The Paraventricular Nucleus of the Thalamus as an Integrating and Relay Node in the Brain Anxiety Network
- Department of Oral Biology, Dr. Gerald Niznick College of Dentistry, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, MB, Canada
The brain anxiety network is composed of a number of interconnected cortical regions that detect threats and execute appropriate defensive responses via projections to the shell of the nucleus accumbens (NAcSh), dorsolateral region of the bed nucleus of the stria terminalis (BSTDL) and lateral region of the central nucleus of the amygdala (CeL). The paraventricular nucleus of the thalamus (PVT) is anatomically positioned to integrate threat- and arousal-related signals from cortex and hypothalamus and then relay these signals to neural circuits in the NAcSh, BSTDL, and CeL that mediate defensive responses. This review describes the anatomical connections of the PVT that support the view that the PVT may be a critical node in the brain anxiety network. Experimental findings are reviewed showing that the arousal peptides orexins (hypocretins) act at the PVT to promote avoidance of potential threats especially following exposure of rats to a single episode of footshocks. Recent anatomical and experimental findings are discussed which show that neurons in the PVT provide divergent projections to subcortical regions that mediate defensive behaviors and that the projection to the NAcSh is critical for the enhanced social avoidance displayed in rats exposed to footshocks. A theoretical model is proposed for how the PVT integrates cortical and hypothalamic signals to modulate the behavioral responses associated with anxiety and other challenging situations.
Introduction
Anxiety is an ethologically advantageous emotion that maximizes survival because it promotes avoidance of potential harm in situations where dangers can emerge quickly (Steimer, 2002; Calhoon and Tye, 2015; LeDoux and Daw, 2018). It is characterized by a state of arousal and hypervigilance in addition to excessive behavioral avoidance of potential threats. Unnecessary levels of anxiety can cause significant distress and a better understanding of how neural circuits in the brain control responses to threats and mediate anxiety is considered important for the development of new treatments (Craske et al., 2017). It is generally accepted that anxiety results from genetic vulnerabilities combined with situational factors like stress and exposure to fear-inducing situations (Nemeroff et al., 2006; Craske and Stein, 2016). There are a number of comprehensive reviews describing the components of the anxiety network (Adhikari, 2014; Calhoon and Tye, 2015; Tovote et al., 2015; LeDoux and Daw, 2018). The network involves a number of interconnected cortical and subcortical regions that evaluate and respond to potential threats (Steimer, 2002; Calhoon and Tye, 2015; LeDoux and Daw, 2018). How these regions function together as a network is poorly understood and is of considerable interest from preclinical and clinical perspectives (Shin and Liberzon, 2010; Adhikari, 2014; Calhoon and Tye, 2015; Fox and Shackman, 2017; Silva and McNaughton, 2019).
The present review presents evidence that the paraventricular nucleus of the thalamus (PVT) may be a critical node in the brain anxiety network. Anatomical details of how the PVT is connected with many components of the brain’s anxiety network are presented as well as recent evidence showing that neurotransmission to the PVT from orexin neurons in the hypothalamus contributes to stress-induced anxiety. The review also discusses recent anatomical evidence that shows that neurons in the PVT provide divergent projections to key striatal-like subcortical regions involved in the various defensive responses. Finally, a model is described that postulates that the PVT integrates and amplifies cortical signals related to threats and relays these signals to activate subcortical circuits that modulate defensive responses including avoidance of potential threats.
Stress and Anxiety
Fear and anxiety are similar types of emotions (LeDoux, 2015; LeDoux and Pine, 2016) that can be inferred in experimental animals from the expression of stereotypical defensive behaviors (LeDoux, 2015; LeDoux and Pine, 2016). Fear is triggered by the presence of an impending threat and is often experimentally defined in rodents as freezing to conditioned cues or contexts. In contrast, anxiety is a response to potential threats and is operationally defined as avoidance of potential risks involving open spaces, bright lights, and novel conspecifics (Steimer, 2002; LeDoux, 2015). An association between stress and anxiety is well-established (Shin and Liberzon, 2010; Bystritsky and Kronemyer, 2014; Daviu et al., 2019) with stress being a contributing factor for most anxiety disorders including social anxiety disorder and posttraumatic stress disorder (PTSD) (Bystritsky and Kronemyer, 2014; Carvajal, 2018). The causal relationship between stress and anxiety is most dramatically exemplified by PTSD where a single but intensely stressful experience can lead to a long-lasting anxiety state in susceptible individuals (Nemeroff et al., 2006; Craske and Stein, 2016). The enduring effect of acute stress on behavior is not unique to humans since exposure of rodents to a single episode of footshocks produces enhanced levels of anxiety, including heightened level of social avoidance that does not appear to be directly dependent of the retrieval of a fear memory (Louvart et al., 2005; Siegmund and Wotjak, 2007; Mikics et al., 2008; Chen et al., 2012).
Brief Overview of the PVT and Its Role in Regulating Behavior
The PVT has received a considerable amount of attention because of its connections with regions of the brain linked to the regulation of emotional and motivated behavior (Groenewegen et al., 1987; Berendse and Groenewegen, 1990, 1991; Berendse et al., 1992; Wright and Groenewegen, 1995, 1996). Tracing studies show that the PVT sends a robust excitatory projection to a continuum in the basal forebrain that includes the shell of the nucleus accumbens (NAcSh), dorsolateral region of the bed nucleus of the stria terminalis (BSTDL) and lateral region of the central nucleus of the amygdala (CeL) consisting of the lateral and capsular subnuclei of the central nucleus of the amygdala (Moga et al., 1995; Parsons et al., 2007; Li and Kirouac, 2008; Vertes and Hoover, 2008; Dong et al., 2017). The BSTDL and CeL are part of a larger macrostructure called the central extended amygdala (cEA) (Heimer and Alheid, 1991; Alheid et al., 1995; de Olmos et al., 2004). The cEA contains striatal-like projection neurons that send fibers to the hypothalamus and brainstem. In addition, the cEA densely innervates the medial extended amygdala (mEA), which consists of the medial regions of the bed nucleus of the stria terminalis and the medial central nucleus of the amygdala (Heimer and Alheid, 1991; Alheid et al., 1995; de Olmos et al., 2004). The mEA is composed of pallidal-like neurons that provide dense descending projections to the somatomotor, visceromotor, and endocrine circuits in the hypothalamus and brainstem known for producing some of the physiological and behavioral responses that make up defensive responses (Petrovich and Swanson, 1997; Cassell et al., 1999; Swanson, 2000). The NAcSh of the ventral striatum is composed of medium spiny neurons that provide fiber projections to the ventral pallidum, lateral hypothalamus, and ventral tegmental area (Heimer et al., 1991; Zahm and Brog, 1992; Zahm, 2006). While the NAcSh is sometimes considered a transitional region between the cEA and the rest of the striatum, it is also appropriate to consider the NAcSh, BSTDL, and CeL as components of a large striatal-like descending macrosystem involved in the regulation of complex behavior (Swanson, 2000; Zahm, 2006). A notable common anatomical feature of this striatal-like macrosystem is an exceptionally dense plexus of PVT fibers (Li and Kirouac, 2008). It is also important to appreciate that PVT neurons project weakly to cortical regions (i.e., prelimbic, infralimbic, anterior insular cortices; ventral subiculum, and the basolateral nucleus of the amygdala) that innervate the same areas of the NAcSh, BSTDL, and CeL that receive fibers from the PVT (Kirouac, 2015). This places the PVT in a position to influence multiple levels of the cortico-subcotical circuits involved in regulating behavior.
The sources of input to the PVT have also been examined (Otake et al., 1995, 2002; Krout and Loewy, 2000a, b; Krout et al., 2002) and a detailed comparative analysis of all afferents to the PVT using retrograde tracing methods indicate that the major sources of inputs originate from neurons in the prefrontal cortex (infralimbic, prelimbic, insular) and the ventral subiculum of the hippocampus (Li and Kirouac, 2012). The robustness of cortical projections to the PVT has also been described using anterograde tracing methods (Sesack et al., 1989; Canteras and Swanson, 1992; Vertes, 2004). In addition, a number of functionally distinct nuclei in the brainstem and hypothalamus known to modulate behavioral states are also significant sources of afferents to the PVT (Otake et al., 1995, 2002; Krout and Loewy, 2000a, b; Krout et al., 2002), but the strength of these inputs often appear to be eclipsed by the comparative strength of the cortical inputs (Li and Kirouac, 2012).
It is also notable that the PVT is an area of the brain consistently identified as being activated during states of behavioral arousal (reviewed in Kirouac, 2015; Millan et al., 2017; Barson et al., 2020; McGinty and Otis, 2020) including those where sensory cues predict rewarding or aversive conditions (Do-Monte et al., 2015; Zhu et al., 2018; Choi et al., 2019). Recording of calcium signals or single unit activity in the PVT of behaving animals exposed to stimuli associated with appetitive or aversive outcomes indicates that PVT neurons respond robustly to novel cues and that these neurons track the saliency of these cues (Do-Monte et al., 2015, 2017; Choi and McNally, 2017; Zhu et al., 2018; Otis et al., 2019). A considerable amount of direct experimental evidence is also available demonstrating that the PVT contributes to conditioned appetitive and defensive behaviors in a projection specific manner (Do-Monte et al., 2015, 2017; Labouebe et al., 2016; Zhu et al., 2016, 2018; Choi and McNally, 2017; Cheng et al., 2018; Choi et al., 2019). The critical question of whether the PVT preferentially promotes appetitive or aversive responses has not been unequivocally resolved (some of the controversies and challenges in studying the role of the PVT in behavior was recently reviewed in McGinty and Otis, 2020). Indeed, the type of influence the PVT has on behavior studied in a laboratory setting may be in part dependent on whether opto- or chemogenetic methods are targeted at a specific projection system. For instance, some studies have shown that PVT neurons that project to the CeL contribute to the behavioral freezing associated with conditioned fear (Do-Monte et al., 2015; Penzo et al., 2015) while others have shown that PVT neurons that project to the NAcSh mediate conditioned sucrose seeking (Labouebe et al., 2016; Cheng et al., 2018). This suggests that PVT neurons that innervate the CeL mediate aversive responses while those that project to the NAcSh mediate appetitive responses. However, this is not supported by other evidence showing that PVT neurons that project to the NAcSh mediate real-time avoidance and conditioned place avoidance (Zhu et al., 2016). It should be clear that our understanding of how the PVT mediates valence-dependent responses is incomplete and is further complicated by the fact that many neurons in the PVT send bifurcating axons that innervate multiple target areas of the forebrain (Unzai et al., 2015; Dong et al., 2017). Divergence of projections from single neurons in the PVT implies that PVT neurons may coordinate behavioral responses by simultaneously engaging multiple subcortical circuits. Furthermore, more recent evidence indicates that the type of influence the PVT has on behavior may be dependent on the type of experimental paradigm being studied and whether competing motivational states are present (Choi and McNally, 2017; Do-Monte et al., 2017; Choi et al., 2019; McGinty and Otis, 2020). Some of the evidence and potential mechanisms by which the PVT influences motivated behavior have been discussed in recent reviews and will not be considered in all of their intricate details here (Kirouac, 2015; Do Monte et al., 2016; Millan et al., 2017). While all neurons in the PVT are presumed to be projection neurons that use excitatory amino acids as their main neurotransmitter (Christie et al., 1987; Frassoni et al., 1997), it is also important to appreciate that the PVT is not a uniform structure (Kirouac, 2015). For example, the anterior (aPVT) and posterior aspect of the PVT (pPVT) are composed of neurons that have preferential efferent targets and different sources of afferent inputs making these two broad regions of the PVT potentially functionally different (Li and Kirouac, 2008, 2012; Kirouac, 2015; Dong et al., 2019).
