- 1Centre de Recherche en Transplantation et Immunologie UMR1064, INSERM, Université de Nantes, Nantes, France
- 2Institut de Transplantation Urologie Néphrologie (ITUN), CHU Nantes, Nantes, France
- 3IHU Cesti, Nantes, France
- 4Labex Immunotherapy Graft Oncology (IGO), Nantes, France
Over the last decade, C-type lectin-like receptors (CTLRs), expressed mostly by myeloid cells, have gained increasing attention for their role in the fine tuning of both innate and adaptive immunity. Not only CTLRs recognize pathogen-derived ligands to protect against infection but also endogenous ligands such as self-carbohydrates, proteins, or lipids to control homeostasis and tissue injury. Interestingly, CTLRs act as antigen-uptake receptors via their carbohydrate-recognition domain for internalization and subsequent presentation to T-cells. Furthermore, CTLRs signal through a complex intracellular network leading to the secretion of a particular set of cytokines that differently polarizes downstream effector T-cell responses according to the ligand and pattern recognition receptor co-engagement. Thus, by orchestrating the balance between inflammatory and resolution pathways, CTLRs are now considered as driving players of sterile inflammation whose dysregulation leads to the development of various pathologies such as autoimmune diseases, allergy, or cancer. For examples, the macrophage-inducible C-type lectin (MINCLE), by sensing glycolipids released during cell-damage, promotes skin allergy and the pathogenesis of experimental autoimmune uveoretinitis. Besides, recent studies described that tumors use physiological process of the CTLRs’ dendritic cell-associated C-type lectin-1 (DECTIN-1) and MINCLE to locally suppress myeloid cell activation and promote immune evasion. Therefore, we aim here to overview the current knowledge of the pivotal role of CTLRs in sterile inflammation with special attention given to the “Dectin-1” and “Dectin-2” families. Moreover, we will discuss the potential of these receptors as promising therapeutic targets to treat a wide range of acute and chronic diseases.
Introduction
C-type lectin receptors (CLRs) are a large family of transmembrane and soluble receptors that contain one or more carbohydrate-recognition domain able to recognize a wide variety of glycans on pathogens or on self-proteins. The hallmark of classical CLRs is the dependence on Ca2+ for glycan recognition. However, many other CLRs lack the coordinated Ca2+ ions and are therefore referred as C-type lectin-like molecules. These C-type lectin-like receptors (CTLRs) are still able to recognize carbohydrates but independently of Ca2+ but also recognize more diverse ligands such as lipids and proteins (1). Of particular interest for their role in coupling both innate and adaptive immunity, are the CTLR genes of the “Dectin-1” and “Dectin-2” families localized on the telomeric region of the natural killer cluster of genes (2, 3). These two groups of CTLRs are expressed mostly by cells of myeloid lineage such as monocytes, macrophages, dendritic cells (DCs), and neutrophils. CTLRs not only serve as antigen-uptake receptors for internalization and presentation to T cells but also trigger multiple signaling pathways leading to NF-κB, type I interferon (IFN), and/or inflammasome activation (1–4). This leads, in turn, to the production of pro- or anti-inflammatory cytokines and chemokines, subsequently fine tuning adaptive immune responses. CTLRs can signal either directly, through integral signaling domains, or indirectly, by associating with adaptor molecules. As illustrated in Figure 1, activation of immune-receptor tyrosine-based activation motif (ITAM) directly or via adaptor proteins such as FcγR, leads to the recruitment of SYK family kinases and the formation of the Card9/Bcl10/Malt1 complex that downstream activates NF-κB pathway and various cellular responses. By contrast, activation of immune-receptor tyrosine-based inhibition motif (ITIM) induces the recruitment and activation of protein tyrosine phosphatases such as SHP-1 and SHP-2 and the dephosphorylation of motifs (1). Consequently, ITIM signaling can inhibit cellular activation mediated by other immunoreceptors to tightly regulate immune response. Such checkpoints allow to prevent uncontrolled immune responses that may lead to harmful, or even fatal, consequences. In addition, some CTLRs were also reported to signal via SYK-independent pathway through the serine/threonine kinase RAF-1 to drive particular Th differentiation (5). Besides, by integrating simultaneous signals from other pattern recognition receptors (PRRs), CTLRs can exert synergistic or antagonistic response to achieve appropriate biological responses (6). This cross talk is regulated by the level and localization of their expression, by their interaction and by their collaborative or conflicting signaling (6, 7). To date, CTLRs “Dectin” families were best known for their involvement in host defense as referred in these excellent reviews (1–4, 8, 9). However, over recent years, these receptors have gained growing interest for their ability to respond also to a wide variety of endogenous ligands (Figure 1). Identification of self-glycans, lipids, or proteins expressed or released by modified or damaged cells reinforced the hypothesis for their implication in sterile inflammation whose dysregulation foster the development of wide range of diseases (10). In this mini review, we aim to focus on some of the CTLRs of the “Dendritic cell-associated C-type lectin (Dectin)” families, discussing the recent discoveries on their implication in the control of tissue injury, autoimmune diseases, or tumorigenesis. In addition, we will underscore their therapeutic potential and impact on human health.
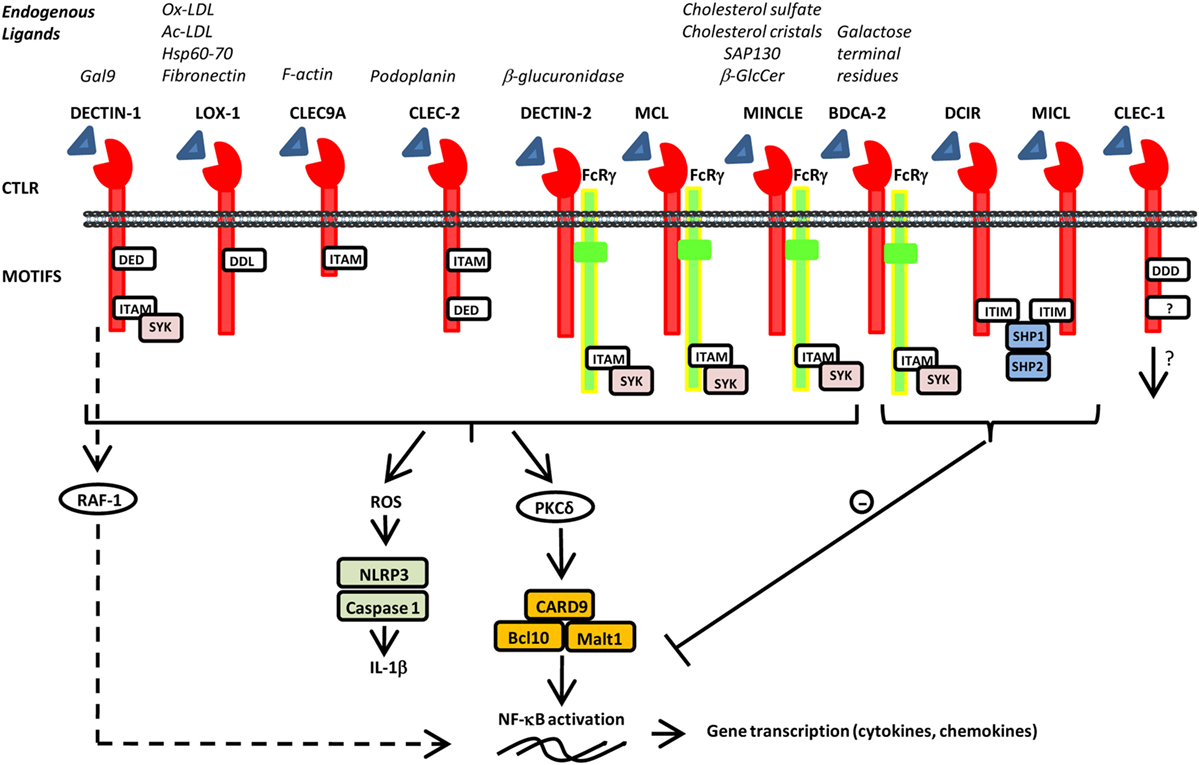
Figure 1. Schematic representation of various C-type lectin-like receptors (CTLRs) and selected endogenous ligands and signals. CTLRs are composed of an extracellular C-type lectin-like domain able to recognize various endogenous ligands and signal directly, through integral motifs in their cytoplasmic tails or indirectly through association with FcRγ. They can also contain a tri-acidic domain DED or DDD important for phagocytosis. Activation of immune-receptor tyrosine-based activation motif (ITAM) leads to the recruitment and activation of SYK family kinases. Subsequent activation of the CARD9–Bcl10–Malt1 complex through PKδ induces NF-κB activation and gene transcription of various cytokine and chemokines. Furthermore, SYK induces reactive oxygen species production and inflammasome activation via NLRP3 and Caspase 1 leading to IL-1β production. Alternative pathway of signalization independently of SYK has been reported for dendritic cell-associated C-type lectin-1 (DECTIN-1) via RAF-1 to finely regulate NF-κB activation. By contrast, activation of immune-receptor tyrosine-based inhibition motif (ITIM) induces the recruitment and activation of protein tyrosine phosphatases such as SHP-1 and SHP-2 and the dephosphorylation of motifs to inhibit cellular activation mediated by other immunoreceptors.
