CD44: molecular interactions, signaling and functions in the nervous system
- Laboratory of Molecular and Systemic Neuromorphology, Nencki Institute of Experimental Biology, Warsaw, Poland
CD44 is the major surface hyaluronan (HA) receptor implicated in intercellular and cell-matrix adhesion, cell migration and signaling. It is a transmembrane, highly glycosylated protein with several isoforms resulting from alternative gene splicing. The CD44 molecule consists of several domains serving different functions: the N-terminal extracellular domain, the stem region, the transmembrane domain and the C-terminal tail. In the nervous system, CD44 expression occurs in both glial and neuronal cells. The role of CD44 in the physiology and pathology of the nervous system is not entirely understood, however, there exists evidence suggesting it might be involved in the axon guidance, cytoplasmic Ca2+ clearance, dendritic arborization, synaptic transmission, epileptogenesis, oligodendrocyte and astrocyte differentiation, post-traumatic brain repair and brain tumour development.
Introduction
CD44 is a transmembrane glycoprotein mediating cell responses to the extracellular microenvironment. Also known as the hermes antigen, Pgp1, MDU3 and Inlu-related p80 glycoprotein, it was first described as a surface molecule present in lymphocytes, thymocytes and granulocytes (Dalchau et al., 1980), and later recognized as a novel human erythrocyte cell surface antigen, lymphocyte homing receptor, and leukocyte surface glycoprotein (Stefanová et al., 1989). Today, CD44 is being described as an adhesion molecule expressed in various cell types, and an important participant in a number of signaling pathways. The present review summarizes the current knowledge on CD44 expression and function in the nervous system.
Structure
CD44 molecule consists of several functional domains. The N-terminal extracellular domain contains motifs serving as docking sites for various ligands—primarily hyaluronan (HA), but also extracellular matrix (ECM) glycoproteins and proteoglycans, growth factors, cytokines and matrix metalloproteinases (Figure 1; Yu and Stamenkovic, 1999; Ponta et al., 2003). The aminoterminal domain of CD44 is separated from the plasma membrane by a short stem structure with proteolytic cleavage sites for such metalloproteinases as disintegrin, metalloproteinase domain-containing proteins 10 and 17 (ADAM-10 and ADAM-17) or membrane type 1-matrix metalloproteinase (MT1–MMP; Okamoto et al., 1999; Nagano and Saya, 2004). The cleavage of CD44 triggers the release of cells bound to HA—a process important in the regulation of cell migration. Notably, the stem region is the variable portion of the protein due to the alternative splicing and insertion of exons 6–15. The C-terminal cytoplasmic tail region plays a crucial role in the CD44 involvement in intracellular signal transduction. It has been shown that it binds to cytoskeletal elements such as ankyrin, the ERM proteins: ezrin, radixin, and moesin, and moesin-ezrin-radixin-like protein (MERLIN; Martin et al., 2003), it also interacts with signaling molecules, namely members of Src family kinases (SFKs) Src, Lck, Fyn and Lyn (Ponta et al., 2003), and activators of small Rho GTPases (Bourguignon et al., 2005). In addition, the cytoplasmic tail of CD44 may be cleaved off by γ-secretase and translocated to the cell nucleus, where it acts as a transcriptional regulator (Nagano and Saya, 2004).
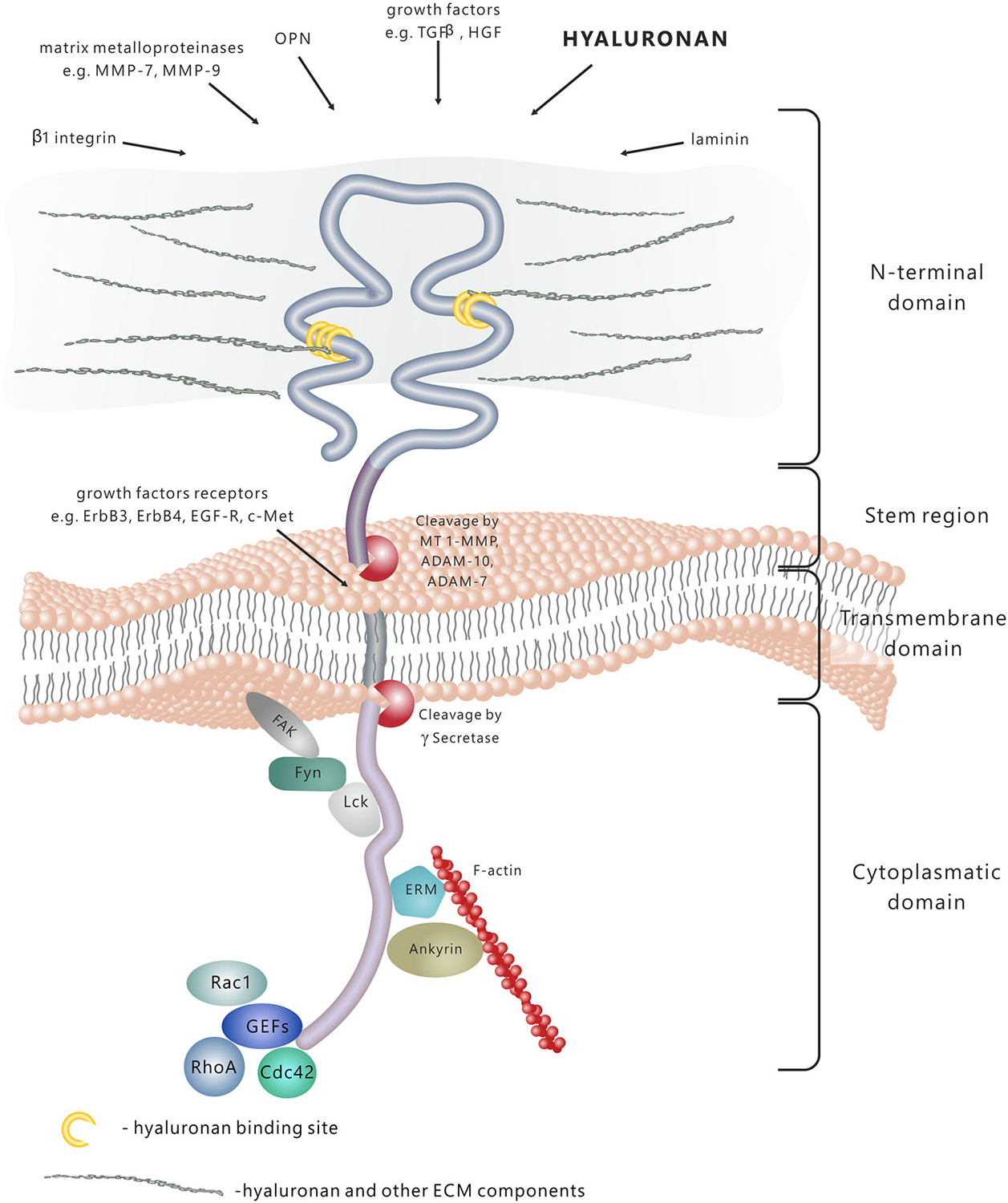
Figure 1. CD44 protein structure and signaling. CD44 is a transmembrane molecule composed of several domains. The N-terminal extracellular domain can bind various ligands, including hyaluronan (HA), extracellular matrix (ECM) glycoproteins and proteoglycans, growth factors, cytokines and matrix metalloproteinases. In result of the proteolytic cleavage within the stem region, the extracellular domain is released into the extracellular space. The transmembrane domain anchors and stabilises the molecule in the plasma membrane. The cytoplasmic domain is responsible for signal transduction through binding to different molecules, including cytoskeleton components, kinases and activators of small Rho GTPases (GEFs-guanine nucleotide exchange factors).