The Brain Anxiety Network
Anxiety states and avoidance of threats are regulated by brain circuits engaged in hierarchical control of defensive strategies in what has been conceptualized as the brain anxiety network (Adhikari, 2014; Calhoon and Tye, 2015; Tovote et al., 2015; LeDoux and Daw, 2018). Potential threats are detected through coordinated activity in a network of interconnected cortical areas that include the basolateral nucleus of the amygdala, ventral hippocampus, and prefrontal cortex (Adhikari, 2014; Calhoon and Tye, 2015; Silva and McNaughton, 2019). It is postulated that this cortical network evaluates potential risks and initiates defensive responses via projections to the subcortical regions associated with the selection of behavioral responses (Kim et al., 2013; Adhikari, 2014; Duvarci and Pare, 2014; Calhoon and Tye, 2015; Fox et al., 2015; Fox and Shackman, 2017). The BSTDL is the part of the cEA that has been most studied for its role in anxiety (Walker et al., 2003, 2009; Kim et al., 2013; Pleil et al., 2015; Normandeau et al., 2018). Optogenetic activation of the BSTDL elicited anxiety in the elevated plus maze (EPM), whereas inhibition has an anxiolytic effect (Kim et al., 2013). The neural connections by which the BSTDL modulates anxiety appear to involve connections to other regions of the bed nucleus of the stria terminalis (i.e., mEA) which exert modulatory effects on anxiety via descending projections to the lateral hypothalamus and ventral tegmental area (Jennings et al., 2013; Kim et al., 2013). The CeL has been primarily investigated for its role in conditioned fear (Kalin et al., 2004; Cai et al., 2012; Ventura-Silva et al., 2013), but recent evidence indicates that the CeL also potentially contributes to anxiety via projections to the BSTDL (Ahrens et al., 2018; Asok et al., 2018a). The NAcSh has been mostly studied for its role in reward and appetitive behaviors (Nicola, 2007; Floresco, 2015; Namburi et al., 2016). However, there is ample evidence that the NAcSh regulates defensive responses (Reynolds and Berridge, 2001, 2002, 2003, 2008; Newton et al., 2002; Barrot et al., 2005; Al-Hasani et al., 2015; Ramirez et al., 2015; Zhu et al., 2016; Anderson et al., 2018; Lee et al., 2018; Piantadosi et al., 2018) including anxiety (Martinez et al., 2002; Lopes et al., 2007, 2012; da Cunha et al., 2008). It is generally accepted that the striatum including the NAcSh integrates cognitive and affective information from the cortex and thalamus in a way that leads to the selection or promotion of an appropriate behavioral response (Nicola, 2007; Floresco, 2015; Namburi et al., 2016) especially where the outcome of an action is ambiguous (Namburi et al., 2016). From this perspective, the NAcSh may contribute to avoidance by biasing motivational/emotional responses in the defensive direction in situations involving both benefits and threats (i.e., approach-avoidance conflicts). The NAcSh is also emerging as an area of the brain critical for social interaction where disruption of normal signaling contributes to social avoidance (Aragona et al., 2006; Christoffel et al., 2011; Chaudhury et al., 2013; Dolen et al., 2013; Gunaydin et al., 2014; Francis et al., 2015; Resendez et al., 2016; Wook Koo et al., 2016; Rademacher et al., 2017; Folkes et al., 2019; Steinman et al., 2019). Our understanding of the neural mechanisms by which the NAcSh mediates the avoidance induced by anxiety is incomplete but is likely to involve projections to the ventral tegmental area and the lateral hypothalamus (Zahm and Brog, 1992; Zahm, 1999, 2000, 2006; da Cunha et al., 2008; Al-Hasani et al., 2015; Zhu et al., 2016) similar to how the BSTDL modulates anxiety-like responses (Jennings et al., 2013; Kim et al., 2013).
Anatomical Connections Between the PVT and the Anxiety Network
Figure 1 illustrates the most prominent connections between the PVT and components of the anxiety network. As shown, the PVT is well-positioned to contribute to the selection of defensive responses via a dense projection to the NAcSh, BSTDL, and CeL (Moga et al., 1995; Parsons et al., 2007; Li and Kirouac, 2008; Vertes and Hoover, 2008; Dong et al., 2017). These striatal-like regions are in turn anatomically positioned to modulate defensive circuits in the mEA, hypothalamus and brainstem. The PVT is also suitably placed to relay arousal related signals to the cortical network involved in threat detection. In addition to being connected with the critical output components of the anxiety network, the PVT is anatomically positioned to integrate and relay a variety of signals known to contribute to anxiety. For example, the prelimbic cortex is a major source of input to the PVT (Sesack et al., 1989; Canteras and Swanson, 1992; Vertes, 2004; Li and Kirouac, 2012) and experimental evidence indicates that the prelimbic cortex not only promotes conditioned fear responses but also contributes to fear generalization and anxiety (Jinks and McGregor, 1997; Lacroix et al., 2000; Sullivan and Gratton, 2002; Shah and Treit, 2003, 2004; Adhikari et al., 2010, 2011; Lisboa et al., 2010; Sotres-Bayon and Quirk, 2010; Xu and Sudhof, 2013; Rozeske et al., 2015; Yamada et al., 2015; Suzuki et al., 2016; Shimizu et al., 2018). It is especially notable that a projection from the prelimbic cortex to the PVT is critical for retrieval of remote fear memories (Padilla-Coreano et al., 2012; Do-Monte et al., 2015; Do Monte et al., 2016). The significance of the latter findings is that fear memories generalize over time in a way that is believe to contribute to the development of PTSD (Liberzon and Abelson, 2016; Asok et al., 2018b). Consequently, the prelimbic cortex may not only relay signals to the PVT directly associated with a previously experienced threat but also any cues that remotely resemble those present at the time of a fear inducing event. The PVT also receives strong input from the infralimbic cortex, insular cortex and the ventral subiculum (Li and Kirouac, 2012), which have been implicated in stress, anxiety and fear generalization (O’Mara et al., 2009; Adhikari et al., 2010; Bi et al., 2013; Berg et al., 2019; Shi et al., 2020).
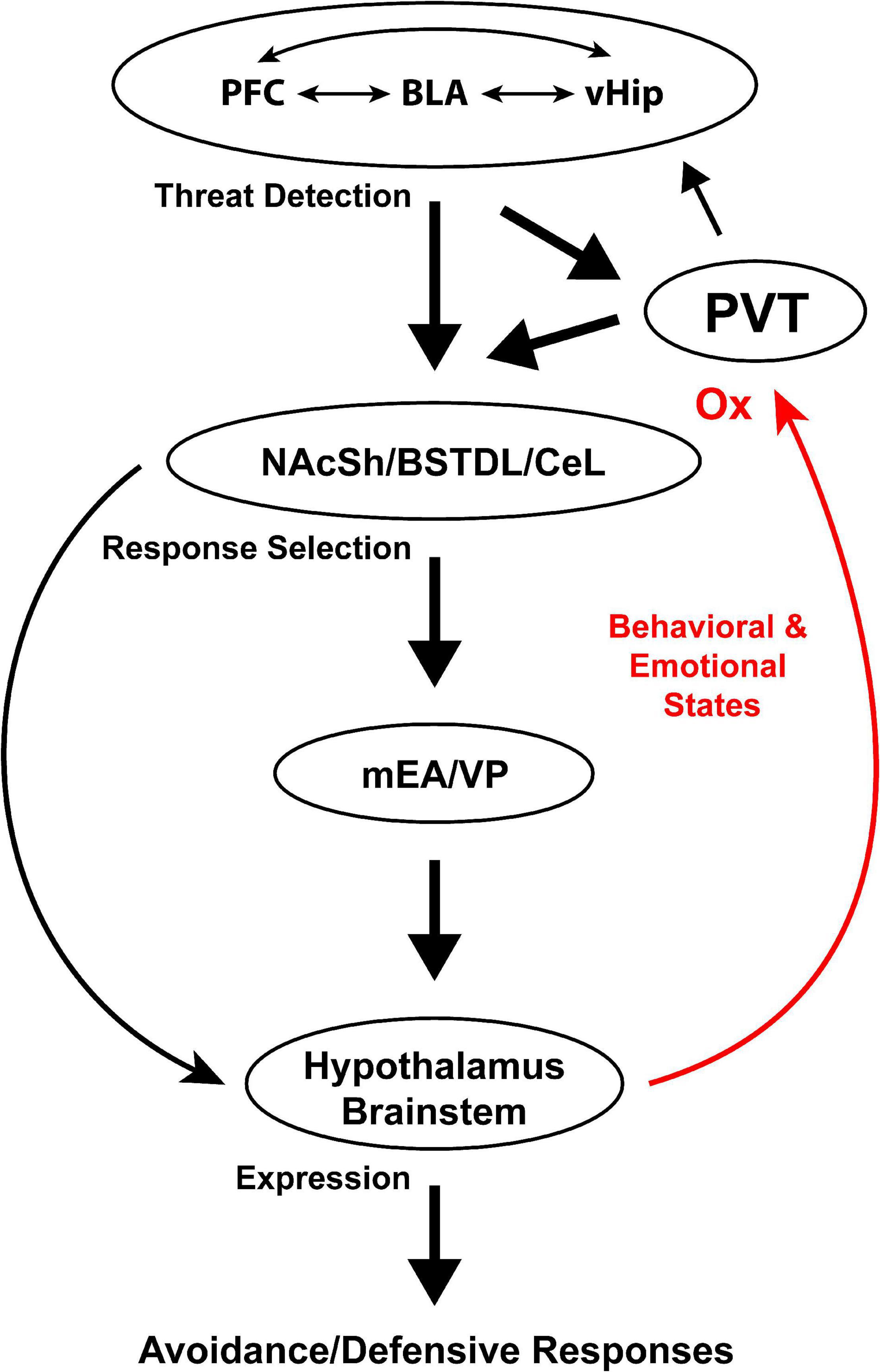
Figure 1. Anatomical connections of the PVT with components of the anxiety network. BLA, basolateral amygdala; BSTDL, dorsolateral region of the bed nucleus of the stria terminalis; CeL, lateral region of the central nucleus of the amygdala; LH, lateral hypothalamus; mEA, medial extended amygdala; NAcSh, shell of the nucleus accumbens; OX, orexins; PFC, prefrontal cortex; PVT, paraventricular nucleus of the thalamus; vHip, ventral hippocampus; VP, ventral pallidum; VTA, ventral tegmental area.
The PVT receives afferents from many regions of the hypothalamus including a significant input from the dorsomedial nucleus of the hypothalamus (Li and Kirouac, 2012). The dorsomedial nucleus of the hypothalamus has been shown to contribute to arousal, stress and anxiety (Fontes et al., 2011; Johnson and Shekhar, 2012) and signals from this region of the hypothalamus to the PVT may be critical for promoting anxiety. In addition, retrograde tracing studies have reported that the PVT receives afferent input from neurons scattered in numerous regions of the brainstem (Krout and Loewy, 2000a, b; Krout et al., 2002). In our comparative analysis of all sources of input to the PVT, regions of the brainstem associated with viscerosensory and motor functions including the periaqueductal gray and parabrachial nucleus were found to be the most prominent source of input to the PVT, whereas adrenergic cell groups including those found in the locus ceruleus and other regions of the brainstem were found to be comparatively minor sources of afferents to the PVT (Kirouac et al., 2006; Li and Kirouac, 2012; Li et al., 2014a). The PVT also receives a relatively weak dopaminergic projection that originates from neurons scattered in the hypothalamus and the ventrolateral periaqueductal gray but not the ventral tegmental area (Li et al., 2014a). It is notable that the PVT receives afferents from neurons scattered in a variety of regions in the hypothalamus and brainstem that can be broadly described as having functions related to the modulation of behavioral states in addition to the relay of viscerosensory and nociceptive information (Li and Kirouac, 2012). It is also of interest that many of the neurons in the brainstem and hypothalamus that innervate the PVT produce neuropeptides that may be involved in signaling states of arousal and stress (Freedman and Cassell, 1991; Battaglia et al., 1992; Otake and Nakamura, 1995; Haskell-Luevano et al., 1999; Kirouac et al., 2005, 2006; Otake, 2005; Clark et al., 2011; Hermes et al., 2013; Lee et al., 2015). For example, orexin peptides have been shown to be involved in the regulation of behavioral states (Peyron et al., 1998; Berridge et al., 2010; Boutrel et al., 2010) including those involving aversive and stressful events (Ida et al., 2000; Zhu et al., 2002; Espana et al., 2003; Winsky-Sommerer et al., 2004; Furlong et al., 2009). As previously reviewed, the bottom-up projections to the PVT are postulated to form an ascending emotional arousal system (van der Werf et al., 2002; Kirouac, 2015).
The PVT as an Emotional Arousal and Stress Responsive Region
Neurons that innervate the PVT originate from regions of the brain that have functions that can be broadly defined as being involved in mediating behavioral or emotional states. For instance, the parabrachial nucleus is a major ascending relay center for viscerosensory and nociceptive information from the body to the forebrain including the PVT (Saper and Loewy, 1980; Gauriau and Bernard, 2002). The hypothalamic inputs originate from neurons located in many regions of the hypothalamus with many of these neurons producing neuropeptides linked to a variety of functions including food intake, arousal, and stress (Freedman and Cassell, 1994; Li and Kirouac, 2012; Colavito et al., 2015; Kirouac, 2015). This suggests that the PVT integrates signals related to a variety of behavioral states including those involved in physiological and psychological challenges. Indeed, many studies have reported that the PVT is activated by exposure of rats to stressful or aversive conditions including restraint (Cullinan et al., 1995; Bhatnagar and Dallman, 1998), tail pinch and footshocks (Smith et al., 1997; Bubser and Deutch, 1999; Yasoshima et al., 2007; Baisley et al., 2011), swimming stress (Cullinan et al., 1995; Zhu et al., 2011), predator scent (Baisley et al., 2011), ultrasonic vocalizations in the dysphoric range (Beckett et al., 1997), aversive visceral stimulation (Yasoshima et al., 2007), and exposure to a context and cues associated with aversive experiences (Beck and Fibiger, 1995; Yasoshima et al., 2007; Padilla-Coreano et al., 2012). Stress-induced activation of the PVT appears to have functional implications since a number of studies have shown that the PVT modulates the neuroendocrine and behavioral responses to chronic stress (Bhatnagar and Dallman, 1998; Bhatnagar et al., 2000, 2002). For example, the pPVT has been shown to be necessary for both the habituation and facilitation of the hypothalamic pituitary axis (HPA) to chronic stress (Bhatnagar and Dallman, 1998, 1999; Bhatnagar et al., 2000, 2002). The HPA response may be facilitated or enhanced in a way that promotes anxiety when an organism encounters novel challenges. In addition, the PVT has been shown to contribute to learned behavioral responses of rodents exposed to aversive and stressful conditions (Li et al., 2011; Padilla-Coreano et al., 2012; Do-Monte et al., 2015; Zhu et al., 2016). More recent evidence indicates that motivational conflicts having both appetitive and aversive consequences selectively activate projection specific neurons in the PVT in a way that promotes a unique behavioral response (Choi and McNally, 2017; Choi et al., 2019). Furthermore, the frustration effect of sucrose reward omission produces a change in how neurons in the PVT that project to the NAcSh or CeL subsequently influence further sucrose seeking (Do-Monte et al., 2017). In summary, the PVT represents a brain region that is generally active during states of high arousal including stressful and aversive conditions as well as the cues previously associated with these conditions (Kirouac, 2015; Do Monte et al., 2016; Barson et al., 2020). This places the PVT in a position to integrate and relay threat- and stress-related signals to the NAcSh and cEA where modulation of local circuits may be involved in the selection of appropriate defensive responses via descending projections. Depending on the situation and proximity of a potential threat, this could involve ceasing normal behavioral activity including all appetitive behaviors, completely stopping all movement (freezing), moving away, or hiding from the perceived threat (Steimer, 2002; Calhoon and Tye, 2015; LeDoux and Daw, 2018).
