Age-related hearing impairment and frailty in Alzheimer's disease: interconnected associations and mechanisms
- 1Neurodegenerative Disease Unit, Department of Basic Medicine, Neuroscience, and Sense Organs, University of Bari Aldo Moro, Bari, Italy
- 2Department of Clinical Research in Neurology, University of Bari Aldo Moro, “Pia Fondazione Cardinale G. Panico”, Tricase, Italy
- 3Geriatric Unit, Laboratory of Gerontology and Geriatrics, Department of Medical Sciences, IRCCS “Casa Sollievo della Sofferenza”, San Giovanni Rotondo, Italy
- 4Geriatric Medicine-Memory Unit and Rare Disease Centre, Interdisciplinary Medicine Department, University of Bari Aldo Moro, Bari, Italy
- 5Research and Development Department, Chiesi Farmaceutici, Parma, Italy
- 6Otolaryngology Unit, Department of Basic Medical Science, Neuroscience and Sensory Organs, University of Bari Aldo Moro, Bari, Italy
- 7Geriatrics Unit, Department of OrthoGeriatrics, Rehabilitation and Stabilitation, Frailty Area, Galliera Hospital NR-HS, Genova, Italy
Among potentially modifiable age-related conditions linked to dementia, Alzheimer's disease (AD), and late-life cognitive disorders, age-related hearing impairment (ARHI) or presbycusis is the most widely diffused sensory disorder and one of the principal causes of chronic disability in older adults (Gates and Mills, 2005). The impairments of peripheral (sensory or strial) and central (predominantly neural) auditory pathways, diagnosed with different procedures, are often variously imbricated in determining ARHI, with mixed clinical findings (Gates and Mills, 2005). A growing body of epidemiological evidence linking ARHI with late-life cognitive disorders (Panza et al., 2015a) suggested the potential for correcting hearing loss so that elders can function better also from a cognitive point of view with appropriate treatment.
ARHI is also a substantial marker for frailty in older age, another age-related clinical condition for identifying older persons at elevated risk for numerous adverse health outcomes such as falls, institutionalization, hospitalization, disability, and death (Rodríguez-Mañas, 2013). Frailty is as a multidimensional syndrome characterized by a nonspecific state of vulnerability, reduced multisystem physiological reserve, and decreased resistance to stressors (Rodríguez-Mañas, 2013). Although there is no consensus regarding the operational definition of frailty, in general, two are the most frequently used approaches: the first is the physical or “phenotypic” model of frailty, while the second is based on deficit accumulation, measured with the so called frailty indexes, and defined as an accumulation of health-related deficits and disorders (Rodríguez-Mañas, 2013). However, also psychological, cognitive and social factors are part of this multidimensional syndrome, with great influence on its definition and treatment. Cognition has already been suggested as a possible component of frailty with increased risk of adverse outcomes. Therefore, the prevention of cognitive-related adverse outcomes including delirium (Eeles et al., 2012) and late-life cognitive disorders (Robertson et al., 2013; Panza et al., 2015b) may be possible also through frailty prevention.
Peripheral Age-related Hearing Impairment, Alzheimer's Disease, and Cognition
In the USA, ARHI is even more prevalent among older adults than previously reported, with the ARHI prevalence that approximately doubles every decade of life from the second through to the seventh decade (Quaranta et al., 2015). At early stage, ARHI typically affects audibility of the higher frequencies (6000 and 8000 Hz), interfering with regular speech comprehension in both quiet and noise, and spreading to the mid and low frequencies over time. Part of the hearing problems are not related to the peripheral deficit of the auditory system but to the central auditory processing (CAP) dysfunction. Subjects with this condition have considerable difficulty in understanding speech in presence of a background noise or in reverberant rooms, but no problem in a quiet environment. Both peripheral and central auditory dysfunctions are therefore relevant to assess a possible influence of ARHI on late-life cognitive disorders.
