+ Tregs and 77% with CD4+ Tregs. Similar differences were observed at a 1:2 ratio in the same experiment and similar results were also obtained with human CD8+ Tregs (28).
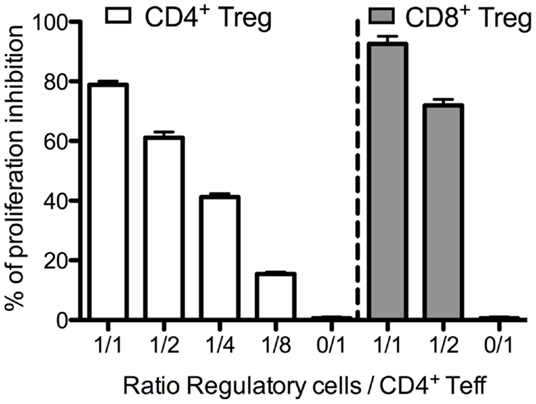
Figure 4. Suppressive activity of murine CD4+ Tregs and CD8+ Tregs. Freshly sorted CD4+GFP− (CD4+ Teffs) cells were cultured with CD4+ Tregs or CD8+ Tregs at various ratios for 3 days, and tritiated thymidine was added for the last 16 h of the culture. Proliferation was assessed by tritiated thymidine incorporation measurement.
In vitro Sensitivity to IL-2 of CD4+ Tregs and CD8+ Tregs
As CD4+ Tregs and CD8+ Tregs constitutively express CD25, included in the high-affinity IL-2 receptor, we investigated their sensitivity to in vitro IL-2 activation. We evaluated the IL-2-induced phosphorylation of STAT5 proteins (pSTAT5) by flow cytometry (Figure 5). CD4+ Tregs are exquisitely sensitive to IL-2 activation, activated with doses 20- to 40-fold lower than those required to activate CD4+ Teffs. It is noteworthy that CD8+ Tregs had the same 20- to 40-fold higher sensitivity to IL-2 activation as CD8+ Teffs, but were 2- to 4-fold less sensitive than CD4+ Tregs (Figure 5A). At 1 IU/mL, the proportion of pSTAT5 positive cells was of 77.7 ± 3.8% in CD4+ Tregs, 29.1 ± 5.2% in CD8+ Tregs, but only 2.9 ± 0.5 and 1.2 ± 0.3% in CD4+ and CD8+ Teffs, respectively. At 10 IU/mL, these proportions were 91.9 ± 1.5% for CD4+ Tregs, 46.0 ± 6.0% for CD8+ Tregs, and only 13.4 ± 2.4% for CD4+ Teffs and 4.7 ± 1.7% for CD8+ Teffs. These differences in IL-2-induced activation in CD4+ Tregs and CD8+ Tregs can be correlated with twofold higher levels of CD25 basal expression in CD4+ Tregs compared with CD8+ Tregs (Figure 5B).
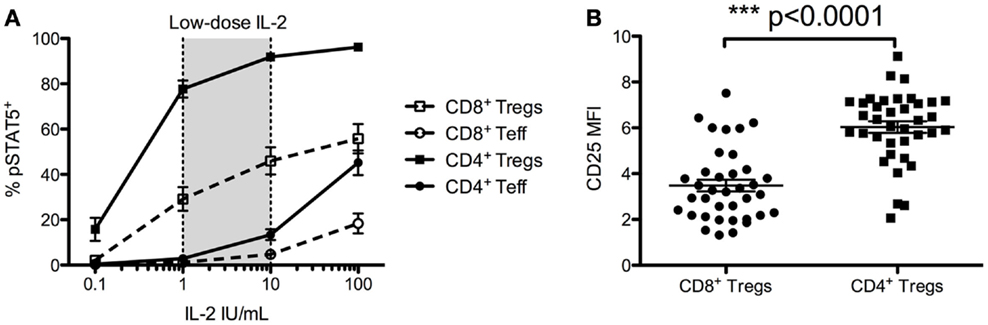
Figure 5. Foxp3+ CD25high CD127low CD4+ T cells (CD4+ Tregs) and Foxp3+ CD25high CD127low CD8+ T cells (CD8+ Tregs) responsiveness to IL-2 and their comparative CD25 cell surface expressions. (A) Total fresh blood from healthy donors was ex vivo stimulated with different doses of IL-2 for 10 min, and STAT5 phosphorylation was measured on different T cell subsets. Results are expressed in mean ± SE, n = 7–22. Low-dose IL-2 has been defined as the dose inducing high STAT5 phosporylation on CD4+ Tregs but not on CD4+ Teff. (B) Mean fluorescence intensity (MFI) of CD25 on CD4+ Tregs and CD8+ Tregs (n = 37) from fresh unstimulated whole blood from healthy donors. Individual values and mean ± SEM are shown.
