- 1Preclinical Department, Faculty of Medicine, Royal College of Medicine Perak, Universiti Kuala Lumpur, Ipoh, Malaysia
- 2Infectious Diseases Laboratory, YR Gaitonde Centre for AIDS Research and Education (YRG CARE), Chennai, India
- 3Laboratory Division, Indian Council of Medical Research-National Institute of Epidemiology (ICMR-NIE), Indian Council of Medical Research, Chennai, India
- 4Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Introduction
The coronavirus disease-19 (COVID-19) pandemic caused by the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) continues to be a global threat (1). Studies have shown that COVID-19 patients also presenting with gastrointestinal symptoms and SARS-CoV-2 RNA has been detected in stool specimens from patients with severe disease (2–4). One of the pressing scientific questions that remain unanswered is, why the elderly population and those with underlying conditions are at more risk of developing severe COVID-19 complications than the younger population. Gastrointestinal manifestations play a major role in exacerbating proinflammatory cytokines due to disturbance of gut lining by SARS-CoV-2. Here, we discuss the possible role of the gut microbiota and the dysbiosis leading to exacerbated COVID-19 severity and cytokine storm.
Gut Immunology and Microbiota
The human body is inhabited by a cornucopia of microorganisms, with a rough estimate of about 38 trillion bacteria and gut remains the most densely and diversely colonized organ (5). The gut microbiota play an arterial role in maintaining immune homeostasis. The mucosal immune system, mainly the mucosa-associated lymphoid tissue such as gut-associated lymphoid tissue (GALT) and bronchial-associated lymphoid tissue, is very important since it acts as the primary line of defense against infections (6). GALT includes Peyer’s patches, appendix and isolated lymphoid follicles of the intestinal mucosa. Crosstalk between immune cells of the GALT and gut microbiota is essential to modulate the immune system. The role of gut microbial products in maintaining the balance between regulatory T cell and effector T cell response has been extensively reviewed (7). Furthermore, Short Chain Fatty Acid (SCFA), a product of commensals’ fermentation of fibre-rich diet, is also essential to facilitate the efficient migration of activated T cells to the intestinal lumen by stimulating the CD103+ Dendritic Cells (DCs) (8). Interestingly, the immune factors and cells from the GALT can be transferred to the bronchial-associated lymphoid tissue through various mechanisms thereby offering protection against respiratory infections (9, 10).
When compared to gut microbiota, the understanding of the microbial community of the lung is relatively less. Evidences from several studies insinuate a vital cross-talk between the intestinal microbiota and the lungs, known as the “gut–lung axis”. A plethora of evidence has supported the connection between gut microbiota and lung immunity. Depletion of gut microbiota has also been linked with the functional impairment of the alveolar macrophages with a reduction in reactive oxygen species-mediated bacterial killing capacity (11). Furthermore, the association between antibiotic-induced disturbance of the gut microbiota and improved survival of Mycobacterium tuberculosis in the lungs with a high chance of disseminated tuberculosis has also been reported (12). The gut-lung axis is reportedly bidirectional, that microbial components like endotoxins and metabolites from the gut can affect the lung through the bloodstream and in case of lung inflammation, the gut microbiota could be impacted as well (13). In one study, after exposure to the influenza virus, lungs-derived CCR9+CD4+ T cells migrate to the intestine where they cause gut microbiotadysbiosis, resulting in aberrant Th17 response with intestinal injury and gasteroenteritis (14).
Gut Microbial Metabolites in Modulation of Immune Responses
Microbial metabolites of gut microbial flora, importantly the short chain fatty acids (SCFAs) such as butyric acid and acetic acid are pivotal in modulating the immune and inflammatory responses (15, 16). These SCFAs can discourage the growth of pathogenic microbes by maintaining acidic pH and mucin production in the intestinal environment (17, 18). They are crucial in maintaining the integrity of gut epithelium to contain leakage and translocation. The SCFAs can also act as inhibitors of histone deacetylase (HDAC) and thereby efficiently hampering excessive inflammatory responses by enhancing the numbers and functions of T helper cells, regulatory T cells and Th17 effector cells (19–22). Also, SCFAs like butyrate demonstrate diverse anti-inflammatory functions by activating G protein-coupled receptors (GPCRs), such as GPR43 and by inhibition of the NF-kB pathway (22–24). Through activation of GPR41, SCFAs have been reported to augment CD8+ T cell functions and butyrate can promote differentiation of regulatory T cells and IL-10/18 producing T cells by activating GPR109A (25, 26).
Interestingly, these SCFAs have been found in minute quantities in the lung compartment as well, thereby indicating a possible link between the gut and the respiratory tract (27). Studies have shown that SCFAs aid formation of progenitors of macrophages and dendritic cells (DCs) in the bone marrow and also via augmenting the function of T cells, they offer defense against airway inflammation and respiratory tract infections (26, 28). In the pathogenesis of chronic obstructive pulmonary disease (COPD), the hypothesis of gut-liver-lung axis has also supported the fascinating role of SCFA. Apart from the SCFAs, other metabolites of the gut flora such as retinoic acid, niacin, lactate, tryptophan, pyruvate and desaminotyrosine have also been reported to have a role in host immunity (25, 29–33).
Gut Dysbiosis and Enhanced Gut Permeability
When there is a change in the composition of gut microbiota, owing to various factors, the normal flora are replaced by pathogenic ones and this phenomenon known as gut dysbiosis, is associated with many diseases (34, 35). Studies have shown associations between change in composition of gut microbiota and respiratory infections (36). Several studies have demonstrated the key role of gut microbiota in the pathogenesis of sepsis and ARDS (37).