“(DECTIN-1)” Family
DECTIN-1 (Alias CLEC7A, CLECSF12, CANDF4, CD369, BGR)
The CTLR, DECTIN-1 has been reported to be enhanced by pro-inflammatory conditions (11, 12) and to be a potent inducer of Th1 and/or Th17 responses in response to pathogens (2). Thereby, pathogenic ligands of DECTIN-1 are currently used to bolster immune responses notably in cancer. For example, administration of β glucans was shown to inhibit tumor growth in murine carcinoma models (13–15), in human melanoma, neuroblastoma, mastocytosis, and lymphoma xenograft models (16, 17) and in ovarian (18, 19), breast (20), lung (14, 21–23), and gastric cancer (19, 24). Mechanistically, β glucans were shown to convert immunosuppressive macrophages into an M1-like antitumoral phenotype (25), to promote NK (26) and CD8+ T cell cytotoxicity (27) as well as a decrease in myeloid-derived suppressor cells and regulatory T cells (13, 28). Interestingly, Zhao et al. recently reported that β glucans upregulate particularly the expression of TNFSF15 and OX40L in DCs in mice, thus promoting efficient Th9 priming and potent anti-melanoma response following vaccination (29). On the contrary, some investigations have described an inhibitory function of DECTIN-1 in sterile inflammation notably during hepatic fibrosis and hepatocellular carcinoma (30). Authors showed that DECTIN-1 inhibit TLR4 signaling and downstream inflammation such as TNFα, IL-6, and chemokines secretion (30). Moreover, DECTIN-1 was reported to be associated with mechanisms of peritumoral immune tolerance by programming suppressive macrophages in pancreatic ductal adenocarcinoma (31). Strikingly, they showed that blockade of DECTIN-1 or its endogenous ligand Galectin-9, both strongly expressed on infiltrating myeloid cells and tumor, delayed tumor progression and extended mice survival. A similar tolerogenic signal of DECTIN-1 has been shown in myeloid cells in response to mucus in the intestine through interaction with Galectin-3 (32). In addition, DECTIN-1-deficient mice were described to exacerbate inflammation in a model of colitis suggesting an important role of DECTIN-1 in gut homeostasis (33). Therefore, DECTIN-1 seems to act as double-edged swords on the regulation of inflammation. Such discrepancy may depend of the type of the response, the nature and the property of the ligands, and of the complex signal network that integrates diverse engaged PRRs.
LOX-1 (Alias OLR-1, CLEC8A)
The CTLR lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) is particularly expressed by endothelial cells and platelets and is upregulated during inflammatory and pathological conditions (34–36). By recognizing oxidized and acetylated low-density lipoproteins, LOX-1 is largely described to play critical functions in vascular diseases, including atherosclerosis (37). However, recent investigations have revealed that LOX-1 is also expressed by human macrophages (38) and DCs (39), and its triggering increases secretion of IL-6 to potentiate B-cell class-switch (39). Moreover, LOX-1 enhances CCR10, APRIL, and BAFF secretion for plasma cell differentiation and migration to mucosal site. In line with these findings, targeting influenza histocompatibility antigen-1 to LOX-1 elicits antigen-specific protective antibody response to virus in macaques suggesting a good candidate for vaccine development (39). Besides, several studies have reported a high expression of LOX-1 in various tumors including gastric (40), colorectal (41), and prostate (42) cancers, which correlates with a poor prognosis in patients (40). Functionally, LOX-1 was shown to promote tumor angiogenesis (42), metastasis (43), and the migration and invasion of gastric cancer cells by notably driving epithelial–mesenchymal transition (40). Interestingly, LOX-1 was recently identified to be particularly expressed by potent polymorphonuclear myeloid-derived suppressor cells from blood and tumor of patients with non-small cell lung or head neck cancer and to be associated with worse survival (44). In fact, LOX-1 expression seems to be upregulated following endoplasmic reticulum stress that occurs during hypoxia or nutrient deprivation inside tumors. These data render this marker an attractive therapeutic target as well as a diagnostic tool for cancer screening (41).
CLEC-1 (Alias CLEC1, CLEC1A)
Although the C-type lectin-like receptor-1 (CLEC-1) was identified a long time ago (45, 46), the downstream signaling and ligand(s) remain uncharacterized (8, 47). We and others described CLEC-1 expression in human and rodent by myeloid cells such as monocytes, DC, and macrophages but also by endothelial cells (8, 9, 46, 48). CLEC-1 expression is decreased by pro-inflammatory stimuli and is enhanced by TGFβ (8, 9, 48). Interestingly, CLEC-1 was found to be expressed mostly intracellular particularly in human endothelial cells and neutrophils (8, 9), suggesting the requirement of particular conditions for cell-surface expression or for recycling from intracellular pools (7). Alternatively, CLEC-1 may play a role in intracellular organelles. Using CLEC-1-deficient rodents, we showed that disruption of CLEC-1 signaling enhances Il12p40 subunit expression in DCs and accordingly exacerbates downstream CD4+ Th1 and Th17 responses following in vivo immunization with exogeneous antigens (9, 48).
CLEC-2 (Alias CLEC2, CLEC2B, CLEC1B)
C-type lectin-like receptor 2 (CLEC-2) is found on platelets and DCs and is largely described for its interaction with its endogeneous ligand podoplanin expressed by lymphatic endothelial cells, myeloid cells, and fibroblast reticular cells (2). The CLEC-2/Podoplanin axis was shown to be critical in platelet activation (49), lymph node microarchitecture (50, 51), reticular network (52), and vascular integrity. Besides, this interaction promotes tumor cell-induced platelet aggregation, tumor growth, and metastasis (53–56) in various types of cancer including brain, lung, and larynx (57–60). Furthermore, CLEC-2 is enhanced by inflammation, promotes DC migration (61) and together with LPS enhances the production of the anti-inflammatory cytokine IL-10 suggesting also a role in the resolution of inflammation (62).
MICL (Alias CLEC12A, DCAL-2, CLL1, CLL-1, KLRL1)
Myeloid inhibitory C-type lectin-like receptor (MICL) is expressed predominantly by granulocytes and monocytes, and its expression is downregulated by pro-inflammatory stimuli (63–65). MICL recruits inhibitory phosphatases and again seems to differently shape T-cell responses according to the cross talk with simultaneous PRR signals. Chen and colleges demonstrated that co-engagement of MICL with TLR4 suppress IL-12 expression in human DCs and downstream Th1 polarization whereas co-engagement with CD40 does the opposite (66). Interestingly, putative endogenous ligands of MICL were identified on various mouse tissues in steady-state conditions, proposing a role for MICL in the control of homeostasis and self-tolerance (65). Corroborating this notion, an inhibitory function for MICL has been put in light in an in vivo model of induced rheumatoid arthritis (67). In an original way, MICL was proposed to modulate myeloid cell activation threshold by acting as an autoantigen during arthritis development (67).
