- 1Department of Rheumatology and Clinical Immunology, Faculty of Medicine, School of Health Sciences, University of Thessaly, Larissa, Greece
- 2Department of Immunology-Histocompatibility, Evangelismos General Hospital, Athens, Greece
- 3Department of Rheumatology, Patras University Hospital, Faculty of Medicine, University of Patras Medical School, Patras, Greece
- 4Institute of Immunology Affiliated to Euroimmun AG, Lübeck, Germany
Epitope mapping of anti-Ro52 antibodies (Abs) has been extensively studied in patients with Sjögren's syndrome (SjS) and systemic lupus erythematosus (SLE). Comprehensive epitope mapping in systemic sclerosis (SSc), where anti-Ro52 antibodies are also frequently detected, has not been performed. The aim of the present study was to fully characterize Ro52 epitopes in anti-Ro52-positive SSc using Ro52 fragments spanning the full antigen. Further analysis was made according to anti-Ro60 status. Epitope mapping was performed in 43 anti-Ro52-positive SSc patients. Seventy eight anti-Ro52-positive pathological controls, including 20 patients with SjS, 28 patients with SLE, 15 patients with dermatomyositis (DM), and 15 patients with primary biliary cholangitis (PBC), and 20 anti-Ro52-negative healthy individuals as normal controls were also tested. Five recombinant Ro52 fragments [Ro52-1 (aa 1-127), Ro52-2 (aa 125-268), Ro52-3 (aa 268-475), Ro52-4 (aa 57-180), and Ro52-5 (aa 181-320) were used to test reactivity by line-immunoassay and in house ELISA. Anti-Ro60 reactivity was tested by ELISA. All anti-Ro52 positive sera reacted with Ro52-2; none recognized Ro52-3. Antibodies against Ro52-1 were less frequently found in SSc than in SjS/SLE (11.6 vs. 41.7%, p = 0.001); and antibodies against Ro52-4 were less frequently found in SSc than in SjS/SLE (27.9 vs. 50%, p = 0.03). In SSc patients, reactivity against Ro52-1 was more frequent in anti-Ro52+/anti-Ro60+ than in anti-Ro52+/anti-Ro60-patients (33.3 vs. 0%, p = 0.003). In this comprehensive analysis of Ro52 epitope mapping in SSc, the coiled coil domain remains the predominant epitope on Ro52. Contrary to SjS and SLE, patients with SSc fail to identify epitopic regions within the N-terminus of the protein, especially if they lack con-current anti-Ro60 reactivity.
Introduction
Anti-Ro52 antibodies (Abs), along with anti-Ro60 or in isolation, are frequently found in patients with autoimmune rheumatic diseases (AIRDs) (1–4). These autoantibodies (autoAbs), originally described in patients with Sjögren's syndrome (SjS), systemic lupus erythematosus (SLE), are detected in other ARDs, as well as in other organ and non-organ specific autoimmune diseases (1–3, 5–7). For instance, we and others reported the presence of anti-Ro52 Abs in ~20–30% of patients with systemic sclerosis (SSc), making it the third most common antibody (Ab) in this disease (8–10). Ro52, originally considered as potential part of the ribonucleoprotein complex, is now well established as member of the tripartite TRIM family (TRIM21). It has been shown that Ro52 (TRIM21) is a cytosolic Fc receptor, bound with high affinity preferentially to IgG, but also to IgA and IgM intra-cytoplasmic receptor of IgG (11, 12). This ability of Ro52 (TRIM21), for simplicity there after mentioned as Ro52, along with its pleiotropic immunomodulatory properties have led us to appreciate the important role of this antigen in regulation of immune-mediated inflammation and regulation of autoreactive immunity (11, 12).
The exact epitopic regions on Ro52 targeted by antigen-specific autoAbs have been extensively studied in SjS and SLE (13–19), but their characterisation in patients with SSc is ill-defined. In SjS and SLE, anti-Ro52 autoAbs mainly target large polypeptidyl sequences in the coiled coil region of the protein (13–19). Linear short sequences within the corresponding epitopes are subdominantly recognized (20). A recent study by Infantino et al (21), using a set of 5 epitopic regions overlapping the whole sequence, has demonstrated reactivity mainly to aa 125-268. Having access to these Ro52 constructs, we considered that it is worth investigating the B-cell epitopes of Ro52 in patients with SSc. Our findings neither refute nor agree with those obtained in SjS and SLE. When anti-Ro52 Ab-positive SSc patients were divided according to con-current anti-Ro60 Abs, different patterns of epitope recognition were found.
Material and Methods
Material
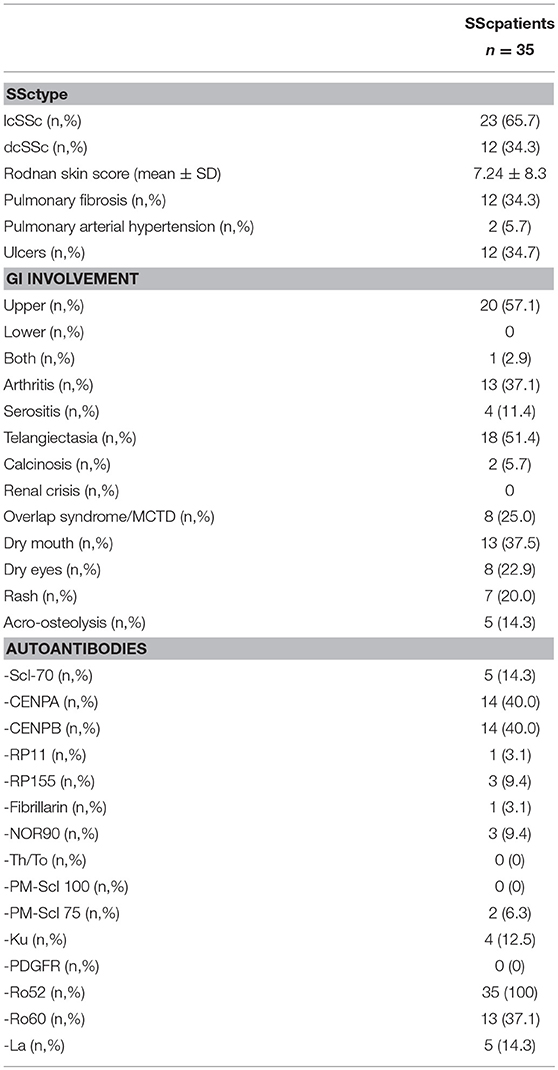
A total of 121 anti Ro52 Ab-positive patients with various autoimmune diseases were analyzed, including 43 patients with SSc (41 females; mean age ±SD: 57.83 ± 12.58 years; disease duration 9.72 ± 7.4 years; 41 ANA positive, median titre 1/160, range 1/80-1/5,120) as the study group (Table 1), 20 patients with SjS (18 females; mean age ±SD: 52 ± 11.2 years), 28 patients with SLE (all females; mean age ±SD: 45 ±14.3 years), 15 with dermatomyositis (DM) (9 females; mean age ±SD: 62.13 ±11.3 years); and 15 patients with primary biliary cholangitis (PBC) (22) (13 females; mean age ±SD: 47.2 ± 13.4 years), as pathological controls. All patients were regularly followed up at the Out-patient Clinic, Department of Rheumatology and Clinical Immunology, University General Hospital of Larissa, in Larissa, Greece (9, 23, 24). A cohort of 10 additional anti-Ro52 Ab-positive SSc patients were also included; these patients were followed up at two other Greek University Hospitals, University of Athens and University of Patras. Diagnosis of SSc was based on the 2013 ACR/EULAR Criteria for the Classification of SSc (25); diagnosis of SjS on the 2016 ACR/EULAR Classification Criteria for primary SjS(26), diagnosis of SLE was based on the 2012 SLICC Criteria (27), and diagnosis of DM was based on the Bohan and Peter Criteria for Polymyositis and Dermatomyositis (28, 29). Diagnosis of PBC was based on the internationally accepted criteria for PBC (22, 30).