While the intestinal barrier prevents the translocation of microbes and their harmful products from the gut lumen to systemic circulation, gut dysbiosis could lead to increased permeability of gut barrier (leaky gut). Gut dysbiosis has been observed to correlate with a decrease in the production of the gut bacteria-derived SCFAs such as butyrate thereby leading to increased gut permeability. This facilitates the translocation of microbiota-derived lipopolysaccharides (LPS), particularly from gram-negative bacteria and inflammatory components to general circulation leading to immune activation and inflammatory responses (38). This immune activation primarily happens via the toll-like receptor 4 (TLR4) and TLR4 activation in immune cells are known to aggravate the inflammatory processes associated with exacerbation of several clinical conditions. Activation of TLR4 by LPS has been shown to worsen the mortality rates in cases of influenza infections (39).
Possible Role of Gut Dysbiosis in Pathophysiology of COVID-19 “Cytokine Storm”
The composition and diversity of gut microbiota are affected by various factors, especially ageing. Age-related imbalance of gut microbiota has been well documented and there are reports on the reduced proportion of probiotic strains like Bifidobacteria, Lactobacillus and bacteria producing SCFAs like butyrate needed for maintaining the integrity of intestinal barrier (40–42). Likewise, there are several evidences supporting the role of gut dysbiosis in ageing-related cardiovascular, renal and metabolic disorders (43, 44).
In case of COVID-19 infection, the disease severity and mortality rates are very high among elderly patients over the age of 65 years, particularly those with pre-existing comorbid conditions such as diabetes, cardiovascular, metabolic and renal disorders (4, 45–49). Immunological aging is reported to be associated with subclinical inflammatory state known as “inflammaging” wherein the Th1 immune responses play a key role, whereas in children there are more Th2 responses, thereby producing less pro-inflammatory molecules. Moreover, alterations in the gut microbiota have been well documented to have an association with respiratory infections (36), inflammatory bowel disease (50), depression (51), type-2 diabetes (52), cardiovascular disease (53) and hypertension (54).
Thus, the high mortality rates among the elderly people and people with underlying medical conditions with COVID-19 possibly point towards the hypothesis that gut microbiota perturbations could influence COVID-19 disease severity and clinical outcome (55, 56). Several studies suggest that mortality associated with COVID-19 are mainly due to the enhanced cytokine and chemokine production contributing to the virally induced hyper-inflammation, referred to as the “cytokine storm” (57–59).
Based on the findings discussed earlier, during conditions like COVID-19, healthy gut microbiota is a requisite to balancing of optimal immune responses preventing an array of excessive inflammatory reactions that could be detrimental. This balance is very crucial that the immune response can have different clinical outcomes and consequences when it is either under reactive or over-reactive.
Bacterial LPS, the Microbe-Associated Molecular Patterns (MAMP) of Gram-negative bacteria can strongly activate the cells of the inflammatory system and the levels of LPS in plasma have been shown to correlate with the degree of intestinal permeability in various conditions. Several studies have demonstrated the association of LPS with T cell activation and elevated pro-inflammatory responses leading to a “cytokine storm” (60).
The chemokine CXCL10 has been observed to play a key role in recruiting of inflammatory cells to the site of inflammation and its role in COVID-19 induced cytokine storm has been shown in both experimental model and patients. A mice-model study using K18 hACE2 transgenic mice infected with SARS-CoV-2revealed significantly pronounced levels of CXCL-10 among those with cytokine storm (61). Studies have demonstrated elevated levels of CXCL10 in COVID-19 patients than healthy controls. Among the COVID-19 patients, the CXCL 10 levels were higher among those required admission to intensive care than those who had less severity (57). This finding supports the possible role of LPS in the severity of COVID-19. Studies also reported increased Levels of IL-1B, IFN-γ, CXCL-10 and CCL2 were also demonstrated as a result Th1 responses (62). Aberrant expressions of a battery of proinflammatory cytokines and chemokines such as IL-6, IFN-α, IFN-γ, IL-1β, IL-12, IL-7, IL-8, IL-9, IL-10, FGF, G-CSF, GM-CSF, IP-10, MCP-1, MIP-1A, MIP1-B, PDGF, IL-18, IL-33, TGF-β, VGEF, CXCL8, CXCL9, CCL2, CCL3, and CCL5 among infected and severe cases of infected patients were also documented (63, 64).
High LPS levels were observed in severe and fatal lung injury cases (65) which signifies that there is indeed a potential implication of LPS in the pathogenesis of the COVID-19 cytokine storm and COVID-19 related microvascular complications which must be investigated. Gut microbiotadysbiosis in some COVID-19 cases may facilitate the translocation of LPS into the portal circulation, which will further stimulate the Kupffer cells residing in the periportal region of the liver, resulting in activation of NF-κB pathway and secretion of TNF-α and IFN-β (66). This effect can cause the hepatic inflammation as well as systemic inflammation especially when LPS reaches the systemic circulation (67, 68). However, in the case of subclinical endotoxemia, that low dose LPS will not be sufficient enough to cause hepatitis but it may cause systemic low-grade inflammation, which can potentiate the effect of cytokine storm and microvascular complications identified in COVID-19 cases. Moreover, proinflammatory effect (IL-8, MCP-1) of low dose LPS on endothelial cells, high sensitivity of vascular smooth muscle cells to the stimulatory action of LPS, the association of endotoxemia with atherosclerosis, and LPS induced insulin resistance effect are considerable factors which could serve as fertile soil for initiating COVID-19 cytokine storm and microvascular injury in COVID-19 cases (69–71). The trigger factor of the cytokine storm may be due to LPS induced CXCL10 expression as discussed above or it may be because of the direct viral effect on the immune system, but the other concept is that low dose LPS can circulate in the plasma in COVID-19 cases with gut dysbiosis and that subclinical endotoxemia can act as a cofactor in facilitating the severe impact of the COVID-19 cytokine storm.