In vivo Sensitivity to IL-2 of CD4+ Tregs and CD8+ Tregs
We next assessed the dynamics of CD4+ Tregs and CD8+ Tregs under low-dose IL-2 treatment, which is known to expand and activate CD4+ Tregs. Seven patients with type 1 diabetes received 3 MIU of IL-2/day for 5 days (34). This led to a significant increase of CD8+ Tregs, which reached 6.7 ± 4.3-fold at day 5 (Figure 6A). This CD8+ Treg expansion was not sustained at day 15, i.e., 10 days after the last IL-2 injection. IL-2 had a less pronounced effect on CD4+ Tregs, reaching a 1.8 ± 0.7-fold expansion at day 5. Nevertheless, and unlike CD8+ Tregs, this increase was sustained until day 15 (1.8 ± 0.6-fold). Interestingly, CD25 mean fluorescence intensity (MFI) was increased both on CD8+ Tregs and CD4+ Tregs after 5-day IL-2 treatment (Figure 6B). This CD25 increase was slightly higher for CD4+ Tregs than for CD8+ Tregs (2.1 ± 0.3 versus 1.6 ± 0.4; p = 0.005).
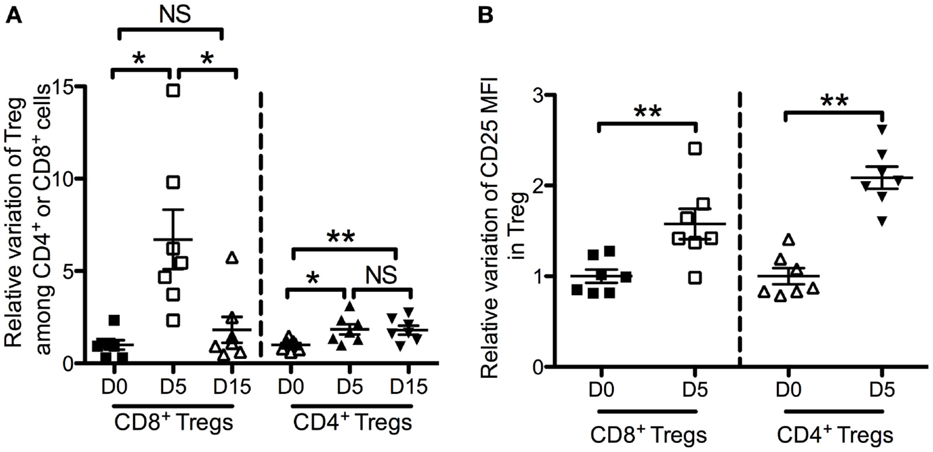
Figure 6. Effects of low-dose IL-2 on Foxp3+ CD25high CD127low CD4+ T cells (CD4+ Tregs) and Foxp3+ CD25high CD127low CD8+ T cells (CD8+ Tregs) in humans. Seven type 1 diabetes patients were treated with 3 MIU IL-2/day for 5 days (34). Time-course changes of CD8+ Tregs and CD4+ Tregs percentages in peripheral blood (A) and of CD25 mean fluorescence intensity (MFI) in CD8+ Tregs and CD4+ Tregs in peripheral blood (B) following a 5-days course of low-dose IL-2. Individual values and mean ± SEM are shown. Results are expressed in fold variation compared with the mean at day 0 of all the samples.
In BALB/c mice injected daily for 5 days with 50,000 or 100,000 IU of IL-2, we observed a similar dose-dependent increase of percentages of CD4+ and CD8+ Tregs in peripheral blood as compared with controls injected with PBS (Figure 7A). A 5-day course of 50,000 IU of IL-2 led to a 1.5 ± 0.3-fold increase of CD4+ Tregs and 1.5 ± 0.6-fold increase of CD8+ Tregs. At 100,000 IU/day, the increase was 1.9 ± 0.1-fold for CD4+ Tregs and 1.8 ± 0.5-fold for CD8+ Tregs. Similarly to the human study, we observed an IL-2 dose-dependent increase of CD25 expression in both CD4+ Tregs and CD8+ Tregs (Figure 7A). The fold increase of CD25 MFI induced by 50,000 or 100,000 IU of IL-2 was 1.4 ± 0.2 or 1.8 ± 0.2 in CD4+ Tregs and 1.3 ± 0.4 or 1.8 ± 0.5 in CD8+ Tregs, respectively. In contrast, FOXP3 expression levels were slightly increased only in CD4+ Tregs (1.1 ± 0.1 both with 50,000 and 100,000 IU of IL-2). No significant differences in FOXP3 expression level were observed in CD8+ Tregs at the two doses.