CLEC9A (Alias DNGR1, DNGR-1, CD370)
CLEC9A is selectively expressed on the mouse subsets of CD8α+ and CD103+ DCs, and on their human BDCA3+ DCs counterparts (68). CLEC9A expression is lost further TLR-induced maturation. Importantly, CLEC9A by recognizing F-actin released by necrotic cells is capable of internalizing bound dead cell-associated antigens for cross-presentation to CD8+ T cells (69–71). Thereby, CLEC9A has been demonstrated to be a powerful target for peptide vaccination to boost antitumor immunity (72, 73). Interestingly, it has recently been shown that necrotic debris that accumulated during atherosclerosis development, trigger through CLEC9A, the downregulation of the anti-inflammatory cytokine IL-10 and the disease progression (74).
“DECTIN-2” Family
DCIR (Alias CLEC4A, CLECSF6, CD367, LLIR)
DC immunoreceptor (DCIR) is expressed on monocytes, neutrophils, DC, and plasmacytoid DCs, and its expression is decreased by pro-inflammatory stimuli (75). The human genome encodes only a single DCIR gene, whereas the mouse genome presents four DCIR-like genes (DCIR1–4) (76). DCIR via its canonical ITIM domain is largely recognized to exert inhibitory cross talk with other PRRs to maintain immune homeostasis and prevent excessive detrimental inflammation and immunopathogenesis (77–79). DCIR inhibits TLR8-induced IL-12 and TNFα production in human moDCs following cross-linking with monoclonal antibody (79). Furthermore, DCIR1 KO mice develop a late spontaneous autoimmune disease associated with elevated levels of autoantibodies, are more susceptible to collagen-induced arthritis, and aggravated experimental autoimmune encephalomyelitis (80, 81). These effects were described to be mediated at least by unrestrained growth of DC population in these mice. However, in support for a role of DCIR in tempering DC activation, a recent study demonstrated that DCIR2 selectively expressed by mouse CD8α− DCs, strongly moderates pro-inflammatory and downstream T-cell responses (82). In vivo, DCIR2-deficient mice are more susceptible to endotoxic shock and aggravate experimental autoimmune encephalomyelitis development by increasing both Th1 and Th17 differentiation. Authors demonstrated that putative endogeneous ligands of DCIR are expressed also on cell surface of DCs. In line with these data, DCIR2 was described in DCs to sustain STAT-1 type I IFN signaling leading to a reduction of IL-12p70 production and Th1 differentiation in response to endogeneous ligand(s) released during cell culture (83). Therefore, by regulating also the IFN responses, DCIR may be a critical player in the control of a number of inflammatory diseases. Interestingly, DCIR was also reported to bind to commensal intestinal microbes (84). However, DCIR-deficient mice only exhibit a slightly increased severity of colitis in a dextran sulfate sodium model (84).
DECTIN-2 (Alias CLEC6A, CLEC4N, CLECSF10)
Several studies suggest a role for DC-associated C-type lectin-2 (DECTIN-2) in the inhibition of sterile inflammation. DECTIN-2 is enhanced in pro-inflammatory conditions and was shown notably to bind to a putative ligand on regulatory CD4+CD25+ T cells to mediate ultraviolet radiation-induced tolerance (85, 86). In addition, DECTIN-2 recognizes glycan mannose on the lysosomal enzyme β-glucuronidase, known to moderate arthritis pathogenesis by preventing accumulation of pro-inflammatory glycosaminoglycans within inflamed joint tissue (87–90). Thus, β-glucuronidase released by dead myeloid cells following tissue damage may act via DECTIN-2 as an inhibitory loop in DCs to temper inflammation (91). Besides, polymorphism of this enzyme was reported to be associated with mucopolysaccharidoses characterized by a pro-inflammatory response (92). In addition, a role for DECTIN-2 in suppression of liver metastasis has been highlighted by its ability to phagocytose cancer cells via CD11b F4/80 Kupffer cells during extravasation step (93).
BDCA-2 (Alias CLEC4C, BDCA2, CD303, CLECSF11, CLECSF7)
Interestingly, blood DC antigen-2 (BDCA-2) is the most specific marker for human plasmacytoid DC but intriguingly is not expressed in mice (94). Expression of BDCA-2 is downregulated following maturation (95). Surprisingly, unlike many other ITAM-coupled receptors, signaling through BDCA-2 inhibits activation of the NF-κB pathway and the production of type I IFNs and cytokines in response to TLR9 ligands or following recognition of galactose terminal residues notably expressed on tumor cells (94, 96, 97). BDCA-2 engagement was also shown to block TRAIL-mediated cytotoxic activity (98). In an interestingly way, BDCA-2 was suggested to function as an Fc receptor by binding glycans on immunoglobulins G (99) and thus, dampens down inflammation in response to rising levels of serum immunoglobulins G.
MINCLE (Alias CLEC4E, CLECSF9)
Macrophage-inducible C-type lectin (MINCLE) is an ITAM-coupled CTLR that forms a heterodimer with the macrophage C-type lectin (MCL). MINCLE expression is enhanced after exposure to pro-inflammatory stimuli or cellular stresses and is translocated to the cell surface via interaction with the stalk region of MCL (100–104). MINCLE was shown to activate in DCs both NF-κB and inflammasome to greatly enhance IL-1β expression in synergy with TLR7/8 (R848) or following in vivo immunization with Freund adjuvant (105, 106). MINCLE senses self-damage by recognizing “unfamiliar” glycolipids that are not present in the extracellular milieu under normal, healthy conditions. For example, MINCLE was reported to bind crystalline cholesterol present in atheriosclerotic plaques that are associated with inflammation and macrophage infiltrates (107). Likewise, as depicted in Figure 2, MINCLE is enhanced on plasmacytoid DCs following skin damage and by recognizing cholesterol sulfate, induces IL-1 α and β secretion, and promotes skin allergy and allergic contact dermatitis (108). Moreover, MINCLE was also reported to bind to the ubiquitous intracellular metabolite β-glucosylceramide released by damaged cells to promote production of pro-inflammatory cytokines by myeloid cells (109). In an opposite way, recent investigations have revealed that MINCLE rather than purely inducing pro-inflammatory responses can also promote the expression of the anti-inflammatory cytokines IL-10 (110). In addition, MINCLE was reported to counter regulate pro-inflammatory signaling pathways mediated by DECTIN-1 to temper IL12p35 production (6, 111). Therefore, MINCLE seems to also exert opposite role on immune responses depending of the ligands and PRR interference. This dual effect is illustrated by the recognition by MINCLE of the spliceosome-associated protein 130 (SAP130), a component of small nuclear ribonucleoprotein released during non homeostatic cell death. On one hand, MINCLE/SAP130 axis was shown to be involved in the pathogenesis of inflammation during tissue damages (112) or ischemia/reperfusion (113, 114) and to contribute to the development of experimental autoimmune uveoretinitis (115). This pro-inflammatory side of MINCLE is supported by a high expression of MINCLE in patients with rheumatoid arthritis (116) and by the link to arthritis of the rat chromosome 4q42 encoding Mincle (117). On the other hand, in the context of cancer, MINCLE/SAP130 axis was reported to be pro-tumorigenic in mouse and human pancreatic ductal adenocarcinoma (118). Both MINCLE and SAP130 released by programmed necrosis are highly expressed in mouse and human carcinoma and as depicted in Figure 2, this interaction leads to an immunosuppressive reprogramming of infiltrating myeloid cells (118). Future research is required to provide insight as to how MINCLE needs to integrate with other PRR signals to differently define the type of immune response.
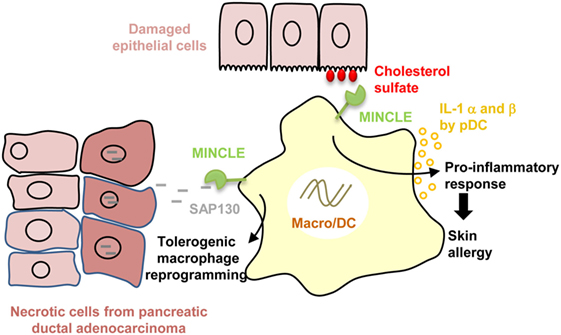
Figure 2. Dual role of macrophage-inducible C-type lectin (MINCLE) in disease pathogenesis. Recognition of cholesterol sulfate by MINCLE whose expression is increased in plasmacytoid dendritic cells (DC) following skin damage, induces IL-1 α and β secretion, and promotes skin allergy and allergic contact dermatitis. By contrast, MINCLE recognition of spliceosome-associated protein 130 (SAP130) released by necrotic cancer cells leads to tolerogenic tumor-infiltrating macrophage reprogramming in pancreatic ductal adenocarcinoma.