The human gene for CD44 consists of 20 exons and is located in the 11p13 locus (Thorne et al., 2004). The most common form of CD44, also called standard CD44 (CD44s), is encoded by exons 1–5, 16–18 and 20. There also exists a large number of CD44 variants (CD44v), generated by extensive alternative splicing and containing the “standard” exons with a combination of exons 6–15 (Ponta et al., 2003). The predominant CD44 isoform expressed in the nervous system is the standard form (Sretavan et al., 1994; Jones et al., 2000; Bouvier-Labit et al., 2002), although the presence of CD44 splice variants has been demonstrated in normal human brain tissue (Kaaijk et al., 1997), as well as in certain primary tumours of the brain and peripheral nerve (Kaaijk et al., 1995; Sherman et al., 1997; Resnick et al., 1999).
Expression and Activation in the Nervous System
Originally, CD44 expression was reported to take place predominantly in the white matter of the human brain (McKenzie et al., 1982; Bignami and Dahl, 1986; Cruz et al., 1986). Likewise, further studies have shown that the source of CD44 protein was glial cells, in particular astrocytes (Girgrah et al., 1991; Vogel et al., 1992). In vitro studies with the use of primary cell cultures from human foetal and adult brain have evidenced the expression of CD44 in astrocytes and oligodendrocytes (Moretto et al., 1993; Bouvier-Labit et al., 2002). The subsequent analysis of developing brain has identified CD44 as a marker for astrocyte-restricted precursor cells (ARPs) committed to give rise to astrocytes in vitro and in vivo, in both rodent and human tissue (Liu et al., 2004). In mouse cerebellum, CD44 expression was observed not only in astrocyte precursor cells, but also in neural stem cells and oligodendrocyte precursor cells (OPCs) at early postnatal stages (Naruse et al., 2013). CD44 expression in glial cells has been further demonstrated by studies examining the role of this molecule in the Schwann cells in developing nerves (Sherman et al., 2000) and at the neuromuscular junction of the adult rat skeletal muscle (Gorlewicz et al., 2009). The immunostaining of surgical specimens of temporal cortex and hippocampus for CD44 revealed astrocyte diversity in the human brain in terms of CD44 expression (Sosunov et al., 2014). High expression of CD44 has been observed in astrocytes with long, unbranched processes and “fibrous”-like astrocytes, whereas the “protoplasmic” astrocytes, which display a “bushy” morphology, exhibit no CD44 expression. Likewise, during development, CD44 expression was limited specifically to Bergmann glia and fibrous astrocytes among three types of astrocytes in cerebellum (Naruse et al., 2013). Interestingly, the expression of CD44 in astrocytes was shut off during postnatal development of the cerebellum.
Although the number of reports point to glial expression of CD44 in the nervous system, and describe adult neurons generally as CD44-negative cells (Vogel et al., 1992; Akiyama et al., 1993; Jones et al., 2000), the neuronal expression has also been observed. Expression of different splice variants of CD44, namely CD44v4, CD44v5 and CD44v10, in neurons (axonal membranes and cytoplasm) of the cerebral cortex, putamen, thalamus, hippocampus, cerebellum and spinal cord, in addition to the expression of CD44s in the white matter astrocytes, has been found in human brain sections (Kaaijk et al., 1997). It has also been shown that the embryonic optic chiasm neurons expressed the standard isoform of CD44 protein (Sretavan et al., 1994), and that strong upregulation of CD44 took place in axotomised facial motoneurons, but not in the gray matter astrocytes (Jones et al., 1997). In yet another study, the cellular location of CD44 in the brain tissue in seven different brain lesion models has been investigated (Jones et al., 2000). The results demonstrated an inducible expression of CD44 in cholinergic neurons of the forebrain, brainstem and spinal cord post distant axotomy, as opposed to its upregulation in astrocytes and other non-neuronal cell types following a more severe trauma involving local disruption of the blood-brain barrier. The antigen has been found in the cell bodies, dendrites and axons of neurons. CD44 immunoreactivity has also been detected in the dentate gyrus inner molecular layer (IML) of the mouse hippocampus after pilocarpine-induced status epilepticus (SE), where it coincided with early mossy fiber sprouting (MFS; Borges et al., 2004). Recently, CD44 mRNA has been detected in the neurons of striatum, extended amygdala and certain hypothalamic, cortical and hippocampal regions of non-stimulated brain (Glezer et al., 2009), as well as in the central respiratory control system (ventral respiratory group—VRG) within the brain stem (Matzke et al., 2007). CD44 expression was also detected in developing cerebellar Purkinje and granule neurons, but was limited to granule neurons in the adult cerebellum (Naruse et al., 2013). The expression of CD44 protein in pyramidal neurons of rat hippocampus and cortex is also developmentally regulated (Skupien et al., 2014). It increases postnatally, peaks around day P10 and then decreases. Notably, electron microscopy analysis revealed the dendritic localization of CD44 immunostaining within the pyramidal neurons of the developing hippocampus. There can be several causes for the apparent discrepancies among the studies describing expression of CD44 in neurons and glia. First, the CD44 expression appears to strongly depend on the developmental stage, especially in neurons. In fact, the studies claiming neurons to be CD44-negative were done mainly in the adult brain (Akiyama et al., 1993; Jones et al., 2000). The discrepancies may result also form differences in sensitivity of detection methods, and the differences in CD44 expression levels in glia (higher expression) and neurons (lower expression). In addition, since CD44 is a subject to extracellular and intramembrane proteolysis, the antibodies against its N-terminus may fail to detect the molecule in cells in which the rate of proteolytic processing is high. Such differences in the CD44 proteolysis, however, remain to be investigated.