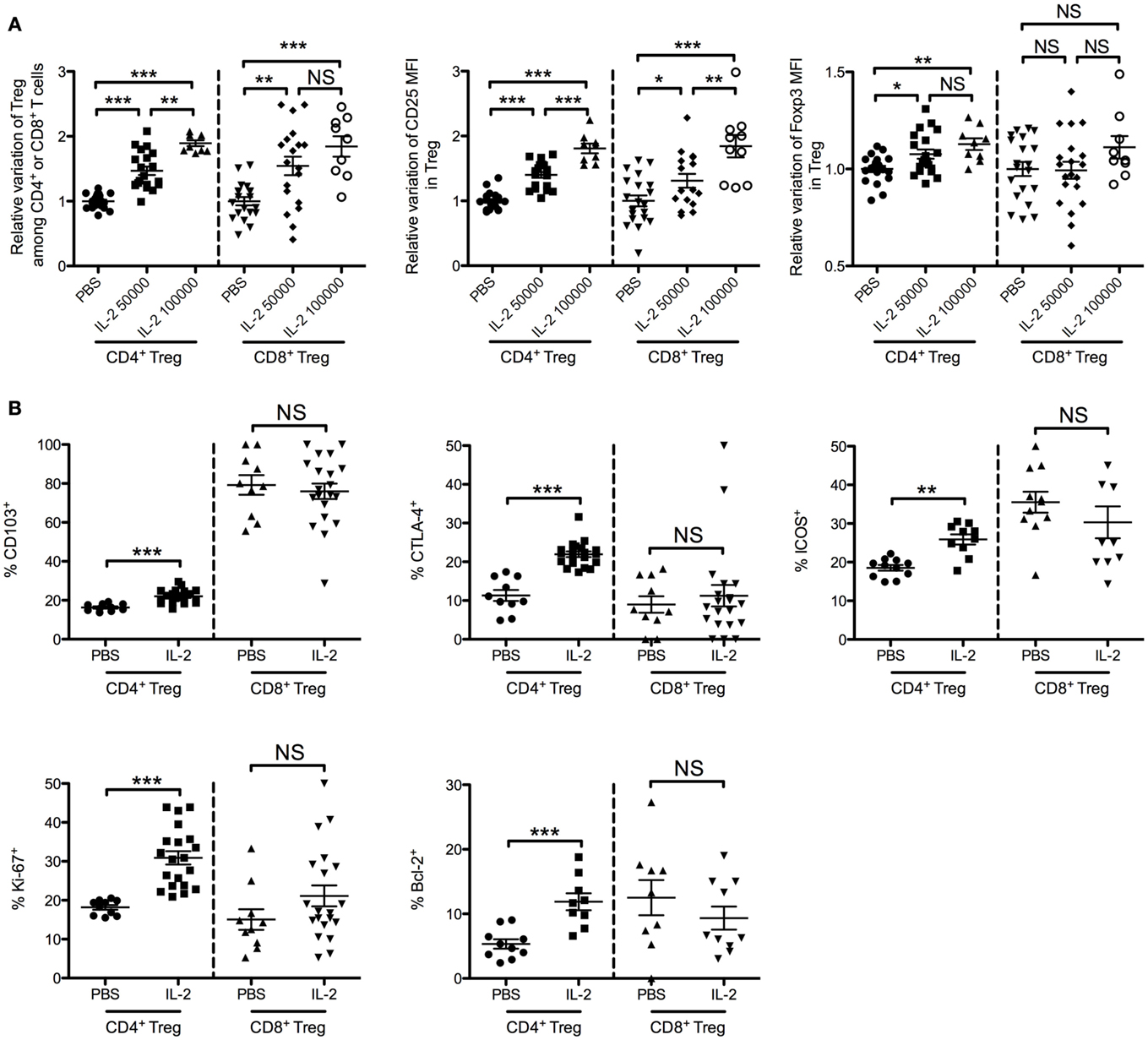
Figure 7. Effects of low-dose IL-2 on CD4+ Tregs and CD8+ Tregs in mice. Eight-week-old female BALB/c mice were injected daily for 5 days with PBS or with 50,000 or 100,000 IU of IL-2, and blood was sampled after the treatment. (A) Relative variations of percentages, CD25 MFI and FOXP3 MFI of CD4+ Tregs and CD8+ Tregs in blood after treatment (pool of three independent experiments). Individual values and mean ± SEM are shown. (B) Phenotypic activation markers of CD4+ Tregs and CD8+ Tregs after 5-day course of PBS (PBS) or 50,000 IU of IL-2 (IL-2) (pool of two independent experiments). Individual values and mean ± SEM are shown.