Therapeutic Potential of CTLRs
Therefore, by their capacity to present antigen and ensure the balance between cellular activation and suppression, CTLRs have emerged as challenging pharmacological targets to treat a wide variety of diseases governed by sterile inflammation including cancers, autoimmune diseases or allergy (1, 119). Ligands such as carbohydrate structures, antibodies, or mimetic peptides could be therapeutically exploited as agonists or antagonists of CTLR signaling. As previously mentioned, the DECTIN-1 agonist β-glucans is used to elicit of potent antitumor immune responses in various types of cancer (14, 16–24). Furthermore, CTLRs such as DEC-205 (120, 121) or CLEC9A (71) have been exploited for the in vivo delivery target of vaccine antigens in cancer (122). In addition, synthetic ligands of MINCLE were generated to specifically enhance immune response (102). Besides, several specific antibodies generated against cancer-specific highly glycosylated podoplanin were shown to efficiently block the CLEC-2/Podoplanin interaction, subsequent platelet aggregation and tumor metastasis (123–128). Importantly, a particular antibody that reacts with podoplanin-expressing cancer cells but not with the one from normal cells has been successfully generated and will be useful for molecular targeting therapy against podoplanin-expressing cancer cells only (126). However, since CTLRs have overlapping ligands that induce distinct and even contrasting immune responses, antibodies targeting specific CTLRs could be more appropriate. Also, inhibitors such as recombinant peptide spanning the CTLR binding region and modulating the receptor–ligand interaction could be considered. Only a few drug-like molecules have been developed for the CTLR family (129) but studies indicate high in silico druggability scores as well as high experimental hit rates from peptide fragment screenings (130, 131).
To conclude, CTLR modulation seems to represent promising strategy for disease management although attempts at identifying endogenous ligands as well as efforts to elucidate their role in sterile inflammation are still warrant.
Author Contributions
The author confirms being the sole contributor of this work and approved it for publication.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
The author is grateful to the support by the “CENTAURE” foundation, “Agence de la Biomédecine 2015–2016,” IHU-Cesti project, and the LabEX IGO program which received French government financial support managed by the National Research Agency via the investment of the future program ANR-10-IBHU-005 and ANR-11-LABX-0016-01.
References
1. Dambuza IM, Brown GD. C-type lectins in immunity: recent developments. Curr Opin Immunol (2015) 32:21–7. doi:10.1016/j.coi.2014.12.002
2. Huysamen C, Brown GD. The fungal pattern recognition receptor, dectin-1, and the associated cluster of C-type lectin-like receptors. FEMS Microbiol Lett (2009) 290(2):121–8. doi:10.1111/j.1574-6968.2008.01418.x
3. Kerscher B, Willment JA, Brown GD. The dectin-2 family of C-type lectin-like receptors: an update. Int Immunol (2013) 25(5):271–7. doi:10.1093/intimm/dxt006
4. Robinson MJ, Sancho D, Slack EC, LeibundGut-Landmann S, Reis e Sousa C. Myeloid C-type lectins in innate immunity. Nat Immunol (2006) 7(12):1258–65. doi:10.1038/ni1417
5. Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Wevers B, Bruijns SC, et al. Dectin-1 directs T helper cell differentiation by controlling noncanonical NF-kappaB activation through Raf-1 and Syk. Nat Immunol (2009) 10(2):203–13. doi:10.1038/ni.1692
6. Ostrop J, Lang R. Contact, collaboration, and conflict: signal integration of Syk-coupled C-type lectin receptors. J Immunol (2017) 198(4):1403–14. doi:10.4049/jimmunol.1601665
7. Garcia-Vallejo JJ, Bloem K, Knippels LM, Garssen J, van Vliet SJ, van Kooyk Y. The consequences of multiple simultaneous C-type lectin-ligand interactions: DCIR alters the endo-lysosomal routing of DC-SIGN. Front Immunol (2015) 6:87. doi:10.3389/fimmu.2015.00087
8. Sattler S, Reiche D, Sturtzel C, Karas I, Richter S, Kalb ML, et al. The human C-type lectin-like receptor CLEC-1 is upregulated by TGF-beta and primarily localized in the endoplasmic membrane compartment. Scand J Immunol (2012) 75(3):282–92. doi:10.1111/j.1365-3083.2011.02665.x
9. Lopez Robles MD, Pallier A, Huchet V, Le Texier L, Remy S, Braudeau C, et al. Cell-surface C-type lectin-like receptor CLEC-1 dampens dendritic cell activation and downstream Th17 responses. Blood Adv (2017) 1(9):557–68. doi:10.1182/bloodadvances.2016002360
10. Garcia-Vallejo JJ, van Kooyk Y. Endogenous ligands for C-type lectin receptors: the true regulators of immune homeostasis. Immunol Rev (2009) 230(1):22–37. doi:10.1111/j.1600-065X.2009.00786.x
11. Rogers H, Williams DW, Feng G-J, Lewis MAO, Wei X-Q. Role of bacterial lipopolysaccharide in enhancing host immune response to Candida albicans. Clin Dev Immunol (2013) 2013:320168. doi:10.1155/2013/320168
12. Willment JA, Lin HH, Reid DM, Taylor PR, Williams DL, Wong SY, et al. Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF-treated macrophages and are negatively regulated by IL-10, dexamethasone, and lipopolysaccharide. J Immunol (2003) 171(9):4569–73. doi:10.4049/jimmunol.171.9.4569
13. Tian J, Ma J, Ma K, Guo H, Baidoo SE, Zhang Y, et al. beta-Glucan enhances antitumor immune responses by regulating differentiation and function of monocytic myeloid-derived suppressor cells. Eur J Immunol (2013) 43(5):1220–30. doi:10.1002/eji.201242841
14. Li B, Cai Y, Qi C, Hansen R, Ding C, Mitchell TC, et al. Orally administered particulate beta-glucan modulates tumor-capturing dendritic cells and improves antitumor T-cell responses in cancer. Clin Cancer Res (2010) 16(21):5153–64. doi:10.1158/1078-0432.CCR-10-0820
15. Masuda Y, Inoue H, Ohta H, Miyake A, Konishi M, Nanba H. Oral administration of soluble beta-glucans extracted from Grifola frondosa induces systemic antitumor immune response and decreases immunosuppression in tumor-bearing mice. Int J Cancer (2013) 133(1):108–19. doi:10.1002/ijc.27999
16. Modak S, Koehne G, Vickers A, O’Reilly RJ, Cheung NK. Rituximab therapy of lymphoma is enhanced by orally administered (1 → 3),(1 → 4)-D-beta-glucan. Leuk Res (2005) 29(6):679–83. doi:10.1016/j.leukres.2004.10.008
17. Tokunaka K, Ohno N, Adachi Y, Miura NN, Yadomae T. Application of Candida solubilized cell wall beta-glucan in antitumor immunotherapy against P815 mastocytoma in mice. Int Immunopharmacol (2002) 2(1):59–67. doi:10.1016/S1567-5769(01)00148-5
18. Inoue M, Tanaka Y, Sugita N, Yamasaki M, Yamanaka T, Minagawa J, et al. Improvement of long-term prognosis in patients with ovarian cancers by adjuvant sizofiran immunotherapy: a prospective randomized controlled study. Biotherapy (1993) 6(1):13–8. doi:10.1007/BF01877381
19. Oba K, Kobayashi M, Matsui T, Kodera Y, Sakamoto J. Individual patient based meta-analysis of lentinan for unresectable/recurrent gastric cancer. Anticancer Res (2009) 29(7):2739–45.