Biological Function in the Nervous System
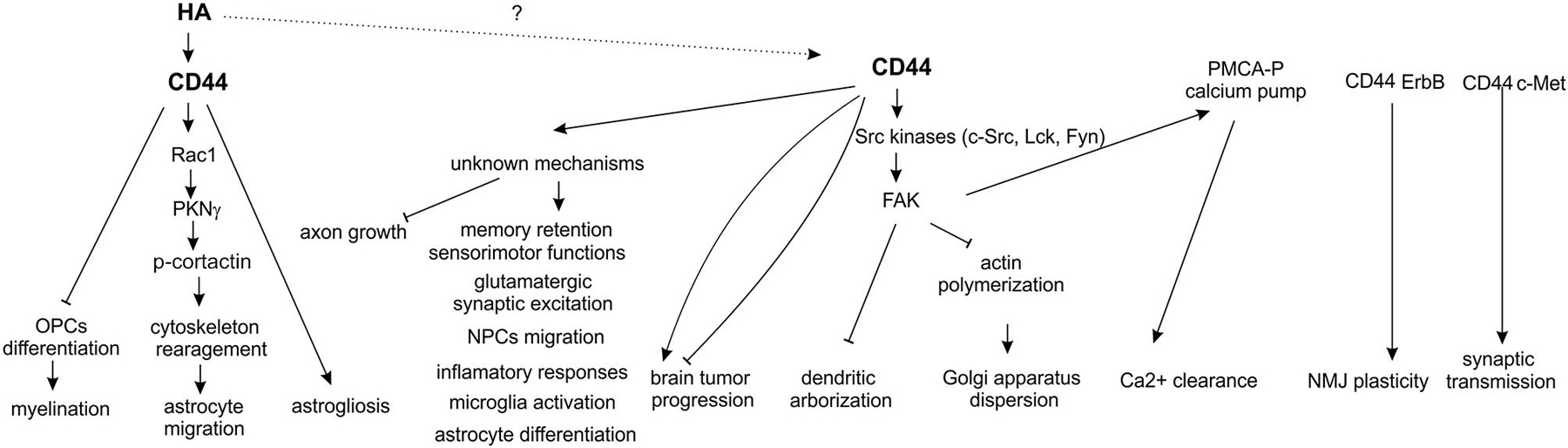
The main function ascribed to CD44 is acting as a receptor for HA—the key component of ECM in the brain playing a crucial role in many physiological and pathological processes, such as the neuronal development, synaptic plasticity (e.g., learning and memory), epileptogenesis, response to injury, neurodegeneration and brain tumour invasion (Kochlamazashvili et al., 2010; Wlodarczyk et al., 2011). The neuronal function of hyaluronic acid receptors, particularly CD44, in the nervous system remains to be elucidated, although there exists evidence that, among other processes, CD44 might be involved in the axon guidance during neuronal development (Figure 2). In vitro studies indicated that CD44 had an inhibitory effect on embryonic retinal axon growth (Sretavan et al., 1994), while experiments using specific anti-CD44 blocking antibodies demonstrated that the functional CD44 molecule in chiasmatic neurons was essential for axon crossing and axon divergence at the mouse optic chiasm (Lin and Chan, 2003). Furthermore, it appears that CD44 participates in the extension of axons from retinal ganglion cells growing on a laminin substrate (Ries et al., 2007).
Recently, a novel signaling pathway has been described in pyramidal neurons of the hippocampus and cerebral cortex, involving CD44 and Src tyrosine kinase-induced cascade, regulating Golgi apparatus morphology and dendritic tree arborization (Skupien et al., 2014). It was shown that the loss of CD44 resulted in an increase in the complexity of dendritic arbors in hippocampal neurons cultured in vitro, and in cortical neurons electroporated in vivo. Moreover, the knockdown of CD44 resulted in structural alteration of the Golgi apparatus, an organelle that is essential for mediating dendritic polarity, growth, and maintenance. Additionally, CD44 interacted with Src kinase in the brain, and the activation of both c-Src and its main substrate, focal adhesion kinase (FAK), was decreased upon CD44 knockdown.
The similar signaling pathway, involving CD44 and plasma membrane Ca2+ ATPase (PMCA), has been described in sensory neurons (Ghosh et al., 2011). The proposed molecular mechanism envisages PMCA as a point of cross-talk enabling the interaction of ECM and CD44, which regulates the Ca2+ signaling through the activation of SFKs (Src family kinases: Lck, Fyn) and FAK cascade. Since Ca2+ clearance from the neuronal cytoplasm regulates a number of Ca2+-dependent processes in neurons, including excitability, plasticity and neurotransmitter release, it appears that CD44 might be involved in all the above processes. Moreover, hyaluronic acid has been implicated in hippocampal synaptic plasticity by modulating postsynaptic L-type voltage-dependent Ca2+ channels (L-VDCCs; Kochlamazashvili et al., 2010). On the other hand, it has been demonstrated that activity of neuronal L-VDCCs is regulated by phosphorylation of its α1c subunit by Src kinase (Bence-Hanulec et al., 2000; Gui et al., 2006). It would be interesting to investigate whether regulation of Src activity by CD44 can be involved in L-VDCCs-dependent synaptic plasticity.
In the nervous system and other tissues, CD44 can act as a co-receptor for receptor tyrosine kinases (RTKs) and serve as a platform for signaling molecule assembling. For instance, in the peripheral nervous system, CD44 enhances the neuregulin signaling by mediating the ErbB receptor heterodimerization in Schwann cells of the developing peripheral nerves (Sherman et al., 2000). In the adult rat nerve-muscle synapse, CD44 present in terminal Schwann cells has been shown to bind to ErbB3 receptors and to be involved in the neuromuscular junction plasticity (Gorlewicz et al., 2009). Moreover, the CD44v6 isoform can act as a co-receptor for c-Met in vitro (Orian-Rousseau et al., 2002). The CD44-c-Met interaction during embryogenesis has been further demonstrated by in vivo studies (Matzke et al., 2007) showing that c-Met is haploinsufficient in the CD44−/− background. The CD44−/− c-Met+/– mice die at birth due to a breathing defect caused by impaired synaptic transmission in the respiratory rhythm generating network, and alterations in the phrenic nerve, while c-Met −/−CD44+/+, and CD44−/− c-Met+/+ mice develop normally and do not exhibit phenotypic abnormalities. The above results suggest that CD44 and c-Met are involved in synaptogenesis and axon myelination in the central and peripheral nervous systems.
It has been shown that CD44-deficient mice were born at the Mendelian ratio without any obvious developmental or neurological defects (Schmits et al., 1997). However, recently, Raber et al, applied a battery of behavioral and cognitive tests to determine if CD44-null mice display cognitive or other neurological disturbances (Raber et al., 2014). The results support an important role for CD44 in locomotor and sensorimotor functions, and in spatial memory retention, but do not specify whether these effects are due to the lack of CD44 in neurons or in glia. Given that CD44 is expressed in neural stem cells implicated in spatial memory (Oishi and Ito-Dufros, 2006; Deng et al., 2009; Naruse et al., 2013) the observed deficits can indicate that CD44 depletion in mice influences adult neurogenesis which in turn affects memory.
Interestingly, Matzke et al. (2007) have shown that the glutamatergic synaptic excitation in CD44 knockout mice was strongly reduced, while no change was detected in the glycinergic and GABAergic synaptic inhibition or in the overall synaptic activity of pre-Bötzinger complex neurons within the brain stem (Matzke et al., 2007). The above observations seem to indicate a potential role of CD44 in synaptic transmission, yet this requires more in-depth research. The absence of overt neurological defects in CD44 knockout mice might be explained by efficient compensation by another protein, all the more so since HA-mediated motility receptor (RHAMM) and intercellular adhesion molecule-1 (ICAM-1) have previously been shown to take over the CD44 role in the CD44 knockout mice (Nedvetzki et al., 2004; Olaku et al., 2011).
Although, the expression of CD44 in astrocytes has been described, the function of the interaction of HA and its receptor in these cells is poorly understood. It has been shown that interaction of hyaluronic acid with CD44 induce Rac 1-dependent PKNγ (protein kinase N-γ) activity which, in turn up-regulates the phosphorylation of the cytoskeletal protein, cortactin. This HA/CD44 interaction with Rac1-PKNγ leads to cytoskeleton activation and enhanced astrocyte migration (Bourguignon et al., 2007). Similarly, in neural precursor cells, overexpression of CD44 protein significantly enhanced the in vitro and in vivo trans-endothelial migration of these cells (Deboux et al., 2013). Moreover, it was shown that CD44 overexpression in glial precursor cells inhibits differentiation towards oligodendrocytes and increases the differentiation into astrocytes (Liu et al., 2004). Accordingly, a high molecular weight form of HA was also shown to inhibit maturation of OPCs into myelin-forming cells (Back et al., 2005).
