Of mice and men: neurogenesis, cognition, and Alzheimer’s disease
- 1Department of Anatomy and Cell Biology, College of Medicine, The University of Illinois at Chicago, Chicago, IL, USA
- 2Department of Neuroscience, Rosalind Franklin University of Medicine and Science, North Chicago, IL, USA
Neural stem cells are maintained in the subgranular layer of the dentate gyrus and in the subventricular zone in the adult mammalian brain throughout life. Neurogenesis is continuous, but its extent is tightly regulated by environmental factors, behavior, hormonal state, age, and brain health. Increasing evidence supports a role for new neurons in cognitive function in rodents. Recent evidence delineates significant similarities and differences between adult neurogenesis in rodents and humans. Being context-dependent, neurogenesis in the human brain might be manifested differently than in the rodent brain. Decline in neurogenesis may play a role in cognitive deterioration, leading to the development of progressive learning and memory disorders, such as Alzheimer’s disease. This review discusses the different observations concerning neurogenesis in the rodent and human brain, and their functional implications for the healthy and diseased brain.
Introduction
In the adult rodent brain, neural stem cells (NSC) in the subventricular zone (SVZ) and the subgranular layer (SGL) of the dentate gyrus (DG) give rise to new neurons and glia throughout life. From the SVZ, neural progenitor cells (NPC) migrate in chains through the rostral migratory stream (RMS), reach the olfactory bulb (OB) and incorporate there as mature neurons (Ihrie and Alvarez-Buylla, 2011). In the SGL, NPC migrate a short distance to the granular cell layer (GCL) of the DG and incorporate there as mature neurons (Yao et al., 2012). Similar observations were reported in the primate brain and in the fetal human brain (Kornack and Rakic, 2001; Pencea et al., 2001; Bedard et al., 2002; Sawamoto et al., 2011; Wang et al., 2011).
It is now established that neurogenesis takes place in the adult human brain. This was first described in the human hippocampus in post-mortem sections of cancer patients that were injected with 5-bromo-2′-deoxyuridine (BrdU; Eriksson et al., 1998). NSC exist in the human brain throughout life. Similar to rodents, human NPC, including those from hippocampus (Johansson et al., 1999; Kukekov et al., 1999; Palmer et al., 2001), SVZ (Johansson et al., 1999; Kukekov et al., 1999; Roy et al., 2000), OB (Pagano et al., 2000), forebrain subcortical white matter (Nunes et al., 2003), cortical and subcortical areas in the temporal lobe (Kirschenbaum et al., 1994), give rise to new neurons and glia. However, the fate and organization of these NPC, the extent of neurogenesis, and its course throughout adulthood are a matter of debate.
Subventricular Zone and Olfactory Bulb
Some studies observed neurogenesis in the OB and neuroblasts in the RMS (Bedard and Parent, 2004) and a remarkable resemblance between the mouse and human RMS through which NPC migrate from the SVZ to the OB during aging (Curtis et al., 2007). However, Wang et al. (2011) find an RMS-like in the adult human brain, but neuroblasts do not seem to get to the OB, and their fate along the ventral olfactory tract is unclear. Additionally, Wang et al. (2011) find only a small number of migratory neuroblasts in the SVZ and RMS and they do not form chains. Instead, possessing the typical migratory morphology, they move along as single cells or as pairs. These migrating neuroblasts express the immature neuronal markers doublecortin (DCX), polysialylated neural cell adhesion molecule (PSA-NCAM) and class III beta-tubulin (Tuj1) and some of them express proliferation markers (e.g., Ki67; Wang et al., 2011). Several studies describe a ribbon of astrocytes that lines the lateral ventricle in the adult human brain (Sanai et al., 2004; Quinones-Hinojosa et al., 2006). Based on proliferating cell nuclear antigen (PCNA) and Ki67 expression, some of these astrocytes seem to proliferate, but do not migrate in chains, and only a small number of them express Tuj1 and exhibit migratory morphology (Sanai et al., 2004; Quinones-Hinojosa et al., 2006). While exhibiting multipotency in vitro, neuroblasts derived from astrocytes in the SVZ do not seem to migrate to the OB (Sanai et al., 2004). Follow up studies suggest that active neurogenesis takes place in the post-natal SVZ up to 6 months of age, and then declines drastically (Sanai et al., 2011). Furthermore, in infants there is an additional migratory stream of DCX(+) cells ending in the ventro-medial pre-frontal cortex (VMPFC). This medial migratory stream (MMS) was observed in human specimens ages 4–6 months but not 8–18 months (Sanai et al., 2011). It should be noted that both the Sanai and Wang studies did not observe any neuroblasts in the adult human OB (Sanai et al., 2011; Wang et al., 2011). Supporting this, examination of neurogenesis in the adult human OB using nuclear 14C levels as a measure of cell birth date reveals neglectable neuronal proliferation (Bergmann et al., 2012). Taken together, it seems that while the number of NPC in the human SVZ seems to be substantial, they do not give rise to new olfactory neurons and their fate is unknown (Figure 1; Table 1).
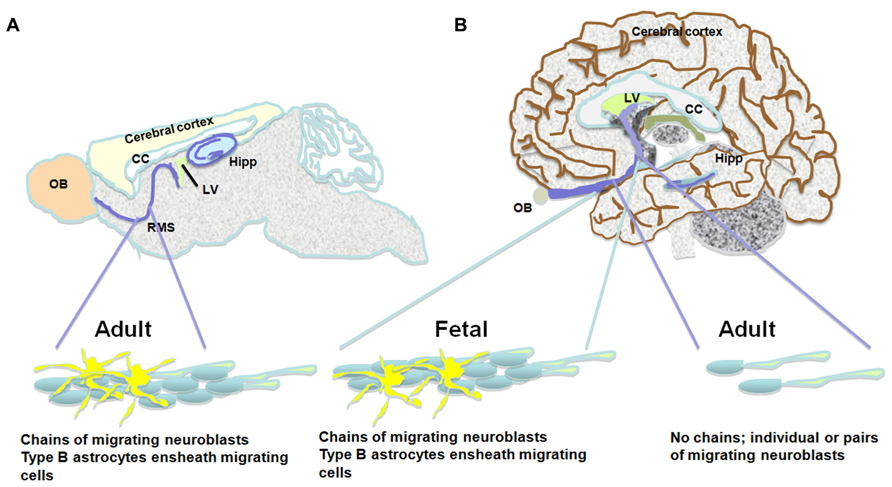
FIGURE 1. The pathway between the subventricular zone and the olfactory bulb in the brains of the adult mouse, the fetal and adult human. Schematic presentation of suggested differences between migration of neuroblasts in the mouse and adult human brain. (A) The rostral migratory stream in the subventricular zone of the mouse brain is composed of chains of migrating neuroblasts ensheathed by type B astrocytes. (B) Similar migratory chains are seen in the fetal human brain. However, the existence of such chains in the adult human is highly controversial. An alternative observation suggests that a low number of neuroblasts migrate toward the olfactory bulb as single cells or in pairs. Both neurogenic niches, the subventricular zone and the subgranular layer of the dentate gyrus are indicated in the scheme.
