Relationship Between Patient Sex and Serum Tumor Necrosis Factor Antagonist Drug and Anti-drug Antibody Concentrations in Inflammatory Bowel Disease; A Nationwide Cohort Study
- 1Division of Gastroenterology, Department of Internal Medicine, Mubarak Alkabeer University Hospital, Kuwait University, Kuwait City, Kuwait
- 2Department of Pharmacy Practice, Faculty of Pharmacy, Kuwait University, Kuwait City, Kuwait
- 3Department of Community Medicine and Behavioral Sciences, Faculty of Medicine, Kuwait University, Kuwait City, Kuwait
- 4Jill Roberts Center for Inflammatory Bowel Disease, Division of Gastroenterology and Hepatology, Department of Medicine, New York Presbyterian-Weill Cornell Medical College, New York, NY, United States
Background: Anti-drug antibodies to infliximab (ATI) and adalimumab (ATA) are associated with loss of response to tumor necrosis factor antagonist (anti-TNF) therapy in inflammatory bowel disease (IBD). We evaluated the relationship between patient sex and serum TNF antagonist drug and antibody concentrations in inflammatory bowel disease.
Methods: A nationwide multicenter retrospective cohort study was conducted by evaluating patients' charts from July 2018 until September 2021. The effect of patient sex on anti-drug antibodies and serum drug concentration in patients with IBD across seven hospitals was investigated. A subgroup analysis also investigated the effect of anti-TNF combination therapy. Geometric means were calculated, and multiple linear regression was used to estimate the adjusted ratio of geometric means (RoGM) and their 95% confidence intervals (CI).
Results: In the total study sample (n = 1093), males receiving infliximab had higher anti-drug antibody concentrations (38.3 vs. 22.3 AU/ml; aRoGM = 1.72, 95% CI: 1.30–2.27, p < 0.001) compared to females. Additionally, infliximab serum drug concentrations among males were lower compared to females (2.6 vs. 4.1 ug/ml; aRoGM = 0.62, 95% CI: 0.44–0.88, p = 0.007). In the subgroup analysis (n = 359), male compared to female patients on combination therapy with infliximab and immunomodulators had similar anti-drug antibody concentrations (30.2 vs. 21.9 AU/ml; aRoGM = 1.38, 95% CI: 0.79–2.40, p = 0.254). There was no difference in the anti-drug antibody and serum drug concentrations among males and females on adalimumab.
Conclusion: In patients receiving infliximab, anti-drug antibodies were higher in males than females. Consistent with this, serum drug concentrations were lower in males than females on infliximab. There was no difference in anti-drug antibody and serum drug concentrations among males and females on adalimumab. In addition, no difference in anti-drug antibodies between males and females receiving anti-TNF combination therapy was observed.
Introduction
Tumor necrosis factor antagonist (anti-TNF) therapies are commonly used for the management of moderate to severe inflammatory bowel disease (IBD) (1–4). However, about one-third of patients treated with anti-TNF therapy develop primary treatment failure (primary non-response), in which a lack of response is observed in induction therapy (5, 6). Furthermore, approximately half of patients with initial response may experience secondary loss of response by losing treatment effect during the maintenance of remission (7). One of the most common causes of treatment failure is immunogenicity, the formation of anti-drug antibodies, which is also associated with low or undetectable drug serum concentrations (8, 9). The serum drug concentrations of anti-TNF therapy might also vary depending on the severity of the disease, degree of inflammation, concurrent use of immunomodulator, patient sex, serum albumin concentration, body mass index (BMI), and genetic factors (7, 8).
Previous data show that anti-drug antibodies exist in over 20% of IBD patients treated with anti-TNF therapy (10). Additionally, patient sex and body weight significantly influence the pharmacokinetics of infliximab as its clearance has been shown to be increased in the presence of anti-drug antibodies and low serum albumin (11). On the other hand, combination therapy, the concurrent administration of an immunomodulator with an anti-TNF, has been associated with improvement in pharmacokinetics by decreasing immunogenicity and increasing serum drug concentrations (1–4). With respect to infliximab, the SONIC and UC-SUCCESS trials demonstrated that the use of infliximab combination therapy is superior to monotherapy in reducing immunogenicity and maintaining remission (12, 13). Conversely, DIAMOND trial and two other meta-analyses, by Kopylov et al. and Chalhoub et al., demonstrated that adalimumab combination therapy is associated with limited impact on maintenance of clinical remission or response (3, 14–16). When considering anti-TNF therapy for pediatric patients with IBD, the ECCO-ESPGHAN guidelines recommend the use of infliximab combination therapy, with an immunomodulator, to reduce the risk of developing anti-drug antibodies to infliximab (ATI). However, for adalimumab, it is preferred to be prescribed as a monotherapy when started as the first anti-TNF agent in children (17, 18). When combination therapy is used in pediatric patients, it is recommended to stop the concomitant use of the immunomodulator after 6–12 months, and the benefits of continuing combination therapy should be weighed against the risk of adverse events (17).
A large-scale real world studies with patient-level data are lacking on the relationship between patient sex and anti-TNF therapy and anti-drug antibody concentrations. Additionally, the impact of combination therapy on anti-TNF pharmacokinetics when accounting for sex has not been described. To address these knowledge gaps, this study utilized a large cohort with patient level data to determine the relationship between patient sex and immunogenicity of infliximab and adalimumab when accounting for important factors such as albumin and concomitant immunomodulator use.