Orexin Neurotransmission to the PVT and Stress-Induced Anxiety
The orexin (hypocretin) peptides are exclusively found in neurons of the lateral and perifornical region of the posterior hypothalamus (Peyron et al., 1998; Sakurai et al., 1998). The bioactive orexin-A (OXA) and orexin-B (OXB) peptides are produced by the cleavage of prepro-orexin (ppOX). Orexins act at G protein-coupled receptors called the orexin-1 receptor (OX1R), which is selective for OXA, and the orexin-2 receptor (OX2R), which is non-selective for OXA and OXB (Sakurai et al., 1998; de Lecea, 2012). Orexin neurons have widespread projections to regions of the brain that regulate arousal and behavioral states (Peyron et al., 1998; Berridge et al., 2010; Boutrel et al., 2010). The PVT contains an especially impressive plexus of orexin fibers (Kirouac et al., 2005, 2006) where orexins act to promote wakefulness (Ren et al., 2018); drug (Matzeu et al., 2014) and food reward seeking (Choi et al., 2010; Meffre et al., 2019); and attribution of salience to reward cues (Haight et al., 2020). Orexin neurons become active when animals are exposed to aversive conditions (Ida et al., 2000; Zhu et al., 2002; Espana et al., 2003; Winsky-Sommerer et al., 2004; Furlong et al., 2009) and experimental evidence indicates that orexins modulate the physiological, hormonal and behavioral responses to stress via action in the brain (Kayaba et al., 2003; Furlong et al., 2009; Zhang et al., 2010; Heydendael et al., 2011; Grafe and Bhatnagar, 2018).
Experimental evidence indicates that orexins are involved in fear and anxiety. For instance, systemic administration of a non-specific orexin receptor antagonist reduces fear potentiated startle and the increases in heart rate and blood pressure that are produced when rats are placed in the context previously associated with footshocks (Furlong et al., 2009; Steiner et al., 2012). Research by our own group found that the level of ppOX mRNA is increased in the hypothalamus of rats that developed anxiety after a single exposure of inescapable footshocks (Chen et al., 2014). The increase in mRNA lasted for a couple of weeks and appeared to be related to an arousal-related increase in orexin neuron activity (Chen et al., 2014). Our group also reported that systemic injections of OX1R or OX2R antagonists as well as a non-specific orexin antagonist in shocked rats reduced contextual fear and the avoidance tendencies that resulted from exposing rodents to inescapable footshocks (Chen et al., 2014; Wang et al., 2017). There is also evidence that the orexin system is critical for the expression of the autonomic and behavioral changes associated with a CO2-panic provocation model of panic anxiety (Bonaventure et al., 2017). Another group has shown that administration of the OXA peptide in the cerebral ventricles elicits anxiety-like behaviors in both mice and rats (Suzuki et al., 2005). There is also preclinical and clinical evidence that an enhanced level of orexin activity may contribute to the higher incidence of anxiety in females (Grafe and Bhatnagar, 2020).
The areas of the brain where the orexin peptides or antagonists act to modulate anxiety and fear remained largely unexplored until recently. Orexin fibers and receptors are found in many of the regions of the anxiety network including the BSTDL and CeL (Peyron et al., 1998; Marcus et al., 2001) and administrations of orexins in these areas of the cEA were reported to produce anxiety-like responses (Lungwitz et al., 2012; Avolio et al., 2014). The PVT contains a relatively high density of orexin fibers compared to what is present in the BSTDL and CeL (Li et al., 2011) and orexin fibers make putative synaptic contacts with neurons that project to the NAcSh (Kirouac et al., 2005; Parsons et al., 2006). These anatomical observations led us to postulate that orexins could modulate anxiety by acting on PVT neurons that innervate the NAcSh and the rest of the cEA (Li and Kirouac, 2008). In a series of investigations, our research group investigated if orexins act at the PVT to modulate anxiety-like behaviors in rats. First, we found that injections of the OXA and OXB peptides in the pPVT region decreased locomotor activity, increased bouts of immobility and avoidance of the center of an open field (Li et al., 2009, 2010a). In another study, we found that injections of the orexin peptides in the pPVT resulted in avoidance of the open arms and increased ethological behaviors in the EPM indicative of an anxiety state (Li et al., 2010b). In contrast to these findings, injections of GABA agonists in the pPVT was reported to decrease the time spent in the open arm of the EPM (Barson and Leibowitz, 2015) indicating that the PVT’s effect on anxiety may be complex and involves multiple neurotransmitters or neuromodulators. We speculated that activation of orexin receptors in the PVT enhances the saliency of threats (e.g., open spaces, novel objects, and bright lights). To further establish that endogenously released orexins modulated anxiety by acting at the PVT, our research group demonstrated that administrations of a specific OX2R antagonist in the pPVT attenuate anxiety-like behaviors in rats that had received footshocks 24 h prior to the EPM test (Li et al., 2010a). It is notable that the anxiolytic effects of the orexin antagonist were only observed in rats that had been previously shocked indicating that anxiogenic effects of orexins are only present in rats exposed to an acute fear-inducing situation. While the pPVT is involved in conditioned fear to discrete auditory cues (Li et al., 2014b; Do-Monte et al., 2015; Penzo et al., 2015), administration of an non-specific orexin antagonist in the pPVT during the fear expression test has no effect on freezing to conditioned tones (Dong et al., 2015). Interestingly, contextual fear expression was also not affected by blocking of orexin receptors in the pPVT while the same treatment decreased social avoidance and anxiety-like responses in the open field (Dong et al., 2015).
In summary, stress and anxiety are complementary states that engage many of the same neural circuits (Bystritsky and Kronemyer, 2014). Orexin neurons are more active under conditions of high arousal including exposure to stressful and aversive situations. High levels of arousal are likely to activate stress-responsive areas of the brain and promote anxiety by increasing the saliency of emotionally relevant cues including potential threats (Mahler et al., 2014). The effect of an acute but intense stress event on orexin neurons has been shown to last for days and up to several weeks (Chen et al., 2014). It is also noteworthy that the actions of orexins on PVT neurons in response to stressful situations or challenges may lead to neuroplastic changes that may make the PVT more sensitive to novel challenges (Heydendael et al., 2011). Accordingly, stress may make orexin neurons more responsive to arousing conditions leading to an enhanced sensitivity of the PVT neurons to novelty and potential threats. The arousal- and threat-related signals may increase the activity of PVT neurons that relay this amplified signal to NAcSh, BSTDL, and CeL.
Neural Pathway for PVT Modulation of Anxiety
There is experimental evidence that the PVT mediates freezing to conditioned tones as well as the immediate anxiogenic effects of footshocks (Li et al., 2014b; Do-Monte et al., 2015; Penzo et al., 2015; Pliota et al., 2018) via a projection to the CeL (Do-Monte et al., 2015; Penzo et al., 2015; Pliota et al., 2018). The pPVT may have a greater influence on fear and anxiety because this region of the PVT projects densely to the BSTDL and CeL (Li and Kirouac, 2008). There is an implicit assumption that a subpopulation of projection-specific neurons in the pPVT may mediate the defensive responses. For example, a PVT-CeL projection may mediate the behavioral freezing link to fear, whereas projections to the NAcSh or BSTDL may mediate the avoidance induced by potential threats. However, the idea that subpopulations of projection-specific neurons mediate unique defensive responses may be an oversimplification because recent anatomical evidence shows that PVT neurons have axons that bifurcate to innervate multiple targets (Unzai et al., 2015; Dong et al., 2017). Indeed, a detailed analysis and mapping of projection neurons in the PVT revealed that most neurons in the PVT innervate the NAcSh and that many of these neurons issue collaterals to the BSTDL and CeL (Dong et al., 2017). Neurons that project to the NAcSh, BSTDL, and CeL are intermixed throughout the aPVT and pPVT and do not form clusters of unique subpopulations of projection specific neurons. One caveat to this statement is that neurons that innervate the core of the nucleus accumbens and the ventromedial region of the shell (vmNAcSh) are located slightly more dorsally and laterally in the PVT, respectively, than those innervating the dorsomedial region of the shell (dmNAcSh). However, there are some notable differences in terms of the number of neurons in the aPVT and pPVT that project to various subcortical regions. As shown in Figure 2, neurons that project to the vmNAcSh and core of the nucleus accumbens are more likely to originate from the pPVT. These pPVT neurons are also more likely to send collaterals that innervate the BSTDL and CeL. In contrast, neurons that innervate the dmNAcSh are more likely to originate in the aPVT and are less likely to project to the BSTDL and CeL. This points to the possibility that PVT neurons that innervate the vmNAcSh along with their collaterals to the BSTDL and CeL may form a projection system that may be involved in mediating aversive or defensive responses. Our group recently tested this hypothesis by examining if chemogenetic inhibition of PVT neurons that project to the vmNAcSh interfered with the lasting behavioral changes produced by exposing rats to a single episode of inescapable foothocks (Dong et al., 2020). An intersectional chemogenetic approach was used to demonstrate that inhibition of PVT neurons that project to the vmNAcSh attenuates the lasting social avoidance that develops following exposure of rats to footshock stress. Interestingly, anxiety-like behaviors in the open field and contextual fear expression were unaffected by the same manipulation. Evidence that the projection to the vmNAcSh was involved was provided by showing that injections in the vmNAcSh of the agonist for a designer receptor exclusively activated by a designer drug (DREADD) had the same effect as systemic injections of the agonist. Furthermore, expression of the immediate early gene cFos was use to show that these effects were mediated by neurons in the NAcSh that contain the opioid peptide dynorphin (Dong et al., 2020). Dynorphin containing medium spiny neurons in the NAcSh have been shown to mediate the aversive effects of stress on behavior (Newton et al., 2002; Barrot et al., 2005; Bruchas et al., 2008; Land et al., 2008; Al-Hasani et al., 2015). The exact region of the NAcSh critical for social avoidance remains unknown because the intersectional DREADDs approach resulted in fibers being transduced in much of the medial NAcSh (Dong et al., 2020). Another unresolved question is whether the anxiety-like behaviors in the open field and/or the freezing associated with contextual fear expression are mediated by fiber collaterals to the BSTDL and CeL that originate from the same PVT-vmNAcSh projection neurons that mediate social avoidance. Indeed, PVT-NAcSh projecting neurons that contribute to social avoidance could also mediate decreases in exploratory behavior and freezing via collaterals to the BSTDL and CeL depending on the situational factors present during the test condition (i.e., presence of a social target, open areas, or shock context). A population of PVT neurons that send divergent projections to the NAcSh, BSTDL, and CeL could provide signals that help coordinate the selection or expression of different defensive responses.
