- 1Department of Gastroenterology and Hepatology, Kindai University Faculty of Medicine, Osaka-Sayama, Japan
- 2Department of Pathology, Kindai University Faculty of Medicine, Osaka-Sayama, Japan
- 3Department of Gastroenterology and Hepatology, Katsuragi Hospital, Kishiwada, Japan
Cap polyposis is a rare gastrointestinal disease characterized by multiple inflammatory polyps located between the distal colon and the rectum. Despite the lack of clarity regarding its pathogenesis, mucosal prolapse, chronic inflammatory responses, and Helicobacter pylori infection are considered key contributors to the development of this disease entity. Although it is now generally accepted that dysbiosis of gut microbiota is associated with intestinal and extra-intestinal diseases, alterations of intestinal microbiota have been poorly defined in cap polyposis. Here, we report a patient with H. pylori-negative cap polyposis who was successfully treated with antibiotics and exhibited dramatic alterations in intestinal microbiota composition after antibiotic treatment. The patient was treated with oral administration of ampicillin and metronidazole and showed regression of cap polyposis 6 months after antibiotic treatment. Fecal microbiota analysis using the next-generation sequencing technology revealed a significant alteration in the intestinal microbiota composition following antibiotic treatment—a marked reduction of Blautia, Dorea, and Sutterella was observed concomitant with a marked increase in Fusobacterium. These data suggest that cap polyposis may originate from dysbiosis and that microbiome-targeted therapy may be useful in this disorder.
Highlights
• Cap polyposis patient.
• Cap polyposis is considered as multiple inflammatory polyps.
• Regression of colonic polyposis after antibiotic treatment.
• Next-generation sequencing reveals dynamic changes in the intestinal microbiota composition following antibiotic treatment.
• Identification of pathogenic bacteria associated with cap polyposis.
Introduction
Cap polyposis is a rare disease characterized by multiple inflammatory polyps that are covered by a cap of fibrinopurulent mucus and are located between the distal colon and the rectum (1–3). Patients usually present with abdominal pain, blood and/or mucus in diarrheal stool, and hypoproteinemia (1–3). Microscopically, the colonic polyps are characterized by a cap of fibrinopurulent exudates, distorted glands, fibromuscular obliteration of the lamina propria with inflammatory cell infiltration (1–3). Although the pathogenesis of cap polyposis remains unknown, the clinical and histopathological features of this disorder resemble those observed in patients with mucosal prolapse syndrome (MPS) (4). Therefore, mucosal prolapse secondary to impaired colonic motility has been considered a possible etiological contributor to cap polyposis (4). Several reports have shown regression of cap polyposis following the use of infliximab (2) or steroid (3), thereby demonstrating the possible involvement of chronic inflammatory responses in the development of cap polyposis. Reportedly, eradication of Helicobacter pylori is effective in the management of cap polyposis (5, 6). However, H. pylori were not detected in the colonic mucosa in any of these cases. Therefore, it is possible that unidentified intestinal bacteria, sensitive to H. pylori-eradication therapy, may contribute to the development of cap polyposis. Considering that eradication of H. pylori causes a significant alteration in the intestinal microbiota composition, these case reports suggest that dysbiosis-related immune responses may underlie the pathogenesis of cap polyposis. However, the intestinal microbiota composition has not been determined in this condition.
We report a patient with H. pylori-negative cap polyposis who was successfully treated using antibiotics. Fecal microbiota analysis using the next-generation sequencing (NGS) technology revealed a significant alteration in the intestinal microbiota composition pre- and post-antibiotic treatment. The results of our study strongly support the contributory role of dysbiosis in the pathogenesis of cap polyposis.
Case Report
An asymptomatic 45-year-old man without a relevant past or family history of gastrointestinal disease underwent a colonoscopic and esophagogastroduodenoscopic examination for the evaluation of a positive fecal occult blood test. Colonoscopic examination revealed multiple sessile polyps in the descending colon, which showed a reddish surface covered by white mucus (Figure 1A).Esophagogastroduodenoscopic examination revealed multiple fundic gland polyps. Serum anti-H. pylori antibody titers were below the detection limit, and serum total protein and albumin levels were within the reference range, as was the complete blood cell count. Endoscopic mucosal resection was performed to determine a histopathological diagnosis of the colonic polyps. The resected specimen showed mucus-containing distorted glands and significant inflammatory cell infiltration with fibrosis in the lamina propria (Figure 1B) and their surface was covered by inflammatory granulation tissue and fibrinopurulent exudate. These endoscopic and histopathological findings were consistent with those typically observed in patients with cap polyposis (1–3). Thus, this patient was diagnosed with cap polyposis without H. pylori infection.