A recent study has reported a significant increase in the permeability of gut epithelial tight junctions in case of severe COVID-19, thereby suggesting a leaky gut situation. The study also noted a steep increase in the level of zonulin, a protein that acts as the physiological mediator of tight junction permeability in the digestive tract. Interestingly, the elevated levels of zonulin were observed to be a marker for increased mortality in severe COVID-19 cases. Measurement of LPS-binding protein, a marker of inflammation also revealed a significant increase among severe COVID-19 cases than milder cases. These findings support the association between severe COVID-19 and gut permeability and microbial translocation (72).
Impact of SARS-CoV-2 Infection on Gut Microbiota
It is interesting to note that the gut-lung axis crosstalk could imply the impact of SARS-CoV-2infection on the quality and composition of gut microbiota as well. Studies have demonstrated alterations in the abundance and composition of fecal bacteria in COVID-19 patients compared to healthy controls. The pattern of the gut microbiota composition was found to be positively correlating with increased expression of IL-18, the proinflammatory cytokine (73).
COVID-19 patients have been reported to have lesser beneficial gut microbiota and harbor more opportunistic pathogens. Interestingly, the severity of COVID-19 was found to correlate positively with the abundant presence of opportunistic pathogens and negatively with an abundance of anti-inflammatory bacterium Facealibacterium prausnitizii (66).
A study has documented the presence of a few bacteria like Streptococcus and Bacteroides to correlate negatively with inflammatory cytokines and positive association with a few other groups of gut microbiota thereby hinting at the potential role of gut microbiota in the predisposition of COVID-19 patients to disease severity. Likewise, in patients with COVID-19, perturbation of enteric RNA and DNA viral flora has been reported and the disease severity was found to be associated with the alternations in gut virome (74).
Interestingly, a recent study that analyzed the levels of 50 gut-associated plasma metabolites using systems biology approach, revealed that most of these metabolites were found to be dysregulated during severe COVID-19 when compared to controls and those with mild disease. The study reports significantly decreased levels of citrulline, an amino acid that is an established marker of gut and enterocyte function. Also, the levels of succinic acid, a well-known marker of gut microbial dysbiosis were observed to be increasing during severe COVID-19 (72).
While discussing on the predisposition of only a certain proportion of COVID-19 patients to develop severe disease, it is of significance to address the gut microbiota–mitochondria crosstalk as well (75). Studies have revealed the role of gut microbiota in influencing various mitochondrial functions including inflammatory cascades mediated by metabolites like SCFA and bile acids. Likewise, mitochondrial functions could alter the gut microflora composition and activity by immunomodulation leading to inflammatory responses during viral infections (76, 77).
Various studies have highlighted the role of administering probiotics and metabolites to retain optimal immune responses and prevent excessive inflammatory responses (31, 78, 79). Studies have demonstrated reduced lung damage caused by viral infection, due to enhanced levels of SCFAs in the blood caused by the change of proportion of Bacteroidetes and Firmicutes attained by food with high-fiber content (23, 26, 80).
A meta-analysis of several randomized clinical trials revealed that people taking probiotics had a 2-fold lower risk of developing upper respiratory tract infections. The study also reported a significant reduction in disease severity among the infected population (81). A study involving 479 adults demonstrated that administration of probiotic bacteria with vitamins and minerals minimized the duration of episodes of common cold and also lowered the days with fever (82). Another study involving 1,783 school children showed a reduction in the incidence of respiratory infection caused by influenza virus following consumption of Lactobacillus sp. (83). Probiotic bacteria have also been shown to enhance the responses of vaccines against respiratory viral infections and recent studies have pointed in this direction suggesting that maintaining the balance of intestinal microbiota may be beneficial to COVID-19 patients and aid in recovery due to improved immune status (10, 84, 85).
Interestingly, another logical reason for altered gut microbiota could be the extensive antibiotics usage in the management of COVID-19 (86). Antibiotics result in dysbiosis and increase susceptibility to new infections and inflammatory disorders.
Thus, understanding the potential mechanisms by which the gut microbiota regulate host immune and inflammatory responses might offer insights on understanding of the pathogenesis of COVID-19 induced cytokine storm and might throw light on interventions by targeting these microbial florae. A recent Chinese study demonstrates construction of a blood proteomic risk score (PRS) for the prediction of COVID-19 progression to a more severe stage. The study findings revealed a strong association between the gut bacteria, the PRS, and the severity of COVID-19 only in older age groups. Additionally, when analyzing a subgroup of about 301 uninfected individuals over a three-year duration, they observed that the changes in gut microbiota occurred before the alteration could reflect in the PRS, indicating that the gut dysbiosis leads to the protein alterations and not the other way around (56). A study has reported dysbiosis of gut microbiota among hospitalized COVID-19 patients wherein there were reduced levels of probiotic bacteria, a lower proportion of beneficial symbionts and a relatively higher proportion of opportunistic pathogens (66, 87). Interestingly, these variations in the composition of gut microbiota were observed to be correlating with the disease severity. Figure 1 represents the schematic representation of the proposed hypothesis of gut microbiota perturbation leading to severe COVID-19 by cytokine storm.