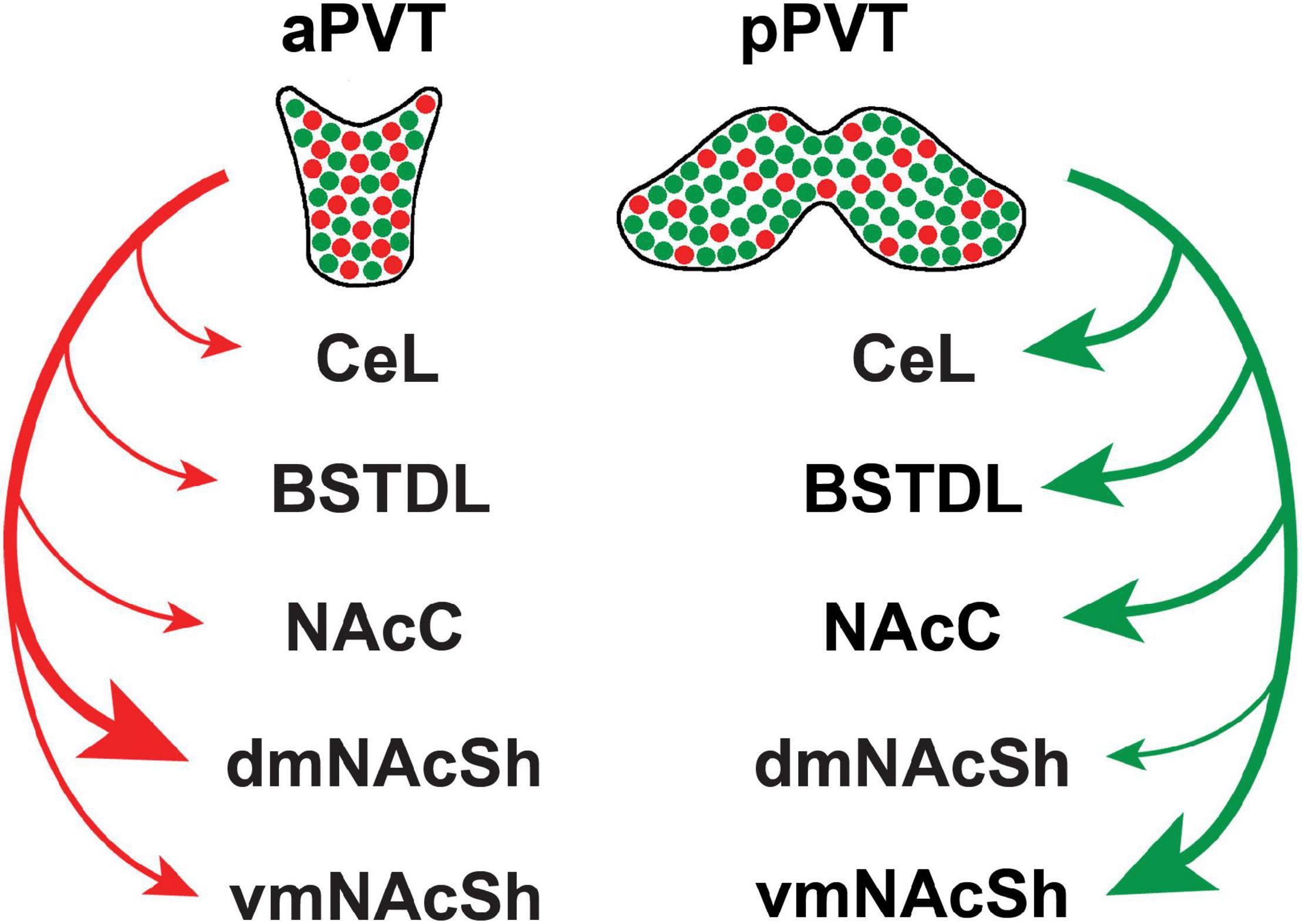
Figure 2. Summary of the efferent projections of the PVT based on recent retrograde tracing experiments involving various combinations of injections of the tracer in subcortical targets of the PVT (Dong et al., 2017). Projections appear to originate from two major population of intermixed neurons that preferentially innervate the dmNAcSh (red) and vmNAcSh (green) along with their collateral projections to other subcortical regions. The size of the arrow is indicative of the strength of the projection based on the number of retrograde-labeled neurons in the PVT from injections of cholera toxin B in the central nucleus of the amygdala (CeL), dorsolateral region of the bed nucleus of the stria terminalis (BSTDL), core of the nucleus accumbens (NacC), dorsomedial aspect of the shell of the nucleus accumbens (dmNAcSh), and ventromedial aspect of the shell of the nucleus accumbens (vmNAcSh). aPVT, anterior aspect of the paraventricular nucleus of the thalamus; pPVT, posterior aspect of the paraventricular nucleus of the thalamus.
Model for How the PVT Contributes to Anxiety
Neurons in the NAcSh and cEA integrate signals from a number of sources including the thalamus, cortex and other areas of the brain resulting in the selection and expression of appropriate behavioral responses via activation of multisynaptic descending pathways (Pennartz et al., 1994; Cardinal et al., 2002; Zahm, 2006; Nicola, 2007; Humphries and Prescott, 2010; Floresco, 2015). This canonical view of the corticostriatal circuits posits that striatal neurons integrate signals and select appropriate responses based on previous learning contingencies and the behavioral state of the organism. An intriguing possibility is that the PVT’s divergent projections could contribute to different types of defensive responses based on situational factors present as well as the emotional or behavioral state of the organism. For example, activation of PVT fibers in the NAcSh could promote social avoidance if a social contact is present, activation of fibers to the BSTDL could support avoidance of open spaces and decrease foraging behavior, whereas activation of PVT fibers to the CeL could support freezing to cues and contexts previously associated with an aversive event. We know from studies using the expression of cFos that PVT neurons are active during states of high arousal including exposure to stressful/aversive conditions and presentation of cues/contexts signaling potential threats (Silveira et al., 2001; Linden et al., 2003, 2005; Salome et al., 2004; Hale et al., 2008; Galvis-Alonso et al., 2010; Kirouac, 2015). As shown in Figure 1, the PVT receives afferents from a number of brain regions involved in arousal and threat detection. Prefrontal cortical areas may relay threat-related signals to the PVT since these cortical areas have been shown to be involved in contextual fear, fear generalization and anxiety (Bermudez-Rattoni et al., 1997; Corcoran and Quirk, 2007; Laurent and Westbrook, 2008; Biedenkapp and Rudy, 2009; Stevenson, 2011; Alves et al., 2013; Kheirbek et al., 2013; Jiang et al., 2014; Rozeske et al., 2015; Wang et al., 2015; Zhang et al., 2015). The prelimbic cortex may be especially critical since it is activated by anxiogenic conditions (Linden et al., 2003, 2005; Hale et al., 2008; Wall et al., 2012) and has been shown to play a role in generating anxiety-like responses (Jinks and McGregor, 1997; Lacroix et al., 2000; Sullivan and Gratton, 2002; Shah and Treit, 2003, 2004; Lisboa et al., 2010; Yamada et al., 2015; Suzuki et al., 2016; Shimizu et al., 2018). The dorsomedial nucleus of the hypothalamus projects significantly to the PVT (Thompson et al., 1996; Li and Kirouac, 2012) and is another potential source of anxiety-related signals because activation of this hypothalamic nucleus has been shown to generate panic and anxiety (Fontes et al., 2011; Johnson and Shekhar, 2012). Finally, the PVT contains a variety of peptidergic fibers that originate from neurons in the hypothalamus and brainstem (Freedman and Cassell, 1994; Otake and Nakamura, 1995; Kirouac et al., 2005, 2006; Otake, 2005). These peptides could signal emotional and behavioral states similar to what has been shown for the orexins (Li et al., 2010a, b; Dong et al., 2015).
Summary and Future Directions
The model emphasizes the hypothesis that the PVT integrates top-down signals related to potential threats with bottom-up signals related to emotional and behavioral states to energize defensive responses by activating descending pathways in the NAcSh and cEA. Cortical areas where the memory of aversive experience is processed and stored would provide the key signals that trigger striatal neurons to generate defensive response. The model advances the view that the PVT receives and integrates threat-related signals from the cortex along with behavioral or emotional state signals from the hypothalamus and brainstem. In this model, the PVT serves to integrate threat and situational information in a way that promotes appropriate defensive responses via its divergent projections to the NAcSh and cEA. The model also proposes that the PVT serves to promote or amplify the influence of the cortex on subcortical regions. The proposed model is focused on how the PVT regulates defensive behaviors. Nonetheless, the model is also pertinent for understanding how the PVT mediates appetitive behaviors. For example, recent evidence shows that signals from orexin and prelimbic cortical neurons converge and act at the PVT to modulate reward seeking responses to cues in a manner similar to what is predicted by the model (Otis et al., 2017, 2019; Campus et al., 2019).
Going forward it will be important to design experiments in which the contribution of the PVT on complex behavior can be examined in experimental situations where both appetitive and aversive outcomes are possible as recently done by some research groups (Do-Monte et al., 2015; Zhu et al., 2018; Choi et al., 2019). It would also be of interest to know if neurons in the PVT affect the behavior produced in the Vogel or the Geller and Seifter conflict tests of anxiety where rodents are punished by electrical shocks when trying to consume food or water (Millan, 2003). It will also be essential for future studies to consider how simultaneous activation of PVT fibers to multiple subcortical target regions affects behavior. This could involve determining how synchronized modulation of different collateral terminal sites affects behavioral responses driven by complex contingencies or behavioral states. A combination of opto- and chemogenetic approaches might be useful despite the technical challenges involved. It will also be of importance to determine how cortical inputs interact with PVT inputs at subcortical levels. For example, are signals from the prefrontal cortex amplified by the PVT in a manner that enhances the threat response driven by activity from the prefrontal cortex to the NAcSh, BSTDL, and CeL? It is clear that there are many challenges in studying the PVT especially when we consider the complexity of the PVT’s connections and the multitude of factors that modulate behavior.
Author Contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Funding
This work was supported by the Canadian Institutes of Health Research (CIHR); Grant No. MOP89758 (to GJK).
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The figures were produced with the help of Sa Li (University of Manitoba).
References
Adhikari, A. (2014). Distributed circuits underlying anxiety. Front. Behav. Neurosci. 8:112. doi: 10.3389/fnbeh.2014.00112
Adhikari, A., Topiwala, M. A., and Gordon, J. A. (2010). Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron 65, 257–269. doi: 10.1016/j.neuron.2009.12.002
Adhikari, A., Topiwala, M. A., and Gordon, J. A. (2011). Single units in the medial prefrontal cortex with anxiety-related firing patterns are preferentially influenced by ventral hippocampal activity. Neuron 71, 898–910. doi: 10.1016/j.neuron.2011.07.027
Ahrens, S., Wu, M. V., Furlan, A., Hwang, G. R., Paik, R., Li, H., et al. (2018). A central extended amygdala circuit that modulates anxiety. J. Neurosci. 38, 5567–5583. doi: 10.1523/jneurosci.0705-18.2018
Al-Hasani, R., Mccall, J. G., Shin, G., Gomez, A. M., Schmitz, G. P., Bernardi, J. M., et al. (2015). Distinct subpopulations of nucleus accumbens dynorphin neurons drive aversion and reward. Neuron 87, 1063–1077. doi: 10.1016/j.neuron.2015.08.019
Alheid, G. F., De Olmos, J. S., and Beltramino, C. A. (eds) (1995). Amygdala and Extended Amygdala. San Diego, CA: Academic.