Materials and Methods
Study Design
A nationwide multicenter retrospective cohort study was conducted to measure the effect of patient sex on anti-TNF anti-drug antibodies and serum drug concentrations in patients with inflammatory bowel disease (IBD). A subgroup analysis was performed at an inflammatory bowel disease center, Mubarak al-Kabeer Hospital, for patients who received either infliximab or adalimumab monotherapy or in combination with an immunomodulator. This study was performed and reported in accordance with Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (19). The study protocol was reviewed and approved by the standing committee for coordination of health and medical research at the ministry of health of Kuwait (IRB 2020/1410).
We included patients that: (1) were previously diagnosed with inflammatory bowel disease (2) had been tested for an anti-drug antibody and/or serum drug trough concentrations reactively or proactively and (3) were receiving an anti-TNF therapy for at least 6 weeks at the time of measurement. (4) who received regular standard dose anti-TNF therapy. Proactive therapeutic drug monitoring (TDM) testing was at week 6 for adalimumab and at week 14 for infliximab. Reactive TDM testing was perfumed at trough levels, before next scheduled dose. Any random testing, beyond the above scheduled times, were excluded. Both serum drug and anti-drug antibody concentrations were measured at the same time. Patients who had past medical history of other autoimmune diseases, such as inflammatory arthritis, or were on immunosuppressant therapies for other non IBD medical conditions were excluded. The data were collected from patients' electronic medical records from seven different hospitals (Mubarak al-Kabeer Hospital, Alamiri Hospital, Farwaniya Hospital, Aladan Hospital, Jahra Hospital, Alsabah Hospital, and Kuwait Oil Company Hospital). Data were collected from July 22nd, 2018 until September 1st, 2021. A subgroup analysis, from the total sample, was performed at an inflammatory bowel disease center,Mubarak al-Kabeer Hospital, for patients who received either infliximab or adalimumab combination or monotherapy.
Patient's characteristics were obtained from patient's electronic medical records including age, body mass index (BMI), type and extent of IBD at the time of serum drug/antibody concentration measurements.
Diagnosis of inflammatory bowel disease (IBD) was made according to the international classification of diseases (ICD-10 version: 2016). Patients were considered to have IBD when they had ICD-10 K50, K50.1, K50.8, K50.9 corresponding to Crohn's disease (CD) and ICD-10 K51, K51.0, K51.2, K51.3, K51.5, K51.8, K51.9 corresponding to ulcerative colitis (UC) (20).
Study Definitions
Patients were considered to have active inflammation if they have one of the following within 14 days from serum drug/antibody concentration measurements: (1) C-reactive protein (CRP) levels above 10 mg/L or (2) stool fecal calprotectin (Fcal) more than 250 ug/g or (3) receiving steroids. Patients were considered to be receiving steroids if they were concomitantly receiving budesonide, methylprednisolone, hydrocortisone, prednisone/prednisolone, or any steroidal agent. Moreover, patients who received an immunomodulator (such as azathioprine, 6-mercaptopurine or methotrexate) concurrently with an anti-TNF therapy were classified to be on combination therapy while patients on infliximab or adalimumab alone were classified to be on monotherapy.
Anti-drug antibody and serum drug concentration samples from all the hospitals were measured at one central immunology laboratory. A drug-tolerant, the homogeneous mobility shift assay (HMSA) was used for all study participants. Anti-drug antibodies were considered detectable at levels >5 AU/ml for infliximab or >10 AU/ml for adalimumab. Additionally, serum drug concentrations/antibody levels were collected only at trough levels, i.e., before the next scheduled dose. A serum drug level of ≥5, and ≥7.5 ug/ml was considered therapeutic for infliximab and adalimumab, respectively. Trough serum drug and antidrug antibody concentrations were performed either reactively, e.g., due to treatment failure, or proactively to optimize therapy, e.g., at week 14 for infliximab, as per each physician clinical judgment and practice.
Outcomes
The primary outcome was to compare anti-drug antibody levels between male and female patients on infliximab and adalimumab. In addition, the association between patient sex and serum drug concentration for infliximab and adalimumab was evaluated.
Secondary outcomes, in sub-analyses, evaluated the impact of combination therapy among male and female patients on infliximab or adalimumab anti-drug antibody levels. The association between serum drug concentration and combination therapy in both sexes was estimated as well. Moreover, combination therapy was compared to monotherapy in terms of anti-drug antibody levels and serum drug concentration.
Statistical Analysis
Analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC, USA). The statistical significance level was set to α = 0.05 for all association analyses. Descriptive analyses were conducted to calculate frequencies and proportions of categorical variables in the total study sample (n = 1,093) and subsample, which was part of the main total sample, (n = 359). To account for skewed distribution of the continuous variables (i.e., serum drug concentrations, and anti-drug antibodies), geometric means were estimated by log10-transformation of the data and subsequently taking the antilog of the calculated means on the transformed scale (21). Correlations between anti-drug antibody and serum drug concentration according to anti-TNF drug type (infliximab and adalimumab) were assessed using Spearman correlation coefficients (r). In the total study sample analysis, the effect of patient sex on anti-drug antibody and serum drug concentrations of both adalimumab and infliximab was assessed. In the subsample analysis, the effect of patient sex on infliximab and adalimumab use in combination or alone on anti-drug antibody levels and serum drug concentrations was assessed.