Fifty anti-Ro52 Ab-negative patients with various AIRDs and other autoimmune diseases, including 12 patients with SSc,10 with SjS, 12 with SLE, 5 with DM, and 11 with PBC, were tested as anti-Ro52 Ab-negative disease controls.
Twenty healthy individuals (all anti-Ro52 Ab-negative) were also tested as normal controls (NCs) (18 females; mean age ±SD: 52.8 ± 10.9 years).
The presence of anti-Ro52 Abs was initially assessed by a line immunoassay (Euroimmun, Lübeck, Germany) and confirmed by an anti-Ro52 specific ELISA (Inova Diagnostics, San Diego, CA).
A written informed consent was obtained by all patients and controls. The study was performed in accordance with the declaration of Helsinki. Patients and NCs participated in the study after approval of the research protocol by the Ethical Committee of the University General Hospital of Larissa, Faculty of Medicine, University of Thessaly, Greece.
Methods
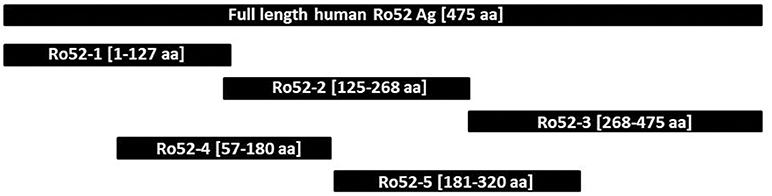
Epitope mapping was performed using a specifically-designed line immunoassay containing five recombinant Ro52 fragments expressed in E coli [Ro52-1 (aa 1-127), Ro52-2 (aa 125-268), Ro52-3 (aa 268-475), Ro52-4 (aa 57-180), and Ro52-5 (aa 181-320) (Figure 1), as described before (21). Ro-52 full-antigen, expressed with the baculovirus system in insect cells, was used as positive control. Titration experiments were performed to establish optimal conditions of experiments. The final concentration of each fragment was established based on ROC curves using four different concentrations (1, 5, 25, 100 μg/ml) tested in 20 anti-Ro52-positive SSc and 20 anti-Ro52-negative NCs. The final concentration of each fragment that gave specificity up to 94% was as follows: 100 μg/ml for Ro52-1 and 25 μg/ml for all other fragments. The specifically designed line strips were incubated with sera (1:100 dilution) on a rocking platform at room temperature for 30 min (21). After the aspiration of the liquid the strips were washed three times in 1.5 ml wash buffer (Euroimmun) for 5 min. Then strips were incubated in alkaline phosphate-labeled anti-human IgG conjugate (Euroimmun) for 30 min, followed by three 5 min washes (21). Finally, strips were incubated in 5-bromo-4-chloro-3-indolyl-phosphate/nitro blue tetrazolium substrate solution (Euroimmun) for 10 min and then washed with distilled water. After being dried, strips were evaluated by the use of EUROLineScan software (Euroimmun) and results were expressed in arbitrary units (AU/ml), as previously described in detail (9, 21, 31). To determine the cut off values of the in house line immunoassays, we tested 70 anti-Ro52 Ab-negative serum samples (39 with various AIRDs, 11 with PBC and 20 NCs, see above). For each fragment the chosen cut off value corresponded to mean+2SD. Based on that, the cut off value was 8 AU/ml for Ro52-1, 10 AU/ml for Ro52-2, 4 AU/ml for Ro52-3, 5 AU/ml for Ro52-4, and 8 AU/ml for Ro52-5 (Supplementary Table 1).
The validity of Ab reactivity to fragments by a line immunoassay was also assessed by an in house ELISA using the same Ro52 fragments testing 32 serum samples (12 randomly selected anti-Ro52 Ab positive SSc patients and 20 anti-Ro52 negative SSc patients), as previously described with slight modifications (32–34). Titration experiments were executed to establish optimal conditions of experiments. Briefly, initially each well was incubated at 20°C for 1 h with 200 μl of blocking buffer (2% BSA in PBS), to block non-specific binding). All reagents were purchased by Sigma Aldrich, until otherwise stated. After a washing step (5 times with PBS-0.1% Tween-20), 100 ml of each Ro52 fragment (final concentration: 25 μg/ml) was added to the wells (diluted in PBS containing 0.1% BSA and 0.1% sodium azide) and incubated at 20°C on shaker for 1 h. Washing was repeated (5x) and following that, 100 μl of each samples at 1/200 dilution (in 2% BSA/PBS containing 0.1% sodium azide) was added and incubated at 20°C on a shaker for 1 h. To ensure consistency, two sera were used as reference controls, including a high titre anti-Ro52 Ab serum from an SLE patient, know to strongly react with the Ro52 full protein, and fragments Ro52-1, Ro52-2, Ro52-4, and Ro52-5, and a NC serum used as negative control totally unreactive against the full Ro52 protein and its fragments. The washing step was repeated and 100 μl of conjugate-1/1000 peroxidase-conjugated goat anti-human (IgG) diluted in 2% BSA/PBS were added to each well and incubated at 20°C for 1 h. After washing steps (5x), 100 μl of TMB substrate (3,3', 5,5;-tetramethylbenzidine) was added and incubated in the dark for 10 min. The reaction was terminated by adding 50 μl of H2SO4. Light absorbance (optical density, OD) was measured against blank well at 450 nm (620 nm as reference wavelength). To determine the cut off value, 20 anti-Ro52 Ab-negative patients (10 with SSc and 10 randomly selected, with other ARDs), were tested with individual Ro52 fragments. Reaction for a given construct exceeded was considered positive when the OD reading of the test serum against the construct exceeded the mean+2SD of the absorbance values of the 32 anti-Ro52 Ab negative controls. To utilize a uniform representation of the absorbance values, the absorbance corresponding to the cut off value was defined as 1 RU/ml.