We also monitored some activation and proliferation markers in CD4+ Tregs and CD8+ Tregs after a 5-day course of 50,000 IU of IL-2. Interestingly, CD103, CTLA-4, ICOS, Ki-67, and Bcl-2 expression levels were increased after IL-2 treatment in CD4+ Tregs but not in CD8+ Tregs (Figure 7B and representative stainings in Figure S2 in Supplementary Material). These results highlight the specific biology of CD8+ Tregs compared with conventional CD4+ Tregs.
Discussion and Conclusion
On Bona Fide CD8+ Tregs
CD8+ Tsups are heterogeneous and often poorly characterized if not controversial cells in mice and humans. We focused our study on CD8+CD25+FOXP3+ T cells, which we call CD8+ Tregs, because they are identified based on the same markers as CD4+ Tregs. CD8+ Tregs are present at steady state at low levels both in human and mouse peripheral blood (about 0.4 and 0.1%, respectively). We previously reported lower percentages of CD8+ Tregs in blood of normal volunteers (0.22 ± 0.1%) (28), but we were using a different flow cytometry staining strategy. Because we optimized our flow cytometry analyses (choice of antibodies, analyses of whole blood with Perfix-NC kit), we believe that our new values accurately reflect their percentages. Natural CD8+CD25+FOXP3+ T cells are also present at very low frequencies (between 0.3 and 1.8% in CD8+ T cells) in blood, LNs, thymus, and spleen from naive non-human primates (37, 38).
We report here that CD8+ Tregs, as CD4+ Tregs, constitutively express CD25 and are sensitive to in vitro IL-2 activation, to which they respond by STAT5 phosphorylation. Like CD4+ Tregs, CD8+ Tregs also express markers associated with CD4+ Treg suppressive activity such as ICOS (39), CD103 (40, 41), and CTLA-4 (42, 43) at higher levels than other CD8+ T cells. It is of note that nearly 80% of CD8+ Tregs are CD103+, a marker associated with suppressive function of CD8+ T cells (21, 40, 44). This phenotype is associated with a high suppressive activity in vitro in classic functional assays. Similar suppressive activities of CD8+ Tregs have also been reported in humans (28) and non-human primates (45). Altogether, CD8+FOXP3+CD25+ T cells share phenotypic and functional characteristics with thymic CD4+ Tregs and are thus bona fide CD8+ Tregs, a designation that should be restricted to this population.
As for CD4+ Tregs, it seems that there could be a population of induced CD8+ Tregs. CD8+Foxp3+ can be induced ex vivo from CD8+Foxp3− cells after culture in the presence of IL-2 and TGF-beta, but are only mildly suppressive (45, 46). Following simian immunodeficiency virus (SIV) infection, these peripheral CD8+CD25+Foxp3+ regulatory T cells are largely induced not only in blood but also in colorectal mucosa (37, 38). These cells have a regulatory phenotype (CD25+, CTLA-4+, CD28+, CD127−) (26, 28) with high proliferative capacities as 60% of induced CD8+ Tregs are Ki-67+. They express low levels of granzyme B and perforin, suggesting that their suppression is not mediated by killing.
On Differences between CD4+ Tregs and CD8+ Tregs
While CD8+ Tregs and CD4+ Tregs share functional suppressive activity, differences in their biology have been reported. IL-2 can bind the high-affinity trimeric IL-2 receptor (IL-2R) (CD25, CD122, and CD132) or the low-affinity dimeric IL-2R (CD122 and CD132) (47, 48). The trimeric IL-2R is constitutively expressed on CD4+ Tregs, but only transiently expressed on CD4+ and CD8+ T cells following T cell receptor (TCR) activation (47). We show here that CD8+ Tregs constitutively express the trimeric IL-2R, but compared with CD4+ Tregs, CD8+ Tregs express fewer CD25 molecules on their cell surface. CD4+ Tregs are two to fourfold more sensitive to IL-2 activation than CD8+ Tregs, although both CD4+ and CD8+ Tregs are equally 20 times more sensitive to IL-2 than their FOXP3− counterparts. However, despite these differences in favor of CD4+ Tregs in vitro, we observed that the sensitivity of CD8+ Tregs to IL-2 in vivo is higher than that of CD4+ Tregs (Figure 6A), a so far unexplained observation. In agreement with our observations, a very large increase of CD8+ Tregs, greater than that for CD4+ Tregs, has been observed in blood of non-human primates following IL-2 treatment (49) in a context of tuberculosis infection.
Upon IL-2 activation, CD25 up-regulation is lower for CD8+ than for CD4+ Tregs. Also, after IL-2 treatment, activation markers such as CD103, CTLA-4, and ICOS, which are increased in CD4+ Tregs, are not increased in CD8+ Tregs, suggesting that IL-2 regulates CD8+ Tregs differently than CD4+ Tregs. Further studies are needed to clarify the molecular effects of IL-2 on CD8+ Tregs.