Association with the Nervous System Pathologies
The limited data from descriptive studies, indicating a widespread upregulation of CD44 expression in neurons and non-neuronal cells post brain injury, suggest an important role of this cell surface glycoprotein in the neuronal, glial and leukocyte response to trauma and in the nervous system repair (Jones et al., 2000; Shin et al., 2005). However, the molecular mechanisms underlying the above processes remain to be elucidated.
Few studies conducted to investigate the CD44 role in epileptogenesis have provided inconsistent results. It has been observed that CD44 was strongly upregulated in the dentate IML 3 days post pilocarpine-induced SE in mice, then it declined over the next 4 weeks (Borges et al., 2004). CD44 appeared to be one of the earliest proteins upregulated in the IML, which coincided with early MFS. On the other hand, repeated kainate injections did not induce any changes in the CD44 expression in the IML, which also correlated with MFS absence in the mice hippocampus. In view of the above, it has been hypothesized that CD44 was involved in the response to axon terminal degeneration and/or neuronal reorganization preceding MFS. Contrary to the above observations, studies on CD44 expression in an in vitro model of MFS have shown that high CD44 expression in the molecular layer coincided with minimal MFS, and that reduced CD44 expression/function following the kainic acid (KA) treatment or use of blocking antibodies was associated with increased MFS (Bausch, 2006). The time course of KA-induced decrease in CD44 expression corresponded with the temporal progression of KA-induced MFS in hippocampal slice cultures, suggesting that reduced CD44 expression might contribute to MFS. HA has been also implicated in epileptogenesis. Studies with the use of microelectrode array recording and Ca2+ imaging in hippocampal neurons cultured in vitro revealed that enzymatic removal of HA by hyaluronidase induced epileptiform activity in neuronal network (Vedunova et al., 2013). Additionally, mice deficient in HA synthase (HAS) genes, especially Has 3 knock-outs, exhibit epileptic phenotype along with a pronounced reduction in the level of tissue HA (Arranz et al., 2014).
Recently, it was shown that the silencing of CD44 expression during the early development of neuronal cells exerted a significant protective effect on young neurons that were exposed to subtoxic conditions, by preventing dendritic shortening induced by glutamate exposure (Skupien et al., 2014). Therefore, CD44 might be a novel therapeutic target in neurological disorders in which alterations in dendritic tree arborization have been observed.
One of a few studies with the use of CD44 knockout mice has provided evidence for the potential role of CD44 in the response to ischaemia in brain tissue (Wang et al., 2002). These findings indicated that CD44 deficiency in mice protected their brain from cerebral ischemia injury, and it has been suggested that this effect might be associated with selective reduction in inflammatory cytokines, for instance interleukin-1b. These results are further supported by studies showing that CD44 expression in activated microglia can be involved in the pathogenesis of neuroinflamatory diseases. Up-regulation of CD44 expression in microglia/macrophages were observed after transient or permanent forebrain ischemia in rats (Wang et al., 2001; Kang et al., 2008), in experimental cryolesions, a model for rat brain injury (Shin et al., 2005) and in mouse model of amyotrophic lateral sclerosis (ALS; Matsumoto et al., 2012).
Increased expression of CD44 and HA accumulation has also been observed in the brain white matter of patients with multiple sclerosis (MS), as compared with the normal brain tissue (Girgrah et al., 1991; Back et al., 2005). Consistently, elevated levels of both CD44 and HA has been detected in areas where there was loss of myelin, in the acute and chronic lesions in mice with experimental autoimmune encephalomyelitis (EAE), a murine model of MS (Back et al., 2005). Transgenic mice that overexpressed CD44 under the control of a myelin-specific promoter had widespread CNS dysmyelination and progressive demyelination that occurred in the absence of an inflammatory response (Tuohy et al., 2004). These findings provide strong evidence that CD44 proteins expressed by oligodendrocytes and Schwann cells, play a role in promoting demyelination. Moreover, HA staining intensity correlated with the levels of CD44 in CD44-overexpressing mice, indicating that HA accumulates in demyelinating CNS lesions as a result, at least in part, of elevated CD44 expression by glial cells (Back et al., 2005). HA inhibits remyelination and OPCs maturation after chemical demyelination of mouse white matter (Back et al., 2005) but, in vitro studies showed that blocked maturation of oligodendrocytes depends on HA interaction with Toll-like receptor 2 (TLR2) rather than with CD44 expressed by OPCs (Sloane et al., 2010). CD44 expression was strongly induced by activated astrocytes surrounding the demyelinated lesions suggesting that CD44 may play a role in facilitating inflammatory responses during reactive gliosis (Girgrah et al., 1991; Haegel et al., 1993). In MS the interactions between astrocytes and lymphocytes occurs during the entry of activated lymphocytes into the CNS, and in the sites of lesions. It was shown that CD44 is involved in direct contacts between T-cells and astrocytes in EAE mice (Haegel et al., 1993). Recently, an increased EAE disease severity and inflammation was demonstrated in CD44-KO mice (Flynn et al., 2013). CD44-deficient mice with EAE had more pro-inflammatory T-cell profile and increased permeability of the brain-blood barrier (BBB) than WT controls. The data suggest that CD44 acts as a negative regulator of inflammation with roles in T-cell differentiation, adhesion and trans-endothelial migration, and BBB permeability.
Protoplasmic astrocytes in patients with Alexander disease, a primary disorder of astrocytes, caused by heterozygous mutations in GFAP (glial fibrillary acidic protein), convert to reactive cells that lost their bushy-like morphology and become multinucleated and hypertrophic (Sosunov et al., 2013). This phenotypic conversion is accompanied by acquiring of CD44 by normally CD44-negative protoplasmic astrocytes of gray matter suggesting that CD44 can play a role in this process e.g., by regulation of changes in astrocyte shape. White matter of patients with vanishing white matter disease, that is associated with maturation defect of astrocytes and oligodendrocytes, is enriched in CD44-expressing astrocyte precursor cells and accumulates HA (Bugiani et al., 2013). Accumulation of HA synthesized by CD44-positive reactive astrocytes was also observed during chronic human neonatal white matter injury (Buser et al., 2012; Back and Rosenberg, 2014).
Chronically elevated CD44 expression and HA accumulation occur in non-human primate CNS with normal aging as a result of age-related astrogliosis and are linked to the aberrant accumulation of OPCs (Cargill et al., 2012). CD44 expression is also highly and persistently upregulated by astrocytes in brains of patients with Alzheimer’s disease (AD; Akiyama et al., 1993).