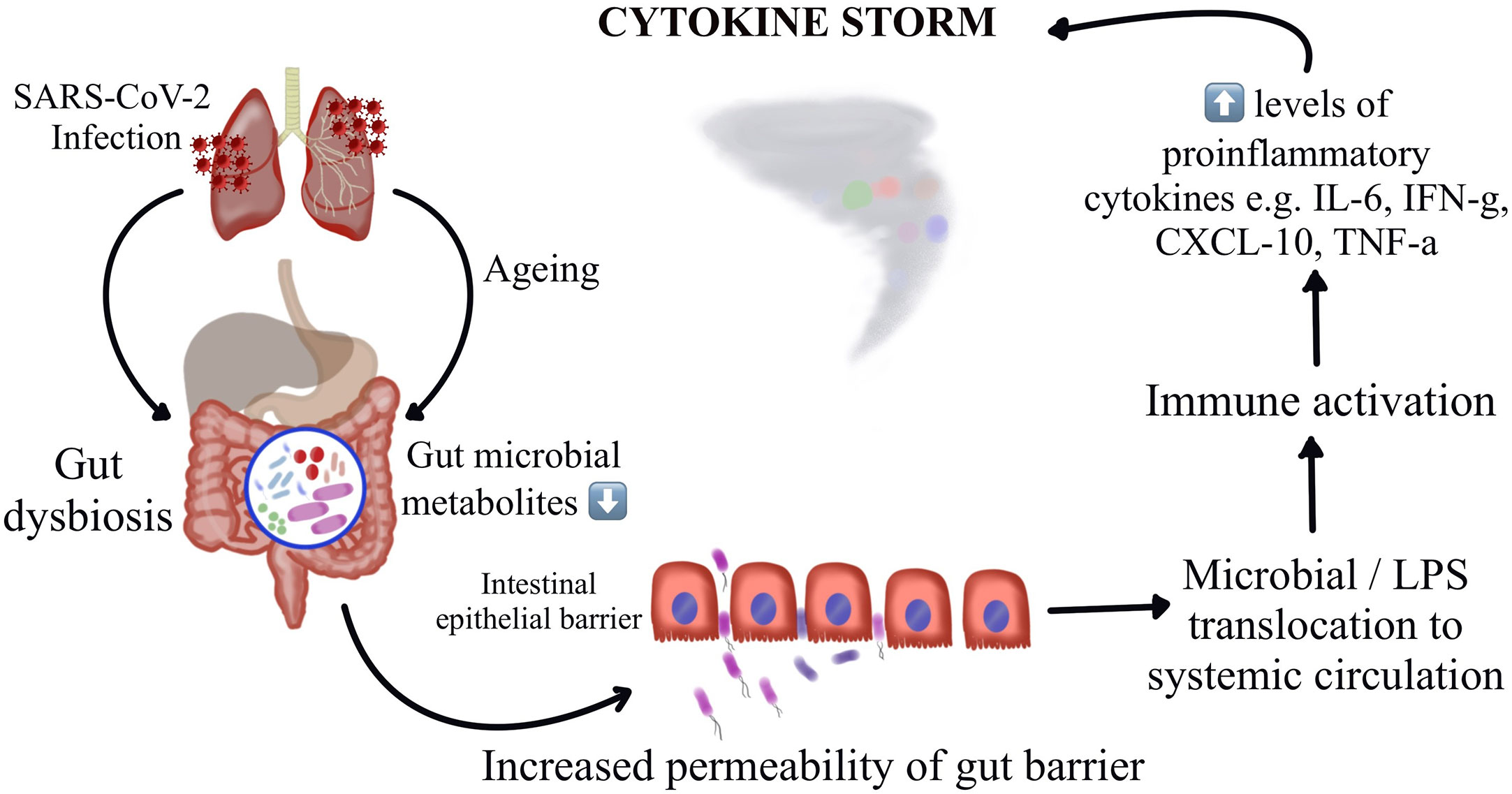
Figure 1 Schematic representation of the proposed hypothesis of gut microbiota perturbation leading to severe COVID-19 by cytokine storm.
Conclusion
As discussed earlier, the immune-gut interaction being well-balanced and bidirectional, the increased inflammation can lead to leaky gut allowing translocation of bacterial toxins and metabolites to the systemic circulation. This can further worsen the septic state of COVID-19 patients. Earlier studies have demonstrated the association between increased intestinal permeability with sepsis and multiple organ failure (88, 89). Microbial translocation due to poor intestinal integrity ensues a secondary infection and bacterial translocation from the gut to lungs can lead to sepsis and acute respiratory distress syndrome (37). Studies have demonstrated the link between the gut and the respiratory tract and their concerted modulation of immune responses and dysbiosis in gut microbiota impacting the respiratory tract (90). Likewise, through the gut-lung axis, viruses causing respiratory infections in lungs have been known to translocate to other organs via systemic circulation. This supports the hypothesis of a disturbed gut microbiota setting stage for disrupted immune homeostasis leading to exacerbation of cytokine storm in COVID-19 patients.
Author Contributions
RV, CS, ZT, MR, SS, and PB led the writing of this opinion article. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge all the health care workers and other frontline workers who are involved in treating and containing of the COVID-19 pandemic.