Importantly, we showed that CD8+ Tregs suppress in vitro effector T cell proliferation as well as or even better than CD4+ Tregs. This experiment has been performed only in mice as for now the low number of bona fide CD8+ Tregs in peripheral blood from human healthy donors does not allow to perform suppressive assays with these cells. However, we have already showed that non-manipulated human cord blood CD8+ Tregs are suppressive (50). It has also been reported that CD8+ Tregs from healthy non-human primates suppress as well as CD4+ Tregs (45). Thus, we can assume that, even if CD8+ Tregs are less numerous than CD4+ Tregs in peripheral blood of human healthy donors, their excellent suppressive activity makes them important cells to consider in the regulation of immune responses.
For now, it has previously shown by others and us that in vivo IL-2 expanded CD4+ Tregs have better in vitro suppressive activities than non-expanded CD4+ Tregs. It remains to evaluate whether this is also true for CD8+ Tregs functions.
On the Possible Role of CD8+ Tregs
Several mechanisms have been implicated in the suppressive activity of suppressive CD8+ T cells, including direct lysis of target cells and secretion of immunosuppressive cytokines (14).
CTLA-4 has been shown to play a role in cell–cell contact-dependent mechanisms of CD4+ Tregs and CD8+ Treg-mediated suppression (51). As CD4+ Tregs, CD8+ Tregs may impede the up-regulation of CD80 and CD86 on immature dendritic cells or down-modulate them on mature dendritic cells (42) via CTLA-4, thereby inhibiting activation of Teffs. Jebbawi et al. (50) described a similar natural CD8+ Treg population from cord blood. This population expresses CTLA-4 and can secrete IL-10 and TGF-beta compared with CD8+CD25− T cells.
CD8+ Tregs have also been implicated in the regulation of human AID, including inflammatory bowel disease (22). Eusebio et al. (52) showed also that asthma patients have fewer CD8+ Tregs in blood than healthy control subjects. Furthermore, FOXP3 mRNA levels of CD8+ T cells were significantly decreased in patients with severe asthma compared with mild to moderate asthma and control patients. Thus, natural CD8+ Tregs could play a major role in control of allergic inflammation.
In cancer, Chaput et al. (28) and Kiniwa et al. (53) demonstrated that CD8+ Tregs from colorectal and prostate tumors have strong immunosuppressive properties, and may contribute to tumoral immune escape and disease progression.
As many groups have reported the presence of CD8+ Tregs after induction either by a drug or by pathological conditions (AIDs, cancer, infection, allergy), it remains to be seen in these models whether they are induced by pathological or natural conditions. In any case, we demonstrate here that bona fide CD8+ Tregs are present at steady state in mice and humans.
Conclusion
Many questions remain, especially about the origin and role of natural CD8+ Tregs in health and disease. Their striking phenotypic similarities with CD4+ Tregs, robust suppressive activity in vitro and exquisite sensitivity to IL-2 warrant further studies to fully appreciate their clinical relevance in AIDs and inflammatory diseases, and in IL-2 treatment efficacy.
Conflict of Interest Statement
Guillaume Churlaud, Michelle Rosenzwajg, Bertrand Bellier, and David Klatzmann are shareholders of ILTOO pharma, a company with the exclusive license for a patent (of which Michelle Rosenzwajg and David Klatzmann are the inventors) owned by the authors’ academic institution and claiming the use of low-dose IL-2 in autoimmune diseases.
Acknowledgments
This work was supported by French state funds managed by the ANR within the Investissements d’Avenir programme under reference ANR-11-IDEX-0004-02. The authors thank Beckman Coulter and especially Fabrice Malergue and Olivier Jaen (Beckman Coulter employees) for their technical support. PSTAT5-PE antibody was kindly provided by Beckman Coulter. The authors also thank Charlotte Pouchy (UPMC UMRS CR7 – INSERM U1135), Michèle Barbie (AP-HP), and Bruno Gouritin (UPMC CNRS UMR 7211) for their technical support in the suppression experiments.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fimmu.2015.00171
References
1. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell (2008) 133:775–87. doi:10.1016/j.cell.2008.05.009
2. Tang XL, Smith TR, Kumar V. Specific control of immunity by regulatory CD8 T cells. Cell Mol Immunol (2005) 2:11–9.
3. Yang M, Rui K, Wang S, Lu L. Regulatory B cells in autoimmune diseases. Cell Mol Immunol (2013) 10:122–32. doi:10.1038/cmi.2012.60
4. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol (1995) 155:1151–64.
5. Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol (2004) 22:531–62. doi:10.1146/annurev.immunol.21.120601.141122
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
6. Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet (2001) 27:20–1. doi:10.1038/83713
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
7. Dinesh RK, Skaggs BJ, La Cava A, Hahn BH, Singh RP. CD8+ Tregs in lupus, autoimmunity, and beyond. Autoimmun Rev (2010) 9:560–8. doi:10.1016/j.autrev.2010.03.006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
8. Pomié C, Ménager-Marcq I, van Meerwijk JPM. Murine CD8+ regulatory T lymphocytes: the new era. Hum Immunol (2008) 69:708–14. doi:10.1016/j.humimm.2008.08.288
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
9. Sinha S, Itani FR, Karandikar NJ. Immune regulation of multiple sclerosis by CD8+ T cells. Immunol Res (2014) 59:254–65. doi:10.1007/s12026-014-8529-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
10. Salgame P, Convit J, Bloom BR. Immunological suppression by human CD8+ T cells is receptor dependent and HLA-DQ restricted. Proc Natl Acad Sci U S A (1991) 88:2598–602. doi:10.1073/pnas.88.6.2598
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
11. Inoue T, Asano Y, Matsuoka S, Furutani-Seiki M, Aizawa S, Nishimura H, et al. Distinction of mouse CD8+ suppressor effector T cell clones from cytotoxic T cell clones by cytokine production and CD45 isoforms. J Immunol (1993) 150:2121–8.
12. Wang YM, Alexander SI. CD8 regulatory T cells: what’s old is now new. Immunol Cell Biol (2009) 87:192–3. doi:10.1038/icb.2009.8
13. Kim H-J, Verbinnen B, Tang X, Lu L, Cantor H. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature (2010) 467:328–32. doi:10.1038/nature09370
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
14. Lu L, Cantor H. Generation and regulation of CD8(+) regulatory T cells. Cell Mol Immunol (2008) 5:401–6. doi:10.1038/cmi.2008.50
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
15. Tsai S, Shameli A, Yamanouchi J, Clemente-Casares X, Wang J, Serra P, et al. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity (2010) 32:568–80. doi:10.1016/j.immuni.2010.03.015
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
16. Suzuki M, Konya C, Goronzy JJ, Weyand CM. Inhibitory CD8+ T cells in autoimmune disease. Hum Immunol (2008) 69:781–9. doi:10.1016/j.humimm.2008.08.283
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
17. Jiang H, Zhang SI, Pernis B. Role of CD8+ T cells in murine experimental allergic encephalomyelitis. Science (1992) 256:1213–5. doi:10.1126/science.256.5060.1213
18. Tada Y, Ho A, Koh DR, Mak TW. Collagen-induced arthritis in CD4- or CD8-deficient mice: CD8+ T cells play a role in initiation and regulate recovery phase of collagen-induced arthritis. J Immunol (1996) 156:4520–6.
19. Filaci G, Bacilieri S, Fravega M, Monetti M, Contini P, Ghio M, et al. Impairment of CD8+ T suppressor cell function in patients with active systemic lupus erythematosus. J Immunol (2001) 166:6452–7. doi:10.4049/jimmunol.166.10.6452
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
20. Tulunay A, Yavuz S, Direskeneli H, Eksioglu-Demiralp E. CD8+CD28-, suppressive T cells in systemic lupus erythematosus. Lupus (2008) 17:630–7. doi:10.1177/0961203308089400
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
21. Zhang L, Bertucci AM, Ramsey-Goldman R, Burt RK, Datta SK. Regulatory T cell (Treg) subsets return in patients with refractory lupus following stem cell transplantation, and TGF-beta-producing CD8+ Treg cells are associated with immunological remission of lupus. J Immunol (2009) 183:6346–58. doi:10.4049/jimmunol.0901773
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
22. Brimnes J, Allez M, Dotan I, Shao L, Nakazawa A, Mayer L. Defects in CD8+ regulatory T cells in the lamina propria of patients with inflammatory bowel disease. J Immunol (2005) 174:5814–22. doi:10.4049/jimmunol.174.9.5814
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
23. Baughman EJ, Mendoza JP, Ortega SB, Ayers CL, Greenberg BM, Frohman EM, et al. Neuroantigen-specific CD8+ regulatory T-cell function is deficient during acute exacerbation of multiple sclerosis. J Autoimmun (2011) 36:115–24. doi:10.1016/j.jaut.2010.12.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
24. Correale J, Villa A. Role of CD8+ CD25+ Foxp3+ regulatory T cells in multiple sclerosis. Ann Neurol (2010) 67:625–38. doi:10.1002/ana.21944
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