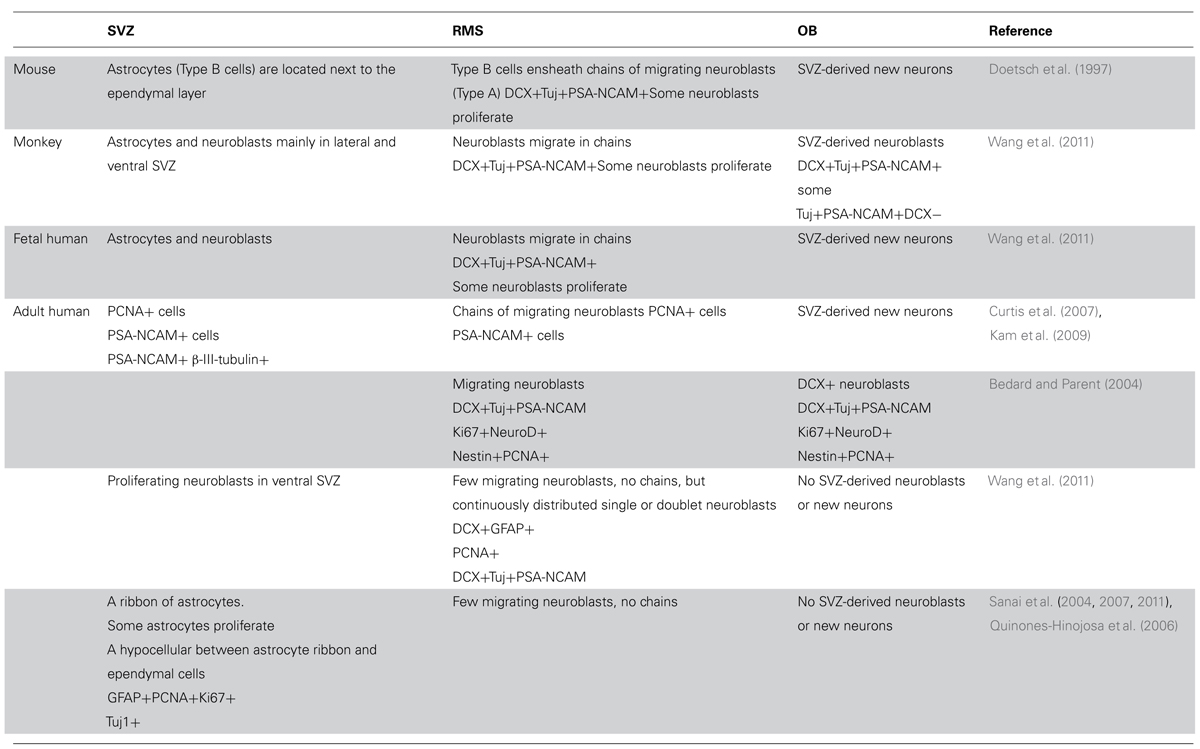
TABLE 1. A summary of observations about the cellular population and its fate in the neurogenic niche of the subventricular zone in the evolutionary path of the mouse, monkey, and human.
Subgranular Layer and Dentate Gyrus
A similar methodology used to assess the generation of hippocampal cells in humans revealed substantial neurogenesis throughout life in the human hippocampus with an estimate of 700 new neurons added to the granular layer of the DG a day (Spalding et al., 2013). This suggests a comparable extent of neurogenesis in humans and rodents and supports a major role for neurogenesis in the human DG. Similar to other mammals, the extent of hippocampal neurogenesis seems to decline exponentially with age in humans (Ninkovic et al., 2007; Imayoshi et al., 2009; Knoth et al., 2010; Spalding et al., 2013). However, a comparative study suggests that long-lived animals (e.g., primates and foxes) have significantly fewer proliferating NPC compared to rodents (Amrein et al., 2011). Additionally, the decline in neurogenesis in early adulthood seems to be greater in the mouse compared to the human hippocampus (Ninkovic et al., 2007; Imayoshi et al., 2009; Spalding et al., 2013). Interestingly, while neuroblasts are detected throughout life, the number of neuroblasts expressing proliferation markers in the human hippocampus declines dramatically in mid-life (Knoth et al., 2010). There is a notable difference in the exchange rate of neurons in the DG between rodents and humans. In rodents, new neurons add to the GCL, rather than replace dying neurons. As a result, the number of granular neurons increases over time (Bayer et al., 1982; Ninkovic et al., 2007; Imayoshi et al., 2009). In humans there is a preferential loss of new neurons and a larger proportion of hippocampal neurons are subject to exchange compared to mice (Ninkovic et al., 2007; Imayoshi et al., 2009; Spalding et al., 2013). Similar to the SVZ, the number of NPC and neuroblasts present in the adult human hippocampus seems to be small compared to the number of these cells post-natally. Intriguingly, the density of neuroblasts in the SVZ is similar to that in the DG in the human brain, and yet, SVZ-derived new neurons are not incorporated in the OB. Taken together, this suggests that the rate of survival of NPC, their recruitment, and neuronal maturation must be substantial in the adult human hippocampus.