Associations of adalimumab and infliximab with log10-transformed anti-drug antibody and serum drug concentration (outcome variables) were evaluated using multiple linear regression models. Associations were assessed in the total sample and stratified by sex. In the total sample analysis, associations were adjusted for the effects of sex and age at assessment. In the subgroup analysis, associations were adjusted for sex, age at assessment, and active inflammation. In the sex stratified analysis, associations were adjusted for the effects of active inflammation and age at assessment. Given that we regressed log10-transformed anti-drug antibody and serum drug concentration, taking the antilog of the linear regression coefficients (β) yields an adjusted ratio of geometric means (aRoGM), not the difference between geometric means. Hence, the related 95% confidence intervals (CIs) represent limits for RoGM with a null value of “1.” Anti-drug antibodies and serum drug concentrations were analyzed as continuous variables while applying log-transformation to account for skewness in the data and maximize the value of the continuous measurements (see Discussion).
Additional analyses were conducted to determine whether albumin can be a confounder of the assessed associations. Albumin was categorized as normal (≥40 g/L) and abnormal (<40 g/L). The association of albumin (normal vs. abnormal) with anti-drug antibodies to infliximab and adalimumab was assessed by applying the Wilcoxon rank sum test. This analysis allowed us to determine whether concentrations of anti-drug antibodies differ across albumin categories. Moreover, albumin was added to the multiple linear regression models to determine if confounding is present.
Results
Demographics
In total, 1,093 patients [567 (51.9%) males] were included in the total study sample analysis and a total of 359 patients [187 (52.1%) males] were included in the subsample analysis. The total study sample and the subsample were similar in all characteristics investigated. Of the total study sample, 42.2% of patients were on infliximab and 57.8% were on adalimumab. Similarly, in the subsample, 40.9 and 59.1% of patients were on infliximab and adalimumab, respectively (Table 1). Among patients in the subsample, 62.9% used anti-TNF combination therapy. Specifically, of the 147 patients on infliximab, 114 (77.6%) were on combination therapy while among the 212 patients on adalimumab, 112 (52.8%) were on combination therapy.
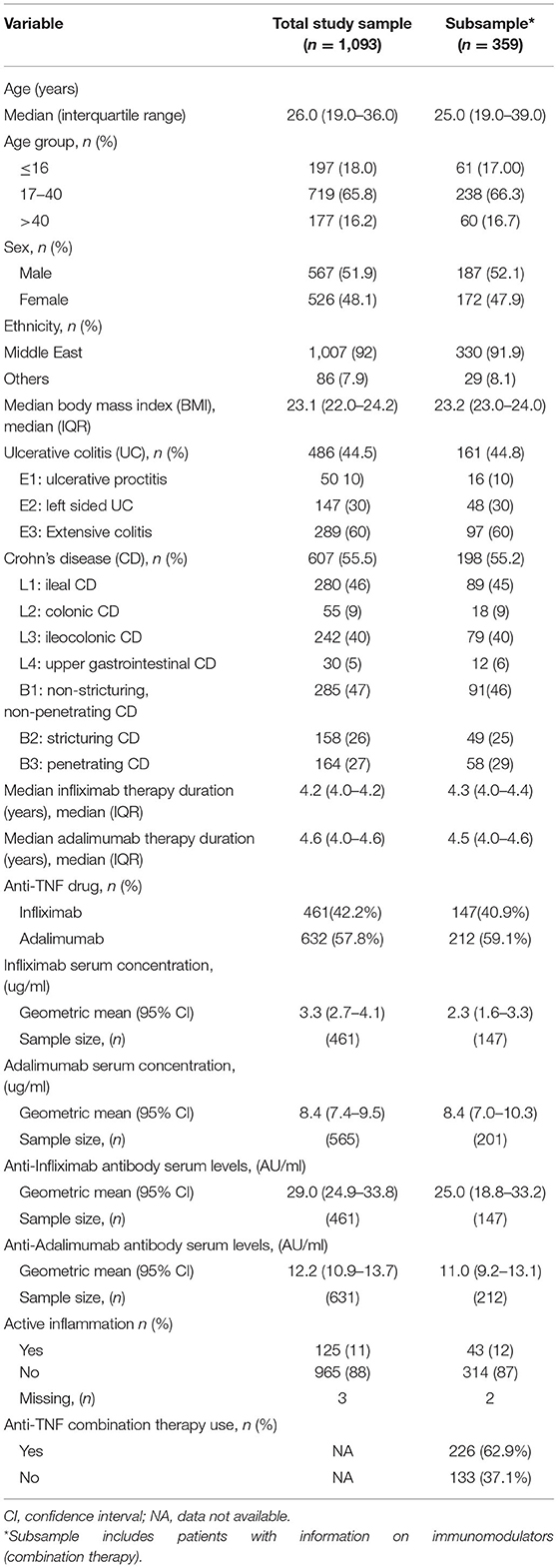
Table 1. Characteristics of total study sample and subsample with information on combination therapy use.
Outcomes
Inter-sex comparisons of anti-drug antibody within anti-TNF therapy are shown in Table 2. Whilst the proportion of male and female patients are similar, male patients on infliximab had higher anti-drug antibody concentrations than female patients in the total sample (38.3 vs. 22.3 AU/ml; aRoGM = 1.72, 95% CI: 1.30–2.27, p < 0.001) (Table 2, Figure 1A) and in the subsample sample (106.5 vs. 24.2 AU/ml; aRoGM = 4.39, 95% CI: 1.58–12.25, p =0.005) (Table 2, Figure 1B). However, male and female patients on infliximab combination therapy in the subsample had similar anti-drug antibody levels (30.2 vs. 21.9 AU/ml; aRoGM = 1.38, 95% CI: 0.79–2.40, p = 0.254) (Table 2, Figure 1B).
