- 1Tsinghua-Peking Center for Life Sciences, School of Medicine, Tsinghua University, Beijing, China
- 2Department of Infectious Diseases, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China
The current outbreak of viral pneumonia, caused by novel coronavirus SARS-CoV-2, is the focus of worldwide attention. The WHO declared the COVID-19 outbreak a pandemic event on Mar 12, 2020, and the number of confirmed cases is still on the rise worldwide. While most infected individuals only experience mild symptoms or may even be asymptomatic, some patients rapidly progress to severe acute respiratory failure with substantial mortality, making it imperative to develop an efficient treatment for severe SARS-CoV-2 pneumonia alongside supportive care. So far, the optimal treatment strategy for severe COVID-19 remains unknown. Intravenous immunoglobulin (IVIg) is a blood product pooled from healthy donors with high concentrations of immunoglobulin G (IgG) and has been used in patients with autoimmune and inflammatory diseases for more than 30 years. In this review, we aim to highlight the known mechanisms of immunomodulatory effects of high-dose IVIg therapy, the immunopathological hypothesis of viral pneumonia, and the clinical evidence of IVIg therapy in viral pneumonia. We then make cautious therapeutic inferences about high-dose IVIg therapy in treating severe COVID-19. These inferences may provide relevant and useful insights in order to aid treatment for COVID-19.
Introduction
Recently, the outbreak of a febrile respiratory disease caused by a novel beta-coronavirus, designated SARS-CoV-2, has become the focus of worldwide attention. The event was declared a Pandemic by the WHO on Mar 12, 2020, and the number of confirmed cases is still climbing worldwide. Typical clinical features of COVID-19 include fever, respiratory symptoms such as dry cough and shortness of breath, and fatigue (1). While the majority of infected individuals experience only mild symptoms or may even be asymptomatic, about 10 to 20% of patients rapidly progress to severe conditions with acute respiratory distress syndrome (ARDS), which can result in shock, sepsis, and multiple organ dysfunction (1, 2). In addition to supportive treatment, several therapeutic approaches have been proposed, such as antiviral therapy, immunomodulators, blood products, and traditional Chinese medicine (TCM) (3, 4). However, the optimal treatment strategy for severe COVID-19 remains debated (5–11). To date, it remains an urgent requirement to identify the most effective treatment for severe and critically ill COVID-19 patients, but a conclusion has not yet been drawn.
The severity of respiratory viral infections depends on a fine balance between the virulence of the pathogen and the inflammatory response of the host's immune system (12). Although an effective immune response is essential to eliminate viral pathogens, clinical observations and experimental data indicate that an excessive host immune response—rather than direct viral damage—primarily account for the pathological injury and clinical deterioration of COVID-19 (12). Inflammatory cytokine storms and lymphopenia are the signature features of patients with severe COVID-19, indicating an intense systemic inflammatory response during SARS-CoV-2 infection (13). Specific adjuvant immunomodulatory treatments have been considered for severe viral infections, but some of these were associated with a worse prognosis. Therefore, it is vital to select appropriate immunomodulators in COVID-19 patients and to carefully assess their benefits and risks (14, 15).
Based on the previous clinical experience in China, it was proposed that early initiation of high-dose intravenous immunoglobulins (IVIg) and low-molecular-weight heparin might be effective in improving the prognosis of severe and critically ill COVID-19 patients (16, 17). The national diagnosis and treatment protocol for COVID-19 (Trial Version 7) and recommendations from the Peking Union Medical College Hospital recommend appropriate application of IVIg therapy in severe and critical COVID-19 cases (4, 18). However, the specific molecular mechanisms and clinical effectiveness of IVIg treatment remain unclear. Here, we review the current knowledge of immunomodulatory mechanisms of high-dose IVIg and discuss its potential role in treating severe viral infection. Moreover, we summarize the clinical efficacy of IVIg therapy in treating severe viral pneumonias such as that caused by SARS, MERS, influenza, and RSV disease, and its current application in COVID-19. A thorough understanding of the immunomodulatory mechanisms of IVIg therapy is crucial for the timing and dosing of early intervention and may provide much-needed insights into the immunomodulatory treatment of severe and critically ill COVID-19 patients.
Immunomodulatory Mechanisms of High-Dose IVIG Therapy
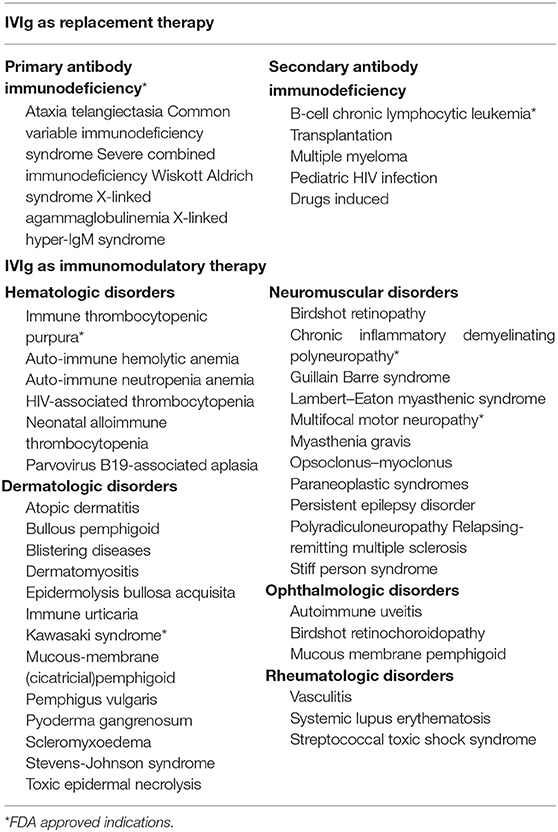
IVIg is a blood preparation isolated and concentrated from healthy donors consisting of over 95% of IgG and trace amounts of IgA or IgM. IVIg has been used in clinical practice for many years. Different doses of IVIg serve diverse medical indications, and the clinical efficacy of IVIg differs with dosage (19). At low and moderate doses, IVIg can be administered as substitutional therapy for primary or acquired immunodeficiencies in order to improve plasma IgG concentrations and provide passive immunity. Apart from this, IVIg can also be used as immunomodulatory therapy at high doses to treat autoimmune or inflammatory disease such as immune thrombocytopenia (ITP) or Kawasaki disease, and has several off-label indications including for hematologic, dermatologic, neuromuscular, and rheumatologic disorders (20) (Table 1).
The underlying molecular mechanisms of IVIg therapy effectiveness are conferred by IgG and its two functional domains, namely the F(ab)′2 fragment (the dimeric antigen-binding fragment), which is responsible for specific antigen binding, and the Fc fragment (crystallizable fragment), responsible for Fc receptor (FcR) and complement binding. Several hypotheses have been proposed to explain the immunomodulatory mechanisms of high-dose IVIg, including the F(ab)′2-mediated and the Fc-mediated mechanisms (Figure 1).
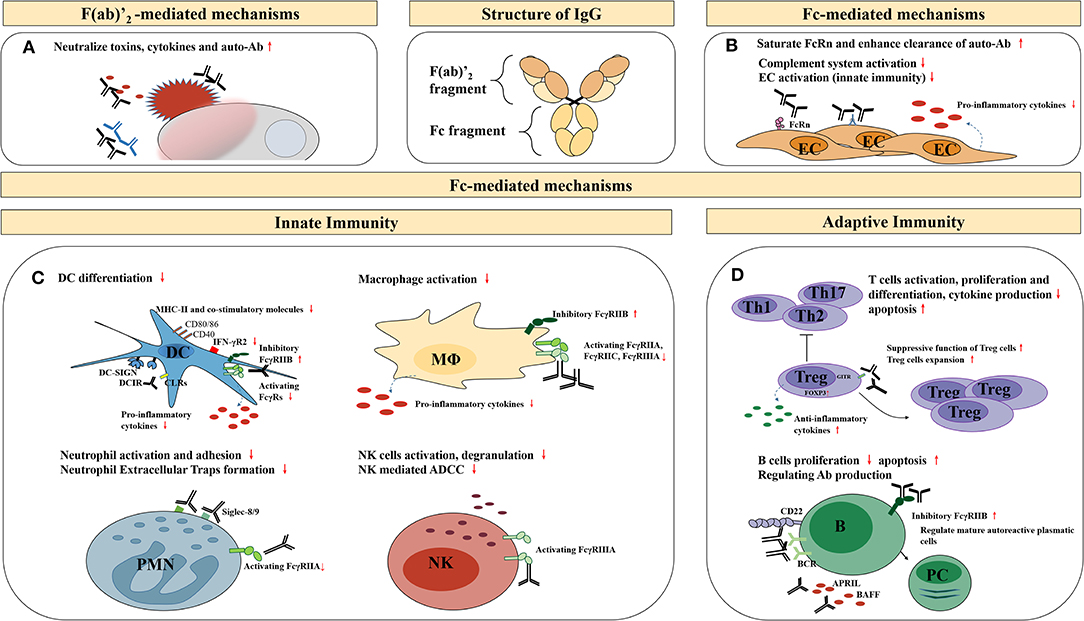
Figure 1. Potential anti-inflammatory, immunomodulatory mechanisms of high-dose IVIg therapy. EC, endothelial cells; DC, dendritic cells; MΦ, macrophages; PMN, granular leukocyte; NK, natural killer cells; ADCC, antibody-dependent cell-mediated cytotoxicity; B, B cells; PC, plasma cells; BCR, B-cell receptors; APRIL, a proliferation-inducing ligand; BAFF, B-cell activating factor of the TNF family. (A) Neutralization of pathogenic antigens through the F(ab)′2-mediated mechanisms; (B) The immunomodulatory effects on endothelial cells through the Fc-mediated mechanisms; (C) The immunomodulatory effects on other innate immune cells through the Fc-mediated mechanisms; (D) The immunomodulatory effects on adaptive immune cells through the Fc-mediated mechanisms.
F(ab)′2-Mediated Mechanisms
Neutralization of Pathogenic Antigens, Including Microorganisms or Toxins
The well-known microbial antigen-specific binding properties of the IgG-F(ab)′2 fragment provide the basis for passive immunity that allows for immediate protection against microbes in immunodeficient patients. In vitro and preclinical experiments confirmed that IVIg contains multivalent pathogen-specific neutralizing IgG antibodies against common opportunistic pathogens and toxins (e.g., Enterococcus spp., Haemophilus influenzae type b, Streptococcus pyogenes, Staphylococcus aureus, cytomegalovirus, and Shiga toxins) (21–26). Variance in pathogen-specific antibody titer and neutralizing activity is usually observed among various IVIg preparations, and is most likely due to differences in the geographical regions where plasma samples were collected, reflecting differences in pathogen exposure (27–29).
Neutralization of Endogenous Antigens Including Inflammatory Cytokines, Chemokines, Complement Fragments, and Apoptosis-Related Molecules
In addition to microbial binding, a wide range of endogenous antigen-specific IgG are also present in IVIg preparations. These endogenous antigens may include: inflammatory cytokines (e.g., IL-1α, IFN-α2a, and GM-CSF), chemokines (e.g., CCR5), apoptosis-related molecules (e.g., Siglec-8, Siglec-9, CD95/Fas, BAFF, and APRIL), complement fragments (C3a and C5a), and anti-idiotypic antibodies (e.g., anti-amyloid β antibodies and anti-coagulation factor VIII antibodies) (30–41). F(ab)′2-mediated neutralization of inflammatory cytokines, chemokines, and complement fragments, along with regulation of immune cell apoptosis might contribute to the conversion of pro-inflammatory to anti-inflammatory conditions. Meanwhile, a possible explanation for the fact that only high-dose IVIg can exert anti-inflammatory effects is that a sufficient amount of antigen-specific IgG is required to achieve therapeutic activity.
However, as previous research revealed that preparations with purified Fc fragments of IgG had an undamped effect compared with intact IgG for some indications, F(ab)′2-mediated mechanisms alone may not be sufficient for the extensive immunomodulatory effects of high-dose IVIg therapy (42–44). Therefore, Fc-mediated mechanisms may play a decisive role in these processes.
Fc-Mediated Mechanisms
The Fc fragment of IgG is responsible for binding to Fc receptors (FcRs) and complement (e.g., C1q, C3b, and C4b). IgG-specific Fc receptors (FcγRs) belong to type I FcRs and are classified into activating FcγRs (FcγRI, FcγRIIA, FcγRIIC, FcγRIIIA, and FcγRIIIB) and an inhibitory FcγR (FcγRIIB), depending on their intracellular motif and the function they mediate. Type II FcRs, including C-type lectins (DC-SIGN, DCIR), type I lectins (CD22), and non-classical FcRs, such as FcRn and FCRL, also act as effector targets of IVIg (Figure 2). The Fc-mediated mechanisms of high-dose IVIg therapy include saturation of activating FcγRs and FcRn, upregulation of the inhibitory FcγRIIB, and scavenging of complement molecules.
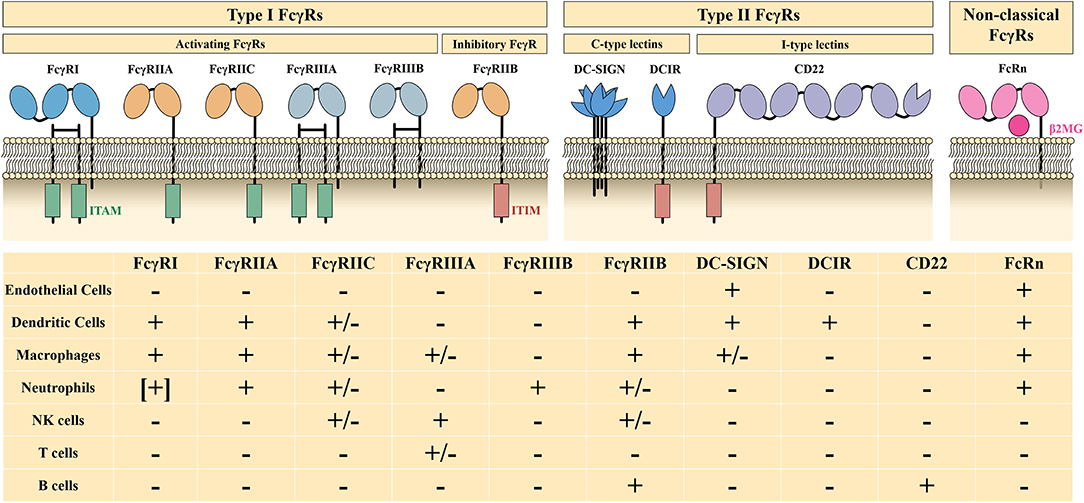
Figure 2. Characteristics and distribution profiles of human FcγRs. ITAM, immunoreceptor tyrosine-based activation motif; ITIM, immunoreceptor tyrosine-based inhibition motif; β2MG, β2-microglobulin; +, generally expressed on cells; –, not expressed on cells; [+], expressed on induced cells, +/–, expressed on specific genotype or subset of cells. Part of this figure has been adapted with permission from Springer Nature Customer Service Centre GmbH: Springer Nature: Nature Reviews Immunology, 13(3):176-89: Intravenous immunoglobulin therapy: how does IgG modulate the immune system?, by Inessa Schwab et al., 2013.