Over two decades ago, a review article with also original findings summarized the first 25 years of research on possible associations between hearing impairment and cognitive dysfunction reviewing a relevant number of reports (Gennis et al., 1991). The cumulative evidence coming from this first series of studies suggested a strong link between peripheral ARHI and cognitive impairment or decline in demented or institutionalized patients, while there was no association in nondemented older subjects (Gennis et al., 1991). More recently, a case-control report on AD patients from a tertiary center (Gold et al., 1996) and a number of cross-sectional (Gussekloo et al., 2005; Lin, 2011) and longitudinal population-based studies (Lin et al., 2004, 2011, 2013; Valentijn et al., 2005; Wallhagen et al., 2008; Gallacher et al., 2012; Kiely et al., 2012) (Table 1) confirmed the interplay among peripheral auditory dysfunction and AD, dementia, and cognitive disorders in older age, with a single exception in a 2-year follow-up of the Australian Longitudinal Study of Aging (Anstey et al., 2001). However, a weak association between hearing loss and memory decline was found in a re-evaluation of the same sample in a longer follow-up of 8 years (Anstey et al., 2003) (Table 1).
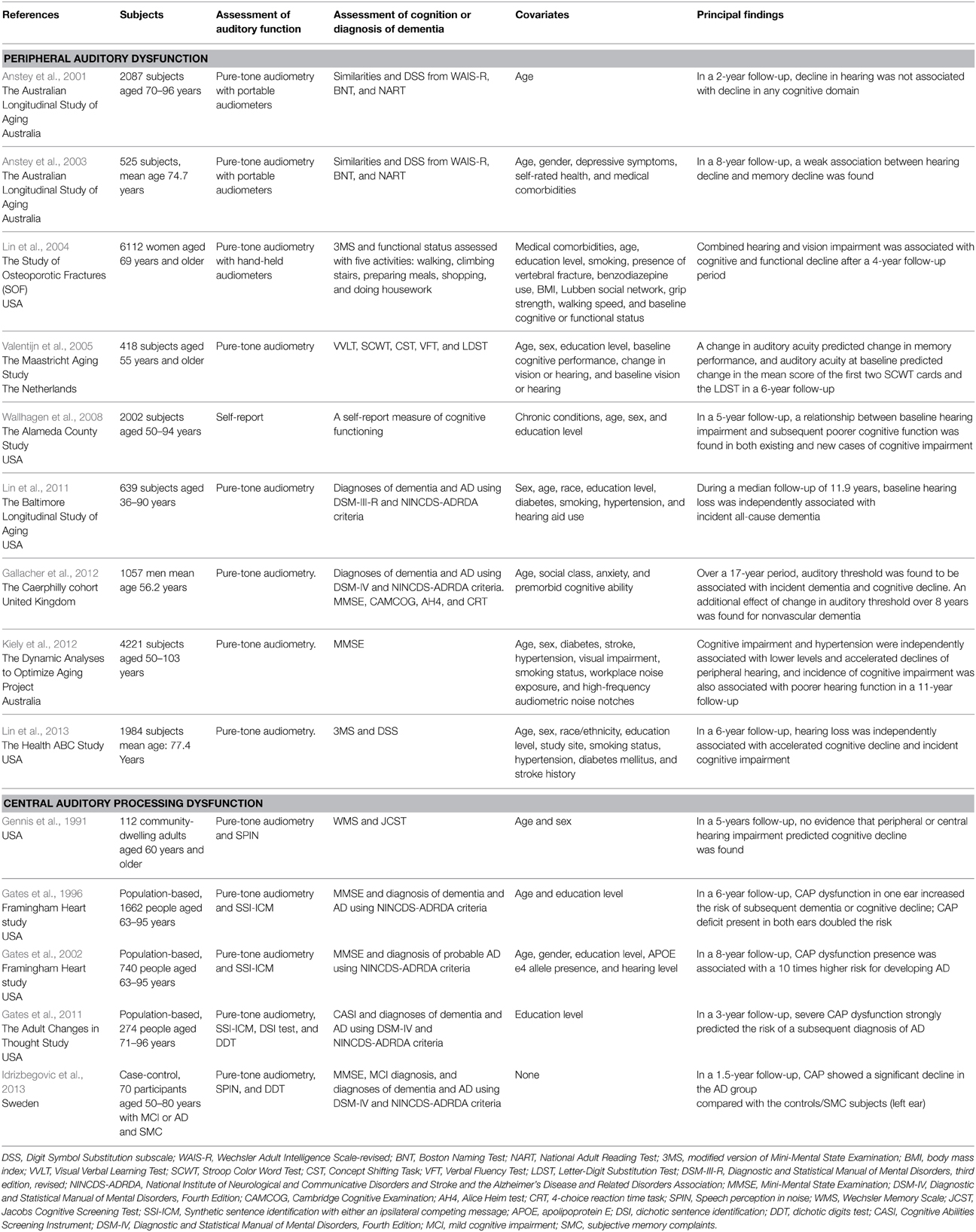
Table 1. Principal case-control and longitudinal population-based studies of peripheral auditory dysfunction and central auditory processing dysfunction in age-related hearing impairment in relation to late-life cognitive decline, incident dementia and Alzheimer's disease (AD).