Alves, F. H., Gomes, F. V., Reis, D. G., Crestani, C. C., Correa, F. M., Guimaraes, F. S., et al. (2013). Involvement of the insular cortex in the consolidation and expression of contextual fear conditioning. Eur. J. Neurosci. 38, 2300–2307. doi: 10.1111/ejn.12210
Anderson, E. M., Sun, H., Guzman, D., Taniguchi, M., Cowan, C. W., Maze, I., et al. (2018). Knockdown of the histone di-methyltransferase G9a in nucleus accumbens shell decreases cocaine self-administration, stress-induced reinstatement, and anxiety. Neuropsychopharmacology 44, 1370–1376. doi: 10.1038/s41386-018-0305-4
Aragona, B. J., Liu, Y., Yu, Y. J., Curtis, J. T., Detwiler, J. M., Insel, T. R., et al. (2006). Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat. Neurosci. 9, 133–139. doi: 10.1038/nn1613
Asok, A., Draper, A., Hoffman, A. F., Schulkin, J., Lupica, C. R., and Rosen, J. B. (2018a). Optogenetic silencing of a corticotropin-releasing factor pathway from the central amygdala to the bed nucleus of the stria terminalis disrupts sustained fear. Mol. Psychiatry 23, 914–922. doi: 10.1038/mp.2017.79
Asok, A., Kandel, E. R., and Rayman, J. B. (2018b). The neurobiology of fear generalization. Front. Behav. Neurosci. 12:329. doi: 10.3389/fnbeh.2018.00329
Avolio, E., Biasone, A., Mele, M., and Alo, R. (2014). Distinct anxiogenic/anxiolytic effects exerted by the hamster lateral amygdalar nucleus injected with ORX-A or ORX-B in the presence of a GABAergic agonist. Neuroreport 25, 932–937. doi: 10.1097/wnr.0000000000000213
Baisley, S. K., Cloninger, C. L., and Bakshi, V. P. (2011). Fos expression following regimens of predator stress versus footshock that differentially affect prepulse inhibition in rats. Physiol. Behav. 104, 796–803. doi: 10.1016/j.physbeh.2011.08.001
Barrot, M., Wallace, D. L., Bolanos, C. A., Graham, D. L., Perrotti, L. I., Neve, R. L., et al. (2005). Regulation of anxiety and initiation of sexual behavior by CREB in the nucleus accumbens. Proc. Natl. Acad. Sci. U S A. 102, 8357–8362. doi: 10.1073/pnas.0500587102
Barson, J. R., and Leibowitz, S. F. (2015). GABA-induced inactivation of dorsal midline thalamic subregions has distinct effects on emotional behaviors. Neurosci. Lett. 609, 92–96. doi: 10.1016/j.neulet.2015.10.029
Barson, J. R., Mack, N. R., and Gao, W. J. (2020). The paraventricular nucleus of the thalamus is an important node in the emotional processing network. Front. Behav. Neurosci. 14:598469. doi: 10.3389/fnbeh.2020.598469
Battaglia, G., Colacitti, C., and Bentivoglio, M. (1992). The relationship of calbindin-containing neurons with substance P, Leu-enkephalin and cholecystokinin fibres: an immunohistochemical study in the rat thalamus. J. Chem. Neuroanat. 5, 453–464. doi: 10.1016/0891-0618(92)90002-8
Beck, C. H., and Fibiger, H. C. (1995). Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J. Neurosci. 15, 709–720. doi: 10.1523/jneurosci.15-01-00709.1995
Beckett, S. R., Duxon, M. S., Aspley, S., and Marsden, C. A. (1997). Central c-fos expression following 20kHz/ultrasound induced defence behaviour in the rat. Brain Res. Bull. 42, 421–426. doi: 10.1016/s0361-9230(96)00332-2
Berendse, H. W., Galis-De Graaf, Y., and Groenewegen, H. J. (1992). Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J. Comp. Neurol. 316, 314–347. doi: 10.1002/cne.903160305
Berendse, H. W., and Groenewegen, H. J. (1990). Organization of the thalamostriatal projections in the rat, with special emphasis on the ventral striatum. J. Comp. Neurol. 299, 187–228. doi: 10.1002/cne.902990206
Berendse, H. W., and Groenewegen, H. J. (1991). Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience 42, 73–102. doi: 10.1016/0306-4522(91)90151-d
Berg, L., Eckardt, J., and Masseck, O. A. (2019). Enhanced activity of pyramidal neurons in the infralimbic cortex drives anxiety behavior. PLoS One 14:e0210949. doi: 10.1371/journal.pone.0210949
Bermudez-Rattoni, F., Introini-Collison, I., Coleman-Mesches, K., and Mcgaugh, J. L. (1997). Insular cortex and amygdala lesions induced after aversive training impair retention: effects of degree of training. Neurobiol. Learn. Mem. 67, 57–63. doi: 10.1006/nlme.1996.3747
Berridge, C. W., Espana, R. A., and Vittoz, N. M. (2010). Hypocretin/orexin in arousal and stress. Brain Res. 1314, 91–102. doi: 10.1016/j.brainres.2009.09.019
Bhatnagar, S., and Dallman, M. (1998). Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience 84, 1025–1039. doi: 10.1016/s0306-4522(97)00577-0
Bhatnagar, S., and Dallman, M. F. (1999). The paraventricular nucleus of the thalamus alters rhythms in core temperature and energy balance in a state-dependent manner. Brain Res. 851, 66–75. doi: 10.1016/s0006-8993(99)02108-3
Bhatnagar, S., Huber, R., Nowak, N., and Trotter, P. (2002). Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J. Neuroendocrinol. 14, 403–410. doi: 10.1046/j.0007-1331.2002.00792.x
Bhatnagar, S., Viau, V., Chu, A., Soriano, L., Meijer, O. C., and Dallman, M. F. (2000). A cholecystokinin-mediated pathway to the paraventricular thalamus is recruited in chronically stressed rats and regulates hypothalamic-pituitary-adrenal function. J. Neurosci. 20, 5564–5573. doi: 10.1523/jneurosci.20-14-05564.2000
Bi, L. L., Wang, J., Luo, Z. Y., Chen, S. P., Geng, F., Chen, Y. H., et al. (2013). Enhanced excitability in the infralimbic cortex produces anxiety-like behaviors. Neuropharmacology 72, 148–156. doi: 10.1016/j.neuropharm.2013.04.048
Biedenkapp, J. C., and Rudy, J. W. (2009). Hippocampal and extrahippocampal systems compete for control of contextual fear: role of ventral subiculum and amygdala. Learn. Mem. 16, 38–45. doi: 10.1101/lm.1099109
Bonaventure, P., Dugovic, C., Shireman, B., Preville, C., Yun, S., Lord, B., et al. (2017). Evaluation of JNJ-54717793 a novel brain penetrant selective orexin 1 receptor antagonist in two rat models of panic attack provocation. Front. Pharmacol. 8:357. doi: 10.3389/fphar.2017.00357
Boutrel, B., Cannella, N., and De Lecea, L. (2010). The role of hypocretin in driving arousal and goal-oriented behaviors. Brain Res. 1314, 103–111. doi: 10.1016/j.brainres.2009.11.054
Bruchas, M. R., Xu, M., and Chavkin, C. (2008). Repeated swim stress induces kappa opioid-mediated activation of extracellular signal-regulated kinase 1/2. Neuroreport 19, 1417–1422. doi: 10.1097/wnr.0b013e32830dd655
Bubser, M., and Deutch, A. Y. (1999). Stress induces Fos expression in neurons of the thalamic paraventricular nucleus that innervate limbic forebrain sites. Synapse 32, 13–22. doi: 10.1002/(sici)1098-2396(199904)32:1<13::aid-syn2>3.0.co;2-r
Bystritsky, A., and Kronemyer, D. (2014). Stress and anxiety: counterpart elements of the stress/anxiety complex. Psychiatr. Clin. North Am. 37, 489–518.
Cai, L., Bakalli, H., and Rinaman, L. (2012). Yohimbine anxiogenesis in the elevated plus maze is disrupted by bilaterally disconnecting the bed nucleus of the stria terminalis from the central nucleus of the amygdala. Neuroscience 223, 200–208. doi: 10.1016/j.neuroscience.2012.08.008
Calhoon, G. G., and Tye, K. M. (2015). Resolving the neural circuits of anxiety. Nat. Neurosci. 18, 1394–1404. doi: 10.1038/nn.4101
Campus, P., Covelo, I. R., Kim, Y., Parsegian, A., Kuhn, B. N., Lopez, S. A., et al. (2019). The paraventricular thalamus is a critical mediator of top-down control of cue-motivated behavior in rats. eLife 8:e49041.
Canteras, N. S., and Swanson, L. W. (1992). Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. J. Comp. Neurol. 324, 180–194. doi: 10.1002/cne.903240204
Cardinal, R. N., Parkinson, J. A., Hall, J., and Everitt, B. J. (2002). Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 26, 321–352. doi: 10.1016/s0149-7634(02)00007-6
Carvajal, C. (2018). Posttraumatic stress disorder as a diagnostic entity - clinical perspectives. Dialogues Clin. Neurosci. 20, 161–168. doi: 10.31887/dcns.2018.20.3/ccarvajal
Cassell, M. D., Freedman, L. J., and Shi, C. (1999). The intrinsic organization of the central extended amygdala. Ann. N. Y. Acad. Sci. 877, 217–241. doi: 10.1111/j.1749-6632.1999.tb09270.x
Chaudhury, D., Walsh, J. J., Friedman, A. K., Juarez, B., Ku, S. M., Koo, J. W., et al. (2013). Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493, 532–536. doi: 10.1038/nature11713
Chen, X., Li, Y., Li, S., and Kirouac, G. J. (2012). Early fear as a predictor of avoidance in a rat model of post-traumatic stress disorder. Behav. Brain Res. 226, 112–117. doi: 10.1016/j.bbr.2011.09.004
Chen, X., Wang, H., Lin, Z., Li, S., Li, Y., Bergen, H. T., et al. (2014). Orexins (hypocretins) contribute to fear and avoidance in rats exposed to a single episode of footshocks. Brain Struct. Funct. 219, 2103–2118. doi: 10.1007/s00429-013-0626-3
Cheng, J., Wang, J., Ma, X., Ullah, R., Shen, Y., and Zhou, Y. D. (2018). Anterior paraventricular thalamus to nucleus accumbens projection is involved in feeding behavior in a novel environment. Front. Mol. Neurosci. 11:202. doi: 10.3389/fnmol.2018.00202
Choi, D. L., Davis, J. F., Fitzgerald, M. E., and Benoit, S. C. (2010). The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience 167, 11–20. doi: 10.1016/j.neuroscience.2010.02.002
Choi, E. A., Jean-Richard-Dit-Bressel, P., Clifford, C. W. G., and Mcnally, G. P. (2019). Paraventricular thalamus controls behavior during motivational conflict. J. Neurosci. 39, 4945–4958. doi: 10.1523/jneurosci.2480-18.2019
Choi, E. A., and McNally, G. P. (2017). Paraventricular thalamus balances danger and reward. J. Neurosci. 37, 3018–3029. doi: 10.1523/jneurosci.3320-16.2017
Christie, M. J., Summers, R. J., Stephenson, J. A., Cook, C. J., and Beart, P. M. (1987). Excitatory amino acid projections to the nucleus accumbens septi in the rat: a retrograde transport study utilizing D[3H]aspartate and [3H]GABA. Neuroscience 22, 425–439. doi: 10.1016/0306-4522(87)90345-9
Christoffel, D. J., Golden, S. A., Dumitriu, D., Robison, A. J., Janssen, W. G., Ahn, H. F., et al. (2011). IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J. Neurosci. 31, 314–321. doi: 10.1523/jneurosci.4763-10.2011
Clark, S. D., Duangdao, D. M., Schulz, S., Zhang, L., Liu, X., Xu, Y. L., et al. (2011). Anatomical characterization of the neuropeptide S system in the mouse brain by in situ hybridization and immunohistochemistry. J. Comp. Neurol. 519, 1867–1893. doi: 10.1002/cne.22606
Colavito, V., Tesoriero, C., Wirtu, A. T., Grassi-Zucconi, G., and Bentivoglio, M. (2015). Limbic thalamus and state-dependent behavior: the paraventricular nucleus of the thalamic midline as a node in circadian timing and sleep/wake-regulatory networks. Neurosci. Biobehav. Rev. 54, 3–17. doi: 10.1016/j.neubiorev.2014.11.021
Corcoran, K. A., and Quirk, G. J. (2007). Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J. Neurosci. 27, 840–844. doi: 10.1523/jneurosci.5327-06.2007
Craske, M. G., Stein, M. B., Eley, T. C., Milad, M. R., Holmes, A., Rapee, R. M., et al. (2017). Anxiety disorders. Nat. Rev. Dis. Primers 3:17024.
Cullinan, W. E., Herman, J. P., Battaglia, D. F., Akil, H., and Watson, S. J. (1995). Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 64, 477–505. doi: 10.1016/0306-4522(94)00355-9
da Cunha, I. C., Lopes, A. P., Steffens, S. M., Ferraz, A., Vargas, J. C., De Lima, T. C., et al. (2008). The microinjection of AMPA receptor antagonist into the accumbens shell, but not into the accumbens core, induces anxiolysis in an animal model of anxiety. Behav. Brain Res. 188, 91–99. doi: 10.1016/j.bbr.2007.10.023
Daviu, N., Bruchas, M. R., Moghaddam, B., Sandi, C., and Beyeler, A. (2019). Neurobiological links between stress and anxiety. Neurobiol. Stress 11:100191. doi: 10.1016/j.ynstr.2019.100191
de Lecea, L. (2012). Hypocretins and the neurobiology of sleep-wake mechanisms. Prog. Brain Res. 198, 15–24. doi: 10.1016/b978-0-444-59489-1.00003-3
de Olmos, J. S., Beltramino, C. A., and Alheid, G. F. (2004). “Amygdala and extended amygdala of the rat: a cytoarchitectonical, fibroarchitectonical, and chemoarchitectonical survey,” in The Rat Nervous System, 3rd Edn, ed. G. Paxinos (New York, NY: Academic Press), 509–603. doi: 10.1016/b978-012547638-6/50020-1
Do Monte, F. H., Quirk, G. J., Li, B., and Penzo, M. A. (2016). Retrieving fear memories, as time goes by. Mol. Psychiatry 21, 1027–1036. doi: 10.1038/mp.2016.78
Dolen, G., Darvishzadeh, A., Huang, K. W., and Malenka, R. C. (2013). Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501, 179–184. doi: 10.1038/nature12518
Do-Monte, F. H., Minier-Toribio, A., Quinones-Laracuente, K., Medina-Colon, E. M., and Quirk, G. J. (2017). Thalamic regulation of sucrose seeking during unexpected reward omission. Neuron 94:e384.
Do-Monte, F. H., Quinones-Laracuente, K., and Quirk, G. J. (2015). A temporal shift in the circuits mediating retrieval of fear memory. Nature 519, 460–463. doi: 10.1038/nature14030
Dong, X., Li, S., and Kirouac, G. (2019). “Extensive divergence of projections from neurons in the paraventricular nucleus of the thalamus to widespread regions of the forebrain,” in Proceedings of the Society for Neuroscience Annual Meeting, (Chicago, IL).