References
1. Ramachandran V, Marimuthu RR, Chinnambedu RS. World War against COVID-19: How strong is our armamentarium? Med J Malaysia (2020) 75:314–5.
2. Jin X, Lian J-S, Hu J-H, Gao J, Zheng L, Zhang Y-M, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut (2020) 69:1002–9. doi: 10.1136/gutjnl-2020-320926
3. D’Amico F, Baumgart DC, Danese S, Peyrin-Biroulet L. Diarrhea During COVID-19 Infection: Pathogenesis, Epidemiology, Prevention, and Management. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc (2020) 18:1663–72. doi: 10.1016/j.cgh.2020.04.001
4. Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med (2020) 382:1199–207. doi: 10.1056/NEJMoa2001316
5. Sender R, Fuchs S, Milo R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell (2016) 164:337–40. doi: 10.1016/j.cell.2016.01.013
6. McGhee JR, Fujihashi K. Inside the mucosal immune system. PloS Biol (2012) 10:e1001397. doi: 10.1371/journal.pbio.1001397
7. Ma H, Tao W, Zhu S. T lymphocytes in the intestinal mucosa: defense and tolerance. Cell Mol Immunol (2019) 16:216–24. doi: 10.1038/s41423-019-0208-2
8. Tan J, McKenzie C, Vuillermin PJ, Goverse G, Vinuesa CG, Mebius RE, et al. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep (2016) 15:2809–24. doi: 10.1016/j.celrep.2016.05.047
9. Qi H, Egen JG, Huang AYC, Germain RN. Extrafollicular activation of lymph node B cells by antigen-bearing dendritic cells. Science (2006) 312:1672–6. doi: 10.1126/science.1125703
10. Samuelson DR, Welsh DA, Shellito JE. Regulation of lung immunity and host defense by the intestinal microbiota. Front Microbiol (2015) 6:1085. doi: 10.3389/fmicb.2015.01085
11. Clarke TB. Early innate immunity to bacterial infection in the lung is regulated systemically by the commensal microbiota via nod-like receptor ligands. Infect Immun (2014) 82:4596–606. doi: 10.1128/IAI.02212-14
12. Khan N, Vidyarthi A, Nadeem S, Negi S, Nair G, Agrewala JN. Alteration in the Gut Microbiota Provokes Susceptibility to Tuberculosis. Front Immunol (2016) 7:529:529. doi: 10.3389/fimmu.2016.00529
13. Dumas A, Bernard L, Poquet Y, Lugo-Villarino G, Neyrolles O. The role of the lung microbiota and the gut-lung axis in respiratory infectious diseases. Cell Microbiol (2018) 20:e12966. doi: 10.1111/cmi.12966
14. Wang J, Li F, Wei H, Lian Z-X, Sun R, Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J Exp Med (2014) 211:2397–410. doi: 10.1084/jem.20140625
15. Budden KF, Gellatly SL, Wood DLA, Cooper MA, Morrison M, Hugenholtz P, et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol (2017) 15:55–63. doi: 10.1038/nrmicro.2016.142
16. Gonçalves P, Araújo JR, Di Santo JP. A Cross-Talk Between Microbiota-Derived Short-Chain Fatty Acids and the Host Mucosal Immune System Regulates Intestinal Homeostasis and Inflammatory Bowel Disease. Inflammation Bowel Dis (2018) 24:558–72. doi: 10.1093/ibd/izx029
17. Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature (2011) 469:543–7. doi: 10.1038/nature09646
18. Jung T-H, Park JH, Jeon W-M, Han K-S. Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutr Res Pract (2015) 9:343–9. doi: 10.4162/nrp.2015.9.4.343
19. Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol (2014) 121:91–119. doi: 10.1016/B978-0-12-800100-4.00003-9
20. Hull EE, Montgomery MR, Leyva KJ. HDAC Inhibitors as Epigenetic Regulators of the Immune System: Impacts on Cancer Therapy and Inflammatory Diseases. BioMed Res Int (2016) 2016:8797206. doi: 10.1155/2016/8797206
21. Husted AS, Trauelsen M, Rudenko O, Hjorth SA, Schwartz TW. GPCR-Mediated Signaling of Metabolites. Cell Metab (2017) 25:777–96. doi: 10.1016/j.cmet.2017.03.008
22. Li M, van Esch BCAM, Wagenaar GTM, Garssen J, Folkerts G, Henricks PAJ. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur J Pharmacol (2018) 831:52–9. doi: 10.1016/j.ejphar.2018.05.003
23. Meijer K, de Vos P, Priebe MG. Butyrate and other short-chain fatty acids as modulators of immunity: what relevance for health? Curr Opin Clin Nutr Metab Care (2010) 13:715–21. doi: 10.1097/MCO.0b013e32833eebe5
24. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature (2013) 504:446–50. doi: 10.1038/nature12721
25. Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, et al. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity (2014) 40:128–39. doi: 10.1016/j.immuni.2013.12.007
26. Trompette A, Gollwitzer ES, Pattaroni C, Lopez-Mejia IC, Riva E, Pernot J, et al. Dietary Fiber Confers Protection against Flu by Shaping Ly6c- Patrolling Monocyte Hematopoiesis and CD8+ T Cell Metabolism. Immunity (2018) 48:992–1005.e8. doi: 10.1016/j.immuni.2018.04.022
27. Dang AT, Marsland BJ. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol (2019) 12:843–50. doi: 10.1038/s41385-019-0160-6
28. Kopf M, Schneider C, Nobs SP. The development and function of lung-resident macrophages and dendritic cells. Nat Immunol (2015) 16:36–44. doi: 10.1038/ni.3052
29. Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol (2016) 16:341–52. doi: 10.1038/nri.2016.42
30. Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, et al. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front Cell Infect Microbiol (2018) 8:13:13. doi: 10.3389/fcimb.2018.00013