Central Auditory Processing Dysfunction, Mild Cognitive Impairment, and Alzheimer's Disease
Age-related decline in CAP appeared not to be an isolated condition, but an entity with a multifactorial nature associated to age- and/or disease-related brain and auditory changes (Gates and Mills, 2005). In fact, CAP dysfunction increases with age (Gates and Mills, 2005), but given the increased incidence of both peripheral ARHI and cognitive impairment in late life, the interpretation of central auditory tests may be difficult. In particular, in the early phase of cognitive decline, the differential impact of the two types of auditory deficits on late-life cognitive disorders may be not easy to determine. Several existing studies mostly focused on the impact of peripheral auditory deficit on late-life cognition, while studies on the link between CAP dysfunction and mild cognitive impairment (MCI) or AD are sparse (Gennis et al., 1991; Kurylo et al., 1993; Gates et al., 1996, 2002, 2008, 2010, 2011; Idrizbegovic et al., 2011, 2013).
Several cross-sectional case-control (Kurylo et al., 1993; Idrizbegovic et al., 2011) and two population-based studies (Gates et al., 2008, 2010) suggested a strong involvement of CAP dysfunction in MCI (Idrizbegovic et al., 2011), dementia (Gates et al., 2010), and AD (Kurylo et al., 1993; Gates et al., 2008; Idrizbegovic et al., 2011). In particular, findings from the Adult Changes in Thought (ACT) Study suggested that CAP disorders may be associated with executive dysfunction in older subject with and without memory loss and dementia (Gates et al., 2010). Therefore, executive control function may be a key factor in the speech-based behavioral tasks evaluating CAP dysfunction because lesions of the central auditory pathway are infrequent in early AD (Kurylo et al., 1993).
A few longitudinal case-control (Idrizbegovic et al., 2013) and population-based studies (Gates et al., 1996, 2002, 2011) suggested that CAP dysfunction in ARHI may be central in determining an increased risk of cognitive decline and incident dementia or AD (Gates et al., 1996), and AD (Gates et al., 2002, 2011) (Table 1). Therefore, deficit in CAP could be an early marker of MCI or AD, with a “gradient” existing in CAP disorders among subjects with subjective memory complaints, MCI, and early AD (Idrizbegovic et al., 2011).
Age-related Hearing Impairment-dementia Link and Frailty
Some factors may be involved in causal mechanistic pathways linking ARHI and cognition, while other factors may constitute shared pathological processes or etiological pathways underlying both ARHI and cognitive disorders in late life. Cognitive testing may be confounded by ARHI in association with poor verbal communication. However, some studies used also nonverbal cognitive tests or were insensitive to the exclusion of subjects with serious hearing loss from the analyses (Anstey et al., 2003; Lin et al., 2013), so not supporting the hypothesis that miscommunication in hearing loss should impair cognitive testing. Furthermore, in older subjects with subclinical cognitive impairment may occur an overdiagnosis of peripheral ARHI, although audiometric testing appeared to be reliable also in patients with early dementia.