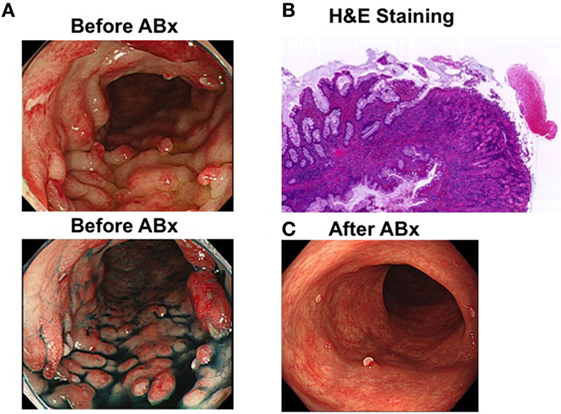
Figure 1. Endoscopic and pathological findings in a patient with cap polyposis. (A) Endoscopic images in a patient with cap polyposis before antibiotic treatment (before ABx). Multiple sessile polyps are observed in the descending colon prior to the initiation of antibiotic treatment. Top and bottom panels show white light endoscopy and chromoendoscopy images, respectively. The polyps appear reddish in color and are covered by white mucus. (B) Microscopic pictures of a patient with cap polyposis. Endoscopic mucosal resection was performed to obtain a histopathological diagnosis. Low magnification revealed inflammatory polyps covered with granulation tissue and fibrinopurulent exudate. Distorted glands were also seen in lamina propria. Hematoxylin and eosin (H&E) staining. Magnification ×40.(C) Endoscopic images obtained from a patient with cap polyposis treated with antibiotics (after ABx). Most of the inflammatory polyps are observed to have disappeared 6 months post-antibiotic treatment.
Helicobacter pylori infection has been considered a possible etiological contributor to the development of cap polyposis because eradication of this organism is observed to cause regression of colonic polyps in some patients (5, 6). However, notably, H. pylori have not been detected in the colonic mucosa in any patient diagnosed with cap polyposis. Thus, gut bacteria sensitive to the antibiotic component of H. pylori-eradication therapy are likely to play a pathogenic role in patients with cap polyposis. Based on this hypothesis, this patient was treated with oral administration of ampicillin (1,500 mg/day) and metronidazole (500 mg/day) for 1 week, and regression of cap polyposis was observed 6 months post-antibiotic treatment (Figure 1C). This clinical course strongly suggests that antibiotic-induced eradication of pathogenic gut bacteria responsible for the development of inflammatory polyps can cause regression of cap polyposis.
Fecal Microbiota Analysis
Stool samples pre- and post-antibiotic treatment were subjected to fecal microbiota analysis, which was performed as previously described (7, 8) to assess any alterations in the intestinal microbiota composition. Ethical approval for this study was granted by a Review Board of the Kindai University Faculty of Medicine. DNA samples extracted from the stool were subjected to polymerase chain reaction for the amplification of the 16S ribosomal RNA (16S rRNA) V3 and V4 regions. Primer sequences are available in our previous report (8). We performed 16S rRNA sequencing using the MiSeq system (Illumina) (7, 8). Trimmomatic, Cutadapt, and Fastq-join programs were used for sequence data processing, and operational taxonomic units (OTUs) were defined using the QIIME program (7, 8). The defined OTUs were subjected to population analysis to identify the bacterial phylum, class, order, family, and genus.
We detected 1,077 OTUs from 3 stool samples obtained pre- and post-antibiotic treatment. No major taxonomic alterations in the microbial communities were observed at the level of the phylum, order, or family (data not shown). Significant changes in the composition of fecal microbiota were noted at the genus level pre- and post-antibiotic treatment (Figure 2A). Blautia and Dorea, which showed a high relative abundance in the feces pre-antibiotic treatment, disappeared 1 week and 6 months post-antibiotic treatment (Figure 2B), and Sutterella disappeared 6 months post-antibiotic treatment. By contrast, the relative abundance of Fusobacterium was observed to have increased post-antibiotic treatment. The relative abundance of Bifidobacterium, Bacteroides, or Veillonella remained largely unchanged pre- and post-antibiotic treatment. This microbiota analysis suggests that regression of cap polyposis following antibiotic treatment is accompanied by a marked decrease in Blautia, Dorea, and Sutterella and a marked increase in Fusobacterium, indicating that cap polyposis might have originated from dysbiosis in this patient.