Dong, X., Li, S., and Kirouac, G. J. (2017). Collateralization of projections from the paraventricular nucleus of the thalamus to the nucleus accumbens, bed nucleus of the stria terminalis, and central nucleus of the amygdala. Brain Struct. Funct. 222, 3927–3943. doi: 10.1007/s00429-017-1445-8
Dong, X., Li, S., and Kirouac, G. J. (2020). A projection from the paraventricular nucleus of the thalamus to the shell of the nucleus accumbens contributes to footshock stress-induced social avoidance. Neurobiol. Stress 13:100266. doi: 10.1016/j.ynstr.2020.100266
Dong, X., Li, Y., and Kirouac, G. J. (2015). Blocking of orexin receptors in the paraventricular nucleus of the thalamus has no effect on the expression of conditioned fear in rats. Front. Behav. Neurosci. 9:161. doi: 10.3389/fnbeh.2015.00161
Duvarci, S., and Pare, D. (2014). Amygdala microcircuits controlling learned fear. Neuron 82, 966–980. doi: 10.1016/j.neuron.2014.04.042
Espana, R. A., Valentino, R. J., and Berridge, C. W. (2003). Fos immunoreactivity in hypocretin-synthesizing and hypocretin-1 receptor-expressing neurons: effects of diurnal and nocturnal spontaneous waking, stress and hypocretin-1 administration. Neuroscience 121, 201–217. doi: 10.1016/s0306-4522(03)00334-8
Floresco, S. B. (2015). The nucleus accumbens: an interface between cognition, emotion, and action. Annu. Rev. Psychol. 66, 25–52. doi: 10.1146/annurev-psych-010213-115159
Folkes, O. M., Baldi, R., Kondev, V., Marcus, D. J., Hartley, N. D., Turner, B. D., et al. (2019). An endocannabinoid-regulated basolateral amygdala-nucleus accumbens circuit modulates sociability. J. Clin. Invest. 130, 1728–1742. doi: 10.1172/jci131752
Fontes, M. A., Xavier, C. H., De Menezes, R. C., and Dimicco, J. A. (2011). The dorsomedial hypothalamus and the central pathways involved in the cardiovascular response to emotional stress. Neuroscience 184, 64–74. doi: 10.1016/j.neuroscience.2011.03.018
Fox, A. S., Oler, J. A., Tromp, Do, P. M., Fudge, J. L., and Kalin, N. H. (2015). Extending the amygdala in theories of threat processing. Trends Neurosci. 38, 319–329. doi: 10.1016/j.tins.2015.03.002
Fox, A. S., and Shackman, A. J. (2017). The central extended amygdala in fear and anxiety: closing the gap between mechanistic and neuroimaging research. Neurosci. Lett. 693, 58–67. doi: 10.1016/j.neulet.2017.11.056
Francis, T. C., Chandra, R., Friend, D. M., Finkel, E., Dayrit, G., Miranda, J., et al. (2015). Nucleus accumbens medium spiny neuron subtypes mediate depression-related outcomes to social defeat stress. Biol. Psychiatry 77, 212–222. doi: 10.1016/j.biopsych.2014.07.021
Frassoni, C., Spreafico, R., and Bentivoglio, M. (1997). Glutamate, aspartate and co-localization with calbindin in the medial thalamus. An immunohistochemical study in the rat. Exp. Brain Res. 115, 95–104. doi: 10.1007/pl00005689
Freedman, L. J., and Cassell, M. D. (1991). Thalamic afferents of the rat infralimbic and lateral agranular cortices. Brain Res. Bull. 26, 957–964. doi: 10.1016/0361-9230(91)90263-j
Freedman, L. J., and Cassell, M. D. (1994). Relationship of thalamic basal forebrain projection neurons to the peptidergic innervation of the midline thalamus. J. Comp. Neurol. 348, 321–342. doi: 10.1002/cne.903480302
Furlong, T. M., Vianna, D. M., Liu, L., and Carrive, P. (2009). Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. Eur. J. Neurosci. 30, 1603–1614. doi: 10.1111/j.1460-9568.2009.06952.x
Galvis-Alonso, O. Y., Garcia, A. M., Orejarena, M. J., Lamprea, M. R., Botelho, S., Conde, C. A., et al. (2010). A combined study of behavior and Fos expression in limbic structures after re-testing Wistar rats in the elevated plus-maze. Brain Res. Bull. 81, 595–599. doi: 10.1016/j.brainresbull.2010.01.007
Gauriau, C., and Bernard, J. F. (2002). Pain pathways and parabrachial circuits in the rat. Exp. Physiol. 87, 251–258. doi: 10.1113/eph8702357
Grafe, L. A., and Bhatnagar, S. (2018). Orexins and stress. Front. Neuroendocrinol. 51:132–145. doi: 10.1016/j.yfrne.2018.06.003
Grafe, L. A., and Bhatnagar, S. (2020). The contribution of orexins to sex differences in the stress response. Brain Res. 1731:145893. doi: 10.1016/j.brainres.2018.07.026
Groenewegen, H. J., Vermeulen-Van Der, Zee, E., Te Kortschot, A., and Witter, M. P. (1987). Organization of the projections from the subiculum to the ventral striatum in the rat. a study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience 23, 103–120. doi: 10.1016/0306-4522(87)90275-2
Gunaydin, L. A., Grosenick, L., Finkelstein, J. C., Kauvar, I. V., Fenno, L. E., Adhikari, A., et al. (2014). Natural neural projection dynamics underlying social behavior. Cell 157, 1535–1551. doi: 10.1016/j.cell.2014.05.017
Haight, J. L., Campus, P., Maria-Rios, C. E., Johnson, A. M., Klumpner, M. S., Kuhn, B. N., et al. (2020). The lateral hypothalamus and orexinergic transmission in the paraventricular thalamus promote the attribution of incentive salience to reward-associated cues. Psychopharmacology (Berl) 237, 3741–3758. doi: 10.1007/s00213-020-05651-4
Hale, M. W., Hay-Schmidt, A., Mikkelsen, J. D., Poulsen, B., Bouwknecht, J. A., Evans, A. K., et al. (2008). Exposure to an open-field arena increases c-Fos expression in a subpopulation of neurons in the dorsal raphe nucleus, including neurons projecting to the basolateral amygdaloid complex. Neuroscience 157, 733–748. doi: 10.1016/j.neuroscience.2008.09.050
Haskell-Luevano, C., Chen, P., Li, C., Chang, K., Smith, M. S., Cameron, J. L., et al. (1999). Characterization of the neuroanatomical distribution of agouti-related protein immunoreactivity in the rhesus monkey and the rat. Endocrinology 140, 1408–1415. doi: 10.1210/endo.140.3.6544
Heimer, L., and Alheid, G. F. (1991). Piecing together the puzzle of basal forebrain anatomy. Adv. Exp. Med. Biol. 295, 1–42. doi: 10.1007/978-1-4757-0145-6_1
Heimer, L., Zahm, D. S., Churchill, L., Kalivas, P. W., and Wohltmann, C. (1991). Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience 41, 89–125. doi: 10.1016/0306-4522(91)90202-y
Hermes, M. L., Kolaj, M., Coderre, E. M., and Renaud, L. P. (2013). Gastrin-releasing peptide acts via postsynaptic BB2 receptors to modulate inward rectifier K+ and TRPV1-like conductances in rat paraventricular thalamic neurons. J. Physiol. 591, 1823–1839. doi: 10.1113/jphysiol.2012.249227
Heydendael, W., Sharma, K., Iyer, V., Luz, S., Piel, D., Beck, S., et al. (2011). Orexins/hypocretins act in the posterior paraventricular thalamic nucleus during repeated stress to regulate facilitation to novel stress. Endocrinology 152, 4738–4752. doi: 10.1210/en.2011-1652
Humphries, M. D., and Prescott, T. J. (2010). The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog. Neurobiol. 90, 385–417. doi: 10.1016/j.pneurobio.2009.11.003
Ida, T., Nakahara, K., Murakami, T., Hanada, R., Nakazato, M., and Murakami, N. (2000). Possible involvement of orexin in the stress reaction in rats. Biochem. Biophys. Res. Commun. 270, 318–323. doi: 10.1006/bbrc.2000.2412
Jennings, J. H., Sparta, D. R., Stamatakis, A. M., Ung, R. L., Pleil, K. E., Kash, T. L., et al. (2013). Distinct extended amygdala circuits for divergent motivational states. Nature 496, 224–228. doi: 10.1038/nature12041
Jiang, Z. C., Pan, Q., Zheng, C., Deng, X. F., Wang, J. Y., and Luo, F. (2014). Inactivation of the prelimbic rather than infralimbic cortex impairs acquisition and expression of formalin-induced conditioned place avoidance. Neurosci. Lett. 569, 89–93. doi: 10.1016/j.neulet.2014.03.074
Jinks, A. L., and McGregor, I. S. (1997). Modulation of anxiety-related behaviours following lesions of the prelimbic or infralimbic cortex in the rat. Brain Res. 772, 181–190. doi: 10.1016/s0006-8993(97)00810-x
Johnson, P. L., and Shekhar, A. (2012). An animal model of panic vulnerability with chronic disinhibition of the dorsomedial/perifornical hypothalamus. Physiol. Behav. 107, 686–698. doi: 10.1016/j.physbeh.2012.03.016
Kalin, N. H., Shelton, S. E., and Davidson, R. J. (2004). The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J. Neurosci. 24, 5506–5515. doi: 10.1523/jneurosci.0292-04.2004
Kayaba, Y., Nakamura, A., Kasuya, Y., Ohuchi, T., Yanagisawa, M., Komuro, I., et al. (2003). Attenuated defense response and low basal blood pressure in orexin knockout mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285, R581–R593.
Kheirbek, M. A., Drew, L. J., Burghardt, N. S., Costantini, D. O., Tannenholz, L., Ahmari, S. E., et al. (2013). Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron 77, 955–968. doi: 10.1016/j.neuron.2012.12.038
Kim, S. Y., Adhikari, A., Lee, S. Y., Marshel, J. H., Kim, C. K., Mallory, C. S., et al. (2013). Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 496, 219–223. doi: 10.1038/nature12018
Kirouac, G. J. (2015). Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neurosci. Biobehav. Rev. 56, 315–329. doi: 10.1016/j.neubiorev.2015.08.005
Kirouac, G. J., Parsons, M. P., and Li, S. (2005). Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res. 1059, 179–188. doi: 10.1016/j.brainres.2005.08.035
Kirouac, G. J., Parsons, M. P., and Li, S. (2006). Innervation of the paraventricular nucleus of the thalamus from cocaine- and amphetamine-regulated transcript (CART) containing neurons of the hypothalamus. J. Comp. Neurol. 497, 155–165. doi: 10.1002/cne.20971
Krout, K. E., Belzer, R. E., and Loewy, A. D. (2002). Brainstem projections to midline and intralaminar thalamic nuclei of the rat. J. Comp. Neurol. 448, 53–101. doi: 10.1002/cne.10236
Krout, K. E., and Loewy, A. D. (2000a). Parabrachial nucleus projections to midline and intralaminar thalamic nuclei of the rat. J. Comp. Neurol. 428, 475–494. doi: 10.1002/1096-9861(20001218)428:3<475::aid-cne6>3.0.co;2-9
Krout, K. E., and Loewy, A. D. (2000b). Periaqueductal gray matter projections to midline and intralaminar thalamic nuclei of the rat. J. Comp. Neurol. 424, 111–141. doi: 10.1002/1096-9861(20000814)424:1<111::aid-cne9>3.0.co;2-3
Labouebe, G., Boutrel, B., Tarussio, D., and Thorens, B. (2016). Glucose-responsive neurons of the paraventricular thalamus control sucrose-seeking behavior. Nat. Neurosci. 19, 999–1002. doi: 10.1038/nn.4331
Lacroix, L., Spinelli, S., Heidbreder, C. A., and Feldon, J. (2000). Differential role of the medial and lateral prefrontal cortices in fear and anxiety. Behav. Neurosci. 114, 1119–1130. doi: 10.1037/0735-7044.114.6.1119
Land, B. B., Bruchas, M. R., Lemos, J. C., Xu, M., Melief, E. J., and Chavkin, C. (2008). The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J. Neurosci. 28, 407–414. doi: 10.1523/jneurosci.4458-07.2008
Laurent, V., and Westbrook, R. F. (2008). Distinct contributions of the basolateral amygdala and the medial prefrontal cortex to learning and relearning extinction of context conditioned fear. Learn. Mem. 15, 657–666. doi: 10.1101/lm.1080108
LeDoux, J., and Daw, N. D. (2018). Surviving threats: neural circuit and computational implications of a new taxonomy of defensive behaviour. Nat. Rev. Neurosci. 19, 269–282. doi: 10.1038/nrn.2018.22
LeDoux, J. E. (2015). Anxious : Using the Brain to Understand and Treat Fear and Anxiety. New York, NY: Viking.