Evidence coming from epidemiological (Fratiglioni et al., 2000; Wilson et al., 2007) and neuropathological studies (Bennett et al., 2006) suggested that social isolation and loneliness, caused by communication impairments in older subjects with ARHI, may lead to cognitive decline and AD. Moreover, also the cognitive reserve concept, often conditioned by communication defects, may account for the link between ARHI and cognition. Cognitive/brain reserve appears to be a buffer against the functional impairments caused by accumulating age-related brain changes or AD-related pathology, so acting as a modulator of the interplay between neuropathology and cognitive outcomes. Increased cognitive load to help compensate auditory processing may reduce the neural resources available to other cognitive processes such as working memory and perceptual speed, increasing the deleterious effects of AD pathology and revealing the earlier clinical symptoms of dementia (Boyle et al., 2008). Furthermore, a key link between communication difficulties, social isolation, and cognitive decline is the reduced capacity to participate in mentally stimulating activities. In fact, cognitive reserve acts as a buffer via engaging in cognitively stimulating behaviors within an enriched environment, so enhancing neuroplasticity, Furthermore, while AD-related neuropathology is absent in the peripheral auditory pathways (Sinha et al., 1993), peripheral ARHI may contribute to loss of gray matter volume in primary auditory cortex (Peelle et al., 2011), accelerated rates of decline in regional brain volumes in the right temporal lobe and whole brain volume (Lin et al., 2014), and variation in the integrity of central auditory white matter tracks (Chang et al., 2004). AD-related neurodegeneration may be also involved in peculiar damage of central auditory nuclei required for higher-order auditory processing (Parvizi et al., 2001). However, more serious effects may be caused by damage to higher-order cortical areas involved in language processing (Kurylo et al., 1993), so not excluding a shared neuropathological origin underlying both ARHI and AD/dementia.
Among possible confounders, common pathological processes, or shared etiological pathways linking ARHI and cognition, frailty syndrome could have a central role. In fact, ARHI may be also a strong indicator for both the most widely diffused models of frailty. In some operational definitions and frailty indexes, ARHI is one of the suggested components of frailty (Frailty Index-Comprehensive Geriatric Assessment, Groningen Frailty Indicator, and Puts model) (Panza et al., 2015a) and it may predict functional decline or incident falls in older adults (Lin and Ferrucci, 2012), some of the health-related adverse outcomes linked to frailty. Among potentially modifiable risk factors, there is a growing body of evidence about the impact of several operational definitions of frailty on late-life cognitive disorders (Robertson et al., 2013; Panza et al., 2015b). In particular, an international consensus group has recently proposed the clinical label “cognitive frailty” for describing the simultaneous presence of both physical frailty and cognitive impairment in nondemented older individuals (Kelaiditi et al., 2013), representing also a possible precursor of neurodegenerative processes and AD. Epidemiological studies strongly suggested that physical frailty may be associated with incident AD and MCI, nonAD dementias, vascular dementia (VaD), AD-related neuropathology, and cognitive impairment and decline in late life (Robertson et al., 2013; Solfrizzi et al., 2013; Panza et al., 2015b), so also validating cognitive frailty as a new clinical condition. Several factors and diseases associated with physical frailty are also related to cognitive impairment, including nutritional factors, metabolic disorders, inflammatory markers, hormones, diabetes mellitus, congestive heart failure, and stroke (Robertson et al., 2013; Panza et al., 2015b), suggesting an underlying and shared pathogenesis probably linked to vascular determinants. In fact, in 2006, the term “cognitive frailty” was firstly used to indicate a specific state of cognitive vulnerability in MCI and related entities exposed to vascular risk with a consequent elevated progression to dementia (Panza et al., 2006). Physical frailty has been proposed also as a prodromal stage of VaD (Robertson et al., 2013; Solfrizzi et al., 2013). This could be therefore a further common pathway explaining the ARHI-frailty-cognition interplay given that another neurobiological process such as vascular disease or shared vascular factors may cause ARHI, frailty, and dementia.