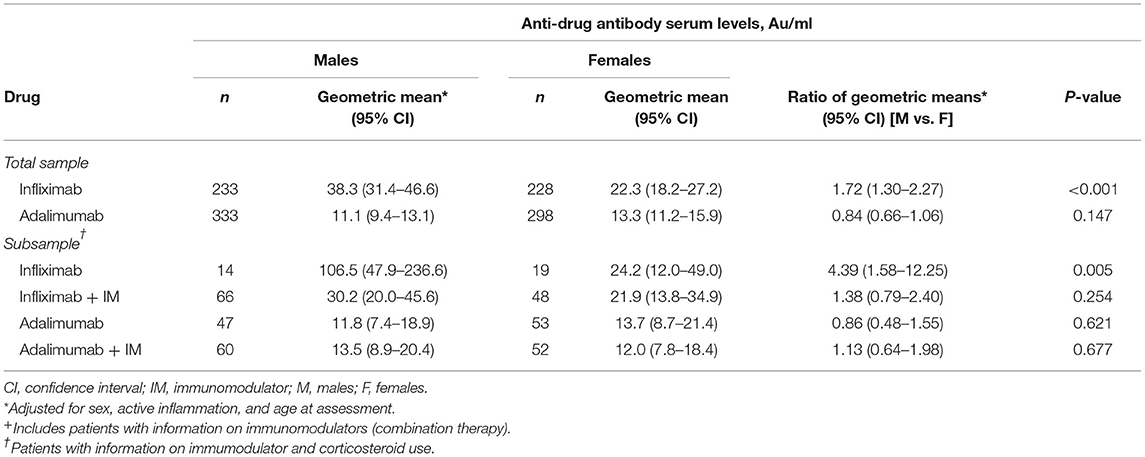
Table 2. Anti-drug antibody serum levels stratified by sex according to monotherapy and combination therapy for infliximab and adalimumab use: inter-sex comparisons.
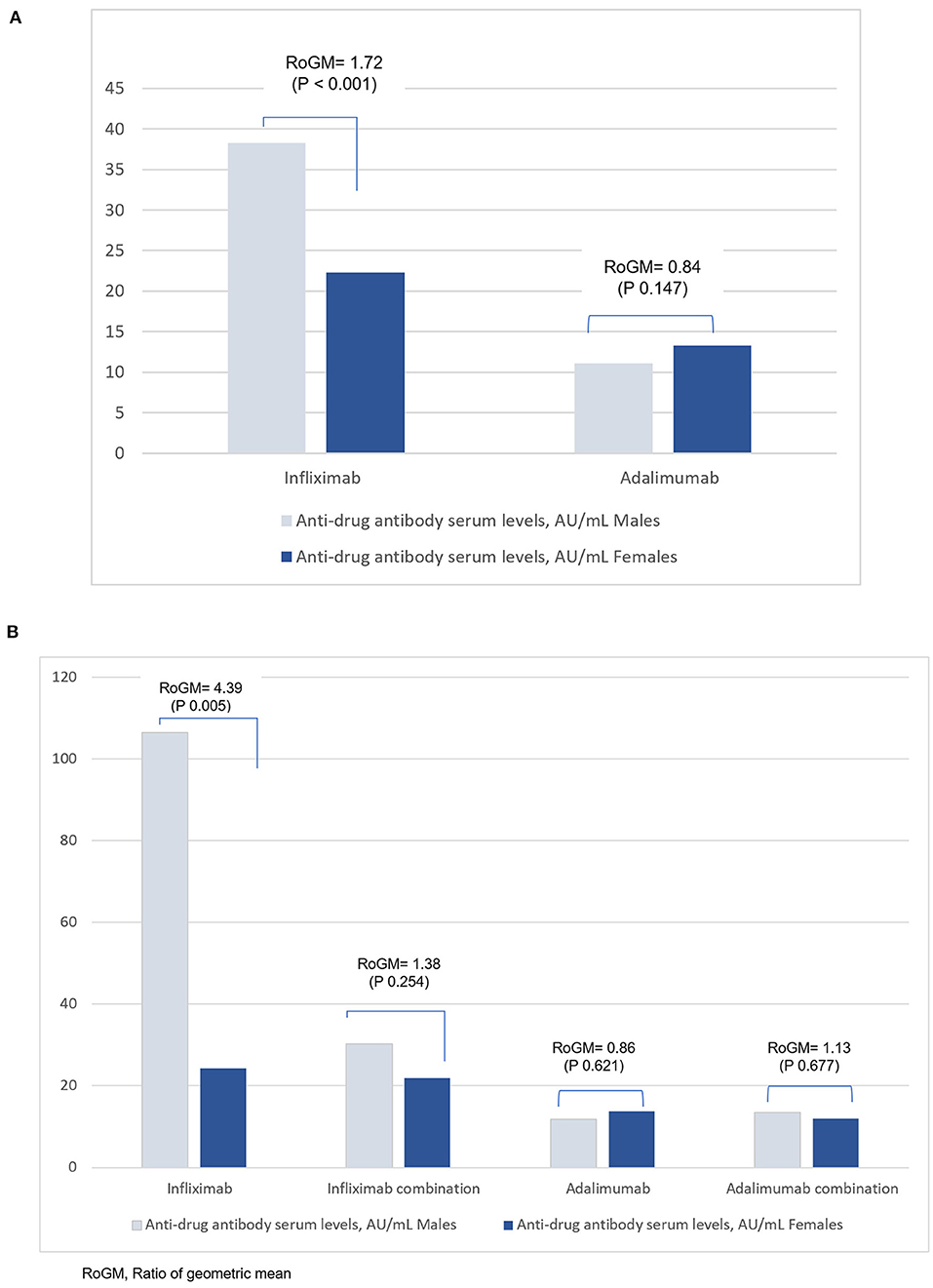
Figure 1. (A) Anti-drug antibody levels in male and female patients with IBD in the total sample analysis for infliximab and adalimumab. The Y-axis corresponds to the geometric mean of the anti-drug antibodies serum levels. (B) Anti-drug antibody serum levels in males and females with IBD in the subgroup analysis for Infliximab and Adalimumab. The Y-axis corresponds to the geometric mean of the anti-drug antibodies serum levels.