Several studies have demonstrated the correlation between high CD44 expression and poor prognosis in patients with brain tumour. CD44s and CD44v are frequently expressed in primary brain tumours and seem to be essential for the invasive growth of various CNS-derived tumour cell types in vitro and in vivo (Kuppner et al., 1992; Nagasaka et al., 1995; Sherman et al., 1997; Breyer et al., 2000; Monaghan et al., 2000; Pusch et al., 2010). The precise mechanism of CD44 action in the development and invasiveness of nervous system tumours has not been elucidated to date. In glioblastoma multiforme (GBM), the most aggressive brain tumour, CD44 inhibits the activation of the mammalian equivalent of Hippo signaling pathway and plays a key role in regulating the stress and apoptotic responses of human GBM cells (Xu et al., 2010). In addition, it has been found that a subset of human GBM cases showed high expression of CD44 in brain tumour stem-like cells (BTSC), and that the growth of these tumours might depend on CD44v6/AKT signaling pathway (Jijiwa et al., 2011). Su and colleagues have demonstrated that CD44 overexpression was induced by the Src kinase activity in malignant peripheral nerve sheath tumour (MPNST) cells, and that it contributed to tumour invasiveness (Su et al., 2003). By contrast, in neuroblastomas, CD44 expression is often low in advanced tumours (Combaret et al., 1997; Gross et al., 1997, 2000; Kramer et al., 1997). Shtivelman and Bishop (1991) have shown that several upstream cis-acting elements contribute to the downregulation of CD44 gene expression in neuroblastoma cells (Shtivelman and Bishop, 1991).
Overall, it appears that both the overexpression and lack of expression of CD44 might be involved in the development and invasiveness of various brain tumour types. The results of the performed studies are consistent with the hypothesis put forward by Herrlich and colleagues, stating that CD44 might play a dual role in cancer pathogenesis, acting as either an oncogene or a tumour-suppressing factor (Herrlich et al., 2000). By binding to growth factors and presenting them to their receptors, CD44 might be involved in the activation of tumour-promoting signaling pathways. On the other hand, in certain conditions, binding of HA might lead to the recruitment of tumour suppressor proteins (e.g., merlin) into the cytoplasmic tail of CD44 and result in cell growth arrest. Clearly, the posed hypothesis is elegant in its attempt to explain the dual role of CD44 in tumorigenesis. Nevertheless, its confirmation requires further research, for instance the identification of individual signaling cascades involved in the above-suggested mechanisms—particularly in the brain tissue.
In conclusion, there is growing evidence that the previously underappreciated CD44 molecule might be a key signal transducer in neurons, where it can act as a c-Met co-receptor to regulate synaptic transmission or through the activation of Src-dependent signaling pathways to influence dendritic arborization and calcium ions clearance. Additionally, CD44 can also modulate many other processes important for neuronal functions summarized in Figure 2, i.e., memory retention and axonal growth, but the exact cellular mechanisms of CD44 action in these phenomena are not discovered yet. One can expect that, at least some of the neuronal functions exerted by CD44, depend on HA binding but still the experimental evidences consistent with this notion are missing. In glial cells, unlike in neurons, binding of HA to CD44 receptor was shown to play an important roles in both physiological (myelination and oligodendrocyte maturation) and pathological (astrogliosis, demyelination) processes. However, except of the well-defined signaling cascade that include CD44/HA-dependent activation of small RhoGTPase Rac1 and PKNγ and subsequent cytoskeletal rearrangement in migrating astrocytes, CD44-related cellular mechanisms in glial cells (astrogliosis, microglia activation, oligodendrocyte and astrocyte maturation, see Figure 2) still remain to be elucidated. Furthermore, explanation of precise molecular mechanisms of CD44 action might potentially form basis for novel therapeutic interventions in a number of CNS disorders.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
JD was supported by the Foundation for Polish Science PARENT-BRIDGE grant, co-financed from the European Union Regional Development Fund; GMW was supported by the National Science Centre grant No: 7873/B/P01/2011/40, and by European Regional Development Fund POIG 01.01.02-00-008/08.
References
Akiyama, H., Tooyama, I., Kawamata, T., Ikeda, K., and McGeer, P. L. (1993). Morphological diversities of CD44 positive astrocytes in the cerebral cortex of normal subjects and patients with Alzheimer’s disease. Brain Res. 632, 249–259. doi: 10.1016/0006-8993(93)91160-t
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Arranz, A. M., Perkins, K. L., Irie, F., Lewis, D. P., Hrabe, J., Xiao, F., et al. (2014). Hyaluronan deficiency due to Has3 knock-out causes altered neuronal activity and seizures via reduction in brain extracellular space. J. Neurosci. 34, 6164–6176. doi: 10.1523/jneurosci.3458-13.2014
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Back, S. A., and Rosenberg, P. A. (2014). Pathophysiology of glia in perinatal white matter injury. Glia 62, 1790–1815. doi: 10.1002/glia.22658
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Back, S. A., Tuohy, T. M., Chen, H., Wallingford, N., Craig, A., Struve, J., et al. (2005). Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat. Med. 11, 966–972. doi: 10.1038/nm1279
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bausch, S. B. (2006). Potential roles for hyaluronan and CD44 in kainic acid-induced mossy fiber sprouting in organotypic hippocampal slice cultures. Neuroscience 143, 339–350. doi: 10.1016/j.neuroscience.2006.07.037
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bence-Hanulec, K. K., Marshall, J., and Blair, L. A. (2000). Potentiation of neuronal L calcium channels by IGF-1 requires phosphorylation of the alpha1 subunit on a specific tyrosine residue. Neuron 27, 121–131. doi: 10.1016/s0896-6273(00)00014-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bignami, A., and Dahl, D. (1986). Brain-specific hyaluronate-binding protein. A product of white matter astrocytes? J. Neurocytol. 15, 671–679. doi: 10.1007/bf01611865
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Borges, K., McDermott, D. L., and Dingledine, R. (2004). Reciprocal changes of CD44 and GAP-43 expression in the dentate gyrus inner molecular layer after status epilepticus in mice. Exp. Neurol. 188, 1–10. doi: 10.1016/j.expneurol.2004.03.019
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bourguignon, L. Y., Gilad, E., Rothman, K., and Peyrollier, K. (2005). Hyaluronan-CD44 interaction with IQGAP1 promotes Cdc42 and ERK signaling, leading to actin binding, Elk-1/estrogen receptor transcriptional activation and ovarian cancer progression. J. Biol. Chem. 280, 11961–11972. doi: 10.3410/f.1023559.281692
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bourguignon, L. Y., Peyrollier, K., Gilad, E., and Brightman, A. (2007). Hyaluronan-CD44 interaction with neural Wiskott-Aldrich syndrome protein (N-WASP) promotes actin polymerization and ErbB2 activation leading to beta-catenin nuclear translocation, transcriptional up-regulation and cell migration in ovarian tumor cells. J. Biol. Chem. 282, 1265–1280. doi: 10.1074/jbc.m604672200