25. Frisullo G, Nociti V, Iorio R, Plantone D, Patanella AK, Tonali PA, et al. CD8(+)Foxp3(+) T cells in peripheral blood of relapsing-remitting multiple sclerosis patients. Hum Immunol (2010) 71:437–41. doi:10.1016/j.humimm.2010.01.024
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
26. Kreft KL, Verbraak E, Wierenga-Wolf AF, Laman JD, Hintzen RQ. Role of CD8 regulatory T-cells in multiple sclerosis. Ann Neurol (2011) 69:593. doi:10.1002/ana.22288
27. Hahn BH, Singh RP, La Cava A, Ebling FM. Tolerogenic treatment of lupus mice with consensus peptide induces Foxp3-expressing, apoptosis-resistant, TGFbeta-secreting CD8+ T cell suppressors. J Immunol (2005) 175:7728–37. doi:10.4049/jimmunol.175.11.7728
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
28. Chaput N, Louafi S, Bardier A, Charlotte F, Vaillant J-C, Ménégaux F, et al. Identification of CD8+CD25+Foxp3+ suppressive T cells in colorectal cancer tissue. Gut (2009) 58:520–9. doi:10.1136/gut.2008.158824
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
29. Singh RP, La Cava A, Wong M, Ebling F, Hahn BH. CD8+ T cell-mediated suppression of autoimmunity in a murine lupus model of peptide-induced immune tolerance depends on Foxp3 expression. J Immunol (2007) 178:7649–57. doi:10.4049/jimmunol.178.12.7649
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
30. Singh RP, La Cava A, Hahn BH. pConsensus peptide induces tolerogenic CD8+ T cells in lupus-prone (NZB x NZW)F1 mice by differentially regulating Foxp3 and PD1 molecules. J Immunol (2008) 180:2069–80. doi:10.4049/jimmunol.180.4.2069
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
31. Malek TR. The biology of interleukin-2. Annu Rev Immunol (2008) 26:453–79. doi:10.1146/annurev.immunol.26.021607.090357
32. Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V, et al. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med (2011) 365:2067–77. doi:10.1056/NEJMoa1105143
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
33. Rosenzwajg M, Churlaud G, Mallone R, Six A, Dérian N, Chaara W, et al. Low-dose interleukin-2 fosters a dose-dependent regulatory T cell tuned milieu in T1D patients. J Autoimmun (2015) 58:48–58. doi:10.1016/j.jaut.2015.01.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
34. Hartemann A, Bensimon G, Payan CA, Jacqueminet S, Bourron O, Nicolas N, et al. Low-dose interleukin 2 in patients with type 1 diabetes: a phase 1/2 randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol (2013) 1:295–305. doi:10.1016/S2213-8587(13)70113-X
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
35. Churlaud G, Jimenez V, Ruberte J, Amadoudji Zin M, Fourcade G, Gottrand G, et al. Sustained stimulation and expansion of Tregs by IL2 control autoimmunity without impairing immune responses to infection, vaccination and cancer. Clin Immunol (2014) 151:114–26. doi:10.1016/j.clim.2014.02.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
36. Dupont G, Demaret J, Venet F, Malergue F, Malcus C, Poitevin-Later F, et al. Comparative dose-responses of recombinant human IL-2 and IL-7 on STAT5 phosphorylation in CD4+FOXP3- cells versus regulatory T cells: a whole blood perspective. Cytokine (2014) 69:146–9. doi:10.1016/j.cyto.2014.05.021
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
37. Nigam P, Velu V, Kannanganat S, Chennareddi L, Kwa S, Siddiqui M, et al. Expansion of FOXP3+ CD8 T cells with suppressive potential in colorectal mucosa following a pathogenic simian immunodeficiency virus infection correlates with diminished antiviral T cell response and viral control. J Immunol (2010) 184:1690–701. doi:10.4049/jimmunol.0902955
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
38. Karlsson I, Malleret B, Brochard P, Delache B, Calvo J, Le Grand R, et al. FoxP3+ CD25+ CD8+ T-cell induction during primary simian immunodeficiency virus infection in cynomolgus macaques correlates with low CD4+ T-cell activation and high viral load. J Virol (2007) 81:13444–55. doi:10.1128/JVI.01619-07
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
39. Ito T, Hanabuchi S, Wang Y-H, Park WR, Arima K, Bover L, et al. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity (2008) 28:870–80. doi:10.1016/j.immuni.2008.03.018
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
40. Koch SD, Uss E, van Lier RAW, ten Berge IJM. Alloantigen-induced regulatory CD8+CD103+ T cells. Hum Immunol (2008) 69:737–44. doi:10.1016/j.humimm.2008.08.281
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