In the subsample, male and female patients on adalimumab alone had similar anti-drug antibody levels (11.8 vs 13.7 AU/ml; aRoGM = 0.86, 95% CI: 0.48–1.55, p = 0.62). Males and females on adalimumab combination therapy had similar anti-drug antibody levels as well (13.5 vs 12 AU/ml; aRoM = 1.13, 95% CI: 0.64–1.98, p = 0.67, Table 2, Figure 1B).
Associations between anti-TNF therapy and serum drug concentrations are shown in Table 3. In the total study sample, male patients had lower infliximab serum drug concentrations compared to female patients (2.6 vs. 4.1 ug/ml; aRoGM = 0.62, 95% CI: 0.44–0.88, p = 0.007). No other between sex differences in serum drug concentrations were observed (Table 3). Serum drug concentrations were similar among patients on infliximab alone compared to those on infliximab combination therapy (Supplementary Table S1). Similarly, no difference in serum drug concentrations was observed among patients on adalimumab alone and those on adalimumab combination therapy (Supplementary Table S1).
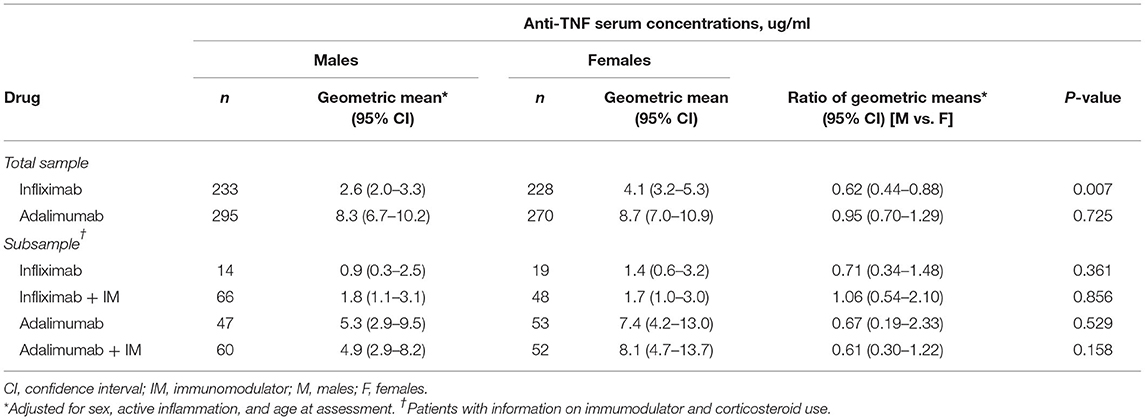
Table 3. Anti-TNF serum concentrations stratified by sex according to monotherapy and combination therapy for infliximab and adalimumab use: inter-sex comparisons.
Anti-drug antibody levels among patients on infliximab combination therapy were significantly lower than in patients on infliximab alone (25.7 vs. 50.8 AU/ml; aRoGM = 0.51, 95% CI: 0.28–0.91, p = 0.023; Supplementary Table S2). In addition, there was no difference in anti-drug antibody between patients on adalimumab combination therapy and those on adalimumab alone (12.7 vs. 12.7 AU/ml; aRoGM = 1.00, 95% CI: 0.66–1.51, p = 0.995; Supplementary Table S2).
In the total study sample, a negative correlation between infliximab serum concentration and anti-drug antibodies to infliximab (ATI) (spearman r = −0.388, p < 0.001) and adalimumab serum concentration and anti-drug antibodies to adalimumab (ATA) (spearman r = −0.415; p < 0.001; Figure 2) was observed.