The Functions of Neurogenesis are Context-Dependent
The differences between rodent and human neurogenesis are not surprising. Phylogenetically, the extent, location, and distribution of adult neurogenesis reflects the distinct physiological provisions of various species and different brain regions (reviewed in Grandel and Brand, 2013). Unlike rodents, which display robust olfactory neurogenesis, olfaction is less consequential in humans, perhaps reflecting reduced demand. Curiously, NPC seem to be present in the adult human SVZ in substantial numbers, suggesting that they play a role in the adult human brain or are a vestigial population. Hippocampal neurogenesis, on the other hand, contributes to highly complex learning and environmental adaptation and this might be fortified in humans. Grandel and Brand (2013) have recently produced a comprehensive review summarizing the comparative aspects of adult neurogenesis among vertebrate species. The process of adult neurogenesis is a trait present in many vertebrate species including stingrays (Dasyatis sabina) indicating that this is an ancient process present even before the divergence of cartilaginous and bony vertebrates (Coggeshall et al., 1978). A correlation can be drawn between neurogenesis and neural function from the extensive work done on songbirds. Robust seasonal neurogenesis is seen in the high vocal center (HCV) nucleus in which fluctuations in neural cell number correlate with seasonal song activity, while in other species of birds, classified as food catching species, behavioral stimulation is manifested by increased hippocampal neurogenesis (Barnea and Pravosudov, 2011). Fluctuations in HCV cell number correlating with learning new songs is well documented in the canary (Serinus canaria) which change their song seasonally. Variations on this theme include the song sparrow (Melospiza melodia), which displays a fixed song repertoire size but shows seasonal modifications (Smith et al., 1997). Furthermore, comparisons within a species suggest a functional link between vocal performance and neural cell number in the HCV. These examples support a role for enhanced neurogenesis in maintaining or supporting complex behaviors. However, it has been reported that zebra finches (Taeniopygia guttata) show only a steady increase in neuronal number independent of the season (Walton et al., 2012). It is notable that this species does not change their song seasonally, perhaps reflecting reduced behavioral plasticity.
One commonality between birds, rodents, and humans is the presence of adult neurogenesis in the DG. As discussed above, neurogenesis in this region is believed to contribute to learning/memory, adaptive behavior, and plasticity. The hippocampus is particularly important for spatial/declarative memories which assist all vertebrate species with environmental complexity and complex social interactions. However, there are species of bats that do not show hippocampal neurogenesis and are highly social animals who live in a complex environment (Amrein et al., 2007). Furthermore, these animals are not deficient in this process as they retain strong olfactory neurogenesis.
Why it might be advantageous to retain neurogenesis in the specific regions of the SGL and SVZ is one area of continued investigation. It is plausible that neurogenesis is optimal for facilitating functions related to areas of particularly high complexity and variability, such as discrete odors and spatial/temporal memory. Based on studies in rodents, new hippocampal neurons play a role in several cognitive functions, such as spatial memory (reviewed in Lazarov et al., 2010) and pattern separation (Sahay et al., 2011). Their enhanced plasticity and distinct characteristics make them suitable for the acquisition of pattern separation and cognitive adaptation to novel experiences (Kempermann et al., 1997). This function requiring the ability to store closely related experiences as separate memories, complements the function of old neurons in the DG in the association of closely related memories (Clelland et al., 2009; Sahay et al., 2011). Several factors may affect and/or reflect differences in the functional significance of neurogenesis in the rodent and human brain. That may include the ratio of the number of new neurons to the number of older neurons, their rate of survival, the frequency of their use or induction, and the recruiting stimuli (Kempermann, 2012). In that regard, recent studies suggest that the extent of human hippocampal neurogenesis may be comparable to that of a middle-aged mouse, thus should be sufficient for cognitive tasks in humans, as it is in the mouse (Spalding et al., 2008, 2013). In the mouse brain, the NSC-progeny ratio in the hippocampus is indicative of the animal’s activity and experience (Dranovsky et al., 2011), suggesting that formation of new neurons and their recruitment is context-dependent.
Alterations in Neurogenesis with Age: From Rodents to Humans
An important debate is over the fate of neurogenesis during the human lifespan. In rodents, adult neurogenesis is present in the aged brain but is dramatically reduced in early adulthood in both the SVZ (Mirich et al., 2002; Shook et al., 2012) and SGL (Kuhn et al., 1996; Cameron and McKay, 1999; Bernal and Peterson, 2004; Bondolfi et al., 2004; Kronenberg et al., 2006; Ben Abdallah et al., 2010; Encinas et al., 2011; Miranda et al., 2012). There is about 80% reduction in neuroblasts during the transition from young adult (2-months) to mid-age (7–9 months) in mice (Demars et al., 2013), and a similar reduction from adult (4-months) to older (12-months) age in rats (Kuhn et al., 1996; Nacher et al., 2003; Rao et al., 2006). After this period of dramatic reductions, the rate of decline is substantially reduced (Rao et al., 2005) though the number of new neurons continues to decline (Demars et al., 2013). This may manifest in deficits in olfactory and hippocampal-dependent function (Bizon et al., 2004; Enwere et al., 2004; Dupret et al., 2008). Nevertheless, the mechanism(s) underlying age-dependent neurogenic decline is controversial. Evidence exists suggesting that the decline is due to reduced number of proliferating and differentiating cells with age (Kuhn et al., 1996; Heine et al., 2004; Rao et al., 2005; Morgenstern et al., 2008; Demars et al., 2013), alterations in NPC cell cycle length in the SGL (Olariu et al., 2007), loss of NSC by their conversion into mature hippocampal astrocytes (Bonaguidi et al., 2011; Encinas et al., 2011), upregulation of signals suppressing self-renewal of NSC (Bonaguidi et al., 2008) or trophic levels (Hattiangady et al., 2005; Shetty et al., 2005; Bernal and Peterson, 2011), and increased NSC quiescence due to a decline in vascularity (Hattiangady and Shetty, 2008; Figure 2). A quantitative inter- and intra-species comparison among rodents, carnivores, and primates suggest an exponential decline in NPC proliferation that is independent on life span, but is chronologically equal (Amrein et al., 2011). Whether a decline in hippocampal neurogenesis takes place at the same pace in the human brain is not clear. An age-dependent decline in expression of proliferation factors in the human hippocampus suggests a decline in the number of proliferating NPC as a function of age (Knoth et al., 2010). Expression of neurogenic markers that are used in rodents for the detection of NPC, such as DCX, are present in the human SGL throughout life. However, the number of DCX decreases as a function of age (Knoth et al., 2010). Assessment of the extent of hippocampal neurogenesis throughout the human life span using nuclear levels of 14C reveals that hippocampal neurogenesis declines dramatically in the first year of life with only a modest decline thereafter (Spalding et al., 2013).