Saturation and Limiting Access of Immune Complexes to Activating FcγRs
Given the central role of activating FcγRs in mediating excessive antibody-dependent effector functions, a possible explanation of the potential anti-inflammatory function of high-dose IVIg lies in that it significantly raises the concentration of IgG above the normal plasma levels and contributes to a functional blockade of FcγRs, limiting access of immune complexes to these activating receptors. Moreover, IgG from IVIg preparations has been proven to be active through inhibitory ITAMi signaling in immune regulation (45).
The role of dimeric IgG in IVIg preparations is emphasized by an enhanced understanding of the optimal IgG form to interact with activating FcγRs. In physiological conditions, monomeric IgG1 is more likely to bind FcγRI with high affinity rather than to other FcγRs with low affinity (46). Although monomeric IgG at therapeutic levels are able to saturate low-affinity FcγR in a dose-dependent manner, it has been suggested that the small amounts of dimeric IgG bind with higher avidity to FcγRs, and therefore constitute the main active component of IVIg mediating FcγR blockade, explaining the immunomodulatory function of high-dose IVIg in ITP, GBS, and CIDP patients (47–51).
Saturation of FcRn and Enhanced Clearance of Pathogenic Antibodies
Endogenous pathogenic autoantibodies, mainly IgG, play a predominant role in the immunopathogenesis of many autoimmune and infectious diseases. A hallmark characteristic of long serum half-life of endogenous pathogenic IgG is mainly due to FcRn. FcRn is a non-classical FcR that can interact with IgG in a pH-dependent manner, which is sufficient in rescuing IgG from lysosomal degradation and recycling it to the cell surface (52, 53). High-dose IVIg therapy leads to an increased clearance rate of monoclonal antibodies and pathogenic IgG in different mouse models, whereas this effect is not observed in FcRn deficient models, indicating that FcRn is involved in therapeutic effects of IVIg therapy (54, 55). Influences of FcRn gene polymorphisms on different IgG kinetics and therapeutic effects of IVIg among patients have been observed (56–58). Pharmacokinetic models predicted that the therapeutic effects of IVIg relate to total serum IgG concentration and FcRn-binding capacity (59). The substantial increase in IgG concentration may saturate FcRn and reduce the half-life of pathogenic antibodies, contributing to the anti-inflammatory mechanism of high-dose IVIg.
Upregulation of the Inhibitory FcγRIIB and Increase in the Activation Threshold
A balance between activating and inhibitory FcγRs is critical for a well-regulated immune response, and a disbalance markedly influences immunopathology in autoimmune and infectious diseases. FcγRIIB is the only ITIM-containing FcγR and negatively regulates many aspects of immune and inflammatory responses. After efficient high-dose IVIg therapy, an upregulation of FcγRIIB on immune cells is considered to contribute to the anti-inflammatory process (43, 60, 61). However, evidence of underlying molecular mechanisms remains sparse and this mechanism of therapeutic action is therefore controversial.
In the last decade, a prevailing theory from murine studies argued that a minor portion of IgG, with specific sialylation of the Asn297-linked glycan structure on the Fc fragment, is essential for anti-inflammatory activities of IVIg via direct interaction with myeloid regulatory cells expressing SIGN-R1 (mice) or DC-SIGN (human) (44, 62, 63). Subsequent research revealed that administration of sialylated IgG resulted in the production of IL-33 from hDC-SIGN+ macrophages or dendritic cells and ensuing expansion of IL-4-producing basophils, and these cytokines led to FcγRIIB expression on effector cells (64, 65). However, other studies have challenged this hypothesis. The evidence of a direct interaction between sialylated IgG and DC-SIGN is not fully supported in the literature (66, 67). Moreover, there is conflicting evidence suggesting that the sialylated Fc fragment of IgG is dispensable for the anti-inflammatory mechanisms of high-dose IVIg (68–72). It should be noted that all evidence of this hypothesis derives from murine studies, which might not be readily translated to human conditions. These controversial results warrant further research to address the mechanisms and molecular basis of high-dose IVIg upregulation of the inhibitory FcγRIIB.
Scavenging of Complement Fragments and Inhibition of Complement System Activation
The complement system is activated through three pathways, namely the classical pathway, the lectin pathway, and the alternative pathway. Besides the F(ab)′2-mediated neutralization of complement C3a and C5a, the interaction between the Fc fragment and complement C1q, C3b, and C4b in a dose-dependent manner, contributes to immunomodulatory effects of IVIg on the classical complement pathway (73–75). The binding site of C3b/C4b is located on the residues 381–390 of the CH3 domain of the IgG Fc fragment, while the residues 318–322 of the CH2 domain are responsible for the binding of C1q (76, 77). The binding domains may react with different N-glycan sialylation patterns on the IgG structure and result in distinct anti-inflammatory effects through the complement pathway (78).
As an extremely complex preparation, IVIg contains a large number of bioactive moieties, and the entirety of effects from IVIg is therefore not fully understood yet. The proposed antigen-specific F(ab)′2-mediated mechanisms and unspecific Fc-mediated mechanisms are not mutually exclusive, but is more likely to regulate the immune system in synergy, giving rise to the immunomodulatory effects of high-dose IVIg in specific clinical settings.
The Immunopathological Hypothesis of Viral Pneumonia
Although an active immune response is essential for pathogen elimination in acute respiratory viral infections, excessive defensive reactions might wreak havoc on healthy cells and tissues. Complications or mortality of respiratory viral infections are often associated with excessive production of pro-inflammatory cytokines and ensuing multiple organ dysfunction. Although the immunopathogenesis of SARS-CoV-2 has not yet been fully described, the histopathological evidence strongly suggests a critical role of an excessive immune response in mediating extensive damage of the lung and other organs, similar to previous observations in SARS, MERS, influenza, and RSV disease, where hyper-inflammatory responses have been shown to be involved in the lung pathology. In this section, we review how a dysfunctional immune response may cause immunopathology in severe viral pneumonia, resulting in the current understanding of IVIg therapy in modulating the hyper-inflammatory conditions.
Cytokine Storm and the Role of IVIg
The cytokine storm syndrome is a form of systemic inflammatory response common to severe acute viral pneumonias, and its presence has also been suggested in severe cases of COVID-19. There is a correlation between severity of the cytokine storm and prognosis of severe illnesses (13). At the initiation of infection, the host cells detect viruses through pattern recognition receptors (PRRs), which in turn triggers an interferon (IFN) response and produces other pro-inflammatory mediators such as cytokines and chemokines, informing both innate and adaptive immune system to respond appropriately to infectious pathogens. A physiological cytokine and chemokine response induced by viruses is a sprawling network, which involves endothelial cells, mononuclear macrophages, dendritic cells, natural killer cells, and lymphocytes, contributing to pathogen clearance and immune protection. However, an uncontrolled positive feedback involving all the relevant players leads to pathogenic hyper-inflammation and can cause extensive damage to tissues.
Endothelial Cells
Endothelial cells can secrete inflammatory mediators, including cytokines, chemokines, histamine, matrix metalloproteinases, and adhesion molecules, which can lead to inflammatory cell infiltration and tissue damage, and plays an essential role in inflammation, hemostasis, and angiogenesis.
SARS-CoV-2 infects host cells using the ACE2 receptor, which is widely expressed on endothelial cells. Endothelial cell infection and endotheliosis have been observed in some COVID-19 patients (79). Direct viral infection of the endothelium may result in extensive endothelial dysfunction and disseminated intravascular coagulation (17, 79). In addition, abnormal levels of complement factors and the deposition of terminal complement complex also suggested that the activation of complement pathways may contribute to endothelial damage and the associated pro-coagulant state in COVID-19 (80, 81).
Although vasculitis and autoantibodies against endothelial cells have been reported and detected in SARS patients, direct endothelial cell infection hasn't been demonstrated yet (82–84). These differences in endothelial infections may contribute to the different immunopathology between SARS-CoV-1 and SARS-CoV-2. In influenza, the central role of the pulmonary endothelium for the regulation of innate immune cell recruitment into the lungs, along with the production of excessive pro-inflammatory cytokines and chemokines, has been demonstrated (85–87).
The anti-inflammatory effect of high-dose IVIg on endothelial cells is mainly inferred from in vitro observations. High-dose IgG has been shown to specifically and completely inhibit TNF-α-induced secretion of pro-inflammatory cytokines (e.g., IL-6, G-CSF, and IL-1β) and production of E-selectin in cultured human coronary artery endothelial cells (88, 89). These effects may predominantly be mediated by F(ab)′2-mediated mechanisms (88, 89). The expression of endothelial adhesion molecules is also reduced by co-culturing endothelial cells with an IVIg preparation (90). Additionally, the inhibitory effects of the classical complement pathway by high-dose IVIg may offer a potential protective effect on virus-induced endothelial damage.
Dendritic Cells
Dendritic cells (DCs) are antigen-presenting cells of the innate immune system. Plasmacytoid dendritic cells (pDCs) are unique sentinel cells that can recognize a virus through PRRs, and enhance the secretion of IFN (91, 92).
Data from COVID-19 patients and a ferret model reveal low levels of IFN with contrastingly high levels of IL-6 and a strong chemokine response, suggesting a similar hyper-inflammatory response pattern to SARS and MERS (93). Several SARS-CoV and MERS-CoV proteins have been shown to antagonize the IFN response in order to reach high viral titers after early infection (94–98). Delayed and massive IFN signaling is suggested to further orchestrate extensive inflammatory monocyte-macrophage responses and induce T cell apoptosis, resulting in a dysfunctional immune response and cytokine storm during SARS-CoV and MERS-CoV infection (99–101).
The immunomodulatory effects of IVIg on DCs differ depending on dosage. IVIg accelerates maturation at physiological doses, but inhibits the maturation, activation, and function at therapeutic doses (102). High-doses IVIg treated immature DCs secrete increased levels of anti-inflammatory cytokines, and decreased levels of pro-inflammatory cytokines (103). C-type lectins expressed on DCs are considered as possible effector targets of IVIg preparations, however, the exact mechanism of action of IVIg on DCs remains controversial. As stated, interactions between Fc-sialylated IgG and DC-SIGN failed to reproduce in human cells, and the expression of FcγRIIB remains stable on DCs after IVIg treatment (104). Another C-type lectin, DRIC, has also been identified to bind to Fc-sialylated IgG and further mediates the induction of Treg cells in a mouse model (105). Additionally, an increased accumulation of lipids and decreased expression of MHC-II, CD40, CD80/CD86, and IFN-γR2 on DCs upon high-doses IVIg treatment might suppress antigen presentation and allogeneic T-cell stimulatory capacity, contributing to a negative regulation of the immune response (104, 106, 107).
Monocyte-Macrophages
Whether infiltrating inflammatory monocyte-macrophages (IMM) are beneficial or deleterious after a viral infection is mainly dependent on their ability to secrete inflammatory cytokines and chemokines. A dysregulated cytokine response can also promote excessive activation of IMM, leading to immunopathological effects on healthy tissues.
Macrophage activation syndrome (MAS) was reported in several COVID-19 patients with severe respiratory failure, and the production of associated pro-inflammatory cytokines (IL-6 and TNFα) might contribute to hyper-inflammatory conditions (108). It was also detected that SARS-CoV-2 infects macrophages and triggers secretion of IL-6, which might contribute to lymphocyte apoptosis, suggesting an excessive infiltration and intense pro-inflammatory activity of IMM in COVID-19 patients (109).
Increased pathogenic IMM influx has been consistently observed in severe or lethal SARS patients, along with mice and Chinese rhesus macaque models (101, 110). The activation of macrophages by SARS-CoV occurs by TLR2 ligand recognition, subsequent activation of the NF-κB pathway and secretion of cytokines, contributing to the immunopathology of SARS (111–113).
High-dose IVIg therapy is widely used for treatment of patients with autoimmune diseases complicated by MAS (114–118). For inhibitory effects of IVIgs, functionally activating FcγRs are required to acquire a cross-tolerant state of mouse macrophages (119). In vitro, IVIg inhibits the production of pro-inflammatory cytokines by M1 macrophages and triggers macrophage polarization via FcγRIII-mediated mechanisms (120). IVIg limits the differentiation of macrophages through inhibition of GM-CSF-driven STAT5 activation by FcγR-dependent mechanisms (119). An increased production of anti-inflammatory cytokines of LPS-stimulated monocytes (especially IL-10) is further enhanced by IVIg through phosphorylation of ERK1/ERK2 and P38/MAPK via FcγRIIA-mediated mechanisms (121).
Neutrophils/Granulocytes
Neutrophils are the most abundant type of granulocytes in peripheral blood and constitute an essential part of innate immunity. The release of cytokines, nitric oxide, reactive oxygen species (ROS), and neutrophil extracellular traps (NET) by neutrophils can help to neutralize pathogens. Paradoxically, however, neutrophil reactivity can also enhance tissue damage in hyper-inflammatory conditions such as severe virus infection.
Excessive neutrophils and NET production are considered a potential predictor of prognosis in influenza (122, 123). Similarly, it has been considered that neutrophils in SARS-CoV-2 may induce NETs and contribute to organ damage (124, 125). High levels of NET biomarkers (e.g., cell-free DNA, myeloperoxidase-DNA, and citrullinated histone H3) are detected in severe COVID-19 patients and may contribute to cytokine storm and respiratory failure (125).
Both Fc and F(ab)′2 fragments account for the anti-inflammatory activity on neutrophils. In patients with Kawasaki disease, IVIg therapy reduces the activation and nitric oxide production of neutrophils (126). In vivo studies revealed that IVIg inhibits neutrophil recruitment and activation through the activation of SHP-1 via FcγRIII-mediated mechanisms (127). Furthermore, IVIg contains anti-Siglec autoantibodies that can regulate neutrophil apoptosis in a dose-dependent cytotoxic manner via F(ab)′2-mediated mechanisms (33, 128, 129). Interestingly, high-dose sulfo-IVIg preparations significantly reduce NET formation in vitro as well as in rat models (130).