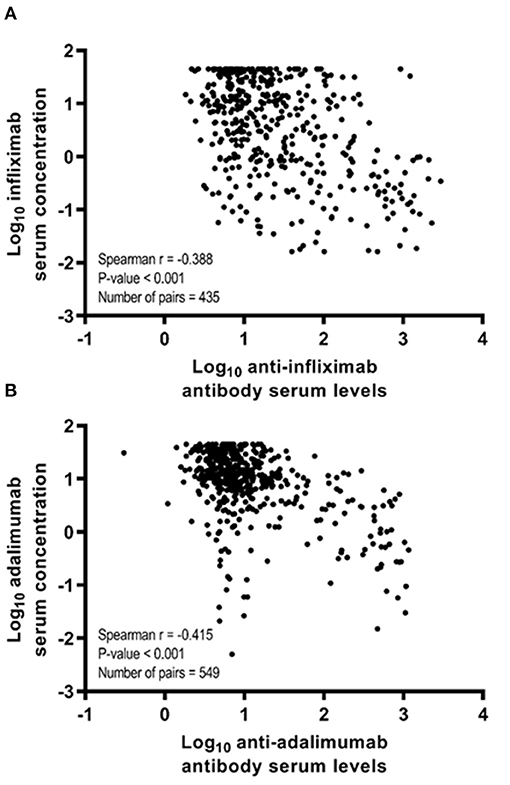
Figure 2. Spearman correlation coefficients (r) between anti-drug antibody levels and anti-TNF therapy serum drug concentration according to anti-TNF therapy in the total study sample. (A) Spearman correlation coefficient between log10-transformed infliximab serum concentration and log10-transformed anti-infliximab antibody serum levels. (B) Spearman correlation coefficient between log10-transformed adalimumab serum concentration and log10-transformed anti-adalimumab antibody serum levels.
In an additional analysis, we assessed the association of serum albumin [≥40 g/L (normal) vs. <40 g/L (abnormal)] with anti-drug antibodies to infliximab and adalimumab. Comparing subjects with normal and abnormal serum albumin levels, we found that average levels of anti-drug antibodies to infliximab (10.6 vs. 19.2 AU/ml, p = 0.158) and anti-drug antibodies to adalimumab (7.5 vs. 7.4 AU/ml, p = 0.991) to be similar across categories of serum albumin. Moreover, when including serum albumin in the multiple linear regression models, the observed associations between male sex and higher anti-drug antibodies to infliximab (aRoGM = 1.79, 95% CI: 1.02–3.11, p = 0.041) and male sex and lower infliximab serum drug concentrations (aRoGM = 0.73, 95% CI: 0.23–0.96, p = 0.046) remained statistically significant. Hence, these additional analyses indicate that albumin does not confound the reported associations.
Discussion
In 1,093 Mediterranean patients with inflammatory bowel disease (IBD), we evaluated the effect of patient sex and combination therapy on anti-drug antibody levels and serum drug concentrations among all patients who were on infliximab or adalimumab. The effect of the patient sex on the anti-drug antibody levels was investigated in both monotherapy and combination therapy in our subgroup analysis. The results showed that serum drug concentrations of infliximab in male patients are commonly lower than female patients. Since weight and sex are somehow correlated, where males generally weigh more than females, it is thought that male patients have higher clearance rates than female patients. These patient factors can affect the pharmacokinetics of infliximab, however; the exact mechanism of this effect is still unknown (7, 11). In our study the median BMI of all patients was within normal range. In a systematic review by Billioud et al. which evaluated loss of response to adalimumab found that male sex was associated with higher likelihood of loss of response and need for dose escalation (22). Moreover, male compared to female patients on infliximab had higher anti-drug antibody concentrations; nonetheless, there is no previously available evidence, to our knowledge, that supports the association between patient sex and presence of anti-drug antibodies. On the other hand, the presence of higher anti-drug antibody levels seems to accelerate the clearance of anti-TNF therapy, which is supported by Fasanmade et al. study that analyzed two randomized-controlled trials (23).
The available data on therapeutic drug response of patients with IBD to medications, stratified by sex, are extremely limited. A recent review by Rustgi et al. suggested the need for further investigation to the role of sex hormones on IBD, to get better therapeutic response for patients with IBD (24). Further studies needed to identify if there is a genetic factor behind a correlation between the patient sex and the anti-drug antibody levels. Two previous studies, by Wilson et al. and Sazonovs et al., identified an association between the genetic variant HLA-DQA1*05 and the formation of anti-drug antibodies, against both infliximab and adalimumab, in patients with Crohn's disease (CD) (25, 26). A study by Sazonovs et al. showed that this variant increased the anti-drug antibodies formation by 2-folds, regardless of the concurrent immunomodulator use (26). They also concluded that to minimize the risk of therapy failure, a pretreatment genetic testing for HLA-DQA1*05 might be helpful in deciding whether to use anti-TNF, or combination therapy in IBD.
As a secondary outcome of our study, the effect of combination therapy was investigated in one inflammatory bowel disease center. The results of our study were similar to the PANTS study, which showed that immunogenicity is more common in patients with CD treated with infliximab than adalimumab, and that the concomitant use of immunomodulator was associated with lower the immunogenicity (27). Moreover, higher drug concentrations and remission rates were found in patients treated with infliximab combination therapy. However, these effects were not shown in patients treated with adalimumab combination therapy, this might be influenced by lower rates of immunogenicity compared to infliximab (27). Furthermore, our study results agree with Hazlewood et al. network meta-analysis, where the effectiveness of immunosuppressants and anti-TNF were found to be comparable, and considered the combination therapy of infliximab with azathioprine and adalimumab monotherapy to be the most effective strategies for inducing and maintaining the remission of CD (28). In addition, a systematic review, by Strand et al., emphasized the role of monitoring both anti-drug antibody levels and serum drug concentrations of the used anti-TNF agent (29). This might be potentially helpful in guiding clinicians to improve anti-TNF therapy management as well as clinical outcomes. It can also reduce risks associated with immunogenicity and help in lowering costs of therapy (29).