LeDoux, J. E., and Pine, D. S. (2016). Using neuroscience to help understand fear and anxiety: a two-system framework. Am. J. Psychiatry 173, 1083–1093. doi: 10.1176/appi.ajp.2016.16030353
Lee, J. S., Lee, E. Y., and Lee, H. S. (2015). Hypothalamic, feeding/arousal-related peptidergic projections to the paraventricular thalamic nucleus in the rat. Brain Res. 1598, 97–113. doi: 10.1016/j.brainres.2014.12.029
Lee, K. M., Coelho, M. A., Class, M. A., Sern, K. R., Bocz, M. D., and Szumlinski, K. K. (2018). mGlu5 receptor blockade within the nucleus accumbens shell reduces behavioral indices of alcohol withdrawal-induced anxiety in mice. Front. Pharmacol. 9:1306. doi: 10.3389/fphar.2018.01306
Li, S., and Kirouac, G. J. (2008). Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J. Comp. Neurol. 506, 263–287. doi: 10.1002/cne.21502
Li, S., and Kirouac, G. J. (2012). Sources of inputs to the anterior and posterior aspects of the paraventricular nucleus of the thalamus. Brain Struct. Funct. 217, 257–273. doi: 10.1007/s00429-011-0360-7
Li, S., Shi, Y., and Kirouac, G. J. (2014a). The hypothalamus and periaqueductal gray are the sources of dopamine fibers in the paraventricular nucleus of the thalamus in the rat. Front. Neuroanat. 8:136. doi: 10.3389/fnana.2014.00136
Li, Y., Dong, X., Li, S., and Kirouac, G. J. (2014b). Lesions of the posterior paraventricular nucleus of the thalamus attenuate fear expression. Front. Behav. Neurosci. 8:94. doi: 10.3389/fnbeh.2014.00094
Li, Y., Li, S., Sui, N., and Kirouac, G. J. (2009). Orexin-A acts on the paraventricular nucleus of the midline thalamus to inhibit locomotor activity in rats. Pharmacol. Biochem. Behav. 93, 506–514. doi: 10.1016/j.pbb.2009.06.017
Li, Y., Li, S., Wei, C., Wang, H., Sui, N., and Kirouac, G. J. (2010a). Changes in emotional behavior produced by orexin microinjections in the paraventricular nucleus of the thalamus. Pharmacol. Biochem. Behav. 95, 121–128. doi: 10.1016/j.pbb.2009.12.016
Li, Y., Li, S., Wei, C., Wang, H., Sui, N., and Kirouac, G. J. (2010b). Orexins in the paraventricular nucleus of the thalamus mediate anxiety-like responses in rats. Psychopharmacology (Berl) 212, 251–265. doi: 10.1007/s00213-010-1948-y
Li, Y., Wang, H., Qi, K., Chen, X., Li, S., Sui, N., et al. (2011). Orexins in the midline thalamus are involved in the expression of conditioned place aversion to morphine withdrawal. Physiol. Behav. 102, 42–50. doi: 10.1016/j.physbeh.2010.10.006
Liberzon, I., and Abelson, J. L. (2016). Context processing and the neurobiology of post-traumatic stress disorder. Neuron 92, 14–30. doi: 10.1016/j.neuron.2016.09.039
Linden, A. M., Baez, M., Bergeron, M., and Schoepp, D. D. (2003). Increased c-Fos expression in the centromedial nucleus of the thalamus in metabotropic glutamate 8 receptor knockout mice following the elevated plus maze test. Neuroscience 121, 167–178. doi: 10.1016/s0306-4522(03)00393-2
Linden, A. M., Shannon, H., Baez, M., Yu, J. L., Koester, A., and Schoepp, D. D. (2005). Anxiolytic-like activity of the mGLU2/3 receptor agonist LY354740 in the elevated plus maze test is disrupted in metabotropic glutamate receptor 2 and 3 knock-out mice. Psychopharmacology (Berl) 179, 284–291. doi: 10.1007/s00213-004-2098-x
Lisboa, S. F., Stecchini, M. F., Correa, F. M., Guimaraes, F. S., and Resstel, L. B. (2010). Different role of the ventral medial prefrontal cortex on modulation of innate and associative learned fear. Neuroscience 171, 760–768. doi: 10.1016/j.neuroscience.2010.09.048
Lopes, A. P., Da Cunha, I. C., Steffens, S. M., Ferraz, A., Vargas, J. C., De Lima, T. C., et al. (2007). GABAA and GABAB agonist microinjections into medial accumbens shell increase feeding and induce anxiolysis in an animal model of anxiety. Behav. Brain Res. 184, 142–149. doi: 10.1016/j.bbr.2007.07.001
Lopes, A. P., Ganzer, L., Borges, A. C., Kochenborger, L., Januario, A. C., Faria, M. S., et al. (2012). Effects of GABA ligands injected into the nucleus accumbens shell on fear/anxiety-like and feeding behaviours in food-deprived rats. Pharmacol. Biochem. Behav. 101, 41–48. doi: 10.1016/j.pbb.2011.11.013
Louvart, H., Maccari, S., Ducrocq, F., Thomas, P., and Darnaudery, M. (2005). Long-term behavioural alterations in female rats after a single intense footshock followed by situational reminders. Psychoneuroendocrinology 30, 316–324. doi: 10.1016/j.psyneuen.2004.09.003
Lungwitz, E. A., Molosh, A., Johnson, P. L., Harvey, B. P., Dirks, R. C., Dietrich, A., et al. (2012). Orexin-A induces anxiety-like behavior through interactions with glutamatergic receptors in the bed nucleus of the stria terminalis of rats. Physiol. Behav. 107, 726–732. doi: 10.1016/j.physbeh.2012.05.019
Mahler, S. V., Moorman, D. E., Smith, R. J., James, M. H., and Aston-Jones, G. (2014). Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat. Neurosci. 17, 1298–1303. doi: 10.1038/nn.3810
Marcus, J. N., Aschkenasi, C. J., Lee, C. E., Chemelli, R. M., Saper, C. B., Yanagisawa, M., et al. (2001). Differential expression of orexin receptors 1 and 2 in the rat brain. J. Comp. Neurol. 435, 6–25. doi: 10.1002/cne.1190
Martinez, G., Ropero, C., Funes, A., Flores, E., Blotta, C., Landa, A. I., et al. (2002). Effects of selective NMDA and non-NMDA blockade in the nucleus accumbens on the plus-maze test. Physiol. Behav. 76, 219–224. doi: 10.1016/s0031-9384(02)00704-7
Matzeu, A., Zamora-Martinez, E. R., and Martin-Fardon, R. (2014). The paraventricular nucleus of the thalamus is recruited by both natural rewards and drugs of abuse: recent evidence of a pivotal role for orexin/hypocretin signaling in this thalamic nucleus in drug-seeking behavior. Front. Behav. Neurosci. 8:117. doi: 10.3389/fnbeh.2014.00117
McGinty, J. F., and Otis, J. M. (2020). Heterogeneity in the paraventricular thalamus: the traffic light of motivated behaviors. Front. Behav. Neurosci. 14:590528. doi: 10.3389/fnbeh.2020.590528
Meffre, J., Sicre, M., Diarra, M., Marchessaux, F., Paleressompoulle, D., and Ambroggi, F. (2019). Orexin in the posterior paraventricular thalamus mediates hunger-related signals in the nucleus accumbens core. Curr. Biol. 29:e3294.
Mikics, E., Baranyi, J., and Haller, J. (2008). Rats exposed to traumatic stress bury unfamiliar objects–a novel measure of hyper-vigilance in PTSD models? Physiol. Behav. 94, 341–348. doi: 10.1016/j.physbeh.2008.01.023
Millan, E. Z., Ong, Z., and Mcnally, G. P. (2017). Paraventricular thalamus: gateway to feeding, appetitive motivation, and drug addiction. Prog. Brain Res. 235, 113–137. doi: 10.1016/bs.pbr.2017.07.006
Millan, M. J. (2003). The neurobiology and control of anxious states. Prog. Neurobiol. 70, 83–244. doi: 10.1016/s0301-0082(03)00087-x
Moga, M. M., Weis, R. P., and Moore, R. Y. (1995). Efferent projections of the paraventricular thalamic nucleus in the rat. J. Comp. Neurol. 359, 221–238. doi: 10.1002/cne.903590204
Namburi, P., Al-Hasani, R., Calhoon, G. G., Bruchas, M. R., and Tye, K. M. (2016). Architectural representation of valence in the limbic system. Neuropsychopharmacology 41, 1697–1715. doi: 10.1038/npp.2015.358
Nemeroff, C. B., Bremner, J. D., Foa, E. B., Mayberg, H. S., North, C. S., and Stein, M. B. (2006). Posttraumatic stress disorder: a state-of-the-science review. J. Psychiatr. Res. 40, 1–21. doi: 10.1002/9781118356142.ch1
Newton, S. S., Thome, J., Wallace, T. L., Shirayama, Y., Schlesinger, L., Sakai, N., et al. (2002). Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J. Neurosci. 22, 10883–10890. doi: 10.1523/jneurosci.22-24-10883.2002
Nicola, S. M. (2007). The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology (Berl) 191, 521–550. doi: 10.1007/s00213-006-0510-4
Normandeau, C. P., Ventura-Silva, A. P., Hawken, E. R., Angelis, S., Sjaarda, C., Liu, X., et al. (2018). A key role for neurotensin in chronic-stress-induced anxiety-like behavior in rats. Neuropsychopharmacology 43, 285–293. doi: 10.1038/npp.2017.134
O’Mara, S. M., Sanchez-Vives, M. V., Brotons-Mas, J. R., and O’hare, E. (2009). Roles for the subiculum in spatial information processing, memory, motivation and the temporal control of behaviour. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 782–790. doi: 10.1016/j.pnpbp.2009.03.040
Otake, K. (2005). Cholecystokinin and substance P immunoreactive projections to the paraventricular thalamic nucleus in the rat. Neurosci. Res. 51, 383–394. doi: 10.1016/j.neures.2004.12.009
Otake, K., Kin, K., and Nakamura, Y. (2002). Fos expression in afferents to the rat midline thalamus following immobilization stress. Neurosci. Res. 43, 269–282. doi: 10.1016/s0168-0102(02)00042-1
Otake, K., and Nakamura, Y. (1995). Sites of origin of corticotropin-releasing factor-like immunoreactive projection fibers to the paraventricular thalamic nucleus in the rat. Neurosci. Lett. 201, 84–86. doi: 10.1016/0304-3940(95)12148-w
Otake, K., Ruggiero, D. A., and Nakamura, Y. (1995). Adrenergic innervation of forebrain neurons that project to the paraventricular thalamic nucleus in the rat. Brain Res. 697, 17–26. doi: 10.1016/0006-8993(95)00749-g
Otis, J. M., Namboodiri, V. M., Matan, A. M., Voets, E. S., Mohorn, E. P., Kosyk, O., et al. (2017). Prefrontal cortex output circuits guide reward seeking through divergent cue encoding. Nature 543, 103–107. doi: 10.1038/nature21376
Otis, J. M., Zhu, M., Namboodiri, V. M. K., Cook, C. A., Kosyk, O., Matan, A. M., et al. (2019). Paraventricular thalamus projection neurons integrate cortical and hypothalamic signals for cue-reward processing. Neuron 103:e424.
Padilla-Coreano, N., Do-Monte, F. H., and Quirk, G. J. (2012). A time-dependent role of midline thalamic nuclei in the retrieval of fear memory. Neuropharmacology 62, 457–463. doi: 10.1016/j.neuropharm.2011.08.037
Parsons, M. P., Li, S., and Kirouac, G. J. (2006). The paraventricular nucleus of the thalamus as an interface between the orexin and CART peptides and the shell of the nucleus accumbens. Synapse 59, 480–490. doi: 10.1002/syn.20264
Parsons, M. P., Li, S., and Kirouac, G. J. (2007). Functional and anatomical connection between the paraventricular nucleus of the thalamus and dopamine fibers of the nucleus accumbens. J. Comp. Neurol. 500, 1050–1063. doi: 10.1002/cne.21224
Pennartz, C. M., Groenewegen, H. J., and Lopes, and Da Silva, F. H. (1994). The nucleus accumbens as a complex of functionally distinct neuronal ensembles: an integration of behavioural, electrophysiological and anatomical data. Prog. Neurobiol. 42, 719–761. doi: 10.1016/0301-0082(94)90025-6
Penzo, M. A., Robert, V., Tucciarone, J., De Bundel, D., Wang, M., Van Aelst, L., et al. (2015). The paraventricular thalamus controls a central amygdala fear circuit. Nature 519, 455–459. doi: 10.1038/nature13978
Petrovich, G. D., and Swanson, L. W. (1997). Projections from the lateral part of the central amygdalar nucleus to the postulated fear conditioning circuit. Brain Res. 763, 247–254. doi: 10.1016/s0006-8993(96)01361-3
Peyron, C., Tighe, D. K., Van Den Pol, A. N., De Lecea, L., Heller, H. C., Sutcliffe, J. G., et al. (1998). Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 18, 9996–10015. doi: 10.1523/jneurosci.18-23-09996.1998
Piantadosi, P. T., Yeates, D. C. M., and Floresco, S. B. (2018). Cooperative and dissociable involvement of the nucleus accumbens core and shell in the promotion and inhibition of actions during active and inhibitory avoidance. Neuropharmacology 138, 57–71. doi: 10.1016/j.neuropharm.2018.05.028
Pleil, K. E., Rinker, J. A., Lowery-Gionta, E. G., Mazzone, C. M., Mccall, N. M., Kendra, A. M., et al. (2015). NPY signaling inhibits extended amygdala CRF neurons to suppress binge alcohol drinking. Nat. Neurosci. 18, 545–552. doi: 10.1038/nn.3972
Pliota, P., Bohm, V., Grossl, F., Griessner, J., Valenti, O., Kraitsy, K., et al. (2018). Stress peptides sensitize fear circuitry to promote passive coping. Mol. Psychiatry 25, 428–441. doi: 10.1038/s41380-018-0089-2
Rademacher, L., Schulte-Ruther, M., Hanewald, B., and Lammertz, S. (2017). Reward: from basic reinforcers to anticipation of social cues. Curr. Top. Behav. Neurosci. 30, 207–221. doi: 10.1007/7854_2015_429
Ramirez, F., Moscarello, J. M., Ledoux, J. E., and Sears, R. M. (2015). Active avoidance requires a serial basal amygdala to nucleus accumbens shell circuit. J. Neurosci. 35, 3470–3477. doi: 10.1523/jneurosci.1331-14.2015
Ren, S., Wang, Y., Yue, F., Cheng, X., Dang, R., Qiao, Q., et al. (2018). The paraventricular thalamus is a critical thalamic area for wakefulness. Science 362, 429–434. doi: 10.1126/science.aat2512
Resendez, S. L., Keyes, P. C., Day, J. J., Hambro, C., Austin, C. J., Maina, F. K., et al. (2016). Dopamine and opioid systems interact within the nucleus accumbens to maintain monogamous pair bonds. eLife 5:e15325.