Statistical Analysis
All results are expressed as percentages (%). To determine cut off values for the line immunoassay ROC analyses were performed and for each Ro52 fragment ab concentration was chosen for a specificity up to 94%. Mean plus 2SD of values of negative patients were used as cut off for each assay (line immunoblotting, ELISA). Differences between groups were tested by chi-square, two-tailed t-test and nonparametric Mann-Whitney test. p-values smaller than or equal to 0.05 were considered significant. The statistical calculations were performed with SPSS statistics 22.
Results
All anti-Ro52 Ab-positive patients reacted against the full Ro52 antigen by a line immunoassay without difference in AU/ml among the various diseases (mean ± SD: 76.55 ± 23.13 AU/ml in SSc compared to 79.8 ± 24.3 AU/ml in SjS; 80.39 ± 28.26 AU/ml in SLE; 81.73 ± 16.93 AU/ml in DM and 83.6 ± 35.2 AU/ml in PBC, p > 0.05 for all) (Figure 2).
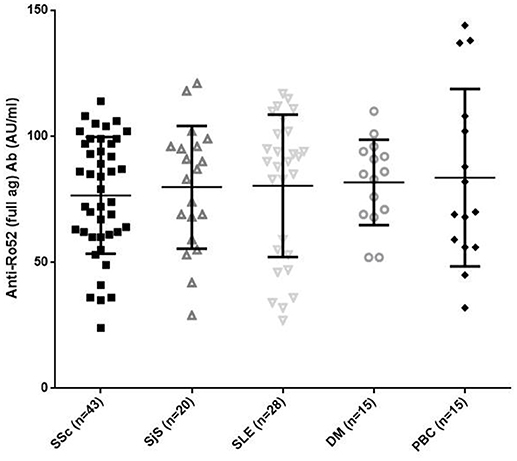
Figure 2. Reactivity in AU/ml against the full length Ro52 antigen in 43 patients with systemic sclerosis (SSc), 20 patients with Sjögren's syndrome (SjS), 28 patients with systemic lupus erythematosus (SLE), 15 patients with DM, and 15 patients with primary biliary cholangitis (PBC). There were no significant differences amongst different diseases. Values are given as symbols. The solid black line at the approximate center of each vertical line is the median. The arms of each line extend with their ends corresponding to 10 and 90% of the values.
Frequency of Ro52 Fragment ab Recognition of SSc and Controls
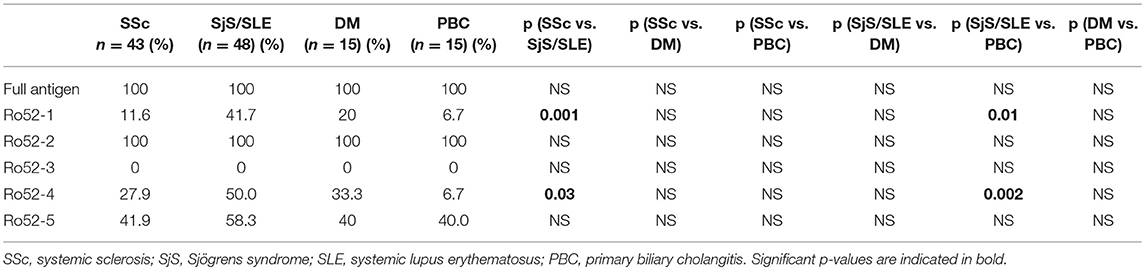
Results of serum reactivity to Ro52 fragments are summarized in Tables 2, 3. Overall, reactivity against fragments Ro52-1, Ro52-2, Ro52-3, Ro52-4, and Ro52-5 in patients with SSc was 11.6, 100, 0, 27.9, and 41.9%, respectively. The respective results in SjS were 40, 100, 0, 40, and 60%; in SLE were 42.9, 100, 0, 57.1, and 57.1%;in DM were 20, 100, 0, 33.3, and 40% and in PBC 6.7, 100, 0, 6.7, and 40%.
According to individual disease, Ab reactivity against fragments Ro52-1, Ro52-4, and Ro52-5 were as follows (SjS and SLE are grouped together as their reactivities to different Ro52 fragments were similar): Abs against Ro52-1 were less frequent in SSc than in SjS/SLE (5/43 [11.6%] vs. 20/48 [41.7%], p = 0.001); Abs against Ro52-4 were less frequent in SSc than in SjS/SLE (12/43 [27.9%] vs. 24/48 [50%], p = 0.03). In addition, Abs against Ro52-1 were also more frequent in SjS/SLE than in PBC (20/48 [41.7%] vs. 1/15 [6.7%], p = 0.01) and Abs against Ro52-4 were more frequent in SjS/SLE than in PBC (24/48 [50%] vs. 1/15 [6.7%], p = 0.002) (Table 2).
