Axonal Non-segregation of the Vesicular Glutamate Transporter VGLUT3 Within Serotonergic Projections in the Mouse Forebrain
- 1Translational Research Institute, Institute of Health and Biomedical Innovation, Queensland University of Technology, Brisbane, QLD, Australia
- 2QIMR Berghofer Medical Research Institute, Institute of Health and Biomedical Innovation, Queensland University of Technology, Brisbane, QLD, Australia
- 3Biologie Integrative et Physiologie, Université Pierre et Marie Curie, Paris, France
A subpopulation of raphe 5-HT neurons expresses the vesicular glutamate transporter VGLUT3 with the co-release of glutamate and serotonin proposed to play a pivotal role in encoding reward- and anxiety-related behaviors. Serotonin axons are identifiable by immunolabeling of either serotonin (5-HT) or the plasma membrane 5-HT transporter (SERT), with SERT labeling demonstrated to be only partially overlapping with 5-HT staining. Studies investigating the colocalization or segregation of VGLUT3 within SERT or 5-HT immunolabeled boutons have led to inconsistent results. Therefore, we combined immunohistochemistry, high resolution confocal imaging, and 3D-reconstruction techniques to map and quantify the distribution of VGLUT3 immunoreactive boutons within 5-HT vs. SERT-positive axons in various regions of the mouse forebrain, including the prefrontal cortex, nucleus accumbens core and shell, bed nucleus of the stria terminalis, dorsal striatum, lateral septum, basolateral and central amygdala, and hippocampus. Our results demonstrate that about 90% of 5-HT boutons are colocalized with SERT in almost all the brain regions studied, which therefore reveals that VGLUT3 and SERT do not segregate. However, in the posterior part of the NAC shell, we confirmed the presence of a subtype of 5-HT immunoreactive axons that lack the SERT. Interestingly, about 90% of the 5-HT/VGLUT3 boutons were labeled for the SERT in this region, suggesting that VGLUT3 is preferentially located in SERT immunoreactive 5-HT boutons. This work demonstrates that VGLUT3 and SERT cannot be used as specific markers to classify the different subtypes of 5-HT axons.
Introduction
Intensive efforts have long been made to understand the complexity of the serotonin (5-HT) system and to identify specific markers for serotonin neuron diversity. Although it is becoming evident that raphe serotonin neurons are morphologically, functionally, and molecularly heterogeneous (Calizo et al., 2011; Kiyasova et al., 2011, 2013; Gaspar and Lillesaar, 2012; Fernandez et al., 2016), the diversity of serotonergic axonal projections to the forebrain is not completely understood.
Pioneer electron or light microscopy and anterograde tracing studies have revealed the existence of 5-HT axon terminals with different sizes, shapes, contents of their small vesicles, and the presence or absence of dense-core vesicles [for review see Descarries et al. (2010)]. In rats, two types of axons were reported, with axons originating from the dorsal raphe showing fine beaded or fusiform varicosities separated by smooth axon segments of variable length (type D), while axons originating from the median raphe displayed large spherical varicosities with fine and smooth inter-varicosity segments (type M) (Kosofsky and Molliver, 1987). In primates, two types of axons were also described, with sparse, small, ovoid, or large, spheroidal varicosities (Hornung et al., 1990). However, it is likely that this axonal morphology classification cannot longer be considered as valid criteria for distinguishing the cellular origin or the chemical identity of the 5-HT neurons. Indeed a chemically defined 5-HT neuron can send several types of axonal projections with different morphologies to different brain regions (Gagnon and Parent, 2014). Hence, research has been rather devoted to studying the molecular or physiological diversity of 5-HT neurons, identifying various 5-HT neuronal subtypes that differentially express the 5-HT1A autoreceptor (Sotelo et al., 1990; Kirby et al., 2003; Bonnavion et al., 2010; Kiyasova et al., 2013; Fernandez et al., 2016), substance P/neurokinin receptor 1 (NK1; Lacoste et al., 2006), galanin and its receptor (Xu and Hökfelt, 1997; Larm et al., 2003), neuronal nitric oxide synthase (nNOS; Xu and Hökfelt, 1997), gamma-aminobutyric acid (GABA)-synthesizing enzyme glutamic acid decarboxylase (GAD; Fu et al., 2010), alpha7 nicotinic receptor (Aznar et al., 2005), MET receptor tyrosine kinase (Kast et al., 2017) or display different pharmacological and electrophysiological properties (Kirby et al., 2003; Hajós et al., 2007; Calizo et al., 2011). This heterogeneity appears to be target-specific (Fernandez et al., 2016; Prouty et al., 2017) and could therefore be used to establish a specific anatomy/function cartography of raphe serotonin sub-systems (Ren et al., 2018).