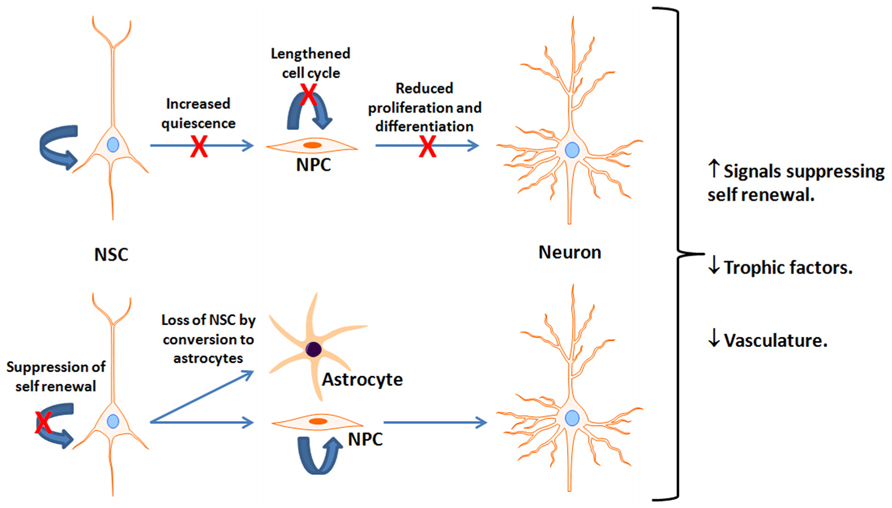
FIGURE 2. Potential mechanisms for reduced neurogenesis with aging. This figure depicts reported age-associated deficits or suppressive factors interfering with neurogenesis. It is unclear which is most prominent and is likely a combination of multiple factors. NSC, Neural stem cells; NPC, neural progenitor cells; X indicates a reduction in the indicated pathway.
Cognitive Consequences of Reduced Neurogenesis with Age
Lesion studies in neurogenic areas using radiation, cytostatic/cytotoxic agents, or transgenic approaches have produced deficits in learning and memory (Shors et al., 2001; Winocur et al., 2006; Dupret et al., 2008; Imayoshi et al., 2008; Kim et al., 2008). Zhang et al. (2008) showed that suppression of neurogenesis produced deficits in hippocampal-dependent learning while not affecting other cognitive domains. A more recent study used both irradiation and genetic ablation of NSC and found that acquisition of avoidance behavior of a shock zone was unimpaired; however, the ability to then adapt and learn the location after changing shock location was impaired (Burghardt et al., 2012). Irradiated mice were impaired in the rotating shock location test only if their initial training was in a fixed shock location. Taken together, this shows that neurogenesis plays a significant role in affecting the ability to distinguish between multiple similar memories.
The connection of neurogenesis to cognition is also supported by the general observation that both hippocampal-dependent memory performance and neurogenesis decline with age. However, a clear and direct link between neurogenesis and learning/memory with aging appears to be complicated. Intra-group comparisons show clear positive correlations between cognitive function and neurogenesis. While performance in hippocampal-dependent learning is clearly reduced with age, the correlation with levels of residual neurogenesis becomes more complicated (reviewed in Couillard-Despres et al., 2011). The extent of neuroblast formation along with survival and differentiation is correlated with age-dependent learning/memory in rats (Drapeau et al., 2003; Driscoll et al., 2006). However, other studies have found that neurogenesis is not correlated or is inversely correlated with memory performance in aged rats (Bizon and Gallagher, 2003; Merrill et al., 2003; Bizon et al., 2004). It is noteworthy that chronic reductions in neurogenesis compromises the morphology and function of other hippocampal areas, such as CA3 (Schloesser et al., 2013), or other brain regions.
Importantly, neither in rodents nor in humans, it is not clear whether the exchange rate or the ratio of new neurons to old neurons changes as a function of age. Current available methodology may not allow such detection. Furthermore, it is not clear what would be the critical neurogenic parameter to reflect age-dependent reduction in neurogenesis that correlates with cognitive decline. Changes have been noted in the volume of the molecular layer of the DG, with the medial layer thinning and the inner layer showing increased volume with age (Rapp et al., 1999). This may simply reflect fewer connections from the entorhinal cortex (medial layer) and a greater level of connection with CA3 of the hippocampus. Similar reorganizations may occur in humans. Studies in adult and elderly people with similar cognitive function have shown reduced activity by functional magnetic resonance imaging (fMRI) in the medial temporal regions while an increase in activity was found in the parietal and prefrontal cortex with age (Burgmans et al., 2010).