Natural Killer Cells
Natural killer (NK) cells are innate lymphocytes that play an essential role in the control of respiratory viral infections. Within days after a respiratory viral infection, NK cells are activated, recruited to the lung, and act as effector cells in a classical FcγR-mediated function, namely antibody-dependent cytotoxicity (ADCC). However, a dysfunction of NK cells may also be responsible for immunopathogenesis during infection.
Increased inhibitory receptor NKG2A expression on NK cells and reduced production of CD107a, IFN-γ, IL-2, and TNF-α, indicate a functional exhaustion of NK cells during SARS-CoV-2 infection, similar to that observed in SARS (131). In patients with severe SARS, the number of NK cells and levels of the functional NK-marker KIR2DL3 were significantly lower than in patients with mild SARS, mycoplasma pneumonia, or healthy controls, manifesting a correlation between the severity of SARS and the number and function of NK cells (132). During influenza virus and RSV infection, virus-induced apoptosis or modulation of NK cell cytotoxicity has also been proposed to serve as a viral evasion strategy, and eventually skew the inflammatory profile of the immune system (133, 134).
After high-dose IVIg therapy, a reduction in number and cytotoxic activity of NK cells is observed in patients with autoimmune diseases (135–137). The dose-dependent FcγRIII blockade and circulating NK cells decline occurred following IVIg treatment, suggesting that inhibition of NK cells by high-dose IVIg is associated with the saturation of activating FcγRs (135). The inhibitory effects of IVIg therapy on NK cells may, however, not impair its functions for controlling viral infections and malignancies (138).
CD4+ T Lymphocytes
Cytokines secreted by different subclasses of CD4+ T cells trigger the immune response to pathogens and maintain immune homeostasis. However, an imbalance of Th1, Th2, Th17, and Treg subclasses is associated with dysfunctional cytokine responses during severe viral infections.
The profiles of serum cytokines revealed an increased concentration of Th17 cells in COVID-19 patients (139, 140). In severe SARS patients, the activation of Th1-related cytokines and chemokines (e.g., IL-1, IL-6, IL-12) was involved in hyper-inflammatory conditions (141). Furthermore, in MERS patients, the significantly increased release of pro-inflammatory cytokines from Th1 and Th17 during the acute phase was considered to be at least partly responsible for the immunopathology (142). Early abundant secretion of Th1- and Th17-related cytokines (e.g., IL-6 and IL-17) has furthermore been associated with complicated infections and mortality in severe influenza patients (143, 144).
In addition to mediating expansion of Tregs, which profoundly impacts immune cascades, high-dose IVIg has also been shown to inhibit the activation and subsequent production of cytokines by Th1 and Th17 cells in several clinical studies and in vitro experiments (145–158). Moreover, F(ab)′2 fragments, rather than Fc fragments, have been shown to retain the function of intact antibodies in inhibiting Th1 and Th17 (158). It has been proposed that IVIg may neutralize sphingosine-1-phosphate (S1P) receptors on the CD4+ T cell and downregulate the S1P1-mTOR signaling axis, thereby inhibiting the differentiation and infiltration of Th1 and Th17 cells while favoring Treg cells (158). Interestingly, monomeric IgA (mIgA) isolated from a IVIg preparation has been revealed to interfere with the STAT3 via its F(ab)′2 fragments and inhibits the differentiation and expansion of Th17 cells (159). It is noted that the proposed mechanism of functional T cell-modulation by IVIg also includes DC-mediated effects; IVIg-primed DCs can steer non-Treg cell precursors toward a Treg differentiation, and C-type lectins expressed on DC cells may be critical in this process (105, 160–162). Novel Treg epitope peptides (Tregitopes) on IgG provide a further explanation of the regulatory effects of IVIg on Treg cells via DC-mediated internalization of IVIg (163). Tregitopes are natural T cell epitopes of IgG, and the presentation of Tregitopes on DCs in the context of MHC-II can lead to the activation and expansion of Treg cells, reinforcing the immunoregulatory effects on other conventional T cells (164, 165).
CD8+ T Lymphocytes
CD8+ T cells contribute to pathogen clearance and immune protection. However, cytokines and cytotoxic granules produced by activated CD8+ T cells may exaggerate cytokine storms and are partially implicated in the immunopathology during respiratory viral infections (166, 167).
Both a substantial reduction of CD8+ T cell counts in peripheral blood, and hyperactivation with extensive expression of surface activation markers (HLA-DR and CD38) and cytotoxic granules in CD8+ T cells are commonly observed in COVID-19 patients (139, 168). An accompanying functional exhaustion of CD8+ T cells is furthermore detected in severe COVID-19 patients (131). Unlike influenza or RSV, the impaired function of CD8+ T cells during SARS-CoV-2 infection suggests that these cells may not be major contributors to the cytokine storm in severe COVID-19 patients (166, 167, 169). However, overactivation and subsequent functional exhaustion of CD8+ T cells may correlate with disease progression (131).
After effective high-dose IVIg therapy, the expression of HLA-DR and the proportion of extensive highly activated Vβ elements of CD8+ T cells decrease in patients with autoimmune diseases (170, 171). In vitro and animal model studies report similar inhibitory effects (172, 173). As no FcγRs are expressed on T cells, the modulation of IVIg on CD8+ T cells may largely depend on specific antibodies present in the IVIg preparation, which directly bind to T cells, or through interactions between APC and TCR signaling (174). For example, it has been shown that the anti-B07.75–84 peptide antibodies in IVIg may be able to inhibit HLA class I–restricted cytotoxicity of CD8+ T cells via their F(ab)′2 fragment (175). Apart from that, a saturation of activating FcγRs on APC results in reduced immune complex internalization and presentation, further contributing to the inhibitory effects of high-dose IVIg on CD8+ T cells (172, 173).
B Lymphocytes
With assistance from helper T cells and stimulation from cytokines, B cells are activated and differentiated into plasma cells to produce antibodies and contribute to humoral immunity (169, 176). Apart from the generation of antibodies, IL-6 produced by activated B cells is also considered as a possible booster of the cytokine storm and immunopathology in autoimmune disease (177, 178).
The rapid reduction of peripheral B cells is also a significant characteristic of severe COIVD-19 patients, yet the exact mechanisms underlying this accelerated lymphocyte loss remain unclear (17, 179). Although there is so far no reliable evidence correlating an overactivation of B cells with the development of cytokine storms during respiratory viral infections, the production of pro-inflammatory cytokines by B cells has been proposed to play a key role in the cytokine cascade in COVID-19 (180–182).
Effects of IVIg on B cells vary with the dosage: at low doses, IVIg induces proliferation of B cells and the production of antibodies (183, 184). On the contrary, high-dose IVIg inhibits the activation of B cells via both F(ab)′2-mediated mechanisms (e.g., neutralizing APRIL, BAFF, and Fas) and Fc-mediated mechanisms acting on inhibitory FcRs (e.g., FcγRIIB and CD22), as well as the TLR-9 signaling cascade.
FcγRIIB is the only classical FcγR expressed on B cells, and its elevated expression levels after effective high-dose IVIg therapy may be involved in inhibitory effects of IVIg on B cells (61, 185, 186). However, IVIg-induced upregulation of FcγRIIB in B cells is independent of classical FcγRIIB signaling intermediates, suggesting that it is likely a consequence rather than a cause of high-dose IVIg therapy (187, 188). It has been proposed that sialylated IgG binds to DC-SIGN in addition to CD23, a C-type lectin which is also a known low-affinity IgE receptor characteristically expressed on B cells, inducing the suppression of B cells (189). However, there has been disagreement as to whether there is a direct interaction between IgG and DC-SIGN/CD23 (67, 190). A further proposed mechanism of action of high-dose IVIg is the interaction between the sialylated Fc fragment of IgG and CD22, an I-type lectin expressed on B cells, which results in the activation of the ITIM signaling cascade, subsequently promoting B cell apoptosis (183, 191, 192). Except for FcRs, the TLR-9 signaling cascade may also be involved in inhibitory effects. IVIg could mimic the effects of MyD88 inhibitors and result in the suppression of the TLR-induced NF-κB signaling pathway and downstream production of cytokines (193, 194).
A “paradoxical” phenomenon has been observed, where large amounts of immature plasma cells were mobilized after high-dose IVIg therapy in patients with autoimmune diseases, suggesting de novo B cell activation. This is consistent with the immunomodulatory functions of IVIg at low doses (184, 195, 196). Although the potential roles of plasma blasts under different disease conditions remain unclear, this phenomenon highlights binary immunomodulatory effects of high-dose IVIg to humoral immunity.
To summarize, the cytokine storm observed during severe viral infection is associated with various immune dysfunctions. Several therapeutic interventions targeting the host immune response have been attempted. Although the proposed mechanisms of high-dose IVIg on the modulation immune cells and cytokine cascades are mainly derived from research into autoimmune diseases, its use in reversing a cytokine storm, in addition to providing passive immunity during severe viral infection, is supported by recent evidence.
Antibody-Dependent Enhancement Phenomenon and the Role of IVIg
The humoral immune response to invading pathogens by producing neutralizing antibodies is crucial in the host adaptive immune system. Paradoxically, antibodies may provide an attractive means for a variety of viruses to enhance viral entry and replication in some cell types under certain conditions. The unique phenomenon that preexisting poor neutralizing antibodies facilitate viral access to FcγRs and lead to enhanced infection or immunopathology is also referred to as antibody-dependent enhancement (ADE). ADE has been implicated widely in flavivirus infections, such as WNV and DENV, and high-dose IVIg is commonly used in treating WNV encephalitis with satisfactory effect. However, little is known about ADE in respiratory virus infection (197–199).
ADE in SARS, MERS, Influenza, and RSV Disease
ADE is currently being considered as a potential contributor to the immunopathology of SARS. People who succumbed to SARS had significantly higher S glycoprotein-specific neutralizing antibodies in serum during the early stage of infection, indicating the possible presence of ADE (200). Similarly, recent results in SARS-CoV-infected Chinese rhesus macaques consistently showed that anti-spike IgG significantly amplified pro-inflammatory cytokine production in activated macrophages and enhanced pulmonary pathology. These effects could be reduced by the FcγRII blocking antibody (110). Similarly, it has been shown that infection of macrophages is enhanced by anti-SARS-CoV spike immune serum but eliminated by the FcγRII antibody, suggesting an FcγRII-dependent ADE of SARS-CoV (201). Molecular signaling analyses investigating FcγRII-mediated infection by SARS-CoV revealed that an intact cytosolic domain of FcγR was required, and FcγRIIA was more prone to ADE than FcγRIIB (202).
Although in vivo evidence of ADE in MERS is lacking, it has been shown that MERS-CoV infections depended on monoclonal antibody (MAb) concentration, binding affinity of MAb for the viral receptor DPP4, and tissue expressions of DPP4 and FcγR, providing a molecular basis of ADE in MERS-CoV (203). Likewise, the presence of sub-neutralizing titers of antibodies is also a contributing factor to the unfavorable outcome in influenza infection. Prophylactic treatment with monoclonal antibodies at a low dose following the H3N2 virus challenge in mice has shown enhanced cellular infiltration and lung pathology compared to the control group (204). In contrast, a high dose prophylaxis showed protective effects (204). Vaccinating pigs against H1N2 resulted in the generation of cross-reactive anti-pH1N1 HA2 antibodies, which exhibited poor neutralizing ability to the HA1 domain and enhanced pH1N1 infection and lung pathology (205). Besides influenza and potentially MERS, the ADE phenomenon has also been observed in RSV infection (206). It has been shown that RSV infections are significantly enhanced in NK cells previously incubated with sub-neutralizing titers of RSV-specific antibodies in vitro, promoting IFN-γ production of NK cells (207). It remains unclear whether such in vitro enhancement of RSV infection has a correlation with in vivo disease. Nevertheless, the Fc-mediated antibody effector functions are believed to participate in the antibody-dependent enhancement of RSV disease (206, 208).
ADE in COVID-19 and the Role of IVIg
To date, the exact role of ADE in SARS-CoV-2 infection remains unclear. Regarding concerns that reappearance of cross-reactive antibodies from other serotypes of coronavirus may enhance the current infection, the risk of ADE needs to be clarified when treating COVID-19 patients (203, 209).
As described above, the occurrence of ADE requires binding of the virion to antibodies at a sub-neutralizing concentration and subsequent uptake by FcγR-bearing cells (210). To inhibit potential phenomenon of ADE in COVID-19, high-dose IVIg therapy is therefore proposed (211). First, it is unlikely to have preexisting anti-SARS-CoV-2 antibodies in IVIg preparations early in the novel epidemic. Additionally, a high concentration of non-neutralizing antibodies, rather than a regular or diluted dose, would integrally saturate activating FcγRs and FcRn, which is beneficial to limit access of immune complexes and enhance clearance of inimical antibodies, to ultimately provide immunomodulatory effects in severe COVID-19 patients (211, 212).
Lessons From Previous and Current Viral Pneumonias
Based on its efficacy and supporting mechanisms in modulating the inflammatory response and improving serum IgG levels, high-dose IVIg therapy is considered for treatment of several severe viral infections. Potential protective effects of IVIg preparations were previously shown in influenza- and RSV-infected animal models (213–218). In this section, we review the previous clinical application of high-dose IVIg therapy in treating viral pneumonia such as SARS, MERS, influenza and RSV disease, as well as its current application in COVID-19 (Table 2).
IVIg in Treating SARS
During the global outbreak of SARS in 2003 caused by SARS-CoV, different therapeutic modalities (e.g., α-interferon, ribavirin, LPV/r, corticosteroids, convalescent plasma, and IVIg) were empirically used (233). Although strong evidence recommending the administration of IVIg therapy is lacking and a previous systemic review concluded that the evidence for efficacy of improving prognosis with IVIg therapy remained inconclusive, some studies commented that patients seemed to improve upon IVIg treatment (233, 234).
In a randomized controlled trial, IVIg therapy efficiently improved the serum IgG concentration of severe SARS patients compared to the control group (222). However, possibly due to the inadequately controlled dosage of glucocorticoids and IVIg therapy, which were given at the discretion of clinicians, there were no significant differences in the fatality rates and the rates of nosocomial infection of severe SARS patients between the IVIg group and the control group (222).