In addition, a subpopulation of dorsal and median raphe 5-HT neurons was found to co-express transcripts of the vesicular glutamate transporter type 3 (VGLUT3; Gras et al., 2002; Hioki et al., 2010), suggesting that 5-HT and glutamate could be stored in the same vesicles and co-released. VGLUT3 protein was also reported to be located in the some 5-HT immunoreactive axonal varicosities in the forebrain (Schäfer et al., 2002; Mintz and Scott, 2006), including the granular cell layer of the olfactory bulb, cerebral cortex, central amygdaloid nuclei, hippocampal CA3 field, dorsolateral septum, and supra-ependymal plexus of the third ventricle (Shutoh et al., 2008). A classification of two serotonergic axons subtypes depending on the presence or absence of VGLUT3 was therefore proposed (Shutoh et al., 2008). It is likely that the co-expression of VGLUT3 and the vesicular monoamine transporter 2 (Vmat2) in serotonin terminals (Schäfer et al., 2002) synergizes the filling of 5-HT and glutamate in the same synaptic vesicles (Amilhon et al., 2010), with 5-HT/glutamate cotransmission proposed to play a pivotal role in the control of reward- and emotion-related neural circuitry (Liu et al., 2014; Sengupta et al., 2017) and their plasticity/adaptability during development or pathological processes (Gagnon and Parent, 2014).
However, some discrepancies have emerged from the aforementioned studies, regarding the total or sparse colocalization of the SERT in 5-HT axon varicosities. While Gagnon and Parent (2014) observed that all 5-HT axon varicosities contain the SERT in rats, two studies have reported a very sparse colocalization of SERT and 5-HT immunolabeling in mice, with VGLUT3 and SERT mostly segregated within 5-HT varicosities (Amilhon et al., 2010; Voisin et al., 2016), especially in the prefrontal cortex, HIP, dorsal striatum, and LS. This data suggests that different subtypes of 5-HT axonal varicosities (SERT-/VGLUT3+ or SERT+/VGLUT3-) may coexist in the mouse forebrain.
In the present study, we therefore investigated the immunohistological distribution of SERT and VGLUT3 within 5-HT axon varicosities in various regions of the mouse forebrain, including the prefrontal cortex, NAc core and NAc shell, BNST, dorsal striatum, LS, BLA and CeA, and HIP. For this, we imaged ±1.55 × 108 μm3 of tissue and 3D-reconstructed ±106 5-HT varicosities to determine the volumetric density and the proportion of varicosities co-labeled with SERT and/or VGLUT3, in each brain region. We found that the great majority (≈90%) of 5-HT varicosities express the SERT in every brain regions analyzed except the posterior shell of the NAc (50%). We herein report that VGLUT3 is preferentially located in SERT+ 5-HT varicosities. Our results demonstrate that VGLUT3 and SERT do not particularly segregate in 5-HT axonal varicosities of the mouse forebrain.
Materials and Methods
Animals
Six 8–10-week-old C57Bl6 mice (3 males, 3 females) were housed in standard ventilated cages in climate-controlled rooms. Food, water, and environmental enrichment were available ad libitum. This study was carried out in accordance with the recommendations of National Health and Medical Research Council (NHMRC) guidelines to promote the well-being of animals used for scientific purposes and the Australian code for the care and use of animals for scientific purposes. The protocol was approved by the Queensland University of Technology Animal Ethics Committee and the University of Queensland Animal Ethics Committee.
Histology
Mice were deeply anesthetized with 100 mg/kg of pentobarbital (Lethobarb, Virbac, Australia) and transcardially perfused with 4% paraformaldehyde (PFA) prior to decapitation. Brains were harvested and post-fixed overnight at 4°C. Forty-micron thick coronal vibratome sections were collected and incubated overnight in blocking solution [2% normal goat serum, 0.3% Triton, and 0.05% Tween 20 in 0.1 M phosphate-buffer saline (PBS)].