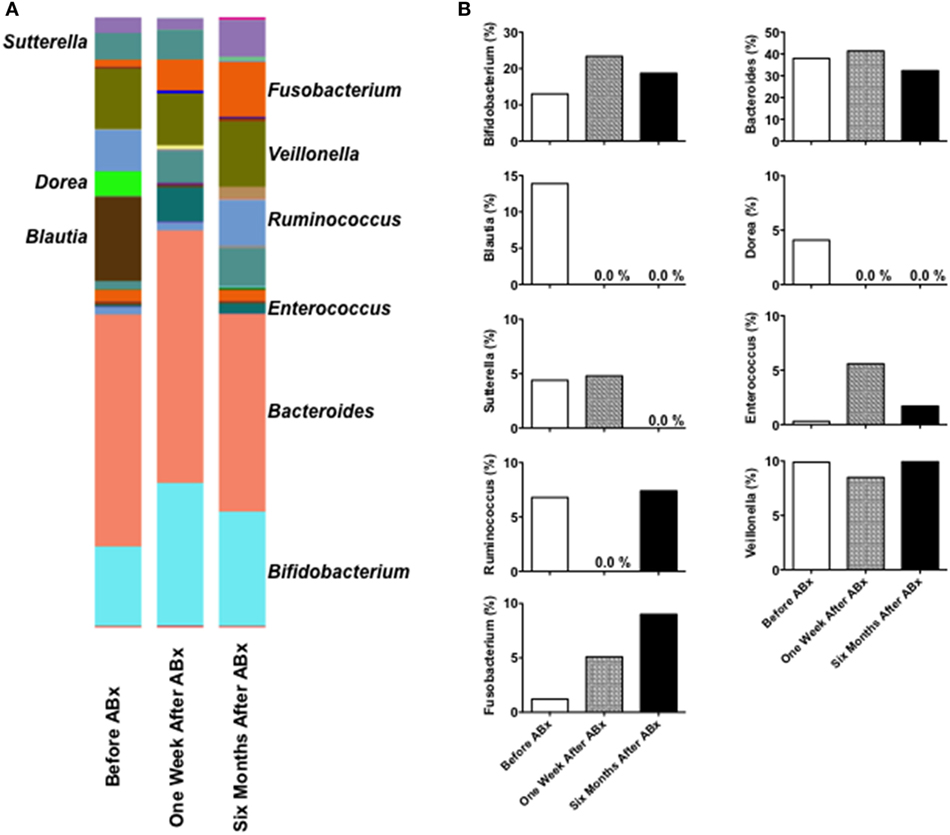
Figure 2. Fecal microbiota analysis in a patient with cap polyposis. (A) Stool samples were obtained from a patient with cap polyposis prior to, 1 week and 6 months post-antibiotic treatment. DNA samples extracted from the stool specimens were subjected to polymerase chain reaction for the amplification of the 16S ribosomal RNA (16S rRNA) V3 and V4 regions. We performed 16S rRNA sequencing using the MiSeq system. The relative abundance of different bacterial taxa at the genus level in each sample has been shown. (B) Comparative analysis of the taxonomic composition of the fecal microbial community at the genus level. Relative abundance of the genera has been shown as a percentage.
Discussion
Recent progress in NGS technology has highlighted the role of an altered intestinal microbiome (dysbiosis) in human diseases (9). Inflammatory bowel disease (IBD) is a prototypical dysbiosis-related disorder mediated by abnormal immune responses to altered intestinal microbiota (9). In this study, we report a patient with cap polyposis in whom antibiotic treatment resulted in regression of multiple inflammatory polyps. Interestingly, regression of cap polyposis was associated with significant alterations in the composition of the intestinal microbiota. Thus, we propose that cap polyposis might be a dysbiosis-related intestinal disorder. It should be noted, however, that we cannot exclude two other possibilities in the regression of cap polyposis. First, spontaneous regression might have occurred in antibiotic treatment-independent manner as previously reported (3). Second, anti-inflammatory responses leading to the regression of cap polyposis might be induced by antibiotic treatment.