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bouvier-Labit, C., Liprandi, A., Monti, G., Pellissier, J. F., and Figarella-Branger, D. (2002). CD44H is expressed by cells of the oligodendrocyte lineage and by oligodendrogliomas in humans. J. Neurooncol. 60, 127–134. doi: 10.1023/A:1020630732625
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Breyer, R., Hussein, S., Radu, D. L., Pütz, K. M., Gunia, S., Hecker, H., et al. (2000). Disruption of intracerebral progression of C6 rat glioblastoma by in vivo treatment with anti-CD44 monoclonal antibody. J. Neurosurg. 92, 140–149. doi: 10.3171/jns.2000.92.1.0140
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bugiani, M., Postma, N., Polder, E., Dieleman, N., Scheffer, P. G., Sim, F. J., et al. (2013). Hyaluronan accumulation and arrested oligodendrocyte progenitor maturation in vanishing white matter disease. Brain 136, 209–222. doi: 10.1093/brain/aws320
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Buser, J. R., Maire, J., Riddle, A., Gong, X., Nguyen, T., Nelson, K., et al. (2012). Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann. Neurol. 71, 93–109. doi: 10.1002/ana.22627
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cargill, R., Kohama, S. G., Struve, J., Su, W., Banine, F., Witkowski, E., et al. (2012). Astrocytes in aged nonhuman primate brain gray matter synthesize excess hyaluronan. Neurobiol. Aging 33, 830.e13–830.e24. doi: 10.1016/j.neurobiolaging.2011.07.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Combaret, V., Gross, N., Lasset, C., Frappaz, D., Beretta-Brognara, C., Philip, T., et al. (1997). Clinical relevance of CD44 cell surface expression and MYCN gene amplification in neuroblastoma. Eur. J. Cancer 33, 2101–2105. doi: 10.1016/s0959-8049(97)00236-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cruz, T. F., Quackenbush, E. J., Letarte, M., and Moscarello, M. A. (1986). Elevated levels of a glycoprotein antigen (P-80) in gray and white matter of brain from victims of multiple sclerosis. Neurochem. Res. 11, 877–889. doi: 10.1007/bf00965211
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dalchau, R., Kirkley, J., and Fabre, J. W. (1980). Monoclonal antibody to a human leukocyte-specific membrane glycoprotein probably homologous to the leukocyte-common (L-C) antigen of the rat. Eur. J. Immunol. 10, 737–744. doi: 10.1002/eji.1830101003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Deboux, C., Ladraa, S., Cazaubon, S., Ghribi-Mallah, S., Weiss, N., Chaverot, N., et al. (2013). Overexpression of CD44 in neural precursor cells improves trans-endothelial migration and facilitates their invasion of perivascular tissues in vivo. PLoS One 8:e57430. doi: 10.1371/journal.pone.0057430
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Deng, W., Saxe, M. D., Gallina, I. S., and Gage, F. H. (2009). Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J. Neurosci. 29, 13532–13542. doi: 10.1523/jneurosci.3362-09.2009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Flynn, K. M., Michaud, M., and Madri, J. A. (2013). CD44 deficiency contributes to enhanced experimental autoimmune encephalomyelitis: a role in immune cells and vascular cells of the blood-brain barrier. Am. J. Pathol. 182, 1322–1336. doi: 10.1016/j.ajpath.2013.01.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ghosh, B., Li, Y., and Thayer, S. A. (2011). Inhibition of the Plasma membrane Ca2+ Pump by CD44 receptor activation of Tyrosine kinases increases the action potential Afterhyperpolarization in sensory neurons. J. Neurosci. 31, 2361–2370. doi: 10.1523/jneurosci.5764-10.2011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Girgrah, N., Letarte, M., Becker, L. E., Cruz, T. F., Theriault, E., and Moscarello, M. A. (1991). Localization of the CD44 glycoprotein to fibrous astrocytes in normal white matter and to reactive astrocytes in active lesions in multiple sclerosis. J. Neuropathol. Exp. Neurol. 50, 779–792. doi: 10.1097/00005072-199111000-00009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Glezer, I., Bittencourt, J. C., and Rivest, S. (2009). Neuronal expression of Cd36, Cd44 and Cd83 antigen transcripts maps to distinct and specific murine brain circuits. J. Comp. Neurol. 517, 906–924. doi: 10.1002/cne.22185
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gorlewicz, A., Wlodarczyk, J., Wilczek, E., Gawlak, M., Cabaj, A., Majczynski, H., et al. (2009). CD44 is expressed in non-myelinating Schwann cells of the adult rat and may play a role in neurodegeneration-induced glial plasticity at the neuromuscular junction. Neurobiol. Dis. 34, 245–258. doi: 10.1016/j.nbd.2009.01.011
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gross, N., Balmas, K., and Brognara, C. B. (1997). Absence of functional CD44 hyaluronan receptor on human NMYC-amplified neuroblastoma cells. Cancer Res. 57, 1387–1393.
Gross, N., Balmas Bourloud, K., and Brognara, C. B. (2000). MYCN-related suppression of functional CD44 expression enhances tumorigenic properties of human neuroblastoma cells. Exp. Cell Res. 260, 396–403. doi: 10.1006/excr.2000.5007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gui, P., Wu, X., Ling, S., Stotz, S. C., Winkfein, R. J., Wilson, E., et al. (2006). Integrin receptor activation triggers converging regulation of Cav1.2 calcium channels by c-Src and protein kinase A pathways. J. Biol. Chem. 281, 14015–14025. doi: 10.1074/jbc.m600433200
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Haegel, H., Tölg, C., Hofmann, M., and Ceredig, R. (1993). Activated mouse astrocytes and T cells express similar CD44 variants. Role of CD44 in astrocyte/T cell binding. J. Cell Biol. 122, 1067–1077. doi: 10.1083/jcb.122.5.1067
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Herrlich, P., Morrison, H., Sleeman, J., Orian-Rousseau, V., Konig, H., Weg-Remers, S., et al. (2000). CD44 acts both as a growth- and invasiveness-promoting molecule and as a tumor-suppressing cofactor. Ann. N Y Acad. Sci. 910, 106–118; discussion 118–120. doi: 10.1111/j.1749-6632.2000.tb06704.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jijiwa, M., Demir, H., Gupta, S., Leung, C., Joshi, K., Orozco, N., et al. (2011). CD44v6 regulates growth of brain tumor stem cells partially through the AKT-mediated pathway. PLoS One 6:e24217. doi: 10.1371/journal.pone.0024217
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jones, L. L., Kreutzberg, G. W., and Raivich, G. (1997). Regulation of CD44 in the regenerating mouse facial motor nucleus. Eur. J. Neurosci. 9, 1854–1863. doi: 10.1111/j.1460-9568.1997.tb00752.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jones, L. L., Liu, Z., Shen, J., Werner, A., Kreutzberg, G. W., and Raivich, G. (2000). Regulation of the cell adhesion molecule CD44 after nerve transection and direct trauma to the mouse brain. J. Comp. Neurol. 426, 468–492. doi: 10.1002/1096-9861(20001023)426:3<468::aid-cne9>3.0.co;2-i
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kaaijk, P., Pals, S. T., Morsink, F., Bosch, D. A., and Troost, D. (1997). Differential expression of CD44 splice variants in the normal human central nervous system. J. Neuroimmunol. 73, 70–76. doi: 10.1016/s0165-5728(96)00167-1
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kaaijk, P., Troost, D., Morsink, F., Keehnen, R. M., Leenstra, S., Bosch, D. A., et al. (1995). Expression of CD44 splice variants in human primary brain tumors. J. Neurooncol. 26, 185–190. doi: 10.1007/bf01052621