Immunohistochemistry
Sections containing the prelimbic cortex (Bregma: +2.46 ± 0.3 mm), the NAC (Bregma: +1.42 ± 0.2 mm), the posterior NAC, the dorsal striatum and the LS (Bregma: +1.00 ± 0.2 mm), the BNST (Bregma: -0.22 ± 0.3 mm), the hippocampus (Bregma: -1–70 ± 0.3 mm), or the amygdala (Bregma: -1.40 ± 0.2 mm) were incubated with primary antibodies diluted in the blocking solution: rat anti-5-HT (Millipore #MAB352, 1:100) for 48 h at room temperature followed by rabbit anti-SERT (Millipore #PC177L, 1:1000) and the KO-validated (Fasano et al., 2017) guinea-pig anti-VGLUT3 (Synaptic System #135204), at 1:500 dilution [as per supplier’s recommendations and other studies (Puighermanal et al., 2017)], overnight at 4 degrees. After three washes in the blocking solution, the slices were incubated for 4 h at room temperature with secondary antibodies diluted in the blocking solution: goat anti-rabbit-Alexa 488, goat anti-guinea pig-Alexa 647 (Thermofisher Scientific, #A11034 and #A21450, 1:500) and goat anti-rat biotinylated (Jackson Laboratory # 112-065-003, 1:200). After three washes in the blocking solution, slices were incubated for 30 min in Streptavidin-Cy3 (Thermofisher Scientific #438315, 1:1000), washed three times in PBS, and mounted in Prolong Gold antifade mountant (Thermofisher Scientific, #P36934).
Imaging and Analysis
Sections (3 sections per animal, n = 6 animals, 18 sections/brain region) were imaged on an Olympus FV3000 using a 60X oil-immersion objective (NA 1.35) with a 2.5x zoom and a z-axis step of 0.3 μm, using sequential scanning. Mosaics of the regions of interest were acquired as depicted in A of Figures 1–7, in OIR file format. The 5-HT immunoreactive boutons were reconstructed in 3D using the surface rendering function with Imaris 9.2.1 (Bitplane), as previously described (Belmer et al., 2016; Tarren et al., 2017). All the images were processed in batch using the same surface thresholding parameters. Mean fluorescence intensities of SERT or VGLUT3 labeling within 5-HT boutons and image volumes were obtained from the surface statistics in Imaris. Since the level of background of confocal images can reach as much as 30% of maximum image intensity (Landmann and Marbet, 2004), we use this threshold as a criteria to define the 5-HT boutons colocalized and non-colocalized with SERT and/or VGLUT3 (i.e., mean intensity >30% or < 30% of the maximum intensity, respectively). For each brain region, the frequency distribution of the colocalized and non-colocalized boutons within each brain region was analyzed using Excel 365, averaged for each animal, and plotted in Graphpad Prism 7.0 (Graph Pad Software Co., San Diego, CA, United States) as replicates (n = 6). Quantification of the degree of colocalization between the different channels in selected brain regions using Mander’s overlap coefficients was performed using Coloc2 plugin in Image J/Fiji (NIH). 3D representation of the proportion of VGLUT3 immunoreactive 5-HT boutons within each brain region was generated using the online Scalable Brain Atlas (Bakker et al., 2015) with a custom color-coded scale (Figure 9).
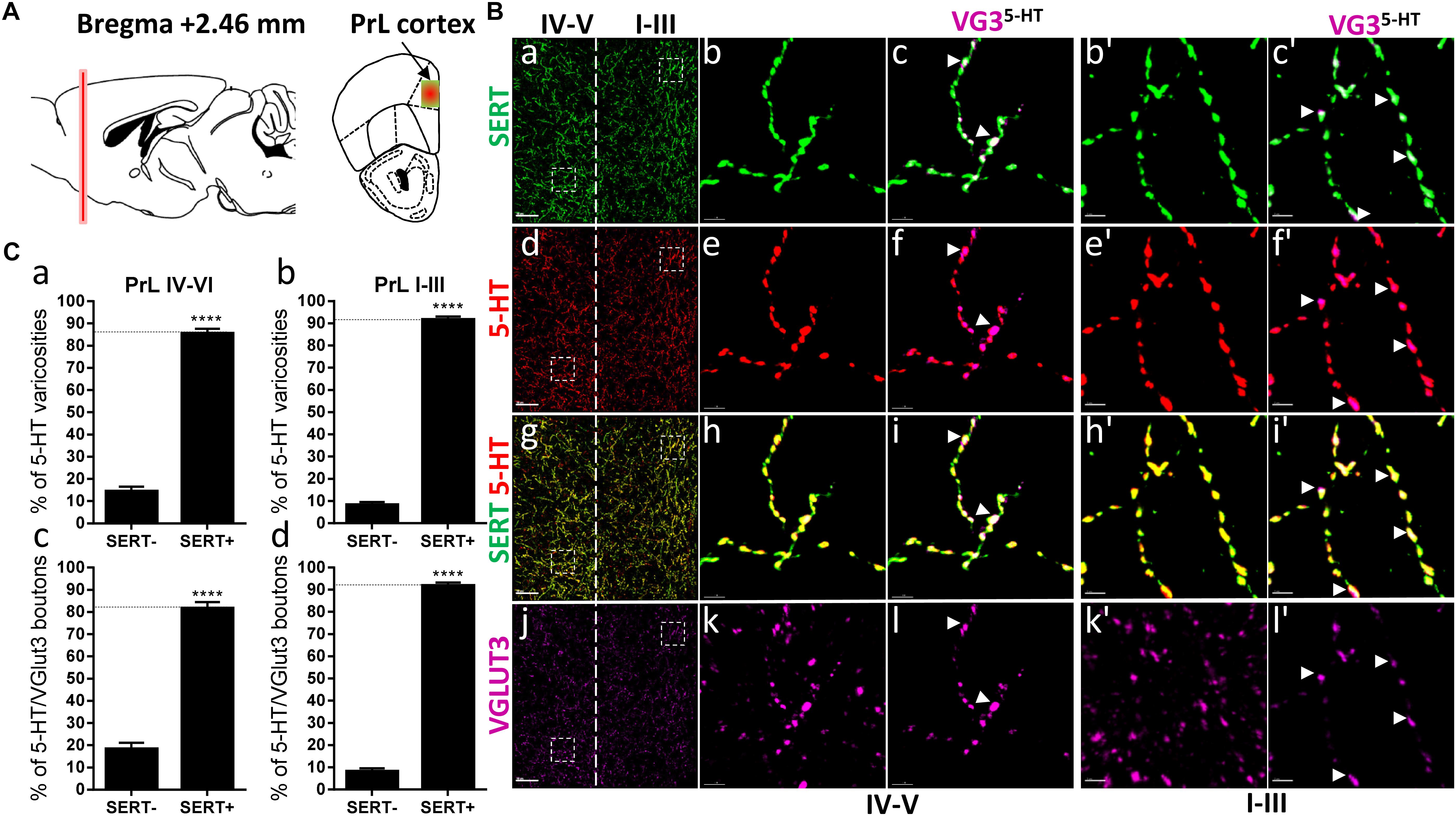
Figure 1. Distribution of VGLUT3+ boutons within 5-HT+ and SERT+ axons in the prelimbic cortex. (A) Schematic drawing showing the location of the acquired micrographs. Prelimbic (PrL) cortex mosaic images of layer I–V were acquired at bregma +2.46 ± 0.2 mm (red vertical line) in the dorsal half of the medial cortex (yellow/red square). (B) Micrograph showing the distribution of the SERT (green, a–c,b’,c’), 5-HT (red, d–f; e’,f’), SERT (green) + 5-HT (red) (g–i, h’,i’), and VGLUT3 (magenta, j–l, k’,l’). Left panel shows lower magnification (a,d,g,j). Scale bar: 50 μm. Middle panel shows higher magnification of the white dash box for layers IV–V (b,c,e,f,h,i,k,l) and right panel shows higher magnification white dash box for layers I–III (b’,c’,e’,f’,h’,i’,k’,l’). Scale bar: 2 μm. The panel labeled VG3-5-HT shows the VGLUT3 boutons co-labeled with SERT (c,c’), 5-HT (f,f’), and SERT+5-HT (i,i’). VGLUT3 puncta located within 5-HT varicosities were isolated using Imaris (l,l’). Arrowheads show 5-HT varicosities collocated with SERT and VGLUT3 (c,c’,f,f’,i,i’,l,l’). (C: a,b) Quantification of the proportion of 5-HT varicosities not co-labeled (SERT-) and co-labeled (SERT+) with SERT in the upper (I–III) or deeper (IV,V) layers of the PrL, showing a great majority of 5-HT varicosities co-labeled with SERT. (c,d) Quantification of the proportion of 5-HT+/VGLUT3+ not co-labeled (SERT-) and co-labeled (SERT+) with SERT in the upper (I–III) or deeper (IV,V) layers of the PrL, showing a great majority of 5-HT+/VGLUT3+ varicosities co-labeled with SERT (t-test, ∗∗∗∗: p < 0.0001).