Conclusions and Future Directions
In recent years, there has been growing attention on the possible correlations between sensorial abnormalities and late-life cognitive disorders. Epidemiological evidence is mainly focused on peripheral auditory disorders and cognition, but in longitudinal population-based studies both peripheral and CAP dysfunctions appear to be associated with incident cognitive impairment and AD and accelerated cognitive decline. While some randomized controlled trials (RCTs) showed improvement in cognitive function or global measures of change in hearing-aid users not cognitively impaired (Mulrow et al., 1990) or with dementia (Allen et al., 2003), determining whether treating hearing loss could delay cognitive decline and dementia remains an open issue. In fact, RCTs with more representative cohorts and technology (i.e., digital hearing aids or cochlear implants), longer follow-up periods, and estimating the effects of hearing rehabilitative interventions on cognitive and global functioning have never been performed (Lin, 2012). At present, use of amplification can be an effective tool for minimizing the perceived disability of older adults and reducing the AD caregiver burden by enhancing the communication abilities of the patients (Palmer et al., 1998). However, hearing aids alone could be not enough to properly manage ARHI, and interventions should be broader, incorporating also concerted counseling, environmental accommodations, and rehabilitative hearing training. Cognition and dementia are causally linked to frailty, and ARHI is also a component of frailty included in several operational definitions. Therefore, frailty could have an important impact in the prevention of late-life cognitive disorders, with nutrition and physical exercise as factors potentially affecting frailty status in advanced age (Clegg et al., 2013). Further investigation on the role of vascular risk on the ARHI-frailty-cognition interplay is warranted to better understand causal mechanisms. Overlaps or interactions among the contributing factors could be investigated supplementing speech-based behavioral measures of CAP with nonbehavioral measures based on electrophysiological studies, and structural, spectroscopic, and functional neuroimaging to detect shared neurobiological markers of ARHI and cognitive decline.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This research was supported by Programmi di Ricerca Scientifica di Rilevante Interesse Nazionale (PRIN) 2009 Grant 2009E4RM4Z.
References
Allen, N. H., Burns, A., Newton, V., Hickson, F., Ramsden, R., Rogers, J., et al. (2003). The effects of improving hearing in dementia. Age Ageing 32, 189–193. doi: 10.1093/ageing/32.2.189
Anstey, K. J., Hofer, S. M., and Luszcz, M. A. (2003). A latent growth curve analysis of late-life sensory and cognitive function over 8 years: evidence for specific and common factors underlying change. Psychol. Aging 18, 714–726. doi: 10.1037/0882-7974.18.4.714
Anstey, K. J., Luszcz, M. A., and Sanchez, L. (2001). Two-year decline in vision but not hearing is associated with memory decline in very old adults in a population-based sample. Gerontology 47, 289–293. doi: 10.1159/000052814
Bennett, D. A., Schneider, J. A., Tang, Y., Arnold, S. E., and Wilson, R. S. (2006). The effect of social networks on the relation between Alzheimer's disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 5, 406–412. doi: 10.1016/S1474-4422(06)70417-3