Neurogenesis and Cognitive Failure in Alzheimer’s Disease
Many of the molecular players in Alzheimer’s disease (AD) are also modulators of neurogenesis. Therefore, it is not surprising that these sets of processes influence each other (reviewed in Lazarov and Marr, 2010; Lazarov et al., 2010). The most prominent players are presenilin-1 (PS1) and soluble amyloid precursor protein α (sAPPα). Mutations in PSEN1 and APP cause familial AD. PS1 regulates NPC differentiation (Gadadhar et al., 2011) while sAPPα regulates NPC proliferation (Caille et al., 2004; Gakhar-Koppole et al., 2008; Rohe et al., 2008; Demars et al., 2011, 2013). Also, PS1 is the catalytic core of the aspartyl protease γ-secretase that cleaves numerous neurogenic substrates including Notch-1. FAD-linked mutations in PS1 have also been found to suppress neurogenesis. α-secretase activities [primarily the ADAM (a disintegrin and metalloprotease) proteases] that produce the sAPPα product from APP also cleave important substrates like Notch-1 and components of epidermal growth factor (EGF) signaling. Furthermore, certain ADAM family members (TACE, ADAM21) are expressed in the SVZ (Yang et al., 2005, 2006; Katakowski et al., 2007). Thus, mutations associated with AD that alter the production of these metabolites or the activities of their processing enzymes can also alter neurogenesis. There are a considerable number of studies that have examined the association of AD pathology with neurogenesis in transgenic mouse models of the disease. Comprehensive summaries can be found elsewhere (Chuang, 2010; Lazarov and Marr, 2010; Winner et al., 2011). Nevertheless, there are a limited number of somewhat contradictory studies addressing the role of neurogenesis in the human disease using post-mortem tissue. Thus, the role of neurogenesis in AD is still a matter of some debate mainly because of lack of evidence that impairments in neurogenesis induce AD-like cognitive deficits, and inversely, that therapy enhancing neurogenic function can ameliorate AD. Importantly, very little information is available about the course and fate of neurogenesis in humans, in normal and pathological aging. In fact, studies in a large cohort of individuals and more substantial experimental tools that will enable the detection of real-time neurogenesis, such as by live imaging, will be required to understand the role of neurogenesis in human cognitive deficit. Based on current observations concerning the differences in adult neurogenesis between mouse and human, these experiments will be instrumental for the determination of the role of neurogenesis in AD.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Amrein, I., Dechmann, D. K., Winter, Y., and Lipp, H. P. (2007). Absent or low rate of adult neurogenesis in the hippocampus of bats (Chiroptera). PLoS ONE 2:e455. doi: 10.1371/journal.pone.0000455
Amrein, I., Isler, K., and Lipp, H. P. (2011). Comparing adult hippocampal neurogenesis in mammalian species and orders: influence of chronological age and life history stage. Eur. J. Neurosci. 34, 978–987. doi: 10.1111/j.1460-9568.2011.07804.x
Barnea, A., and Pravosudov, V. (2011). Birds as a model to study adult neurogenesis: bridging evolutionary, comparative and neuroethological approaches. Eur. J. Neurosci. 34, 884–907. doi: 10.1111/j.1460-9568.2011.07851.x
Bayer, S. A., Yackel, J. W., and Puri, P. S. (1982). Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science 216, 890–892. doi: 10.1126/science.7079742
Bedard, A., Levesque, M., Bernier, P. J., and Parent, A. (2002). The rostral migratory stream in adult squirrel monkeys: contribution of new neurons to the olfactory tubercle and involvement of the antiapoptotic protein Bcl-2. Eur. J. Neurosci. 16, 1917–1924. doi: 10.1046/j.1460-9568.2002.02263.x
Bedard, A., and Parent, A. (2004). Evidence of newly generated neurons in the human olfactory bulb. Brain Res. Dev. Brain Res. 151, 159–168. doi: 10.1016/j.devbrainres.2004.03.021
Ben Abdallah, N. M., Slomianka, L., Vyssotski, A. L., and Lipp, H. P. (2010). Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol. Aging 31, 151–161. doi: 10.1016/j.neurobiolaging.2008.03.002
Bergmann, O., Liebl, J., Bernard, S., Alkass, K., Yeung, M. S., Steier, P., et al. (2012). The age of olfactory bulb neurons in humans. Neuron 74, 634–639. doi: 10.1016/j.neuron.2012.03.030
Bernal, G. M., and Peterson, D. A. (2004). Neural stem cells as therapeutic agents for age-related brain repair. Aging Cell 3, 345–351. doi: 10.1111/j.1474-9728.2004.00132.x
Bernal, G. M., and Peterson, D. A. (2011). Phenotypic and gene expression modification with normal brain aging in GFAP-positive astrocytes and neural stem cells. Aging Cell 10, 466–482. doi: 10.1111/j.1474-9726.2011.00694.x
Bizon, J. L., and Gallagher, M. (2003). Production of new cells in the rat dentate gyrus over the lifespan: relation to cognitive decline. Eur. J. Neurosci. 18, 215–219. doi: 10.1046/j.1460-9568.2003.02733.x
Bizon, J. L., Lee, H. J., and Gallagher, M. (2004). Neurogenesis in a rat model of age-related cognitive decline. Aging Cell 3, 227–234. doi: 10.1111/j.1474-9728.2004.00099.x
Bonaguidi, M. A., Peng, C. Y., Mcguire, T., Falciglia, G., Gobeske, K. T., Czeisler, C., et al. (2008). Noggin expands neural stem cells in the adult hippocampus. J. Neurosci. 28, 9194–9204. doi: 10.1523/JNEUROSCI.3314-07.2008
Bonaguidi, M. A., Wheeler, M. A., Shapiro, J. S., Stadel, R. P., Sun, G. J., Ming, G. L., et al. (2011). In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 145, 1142–1155. doi: 10.1016/j.cell.2011.05.024
Bondolfi, L., Ermini, F., Long, J. M., Ingram, D. K., and Jucker, M. (2004). Impact of age and caloric restriction on neurogenesis in the dentate gyrus of C57BL/6 mice. Neurobiol. Aging 25, 333–340. doi: 10.1016/S0197-4580(03)00083-6
Burghardt, N. S., Park, E. H., Hen, R., and Fenton, A. A. (2012). Adult-born hippocampal neurons promote cognitive flexibility in mice. Hippocampus 22, 1795–1808. doi: 10.1002/hipo.22013
Burgmans, S., Van Boxtel, M. P., Vuurman, E. F., Evers, E. A., and Jolles, J. (2010). Increased neural activation during picture encoding and retrieval in 60-year-olds compared to 20-year-olds. Neuropsychologia 48, 2188–2197. doi: 10.1016/j.neuropsychologia.2010.04.011
Caille, I., Allinquant, B., Dupont, E., Bouillot, C., Langer, A., Muller, U., et al. (2004). Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development 131, 2173–2181. doi: 10.1242/dev.01103
Cameron, H. A., and McKay, R. D. (1999). Restoring production of hippocampal neurons in old age. Nat. Neurosci. 2, 894–897. doi: 10.1038/13197
Chuang, T. T. (2010). Neurogenesis in mouse models of Alzheimer’s disease. Biochim. Biophys. Acta 1802, 872–880. doi: 10.1016/j.bbadis.2009.12.008
Clelland, C. D., Choi, M., Romberg, C., Clemenson, G. D. Jr., Fragniere, A., Tyers, P., et al. (2009). A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–213. doi: 10.1126/science.1173215
Coggeshall, R. E., Leonard, R. B., Applebaum, M. L., and Willis, W. D. (1978). Organization of peripheral nerves and spinal roots of the Atlantic stingray, Dasyatis sabina. J. Neurophysiol. 41, 97–107.