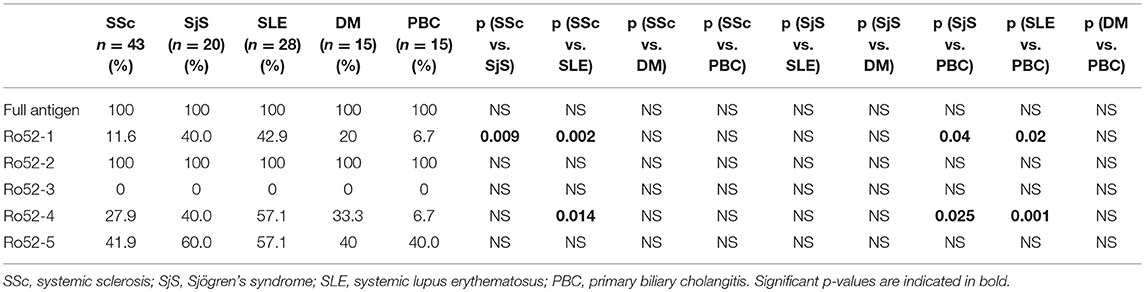
Comparison of reactivities against Ro52 fragments between SSc and separate SLE or SjS patient groups are shown in Table 3. In particular, SSc patients were less frequently reactive against Ro52-1 than SjS (5/43 [11.6%] vs. 8/20 [40.0%], p = 0.0095) and SLE patients (5/43 [20.9%] vs. 12/28 [42.9%], p = 0.002). PBC patients were also less frequently reactive against Ro52-1 than SjS (1/15 [6.7%] vs. 8/20 [40.0%], p = 0.04) and SLE patients (1/15 [6.7%] vs. 16/28 [57.1%], p = 0.02). Moreover, SSc patients were less frequently reactive against Ro52-4 than SLE (12/43 [27.9%] vs. 16/28 [57.1%], p = 0.014) and this was also the case for PBC compared to SjS (1/15 [6.7%] vs. 8/20 [40.0%], p = 0.025) and SLE patients (1/15 [6.7%] vs. 16/28 [571%], p = 0.001) (Table 3).
Ab reactivity to individual Ro52 fragments by line immunoassay correlated with Ab binding of the same fragments when tested by in house ELISA (r = 0.95, p < 0.001 for Ro52-1; r = 0.783, p = 0.003 for Ro52-2; r = 0.485, p = 0.11 for Ro52-3; r = 0.729, p = 0.007 for Ro52-4; r = 0.784, p = 0.003 for Ro52-5) (Supplementary Figure 1). All sera tested negative for Ro52 fragments by line immunoassay were also negative by ELISA.
Magnitude of Ab Reactivity Against Ro52 Fragments in SSc and Controls
The magnitude of Ab reactivity to individual Ro52 fragments is illustrated in Figure 3. Ab reactivity to Ro52-2 was lower in SSc compared to SjS (59.62 ± 26.21 AU/ml vs. 78.75 ± 25.66 AU/ml; p = 0.009), SLE (79.46 ± 30.92 AU/ml; p = 0.007), and PBC (90.06 ± 32.31 AU/ml, p = 0.004) patients. Reactivity against Ro52-4 was lower in SSc (4.88 ± 5.75 AU/ml) than in PBC (1.26 ± 2.15 AU/ml; p = 0.001) (Figure 3).
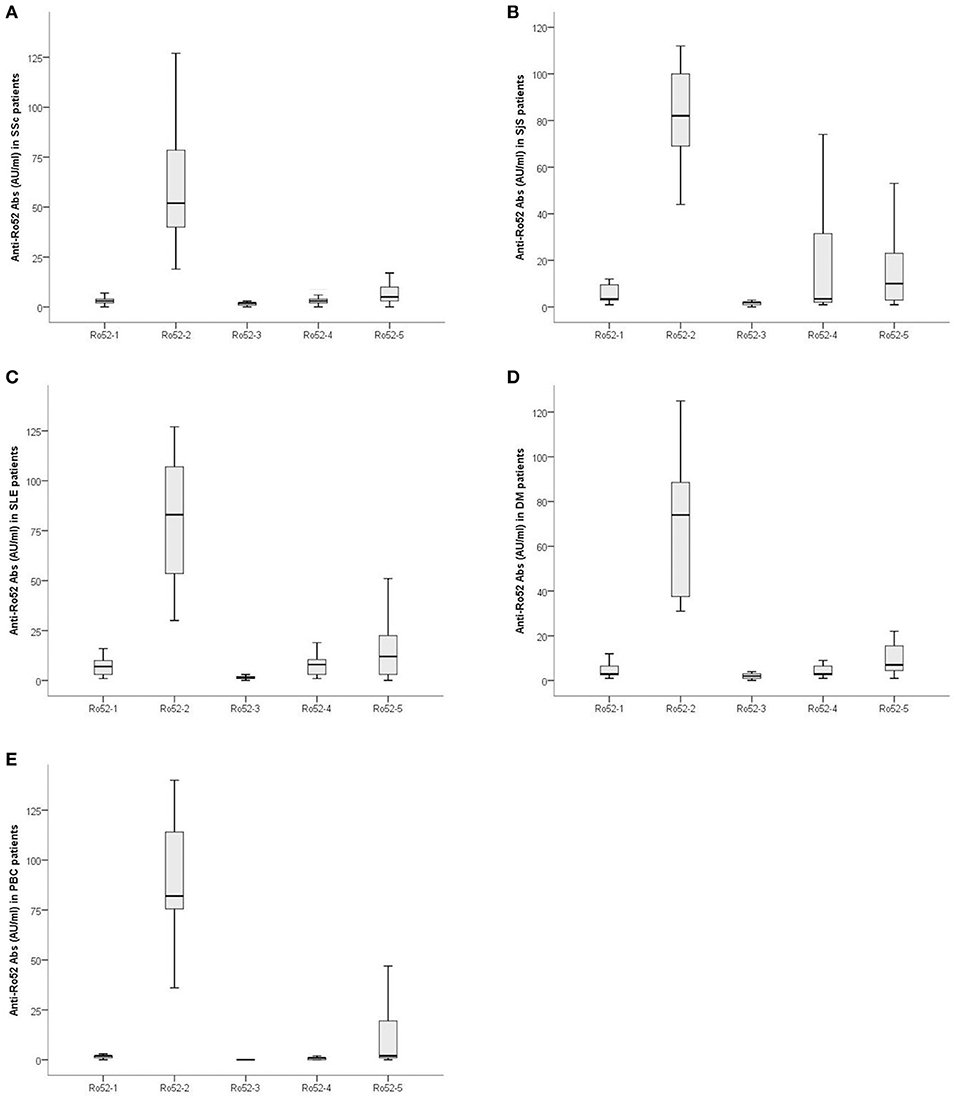
Figure 3. Magnitude of Ab reactivity to individual Ro52 fragments in anti-Ro52-Ab-positive patients: (A) 43 patients with Systemic Sclerosis (SSc), (B) 20 patients with Sjögren's syndrome (SjS), (C) 28 patients with systemic lupus erythematosus (SLE), (D) 15 patients with dermatomyositis (DM) and (E) 15 patients with primary biliary cholangitis (PBC). Values are given as box plots which represent interquartile ranges and the solid black line at the approximate center of each box is the median. The arms of each box extend with their ends corresponding to 10 and 90% of the value.