20. Demir G, Klein HO, Mandel-Molinas N, Tuzuner N. Beta glucan induces proliferation and activation of monocytes in peripheral blood of patients with advanced breast cancer. Int Immunopharmacol (2007) 7(1):113–6. doi:10.1016/j.intimp.2006.08.011
21. Suzuki I, Sakurai T, Hashimoto K, Oikawa S, Masuda A, Ohsawa M, et al. Inhibition of experimental pulmonary metastasis of Lewis lung carcinoma by orally administered beta-glucan in mice. Chem Pharm Bull (Tokyo) (1991) 39(6):1606–8. doi:10.1248/cpb.39.1606
22. Murphy EA, Davis JM, Brown AS, Carmichael MD, Mayer EP, Ghaffar A. Effects of moderate exercise and oat beta-glucan on lung tumor metastases and macrophage antitumor cytotoxicity. J Appl Physiol (1985) (2004) 97(3):955–9. doi:10.1152/japplphysiol.00252.2004
23. Zhong W, Hansen R, Li B, Cai Y, Salvador C, Moore GD, et al. Effect of yeast-derived beta-glucan in conjunction with bevacizumab for the treatment of human lung adenocarcinoma in subcutaneous and orthotopic xenograft models. J Immunother (2009) 32(7):703–12. doi:10.1097/CJI.0b013e3181ad3fcf
24. Hazama S, Watanabe S, Ohashi M, Yagi M, Suzuki M, Matsuda K, et al. Efficacy of orally administered superfine dispersed lentinan (beta-1,3-glucan) for the treatment of advanced colorectal cancer. Anticancer Res (2009) 29(7):2611–7.
25. Liu M, Luo F, Ding C, Albeituni S, Hu X, Ma Y, et al. Dectin-1 activation by a natural product beta-glucan converts immunosuppressive macrophages into an M1-like phenotype. J Immunol (2015) 195(10):5055–65. doi:10.4049/jimmunol.1501158
26. Chiba S, Ikushima H, Ueki H, Yanai H, Kimura Y, Hangai S, et al. Recognition of tumor cells by dectin-1 orchestrates innate immune cells for anti-tumor responses. Elife (2014) 3:e04177. doi:10.7554/eLife.04177
27. Haas T, Heidegger S, Wintges A, Bscheider M, Bek S, Fischer JC, et al. Card9 controls dectin-1-induced T-cell cytotoxicity and tumor growth in mice. Eur J Immunol (2017) 47(5):872–9. doi:10.1002/eji.201646775
28. Hong F, Yan J, Baran JT, Allendorf DJ, Hansen RD, Ostroff GR, et al. Mechanism by which orally administered beta-1,3-glucans enhance the tumoricidal activity of antitumor monoclonal antibodies in murine tumor models. J Immunol (2004) 173(2):797–806. doi:10.4049/jimmunol.173.2.797
29. Zhao Y, Chu X, Chen J, Wang Y, Gao S, Jiang Y, et al. Dectin-1-activated dendritic cells trigger potent antitumour immunity through the induction of Th9 cells. Nat Commun (2016) 7:12368. doi:10.1038/ncomms12368
30. Seifert L, Deutsch M, Alothman S, Alqunaibit D, Werba G, Pansari M, et al. Dectin-1 regulates hepatic fibrosis and hepatocarcinogenesis by suppressing TLR4 signaling pathways. Cell Rep (2015) 13(9):1909–21. doi:10.1016/j.celrep.2015.10.058
31. Daley D, Mani VR, Mohan N, Akkad N, Ochi A, Heindel DW, et al. Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat Med (2017) 23(5):556–67. doi:10.1038/nm.4314
32. Shan M, Gentile M, Yeiser JR, Walland AC, Bornstein VU, Chen K, et al. Mucus enhances gut homeostasis and oral tolerance by delivering immunoregulatory signals. Science (2013) 342(6157):447–53. doi:10.1126/science.1237910
33. Iliev ID, Funari VA, Taylor KD, Nguyen Q, Reyes CN, Strom SP, et al. Interactions between commensal fungi and the C-type lectin receptor dectin-1 influence colitis. Science (2012) 336(6086):1314–7. doi:10.1126/science.1221789
34. Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature (1997) 386(6620):73–7. doi:10.1038/386073a0
35. Aoyama T, Chen M, Fujiwara H, Masaki T, Sawamura T. LOX-1 mediates lysophosphatidylcholine-induced oxidized LDL uptake in smooth muscle cells. FEBS Lett (2000) 467(2–3):217–20. doi:10.1016/S0014-5793(00)01154-6
36. Draude G, Lorenz RL. TGF-beta1 downregulates CD36 and scavenger receptor A but upregulates LOX-1 in human macrophages. Am J Physiol Heart Circ Physiol (2000) 278(4):H1042–8. doi:10.1152/ajpheart.2000.278.4.H1042
37. Chen M, Masaki T, Sawamura T. LOX-1, the receptor for oxidized low-density lipoprotein identified from endothelial cells: implications in endothelial dysfunction and atherosclerosis. Pharmacol Ther (2002) 95(1):89–100. doi:10.1016/S0163-7258(02)00236-X
38. Yoshida H, Kondratenko N, Green S, Steinberg D, Quehenberger O. Identification of the lectin-like receptor for oxidized low-density lipoprotein in human macrophages and its potential role as a scavenger receptor. Biochem J (1998) 334(Pt 1):9–13. doi:10.1042/bj3340009
39. Joo H, Li D, Dullaers M, Kim TW, Duluc D, Upchurch K, et al. C-type lectin-like receptor LOX-1 promotes dendritic cell-mediated class-switched B cell responses. Immunity (2014) 41(4):592–604. doi:10.1016/j.immuni.2014.09.009
40. Li C, Zhang J, Wu H, Li L, Yang C, Song S, et al. Lectin-like oxidized low-density lipoprotein receptor-1 facilitates metastasis of gastric cancer through driving epithelial-mesenchymal transition and PI3K/Akt/GSK3beta activation. Sci Rep (2017) 7:45275. doi:10.1038/srep45275
41. Murdocca M, Mango R, Pucci S, Biocca S, Testa B, Capuano R, et al. The lectin-like oxidized LDL receptor-1: a new potential molecular target in colorectal cancer. Oncotarget (2016) 7(12):14765–80. doi:10.18632/oncotarget.7430
42. Gonzalez-Chavarria I, Cerro RP, Parra NP, Sandoval FA, Zuniga FA, Omazabal VA, et al. Lectin-like oxidized LDL receptor-1 is an enhancer of tumor angiogenesis in human prostate cancer cells. PLoS One (2014) 9(8):e106219. doi:10.1371/journal.pone.0106219
43. Liang M, Zhang P, Fu J. Up-regulation of LOX-1 expression by TNF-alpha promotes trans-endothelial migration of MDA-MB-231 breast cancer cells. Cancer Lett (2007) 258(1):31–7. doi:10.1016/j.canlet.2007.08.003
44. Condamine T, Dominguez GA, Youn J-I, Kossenkov AV, Mony S, Alicea-Torres K, et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol (2016) 1(2):aaf8943. doi:10.1126/sciimmunol.aaf8943
45. Colonna M, Samaridis J, Angman L. Molecular characterization of two novel C-type lectin-like receptors, one of which is selectively expressed in human dendritic cells. Eur J Immunol (2000) 30(2):697–704. doi:10.1002/1521-4141(200002)30:2<697::AID-IMMU697>3.0.CO;2-M
46. Sobanov Y, Bernreiter A, Derdak S, Mechtcheriakova D, Schweighofer B, Duchler M, et al. A novel cluster of lectin-like receptor genes expressed in monocytic, dendritic and endothelial cells maps close to the NK receptor genes in the human NK gene complex. Eur J Immunol (2001) 31(12):3493–503. doi:10.1002/1521-4141(200112)31:12<3493::AID-IMMU3493>3.0.CO;2-9