Couillard-Despres, S., Iglseder, B., and Aigner, L. (2011). Neurogenesis, cellular plasticity and cognition: the impact of stem cells in the adult and aging brain–a mini-review. Gerontology 57, 559–564. doi: 10.1159/000323481
Curtis, M. A., Kam, M., Nannmark, U., Anderson, M. F., Axell, M. Z., Wikkelso, C., et al. (2007). Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science 315, 1243–1249. doi: 10.1126/science.1136281
Demars, M. P., Bartholomew, A., Strakova, Z., and Lazarov, O. (2011). Soluble amyloid precursor protein: a novel proliferation factor of adult progenitor cells of ectodermal and mesodermal origin. Stem Cell Res. Ther. 2, 36. doi: 10.1186/scrt77
Demars, M. P., Hollands, C., Zhao, K. D., and Lazarov, O. (2013). Soluble amyloid precursor protein-alpha rescues age-linked decline in neural progenitor cell proliferation. Neurobiol. Aging 34, 2431–2440. doi: 10.1016/j.neurobiolaging.2013.04.016
Doetsch, F., Garcia-Verdugo, J. M., and Alvarez-Buylla, A. (1997). Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 17, 5046–5061.
Dranovsky, A., Picchini, A. M., Moadel, T., Sisti, A. C., Yamada, A., Kimura, S., et al. (2011). Experience dictates stem cell fate in the adult hippocampus. Neuron 70, 908–923. doi: 10.1016/j.neuron.2011.05.022
Drapeau, E., Mayo, W., Aurousseau, C., Le Moal, M., Piazza, P. V., and Abrous, D. N. (2003). Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc. Natl. Acad. Sci. U.S.A. 100, 14385–14390. doi: 10.1073/pnas.2334169100
Driscoll, I., Howard, S. R., Stone, J. C., Monfils, M. H., Tomanek, B., Brooks, W. M., et al. (2006). The aging hippocampus: a multi-level analysis in the rat. Neuroscience 139, 1173–1185. doi: 10.1016/j.neuroscience.2006.01.040
Dupret, D., Revest, J. M., Koehl, M., Ichas, F., De Giorgi, F., Costet, P., et al. (2008). Spatial relational memory requires hippocampal adult neurogenesis. PLoS ONE 3:e1959. doi: 10.1371/journal.pone.0001959
Encinas, J. M., Michurina, T. V., Peunova, N., Park, J. H., Tordo, J., Peterson, D. A., et al. (2011). Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 8, 566–579. doi: 10.1016/j.stem.2011.03.010
Enwere, E., Shingo, T., Gregg, C., Fujikawa, H., Ohta, S., and Weiss, S. (2004). Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J. Neurosci. 24, 8354–8365. doi: 10.1523/JNEUROSCI.2751-04.2004
Eriksson, P. S., Perfilieva, E., Bjork-Eriksson, T., Alborn, A. M., Nordborg, C., Peterson, D. A., et al. (1998). Neurogenesis in the adult human hippocampus. Nat. Med. 4, 1313–1317. doi: 10.1038/3305
Gadadhar, A., Marr, R. A., and Lazarov, O. (2011). Presenilin-1 regulates neural progenitor cell differentiation in the adult brain. J. Neurosci. 31, 2615–2623. doi: 10.1523/JNEUROSCI.4767-10.2011
Gakhar-Koppole, N., Hundeshagen, P., Mandl, C., Weyer, S. W., Allinquant, B., Muller, U., et al. (2008). Activity requires soluble amyloid precursor protein alpha to promote neurite outgrowth in neural stem cell-derived neurons via activation of the MAPK pathway. Eur. J. Neurosci. 28, 871–882. doi: 10.1111/j.1460-9568.2008.06398.x
Grandel, H., and Brand, M. (2013). Comparative aspects of adult neural stem cell activity in vertebrates. Dev. Genes Evol. 223, 131–147. doi: 10.1007/s00427-012-0425-5
Hattiangady, B., Rao, M. S., Shetty, G. A., and Shetty, A. K. (2005). Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp. Neurol. 195, 353–371. doi: 10.1016/j.expneurol.2005.05. 014
Hattiangady, B., and Shetty, A. K. (2008). Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol. Aging 29, 129–147. doi: 10.1016/j.neurobiolaging.2006.09.015
Heine, V. M., Maslam, S., Joels, M., and Lucassen, P. J. (2004). Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus–pituitary–adrenal axis activation. Neurobiol. Aging 25, 361–375. doi: 10.1016/S0197-4580(03)00090-3
Ihrie, R. A., and Alvarez-Buylla, A. (2011). Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain. Neuron 70, 674–686. doi: 10.1016/j.neuron.2011.05.004
Imayoshi, I., Sakamoto, M., Ohtsuka, T., and Kageyama, R. (2009). Continuous neurogenesis in the adult brain. Dev. Growth Differ. 51, 379–386. doi: 10.1111/j.1440-169X.2009.01094.x
Imayoshi, I., Sakamoto, M., Ohtsuka, T., Takao, K., Miyakawa, T., Yamaguchi, M., et al. (2008). Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat. Neurosci. 11, 1153–1161. doi: 10.1038/nn.2185
Johansson, C. B., Svensson, M., Wallstedt, L., Janson, A. M., and Frisen, J. (1999). Neural stem cells in the adult human brain. Exp. Cell Res. 253, 733–736. doi: 10.1006/excr.1999.4678
Kam, M., Curtis, M. A., Mcglashan, S. R., Connor, B., Nannmark, U., and Faull, R. L. (2009). The cellular composition and morphological organization of the rostral migratory stream in the adult human brain. J. Chem. Neuroanat. 37, 196–205. doi: 10.1016/j.jchemneu.2008.12.009
Katakowski, M., Chen, J., Zhang, Z. G., Santra, M., Wang, Y., and Chopp, M. (2007). Stroke-induced subventricular zone proliferation is promoted by tumor necrosis factor-alpha-converting enzyme protease activity. J. Cereb. Blood Flow Metab. 27, 669–678.