Ro52 Epitope Recognition in anti-Ro52+/anti-Ro60+ (double Positive) and anti-Ro52+/anti-Ro60- Patients
When anti-Ro52 Ab-positive patients were divided in anti-Ro52+/anti-CEN+ and anti-Ro52+/anti-CEN- no differences were found in epitope recognition patterns. Similarly, comparisons between subgrouping anti-Ro52+/anti-Scl70+ and anti-Ro52+/anti-Scl70- did not reveal statistically significant differences.
When anti-Ro52 positive patients were divided in anti-Ro52+/anti-Ro60+ (double positive) and anti-Ro52+/anti-Ro60–, statistically significant differences amongst diseases were found. In SSc patients (n = 43), reactivity against Ro52-1 was more frequent in anti-Ro52+/anti-Ro60+ patients than anti-Ro52+/anti-Ro60– (5/15 [33.3%] vs. 0/28 [0%], p = 0.003) (Table 4).
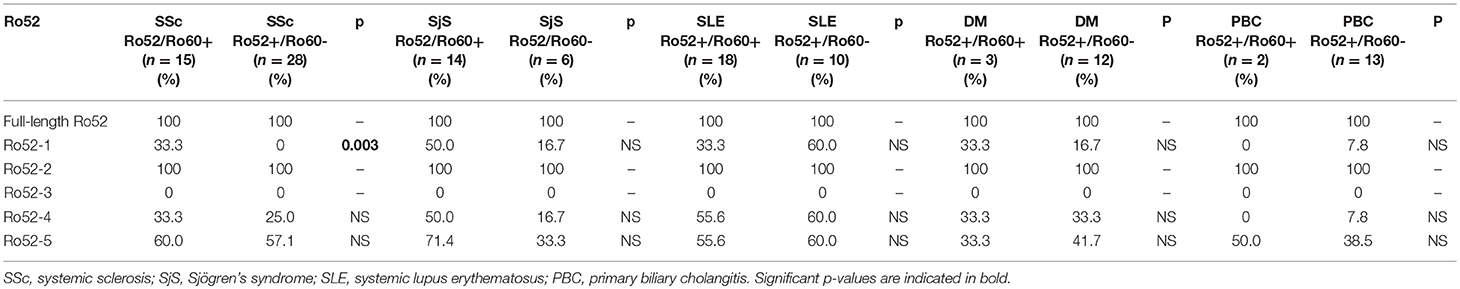
Table 4. Ab reactivity against Ro52 fragments in patients subdivided toanti-Ro52+/anti-Ro60+ and anti-Ro52+/anti-Ro60–.
Comparing reactivity against various Ro52 fragments in anti-Ro52+/anti-Ro60+ patients among various diseases, no statistically significant difference was detected. On the contrary, the same comparison in anti-Ro52+/anti-Ro60– patients among various diseases showed that reactivity against Ro52-1 was less frequent in SSc compared to SLE (0/28 [0%] vs. 6/10, [60%], p = 0.001); Similarly, reactivity against Ro52-4 was less frequent in SSc than in SLE (5/28 [17.9%] vs. 6/10 [60%], p = 0.019) patients.
In anti-Ro52-positive SSc patients, the clinical and immunological characteristics between anti-Ro52+/anti-Ro60– and anti-Ro52+/anti-Ro60+ SSc patients did not reveal any statistically significant differences (Supplementary Table 2). Similarly, clinical and immunological features were not statistically different when SSc patients were divided according to reactivity to specific Ro52 (Ro52-1, Ro52-4, Ro52-5) fragments.
Discussion
This is the first comprehensive analysis of B-cell epitope mapping of anti-Ro52 Abs in patients with SSc using large polypeptidyl fragments spanning the whole Ro52 antigen. Our data show that, as in other AIRDs, such as SjS and SLE (13–19), the dominant epitopic region universally recognized by anti-Ro52 Abs in SSc is that lying within the coiled coil domain of the protein (aa 125-268) (13, 21). Lack of Ab binding of a sequence spanning the C-terminus of the antigen, reported in SjS and SLE is also confirmed in the present study (13–19, 21). However, our study revealed novel findings: patients with SSc less frequently recognize Ro52-1 compared to SLE patients. More importantly, anti-Ro60+ SSc patients showed a distinct, previously unrecognized epitopic pattern, characterized by broad recognition of Ro52 epitopes (including Ro52-1, Ro52-2, Ro52-4, and Ro52-5) compared to anti-Ro60- SSc patients where reactivity by large is restricted to Ro52-2.
In particular, SSc sera were less frequently reactive to Ro52-1 -the N-terminus fragment spanning aa 1-127—than combined SjS/SLE sera (11.6 vs. 41.7%). In a similar vein, Abs against Ro52-4 (aa 57-180)—which partly overlaps with Ro52-1 were less frequently found in SSc than in SjS/SLE (27.9 vs. 50%). This led us to assume that, while the overlapping region contains an epitope (or epitopes) of anti-Ro52 in SjS and SLE such an epitope recognition is absent, at least in part in SSc. Why Ab responses against specific Ro52 fragments are different in frequency and strength among various autoimmune diseases is not an easy task to address (13, 35–38). We can only speculate that the exact mechanisms which are responsible for the induction of anti-Ro52 Ab responses in SSc somewhat differ from those operating in SLE and SjS. It should be noted that in SSc anti-Ro52 Abs less frequently co-exist with anti-Ro60 Abs compared to SLE and SjS, which usually have both autoAb specificities (8, 10, 39). A similar to SSc pattern of less frequently recognition of Ro52-1 and Ro52-4 was also seen in PBC, suggesting that a common (or similar) mechanism of autoAbs production for both diseases may be in operation (40).