41. Allakhverdi Z, Fitzpatrick D, Boisvert A, Baba N, Bouguermouh S, Sarfati M, et al. Expression of CD103 identifies human regulatory T-cell subsets. J Allergy Clin Immunol (2006) 118:1342–9. doi:10.1016/j.jaci.2006.07.034
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
42. Oderup C, Cederbom L, Makowska A, Cilio CM, Ivars F. Cytotoxic T lymphocyte antigen-4-dependent down-modulation of costimulatory molecules on dendritic cells in CD4+ CD25+ regulatory T-cell-mediated suppression. Immunology (2006) 118:240–9. doi:10.1111/j.1365-2567.2006.02362.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
43. Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med (2000) 192:303–10. doi:10.1084/jem.192.2.303
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
44. Keino H, Masli S, Sasaki S, Streilein JW, Stein-Streilein J. CD8+ T regulatory cells use a novel genetic program that includes CD103 to suppress Th1 immunity in eye-derived tolerance. Invest Ophthalmol Vis Sci (2006) 47:1533–42. doi:10.1167/iovs.04-1454
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
45. Aoyama A, Klarin D, Yamada Y, Boskovic S, Nadazdin O, Kawai K, et al. Low-dose IL-2 for In vivo expansion of CD4+ and CD8+ regulatory T cells in nonhuman primates. Am J Transplant (2012) 12:2532–7. doi:10.1111/j.1600-6143.2012.04133.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
46. Mayer CT, Floess S, Baru AM, Lahl K, Huehn J, Sparwasser T. CD8+ Foxp3+ T cells share developmental and phenotypic features with classical CD4+ Foxp3+ regulatory T cells but lack potent suppressive activity. Eur J Immunol (2011) 41:716–25. doi:10.1002/eji.201040913
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
47. Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol (2012) 12:180–90. doi:10.1038/nri3156
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
48. Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity (2010) 33:153–65. doi:10.1016/j.immuni.2010.08.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
49. Chen CY, Huang D, Yao S, Halliday L, Zeng G, Wang RC, et al. IL-2 simultaneously expands Foxp3+ T regulatory and T effector cells and confers resistance to severe tuberculosis (TB): implicative Treg-T effector cooperation in immunity to TB. J Immunol (2012) 188:4278–88. doi:10.4049/jimmunol.1101291
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
50. Jebbawi F, Fayyad-Kazan H, Merimi M, Lewalle P, Verougstraete J-C, Leo O, et al. A microRNA profile of human CD8. J Transl Med (2014) 12:218. doi:10.1186/s12967-014-0218-x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
51. Cosmi L, Liotta F, Lazzeri E, Francalanci M, Angeli R, Mazzinghi B, et al. Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood (2003) 102:4107–14. doi:10.1182/blood-2003-04-1320
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
52. Eusebio M, Kraszula L, Kupczyk M, Kuna P, Pietruczuk M. Low frequency of CD8+CD25+FOXP3(BRIGHT) T cells and FOXP3 mRNA expression in the peripheral blood of allergic asthma patients. J Biol Regul Homeost Agents (2012) 26:211–20.
53. Kiniwa Y, Miyahara Y, Wang HY, Peng W, Peng G, Wheeler TM, et al. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res (2007) 13:6947–58. doi:10.1158/1078-0432.CCR-07-0842
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: immunological tolerance, immunotherapy, autoimmunity, T cell biology, immune response
Citation: Churlaud G, Pitoiset F, Jebbawi F, Lorenzon R, Bellier B, Rosenzwajg M and Klatzmann D (2015) Human and mouse CD8+CD25+FOXP3+ regulatory T cells at steady state and during interleukin-2 therapy. Front. Immunol. 6:171. doi: 10.3389/fimmu.2015.00171
Received: 28 November 2014; Accepted: 27 March 2015;
Published online: 15 April 2015.
Edited by:
Anne L. Astier, University of Edinburgh, UKReviewed by:
Carole Guillonneau, Centre national de la recherche scientifique (CNRS), FranceMargarita Dominguez-Villar, Yale School of Medicine, USA
Copyright: © 2015 Churlaud, Pitoiset, Jebbawi, Lorenzon, Bellier, Rosenzwajg and Klatzmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David Klatzmann, Department of Inflammation-Immunopathology-Biotherapy (I2B), Clinical Investigation Center for Biotherapies (CIC-BTi), Hôpital Pitié-Salpêtrière, Assistance Publique Hôpitaux de Paris (AP-HP), 83 Bd de l’Hôpital, Paris F-75013, France e-mail: david.klatzmann@upmc.fr
† Guillaume Churlaud and Fabien Pitoiset have contributed equally to this work.
 Guillaume Churlaud
Guillaume Churlaud Fabien Pitoiset
Fabien Pitoiset Fadi Jebbawi
Fadi Jebbawi Roberta Lorenzon1,2,3
Roberta Lorenzon1,2,3 Bertrand Bellier
Bertrand Bellier David Klatzmann
David Klatzmann