Kempermann, G. (2012). New neurons for ‘survival of the fittest’. Nat. Rev. Neurosci. 13, 727–736. doi: 10.1038/nrn3319
Kempermann, G., Kuhn, H. G., and Gage, F. H. (1997). More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–495. doi: 10.1038/386493a0
Kim, J. S., Lee, H. J., Kim, J. C., Kang, S. S., Bae, C. S., Shin, T., et al. (2008). Transient impairment of hippocampus-dependent learning and memory in relatively low-dose of acute radiation syndrome is associated with inhibition of hippocampal neurogenesis. J. Radiat. Res. 49, 517–526. doi: 10.1269/jrr. 08020
Kirschenbaum, B., Nedergaard, M., Preuss, A., Barami, K., Fraser, R. A., and Goldman, S. A. (1994). In vitro neuronal production and differentiation by precursor cells derived from the adult human forebrain. Cereb. Cortex 4, 576–589. doi: 10.1093/cercor/4.6.576
Knoth, R., Singec, I., Ditter, M., Pantazis, G., Capetian, P., Meyer, R. P., et al. (2010). Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS ONE 5:e8809. doi: 10.1371/journal.pone.0008809
Kornack, D. R., and Rakic, P. (2001). The generation, migration, and differentiation of olfactory neurons in the adult primate brain. Proc. Natl. Acad. Sci. U.S.A. 98, 4752–4757. doi: 10.1073/pnas.081074998
Kronenberg, G., Bick-Sander, A., Bunk, E., Wolf, C., Ehninger, D., and Kempermann, G. (2006). Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol. Aging 27, 1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016
Kuhn, H. G., Dickinson-Anson, H., and Gage, F. H. (1996). Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 16, 2027–2033.
Kukekov, V. G., Laywell, E. D., Suslov, O., Davies, K., Scheffler, B., Thomas, L. B., et al. (1999). Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp. Neurol. 156, 333–344. doi: 10.1006/exnr.1999.7028
Lazarov, O., and Marr, R. A. (2010). Neurogenesis and Alzheimer’s disease: at the crossroads. Exp. Neurol. 223, 267–281. doi: 10.1016/j.expneurol.2009.08.009
Lazarov, O., Mattson, M. P., Peterson, D. A., Pimplikar, S. W., and Van Praag, H. (2010). When neurogenesis encounters aging and disease. Trends Neurosci. 33, 569–579. doi: 10.1016/j.tins.2010.09.003
Merrill, D. A., Karim, R., Darraq, M., Chiba, A. A., and Tuszynski, M. H. (2003). Hippocampal cell genesis does not correlate with spatial learning ability in aged rats. J. Comp. Neurol. 459, 201–207. doi: 10.1002/cne.10616
Miranda, C. J., Braun, L., Jiang, Y., Hester, M. E., Zhang, L., Riolo, M., et al. (2012). Aging brain microenvironment decreases hippocampal neurogenesis through Wnt-mediated survivin signaling. Aging Cell 11, 542–552. doi: 10.1111/j.1474-9726.2012.00816.x
Mirich, J. M., Williams, N. C., Berlau, D. J., and Brunjes, P. C. (2002). Comparative study of aging in the mouse olfactory bulb. J. Comp. Neurol. 454, 361–372. doi: 10.1002/cne. 10426
Morgenstern, N. A., Lombardi, G., and Schinder, A. F. (2008). Newborn granule cells in the ageing dentate gyrus. J. Physiol. 586, 3751–3757. doi: 10.1113/jphysiol.2008.154807
Nacher, J., Alonso-Llosa, G., Rosell, D. R., and Mcewen, B. S. (2003). NMDA receptor antagonist treatment increases the production of new neurons in the aged rat hippocampus. Neurobiol. Aging 24, 273–284. doi: 10.1016/S0197-4580(02)00096-9
Ninkovic, J., Mori, T., and Gotz, M. (2007). Distinct modes of neuron addition in adult mouse neurogenesis. J. Neurosci. 27, 10906–10911. doi: 10.1523/JNEUROSCI.2572-07.2007
Nunes, M. C., Roy, N. S., Keyoung, H. M., Goodman, R. R., Mckhann, G. II, Jiang, L., et al. (2003). Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat. Med. 9, 439–447. doi: 10.1038/nm837
Olariu, A., Cleaver, K. M., and Cameron, H. A. (2007). Decreased neurogenesis in aged rats results from loss of granule cell precursors without lengthening of the cell cycle. J. Comp. Neurol. 501, 659–667. doi: 10.1002/cne.21268
Pagano, S. F., Impagnatiello, F., Girelli, M., Cova, L., Grioni, E., Onofri, M., et al. (2000). Isolation and characterization of neural stem cells from the adult human olfactory bulb. Stem Cells 18, 295–300. doi: 10.1634/stemcells.18-4-295
Palmer, T. D., Schwartz, P. H., Taupin, P., Kaspar, B., Stein, S. A., and Gage, F. H. (2001). Cell culture. Progenitor cells from human brain after death. Nature 411, 42–43. doi: 10.1038/3507 5141
Pencea, V., Bingaman, K. D., Freedman, L. J., and Luskin, M. B. (2001). Neurogenesis in the subventricular zone and rostral migratory stream of the neonatal and adult primate forebrain. Exp. Neurol. 172, 1–16. doi: 10.1006/exnr.2001.7768
Quinones-Hinojosa, A., Sanai, N., Soriano-Navarro, M., Gonzalez-Perez, O., Mirzadeh, Z., Gil-Perotin, S., et al. (2006). Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. J. Comp. Neurol. 494, 415–434. doi: 10.1002/cne.20798
Rao, M. S., Hattiangady, B., Abdel-Rahman, A., Stanley, D. P., and Shetty, A. K. (2005). Newly born cells in the ageing dentate gyrus display normal migration, survival and neuronal fate choice but endure retarded early maturation. Eur. J. Neurosci. 21, 464–476. doi: 10.1111/j.1460-9568.2005.03853.x
Rao, M. S., Hattiangady, B., and Shetty, A. K. (2006). The window and mechanisms of major age-related decline in the production of new neurons within the dentate gyrus of the hippocampus. Aging Cell 5, 545–558. doi: 10.1111/j.1474-9726.2006.00243.x
Rapp, P. R., Stack, E. C., and Gallagher, M. (1999). Morphometric studies of the aged hippocampus: I. Volumetric analysis in behaviorally characterized rats. J. Comp. Neurol. 403, 459–470. doi: 10.1002/(SICI)1096-9861(19990125)403:4<459::AID-CNE3>3.0.CO;2-9