The increased frequency of reactivity against Ro52-1 and Ro52-5 in anti-Ro52/anti-Ro60 double positive patients than in anti-Ro52+/anti-Ro60– SSc patients is difficult to explain. Currently, it is not known why some patients have reactivities to Ro52 alone, Ro60 alone or both (41). The two autoantigens are structurally unrelated but—immunologically—interrelated since anti-Ro52 and anti-Ro60 immune responses tend to co-exist (42, 43). However, anti-Ro52 autoAbs can be present without ever anti-Ro60 reactivity in many autoimmune diseases. Mechanisms, such as epitope spreading and exposure to cryptic epitopes in double positive sera at very early stages of disease may account for con-current reactivity (35–37, 44, 45). The clinical significance of epitopic recognition is underlined in experimental diseases, where the clinical phenotype largely depends on the Ro52 domain, used as an immunogen. For instance, Sroka et al. (46) have recently shown that only immunization with the coiled coil Ro52 domain and its subsequent immune response against the coiled coil Ro52 domain can induce salivary gland dysfunction (46). However, we were unable to find specific associations between clinical features and epitope profiling. The relatively recent demonstration of the true nature of the Ro52 antigen and its pleiotropic key role for signal transduction, in adaptive and innate immunity as member of the TRIM family of proteins may explain (at least in part) some of these attributes (47–49). Ro52 (TRIM21) is an intra-cytoplasmic receptor of IgG and epitope spreading mechanisms involving regions corresponding to dominant or subdominant epitopes of Ro52 and Ro60 may account for the observed distinct Ab recognition against the two antigens (11, 12).
Our data suggests that epitope mapping of anti-Ro52 Abs in systemic sclerosis reveals a common denominator, the coiled-coil related epitope which, similarly to SjS, SLE, and other autoimmune diseases, is universally reactive. The N-terminus region spanned by aa 57-180 is a dominant epitope in anti-Ro52+/Ro60+ SSc patients but not in Ro52+/Ro60- SSc patients suggesting that Ro60 directly or indirectly is involved in the shaping of the epitopic repertoire of anti-Ro52 Abs, a finding which warranties further investigation(35, 36). Understanding the mechanisms responsible for the breaking of tolerance to Ro52 in SSc may shed a light not only for the understanding of the pathogenesis of this disease but also for those of SjS and SLE, positioning Ro60 as a key player. By no means our study or other studies of this kind (21) can address the key question that arises. Are these data epiphenomenal or do they really play a role in the induction of anti-Ro52 or anti-Ro60 in SSc, other AIRDs or indeed in other autoimmune diseases? Nevertheless, our data provide the impetus for subsequent studies performed in serum samples from patients on a large scale, as well as in experimental models of the disease.
Author Contributions
Each authors named as an author has made substantial contributions to the conception, design of the study, or acquisition, analysis, and interpretation of data. AG, CL, MGM performed experiments; TSc, WM prepared antigenic preparations; TS, CK, DD and LIS performed clinical assessments; TS, AG, AT, and CK prepared clinical and laboratory datasets; AG and CL analyzed the data; AG, LIS and DPB drafted the manuscript; DPB and LIS supervised the project and designed the experimental work; DPB had the original idea. All authors approved the final version of the manuscript.
Funding
The Authors did not receive any external funding. All necessary reagents/consumables were purchased by Grant code#2610, Research Committee, University of Thessaly.
Conflict of Interest Statement
TSc and WM are employees of Euroimmun, Germany.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Prof. Stamatis-Nick Liossis (University of Patras, Greece) for clinical assessments and for providing serum samples from systemic sclerosis patients.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2018.02835/full#supplementary-material
Supplementary Figure 1. Correlation of line immunobloding readings and ELISA absorbance values testing of individual fragments from patients with systemic sclerosis. The immunobloting data were confirmed in a representative set of sera from 12 anti-Ro52 Ab positive SSc patients and 20 anti-Ro52 Ab negative controls (Data not included). A statistically significant correlation is noted for all fragments between the two assays readouts.
Supplementary Table 1. Representation of the alternative cut off calculation, by the mean plus two standard deviations of the reactivity values of anti-Ro52 negative patients with autoimmune rheumatic diseases.
Supplementary Table 2. Main demographic, clinical and immunological parameters in anti- Ro52+/Ro60+ and Ro52+/Ro60- patients with SSc.
Abbreviations
Ab, antibody; AutoAb, autoantibody; ARD, autoimmune rheumatic diseases; PBC, primary biliary cholangitis; Sjögren's syndrome; SLE, systemic lupus erythematosus; SSc, systemic sclerosis.
References
1. Lee AYS. A review of the role and clinical utility of anti-Ro52/TRIM21 in systemic autoimmunity. Rheumatol Int. (2017) 37:1323–33. doi: 10.1007/s00296–017-3718–1
2. Menendez A, Gomez J, Escanlar E, Caminal-Montero L, Mozo L. Clinical associations of anti-SSA/Ro60 and anti-Ro52/TRIM21 antibodies: Diagnostic utility of their separate detection. Autoimmunity (2013) 46:32–9. doi: 10.3109/08916934.2012.732131
3. Murng SHK, Thomas M. Clinical associations of the positive anti Ro52 without Ro60 autoantibodies: undifferentiated connective tissue diseases. J Clin Pathol. (2018) 71:12–9. doi: 10.1136/jclinpath-2015–203587
4. Kvarnstrom M, Dzikaite-Ottosson V, Ottosson L, Gustafsson JT, Gunnarsson I, Svenungsson E, et al. Autoantibodies to the functionally active RING-domain of Ro52/SSA are associated with disease activity in patients with lupus. Lupus (2013) 22:477–85. doi: 10.1177/0961203313479420
5. Infantino M, Manfredi M, Grossi V, Benucci M, Morozzi G, Tonutti E, et al. An effective algorithm for the serological diagnosis of idiopathic inflammatory myopathies: the key role of anti-Ro52 antibodies. Clin Chim Acta (2017) 475:15–9. doi: 10.1016/j.cca.2017.10.002
6. Zachou K, Gampeta S, Gatselis NK, Oikonomou K, Goulis J, Manoussakis MN, et al. Anti-SLA/LP alone or in combination with anti-Ro52 and fine specificity of anti-Ro52 antibodies in patients with autoimmune hepatitis. Liver Int. (2015) 35:660–72. doi: 10.1111/liv.12658
7. Granito A, Muratori P, Muratori L, Pappas G, Cassani F, Worthington J, et al. Antibodies to SS-A/Ro-52kD and centromere in autoimmune liver disease: a clue to diagnosis and prognosis of primary biliary cirrhosis. Aliment Pharmacol Ther. (2007) 26:831–8. doi: 10.1111/j.1365–2036.2007.03433.x
8. Hudson M, Pope J, Mahler M, Tatibouet S, Steele R, Baron M, et al. Clinical significance of antibodies to Ro52/TRIM21 in systemic sclerosis. Arthritis Res Ther. (2012) 14:R50. doi: 10.1186/ar3763
9. Liaskos C, Marou E, Simopoulou T, Barmakoudi M, Efthymiou G, Scheper T, et al. Disease-related autoantibody profile in patients with systemic sclerosis. Autoimmunity (2017) 50:414–21. doi: 10.1080/08916934.2017.1357699
10. Wodkowski M, Hudson M, Proudman S, Walker J, Stevens W, Nikpour M, et al. Monospecific anti-Ro52/TRIM21 antibodies in a tri-nation cohort of 1574 systemic sclerosis subjects: evidence of an association with interstitial lung disease and worse survival. Clin Exp Rheumatol. (2015) 33(4 Suppl 91):S131–5.