Apart from hypogammaglobulinemia, two further common features of severe SARS are leukopenia and thrombocytopenia, and these features seemed to improve in SARS patients upon IVIg therapy (223, 234). The peripheral WBC and platelet count of severe SARS patients significantly increased after receiving IVIg therapy without steroids, suggesting a potential role of IVIg in controlling leukopenia and thrombocytopenia in SARS patients (224). Furthermore, significant clinical improvement in pediatric SARS patients was noted after IVIg therapy (224). A declining WBC significantly recovered after IVIg therapy, and the temperature of most patients normalized within 3 days. Compared to the historical controlled group, a significantly shorter time of chest radiographic absorption in these patients was observed and indicated the potential efficacy of IVIg in clinical improvement and absorption of lung lesions.
Interestingly, an IgM-enriched IVIg preparation also showed significant beneficial effects in deteriorating SARS patients who failed corticosteroid and ribavirin treatment (225). A significant improvement regarding the oxygen requirements and radiographic scores were observed after pentaglobin therapy, and most patients recovered. It has been proposed that abnormal cytokine levels (e.g., IL-6 and TNF-α) are correlated with poor prognosis in SARS patients, and that the inhibitory effects of pentaglobin on cytokine release might represent an essential mechanism of action in the treatment of SARS (225, 235).
IVIg in Treating MERS
MERS is a highly fatal respiratory disease caused by MERS-CoV, with two major historic outbreaks in 2012 and 2015. The rapid deterioration of health in a large number of patients made it unrealistic and unethical to perform randomized controlled treatment trials. Therefore, the current availability of strong evidence is minimal, and only a few case series reported administration of IVIg late in the course of MERS. These studies discussed the possible efficacy of IVIg in reversing severe thrombocytopenia through immunomodulatory mechanisms (226, 236).
IVIg in Treating Influenza
Although influenza has been around for centuries, severe influenza remains a health challenge for humans. The complications or deaths are usually associated with the extensive induction of pro-inflammatory cytokines in severely affected influenza patients (166). Immunomodulatory strategies or treatments, including amongst others corticosteroids, PPARs agonists, S1P1 receptor 1 agonists, antioxidants, and IVIg therapy, have been widely considered as adjunctive treatments for cytokine storms in influenza (237).
In a previous randomized controlled trial, patients infected with the severe 2009 pandemic influenza A (H1N1) were randomized to receive 0.4 g/kg for one dose of Flu-IVIg (anti-influenza hyperimmune intravenous immunoglobulin with high HAI antibodies level) or IVIg therapy (227). Patients who received Flu-IVIg showed a greater rate of viral load reduction than the IVIg group. However, there was no significant difference in the cytokine profiles (e.g., IFN-α2, IL-1α, IL-6, IL-10, IL-15, IL-1ra, MCP-1, MIP-1α, GM-CSF, TNF-α) between the IVIg and Flu-IVIg group on day 5. Subgroup multivariate analysis of patients receiving treatment within 5 days of symptom onset revealed that Flu-IVIg therapy, rather than IVIg therapy, was the only factor that independently reduced mortality.
In contrast, other RCTs using IVIg treatment at high doses have shown improved survival. In two similar studies, pediatric patients with the severe 2009 pandemic influenza A (H1N1) were randomized to receive standard care or standard care plus 1 g/kg/d high-dose IVIg for 2 days (228, 229). Significant clinical improvement with regards to temperature normalization, reduction of cough, rhinorrhea, tachypnea, respiratory sounds (namely wheeze or rhonchi), and chest radiographic lesions were observed in the IVIg group compared to the control group, revealing a possible clinical benefit of high-dose IVIg therapy for the improvement of clinical symptoms in pediatric patients with severe influenza A H1N1 (228, 229).
IVIg in Treating RSV Infection
RSV is a major respiratory pathogen that causes extensive respiratory symptoms, including both upper and severe lower respiratory tract infection (URTI/LRTI) in infants, the elderly, and immunocompromised individuals (238). Available therapies are limited to RBV, IVIg, and palivizumab, but the use of IVIg for treatment of RSV infections remains controversial (239).
A study showed that hypogammaglobulinemia was a significant risk factor for fatal outcomes of RSV infections in a hematology and transplant unit, and treatment with IVIg at regular doses was unable to reverse the poor prognosis (230). By contrast, oral RBV in combination with high-dose IVIg therapy (rather than lower doses), have been potentially attributed to the improvement of survival in RSV-infected allogeneic hematopoietic stem cell transplanted (HSCT) patients (231). The dosage of IVIg might be the critical factor for efficacy of therapy, since the combination of high-dose IVIg and aerosolized RBV therapy has also been shown to be a safe and promising approach to prevent progression of RSV infections immunocompromised patients (232).
Several published studies suggest that a combined therapy of RBV and IVIg improves the outcome of HSCT and leukemia patients with RSV-URTI, and additional recommendations are given for high-risk patients with RSV-LRTI (240–242). However, it is noted that most studies on IVIg efficacy are limited to pediatric or HSCT patients, which may not explicitly be recommended in all situations (243).
In summary, IVIg therapy exhibits different levels of potential clinical benefits in SARS, MERS, influenza, and RSV infections, though currently there is no high-level evidence to support IVIg use in these infections. Of note, the presented results are partly limited to pediatric and immunocompromised patients, and the clinical benefits of IVIg therapy may vary in different patient populations (e.g., infants, the elderly, and immunocompromised individuals). It is also noteworthy that most studies for these viral infections show possible benefits from IVIg therapy at higher doses, which indicates that a high dosage might be key for clinical efficacy. Considering the immunomodulatory effects of high-dose IVIg on the excessive immune cascade, high-dose IVIg therapy, as stated above, may be considered a potential adjunctive treatment for severe COVID-19 patients.
IVIg in Treating COVID-19
The national diagnosis and treatment protocol for COVID-19 (Trial Version 7) and recommendations from the Peking Union Medical College Hospital have suggested the application of IVIg therapy in severe and critically ill COVID-19 patients (4, 18). Several observational and interventional studies were conducted to evaluate the efficacy of IVIg.
A retrospective study (n = 58) in China included severe and critical ill COVID-19 patients that were administered IVIg therapy after admission (219). IVIg therapy was given at 20 g daily, and 23 of 58 (39.6%) patients died within 28 days. For retrospective analysis, patients were split into two groups, based on whether they received IVIg administration within or after 48 h of admission to the intensive care unit (ICU). The results showed that application of IVIg within 48 h of admission to ICU reduced the use of mechanical ventilation, shortened duration of ICU and hospital stay, and improved 28-day survival (219). The 28-day mortality of the two groups (IVIg within or after 48 h) was 23.3% (within 48 h) and 57.1% (after 48 h), indicating that early initiation of high-dose IVIg therapy may be beneficial in severe COVID-19 patients. On the contrary, there were no significant effects of IVIg therapy on the survival of severe COVID-19 patients who developed ARDS in another study; here, the insufficient effects of IVIg therapy may potentially be a result of timing and dosage of IVIg administration (220).
In another study, a combination therapy of a high dose of IVIg (20 g/d) and corticosteroid (160 mg/d) succeeded in reversing the deteriorating condition of severe COVID-19 patients who had previously failed low-dose IVIg (10 g/d) and corticosteroid treatment (221). However, as this study investigated a combined therapy, it is not possible to estimate the exact effect of high IVIg doses in this group of patients.
The administration of IVIg therapy (25 g/d for 5 days) at the time of initiation of respiratory distress of severe COVID-19 patients may improve the prognosis (16). It is noted that only one of three patients received corticosteroids, and these observations suggest that high-dose IVIg therapy may be the main contributor to successful recovery from deteriorating conditions. However, more controlled trials of IVIg therapy are needed to provide strong evidence for its beneficial effects in COVID-19.
Based on these clinical observations, the administration of high-dose IVIg therapy at the appropriate point may be able to prevent disease progression and improve the prognosis of patients with severe COVID-19. Currently ongoing randomized controlled trials of high-dose IVIg therapy in severe COVID-19 patients are evaluating the benefit of IVIg compared to standard care (NCT04261426 and NCT04350580) and will be able to further provide more information about the clinical effects of IVIg therapy in COVID-19.
Although a large number of clinical trials have demonstrated that IVIg therapy is well-tolerated, various side effects have been reported (244). The majority of these effects are mild and transient, yet clinicians need to be vigilant about rare but serious adverse effects such as aseptic meningitis, renal impairment, thrombosis, and hemolytic anemia (244). It is noted that thrombosis is common in COVID-19, and whether high-dose IVIg therapy would further increase the risk of thrombosis remains unclear (17, 245). However, the combined treatment of low molecular weight heparin and high-dose IVIg therapy at 0.3–0.5 g/kg/d for 5 days in the early phase has been suggested to have proper efficacy in treating severe COVID-19 patients (17).
Conclusion
The IVIg preparation is a widely used pooled human blood product that can provide passive immunity and modulate the immune functions. Although, for COIVD-19 the pathogen-specific effects of IVIg are not relevant yet, since available preparations were collected from healthy donors without pre-existing immunity early before the pandemic began. The anti-inflammatory and immunomodulatory effects on the various immune cells of high-dose IVIg may account for its clinical benefits. Based on these potential supportive F(ab)′2 and Fc mediated mechanisms and the known clinical effects in treating severe virus pneumonia such as SARS, MERS, influenza, and RSV disease, the early application of high-dose IVIg therapy may be considered in the management of severe COVID-19 patients. Currently, limited clinical practice of high-dose IVIg in treating SARS-CoV-2 infection has been reported to show potential clinical benefits. Still, more research is needed but these inferences may provide relevant and useful insights and help in confronting the COVID-19 epidemic.
Author Contributions
XL conceived and wrote the manuscript and prepared figures. WC and TL contributed to the modification and revision of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). (2020). Available oline at: https://www.who.int/publications-detail/report-of-the-who-china-joint-mission-on-coronavirus-disease-2019-(covid-19) (2020) (accessed March 12, 2020).
2. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
3. Md Insiat Islam R. Current drugs with potential for treatment of COVID-19: a literature review. J Pharm Pharm Sci. (2020) 23:58–64. doi: 10.18433/jpps31002
4. CHINA National Health Commission. Diagnosis and Treatment of Pneumonia Infected by Novel Coronavirus (Trial Version 7). (2020). Available oline at: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml (accessed March 4, 2020).
5. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. (2020) 395:473–5. doi: 10.1016/S0140-6736(20)30317-2
6. McIntosh JJ. Corticosteroid guidance for pregnancy during COVID-19 pandemic. Am J Perinatol. (2020) 37:809–12. doi: 10.1055/s-0040-1709684
7. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. (2020) 395:1569–78. doi: 10.1016/S0140-6736(20)31022-9.
8. Magagnoli J, Narendran S, Pereira F, Cummings TH, Hardin JW, Sutton SS, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid19. Med. doi: 10.1016/j.medj.2020.06.001. [Epub ahead of print].
9. Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. (2020) 369:m1849. doi: 10.1136/bmj.m1849
10. Chen C, Zhang Y, Huang J, Yin P, Cheng Z, Wu J, et al. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv [Preprint]. (2020). doi: 10.1101/2020.03.17.20037432
11. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. (2020) 382:1787–99. doi: 10.1056/NEJMc2008043
12. Newton AH, Cardani A, Braciale TJ. The host immune response in respiratory virus infection: balancing virus clearance and immunopathology. Semin Immunopathol. (2016) 38:471–82. doi: 10.1007/s00281-016-0558-0
13. Vaninov N. In the eye of the COVID-19 cytokine storm. Nat Rev Immunol. (2020) 20:277. doi: 10.1038/s41577-020-0305-6
14. Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin Immunol. (2020) 214:108393. doi: 10.1016/j.clim.2020.108393
15. Hui DS, Lee N, Chan PK, Beigel JH. The role of adjuvant immunomodulatory agents for treatment of severe influenza. Antiviral Res. (2018) 150:202–16. doi: 10.1016/j.antiviral.2018.01.002
16. Cao W, Liu X, Bai T, Fan H, Hong K, Song H, et al. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with Coronavirus Disease 2019. Open Forum Infect Dis. (2020) 7:ofaa102. doi: 10.1093/ofid/ofaa102
17. Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. (2020) 9:727–32. doi: 10.1080/22221751.2020.1746199
18. Li T. Diagnosis and clinical management of severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) infection: an operational recommendation of Peking Union Medical College Hospital (V2.0). Emerg Microbes Infect. (2020) 9:582–5. doi: 10.1080/22221751.2020.1735265
19. Kerr J, Quinti I, Eibl M, Chapel H, Späth PJ, Sewell WA, et al. Is dosing of therapeutic immunoglobulins optimal? A review of a three-decade long debate in europe. Front Immunol. (2014) 5:629. doi: 10.3389/fimmu.2014.00629
20. Kivity S, Katz U, Daniel N, Nussinovitch U, Papageorgiou N, Shoenfeld Y. Evidence for the use of intravenous immunoglobulins–a review of the literature. Clin Rev Allergy Immunol. (2010) 38:201–69. doi: 10.1007/s12016-009-8155-9
21. Reglinski M, Gierula M, Lynskey NN, Edwards RJ, Sriskandan S. Identification of the Streptococcus pyogenes surface antigens recognised by pooled human immunoglobulin. Sci Rep. (2015) 5:15825. doi: 10.1038/srep15825
22. Mikolajczyk MG, Concepcion NF, Wang T, Frazier D, Golding B, Frasch CE, et al. Characterization of antibodies to capsular polysaccharide antigens of Haemophilus influenzae type b and Streptococcus pneumoniae in human immune globulin intravenous preparations. Clin Diagn Lab Immunol. (2004) 11:1158–64. doi: 10.1128/CDLI.11.6.1158-1164.2004
23. Diep BA, Le VT, Badiou C, Le HN, Pinheiro MG, Duong AH, et al. IVIG-mediated protection against necrotizing pneumonia caused by MRSA. Sci Transl Med. (2016) 8:357ra124. doi: 10.1126/scitranslmed.aag1153
24. Farag N, Mahran L, Abou-Aisha K, El-Azizi M. Assessment of the efficacy of polyclonal intravenous immunoglobulin G (IVIG) against the infectivity of clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA) in vitro and in vivo. Eur J Clin Microbiol Infect Dis. (2013) 32:1149–60. doi: 10.1007/s10096-013-1861-5
25. Reglinski M, Sriskandan S. Treatment potential of pathogen-reactive antibodies sequentially purified from pooled human immunoglobulin. BMC Res Notes. (2019) 12:228. doi: 10.1186/s13104-019-4262-8
26. Schampera MS, Schweinzer K, Abele H, Kagan KO, Klein R, Rettig I, et al. Comparison of cytomegalovirus (CMV)-specific neutralization capacity of hyperimmunoglobulin (HIG) versus standard intravenous immunoglobulin (IVIG) preparations: impact of CMV IgG normalization. J Clin Virol. (2017) 90:40–5. doi: 10.1016/j.jcv.2017.03.005
27. Schrage B, Duan G, Yang LP, Fraser JD, Proft T. Different preparations of intravenous immunoglobulin vary in their efficacy to neutralize streptococcal superantigens: implications for treatment of streptococcal toxic shock syndrome. Clin Infect Dis. (2006) 43:743–6. doi: 10.1086/507037
28. Wood JB, Jones LS, Soper NR, Nagarsheth M, Creech CB, Thomsen IP. Commercial intravenous immunoglobulin preparations contain functional neutralizing antibodies against the Staphylococcus aureus leukocidin LukAB (LukGH). Antimicrob Agents Chemother. (2017) 61:e00968–17. doi: 10.1128/AAC.00968-17
29. Ye S, Li D, Liu F, Lei M, Jiang P, Wang Z, et al. In vitro evaluation of the biological activities of IgG in seven Chinese intravenous immunoglobulin preparations. J Pharm Biomed Anal. (2018) 151:317–23. doi: 10.1016/j.jpba.2018.01.021
30. Wadhwa M, Meager A, Dilger P, Bird C, Dolman C, Das RG, et al. Neutralizing antibodies to granulocyte-macrophage colony-stimulating factor, interleukin-1alpha and interferon-alpha but not other cytokines in human immunoglobulin preparations. Immunology. (2000) 99:113–23. doi: 10.1046/j.1365-2567.2000.00949.x
31. Bouhlal H, Hocini H, Quillent-Gregoire C, Donkova V, Rose S, Amara A, et al. Antibodies to C-C chemokine receptor 5 in normal human IgG block infection of macrophages and lymphocytes with primary R5-tropic strains of HIV-1. J Immunol. (2001) 166:7606–11. doi: 10.4049/jimmunol.166.12.7606
32. von Gunten S, Vogel M, Schaub A, Stadler BM, Miescher S, Crocker PR, et al. Intravenous immunoglobulin preparations contain anti-Siglec-8 autoantibodies. J Allergy Clin Immunol. (2007) 119:1005–11. doi: 10.1016/j.jaci.2007.01.023
33. Schaub A, von Gunten S, Vogel M, Wymann S, Rüegsegger M, Stadler BM, et al. Dimeric IVIG contains natural anti-Siglec-9 autoantibodies and their anti-idiotypes. Allergy. (2011) 66:1030–7. doi: 10.1111/j.1398-9995.2011.02579.x
34. Altznauer F, von Gunten S, Spath P, Simon HU. Concurrent presence of agonistic and antagonistic anti-CD95 autoantibodies in intravenous Ig preparations. J Allergy Clin Immunol. (2003) 112:1185–90. doi: 10.1016/j.jaci.2003.09.045
35. Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, et al. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science. (1998) 282:490–3. doi: 10.1126/science.282.5388.490
36. Le Pottier L, Bendaoud B, Dueymes M, Daridon C, Youinou P, Shoenfeld Y, et al. BAFF, a new target for intravenous immunoglobulin in autoimmunity and cancer. J Clin Immunol. (2007) 27:257–65. doi: 10.1007/s10875-007-9082-2
37. Le Pottier L, Sapir T, Bendaoud B, Youinou P, Shoenfeld Y, Pers J-O. Intravenous immunoglobulin and cytokines: focus on tumor necrosis factor family members BAFF and APRIL. Ann N Y Acad Sci. (2007) 1110:426–32. doi: 10.1196/annals.1423.044
38. Loeffler DA, Klaver AC, Coffey MP. Abeta anti-idiotypic antibodies are present in intravenous immunoglobulin and are produced in mice following its administration. Autoimmunity. (2015) 48:196–200. doi: 10.3109/08916934.2014.983265
39. Sultan Y, Kazatchkine MD, Nydegger U, Rossi F, Dietrich G, Algiman M. Intravenous immunoglobulin in the treatment of spontaneously acquired factor VIII:C inhibitors. Am J Med. (1991) 91:35s−9s. doi: 10.1016/S0002-9343(91)80147-E
40. Basta M, Van Goor F, Luccioli S, Billings EM, Vortmeyer AO, Baranyi L, et al. F(ab)′2-mediated neutralization of C3a and C5a anaphylatoxins: a novel effector function of immunoglobulins. Nat Med. (2003) 9:431–8. doi: 10.1038/nm836
41. Gong B, Levine S, Barnum SR, Pasinetti GM. Role of complement systems in IVIG mediated attenuation of cognitive deterioration in Alzheimer's disease. Curr Alzheimer Res. (2014) 11:637–44. doi: 10.2174/1567205011666140812113707
42. Debre M, Bonnet MC, Fridman WH, Carosella E, Philippe N, Reinert P, et al. Infusion of Fc gamma fragments for treatment of children with acute immune thrombocytopenic purpura. Lancet. (1993) 342:945–9. doi: 10.1016/0140-6736(93)92000-J
43. Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. (2001) 291:484–6. doi: 10.1126/science.291.5503.484
44. Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. (2006) 313:670–3. doi: 10.1126/science.1129594
45. Aloulou M, Ben Mkaddem S, Biarnes-Pelicot M, Boussetta T, Souchet H, Rossato E, et al. IgG1 and IVIg induce inhibitory ITAM signaling through FcgammaRIII controlling inflammatory responses. Blood. (2012) 119:3084–96. doi: 10.1182/blood-2011-08-376046
46. Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. (2009) 113:3716–25. doi: 10.1182/blood-2008-09-179754
47. van Mirre E, Teeling JL, van der Meer JW, Bleeker WK, Hack CE. Monomeric IgG in intravenous Ig preparations is a functional antagonist of FcgammaRII and FcgammaRIIIb. J Immunol. (2004) 173:332–9. doi: 10.4049/jimmunol.173.1.332
48. Teeling JL, Jansen-Hendriks T, Kuijpers TW, de Haas M, van de Winkel JG, Hack CE, et al. Therapeutic efficacy of intravenous immunoglobulin preparations depends on the immunoglobulin G dimers: studies in experimental immune thrombocytopenia. Blood. (2001) 98:1095–9. doi: 10.1182/blood.V98.4.1095
49. Augener W, Friedmann B, Brittinger G. Are aggregates of IgG the effective part of high-dose immunoglobulin therapy in adult idiopathic thrombocytopenic purpura (ITP)? Blut. (1985) 50:249–52. doi: 10.1007/BF00320302
50. Svacina MKR, Roth P, Bobylev I, Sprenger A, Zhang G, Sheikh KA, et al. Changes of serum IgG dimer levels after treatment with IVIg in Guillain-Barre syndrome. J Neuroimmune Pharmacol. (2019) 14:642–8. doi: 10.1007/s11481-019-09871-0
51. Ritter C, Bobylev I, Lehmann HC. Chronic inflammatory demyelinating polyneuropathy (CIDP): change of serum IgG dimer levels during treatment with intravenous immunoglobulins. J Neuroinflammation. (2015) 12:148. doi: 10.1186/s12974-015-0361-1
52. Mackness BC, Jaworski JA, Boudanova E, Park A, Valente D, Mauriac C, et al. Antibody Fc engineering for enhanced neonatal Fc receptor binding and prolonged circulation half-life. MAbs. (2019) 11:1276–88. doi: 10.1080/19420862.2019.1633883
53. Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. (2007) 7:715–25. doi: 10.1038/nri2155
54. Hansen RJ, Balthasar JP. Intravenous immunoglobulin mediates an increase in anti-platelet antibody clearance via the FcRn receptor. Thromb Haemost. (2002) 88:898–9. doi: 10.1055/s-0037-1613331
55. Li N, Zhao M, Hilario-Vargas J, Prisayanh P, Warren S, Diaz LA, et al. Complete FcRn dependence for intravenous Ig therapy in autoimmune skin blistering diseases. J Clin Invest. (2005) 115:3440–50. doi: 10.1172/JCI24394
56. Litzman J. Influence of FCRN expression on lung decline and intravenous immunoglobulin catabolism in common variable immunodeficiency patients. Clin Exp Immunol. (2014) 178(Suppl. 1):103–4. doi: 10.1111/cei.12529
57. Passot C, Azzopardi N, Renault S, Baroukh N, Arnoult C, Ohresser M, et al. Influence of FCGRT gene polymorphisms on pharmacokinetics of therapeutic antibodies. MAbs. (2013) 5:614–9. doi: 10.4161/mabs.24815
58. Gouilleux-Gruart V, Chapel H, Chevret S, Lucas M, Malphettes M, Fieschi C, et al. Efficiency of immunoglobulin G replacement therapy in common variable immunodeficiency: correlations with clinical phenotype and polymorphism of the neonatal Fc receptor. Clin Exp Immunol. (2013) 171:186–94. doi: 10.1111/cei.12002
59. Xiao JJ. Pharmacokinetic models for FcRn-mediated IgG disposition. J Biomed Biotechnol. (2012) 2012:282989. doi: 10.1155/2012/282989
60. Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. (1996) 379:346–9. doi: 10.1038/379346a0
61. Tackenberg B, Jelcic I, Baerenwaldt A, Oertel WH, Sommer N, Nimmerjahn F, et al. Impaired inhibitory Fcgamma receptor IIB expression on B cells in chronic inflammatory demyelinating polyneuropathy. Proc Natl Acad Sci USA. (2009) 106:4788–92. doi: 10.1073/pnas.0807319106
62. Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. (2008) 320:373–6. doi: 10.1126/science.1154315
63. Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci USA. (2008) 105:19571–8. doi: 10.1073/pnas.0810163105
64. Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. (2011) 475:110–3. doi: 10.1038/nature10134
65. Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat Rev Immunol. (2013) 13:176–89. doi: 10.1038/nri3401
66. Yu X, Vasiljevic S, Mitchell DA, Crispin M, Scanlan CN. Dissecting the molecular mechanism of IVIg therapy: the interaction between serum IgG and DC-SIGN is independent of antibody glycoform or Fc domain. J Mol Biol. (2013) 425:1253–8. doi: 10.1016/j.jmb.2013.02.006
67. Temming AR, Dekkers G, van de Bovenkamp FS, Plomp HR, Bentlage AEH, Szittner Z, et al. Human DC-SIGN and CD23 do not interact with human IgG. Sci Rep. (2019) 9:9995. doi: 10.1038/s41598-019-46484-2
68. Bayry J, Bansal K, Kazatchkine MD, Kaveri SV. DC-SIGN and alpha2,6-sialylated IgG Fc interaction is dispensable for the anti-inflammatory activity of IVIg on human dendritic cells. Proc Natl Acad Sci USA. (2009) 106:E25. doi: 10.1073/pnas.0900016106
69. Kasermann F, Boerema DJ, Ruegsegger M, Hofmann A, Wymann S, Zuercher AW, et al. Analysis and functional consequences of increased Fab-sialylation of intravenous immunoglobulin (IVIG) after lectin fractionation. PLoS ONE. (2012) 7:e37243. doi: 10.1371/journal.pone.0037243
70. Campbell IK, Miescher S, Branch DR, Mott PJ, Lazarus AH, Han D, et al. Therapeutic effect of IVIG on inflammatory arthritis in mice is dependent on the Fc portion and independent of sialylation or basophils. J Immunol. (2014) 192:5031–8. doi: 10.4049/jimmunol.1301611
71. Nagelkerke SQ, Dekkers G, Kustiawan I, van de Bovenkamp FS, Geissler J, Plomp R, et al. Inhibition of FcgammaR-mediated phagocytosis by IVIg is independent of IgG-Fc sialylation and FcgammaRIIb in human macrophages. Blood. (2014) 124:3709–18. doi: 10.1182/blood-2014-05-576835
72. Leontyev D, Katsman Y, Ma XZ, Miescher S, Kasermann F, Branch DR. Sialylation-independent mechanism involved in the amelioration of murine immune thrombocytopenia using intravenous gammaglobulin. Transfusion. (2012) 52:1799–805. doi: 10.1111/j.1537-2995.2011.03517.x
73. Basta M, Dalakas MC. High-dose intravenous immunoglobulin exerts its beneficial effect in patients with dermatomyositis by blocking endomysial deposition of activated complement fragments. J Clin Invest. (1994) 94:1729–35. doi: 10.1172/JCI117520