31. Levy M, Thaiss CA, Elinav E. Metabolites: messengers between the microbiota and the immune system. Genes Dev (2016) 30:1589–97. doi: 10.1101/gad.284091.116
32. Morita N, Umemoto E, Fujita S, Hayashi A, Kikuta J, Kimura I, et al. GPR31-dependent dendrite protrusion of intestinal CX3CR1+ cells by bacterial metabolites. Nature (2019) 566:110–4. doi: 10.1038/s41586-019-0884-1
33. Steed AL, Christophi GP, Kaiko GE, Sun L, Goodwin VM, Jain U, et al. The microbial metabolite desaminotyrosine protects from influenza through type I interferon. Science (2017) 357:498–502. doi: 10.1126/science.aam5336
34. Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic effects on host-microbiota mutualism. Nat Rev Microbiol (2011) 9:233–43. doi: 10.1038/nrmicro2536
35. Mosca A, Leclerc M, Hugot JP. Gut Microbiota Diversity and Human Diseases: Should We Reintroduce Key Predators in Our Ecosystem? Front Microbiol (2016) 7:455:455. doi: 10.3389/fmicb.2016.00455
36. Groves HT, Higham SL, Moffatt MF, Cox MJ, Tregoning JS. Respiratory Viral Infection Alters the Gut Microbiota by Inducing Inappetence. mBio (2020) 11:1. doi: 10.1128/mBio.03236-19
37. Dickson RP. The microbiome and critical illness. Lancet Respir Med (2016) 4:59–72. doi: 10.1016/S2213-2600(15)00427-0
38. Fernandes R, Viana SD, Nunes S, Reis F. Diabetic gut microbiota dysbiosis as an inflammaging and immunosenescence condition that fosters progression of retinopathy and nephropathy. Biochim Biophys Acta Mol Basis Dis (2019) 1865:1876–97. doi: 10.1016/j.bbadis.2018.09.032
39. Perrin-Cocon L, Aublin-Gex A, Sestito SE, Shirey KA, Patel MC, André P, et al. TLR4 antagonist FP7 inhibits LPS-induced cytokine production and glycolytic reprogramming in dendritic cells, and protects mice from lethal influenza infection. Sci Rep (2017) 7:40791. doi: 10.1038/srep40791
40. Mangiola F, Nicoletti A, Gasbarrini A, Ponziani FR. Gut microbiota and aging. Eur Rev Med Pharmacol Sci (2018) 22:7404–13. doi: 10.26355/eurrev_201811_16280
41. Nagpal R, Mainali R, Ahmadi S, Wang S, Singh R, Kavanagh K, et al. Gut microbiome and aging: Physiological and mechanistic insights. Nutr Healthy Aging (2018) 4:267–85. doi: 10.3233/NHA-170030
42. Aleman FDD, Valenzano DR. Microbiome evolution during host aging. PloS Pathog (2019) 15:e1007727. doi: 10.1371/journal.ppat.1007727
43. Abenavoli L, Scarpellini E, Colica C, Boccuto L, Salehi B, Sharifi-Rad J, et al. Gut Microbiota and Obesity: A Role for Probiotics. Nutrients (2019) 11:11. doi: 10.3390/nu11112690
44. Sanchez-Rodriguez E, Egea-Zorrilla A, Plaza-Díaz J, Aragón-Vela J, Muñoz-Quezada S, Tercedor-Sánchez L, et al. The Gut Microbiota and Its Implication in the Development of Atherosclerosis and Related Cardiovascular Diseases. Nutrients (2020) 12(3):605. doi: 10.3390/nu12030605
45. Du R-H, Liang L-R, Yang C-Q, Wang W, Cao T-Z, Li M, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J (2020) 56:3. doi: 10.1183/13993003.00524-2020
46. Roncon L, Zuin M, Rigatelli G, Zuliani G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J Clin Virol Off Publ Pan Am Soc Clin Virol (2020) 127:104354. doi: 10.1016/j.jcv.2020.104354
47. Shi Q, Zhang X, Jiang F, Zhang X, Hu N, Bimu C, et al. Clinical Characteristics and Risk Factors for Mortality of COVID-19 Patients With Diabetes in Wuhan, China: A Two-Center, Retrospective Study. Diabetes Care (2020) 43:1382–91. doi: 10.2337/dc20-0598
48. Wang X, Fang X, Cai Z, Wu X, Gao X, Min J, et al. Comorbid Chronic Diseases and Acute Organ Injuries Are Strongly Correlated with Disease Severity and Mortality among COVID-19 Patients: A Systemic Review and Meta-Analysis. Res Wash DC (2020) 2020:2402961. doi: 10.34133/2020/2402961
49. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet Lond Engl (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
50. Khan I, Ullah N, Zha L, Bai Y, Khan A, Zhao T, et al. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens (2019) 8:126. doi: 10.3390/pathogens8030126
51. Zalar B, Haslberger A, Peterlin B. THE ROLE OF MICROBIOTA IN DEPRESSION - A BRIEF REVIEW. Psychiatr Danub (2018) 30:136–41. doi: 10.24869/psyd.2018.136
52. Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, et al. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine (2020) 51:102590. doi: 10.1016/j.ebiom.2019.11.051
53. Tang WHW, Kitai T, Hazen SL. Gut Microbiota in Cardiovascular Health and Disease. Circ Res (2017) 120:1183–96. doi: 10.1161/CIRCRESAHA.117.309715
54. Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, et al. Gut Dysbiosis Is Linked to Hypertension. Hypertension (2015) 65:1331–40. doi: 10.1161/HYPERTENSIONAHA.115.05315
55. Villapol S. Gastrointestinal symptoms associated with COVID-19: impact on the gut microbiome. Transl Res (2020) 226:57–69. doi: 10.1016/j.trsl.2020.08.004
56. Gou W, Fu Y, Yue L, Chen G, Cai X, Shuai M, et al. Gut microbiota may underlie the predisposition of healthy individuals to COVID-19. medRxiv (2020) 2020.04.22.20076091. doi: 10.1101/2020.04.22.20076091.
57. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
58. Kalantar-Zadeh K, Ward SA, Kalantar-Zadeh K, El-Omar EM. Considering the Effects of Microbiome and Diet on SARS-CoV-2 Infection: Nanotechnology Roles. ACS Nano (2020) 14:5179–82. doi: 10.1021/acsnano.0c03402
59. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med (2020) 46:846–8. doi: 10.1007/s00134-020-05991-x
60. Santos-Oliveira JR, Regis EG, Leal CRB, Cunha RV, Bozza PT, Da-Cruz AM. Evidence that lipopolisaccharide may contribute to the cytokine storm and cellular activation in patients with visceral leishmaniasis. PloS Negl Trop Dis (2011) 5:e1198. doi: 10.1371/journal.pntd.0001198
61. Oladunni FS, Park J-G, Pino P-A, Gonzalez O, Akhter A, Allué-Guardia A, et al. Lethality of SARS-CoV-2 infection in K18 human angiotensin converting enzyme 2 transgenic mice. Nat Commun (2020) 11(1):1–17. doi: 10.1038/s41467-020-19891-7
62. Zhang C, Wu Z, Li J-W, Zhao H, Wang G-Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents (2020) 55:105954. doi: 10.1016/j.ijantimicag.2020.105954
63. Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev (2020) 53:25–32. doi: 10.1016/j.cytogfr.2020.05.003
64. Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 Cytokine Storm; What We Know So Far. Front Immunol (2020) 11:1446. doi: 10.3389/fimmu.2020.01446
65. Li Y, Zeng Z, Li Y, Huang W, Zhou M, Zhang X, et al. Angiotensin-Converting Enzyme Inhibition Attenuates Lipopolysaccharide-Induced Lung Injury by Regulating the Balance Between Angiotensin-Converting Enzyme and Angiotensin-Converting Enzyme 2 and Inhibiting Mitogen-Activated Protein Kinase Activation. Shock (2015) 43:395–404. doi: 10.1097/SHK.0000000000000302
66. Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, et al. Alterations in Gut Microbiota of Patients With COVID-19 During Time of Hospitalization. Gastroenterology (2020). doi: 10.1053/j.gastro.2020.05.048
67. Tang T, Sui Y, Lian M, Li Z, Hua J. Pro-inflammatory activated Kupffer cells by lipids induce hepatic NKT cells deficiency through activation-induced cell death. PloS One (2013) 8:e81949. doi: 10.1371/journal.pone.0081949
68. Kawaratani H, Tsujimoto T, Douhara A, Takaya H, Moriya K, Namisaki T, et al. The effect of inflammatory cytokines in alcoholic liver disease. Mediators Inflammation (2013) 2013:495156. doi: 10.1155/2013/495156
69. Eggesbø JB, Hjermann I, Ovstebø R, Joø GB, Kierulf P. LPS induced procoagulant activity and plasminogen activator activity in mononuclear cells from persons with high or low levels of HDL lipoprotein. Thromb Res (1995) 77:441–52. doi: 10.1016/0049-3848(95)93880-9
70. Stoll LL, Denning GM, Li W-G, Rice JB, Harrelson AL, Romig SA, et al. Regulation of endotoxin-induced proinflammatory activation in human coronary artery cells: expression of functional membrane-bound CD14 by human coronary artery smooth muscle cells. J Immunol Baltim Md 1950 (2004) 173:1336–43. doi: 10.4049/jimmunol.173.2.1336
71. Szeto C-C, Kwan BC-H, Chow K-M, Lai K-B, Chung K-Y, Leung C-B, et al. Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin J Am Soc Nephrol CJASN (2008) 3:431–6. doi: 10.2215/CJN.03600807
72. Giron LB, Dweep H, Yin X, Wang H, Damra M, Goldman AR, et al. Severe COVID-19 Is Fueled by Disrupted Gut Barrier Integrity. medRxiv (2020). doi: 10.1101/2020.11.13.20231209
73. Tao W, Zhang G, Wang X, Guo M, Zeng W, Xu Z, et al. Analysis of the intestinal microbiota in COVID-19 patients and its correlation with the inflammatory factor IL-18. Med Microecol (2020) 5:100023. doi: 10.1016/j.medmic.2020.100023
74. Zuo T, Liu Q, Zhang F, Yeoh YK, Wan Y, Zhan H, et al. Temporal Landscape of Human Gut RNA and DNA Viromes in SARS-CoV-2 Infection and Severity. (2020) Med Microecol 5:100023. doi: 10.21203/rs.3.rs-66879/v1
75. Saleh J, Peyssonnaux C, Singh KK, Edeas M. Mitochondria and microbiota dysfunction in COVID-19 pathogenesis. Mitochondrion (2020) 54:1–7. doi: 10.1016/j.mito.2020.06.008