11. Rhodes DA, Isenberg DA. TRIM21 and the function of antibodies inside cells. Trends Immunol. (2017) 38:916–26. doi: 10.1016/j.it.2017.07.005
12. Oke V, Wahren-Herlenius M. The immunobiology of Ro52 (TRIM21) in autoimmunity: a critical review. J Autoimmun. (2012) 39:77–82. doi: 10.1016/j.jaut.2012.01.014
13. Wahren-Herlenius M, Muller S, Isenberg D. Analysis of B-cell epitopes of the Ro/SS-A autoantigen. Immunol Today (1999) 20:234–40.
14. Bozic B, Pruijn GJ, Rozman B, van Venrooij WJ. Sera from patients with rheumatic diseases recognize different epitope regions on the 52-kD Ro/SS-A protein. Clin Exp Immunol. (1993) 94:227–35.
15. Blange I, Ringertz NR, Pettersson I. Identification of antigenic regions of the human 52kD Ro/SS-A protein recognized by patient sera. J Autoimmun. (1994) 7:263–74. doi: 10.1006/jaut.1994.1020
16. Buyon JP, Slade SG, Reveille JD, Hamel JC, Chan EK. Autoantibody responses to the “native” 52-kDa SS-A/Ro protein in neonatal lupus syndromes, systemic lupus erythematosus, and Sjogren's syndrome. J Immunol. (1994) 152:3675–84.
17. McCauliffe DP, Yin H, Wang LX, Lucas L. Autoimmune sera react with multiple epitopes on recombinant 52 and 60 kDa Ro(SSA) proteins. J Rheumatol. (1994) 21:1073–80.
18. Kato T, Sasakawa H, Suzuki S, Shirako M, Tashiro F, Nishioka K, et al. Autoepitopes of the 52-kd SS-A/Ro molecule. Arthritis Rheum. (1995) 38:990–8.
19. Dorner T, Feist E, Wagenmann A, Kato T, Yamamoto K, Nishioka K, et al. Anti-52 kDa Ro(SSA) autoantibodies in different autoimmune diseases preferentially recognize epitopes on the central region of the antigen. J Rheumatol. (1996) 23:462–8.
20. Ricchiuti V, Briand JP, Meyer O, Isenberg DA, Pruijn G, Muller S. Epitope mapping with synthetic peptides of 52-kD SSA/Ro protein reveals heterogeneous antibody profiles in human autoimmune sera. Clin Exp Immunol. (1994) 95:397–407.
21. Infantino M, Meacci F, Grossi V, Benucci M, Morozzi G, Tonutti E, et al. Serological epitope profile of anti-Ro52-positive patients with systemic autoimmune rheumatic diseases. Arthritis Res Ther. (2015) 17:365. doi: 10.1186/s13075–015-0871–3
22. Beuers U, Gershwin ME, Gish RG, Invernizzi P, Jones DE, Lindor K, et al. Changing nomenclature for PBC: from ‘cirrhosis’ to ‘cholangitis’. Gastroenterology (2015) 149:1627–9. doi: 10.1053/j.gastro.2015.08.031
23. Mavropoulos A, Simopoulou T, Varna A, Liaskos C, Katsiari CG, Bogdanos DP, et al. Breg Cells Are Numerically Decreased and Functionally Impaired in Patients With Systemic Sclerosis. Arthritis Rheumatol. (2016) 68:494–504. doi: 10.1002/art.39437
24. Mavropoulos A, Liaskos C, Simopoulou T, Bogdanos DP, Sakkas LI. IL-10-producing regulatory B cells (B10 cells), IL-17+ T cells and autoantibodies in systemic sclerosis. Clin Immunol. (2017) 184:26–32. doi: 10.1016/j.clim.2017.04.013
25. van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. Classification criteria for systemic sclerosis: an ACR-EULAR collaborative initiative. Arthritis Rheum. (2013) 65:2737–47. doi: 10.1002/art.38098
26. Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 ACR-EULAR classification criteria for primary Sjögren's syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. (2017) 69:35–45. doi: 10.1002/art.39859
27. Petri M, Orbai A-M, Alarcón GS, Gordon C, Merrill JT, Fortin PR, et al. Derivation and validation of systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. (2012) 64:2677–86. doi: 10.1002/art.34473
28. Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). NEJM (1975) 292:403–7. doi: 10.1056/nejm197502202920807
29. Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). NEJM (1975) 292:344–7. doi: 10.1056/nejm197502132920706
30. Kaplan MM, Gershwin ME. Primary biliary cirrhosis. NEJM (2005) 353:1261–73. doi: 10.1056/NEJMra043898
31. Christos Liaskos EM, Simopoulou T, Gkoutzourelas A, Barmakoudi M, Efthymiou G, Scheper T, et al. Multiparametric autoantibody profiling of patients with systemic sclerosis in Greece. Mediterr J Rheumatol. (2018) 29:120–6. doi: 10.31138/mjr.29.3.120
32. Bogdanos DP, Baum H, Sharma UC, Grasso A, Ma Y, Burroughs AK, et al. Antibodies against homologous microbial caseinolytic proteases P characterise primary biliary cirrhosis. J Hepatol. (2002) 36:14–21. doi: 10.1016/S0168-8278(01)00252-5
33. Gregorio GV, Choudhuri K, Ma Y, Pensati P, Iorio R, Grant P, et al. Mimicry between the hepatitis C virus polyprotein and antigenic targets of nuclear and smooth muscle antibodies in chronic hepatitis C virus infection. Clin Exp Immunol. (2003) 133:404–13. doi: 10.1046/j.1365-2249.2003.02229.x
34. Polymeros D, Bogdanos DP, Day R, Arioli D, Vergani D, Forbes A. Does cross-reactivity between mycobacterium avium paratuberculosis and human intestinal antigens characterize Crohn's disease? Gastroenterology (2006) 131:85–96. doi: 10.1053/j.gastro.2006.04.021
35. Tseng CE, Chan EK, Miranda E, Gross M, Di Donato F, Buyon JP. The 52-kd protein as a target of intermolecular spreading of the immune response to components of the SS-A/Ro-SS-B/La complex. Arthritis Rheum. (1997) 40:936–44.