Mucosal prolapse syndrome and cap polyposis share endoscopic findings in that reddish elevated mucus-covered lesions are common to both conditions (4). Histopathologically, both disorders are characterized by findings of superficial erosions covered by inflammatory granulation tissue, distorted and elongated glands, and fibromuscular obliteration of the lamina propria—all these being typical findings observed in our patient. These clinical and histopathological similarities lead us to the conclusion that MPS and cap polyposis share common pathogenetic mechanisms—mucosal prolapse secondary to impaired colonic motility noted in MPS has been considered a possible etiological factor in cap polyposis. However, MPS and cap polyposis differ in terms of treatment because suppression of a chronic inflammatory response using steroid or infliximab is often effective only in the latter (2, 3). Moreover, antibiotic-induced eradication of H. pylori or as yet unidentified bacteria can lead to regression of cap polyposis (5, 6). These reports strongly indicate the role of chronic immune reactions toward intestinal microflora in the pathogenesis of cap polyposis. Therefore, cap polyposis might be caused by impaired host-bacterial mutualism. We have demonstrated that antibiotic treatment leads to regression of cap polyposis through significant alterations in fecal microbiota composition. Our results strongly support the idea that impaired host-bacterial mutualism caused by dysbiosis underlies the pathogenesis of cap polyposis. Further studies are warranted to assess the intestinal microbiota composition in a larger number of samples with cap polyposis to validate our results.
Significant changes in fecal microbiota composition were observed at the genus levels pre- and post-antibiotic treatment. The relative abundance of Blautia, Dorea, and Sutterella was markedly decreased 6 months post-antibiotic treatment compared to the pre-antibiotic treatment finding, whereas the relative abundance of Fusobacterium was markedly increased post-antibiotic treatment. Thus, disappearance of Blautia, Dorea, and Sutterella and colonization of Fusobacterium were observed to be associated with regression of cap polyposis. Therefore, Blautia, Dorea, and Sutterella, and Fusobacterium might be considered pathogenic and beneficial bacteria, respectively, for cap polyposis. Consistent with these results, Nishino et al. have shown that mucosal microbiota composition in those with ulcerative colitis is characterized by a greater abundance of Blautia (10). An increased abundance of Dorea has been detected in the normal colonic mucosa in patients with colorectal adenomas (11). However, an increased percentage of Blautia and Dorea observed in the gut mucosal microbiota composition is reportedly associated with remission after surgery in IBD patients (12, 13). Mukhopadhya et al. have reported that Sutterella is unlikely to play a role in the pathogenesis of IBD (14). Moreover, Fusobacterium, which demonstrated higher relative abundance post-antibiotic treatment in this patient, has been shown to promote colorectal tumor growth (15). Although the discrepancy between our data and previous reports remains unexplained, it could be attributed to a likely difference between fecal and mucosal microbiota composition. Identification of pathogenic or beneficial bacteria for cap polyposis requires future studies that assess microbiota composition in a larger number of patients with cap polyposis. Fecal microbiota analyses in a large number of patients with cap polyposis are necessary to confirm the involvement of dysbiosis and to determine the sensitivity to antibiotic treatment in this disorder.
Concluding Remarks
To our knowledge, this is the first report describing intestinal microbiota analysis in a patient with cap polyposis. Our results strongly suggest that cap polyposis may originate from dysbiosis and that microbiome-targeted therapy may be useful in this disorder.
Ethics Statement
This study was carried out in accordance with the recommendations of a Review Board of the Kindai University Faculty of Medicine with written informed consent from the patient. The patient gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by a Review Board of the Kindai University Faculty of Medicine.
Author Contributions
KO, TW, YK, AO, HS, and KF took care of the patient. KO, TW, KM, KK, KY, MT, SH, and TS wrote the manuscript. TT performed pathological examinations. KO, TW, and NN performed experiments. MK supervised the research. All the coauthors checked the final version of the manuscript before the submission.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer MM and handling Editor declared their shared affiliation.
Funding
This work was supported by the Naito Foundation, SENSHIN Medical Research Foundation, the Yakult Bio-Science Foundation, the Smoking Research Foundation, the Kobayashi Foundation for Cancer Research, and the Takeda Science Foundation.
Abbreviations
IBD, inflammatory bowel disease; MPS, mucosal prolapse syndrome; NGS, next-generation sequencing; OTU, operational taxonomic unit; rRNA, ribosomal RNA.