We did not analyze anti-drug antibodies and drug concentrations as dichotomous/categorical variables (e.g., normal/low vs. abnormal/high), such categorization is helpful in clinical practice, but has some drawbacks. Loss of information, reduced statistical power, underestimating the true variability in the data, and residual confounding are the major issues with categorization of continuous variables in clinical research (30). Given the previous drawbacks, we have analyzed the outcome variables (i.e., anti-drug antibodies and serum drug concentrations) as continuous variables.
Our study has several strengths. It is a nationwide multi center study that involved all hospitals in the country where therapeutic drug monitoring (TDM) testing is done. It is well designed with over 3 years total of all available data of eligible patients. It also addresses a gap in knowledge and encourage future research in this area.
However, there were some limitations to our study. Being a retrospective cohort, there might be some confounders, such as those on monotherapy could have been on combination therapy previously and then were discontinued due to adequate serum drug concentration and low anti-drug antibodies. In addition, patients' adherence to anti-TNF therapy at regular intervals could not be evaluated. Moreover, endoscopic and clinical targets were not studied; however, we controlled for objective inflammatory markers (CRP, Fcal, and steroids use). Combination therapy was only assessed at one center due to lack of data from other centers. Finally, the proportion of therapeutic drug monitoring (TDM) tests that were done reactively vs. proactively was not assessed.
In conclusion, anti-drug antibodies to infliximab (ATI) were higher in males than females whereas anti-drug antibodies to adalimumab (ATA) were similar in both sexes. In addition, male patients had lower infliximab serum drug concentrations compared to female patients while no sex differences was observed in adalimumab serum drug concentrations. Moreover, combination therapy was more effective than monotherapy in reducing ATI, but not better than monotherapy in reducing ATA. However, male and female patients on infliximab combination therapy had similar anti-drug antibody levels. Future studies are needed to assess the effect of patient sex, i.e., sex hormones, on anti-TNF anti-drug antibody and serum drug concentrations in patients with inflammatory bowel disease.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The study protocol was reviewed and approved by the standing committee for coordination of health and medical research at the ministry of health of Kuwait (IRB 2020/1410). Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author Contributions
MS: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and submission of the manuscript. HA, IA, GA, and AnA: acquisition of data and drafting of the manuscript. AhA: drafting of the manuscript. AZ: statistical analysis, analysis and interpretation of data. RB: critical revision of the manuscript for important intellectual content, study supervision, and responsible for the overall work as a guarantor. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.801532/full#supplementary-material
References
1. Feuerstein JD, Ho EY, Shmidt E, Singh H, Falck-Ytter Y, Sultan S, et al. AGA clinical practice guidelines on the medical management of moderate to severe luminal and perianal fistulizing crohn's disease. Gastroenterology. (2021) 160:2496–508. doi: 10.1053/j.gastro.2021.04.022
2. Feuerstein JD, Isaacs KL, Schneider Y, Siddique SM, Falck-Ytter Y, Singh S, et al. Clinical practice guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology. (2020) 158:1450–61. doi: 10.1053/j.gastro.2020.01.006
3. Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, et al. ECCO guidelines on therapeutics in crohn's disease: medical treatment. J Crohns Colitis. (2020) 14:4–22. doi: 10.1093/ecco-jcc/jjz180
4. Harbord M, Eliakim R, Bettenworth D, Karmiris K, Katsanos K, Kopylov U, et al. Third european evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. (2017) 11:769–84. doi: 10.1093/ecco-jcc/jjx009
5. Papamichael K, Vande Casteele N, Ferrante M, Gils A, Cheifetz AS. Therapeutic drug monitoring during induction of anti-tumor necrosis factor therapy in inflammatory bowel disease: defining a therapeutic drug window. Inflamm Bowel Dis. (2017) 23:1510–5. doi: 10.1097/MIB.0000000000001231
6. Papamichael K, Cheifetz AS. Use of anti-TNF drug levels to optimise patient management. Frontline Gastroenterol. (2016) 7:289–300. doi: 10.1136/flgastro-2016-100685
7. Steenholdt C, Bendtzen K, Brynskov J, Ainsworth MA. Optimizing treatment with TNF inhibitors in inflammatory bowel disease by monitoring drug levels and antidrug antibodies. Inflamm Bowel Dis. (2016) 22:1999–2015. doi: 10.1097/MIB.0000000000000772
8. Vermeire S, Gils A, Accossato P, Lula S, Marren A. Immunogenicity of biologics in inflammatory bowel disease. TAG. (2018) 11:1756283x17750355. doi: 10.1177/1756283X17750355
9. Feuerstein JD, Nguyen GC, Kupfer SS, Falck-Ytter Y, Singh S. American gastroenterological association institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. (2017) 153:827–34. doi: 10.1053/j.gastro.2017.07.032