74. Lutz HU, Stammler P, Bianchi V, Trueb RM, Hunziker T, Burger R, et al. Intravenously applied IgG stimulates complement attenuation in a complement-dependent autoimmune disease at the amplifying C3 convertase level. Blood. (2004) 103:465–72. doi: 10.1182/blood-2003-05-1530
75. Arumugam TV, Tang SC, Lathia JD, Cheng A, Mughal MR, Chigurupati S, et al. Intravenous immunoglobulin (IVIG) protects the brain against experimental stroke by preventing complement-mediated neuronal cell death. Proc Natl Acad Sci USA. (2007) 104:14104–9. doi: 10.1073/pnas.0700506104
76. Frank MM, Miletic VD, Jiang H. Immunoglobulin in the control of complement action. Immunol Res. (2000) 22:137–46. doi: 10.1385/IR:22:2-3:137
77. Duncan AR, Winter G. The binding site for C1q on IgG. Nature. (1988) 332:738–40. doi: 10.1038/332738a0
78. Peschke B, Keller CW, Weber P, Quast I, Lunemann JD. Fc-Galactosylation of human immunoglobulin gamma isotypes improves C1q binding and enhances complement-dependent cytotoxicity. Front Immunol. (2017) 8:646. doi: 10.3389/fimmu.2017.00646
79. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. (2020) 395:1417–141. doi: 10.1016/S0140-6736(20)30937-5
80. Magro C, Mulvey JJ, Berlin D, Nuovo G, Salvatore S, Harp J, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. (2020) 220:1–13. doi: 10.1016/j.trsl.2020.04.007
81. Chen F, Liu ZS, Zhang FR, Xiong RH, Chen Y, Cheng XF, et al. [First case of critical novel coronavirus pneumonia in Chinese children]. Zhonghua Er Ke Za Zhi. (2020) 58:E005. doi: 10.3760/cma.j.issn.0578-1310.2020.0005
82. Ding Y, Wang H, Shen H, Li Z, Geng J, Han H, et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. (2003) 200:282–9. doi: 10.1002/path.1440
83. To KF, Lo AW. Exploring the pathogenesis of severe acute respiratory syndrome (SARS): the tissue distribution of the coronavirus (SARS-CoV) and its putative receptor, angiotensin-converting enzyme 2 (ACE2). J Pathol. (2004) 203:740–3. doi: 10.1002/path.1597
84. Yang YH, Huang YH, Chuang YH, Peng CM, Wang LC, Lin YT, et al. Autoantibodies against human epithelial cells and endothelial cells after severe acute respiratory syndrome (SARS)-associated coronavirus infection. J Med Virol. (2005) 77:1–7. doi: 10.1002/jmv.20407
85. Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. (2011) 146:980–91. doi: 10.1016/j.cell.2011.08.015
86. Wang S, Le TQ, Kurihara N, Chida J, Cisse Y, Yano M, et al. Influenza virus-cytokine-protease cycle in the pathogenesis of vascular hyperpermeability in severe influenza. J Infect Dis. (2010) 202:991–1001. doi: 10.1086/656044
87. Short KR, Veldhuis Kroeze EJ, Reperant LA, Richard M, Kuiken T. Influenza virus and endothelial cells: a species specific relationship. Front Microbiol. (2014) 5:653. doi: 10.3389/fmicb.2014.00653
88. Matsuda A, Morita H, Unno H, Saito H, Matsumoto K, Hirao Y, et al. Anti-inflammatory effects of high-dose IgG on TNF-alpha-activated human coronary artery endothelial cells. Eur J Immunol. (2012) 42:2121–31. doi: 10.1002/eji.201242398
89. Makata H, Ichiyama T, Uchi R, Takekawa T, Matsubara T, Furukawa S. Anti-inflammatory effect of intravenous immunoglobulin in comparison with dexamethasone in vitro: implication for treatment of Kawasaki disease. Naunyn Schmiedebergs Arch Pharmacol. (2006) 373:325–32. doi: 10.1007/s00210-006-0084-z
90. Radder CM, Beekhuizen H, Kanhai HH, Brand A. Effect of maternal anti-HPA-1a antibodies and polyclonal IVIG on the activation status of vascular endothelial cells. Clin Exp Immunol. (2004) 137:216–22. doi: 10.1111/j.1365-2249.2004.02496.x
91. Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. (2005) 434:772–7. doi: 10.1038/nature03464
92. Honda K, Ohba Y, Yanai H, Negishi H, Mizutani T, Takaoka A, et al. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. (2005) 434:1035–40. doi: 10.1038/nature03547
93. Blanco-Melo D. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. (2020) 181:1036–45.e9. doi: 10.1016/j.cell.2020.04.026
94. Frieman M, Yount B, Heise M, Kopecky-Bromberg SA, Palese P, Baric RS. Severe acute respiratory syndrome coronavirus ORF6 antagonizes STAT1 function by sequestering nuclear import factors on the rough endoplasmic reticulum/Golgi membrane. J Virol. (2007) 81:9812–24. doi: 10.1128/JVI.01012-07
95. Narayanan K, Huang C, Lokugamage K, Kamitani W, Ikegami T, Tseng CT, et al. Severe acute respiratory syndrome coronavirus nsp1 suppresses host gene expression, including that of type I interferon, in infected cells. J Virol. (2008) 82:4471–9. doi: 10.1128/JVI.02472-07
96. Sun L, Xing Y, Chen X, Zheng Y, Yang Y, Nichols DB, et al. Coronavirus papain-like proteases negatively regulate antiviral innate immune response through disruption of STING-mediated signaling. PLoS ONE. (2012) 7:e30802. doi: 10.1371/journal.pone.0030802
97. Kopecky-Bromberg SA, Martínez-Sobrido L, Frieman M, Baric RA, Palese P. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J Virol. (2007) 81:548–57. doi: 10.1128/JVI.01782-06
98. Menachery VD, Mitchell HD, Cockrell AS, Gralinski LE, Yount BL Jr, Graham RL, et al. MERS-CoV accessory ORFs play key role for infection and pathogenesis. mBio. (2017) 8:e00665–17. doi: 10.1128/mBio.00665-17
99. Scheuplein VA, Seifried J, Malczyk AH, Miller L, Hocker L, Vergara-Alert J, et al. High secretion of interferons by human plasmacytoid dendritic cells upon recognition of Middle East respiratory syndrome coronavirus. J Virol. (2015) 89:3859–69. doi: 10.1128/JVI.03607-14
100. Cervantes-Barragan L, Zust R, Weber F, Spiegel M, Lang KS, Akira S, et al. Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood. (2007) 109:1131–7. doi: 10.1182/blood-2006-05-023770
101. Channappanavar R, Fehr AR, Vijay R, Mack M, Zhao J, Meyerholz DK, et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. (2016) 19:181–93. doi: 10.1016/j.chom.2016.01.007
102. Qian J, Wang L, Yuan X, Wang L, Chen T. Dose-related regulatory effect of intravenous immunoglobulin on dendritic cells-mediated immune response. Immunopharmacol Immunotoxicol. (2014) 36:33–42. doi: 10.3109/08923973.2013.864668
103. Bayry J, Lacroix-Desmazes S, Carbonneil C, Misra N, Donkova V, Pashov A, et al. Inhibition of maturation and function of dendritic cells by intravenous immunoglobulin. Blood. (2003) 101:758–65. doi: 10.1182/blood-2002-05-1447
104. Tjon AS, van Gent R, Jaadar H, Martin van Hagen P, Mancham S, van der Laan LJ, et al. Intravenous immunoglobulin treatment in humans suppresses dendritic cell function via stimulation of IL-4 and IL-13 production. J Immunol. (2014) 192:5625–34. doi: 10.4049/jimmunol.1301260
105. Massoud AH, Yona M, Xue D, Chouiali F, Alturaihi H, Ablona A, et al. Dendritic cell immunoreceptor: a novel receptor for intravenous immunoglobulin mediates induction of regulatory T cells. J Allergy Clin Immunol. (2014) 133:853–63.e5. doi: 10.1016/j.jaci.2013.09.029
106. Othy S, Bruneval P, Topcu S, Dugail I, Delers F, Lacroix-Desmazes S, et al. Effect of IVIg on human dendritic cell-mediated antigen uptake and presentation: role of lipid accumulation. J Autoimmun. (2012) 39:168–72. doi: 10.1016/j.jaut.2012.05.013
107. Tha-In T, Metselaar HJ, Tilanus HW, Boor PP, Mancham S, Kuipers EJ, et al. Superior immunomodulatory effects of intravenous immunoglobulins on human T-cells and dendritic cells: comparison to calcineurin inhibitors. Transplantation. (2006) 81:1725–34. doi: 10.1097/01.tp.0000226073.20185.b1
108. Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. (2020) 27:992–1000.e3. doi: 10.1016/j.chom.2020.04.009
109. Chen Y, Feng Z, Diao B, Wang R, Wang G, Wang C, et al. The novel severe acute respiratory syndrome Coronavirus 2 (SARS-CoV-2) directly decimates human spleens and lymph nodes. medRxiv [Preprint]. (2020). doi: 10.1101/2020.03.27.20045427
110. Liu L, Wei Q, Lin Q, Fang J, Wang H, Kwok H, et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. (2019) 4:e123158. doi: 10.1172/jci.insight.123158
111. Cheung CY, Poon LL, Ng IH, Luk W, Sia SF, Wu MH, et al. Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J Virol. (2005) 79:7819–26. doi: 10.1128/JVI.79.12.7819-7826.2005
112. Wang W, Ye L, Ye L, Li B, Gao B, Zeng Y, et al. Up-regulation of IL-6 and TNF-alpha induced by SARS-coronavirus spike protein in murine macrophages via NF-kappaB pathway. Virus Res. (2007) 128:1–8. doi: 10.1016/j.virusres.2007.02.007
113. Dosch SF, Mahajan SD, Collins AR. SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-kappaB pathway in human monocyte macrophages in vitro. Virus Res. (2009) 142:19–27. doi: 10.1016/j.virusres.2009.01.005
114. Rhoades CJ, Williams MA, Kelsey SM, Newland AC. Monocyte-macrophage system as targets for immunomodulation by intravenous immunoglobulin. Blood Rev. (2000) 14:14–30. doi: 10.1054/blre.1999.0121
115. Liu AC, Yang Y, Li MT, Jia Y, Chen S, Ye S, et al. Macrophage activation syndrome in systemic lupus erythematosus: a multicenter, case-control study in China. Clin Rheumatol. (2018) 37:93–100. doi: 10.1007/s10067-017-3625-6
116. Yao HH, Wang YN, Zhang X, Zhao JX, Jia Y, Wang Z, et al. [Clinical characteristics and treatment outcomes of macrophage activation syndrome in adults: a case series of 67 patients]. Beijing Da Xue Xue Bao Yi Xue Ban. (2019) 51:996–1002.
117. Jin P, Luo Y, Liu X, Xu J, Liu C. Kawasaki disease complicated with macrophage activation syndrome: case reports and literature review. Front Pediatr. (2019) 7:423. doi: 10.3389/fped.2019.00423
118. Sen ES, Clarke SL, Ramanan AV. Macrophage activation syndrome. Indian J Pediatr. (2016) 83:248–53. doi: 10.1007/s12098-015-1877-1
119. Dominguez-Soto A, Simon-Fuentes M, de Las Casas-Engel M, Cuevas VD, Lopez-Bravo M, Dominguez-Andres J, et al. IVIg promote cross-tolerance against inflammatory stimuli in vitro and in vivo. J Immunol. (2018) 201:41–52. doi: 10.4049/jimmunol.1701093
120. Dominguez-Soto A, de las Casas-Engel M, Bragado R, Medina-Echeverz J, Aragoneses-Fenoll L, Martin-Gayo E, et al. Intravenous immunoglobulin promotes antitumor responses by modulating macrophage polarization. J Immunol. (2014) 193:5181–9. doi: 10.4049/jimmunol.1303375
121. Kozicky LK, Menzies SC, Zhao ZY, Vira T, Harnden K, Safari K, et al. IVIg and LPS co-stimulation induces IL-10 production by human monocytes, which is compromised by an FcgammaRIIA disease-associated gene variant. Front Immunol. (2018) 9:2676. doi: 10.3389/fimmu.2018.02676
122. Narasaraju T, Yang E, Samy RP, Ng HH, Poh WP, Liew AA, et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. (2011) 179:199–210. doi: 10.1016/j.ajpath.2011.03.013
123. Zhu L, Liu L, Zhang Y, Pu L, Liu J, Li X, et al. High level of neutrophil extracellular traps correlates with poor prognosis of severe influenza a infection. J Infect Dis. (2018) 217:428–37. doi: 10.1093/infdis/jix475
124. Barnes BJ, Adrover JM, Baxter-Stoltzfus A, Borczuk A, Cools-Lartigue J, Crawford JM, et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med. (2020) 217:e20200652. doi: 10.1084/jem.20200652
125. Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. (2020) 5:138999. doi: 10.1101/2020.04.30.20086736
126. Wang C-L, Wu Y-T, Lee C-J, Liu H-C, Huang L-T, Yang KD. Decreased nitric oxide production after intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr. (2002) 141:560–5. doi: 10.1067/mpd.2002.127505
127. Jang J-E, Hidalgo A, Frenette PS. Intravenous immunoglobulins modulate neutrophil activation and vascular injury through FcγRIII and SHP-1. Circul Res. (2012) 110:1057–66. doi: 10.1161/CIRCRESAHA.112.266411
128. Schneider C, Wicki S, Graeter S, Timcheva TM, Keller CW, Quast I, et al. IVIG regulates the survival of human but not mouse neutrophils. Sci Rep. (2017) 7:1296. doi: 10.1038/s41598-017-01404-0
129. Graeter S, Simon H-U, von Gunten S. Granulocyte death mediated by specific antibodies in intravenous immunoglobulin (IVIG). Pharmacol Res. (2019) 154:104168. doi: 10.1016/j.phrs.2019.02.007
130. Uozumi R, Iguchi R, Masuda S, Nishibata Y, Nakazawa D, Tomaru U, et al. Pharmaceutical immunoglobulins reduce neutrophil extracellular trap formation and ameliorate the development of MPO-ANCA-associated vasculitis. Mod Rheumatol. (2019) 30:1–7. doi: 10.1080/14397595.2019.1602292
131. Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol. (2020) 17:533–5. doi: 10.1038/s41423-020-0402-2
132. National Research Project for SARS BG. The involvement of natural killer cells in the pathogenesis of severe acute respiratory syndrome. Am J Clin Pathol. (2004) 121:507–11. doi: 10.1309/WPK7Y2XKNF4CBF3R
133. van Erp EA, van Kampen MR, van Kasteren PB, de Wit J. Viral infection of human natural killer cells. Viruses. (2019) 11:243. doi: 10.3390/v11030243
134. Mao H, Tu W, Qin G, Law HK, Sia SF, Chan PL, et al. Influenza virus directly infects human natural killer cells and induces cell apoptosis. J Virol. (2009) 83:9215–22. doi: 10.1128/JVI.00805-09
135. Bohn AB, Nederby L, Harbo T, Skovbo A, Vorup-Jensen T, Krog J, et al. The effect of IgG levels on the number of natural killer cells and their Fc receptors in chronic inflammatory demyelinating polyradiculoneuropathy. Eur J Neurol. (2011) 18:919–24. doi: 10.1111/j.1468-1331.2010.03333.x
136. Ebbo M, Audonnet S, Grados A, Benarous L, Mahevas M, Godeau B, et al. NK cell compartment in the peripheral blood and spleen in adult patients with primary immune thrombocytopenia. Clin Immunol. (2017) 177:18–28. doi: 10.1016/j.clim.2015.11.005
137. Ahmadi M, Ghaebi M, Abdolmohammadi-Vahid S, Abbaspour-Aghdam S, Hamdi K, Abdollahi-Fard S, et al. NK cell frequency and cytotoxicity in correlation to pregnancy outcome and response to IVIG therapy among women with recurrent pregnancy loss. J Cell Physiol. (2019) 234:9428–37. doi: 10.1002/jcp.27627
138. Papaserafeim M, Jandus P, Ferfoglia RI, Nieke JP, Vonarburg C, Spirig R, et al. Effect of intravenous immunoglobulin G therapy on natural killer cell function related to Fc gamma receptor gene expression. J Allergy Clin Immunol. (2020). doi: 10.1016/j.jaci.2020.04.001. [Epub ahead of print].
139. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. (2020) 8:420–2. doi: 10.1016/S2213-2600(20)30076-X
140. Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. (2020) 53:368–70. doi: 10.1016/j.jmii.2020.03.005
141. Wong CK, Lam CW, Wu AK, Ip WK, Lee NL, Chan IH, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. (2004) 136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x
142. Mahallawi WH Khabour OF Zhang Q Makhdoum HM Suliman BA. MERS-CoV infection in humans is associated with a pro-inflammatory Th1 and Th17 cytokine profile. Cytokine. (2018) 104:8–13. doi: 10.1016/j.cyto.2018.01.025
143. Sarda C, Palma P, Rello J. Severe influenza: overview in critically ill patients. Curr Opin Crit Care. (2019) 25:449–57. doi: 10.1097/MCC.0000000000000638
144. Bermejo-Martin JF, Ortiz de Lejarazu R, Pumarola T, Rello J, Almansa R, Ramirez P, et al. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit Care. (2009) 13:R201. doi: 10.1186/cc8208
145. Kwak-Kim J, Lee SK, Gilman-Sachs A. Elevated Th1/Th2 cell ratios in a pregnant woman with a history of RSA, secondary Sjogren's syndrome and rheumatoid arthritis complicated with one fetal demise of twin pregnancy. Am J Reprod Immunol. (2007) 58:325–9. doi: 10.1111/j.1600-0897.2007.00506.x
146. Wang Y, Wang W, Gong F, Fu S, Zhang Q, Hu J, et al. Evaluation of intravenous immunoglobulin resistance and coronary artery lesions in relation to Th1/Th2 cytokine profiles in patients with Kawasaki disease. Arthritis Rheum. (2013) 65:805–14. doi: 10.1002/art.37815
147. Graphou O, Chioti A, Pantazi A, Tsukoura C, Kontopoulou V, Guorgiadou E, et al. Effect of intravenous immunoglobulin treatment on the Th1/Th2 balance in women with recurrent spontaneous abortions. Am J Reprod Immunol. (2003) 49:21–9. doi: 10.1034/j.1600-0897.2003.01169.x
148. Mouzaki A, Theodoropoulou M, Gianakopoulos I, Vlaha V, Kyrtsonis MC, Maniatis A. Expression patterns of Th1 and Th2 cytokine genes in childhood idiopathic thrombocytopenic purpura (ITP) at presentation and their modulation by intravenous immunoglobulin G (IVIg) treatment: their role in prognosis. Blood. (2002) 100:1774–9. doi: 10.1182/blood.V100.5.1774.h81702001774_1774_1779
149. Ahmadi M, Abdolmohammadi-Vahid S, Ghaebi M, Aghebati-Maleki L, Afkham A, Danaii S, et al. Effect of Intravenous immunoglobulin on Th1 and Th2 lymphocytes and improvement of pregnancy outcome in recurrent pregnancy loss (RPL). Biomed Pharmacother. (2017) 92:1095–102. doi: 10.1016/j.biopha.2017.06.001
150. Jafarzadeh S, Ahmadi M, Dolati S, Aghebati-Maleki L, Eghbal-Fard S, Kamrani A, et al. Intravenous immunoglobulin G treatment increases live birth rate in women with recurrent miscarriage and modulates regulatory and exhausted regulatory T cells frequency and function. J Cell Biochem. (2019) 120:5424–34. doi: 10.1002/jcb.27821
151. Muyayalo KP, Li Z-H, Mor G, Liao A-H. Modulatory effect of intravenous immunoglobulin on Th17/Treg cell balance in women with unexplained recurrent spontaneous abortion. Am J Reprod Immunol. (2018) 80:e13018. doi: 10.1111/aji.13018
152. Ahmadi M, Aghdam SA, Nouri M, Babaloo Z, Farzadi L, Ghasemzadeh A, et al. Intravenous immunoglobulin (IVIG) treatment modulates peripheral blood Th17 and regulatory T cells in recurrent miscarriage patients: non randomized, open-label clinical trial. Immunol Lett. (2017) 192:12–9. doi: 10.1016/j.imlet.2017.10.003
153. Ahmadi M, Abdolmohammadi-Vahid S, Ghaebi M, Aghebati-Maleki L, Dolati S, Farzadi L, et al. Regulatory T cells improve pregnancy rate in RIF patients after additional IVIG treatment. Syst Biol Reprod Med. (2017) 63:350–9. doi: 10.1080/19396368.2017.1390007
154. Guo MM, Tseng WN, Ko CH, Pan HM, Hsieh KS, Kuo HC. Th17- and Treg-related cytokine and mRNA expression are associated with acute and resolving Kawasaki disease. Allergy. (2015) 70:310–8. doi: 10.1111/all.12558
155. Maddur MS, Rabin M, Hegde P, Bolgert F, Guy M, Vallat JM, et al. Intravenous immunoglobulin exerts reciprocal regulation of Th1/Th17 cells and regulatory T cells in Guillain-Barre syndrome patients. Immunol Res. (2014) 60:320–9. doi: 10.1007/s12026-014-8580-6
156. Tsurikisawa N, Saito H, Oshikata C, Tsuburai T, Akiyama K. High-dose intravenous immunoglobulin therapy for eosinophilic granulomatosis with polyangiitis. Clin Transl Allergy. (2014) 4:38. doi: 10.1186/2045-7022-4-38
157. Othy S, Topcu S, Saha C, Kothapalli P, Lacroix-Desmazes S, Kasermann F, et al. Sialylation may be dispensable for reciprocal modulation of helper T cells by intravenous immunoglobulin. Eur J Immunol. (2014) 44:2059–63. doi: 10.1002/eji.201444440
158. Othy S, Hegde P, Topçu S, Sharma M, Maddur MS, Lacroix-Desmazes S, et al. Intravenous gammaglobulin inhibits encephalitogenic potential of pathogenic T cells and interferes with their trafficking to the central nervous system, implicating sphingosine-1 phosphate receptor 1-mammalian target of rapamycin axis. J Immunol. (2013) 190:4535–41. doi: 10.4049/jimmunol.1201965
159. Saha C, Das M, Patil V, Stephen-Victor E, Sharma M, Wymann S, et al. Monomeric immunoglobulin a from plasma inhibits human th17 responses in vitro independent of FcalphaRI and DC-SIGN. Front Immunol. (2017) 8:275. doi: 10.3389/fimmu.2017.00275
160. Massoud AH, Guay J, Shalaby KH, Bjur E, Ablona A, Chan D, et al. Intravenous immunoglobulin attenuates airway inflammation through induction of forkhead box protein 3-positive regulatory T cells. J Allergy Clin Immunol. (2012) 129:1656–65.e3. doi: 10.1016/j.jaci.2012.02.050
161. Trinath J, Hegde P, Sharma M, Maddur MS, Rabin M, Vallat JM, et al. Intravenous immunoglobulin expands regulatory T cells via induction of cyclooxygenase-2-dependent prostaglandin E2 in human dendritic cells. Blood. (2013) 122:1419–27. doi: 10.1182/blood-2012-11-468264
162. Wiedeman AE, Santer DM, Yan W, Miescher S, Kasermann F, Elkon KB. Contrasting mechanisms of interferon-alpha inhibition by intravenous immunoglobulin after induction by immune complexes versus Toll-like receptor agonists. Arthritis Rheum. (2013) 65:2713–23. doi: 10.1002/art.38082
163. Cousens LP, Tassone R, Mazer BD, Ramachandiran V, Scott DW, De Groot AS. Tregitope update: mechanism of action parallels IVIg. Autoimmun Rev. (2013) 12:436–43. doi: 10.1016/j.autrev.2012.08.017
164. Ballow M. Mechanisms of immune regulation by IVIG. Curr Opin Allergy Clin Immunol. (2014) 14:509–15. doi: 10.1097/ACI.0000000000000116
165. De Groot AS, Cousens L, Mingozzi F, Martin W. Tregitope peptides: the active pharmaceutical ingredient of IVIG? Clin Dev Immunol. (2013) 2013:493138. doi: 10.1155/2013/493138
166. Guo XJ, Thomas PG. New fronts emerge in the influenza cytokine storm. Semin Immunopathol. (2017) 39:541–50. doi: 10.1007/s00281-017-0636-y
167. Walsh KB, Teijaro JR, Brock LG, Fremgen DM, Collins PL, Rosen H, et al. Animal model of respiratory syncytial virus: CD8+ T cells cause a cytokine storm that is chemically tractable by sphingosine-1-phosphate 1 receptor agonist therapy. J Virol. (2014) 88:6281–93. doi: 10.1128/JVI.00464-14
168. Ganji A, Farahani I, Khansarinejad B, Ghazavi A, Mosayebi G. Increased expression of CD8 marker on T-cells in COVID-19 patients. Blood Cells Mol Dis. (2020) 83:102437. doi: 10.1016/j.bcmd.2020.102437
169. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. (2020) 55:105954. doi: 10.1016/j.ijantimicag.2020.105954
170. Ye Q, Gong F-Q, Shang S-Q, Hu J. Intravenous immunoglobulin treatment responsiveness depends on the degree of CD8+ T cell activation in Kawasaki disease. Clin Immunol. (2016) 171:25–31. doi: 10.1016/j.clim.2016.08.012
171. Mausberg AK, Dorok M, Stettner M, Muller M, Hartung HP, Dehmel T, et al. Recovery of the T-cell repertoire in CIDP by IV immunoglobulins. Neurology. (2013) 80:296–303. doi: 10.1212/WNL.0b013e31827debad
172. Trepanier P, Bazin R. Intravenous immunoglobulin (IVIg) inhibits CD8 cytotoxic T-cell activation. Blood. (2012) 120:2769–70. doi: 10.1182/blood-2012-07-445007
173. Trépanier P, Chabot D, Bazin R. Intravenous immunoglobulin modulates the expansion and cytotoxicity of CD8+ T cells. Immunology. (2014) 141:233–41. doi: 10.1111/imm.12189
174. Tawfik DS, Cowan KR, Walsh AM, Hamilton WS, Goldman FD. Exogenous immunoglobulin downregulates T-cell receptor signaling and cytokine production. Pediatr Allergy Immunol. (2012) 23:88–95. doi: 10.1111/j.1399-3038.2010.01129.x
175. Kaveri S, Vassilev T, Hurez V, Lengagne R, Lefranc C, Cot S, et al. Antibodies to a conserved region of HLA class I molecules, capable of modulating CD8 T cell-mediated function, are present in pooled normal immunoglobulin for therapeutic use. J Clin Invest. (1996) 97:865–9. doi: 10.1172/JCI118488
176. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. (2014) 6:a016295. doi: 10.1101/cshperspect.a016295
177. Pistoia V, Corcione A. Relationships between B cell cytokine production in secondary lymphoid follicles and apoptosis of germinal center B lymphocytes. Stem Cells. (1995) 13:487–500. doi: 10.1002/stem.5530130506
178. Arkatkar T, Du SW, Jacobs HM, Dam EM, Hou B, Buckner JH, et al. B cell-derived IL-6 initiates spontaneous germinal center formation during systemic autoimmunity. J Exp Med. (2017) 214:3207–17. doi: 10.1084/jem.20170580
179. Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. (2020). doi: 10.1093/cid/ciaa248. [Epub ahead of print].
180. Wen W, Su W, Tang H, Le W, Zhang X, Zheng Y, et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. medRxiv [Preprint]. (2020). doi: 10.1101/2020.03.23.20039362
181. Quinti I, Lougaris V, Milito C, Cinetto F, Pecoraro A, Mezzaroma I, et al. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J Allergy Clin Immunol. (2020) 146: 211–3.e4. doi: 10.1016/j.jaci.2020.04.013
182. Soresina A, Moratto D, Chiarini M, Paolillo C, Baresi G, Foca E, et al. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr Allergy Immunol. (2020). doi: 10.1111/pai.13263. [Epub ahead of print].
183. Seite JF, Hillion S, Harbonnier T, Pers JO. Review: intravenous immunoglobulin and B cells: when the product regulates the producer. Arthritis Rheumatol. (2015) 67:595–603. doi: 10.1002/art.38910
184. Bayry J, Fournier EM, Maddur MS, Vani J, Wootla B, Siberil S, et al. Intravenous immunoglobulin induces proliferation and immunoglobulin synthesis from B cells of patients with common variable immunodeficiency: a mechanism underlying the beneficial effect of IVIg in primary immunodeficiencies. J Autoimmun. (2011) 36:9–15. doi: 10.1016/j.jaut.2010.09.006
185. Nikolova KA, Tchorbanov AI, Djoumerska-Alexieva IK, Nikolova M, Vassilev TL. Intravenous immunoglobulin up-regulates the expression of the inhibitory FcgammaIIB receptor on B cells. Immunol Cell Biol. (2009) 87:529–33. doi: 10.1038/icb.2009.36
186. Jin J, Gong J, Lin B, Li Y, He Q. FcgammaRIIb expression on B cells is associated with treatment efficacy for acute rejection after kidney transplantation. Mol Immunol. (2017) 85:283–92. doi: 10.1016/j.molimm.2017.03.006
187. Crow AR, Song S, Freedman J, Helgason CD, Humphries RK, Siminovitch KA, et al. IVIg-mediated amelioration of murine ITP via FcgammaRIIB is independent of SHIP1, SHP-1, and Btk activity. Blood. (2003) 102:558–60. doi: 10.1182/blood-2003-01-0023
188. Zhuang Q, Bisotto S, Fixman ED, Mazer B. Suppression of IL-4- and CD40-induced B-lymphocyte activation by intravenous immunoglobulin is not mediated through the inhibitory IgG receptor FcgammaRIIb. J Allergy Clin Immunol. (2002) 110:480–3. doi: 10.1067/mai.2002.127284
189. Sondermann P, Pincetic A, Maamary J, Lammens K, Ravetch JV. General mechanism for modulating immunoglobulin effector function. Proc Natl Acad Sci USA. (2013) 110:9868–72. doi: 10.1073/pnas.1307864110
190. Crispin M, Yu X, Bowden TA. Crystal structure of sialylated IgG Fc: implications for the mechanism of intravenous immunoglobulin therapy. Proc Natl Acad Sci USA. (2013) 110:E3544–6. doi: 10.1073/pnas.1310657110
191. Seite JF, Cornec D, Renaudineau Y, Youinou P, Mageed RA, Hillion S. IVIg modulates BCR signaling through CD22 and promotes apoptosis in mature human B lymphocytes. Blood. (2010) 116:1698–704. doi: 10.1182/blood-2009-12-261461
192. Blank M, Shoenfeld Y. Sialic acid-IVIg targeting CD22. Blood. (2010) 116:1630–2. doi: 10.1182/blood-2010-06-289892
193. Kessel A, Peri R, Haj T, Snir A, Slobodin G, Sabo E, et al. IVIg attenuates TLR-9 activation in B cells from SLE patients. J Clin Immunol. (2011) 31:30–8. doi: 10.1007/s10875-010-9469-3
194. Séité JF Guerrier T Cornec D Jamin C Youinou P Hillion S. TLR9 responses of B cells are repressed by intravenous immunoglobulin through the recruitment of phosphatase. J Autoimmun. (2011) 37:190–7. doi: 10.1016/j.jaut.2011.05.014
195. Mori I, Parizot C, Dorgham K, Demeret S, Amoura Z, Bolgert F, et al. Prominent plasmacytosis following intravenous immunoglobulin correlates with clinical improvement in Guillain-Barré syndrome. PloS ONE. (2008) 3:e2109. doi: 10.1371/journal.pone.0002109
196. Brem MD, Jacobs BC, van Rijs W, Fokkink WJR, Tio-Gillen AP, Walgaard C, et al. IVIg-induced plasmablasts in patients with Guillain-Barre syndrome. Ann Clin Transl Neurol. (2019) 6:129–43. doi: 10.1002/acn3.687
197. Khandia R, Munjal A, Dhama K, Karthik K, Tiwari R, Malik YS, et al. Modulation of dengue/zika virus pathogenicity by antibody-dependent enhancement and strategies to protect against enhancement in zika virus infection. Front Immunol. (2018) 9:597. doi: 10.3389/fimmu.2018.00597
198. Rulli NE, Suhrbier A, Hueston L, Heise MT, Tupanceska D, Zaid A, et al. Ross River virus: molecular and cellular aspects of disease pathogenesis. Pharmacol Ther. (2005) 107:329–42. doi: 10.1016/j.pharmthera.2005.03.006
199. Taylor A, Foo SS, Bruzzone R, Dinh LV, King NJ, Mahalingam S. Fc receptors in antibody-dependent enhancement of viral infections. Immunol Rev. (2015) 268:340–64. doi: 10.1111/imr.12367
200. Zhang L, Zhang F, Yu W, He T, Yu J, Yi CE, et al. Antibody responses against SARS coronavirus are correlated with disease outcome of infected individuals. J Med Virol. (2006) 78:1–8. doi: 10.1002/jmv.20499
201. Leung H-l. Mechanism of antibody-dependent enhancement in severe acute respiratory syndrome coronavirus infection (dissertation). The University of Hong Kong, Hong Kong.