Boyle, P. A., Wilson, R. S., Schneider, J. A., Bienias, J. L., and Bennett, D. A. (2008). Processing resources reduce the effect of Alzheimer pathology on other cognitive systems. Neurology 70, 1534–1542. doi: 10.1212/01.wnl.0000304345.14212.38
Chang, Y., Lee, S. H., Lee, Y. J., Hwang, M. J., Bae, S. J., Kim, M. N., Lee, J., et al. (2004). Auditory neural pathway evaluation on sensorineural hearing loss using diffusion tensor imaging. Neuroreport 15, 1699–1703. doi: 10.1097/01.wnr.0000134584.10207.1a
Clegg, A., Young, J., Iliffe, S., Rikkert, M. O., and Rockwood, K. (2013). Frailty in elderly people. Lancet 381, 752–762. doi: 10.1016/S0140-6736(12)62167-9
Eeles, E. M., White, S. V., O'Mahony, S. M., Bayer, A. J., and Hubbard, R. E. (2012). The impact of frailty and delirium on mortality in older inpatients. Age Ageing 41, 412–416. doi: 10.1093/ageing/afs021
Fratiglioni, L., Wang, H. X., Ericsson, K., Maytan, M., and Winblad, B. (2000). Influence of social network on occurrence of dementia: a community-based longitudinal study. Lancet 355, 1315–1319. doi: 10.1016/S0140-6736(00)02113-9
Gallacher, J., Ilubaera, V., Ben-Shlomo, Y., Bayer, A., Fish, M., Babisch, W., et al. (2012). Auditory threshold, phonologic demand, and incident dementia. Neurology 79, 1583–1590. doi: 10.1212/WNL.0b013e31826e263d
Gates, G. A., Anderson, M. L., Feeney, M. P., McCurry, S. M., and Larson, E. B. (2008). Central auditory dysfunction in older persons with memory impairment or Alzheimer dementia. Arch. Otolaryngol. Head Neck Surg. 134, 771–777. doi: 10.1001/archotol.134.7.771
Gates, G. A., Anderson, M. L., McCurry, S. M., Feeney, M. P., and Larson, E. B. (2011). Central auditory dysfunction as a harbinger of Alzheimer dementia. Arch. Otolaryngol. Head Neck Surg. 137, 390–395. doi: 10.1001/archoto.2011.28
Gates, G. A., Beiser, A., Rees, T. S., D'Agostino, R. B., and Wolf, P. A. (2002). Central auditory dysfunction may precede the onset of clinical dementia in people with probable Alzheimer's disease. J. Am. Geriatr. Soc. 50, 482–488. doi: 10.1046/j.1532-5415.2002.50114.x
Gates, G. A., Cobb, J. L., Linn, R. T., Rees, T., Wolf, P. A., and D'Agostino, R. B. (1996). Central auditory dysfunction, cognitive dysfunction, and dementia in older people. Arch. Otolaryngol. Head Neck Surg. 122, 161–167. doi: 10.1001/archotol.1996.01890140047010
Gates, G. A., Gibbons, L. E., McCurry, S. M., Crane, P. K., Feeney, M. P., and Larson, E. B. (2010). Executive dysfunction and presbycusis in older persons with and without memory loss and dementia. Cogn. Behav. Neurol. 23, 218–223. doi: 10.1097/WNN.0b013e3181d748d7
Gates, G. A., and Mills, J. H. (2005). Presbycusis. Lancet 366, 1111–1120. doi: 10.1016/S0140-6736(05)67423-5
Gennis, V., Garry, P. J., Haaland, K. Y., Yeo, R. A., and Goodwin, J. S. (1991). Hearing and cognition in the elderly. New findings and a review of the literature. Arch. Intern. Med. 151, 2259–2264. doi: 10.1001/archinte.1991.00400110105021
Gold, M., Lightfoot, L. A., and Hnath-Chisolm, T. (1996). Hearing loss in a memory disorders clinic. A specially vulnerable population. Arch. Neurol. 53, 922–928. doi: 10.1001/archneur.1996.00550090134019
Gussekloo, J., de Craen, A. J., Oduber, C., van Boxtel, M. P., and Westendorp, R. G. (2005). Sensory impairment and cognitive functioning in oldest-old subjects: the Leiden 85+ Study. Am. J. Geriatr. Psychiatry 13, 781–786. doi: 10.1176/appi.ajgp.13.9.781