10. Battat R, Lukin D, Scherl EJ, Pola S, Kumar A, Okada L, et al. Immunogenicity of tumor necrosis factor antagonists and effect of dose escalation on anti-drug antibodies and serum drug concentrations in inflammatory bowel disease. Inflamm Bowel Dis. (2020) 27:1443–51. doi: 10.1093/ibd/izaa313
11. Ordás I, Mould DR, Feagan BG, Sandborn WJ. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther. (2012) 91:635–46. doi: 10.1038/clpt.2011.328
12. Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. (2010) 362:1383–95. doi: 10.1056/NEJMoa0904492
13. Panaccione R, Ghosh S, Middleton S, Márquez JR, Scott BB, Flint L, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology. (2014) 146:392-400.e3. doi: 10.1053/j.gastro.2013.10.052
14. Matsumoto T, Motoya S, Watanabe K, Hisamatsu T, Nakase H, Yoshimura N, et al. Adalimumab monotherapy and a combination with azathioprine for crohn's disease: a prospective, randomized trial. J Crohns Colitis. (2016) 10:1259–66. doi: 10.1093/ecco-jcc/jjw152
15. Kopylov U, Al-Taweel T, Yaghoobi M, Nauche B, Bitton A, Lakatos PL, et al. Adalimumab monotherapy versus combination therapy with immunomodulators in patients with Crohn's disease: a systematic review and meta-analysis. J Crohns Colitis. (2014) 8:1632–41. doi: 10.1016/j.crohns.2014.07.003
16. Chalhoub JM, Rimmani HH, Gumaste VV, Sharara AI. Systematic review and meta-analysis: adalimumab monotherapy versus combination therapy with immunomodulators for induction and maintenance of remission and response in patients with crohn's disease. Inflamm Bowel Dis. (2017) 23:1316–27. doi: 10.1097/MIB.0000000000001203
17. van Rheenen PF, Aloi M, Assa A, Bronsky J, Escher JC, Fagerberg UL, et al. The Medical Management of Paediatric Crohn's Disease: an ECCO-ESPGHAN Guideline Update. J Crohns Colitis. (2020) jjaa161. doi: 10.1093/ecco-jcc/jjaa161
18. Turner D, Ruemmele FM, Orlanski-Meyer E, Griffiths AM, de Carpi JM, Bronsky J, et al. Management of paediatric ulcerative colitis, part 1: ambulatory care-an evidence-based guideline from European Crohn's and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. (2018) 67:257–91. doi: 10.1097/MPG.0000000000002035
19. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. (2007) 370:1453–7. doi: 10.1016/S0140-6736(07)61602-X
20. WHO. International Statistical Classification of Diseases and Related Health Problems 10th Revision (2016).
21. Bland JM, Altman DG. The use of transformation when comparing two means. BMJ. (1996) 312:1153. doi: 10.1136/bmj.312.7039.1153
22. Billioud V, Sandborn WJ, Peyrin-Biroulet L. Loss of response and need for adalimumab dose intensification in Crohn's disease: a systematic review. Am J Gastroenterol. (2011) 106:674–84. doi: 10.1038/ajg.2011.60
23. Fasanmade AA, Adedokun OJ, Ford J, Hernandez D, Johanns J, Hu C, et al. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur J Clin Pharmacol. (2009) 65:1211–28. doi: 10.1007/s00228-009-0718-4
24. Rustgi SD, Kayal M, Shah SC. Sex-based differences in inflammatory bowel diseases: a review. Therap Adv Gastroenterol. (2020) 13:1756284820915043. doi: 10.1177/1756284820915043
25. Wilson A, Peel C, Wang Q, Pananos AD, Kim RB. HLADQA1*05 genotype predicts anti-drug antibody formation and loss of response during infliximab therapy for inflammatory bowel disease. Aliment Pharmacol Ther. (2020) 51:356–63. doi: 10.1111/apt.15563
26. Sazonovs A, Kennedy NA, Moutsianas L, Heap GA, Rice DL, Reppell M, et al. HLA-DQA1*05 carriage associated with development of anti-drug antibodies to infliximab and adalimumab in patients with crohn's disease. Gastroenterology. (2020) 158:189–99. doi: 10.1053/j.gastro.2019.09.041
27. Kennedy NA, Heap GA, Green HD, Hamilton B, Bewshea C, Walker GJ, et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn's disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. (2019) 4:341–53. doi: 10.1016/S2468-1253(19)30012-3
28. Hazlewood GS, Rezaie A, Borman M, Panaccione R, Ghosh S, Seow CH, et al. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn's disease: a network meta-analysis. Gastroenterology. (2015) 148:344–54.e5. doi: 10.1053/j.gastro.2014.10.011
29. Strand V, Balsa A, Al-Saleh J, Barile-Fabris L, Horiuchi T, Takeuchi T, et al. Immunogenicity of biologics in chronic inflammatory diseases: a systematic review. BioDrugs. (2017) 31:299–316. doi: 10.1007/s40259-017-0231-8
Keywords: IBD, TDM (therapeutic drug monitoring), anti-TNF agent, sex, immunogenicity
Citation: Shehab M, Alasfour H, Abdullah I, Alhendi G, Alhadab A, Alfadhli A, Ziyab AH and Battat R (2021) Relationship Between Patient Sex and Serum Tumor Necrosis Factor Antagonist Drug and Anti-drug Antibody Concentrations in Inflammatory Bowel Disease; A Nationwide Cohort Study. Front. Med. 8:801532. doi: 10.3389/fmed.2021.801532
Received: 25 October 2021; Accepted: 06 December 2021;
Published: 23 December 2021.
Edited by:
Antonietta G. Gravina, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Borja Hernández-Breijo, University Hospital La Paz Research Institute (IdiPAZ), SpainIvan Guerra, Fuenlabrada University Hospital, Spain
Copyright © 2021 Shehab, Alasfour, Abdullah, Alhendi, Alhadab, Alfadhli, Ziyab and Battat. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad Shehab, dr_mshehab@hotmail.com
 Mohammad Shehab
Mohammad Shehab Hajer Alasfour2
Hajer Alasfour2  Israa Abdullah
Israa Abdullah