Rohe, M., Carlo, A. S., Breyhan, H., Sporbert, A., Militz, D., Schmidt, V., et al. (2008). Sortilin-related receptor with A-type repeats (SORLA) affects the amyloid precursor protein-dependent stimulation of ERK signaling and adult neurogenesis. J. Biol. Chem. 283, 14826–14834. doi: 10.1074/jbc.M710574200
Roy, N. S., Benraiss, A., Wang, S., Fraser, R. A., Goodman, R., Couldwell, W. T., et al. (2000). Promoter-targeted selection and isolation of neural progenitor cells from the adult human ventricular zone. J. Neurosci. Res. 59, 321–331. doi: 10.1002/(SICI)1097-4547(20000201)59:3<321::AID-JNR5>3.0.CO;2-9
Sahay, A., Scobie, K. N., Hill, A. S., O’carroll, C. M., Kheirbek, M. A., Burghardt, N. S., et al. (2011). Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472, 466–470. doi: 10.1038/nature09817
Sanai, N., Berger, M. S., Garcia-Verdugo, J. M., and Alvarez-Buylla, A. (2007). Comment on “Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension”. Science 318, 393; author reply 393. doi: 10.1126/science.1145164
Sanai, N., Nguyen, T., Ihrie, R. A., Mirzadeh, Z., Tsai, H. H., Wong, M., et al. (2011). Corridors of migrating neurons in the human brain and their decline during infancy. Nature 478, 382–386. doi: 10.1038/nature10487
Sanai, N., Tramontin, A. D., Quinones-Hinojosa, A., Barbaro, N. M., Gupta, N., Kunwar, S., et al. (2004). Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 427, 740–744. doi: 10.1038/nature02301
Sawamoto, K., Hirota, Y., Alfaro-Cervello, C., Soriano-Navarro, M., He, X., Hayakawa-Yano, Y., et al. (2011). Cellular composition and organization of the subventricular zone and rostral migratory stream in the adult and neonatal common marmoset brain. J. Comp. Neurol. 519, 690–713. doi: 10.1002/cne. 22543
Schloesser, R. J., Jimenez, D. V., Hardy, N. F., Paredes, D., Catlow, B. J., Manji, H. K., et al. (2013). Atrophy of pyramidal neurons and increased stress-induced glutamate levels in CA3 following chronic suppression of adult neurogenesis. Brain Struct. Funct. doi: 10.1007/s00429-013-0532-8 [Epub ahead of print].
Shetty, A. K., Hattiangady, B., and Shetty, G. A. (2005). Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia 51, 173–186. doi: 10.1002/glia.20187
Shook, B. A., Manz, D. H., Peters, J. J., Kang, S., and Conover, J. C. (2012). Spatiotemporal changes to the subventricular zone stem cell pool through aging. J. Neurosci. 32, 6947–6956. doi: 10.1523/JNEUROSCI.5987-11.2012
Shors, T. J., Miesegaes, G., Beylin, A., Zhao, M., Rydel, T., and Gould, E. (2001). Neurogenesis in the adult is involved in the formation of trace memories. Nature 410, 372–376. doi: 10.1038/35066584
Smith, G. T., Brenowitz, E. A., Beecher, M. D., and Wingfield, J. C. (1997). Seasonal changes in testosterone, neural attributes of song control nuclei, and song structure in wild songbirds. J. Neurosci. 17, 6001–6010.
Spalding, K. L., Arner, E., Westermark, P. O., Bernard, S., Buchholz, B. A., Bergmann, O., et al. (2008). Dynamics of fat cell turnover in humans. Nature 453, 783–787. doi: 10.1038/nature06902
Spalding, K. L., Bergmann, O., Alkass, K., Bernard, S., Salehpour, M., Huttner, H. B., et al. (2013). Dynamics of hippocampal neurogenesis in adult humans. Cell 153, 1219–1227. doi: 10.1016/j.cell.2013.05.002
Walton, C., Pariser, E., and Nottebohm, F. (2012). The zebra finch paradox: song is little changed, but number of neurons doubles. J. Neurosci. 32, 761–774. doi: 10.1523/JNEUROSCI.3434-11.2012
Wang, C., Liu, F., Liu, Y. Y., Zhao, C. H., You, Y., Wang, L., et al. (2011). Identification and characterization of neuroblasts in the subventricular zone and rostral migratory stream of the adult human brain. Cell Res. 21, 1534–1550. doi: 10.1038/cr.2011.83
Winner, B., Kohl, Z., and Gage, F. H. (2011). Neurodegenerative disease and adult neurogenesis. Eur. J. Neurosci. 33, 1139–1151. doi: 10.1111/j.1460-9568.2011.07613.x
Winocur, G., Wojtowicz, J. M., Sekeres, M., Snyder, J. S., and Wang, S. (2006). Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus 16, 296–304. doi: 10.1002/hipo.20163
Yang, P., Baker, K. A., and Hagg, T. (2005). A disintegrin and metalloprotease 21 (ADAM21) is associated with neurogenesis and axonal growth in developing and adult rodent CNS. J. Comp. Neurol. 490, 163–179. doi: 10.1002/cne.20659
Yang, P., Baker, K. A., and Hagg, T. (2006). The ADAMs family: coordinators of nervous system development, plasticity and repair. Prog. Neurobiol. 79, 73–94. doi: 10.1016/j.pneurobio.2006.05.001
Yao, J., Mu, Y., and Gage, F. H. (2012). Neural stem cells: mechanisms and modeling. Protein Cell 3, 251–261. doi: 10.1007/s13238-012-2033-6
Keywords: cognition, learning and memory, aging, Alzheimer’s disease, neurodegenerative disease
Citation: Lazarov O and Marr RA (2013) Of mice and men: neurogenesis, cognition, and Alzheimer’s disease. Front. Aging Neurosci. 5:43. doi: 10.3389/fnagi.2013.00043
Received: 07 June 2013; Paper pending published: 06 July 2013;
Accepted: 04 August 2013; Published online: 27 August 2013.
Edited by:
Philip B. Gorelick, University of Chicago, USAReviewed by:
Ashok K. Shetty, Texas A&M Health Science Center, USAPhilip B. Gorelick, University of Chicago, USA
Copyright © 2013 Lazarov and Marr. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Orly Lazarov, Department of Anatomy and Cell Biology, College of Medicine, The University of Illinois at Chicago, 808 South Wood Street, Chicago, IL 60612, USA e-mail: olazarov@uic.edu; Robert A. Marr, Department of Neuroscience, Rosalind Franklin University of Medicine and Science, 3333 Green Bay Road, North Chicago, IL 60064, USA e-mail: robert.marr@rosalindfranklin.edu