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kang, W. S., Choi, J. S., Shin, Y. J., Kim, H. Y., Cha, J. H., Lee, J. Y., et al. (2008). Differential regulation of osteopontin receptors, CD44 and the alpha(v) and beta(3) integrin subunits, in the rat hippocampus following transient forebrain ischemia. Brain Res. 1228, 208–216. doi: 10.1016/j.brainres.2008.06.106
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kochlamazashvili, G., Henneberger, C., Bukalo, O., Dvoretskova, E., Senkov, O., Lievens, P. M., et al. (2010). The extracellular matrix molecule hyaluronic acid regulates hippocampal synaptic plasticity by modulating postsynaptic L-type Ca(2+) channels. Neuron 67, 116–128. doi: 10.1016/j.neuron.2010.05.030
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kramer, K., Cheung, N. K., Gerald, W. L., LaQuaglia, M., Kushner, B. H., LeClerc, J. M., et al. (1997). Correlation of MYCN amplification, Trk-A and CD44 expression with clinical stage in 250 patients with neuroblastoma. Eur. J. Cancer 33, 2098–2100. doi: 10.1016/s0959-8049(97)00211-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kuppner, M. C., Van Meir, E., Gauthier, T., Hamou, M. F., and de Tribolet, N. (1992). Differential expression of the CD44 molecule in human brain tumours. Int. J. Cancer 50, 572–577. doi: 10.1002/ijc.2910500414
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lin, L., and Chan, S. O. (2003). Perturbation of CD44 function affects chiasmatic routing of retinal axons in brain slice preparations of the mouse retinofugal pathway. Eur. J. Neurosci. 17, 2299–2312. doi: 10.1046/j.1460-9568.2003.02686.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Liu, Y., Han, S. S., Wu, Y., Tuohy, T. M., Xue, H., Cai, J., et al. (2004). CD44 expression identifies astrocyte-restricted precursor cells. Dev. Biol. 276, 31–46. doi: 10.1016/j.ydbio.2004.08.018
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Martin, T. A., Harrison, G., Mansel, R. E., and Jiang, W. G. (2003). The role of the CD44/ezrin complex in cancer metastasis. Crit. Rev. Oncol. Hematol. 46, 165–186. doi: 10.1016/s1040-8428(02)00172-5
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Matsumoto, T., Imagama, S., Hirano, K., Ohgomori, T., Natori, T., Kobayashi, K., et al. (2012). CD44 expression in astrocytes and microglia is associated with ALS progression in a mouse model. Neurosci. Lett. 520, 115–120. doi: 10.1016/j.neulet.2012.05.048
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Matzke, A., Sargsyan, V., Holtmann, B., Aramuni, G., Asan, E., Sendtner, M., et al. (2007). Haploinsufficiency of c-Met in cd44−/− mice identifies a collaboration of CD44 and c-Met in vivo. Mol. Cell. Biol. 27, 8797–8806. doi: 10.1128/mcb.01355-07
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
McKenzie, J. L., Dalchau, R., and Fabre, J. W. (1982). Biochemical characterisation and localization in brain of a human brain-leucocyte membrane glycoprotein recognised by a monoclonal antibody. J. Neurochem. 39, 1461–1466. doi: 10.1111/j.1471-4159.1982.tb12592.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Monaghan, M., Mulligan, K. A., Gillespie, H., Trimble, A., Winter, P., Johnston, P. G., et al. (2000). Epidermal growth factor up-regulates CD44-dependent astrocytoma invasion in vitro. J. Pathol. 192, 519–525. doi: 10.1002/1096-9896(2000)9999:9999<::aid-path784>3.3.co;2-d
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Moretto, G., Xu, R. Y., and Kim, S. U. (1993). CD44 expression in human astrocytes and oligodendrocytes in culture. J. Neuropathol. Exp. Neurol. 52, 419–423. doi: 10.1097/00005072-199307000-00009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nagano, O., and Saya, H. (2004). Mechanism and biological significance of CD44 cleavage. Cancer Sci. 95, 930–935. doi: 10.1111/j.1349-7006.2004.tb03179.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nagasaka, S., Tanabe, K. K., Bruner, J. M., Saya, H., Sawaya, R. E., and Morrison, R. S. (1995). Alternative RNA splicing of the hyaluronic acid receptor CD44 in the normal human brain and in brain tumors. J. Neurosurg. 82, 858–863. doi: 10.3171/jns.1995.82.5.0858
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Naruse, M., Shibasaki, K., Yokoyama, S., Kurachi, M., and Ishizaki, Y. (2013). Dynamic changes of CD44 expression from progenitors to subpopulations of astrocytes and neurons in developing cerebellum. PLoS One 8:e53109. doi: 10.1371/journal.pone.0053109
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Nedvetzki, S., Gonen, E., Assayag, N., Reich, R., Williams, R. O., Thurmond, R. L., et al. (2004). RHAMM, a receptor for hyaluronan-mediated motility, compensates for CD44 in inflamed CD44-knockout mice: a different interpretation of redundancy. Proc. Natl. Acad. Sci. U S A 101, 18081–18086. doi: 10.1073/pnas.0407378102
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Oishi, K., and Ito-Dufros, Y. (2006). Angiogenic potential of CD44+ CD90+ multipotent CNS stem cells in vitro. Biochem. Biophys. Res. Commun. 349, 1065–1072. doi: 10.1016/j.bbrc.2006.08.135
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Okamoto, I., Kawano, Y., Tsuiki, H., Sasaki, J., Nakao, M., Matsumoto, M., et al. (1999). CD44 cleavage induced by a membrane-associated metalloprotease plays a critical role in tumor cell migration. Oncogene 18, 1435–1446. doi: 10.1038/sj.onc.1202447
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Olaku, V., Matzke, A., Mitchell, C., Hasenauer, S., Sakkaravarthi, A., Pace, G., et al. (2011). c-Met recruits ICAM-1 as a coreceptor to compensate for the loss of CD44 in Cd44 null mice. Mol. Biol. Cell 22, 2777–2786. doi: 10.1091/mbc.E11-02-0134
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Orian-Rousseau, V., Chen, L., Sleeman, J. P., Herrlich, P., and Ponta, H. (2002). CD44 is required for two consecutive steps in HGF/c-Met signaling. Genes Dev. 16, 3074–3086. doi: 10.1101/gad.242602
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ponta, H., Sherman, L., and Herrlich, P. A. (2003). CD44: from adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 4, 33–45. doi: 10.1038/nrm1004
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pusch, A., Boeckenhoff, A., Glaser, T., Kaminski, T., Kirfel, G., Hans, M., et al. (2010). CD44 and hyaluronan promote invasive growth of B35 neuroblastoma cells into the brain. Biochim. Biophys. Acta 1803, 261–274. doi: 10.1016/j.bbamcr.2009.12.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Raber, J., Olsen, R. H., Su, W., Foster, S., Xing, R., Acevedo, S. F., et al. (2014). CD44 is required for spatial memory retention and sensorimotor functions. Behav. Brain Res. 275, 146–149. doi: 10.1016/j.bbr.2014.09.010
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Resnick, D. K., Resnick, N. M., Welch, W. C., and Cooper, D. L. (1999). Differential expressions of CD44 variants in tumors affecting the central nervous system. Mol. Diagn. 4, 219–232. doi: 10.1016/s1084-8592(99)80025-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ries, A., Goldberg, J. L., and Grimpe, B. (2007). A novel biological function for CD44 in axon growth of retinal ganglion cells identified by a bioinformatics approach. J. Neurochem. 103, 1491–1505. doi: 10.1111/j.1471-4159.2007.04858.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Schmits, R., Filmus, J., Gerwin, N., Senaldi, G., Kiefer, F., Kundig, T., et al. (1997). CD44 regulates hematopoietic progenitor distribution, granuloma formation and tumorigenicity. Blood 90, 2217–2233.