47. Flornes LM, Nylenna O, Saether PC, Daws MR, Dissen E, Fossum S. The complete inventory of receptors encoded by the rat natural killer cell gene complex. Immunogenetics (2010) 62(8):521–30. doi:10.1007/s00251-010-0455-y
48. Thebault P, Lhermite N, Tilly G, Le Texier L, Quillard T, Heslan M, et al. The C-type lectin-like receptor CLEC-1, expressed by myeloid cells and endothelial cells, is up-regulated by immunoregulatory mediators and moderates T cell activation. J Immunol (2009) 183(5):3099–108. doi:10.4049/jimmunol.0803767
49. Suzuki-Inoue K, Inoue O, Ozaki Y. Novel platelet activation receptor CLEC-2: from discovery to prospects. J Thromb Haemost (2011) 9(Suppl 1):44–55. doi:10.1111/j.1538-7836.2011.04335.x
50. Herzog BH, Fu J, Wilson SJ, Hess PR, Sen A, McDaniel JM, et al. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature (2013) 502(7469):105–9. doi:10.1038/nature12501
51. Benezech C, Nayar S, Finney BA, Withers DR, Lowe K, Desanti GE, et al. CLEC-2 is required for development and maintenance of lymph nodes. Blood (2014) 123(20):3200–7. doi:10.1182/blood-2013-03-489286
52. Acton SE, Farrugia AJ, Astarita JL, Mourao-Sa D, Jenkins RP, Nye E, et al. Dendritic cells control fibroblastic reticular network tension and lymph node expansion. Nature (2014) 514(7523):498–502. doi:10.1038/nature13814
53. Suzuki-Inoue K, Kato Y, Inoue O, Kaneko MK, Mishima K, Yatomi Y, et al. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J Biol Chem (2007) 282(36):25993–6001. doi:10.1074/jbc.M702327200
54. Christou CM, Pearce AC, Watson AA, Mistry AR, Pollitt AY, Fenton-May AE, et al. Renal cells activate the platelet receptor CLEC-2 through podoplanin. Biochem J (2008) 411(1):133–40. doi:10.1042/BJ20071216
55. Kato Y, Kaneko MK, Kunita A, Ito H, Kameyama A, Ogasawara S, et al. Molecular analysis of the pathophysiological binding of the platelet aggregation-inducing factor podoplanin to the C-type lectin-like receptor CLEC-2. Cancer Sci (2008) 99(1):54–61. doi:10.1111/j.1349-7006.2007.00634.x
56. Lowe KL, Navarro-Nunez L, Watson SP. Platelet CLEC-2 and podoplanin in cancer metastasis. Thromb Res (2012) 129(Suppl 1):S30–7. doi:10.1016/S0049-3848(12)70013-0
57. Mishima K, Kato Y, Kaneko MK, Nishikawa R, Hirose T, Matsutani M. Increased expression of podoplanin in malignant astrocytic tumors as a novel molecular marker of malignant progression. Acta Neuropathol (2006) 111(5):483–8. doi:10.1007/s00401-006-0063-y
58. Kato Y, Fujita N, Kunita A, Sato S, Kaneko M, Osawa M, et al. Molecular identification of Aggrus/T1alpha as a platelet aggregation-inducing factor expressed in colorectal tumors. J Biol Chem (2003) 278(51):51599–605. doi:10.1074/jbc.M309935200
59. Rodrigo JP, Garcia-Carracedo D, Gonzalez MV, Mancebo G, Fresno MF, Garcia-Pedrero J. Podoplanin expression in the development and progression of laryngeal squamous cell carcinomas. Mol Cancer (2010) 9:48. doi:10.1186/1476-4598-9-48
60. Kato Y, Kaneko M, Sata M, Fujita N, Tsuruo T, Osawa M. Enhanced expression of Aggrus (T1alpha/podoplanin), a platelet-aggregation-inducing factor in lung squamous cell carcinoma. Tumour Biol (2005) 26(4):195–200. doi:10.1159/000086952
61. Acton SE, Astarita JL, Malhotra D, Lukacs-Kornek V, Franz B, Hess PR, et al. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the C-type lectin receptor CLEC-2. Immunity (2012) 37(2):276–89. doi:10.1016/j.immuni.2012.05.022
62. Mourao-Sa D, Robinson MJ, Zelenay S, Sancho D, Chakravarty P, Larsen R, et al. CLEC-2 signaling via Syk in myeloid cells can regulate inflammatory responses. Eur J Immunol (2011) 41(10):3040–53. doi:10.1002/eji.201141641
63. Marshall AS, Willment JA, Lin HH, Williams DL, Gordon S, Brown GD. Identification and characterization of a novel human myeloid inhibitory C-type lectin-like receptor (MICL) that is predominantly expressed on granulocytes and monocytes. J Biol Chem (2004) 279(15):14792–802. doi:10.1074/jbc.M313127200
64. Marshall AS, Willment JA, Pyz E, Dennehy KM, Reid DM, Dri P, et al. Human MICL (CLEC12A) is differentially glycosylated and is down-regulated following cellular activation. Eur J Immunol (2006) 36(8):2159–69. doi:10.1002/eji.200535628
65. Pyz E, Huysamen C, Marshall AS, Gordon S, Taylor PR, Brown GD. Characterisation of murine MICL (CLEC12A) and evidence for an endogenous ligand. Eur J Immunol (2008) 38(4):1157–63. doi:10.1002/eji.200738057
66. Chen CH, Floyd H, Olson NE, Magaletti D, Li C, Draves K, et al. Dendritic-cell-associated C-type lectin 2 (DCAL-2) alters dendritic-cell maturation and cytokine production. Blood (2006) 107(4):1459–67. doi:10.1182/blood-2005-08-3264
67. Redelinghuys P, Whitehead L, Augello A, Drummond RA, Levesque JM, Vautier S, et al. MICL controls inflammation in rheumatoid arthritis. Ann Rheum Dis (2016) 75(7):1386–91. doi:10.1136/annrheumdis-2014-206644
68. Schreibelt G, Klinkenberg LJ, Cruz LJ, Tacken PJ, Tel J, Kreutz M, et al. The C-type lectin receptor CLEC9A mediates antigen uptake and (cross-) presentation by human blood BDCA3+ myeloid dendritic cells. Blood (2012) 119(10):2284–92. doi:10.1182/blood-2011-08-373944
69. Ahrens S, Zelenay S, Sancho D, Hanc P, Kjaer S, Feest C, et al. F-actin is an evolutionarily conserved damage-associated molecular pattern recognized by DNGR-1, a receptor for dead cells. Immunity (2012) 36(4):635–45. doi:10.1016/j.immuni.2012.03.008
70. Zhang JG, Czabotar PE, Policheni AN, Caminschi I, Wan SS, Kitsoulis S, et al. The dendritic cell receptor Clec9A binds damaged cells via exposed actin filaments. Immunity (2012) 36(4):646–57. doi:10.1016/j.immuni.2012.03.009
71. Sancho D, Mourao-Sa D, Joffre OP, Schulz O, Rogers NC, Pennington DJ, et al. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest (2008) 118(6):2098–110. doi:10.1172/JCI34584
72. Yan Z, Wu Y, Du J, Li G, Wang S, Cao W, et al. A novel peptide targeting Clec9a on dendritic cell for cancer immunotherapy. Oncotarget (2016) 7(26):40437–50. doi:10.18632/oncotarget.9624
73. Tullett KM, Leal Rojas IM, Minoda Y, Tan PS, Zhang JG, Smith C, et al. Targeting CLEC9A delivers antigen to human CD141+ DC for CD4+ and CD8+T cell recognition. JCI Insight (2016) 1(7):e87102. doi:10.1172/jci.insight.87102
74. Haddad Y, Lahoute C, Clement M, Laurans L, Metghalchi S, Zeboudj L, et al. The dendritic cell receptor DNGR-1 promotes the development of atherosclerosis in mice. Circ Res (2017) 121(3):234–43. doi:10.1161/CIRCRESAHA.117.310960
75. Bates EE, Fournier N, Garcia E, Valladeau J, Durand I, Pin JJ, et al. APCs express DCIR, a novel C-type lectin surface receptor containing an immunoreceptor tyrosine-based inhibitory motif. J Immunol (1999) 163(4):1973–83.