202. Yip MS, Leung NH, Cheung CY, Li PH, Lee HH, Daëron M, et al. Antibody-dependent infection of human macrophages by severe acute respiratory syndrome coronavirus. Virol J. (2014) 11:82. doi: 10.1186/1743-422X-11-82
203. Wan Y, Shang J, Sun S, Tai W, Chen J, Geng Q, et al. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J Virol. (2020) 94:e02015-19. doi: 10.1128/JVI.02015-19
204. Winarski KL, Tang J, Klenow L, Lee J, Coyle EM, Manischewitz J, et al. Antibody-dependent enhancement of influenza disease promoted by increase in hemagglutinin stem flexibility and virus fusion kinetics. Proc Natl Acad Sci USA. (2019) 116:15194–9. doi: 10.1073/pnas.1821317116
205. Khurana S, Loving CL, Manischewitz J, King LR, Gauger PC, Henningson J, et al. Vaccine-induced anti-HA2 antibodies promote virus fusion and enhance influenza virus respiratory disease. Sci Transl Med. (2013) 5:200ra114. doi: 10.1126/scitranslmed.3006366
206. van Erp EA, Luytjes W, Ferwerda G, van Kasteren PB. Fc-Mediated antibody effector functions during respiratory syncytial virus infection and disease. Front Immunol. (2019) 10:548. doi: 10.3389/fimmu.2019.00548
207. van Erp EA, Feyaerts D, Duijst M, Mulder HL, Wicht O, Luytjes W, et al. Respiratory syncytial virus infects primary neonatal and adult natural killer cells and affects their antiviral effector function. J Infect Dis. (2019) 219:723–33. doi: 10.1093/infdis/jiy566
208. van Erp EA, van Kasteren PB, Guichelaar T, Ahout IML, de Haan CAM, Luytjes W, et al. In vitro enhancement of respiratory syncytial virus infection by maternal antibodies does not explain disease severity in infants. J Virol. (2017) 91:e00851-17. doi: 10.1128/JVI.00851-17
209. Iwasaki A, Yang Y. The potential danger of suboptimal antibody responses in COVID-19. Nat Rev Immunol. (2020) 20:339–41. doi: 10.1038/s41577-020-0321-6
210. Smatti MK, Al Thani AA, Yassine HM. Viral-induced enhanced disease illness. Front Microbiol. (2018) 9:2991. doi: 10.3389/fmicb.2018.02991
211. Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. (2020). doi: 10.1007/s12250-020-00207-4. [Epub ahead of print].
212. de Alwis R, Chen S, Gan ES, Ooi EE. Impact of immune enhancement on Covid-19 polyclonal hyperimmune globulin therapy and vaccine development. EBioMedicine. (2020) 55:102768. doi: 10.1016/j.ebiom.2020.102768
213. Ramisse F, Deramoudt FX, Szatanik M, Bianchi A, Binder P, Hannoun C, et al. Effective prophylaxis of influenza A virus pneumonia in mice by topical passive immunotherapy with polyvalent human immunoglobulins or F(ab')2 fragments. Clin Exp Immunol. (1998) 111:583–7. doi: 10.1046/j.1365-2249.1998.00538.x
214. Rockman S, Lowther S, Camuglia S, Vandenberg K, Taylor S, Fabri L, et al. Intravenous immunoglobulin protects against severe pandemic influenza infection. EBioMedicine. (2017) 19:119–27. doi: 10.1016/j.ebiom.2017.04.010
215. Hemming VG, Prince GA, London WT, Baron PA, Brown R, Chanock RM. Topically administered immunoglobulin reduces pulmonary respiratory syncytial virus shedding in owl monkeys. Antimicrob Agents Chemother. (1988) 32:1269–70. doi: 10.1128/AAC.32.8.1269
216. Hemming VG, Prince GA, Horswood RL, London WJ, Murphy BR, Walsh EE, et al. Studies of passive immunotherapy for infections of respiratory syncytial virus in the respiratory tract of a primate model. J Infect Dis. (1985) 152:1083–7. doi: 10.1093/infdis/152.5.1083
217. Hemming VG, Prince GA. Immunoprophylaxis of infections with respiratory syncytial virus: observations and hypothesis. Rev Infect Dis. (1990) 12(Suppl. 4):S470–4. doi: 10.1093/clinids/12.Supplement_4.S470
218. Prince GA, Hemming VG, Horswood RL, Baron PA, Chanock RM. Effectiveness of topically administered neutralizing antibodies in experimental immunotherapy of respiratory syncytial virus infection in cotton rats. J Virol. (1987) 61:1851–4. doi: 10.1128/JVI.61.6.1851-1854.1987
219. Xie Y, Cao S, Li Q, Chen E, Dong H, Zhang W, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. (2020). doi: 10.1016/j.jinf.2020.03.044. [Epub ahead of print].
220. Liu Y, Sun W, Li J, Chen L, Wang Y, Zhang L, et al. Clinical features and progression of acute respiratory distress syndrome in coronavirus disease 2019. medRxiv [Preprint]. (2020). doi: 10.1101/2020.02.17.20024166
221. Zhou Z-G, Xie S-M, Zhang J, Zheng F, Jiang D-X, Li K-Y, et al. Short-term moderate-dose corticosteroid plus immunoglobulin effectively reverses COVID-19 patients who have failed low-dose therapy. Preprints. (2020). doi: 10.20944/preprints202003.0065.v1
222. Wu L, Xiao Z, Li Y. [Intravenous Immunoglobulin as adjunction treatment in treating 23 severe infectious atypical pneumonia patients]. Jo Jinan Univ. (2005) 26:826–8.
223. Wang J-T, Sheng W-H, Fang C-T, Chen Y-C, Wang J-L, Yu C-J, et al. Clinical manifestations, laboratory findings, and treatment outcomes of SARS patients. Emerg Infect Dis. (2004) 10:818–24. doi: 10.3201/eid1005.030640
224. Zeng H, Liu L, Wang B, Lu H. [Clinical analysis of intravenous immunoglobulin in children with severe acute respiratory syndrome]. Lin Chuang Er Ke Za Zhi. (2003) 21.
225. Ho JC, Wu AY, Lam B, Ooi GC, Khong PL, Ho PL, et al. Pentaglobin in steroid-resistant severe acute respiratory syndrome. Int J Tuberc Lung Dis. (2004) 8:1173–9.
226. Kapoor M, Pringle K, Kumar A, Dearth S, Liu L, Lovchik J, et al. Clinical and laboratory findings of the first imported case of Middle East respiratory syndrome coronavirus to the United States. Clin Infect Dis. (2014) 59:1511–8. doi: 10.1093/cid/ciu635
227. Hung IFN, To KKW, Lee CK, Lee KL, Yan WW, Chan K, et al. Hyperimmune IV immunoglobulin treatment: a multicenter double-blind randomized controlled trial for patients with severe 2009 influenza A(H1N1) infection. Chest. (2013) 144:464–73. doi: 10.1378/chest.12-2907
228. Lifeng H. [High-dose intravenous immunoglobulin for children with severe H1N1 influenza infection]. Int Med Health Guidance News. (2010) 1729–32.
229. CHEN Zhi-feng LVB, MO Wei-xiong. [Clinical observation on intravenous therapy with human immunoglobulin in severe H1N1 influenza in children]. Int Med Health Guidance News. (2013) 2171–3.
230. Lehners N, Schnitzler P, Geis S, Puthenparambil J, Benz MA, Alber B, et al. Risk factors and containment of respiratory syncytial virus outbreak in a hematology and transplant unit. Bone Marrow Transplant. (2013) 48:1548–53. doi: 10.1038/bmt.2013.94
231. Balassa K, Salisbury R, Watson E, Lubowiecki M, Tseu B, Maouche N, et al. Treatment stratification of respiratory syncytial virus infection in allogeneic stem cell transplantation. J Infect. (2019) 78:461–7. doi: 10.1016/j.jinf.2019.04.004
232. Ghosh S, Champlin RE, Englund J, Giralt SA, Rolston K, Raad I, et al. Respiratory syncytial virus upper respiratory tract illnesses in adult blood and marrow transplant recipients: combination therapy with aerosolized ribavirin and intravenous immunoglobulin. Bone Marrow Transplant. (2000) 25:751–5. doi: 10.1038/sj.bmt.1702228
233. Lai ST. Treatment of severe acute respiratory syndrome. Eur J Clin Microbiol Infect Dis. (2005) 24:583–91. doi: 10.1007/s10096-005-0004-z
234. Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. (2006) 3:e343. doi: 10.1371/journal.pmed.0030343
235. Mándi Y, Farkas G, Ocsovszky I. Effects of pentoxifyllin and pentaglobinO on TNF and IL-6 production in septic patients. Acta Microbiol Immunol Hung. (1995) 42:301–8.
236. Arabi YM, Arifi AA, Balkhy HH, Najm H, Aldawood AS, Ghabashi A, et al. Clinical course and outcomes of critically ill patients with Middle East respiratory syndrome coronavirus infection. Ann Intern Med. (2014) 160:389–97. doi: 10.7326/M13-2486
237. Liu Q, Zhou Y-h, Yang Z-q. The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell Mol Immunol. (2016) 13:3–10. doi: 10.1038/cmi.2015.74
238. Jorquera PA, Tripp RA. Respiratory syncytial virus: prospects for new and emerging therapeutics. Expert Rev Respir Med. (2017) 11:609–15. doi: 10.1080/17476348.2017.1338567
239. Shah JN, Chemaly RF. Management of RSV infections in adult recipients of hematopoietic stem cell transplantation. Blood. (2011) 117:2755–63. doi: 10.1182/blood-2010-08-263400
240. Shah DP, Ghantoji SS, Shah JN, El Taoum KK, Jiang Y, Popat U, et al. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J Antimicrob Chemother. (2013) 68:1872–80. doi: 10.1093/jac/dkt111
241. Hirsch HH, Martino R, Ward KN, Boeckh M, Einsele H, Ljungman P. Fourth European Conference on Infections in Leukaemia (ECIL-4): guidelines for diagnosis and treatment of human respiratory syncytial virus, parainfluenza virus, metapneumovirus, rhinovirus, and coronavirus. Clin Infect Dis. (2013) 56:258–66. doi: 10.1093/cid/cis844
242. Gorcea CM, Tholouli E, Turner A, Saif M, Davies E, Battersby E, et al. Effective use of oral ribavirin for respiratory syncytial viral infections in allogeneic haematopoietic stem cell transplant recipients. J Hosp Infect. (2017) 95:214–7. doi: 10.1016/j.jhin.2016.11.012
243. von Lilienfeld-Toal M, Berger A, Christopeit M, Hentrich M, Heussel CP, Kalkreuth J, et al. Community acquired respiratory virus infections in cancer patients-Guideline on diagnosis and management by the Infectious Diseases Working Party of the German Society for haematology and Medical Oncology. Eur J Cancer. (2016) 67:200–12. doi: 10.1016/j.ejca.2016.08.015
244. Guo Y, Tian X, Wang X, Xiao Z. Adverse effects of immunoglobulin therapy. Front Immunol. (2018) 9:1299. doi: 10.3389/fimmu.2018.01299
Keywords: intravenous immunoglobulin, SARS-CoV-2, COVID-19, viral pneumonia, mechanism of action, therapeutic inference
Citation: Liu X, Cao W and Li T (2020) High-Dose Intravenous Immunoglobulins in the Treatment of Severe Acute Viral Pneumonia: The Known Mechanisms and Clinical Effects. Front. Immunol. 11:1660. doi: 10.3389/fimmu.2020.01660
Received: 24 February 2020; Accepted: 22 June 2020;
Published: 14 July 2020.
Edited by:
Christoph T. Berger, University of Basel, SwitzerlandReviewed by:
Hillary Anne Vanderven, James Cook University, AustraliaCécile Aubron, Université de Bretagne Occidentale, France
Copyright © 2020 Liu, Cao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Taisheng Li, litsh@263.net
 Xiaosheng Liu
Xiaosheng Liu Wei Cao
Wei Cao Taisheng Li1,2*
Taisheng Li1,2*