Idrizbegovic, E., Hederstierna, C., Dahlquist, M., Kämpfe Nordström, C., Jelic, V., and Rosenhall, U. (2011). Central auditory function in early Alzheimer's disease and in mild cognitive impairment. Age Ageing 40, 249–254. doi: 10.1093/ageing/afq168
Idrizbegovic, E., Hederstierna, C., Dahlquist, M., and Rosenhall, U. (2013). Short-term longitudinal study of central auditory function in Alzheimer's disease and mild cognitive impairment. Dement. Geriatr. Cogn. Dis. Extra 3, 468–471. doi: 10.1159/000355371
Kelaiditi, E., Cesari, M., Canevelli, M., van Kan, G. A., Ousset, P. J., Gillette-Guyonnet, S., et al. (2013). Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J. Nutr. Health Aging 17, 726–734. doi: 10.1007/s12603-013-0367-2
Kiely, K. M., Gopinath, B., Mitchell, P., Luszcz, M., and Anstey, K. J. (2012). Cognitive, health, and sociodemographic predictors of longitudinal decline in hearing acuity among older adults. J. Gerontol. A Biol. Sci. Med. Sci. 67, 997–1003. doi: 10.1093/gerona/gls066
Kurylo, D. D., Corkin, S., Allard, T., Zatorre, R. J., and Growdon, J. H. (1993). Auditory function in Alzheimer's disease. Neurology 43, 1893–1899. doi: 10.1212/WNL.43.10.1893
Lin, F. R. (2011). Hearing loss and cognition among older adults in the United States. J. Gerontol. A Biol. Sci. Med. Sci. 66, 1131–1136. doi: 10.1093/gerona/glr115
Lin, F. R. (2012). Hearing loss in older adults: who's listening? JAMA 307, 1147–1148. doi: 10.1001/jama.2012.321
Lin, F. R., and Ferrucci, L. (2012). Hearing loss and falls among older adults in the United States. Arch. Intern. Med. 172, 369–371. doi: 10.1001/archinternmed.2011.728
Lin, F. R., Ferrucci, L., An, Y., Goh, J. O., Doshi, J., Metter, E. J., et al. (2014). Association of hearing impairment with brain volume changes in older adults. Neuroimage 90, 84–92. doi: 10.1016/j.neuroimage.2013.12.059
Lin, F. R., Metter, E. J., O'Brien, R. J., Resnick, S. M., Zonderman, A. B., and Ferrucci, L. (2011). Hearing loss and incident dementia. Arch. Neurol. 68, 214–220. doi: 10.1001/archneurol.2010.362
Lin, F. R., Yaffe, K., Xia, J., Xue, Q. L., Harris, T. B., Purchase-Helzner, E., et al. (2013). Hearing loss and cognitive decline in older adults. JAMA Intern. Med. 173, 293–299. doi: 10.1001/jamainternmed.2013.1868
Lin, M. Y., Gutierrez, P. R., Stone, K. L., Yaffe, K., Ensrud, K. E., Fink, H. A., et al. (2004). Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J. Am. Geriatr. Soc. 52, 1996–2002. doi: 10.1111/j.1532-5415.2004.52554.x
Mulrow, C. D., Aguilar, C., Endicott, J. E., Tuley, M. R., Velez, R., Charlip, W. S., et al. (1990). Quality-of-Life changes and hearing impairment: a randomized trial. Ann. Intern. Med. 113, 188–194. doi: 10.7326/0003-4819-113-3-188
Palmer, C. V., Adams, S. W., Durrant, J. D., Bourgeois, M., and Rossi, M. (1998). Managing hearing loss in a patient with Alzheimer disease. J. Am. Acad. Audiol. 9, 275–284.
Panza, F., D'Introno, A., Colacicco, A. M., Capurso, C., Parigi, A. D., Capurso, S. A., et al. (2006). Cognitive frailty: predementia syndrome and vascular risk factors. Neurobiol. Aging 27, 933–940. doi: 10.1016/j.neurobiolaging.2005.05.008
Panza, F., Solfrizzi, V., Barulli, M. R., Santamato, A., Seripa, D., Pilotto, A., et al. (2015b). Cognitive frailty - epidemiological and neurobiological evidence of an age-related clinical condition: a systematic review. Rejuvenation Res. doi: 10.1089/rej.2014.1637. [Epub ahead of print].