References
1. Kini GP, Murray I, Champion-Young J, Lau M, Katta V, Thorn M, et al. Cap polyposis mistaken for Crohn’s disease: case report and review of literature. J Crohns Colitis (2013) 7(3):e108–11. doi:10.1016/j.crohns.2012.06.005
2. Bookman ID, Redston MS, Greenberg GR. Successful treatment of cap polyposis with infliximab. Gastroenterology (2004) 126(7):1868–71. doi:10.1053/j.gastro.2004.03.007
3. Chang HS, Yang SK, Kim MJ, Ye BD, Byeon JS, Myung SJ, et al. Long-term outcome of cap polyposis, with special reference to the effects of steroid therapy. Gastrointest Endosc (2012) 75(1):211–6. doi:10.1016/j.gie.2011.08.027
4. Oriuchi T, Kinouchi Y, Kimura M, Hiwatashi N, Hayakawa T, Watanabe H, et al. Successful treatment of cap polyposis by avoidance of intraluminal trauma: clues to pathogenesis. Am J Gastroenterol (2000) 95(8):2095–8. doi:10.1111/j.1572-0241.2000.02277.x
5. Akamatsu T, Nakamura N, Kawamura Y, Shinji A, Tateiwa N, Ochi Y, et al. Possible relationship between Helicobacter pylori infection and cap polyposis of the colon. Helicobacter (2004) 9(6):651–6. doi:10.1111/j.1083-4389.2004.00273.x
6. Oiya H, Okawa K, Aoki T, Nebiki H, Inoue T. Cap polyposis cured by Helicobacter pylori eradication therapy. J Gastroenterol (2002) 37(6):463–6. doi:10.1007/s005350200067
7. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods (2010) 7(5):335–6. doi:10.1038/nmeth.f.303
8. Nishiyama H, Nagai T, Kudo M, Okazaki Y, Azuma Y, Watanabe T, et al. Supplementation of pancreatic digestive enzymes alters the composition of intestinal microbiota in mice. Biochem Biophys Res Commun (2018) 495(1):273–9. doi:10.1016/j.bbrc.2017.10.130
9. Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol (2017) 17(4):219–32. doi:10.1038/nri.2017.7
10. Nishino K, Nishida A, Inoue R, Kawada Y, Ohno M, Sakai S, et al. Analysis of endoscopic brush samples identified mucosa-associated dysbiosis in inflammatory bowel disease. J Gastroenterol (2017) 53(1). doi:10.1007/s00535-017-1384-4
11. Shen XJ, Rawls JF, Randall T, Burcal L, Mpande CN, Jenkins N, et al. Molecular characterization of mucosal adherent bacteria and associations with colorectal adenomas. Gut Microbes (2010) 1(3):138–47. doi:10.4161/gmic.1.3.12360
12. Mondot S, Lepage P, Seksik P, Allez M, Treton X, Bouhnik Y, et al. Structural robustness of the gut mucosal microbiota is associated with Crohn’s disease remission after surgery. Gut (2016) 65(6):954–62. doi:10.1136/gutjnl-2015-309184
13. Tyler AD, Knox N, Kabakchiev B, Milgrom R, Kirsch R, Cohen Z, et al. Characterization of the gut-associated microbiome in inflammatory pouch complications following ileal pouch-anal anastomosis. PLoS One (2013) 8(9):e66934. doi:10.1371/journal.pone.0066934
14. Mukhopadhya I, Hansen R, Nicholl CE, Alhaidan YA, Thomson JM, Berry SH, et al. A comprehensive evaluation of colonic mucosal isolates of Sutterella wadsworthensis from inflammatory bowel disease. PLoS One (2011) 6(10):e27076. doi:10.1371/journal.pone.0027076
Keywords: cap polyposis, intestinal microbiota, next-generation sequencing, inflammation, antibiotics
Citation: Okamoto K, Watanabe T, Komeda Y, Okamoto A, Minaga K, Kamata K, Yamao K, Takenaka M, Hagiwara S, Sakurai T, Tanaka T, Sakamoto H, Fujimoto K, Nishida N and Kudo M (2018) Dysbiosis-Associated Polyposis of the Colon—Cap Polyposis. Front. Immunol. 9:918. doi: 10.3389/fimmu.2018.00918
Received: 18 January 2018; Accepted: 13 April 2018;
Published: 07 May 2018
Edited by:
Ashutosh K. Mangalam, University of Iowa, United StatesReviewed by:
Anastasia Sobolewski, University of East Anglia, United KingdomMohamad Mokadem, University of Iowa, United States
Copyright: © 2018 Okamoto, Watanabe, Komeda, Okamoto, Minaga, Kamata, Yamao, Takenaka, Hagiwara, Sakurai, Tanaka, Sakamoto, Fujimoto, Nishida and Kudo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tomohiro Watanabe, tomohiro@med.kindai.ac.jp
 Kazuki Okamoto1
Kazuki Okamoto1 Tomohiro Watanabe
Tomohiro Watanabe Naoshi Nishida
Naoshi Nishida