36. Keech CL, Gordon TP, McCluskey J. The immune response to 52-kDa Ro and 60-kDa Ro is linked in experimental autoimmunity. J Immunol. (1996) 157:3694–9.
37. Topfer F, Gordon T, McCluskey J. Intra- and intermolecular spreading of autoimmunity involving the nuclear self-antigens La (SS-B) and Ro (SS-A). Proc Natl Acad Sci USA. (1995) 92:875–9.
38. Kinoshita G, Keech CL, Sontheimer RD, Purcell A, McCluskey J, Gordon TP. Spreading of the immune response from 52 kDaRo and 60 kDaRo to calreticulin in experimental autoimmunity. Lupus (1998) 7:7–11. doi: 10.1191/096120398678919606
39. Fujimoto M, Shimozuma M, Yazawa N, Kubo M, Ihn H, Sato S, et al. Prevalence and clinical relevance of 52-kDa and 60-kDa Ro/SS-A autoantibodies in Japanese patients with systemic sclerosis. Ann Rheum Dis. (1997) 56:667–70.
40. Dorner T, Feist E, Held C, Conrad K, Burmester GR, Hiepe F. Differential recognition of the 52-kd Ro(SS-A) antigen by sera from patients with primary biliary cirrhosis and primary Sjogren's syndrome. Hepatology (1996) 24:1404–7. doi: 10.1002/hep.510240616
41. Ben-Chetrit E, Chan EK, Sullivan KF, Tan EM. A 52-kD protein is a novel component of the SS-A/Ro antigenic particle. J Exp Med. (1988) 167:1560–71.
42. Espinosa A, Dardalhon V, Brauner S, Ambrosi A, Higgs R, Quintana FJ, et al. Loss of the lupus autoantigen Ro52/Trim21 induces tissue inflammation and systemic autoimmunity by disregulating the IL-23-Th17 pathway. J Exp Med. (2009) 206:1661–71. doi: 10.1084/jem.20090585
43. Slobbe RL, Pruijn GJ, Damen WG, van der Kemp JW, van Venrooij WJ. Detection and occurrence of the 60- and 52-kD Ro (SS-A) antigens and of autoantibodies against these proteins. Clin Exp Immunol. (1991) 86:99–105.
44. Sakkas LI, Bogdanos DP. Systemic sclerosis: New evidence re-enforces the role of B cells. Autoimmun Rev. (2016) 15:155–61. doi: 10.1016/j.autrev.2015.10.005
45. Ricchiuti V, Pruijn GJ, Thijssen JP, van Venrooij WJ, Muller S. Accessibility of epitopes on the 52-kD Ro/SSA protein (Ro52) and on the RoRNP associated Ro52 protein as determined by anti-peptide antibodies. J Autoimmun. (1997) 10:181–91. doi: 10.1006/jaut.1996.0122
46. Sroka M, Bagavant H, Biswas I, Ballard A, Deshmukh US. Immune response against the coiled coil domain of Sjogren's syndrome associated autoantigen Ro52 induces salivary gland dysfunction. Clin Exp Rheum. (2018) 36 (Suppl. 112):41–6. doi: 10.3390/ijms19102935
47. Foss S, Watkinson R, Sandlie I, James LC, Andersen JT. TRIM21: a cytosolic Fc receptor with broad antibody isotype specificity. Immunol Rev. (2015) 268:328–39. doi: 10.1111/imr.12363
48. Yang Y, Eversole T, Lee DJ, Sontheimer RD, Capra JD. Protein-protein interactions between native Ro52 and immunoglobulin G heavy chain. Scand J Immunol. (1999) 49:620–8.
Keywords: autoantibody, autoimmunity, autoimmune rheumatic diseases, epitope, SS-A
Citation: Gkoutzourelas A, Liaskos C, Mytilinaiou MG, Simopoulou T, Katsiari C, Tsirogianni A, Daoussis D, Scheper T, Meyer W, Bogdanos DP and Sakkas LI (2018) Anti-Ro60 Seropositivity Determines Anti-Ro52 Epitope Mapping in Patients With Systemic Sclerosis. Front. Immunol. 9:2835. doi: 10.3389/fimmu.2018.02835
Received: 11 June 2018; Accepted: 16 November 2018;
Published: 07 December 2018.
Edited by:
Philippe Guilpain, Université de Montpellier, FranceReviewed by:
Thierry Vincent, Hôpital Saint Eloi, FranceCarsten Grötzinger, Charité Universitätsmedizin Berlin, Germany
Batteux Frederic, Université Paris Descartes, France
Copyright © 2018 Gkoutzourelas, Liaskos, Mytilinaiou, Simopoulou, Katsiari, Tsirogianni, Daoussis, Scheper, Meyer, Bogdanos and Sakkas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dimitrios P. Bogdanos, bogdanos@med.uth.gr
 Athanasios Gkoutzourelas
Athanasios Gkoutzourelas Christos Liaskos
Christos Liaskos Maria G. Mytilinaiou
Maria G. Mytilinaiou Theodora Simopoulou
Theodora Simopoulou Christina Katsiari1
Christina Katsiari1 Dimitrios P. Bogdanos
Dimitrios P. Bogdanos Lazaros I. Sakkas
Lazaros I. Sakkas