Sherman, L., Jacoby, L. B., Lampe, J., Pelton, P., Aguzzi, A., Herrlich, P., et al. (1997). CD44 expression is aberrant in benign Schwann cell tumors possessing mutations in the neurofibromatosis type 2, but not type 1, gene. Cancer Res. 57, 4889–4897.
Sherman, L. S., Rizvi, T. A., Karyala, S., and Ratner, N. (2000). CD44 enhances neuregulin signaling by Schwann cells. J. Cell Biol. 150, 1071–1084. doi: 10.1083/jcb.150.5.1071
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Shin, T., Ahn, M., Kim, H., Moon, C., Kang, T. Y., Lee, J. M., et al. (2005). Temporal expression of osteopontin and CD44 in rat brains with experimental cryolesions. Brain Res. 1041, 95–101. doi: 10.1016/j.brainres.2005.02.019
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Shtivelman, E., and Bishop, J. M. (1991). Expression of CD44 is repressed in neuroblastoma cells. Mol. Cell. Biol. 11, 5446–5453.
Skupien, A., Konopka, A., Trzaskoma, P., Labus, J., Gorlewicz, A., Swiech, L., et al. (2014). CD44 regulates dendrite morphogenesis through Src tyrosine kinase-dependent positioning of the Golgi. J. Cell Sci. 127, 5038–5051. doi: 10.1242/jcs.154542
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sloane, J. A., Batt, C., Ma, Y., Harris, Z. M., Trapp, B., and Vartanian, T. (2010). Hyaluronan blocks oligodendrocyte progenitor maturation and remyelination through TLR2. Proc. Natl. Acad. Sci. U S A 107, 11555–11560. doi: 10.1073/pnas.1006496107
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sosunov, A. A., Guilfoyle, E., Wu, X., McKhann, G. M. 2nd, and Goldman, J. E. (2013). Phenotypic conversions of “protoplasmic” to “reactive” astrocytes in Alexander disease. J. Neurosci. 33, 7439–7450. doi: 10.1523/JNEUROSCI.4506-12.2013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sosunov, A. A., Wu, X., Tsankova, N. M., Guilfoyle, E., McKhann, G. M. 2nd, and Goldman, J. E. (2014). Phenotypic heterogeneity and plasticity of isocortical and hippocampal astrocytes in the human brain. J. Neurosci. 34, 2285–2298. doi: 10.1523/JNEUROSCI.4037-13.2014
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sretavan, D. W., Feng, L., Pure, E., and Reichardt, L. F. (1994). Embryonic neurons of the developing optic chiasm express L1 and CD44, cell surface molecules with opposing effects on retinal axon growth. Neuron 12, 957–975. doi: 10.1016/0896-6273(94)90307-7
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stefanová, I., Hilgert, I., Bazil, V., Kristofová, H., and Horejsí, V. (1989). Human leucocyte surface glycoprotein CDw44 and lymphocyte homing receptor are identical molecules. Immunogenetics 29, 402–404. doi: 10.1007/bf00375869
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Su, W., Sin, M., Darrow, A., and Sherman, L. S. (2003). Malignant peripheral nerve sheath tumor cell invasion is facilitated by Src and aberrant CD44 expression. Glia 42, 350–358. doi: 10.1002/glia.10206
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Thorne, R. F., Legg, J. W., and Isacke, C. M. (2004). The role of the CD44 transmembrane and cytoplasmic domains in co-ordinating adhesive and signalling events. J. Cell Sci. 117, 373–380. doi: 10.1242/jcs.00954
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tuohy, T. M., Wallingford, N., Liu, Y., Chan, F. H., Rizvi, T., Xing, R., et al. (2004). CD44 overexpression by oligodendrocytes: a novel mouse model of inflammation-independent demyelination and dysmyelination. Glia 47, 335–345. doi: 10.1002/glia.20042
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vedunova, M., Sakharnova, T., Mitroshina, E., Perminova, M., Pimashkin, A., Zakharov, Y., et al. (2013). Seizure-like activity in hyaluronidase-treated dissociated hippocampal cultures. Front. Cell. Neurosci. 7:149. doi: 10.3389/fncel.2013.00149
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vogel, H., Butcher, E. C., and Picker, L. J. (1992). H-CAM expression in the human nervous system: evidence for a role in diverse glial interactions. J. Neurocytol. 21, 363–373. doi: 10.1007/bf01191704
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wang, X., Xu, L., Wang, H., Zhan, Y., Puré, E., and Feuerstein, G. Z. (2002). CD44 deficiency in mice protects brain from cerebral ischemia injury. J. Neurochem. 83, 1172–1179. doi: 10.1046/j.1471-4159.2002.01225.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wang, H., Zhan, Y., Xu, L., Feuerstein, G. Z., and Wang, X. (2001). Use of suppression subtractive hybridization for differential gene expression in stroke: discovery of CD44 gene expression and localization in permanent focal stroke in rats. Stroke 32, 1020–1027. doi: 10.1161/01.str.32.4.1020
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wlodarczyk, J., Mukhina, I., Kaczmarek, L., and Dityatev, A. (2011). Extracellular matrix molecules, their receptors and secreted proteases in synaptic plasticity. Dev. Neurobiol. 71, 1040–1053. doi: 10.1002/dneu.20958
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Xu, Y., Stamenkovic, I., and Yu, Q. (2010). CD44 attenuates activation of the hippo signaling pathway and is a prime therapeutic target for glioblastoma. Cancer Res. 70, 2455–2464. doi: 10.1158/0008-5472.can-09-2505
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yu, Q., and Stamenkovic, I. (1999). Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev. 13, 35–48. doi: 10.1101/gad.13.1.35
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: CD44, adhesion molecule, hyaluronan receptor, extracellular matrix receptor
Citation: Dzwonek J and Wilczynski GM (2015) CD44: molecular interactions, signaling and functions in the nervous system. Front. Cell. Neurosci. 9:175. doi: 10.3389/fncel.2015.00175
Received: 30 January 2015; Accepted: 20 April 2015;
Published online: 07 May 2015.
Edited by:
Jerzy W. Mozrzymas, Wroclaw Medical University, PolandReviewed by:
Alexander Dityatev, German Center for Neurodegenerative Diseases, GermanyLarry Scott Sherman, Oregon Health and Science Univeristy, USA
Copyright © 2015 Dzwonek and Wilczynski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joanna Dzwonek and Grzegorz M. Wilczynski, Laboratory of Molecular and Systemic Neuromorphology, Nencki Institute of Experimental Biology, ul. Pasteura 3, Warsaw 02-093, Poland, j.dzwonek@nencki.gov.pl;
g.wilczynski@nencki.gov.pl
 Joanna Dzwonek*
Joanna Dzwonek*  Grzegorz M. Wilczynski
Grzegorz M. Wilczynski