76. Flornes LM, Bryceson YT, Spurkland A, Lorentzen JC, Dissen E, Fossum S. Identification of lectin-like receptors expressed by antigen presenting cells and neutrophils and their mapping to a novel gene complex. Immunogenetics (2004) 56(7):506–17. doi:10.1007/s00251-004-0714-x
77. Lambert AA, Barabe F, Gilbert C, Tremblay MJ. DCIR-mediated enhancement of HIV-1 infection requires the ITIM-associated signal transduction pathway. Blood (2011) 117(24):6589–99. doi:10.1182/blood-2011-01-331363
78. Massoud AH, Yona M, Xue D, Chouiali F, Alturaihi H, Ablona A, et al. Dendritic cell immunoreceptor: a novel receptor for intravenous immunoglobulin mediates induction of regulatory T cells. J Allergy Clin Immunol (2014) 133(3):853–63.e5. doi:10.1016/j.jaci.2013.09.029
79. Meyer-Wentrup F, Cambi A, Joosten B, Looman MW, de Vries IJ, Figdor CG, et al. DCIR is endocytosed into human dendritic cells and inhibits TLR8-mediated cytokine production. J Leukoc Biol (2009) 85(3):518–25. doi:10.1189/jlb.0608352
80. Fujikado N, Saijo S, Yonezawa T, Shimamori K, Ishii A, Sugai S, et al. DCIR deficiency causes development of autoimmune diseases in mice due to excess expansion of dendritic cells. Nat Med (2008) 14(2):176–80. doi:10.1038/nm1697
81. Seno A, Maruhashi T, Kaifu T, Yabe R, Fujikado N, Ma G, et al. Exacerbation of experimental autoimmune encephalomyelitis in mice deficient for DCIR, an inhibitory C-type lectin receptor. Exp Anim (2015) 64(2):109–19. doi:10.1538/expanim.14-0079
82. Uto T, Fukaya T, Takagi H, Arimura K, Nakamura T, Kojima N, et al. Clec4A4 is a regulatory receptor for dendritic cells that impairs inflammation and T-cell immunity. Nat Commun (2016) 7:11273. doi:10.1038/ncomms11273
83. Troegeler A, Mercier I, Cougoule C, Pietretti D, Colom A, Duval C, et al. C-type lectin receptor DCIR modulates immunity to tuberculosis by sustaining type I interferon signaling in dendritic cells. Proc Natl Acad Sci U S A (2017) 114(4):E540–9. doi:10.1073/pnas.1613254114
84. Hütter J, Eriksson M, Johannssen T, Klopfleisch R, von Smolinski D, Gruber AD, et al. Role of the C-type lectin receptors MCL and DCIR in experimental colitis. PLoS One (2014) 9(7):e103281. doi:10.1371/journal.pone.0103281
85. Taylor PR, Reid DM, Heinsbroek SEM, Brown GD, Gordon S, Wong SYC. Dectin-2 is predominantly myeloid restricted and exhibits unique activation-dependent expression on maturing inflammatory monocytes elicited in vivo. Eur J Immunol (2005) 35(7):2163–74. doi:10.1002/eji.200425785
86. Aragane Y, Maeda A, Schwarz A, Tezuka T, Ariizumi K, Schwarz T. Involvement of dectin-2 in ultraviolet radiation-induced tolerance. J Immunol (2003) 171(7):3801–7. doi:10.4049/jimmunol.171.7.3801
87. Depke M, Breitbach K, Dinh Hoang Dang K, Brinkmann L, Salazar MG, Dhople VM, et al. Bone marrow-derived macrophages from BALB/c and C57BL/6 mice fundamentally differ in their respiratory chain complex proteins, lysosomal enzymes and components of antioxidant stress systems. J Proteomics (2014) 103:72–86. doi:10.1016/j.jprot.2014.03.027
88. Mori D, Shibata K, Yamasaki S. C-type lectin receptor dectin-2 binds to an endogenous protein beta-glucuronidase on dendritic cells. PLoS One (2017) 12(1):e0169562. doi:10.1371/journal.pone.0169562
89. McGreal EP, Rosas M, Brown GD, Zamze S, Wong SY, Gordon S, et al. The carbohydrate-recognition domain of dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology (2006) 16(5):422–30. doi:10.1093/glycob/cwj077
90. Robinson MJ, Osorio F, Rosas M, Freitas RP, Schweighoffer E, Gross O, et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med (2009) 206(9):2037–51. doi:10.1084/jem.20082818
91. Bramwell KK, Ma Y, Weis JH, Chen X, Zachary JF, Teuscher C, et al. Lysosomal beta-glucuronidase regulates Lyme and rheumatoid arthritis severity. J Clin Invest (2014) 124(1):311–20. doi:10.1172/JCI72339
92. Tomatsu S, Montano AM, Dung VC, Grubb JH, Sly WS. Mutations and polymorphisms in GUSB gene in mucopolysaccharidosis VII (sly syndrome). Hum Mutat (2009) 30(4):511–9. doi:10.1002/humu.20828
93. Kimura Y, Inoue A, Hangai S, Saijo S, Negishi H, Nishio J, et al. The innate immune receptor dectin-2 mediates the phagocytosis of cancer cells by Kupffer cells for the suppression of liver metastasis. Proc Natl Acad Sci U S A (2016) 113(49):14097–102. doi:10.1073/pnas.1617903113
94. Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med (2001) 194(12):1823–34. doi:10.1084/jem.194.12.1823
95. Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, et al. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol (2000) 165(11):6037–46. doi:10.4049/jimmunol.165.11.6037
96. Rock J, Schneider E, Grun JR, Grutzkau A, Kuppers R, Schmitz J, et al. CD303 (BDCA-2) signals in plasmacytoid dendritic cells via a BCR-like signalosome involving Syk, Slp65 and PLCgamma2. Eur J Immunol (2007) 37(12):3564–75. doi:10.1002/eji.200737711
97. Riboldi E, Daniele R, Parola C, Inforzato A, Arnold PL, Bosisio D, et al. Human C-type lectin domain family 4, member C (CLEC4C/BDCA-2/CD303) is a receptor for asialo-galactosyl-oligosaccharides. J Biol Chem (2011) 286(41):35329–33. doi:10.1074/jbc.C111.290494
98. Riboldi E, Daniele R, Cassatella MA, Sozzani S, Bosisio D. Engagement of BDCA-2 blocks TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. Immunobiology (2009) 214(9–10):868–76. doi:10.1016/j.imbio.2009.06.016
99. Jegouzo SA, Feinberg H, Dungarwalla T, Drickamer K, Weis WI, Taylor ME. A novel mechanism for binding of galactose-terminated glycans by the C-type carbohydrate recognition domain in blood dendritic cell antigen 2. J Biol Chem (2015) 290(27):16759–71. doi:10.1074/jbc.M115.660613
100. Matsumoto M, Tanaka T, Kaisho T, Sanjo H, Copeland NG, Gilbert DJ, et al. A novel LPS-inducible C-type lectin is a transcriptional target of NF-IL6 in macrophages. J Immunol (1999) 163(9):5039–48.