Reynolds, S. M., and Berridge, K. C. (2001). Fear and feeding in the nucleus accumbens shell: rostrocaudal segregation of GABA-elicited defensive behavior versus eating behavior. J. Neurosci. 21, 3261–3270. doi: 10.1523/jneurosci.21-09-03261.2001
Reynolds, S. M., and Berridge, K. C. (2002). Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking”/”disliking” reactions, place preference/avoidance, and fear. J. Neurosci. 22, 7308–7320. doi: 10.1523/jneurosci.22-16-07308.2002
Reynolds, S. M., and Berridge, K. C. (2003). Glutamate motivational ensembles in nucleus accumbens: rostrocaudal shell gradients of fear and feeding. Eur. J. Neurosci. 17, 2187–2200. doi: 10.1046/j.1460-9568.2003.02642.x
Reynolds, S. M., and Berridge, K. C. (2008). Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nat. Neurosci. 11, 423–425. doi: 10.1038/nn2061
Rozeske, R. R., Valerio, S., Chaudun, F., and Herry, C. (2015). Prefrontal neuronal circuits of contextual fear conditioning. Genes Brain Behav. 14, 22–36. doi: 10.1111/gbb.12181
Sakurai, T., Amemiya, A., Ishii, M., Matsuzaki, I., Chemelli, R. M., Tanaka, H., et al. (1998). Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92, 573–585. doi: 10.1016/s0092-8674(00)80949-6
Salome, N., Salchner, P., Viltart, O., Sequeira, H., Wigger, A., Landgraf, R., et al. (2004). Neurobiological correlates of high (HAB) versus low anxiety-related behavior (LAB): differential Fos expression in HAB and LAB rats. Biol. Psychiatry 55, 715–723. doi: 10.1016/j.biopsych.2003.10.021
Saper, C. B., and Loewy, A. D. (1980). Efferent connections of the parabrachial nucleus in the rat. Brain Res. 197, 291–317. doi: 10.1016/0006-8993(80)91117-8
Sesack, S. R., Deutch, A. Y., Roth, R. H., and Bunney, B. S. (1989). Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J. Comp. Neurol. 290, 213–242. doi: 10.1002/cne.902900205
Shah, A. A., and Treit, D. (2003). Excitotoxic lesions of the medial prefrontal cortex attenuate fear responses in the elevated-plus maze, social interaction and shock probe burying tests. Brain Res. 969, 183–194. doi: 10.1016/s0006-8993(03)02299-6
Shah, A. A., and Treit, D. (2004). Infusions of midazolam into the medial prefrontal cortex produce anxiolytic effects in the elevated plus-maze and shock-probe burying tests. Brain Res. 996, 31–40. doi: 10.1016/j.brainres.2003.10.015
Shi, T., Feng, S., Wei, M., and Zhou, W. (2020). Role of the anterior agranular insular cortex in the modulation of fear and anxiety. Brain Res. Bull. 155, 174–183. doi: 10.1016/j.brainresbull.2019.12.003
Shimizu, T., Minami, C., and Mitani, A. (2018). Effect of electrical stimulation of the infralimbic and prelimbic cortices on anxiolytic-like behavior of rats during the elevated plus-maze test, with particular reference to multiunit recording of the behavior-associated neural activity. Behav. Brain Res. 353, 168–175. doi: 10.1016/j.bbr.2018.07.005
Shin, L. M., and Liberzon, I. (2010). The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35, 169–191. doi: 10.1038/npp.2009.83
Siegmund, A., and Wotjak, C. T. (2007). A mouse model of posttraumatic stress disorder that distinguishes between conditioned and sensitised fear. J. Psychiatr. Res. 41, 848–860. doi: 10.1016/j.jpsychires.2006.07.017
Silva, C., and McNaughton, N. (2019). Are periaqueductal gray and dorsal raphe the foundation of appetitive and aversive control? a comprehensive review. Prog. Neurobiol. 177, 33–72. doi: 10.1016/j.pneurobio.2019.02.001
Silveira, M. C., Zangrossi, H., De Barros Viana, M., Silveira, R., and Graeff, F. G. (2001). Differential expression of Fos protein in the rat brain induced by performance of avoidance or escape in the elevated T-maze. Behav. Brain Res. 126, 13–21. doi: 10.1016/s0166-4328(01)00233-9
Smith, W. J., Stewart, J., and Pfaus, J. G. (1997). Tail pinch induces fos immunoreactivity within several regions of the male rat brain: effects of age. Physiol. Behav. 61, 717–723. doi: 10.1016/s0031-9384(96)00524-0
Sotres-Bayon, F., and Quirk, G. J. (2010). Prefrontal control of fear: more than just extinction. Curr. Opin. Neurobiol. 20, 231–235. doi: 10.1016/j.conb.2010.02.005
Steimer, T. (2002). The biology of fear- and anxiety-related behaviors. Dial. Clin. Neurosci. 4, 231–249. doi: 10.31887/dcns.2002.4.3/tsteimer
Steiner, M. A., Lecourt, H., and Jenck, F. (2012). The brain orexin system and almorexant in fear-conditioned startle reactions in the rat. Psychopharmacology (Berl) 223, 465–475. doi: 10.1007/s00213-012-2736-7
Steinman, M. Q., Duque-Wilckens, N., and Trainor, B. C. (2019). Complementary neural circuits for divergent effects of oxytocin: social approach versus social anxiety. Biol. Psychiatry 85, 792–801. doi: 10.1016/j.biopsych.2018.10.008
Stevenson, C. W. (2011). Role of amygdala-prefrontal cortex circuitry in regulating the expression of contextual fear memory. Neurobiol. Learn. Mem. 96, 315–323. doi: 10.1016/j.nlm.2011.06.005
Sullivan, R. M., and Gratton, A. (2002). Behavioral effects of excitotoxic lesions of ventral medial prefrontal cortex in the rat are hemisphere-dependent. Brain Res. 927, 69–79. doi: 10.1016/s0006-8993(01)03328-5
Suzuki, M., Beuckmann, C. T., Shikata, K., Ogura, H., and Sawai, T. (2005). Orexin-A (hypocretin-1) is possibly involved in generation of anxiety-like behavior. Brain Res. 1044, 116–121. doi: 10.1016/j.brainres.2005.03.002
Suzuki, S., Saitoh, A., Ohashi, M., Yamada, M., Oka, J., and Yamada, M. (2016). The infralimbic and prelimbic medial prefrontal cortices have differential functions in the expression of anxiety-like behaviors in mice. Behav. Brain Res. 304, 120–124. doi: 10.1016/j.bbr.2016.01.044
Swanson, L. W. (2000). Cerebral hemisphere regulation of motivated behavior. Brain Res. 886, 113–164. doi: 10.1016/s0006-8993(00)02905-x
Thompson, R. H., Canteras, N. S., and Swanson, L. W. (1996). Organization of projections from the dorsomedial nucleus of the hypothalamus: a PHA-L study in the rat. J. Comp. Neurol. 376, 143–173. doi: 10.1002/(sici)1096-9861(19961202)376:1<143::aid-cne9>3.0.co;2-3
Tovote, P., Fadok, J. P., and Luthi, A. (2015). Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci. 16, 317–331. doi: 10.1038/nrn3945
Unzai, T., Kuramoto, E., Kaneko, T., and Fujiyama, F. (2015). Quantitative analyses of the projection of individual neurons from the midline thalamic nuclei to the striosome and matrix compartments of the rat striatum. Cereb. Cortex 27, 1164–1181.
van der Werf, Y. D., Witter, M. P., and Groenewegen, H. J. (2002). The intralaminar and midline nuclei of the thalamus. anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res. Brain Res. Rev. 39, 107–140. doi: 10.1016/s0165-0173(02)00181-9
Ventura-Silva, A. P., Melo, A., Ferreira, A. C., Carvalho, M. M., Campos, F. L., Sousa, N., et al. (2013). Excitotoxic lesions in the central nucleus of the amygdala attenuate stress-induced anxiety behavior. Front. Behav. Neurosci. 7:32. doi: 10.3389/fnbeh.2013.00032
Vertes, R. P. (2004). Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51, 32–58. doi: 10.1002/syn.10279
Vertes, R. P., and Hoover, W. B. (2008). Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J. Comp. Neurol. 508, 212–237. doi: 10.1002/cne.21679
Walker, D. L., Miles, L. A., and Davis, M. (2009). Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 1291–1308. doi: 10.1016/j.pnpbp.2009.06.022
Walker, D. L., Toufexis, D. J., and Davis, M. (2003). Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur. J. Pharmacol. 463, 199–216. doi: 10.1016/s0014-2999(03)01282-2
Wall, V. L., Fischer, E. K., and Bland, S. T. (2012). Isolation rearing attenuates social interaction-induced expression of immediate early gene protein products in the medial prefrontal cortex of male and female rats. Physiol. Behav. 107, 440–450. doi: 10.1016/j.physbeh.2012.09.002
Wang, H., Li, S., and Kirouac, G. J. (2017). Role of the orexin (hypocretin) system in contextual fear conditioning in rats. Behav. Brain Res. 316, 47–53. doi: 10.1016/j.bbr.2016.08.052
Wang, J., Bast, T., Wang, Y. C., and Zhang, W. N. (2015). Hippocampus and two-way active avoidance conditioning: contrasting effects of cytotoxic lesion and temporary inactivation. Hippocampus 25, 1517–1531. doi: 10.1002/hipo.22471
Winsky-Sommerer, R., Yamanaka, A., Diano, S., Borok, E., Roberts, A. J., Sakurai, T., et al. (2004). Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J. Neurosci. 24, 11439–11448. doi: 10.1523/jneurosci.3459-04.2004
Wook Koo, J., Labonte, B., Engmann, O., Calipari, E. S., Juarez, B., Lorsch, Z., et al. (2016). Essential role of mesolimbic brain-derived neurotrophic factor in chronic social stress-induced depressive behaviors. Biol. Psychiatry 80, 469–478. doi: 10.1016/j.biopsych.2015.12.009
Wright, C. I., and Groenewegen, H. J. (1995). Patterns of convergence and segregation in the medial nucleus accumbens of the rat: relationships of prefrontal cortical, midline thalamic, and basal amygdaloid afferents. J. Comp. Neurol. 361, 383–403. doi: 10.1002/cne.903610304
Wright, C. I., and Groenewegen, H. J. (1996). Patterns of overlap and segregation between insular cortical, intermediodorsal thalamic and basal amygdaloid afferents in the nucleus accumbens of the rat. Neuroscience 73, 359–373. doi: 10.1016/0306-4522(95)00592-7
Xu, W., and Sudhof, T. C. (2013). A neural circuit for memory specificity and generalization. Science 339, 1290–1295. doi: 10.1126/science.1229534
Yamada, M., Saitoh, A., Ohashi, M., Suzuki, S., Oka, J., and Yamada, M. (2015). Induction of c-Fos immunoreactivity in the amygdala of mice expressing anxiety-like behavior after local perfusion of veratrine in the prelimbic medial prefrontal cortex. J. Neural. Transm. (Vienna) 122, 1203–1207. doi: 10.1007/s00702-015-1373-9
Yasoshima, Y., Scott, T. R., and Yamamoto, T. (2007). Differential activation of anterior and midline thalamic nuclei following retrieval of aversively motivated learning tasks. Neuroscience 146, 922–930. doi: 10.1016/j.neuroscience.2007.02.044
Zahm, D. S. (1999). Functional-anatomical implications of the nucleus accumbens core and shell subterritories. Ann. N. Y. Acad. Sci. 877, 113–128. doi: 10.1111/j.1749-6632.1999.tb09264.x
Zahm, D. S. (2000). An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci. Biobehav. Rev. 24, 85–105. doi: 10.1016/s0149-7634(99)00065-2
Zahm, D. S. (2006). The evolving theory of basal forebrain functional-anatomical ‘macrosystems’. Neurosci. Biobehav. Rev. 30, 148–172. doi: 10.1016/j.neubiorev.2005.06.003
Zahm, D. S., and Brog, J. S. (1992). On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience 50, 751–767. doi: 10.1016/0306-4522(92)90202-d
Zhang, W., Sunanaga, J., Takahashi, Y., Mori, T., Sakurai, T., Kanmura, Y., et al. (2010). Orexin neurons are indispensable for stress-induced thermogenesis in mice. J. Physiol. 588, 4117–4129. doi: 10.1113/jphysiol.2010.195099
Zhang, Z., Gadotti, V. M., Chen, L., Souza, I. A., Stemkowski, P. L., and Zamponi, G. W. (2015). Role of prelimbic GABAergic circuits in sensory and emotional aspects of neuropathic pain. Cell Rep. 12, 752–759. doi: 10.1016/j.celrep.2015.07.001
Zhu, L., Onaka, T., Sakurai, T., and Yada, T. (2002). Activation of orexin neurones after noxious but not conditioned fear stimuli in rats. Neuroreport 13, 1351–1353. doi: 10.1097/00001756-200207190-00027
Zhu, L., Wu, L., Yu, B., and Liu, X. (2011). The participation of a neurocircuit from the paraventricular thalamus to amygdala in the depressive like behavior. Neurosci. Lett. 488, 81–86. doi: 10.1016/j.neulet.2010.11.007
Zhu, Y., Nachtrab, G., Keyes, P. C., Allen, W. E., Luo, L., and Chen, X. (2018). Dynamic salience processing in paraventricular thalamus gates associative learning. Science 362, 423–429. doi: 10.1126/science.aat0481
Keywords: anxiety, paraventricular nucleus, thalamus, stress, nucleus accumbens, extended amygdala
Citation: Kirouac GJ (2021) The Paraventricular Nucleus of the Thalamus as an Integrating and Relay Node in the Brain Anxiety Network. Front. Behav. Neurosci. 15:627633. doi: 10.3389/fnbeh.2021.627633
Received: 09 November 2020; Accepted: 28 January 2021;
Published: 24 February 2021.
Edited by:
Nuno Sousa, University of Minho, PortugalReviewed by:
Shelly Beth Flagel, University of Michigan, United StatesMaria M. Diehl, Kansas State University, United States
Copyright © 2021 Kirouac. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gilbert J. Kirouac, Gilbert.Kirouac@umanitoba.ca
 Gilbert J. Kirouac
Gilbert J. Kirouac