76. Durand P-Y, Nicco C, Serteyn D, Attaf D, Edeas M. Microbiota Quality and Mitochondrial Activity Link with Occurrence of Muscle Cramps in Hemodialysis Patients using Citrate Dialysate: A Pilot Study. Blood Purif (2018) 46:301–8. doi: 10.1159/000490612
77. Mottawea W, Chiang C-K, Mühlbauer M, Starr AE, Butcher J, Abujamel T, et al. Altered intestinal microbiota–host mitochondria crosstalk in new onset Crohn’s disease. Nat Commun (2016) 7:13419. doi: 10.1038/ncomms13419
78. Hanada S, Pirzadeh M, Carver KY, Deng JC. Respiratory Viral Infection-Induced Microbiome Alterations and Secondary Bacterial Pneumonia. Front Immunol (2018) 9:2640:2640. doi: 10.3389/fimmu.2018.02640
79. Descamps HC, Herrmann B, Wiredu D, Thaiss CA. The path toward using microbial metabolites as therapies. EBioMedicine (2019) 44:747–54. doi: 10.1016/j.ebiom.2019.05.063
80. den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud D-J, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res (2013) 54:2325–40. doi: 10.1194/jlr.R036012
81. Hao Q, Dong BR, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev (2015) 54:CD006895. doi: 10.1002/14651858.CD006895.pub3
82. de Vrese M, Winkler P, Rautenberg P, Harder T, Noah C, Laue C, et al. Probiotic bacteria reduced duration and severity but not the incidence of common cold episodes in a double blind, randomized, controlled trial. Vaccine (2006) 24:6670–4. doi: 10.1016/j.vaccine.2006.05.048
83. Waki N, Matsumoto M, Fukui Y, Suganuma H. Effects of probiotic Lactobacillus brevis KB290 on incidence of influenza infection among schoolchildren: an open-label pilot study. Lett Appl Microbiol (2014) 59:565–71. doi: 10.1111/lam.12340
84. Lei H, Xu Y, Chen J, Wei X, Lam DM-K. Immunoprotection against influenza H5N1 virus by oral administration of enteric-coated recombinant Lactococcus lactis mini-capsules. Virology (2010) 407:319–24. doi: 10.1016/j.virol.2010.08.007
85. Wang Y, Qin S, Jia J, Huang L, Li F, Jin F, et al. Intestinal Microbiota-Associated Metabolites: Crucial Factors in the Effectiveness of Herbal Medicines and Diet Therapies. Front Physiol (2019) 10:1343. doi: 10.3389/fphys.2019.01343
86. Guan W, Liang W, Zhao Y, Liang H, Chen Z, Li Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J (2020) 55. doi: 10.1183/13993003.00547-2020
87. Gu S, Chen Y, Wu Z, Chen Y, Gao H, Lv L, et al. Alterations of the Gut Microbiota in Patients with COVID-19 or H1N1 Influenza. Clin Infect Dis Off Publ Infect Dis Soc Am (2020) 55:5. doi: 10.1093/cid/ciaa709
88. Doig CJ, Sutherland LR, Sandham JD, Fick GH, Verhoef M, Meddings JB. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am J Respir Crit Care Med (1998) 158:444–51. doi: 10.1164/ajrccm.158.2.9710092
89. Deitch EA. Gut-Origin sepsis; evolution of a concept. Surg J R Coll Surg Edinb Irel (2012) 10:350–6. doi: 10.1016/j.surge.2012.03.003
Keywords: COVID-19 and gut microbiome, COVID-19 inflammation, Severe Acute Respiratory Syndrome Coronavirus-2, gut dysbiosis in COVID-19, microbial translocation in COVID-19, cytokine storm in SARS-CoV-2, microbiome and cytokine storm
Citation: Vignesh R, Swathirajan CR, Tun ZH, Rameshkumar MR, Solomon SS and Balakrishnan P (2021) Could Perturbation of Gut Microbiota Possibly Exacerbate the Severity of COVID-19 via Cytokine Storm? Front. Immunol. 11:607734. doi: 10.3389/fimmu.2020.607734
Received: 18 September 2020; Accepted: 18 December 2020;
Published: 25 January 2021.
Edited by:
Fabrice Cognasse, Groupe Sur L’immunité Des Muqueuses Et Agents Pathogènes (GIMAP), FranceReviewed by:
Francesca Romana Ponziani, Hepatology Fondazione Policlinico Universitario Agostino Gemelli IRCCS, ItalyMarvin Edeas, INSERM U1016 Institut Cochin, France
Copyright © 2021 Vignesh, Swathirajan, Tun, Rameshkumar, Solomon and Balakrishnan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pachamuthu Balakrishnan, bala@yrgcare.org
 Ramachandran Vignesh
Ramachandran Vignesh Chinnambedu Ravichandran Swathirajan
Chinnambedu Ravichandran Swathirajan Zaw Htet Tun
Zaw Htet Tun Marimuthu Ragavan Rameshkumar
Marimuthu Ragavan Rameshkumar Sunil Suhas Solomon2,4
Sunil Suhas Solomon2,4 Pachamuthu Balakrishnan
Pachamuthu Balakrishnan