101. Lobato-Pascual A, Saether PC, Fossum S, Dissen E, Daws MR. Mincle, the receptor for mycobacterial cord factor, forms a functional receptor complex with MCL and FcepsilonRI-gamma. Eur J Immunol (2013) 43(12):3167–74. doi:10.1002/eji.201343752
102. Ostrop J, Jozefowski K, Zimmermann S, Hofmann K, Strasser E, Lepenies B, et al. Contribution of MINCLE-SYK signaling to activation of primary human APCs by mycobacterial cord factor and the novel adjuvant TDB. J Immunol (2015) 195(5):2417–28. doi:10.4049/jimmunol.1500102
103. Hupfer T, Schick J, Jozefowski K, Voehringer D, Ostrop J, Lang R. Stat6-dependent inhibition of MINCLE expression in mouse and human antigen-presenting cells by the Th2 cytokine IL-4. Front Immunol (2016) 7:423. doi:10.3389/fimmu.2016.00423
104. Miyake Y, Masatsugu OH, Yamasaki S. C-type lectin receptor MCL facilitates MINCLE expression and signaling through complex formation. J Immunol (2015) 194(11):5366–74. doi:10.4049/jimmunol.1402429
105. van Haren SD, Dowling DJ, Foppen W, Christensen D, Andersen P, Reed SG, et al. Age-specific adjuvant synergy: dual TLR7/8 and MINCLE activation of human newborn dendritic cells enables Th1 polarization. J Immunol (2016) 197(11):4413–24. doi:10.4049/jimmunol.1600282
106. Shenderov K, Barber DL, Mayer-Barber KD, Gurcha SS, Jankovic D, Feng CG, et al. Cord factor and peptidoglycan recapitulate the Th17-promoting adjuvant activity of mycobacteria through mincle/CARD9 signaling and the inflammasome. J Immunol (2013) 190(11):5722–30. doi:10.4049/jimmunol.1203343
107. Kiyotake R, Oh-Hora M, Ishikawa E, Miyamoto T, Ishibashi T, Yamasaki S. Human MINCLE binds to cholesterol crystals and triggers innate immune responses. J Biol Chem (2015) 290(42):25322–32. doi:10.1074/jbc.M115.645234
108. Kostarnoy AV, Gancheva PG, Lepenies B, Tukhvatulin AI, Dzharullaeva AS, Polyakov NB, et al. Receptor MINCLE promotes skin allergies and is capable of recognizing cholesterol sulfate. Proc Natl Acad Sci U S A (2017) 114(13):E2758–65. doi:10.1073/pnas.1611665114
109. Nagata M, Izumi Y, Ishikawa E, Kiyotake R, Doi R, Iwai S, et al. Intracellular metabolite beta-glucosylceramide is an endogenous MINCLE ligand possessing immunostimulatory activity. Proc Natl Acad Sci U S A (2017) 114(16):E3285–94. doi:10.1073/pnas.1618133114
110. Patin EC, Willcocks S, Orr S, Ward TH, Lang R, Schaible UE. Mincle-mediated anti-inflammatory IL-10 response counter-regulates IL-12 in vitro. Innate Immun (2016) 22(3):181–5. doi:10.1177/1753425916636671
111. Wevers BA, Kaptein TM, Zijlstra-Willems EM, Theelen B, Boekhout T, Geijtenbeek TB, et al. Fungal engagement of the C-type lectin MINCLE suppresses dectin-1-induced antifungal immunity. Cell Host Microbe (2014) 15(4):494–505. doi:10.1016/j.chom.2014.03.008
112. Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. MINCLE is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol (2008) 9(10):1179–88. doi:10.1038/ni.1651
113. Suzuki Y, Nakano Y, Mishiro K, Takagi T, Tsuruma K, Nakamura M, et al. Involvement of MINCLE and Syk in the changes to innate immunity after ischemic stroke. Sci Rep (2013) 3:3177. doi:10.1038/srep03177
114. Arumugam TV, Manzanero S, Furtado M, Biggins PJ, Hsieh YH, Gelderblom M, et al. An atypical role for the myeloid receptor MINCLE in central nervous system injury. J Cereb Blood Flow Metab (2017) 37(6):2098–111. doi:10.1177/0271678X16661201
115. Lee EJ, Brown BR, Vance EE, Snow PE, Silver PB, Heinrichs D, et al. MINCLE activation and the Syk/Card9 signaling axis are central to the development of autoimmune disease of the eye. J Immunol (2016) 196(7):3148–58. doi:10.4049/jimmunol.1502355
116. Nakamura N, Shimaoka Y, Tougan T, Onda H, Okuzaki D, Zhao H, et al. Isolation and expression profiling of genes upregulated in bone marrow-derived mononuclear cells of rheumatoid arthritis patients. DNA Res (2006) 13(4):169–83. doi:10.1093/dnares/dsl006
117. Ribbhammar U, Flornes L, Backdahl L, Luthman H, Fossum S, Lorentzen JC. High resolution mapping of an arthritis susceptibility locus on rat chromosome 4, and characterization of regulated phenotypes. Hum Mol Genet (2003) 12(17):2087–96. doi:10.1093/hmg/ddg224
118. Seifert L, Werba G, Tiwari S, Giao Ly NN, Alothman S, Alqunaibit D, et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and mincle-induced immune suppression. Nature (2016) 532(7598):245–9. doi:10.1038/nature17403
119. Yan H, Kamiya T, Suabjakyong P, Tsuji NM. Targeting C-type lectin receptors for cancer immunity. Front Immunol (2015) 6:408. doi:10.3389/fimmu.2015.00408
120. Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med (2002) 196(12):1627–38. doi:10.1084/jem.20021598
121. Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med (2004) 199(6):815–24. doi:10.1084/jem.20032220
122. Temizoz B, Kuroda E, Ishii KJ. Vaccine adjuvants as potential cancer immunotherapeutics. Int Immunol (2016) 28(7):329–38. doi:10.1093/intimm/dxw015
123. Takagi S, Sato S, Oh-hara T, Takami M, Koike S, Mishima Y, et al. Platelets promote tumor growth and metastasis via direct interaction between Aggrus/podoplanin and CLEC-2. PLoS One (2013) 8(8):e73609. doi:10.1371/journal.pone.0073609
124. Kaneko MK, Nakamura T, Honma R, Ogasawara S, Fujii Y, Abe S, et al. Development and characterization of anti-glycopeptide monoclonal antibodies against human podoplanin, using glycan-deficient cell lines generated by CRISPR/Cas9 and TALEN. Cancer Med (2017) 6(2):382–96. doi:10.1002/cam4.954
125. Kaneko MK, Yamada S, Nakamura T, Abe S, Nishioka Y, Kunita A, et al. Antitumor activity of chLpMab-2, a human-mouse chimeric cancer-specific antihuman podoplanin antibody, via antibody-dependent cellular cytotoxicity. Cancer Med (2017) 6(4):768–77. doi:10.1002/cam4.1049
126. Kato Y, Kaneko MK. A cancer-specific monoclonal antibody recognizes the aberrantly glycosylated podoplanin. Sci Rep (2014) 4:5924. doi:10.1038/srep05924
127. Chang Y-W, Hsieh P-W, Chang Y-T, Lu M-H, Huang T-F, Chong K-Y, et al. Identification of a novel platelet antagonist that binds to CLEC-2 and suppresses podoplanin-induced platelet aggregation and cancer metastasis. Oncotarget (2015) 6(40):42733–48. doi:10.18632/oncotarget.5811
128. Shirai T, Inoue O, Tamura S, Tsukiji N, Sasaki T, Endo H, et al. C-type lectin-like receptor 2 promotes hematogenous tumor metastasis and prothrombotic state in tumor-bearing mice. J Thromb Haemost (2017) 15(3):513–25. doi:10.1111/jth.13604
129. Lang R, Schoenen H, Desel C. Targeting Syk-Card9-activating C-type lectin receptors by vaccine adjuvants: findings, implications and open questions. Immunobiology (2011) 216(11):1184–91. doi:10.1016/j.imbio.2011.06.005
130. Chang J, Patton JT, Sarkar A, Ernst B, Magnani JL, Frenette PS. GMI-1070, a novel pan-selectin antagonist, reverses acute vascular occlusions in sickle cell mice. Blood (2010) 116(10):1779–86. doi:10.1182/blood-2009-12-260513
Keywords: C-type lectin-like receptors, sterile inflammation, autoimmune diseases, tissue injury, cancer
Citation: Chiffoleau E (2018) C-Type Lectin-Like Receptors As Emerging Orchestrators of Sterile Inflammation Represent Potential Therapeutic Targets. Front. Immunol. 9:227. doi: 10.3389/fimmu.2018.00227
Received: 13 October 2017; Accepted: 26 January 2018;
Published: 15 February 2018
Edited by:
Bernd Lepenies, University of Veterinary Medicine, GermanyReviewed by:
Luisa Martinez-Pomares, University of Nottingham, United KingdomTaruna Madan, National Institute for Research in Reproductive Health, India
Copyright: © 2018 Chiffoleau. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elise Chiffoleau, elise.chiffoleau@univ-nantes.fr
 Elise Chiffoleau
Elise Chiffoleau