Panza, F., Solfrizzi, V., and Logroscino, G. (2015a). Age-related hearing impairment-a risk factor and frailty marker for dementia and AD. Nat. Rev. Neurol. 11, 166–175. doi: 10.1038/nrneurol.2015.12
Parvizi, J., Van Hoesen, G. W., and Damasio, A. (2001). The selective vulnerability of brainstem nuclei to Alzheimer's disease. Ann. Neurol. 49, 53–66. doi: 10.1002/1531-8249(200101)49:1<53::AID-ANA30>3.0.CO;2-Q
Peelle, J. E., Troiani, V., Grossman, M., and Wingfield, A. (2011). Hearing loss in older adults affects neural systems supporting speech comprehension. J. Neurosci. 31, 12638–12643. doi: 10.1523/JNEUROSCI.2559-11.2011
Quaranta, N., Coppola, F., Casulli, M., Barulli, M. R., Panza, F., Tortelli, R., et al. (2015). Epidemiology of age related hearing loss: a review. Hearing Balance Commun. 17, 77–81. doi: 10.3109/21695717.2014.994869
Robertson, D. A., Savva, G. M., and Kenny, R. A. (2013). Frailty and cognitive impairment-A review of the evidence and causal mechanisms. Ageing Res. Rev. 12, 840–851. doi: 10.1016/j.arr.2013.06.004
Rodríguez-Mañas, L., Féart, C., Mann, G., Viña, J., Chatterji, S., Chodzko-Zajko, W., et al. (2013). Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J. Gerontol. A Biol. Sci. Med. Sci. 68, 62–67. doi: 10.1093/gerona/gls119
Sinha, U. K., Hollen, K. M., Rodriguez, R., and Miller, C. A. (1993). Auditory system degeneration in Alzheimer's disease. Neurology 43, 779–785. doi: 10.1212/WNL.43.4.779
Solfrizzi, V., Scafato, E., Frisardi, V., Seripa, D., Logroscino, G., Maggi, S., et al. (2013). Frailty syndrome and the risk of vascular dementia: the Italian Longitudinal Study on Aging. Alzheimers Dement. 9, 113–122. doi: 10.1016/j.jalz.2011.09.223
Valentijn, S. A., van Boxtel, M. P., van Hooren, S. A., Bosma, H., Beckers, H. J., Ponds, R. W., et al. (2005). Change in sensory functioning predicts change in cognitive functioning: results from a 6-year follow-up in the maastricht aging study. J. Am. Geriatr. Soc. 53, 374–380. doi: 10.1111/j.1532-5415.2005.53152.x
Wallhagen, M. I., Strawbridge, W. J., and Shema, S. J. (2008). The relationship between hearing impairment and cognitive function: a 5-year longitudinal study. Res. Gerontol. Nurs. 1, 80–86. doi: 10.3928/19404921-20080401-08
Keywords: Alzheimer's disease, dementia, mild cognitive impairment, age-related hearing impairment, peripheral auditory dysfunction, central auditory processing deficit, frailty syndrome
Citation: Panza F, Solfrizzi V, Seripa D, Imbimbo BP, Capozzo R, Quaranta N, Pilotto A and Logroscino G (2015) Age-related hearing impairment and frailty in Alzheimer's disease: interconnected associations and mechanisms. Front. Aging Neurosci. 7:113. doi: 10.3389/fnagi.2015.00113
Received: 06 March 2015; Accepted: 29 May 2015;
Published: 09 June 2015.
Edited by:
Kaarin J. Anstey, Australian National University College of Medicine, Biology and Environment, AustraliaReviewed by:
Kim Matthew Kiely, The Australian National University, AustraliaCopyright © 2015 Panza, Solfrizzi, Seripa, Imbimbo, Capozzo, Quaranta, Pilotto and Logroscino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Panza, geriat.dot@geriatria.uniba.it
†These authors have contributed equally to this work.
 Francesco Panza
Francesco Panza Vincenzo Solfrizzi
Vincenzo Solfrizzi Davide Seripa
Davide Seripa Bruno P. Imbimbo
Bruno P. Imbimbo Rosa Capozzo1,2
Rosa Capozzo1,2  Alberto Pilotto
Alberto Pilotto Giancarlo Logroscino
Giancarlo Logroscino