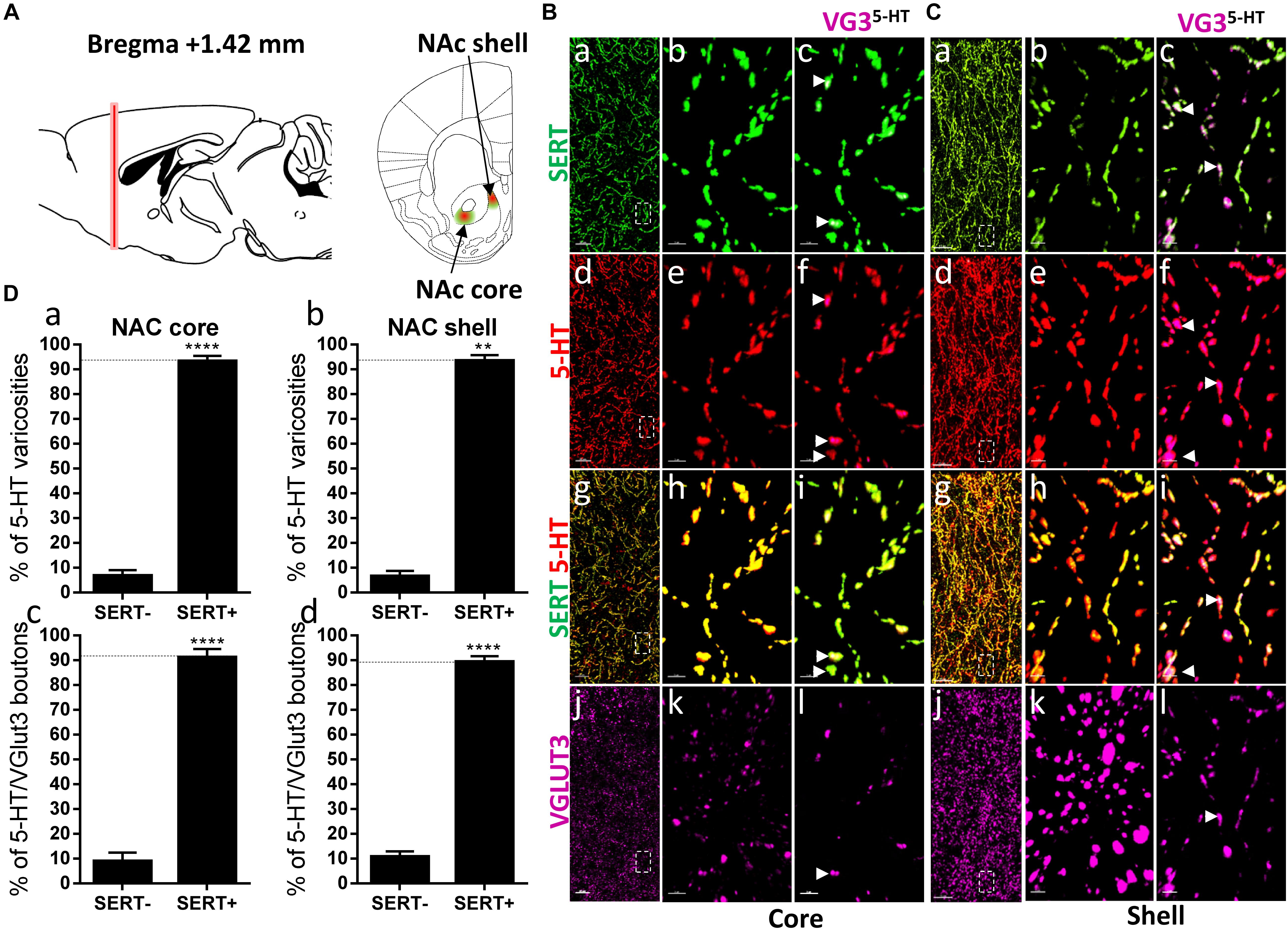
Figure 2. Distribution of VGLUT3+ boutons within 5-HT+ and SERT+ axons in the nucleus accumbens. (A) Schematic drawing showing the location of the acquired micrographs. Nucleus accumbens (NAc) mosaic images were acquired at bregma +1.42 ± 0.2 mm (red vertical line) in the dorsomedial shell and the core (ventrolateral to the anterior commissure) of the nucleus accumbens (green/red areas). (B) Micrograph showing the distribution of the SERT (green, a–c), 5-HT (red, d–f), SERT (green) + 5-HT (red) (g–i), and VGLUT3 (magenta, j–l) in the NAc core. Left panel (a,d,g,j) shows lower magnification. Scale bar: 50 μm. Middle (b,e,h,k) and right (c,f,i,l) panels show higher magnification of the white dashed box in the left panel (a,d,g,j). Scale bar: 2 μm. The right panel shows the VGLUT3 boutons co-labeled with SERT (c), 5-HT (f), and SERT + 5-HT (i). VGLUT3 puncta located within 5-HT varicosities were isolated using Imaris (l). Arrowheads show 5-HT varicosities collocated with SERT and VGLUT3 (c,f,i,l). (C) Micrograph showing the distribution of the SERT (green, a–c), 5-HT (red, d–f), SERT (green) + 5-HT (red) (g–i), and VGLUT3 (magenta, j–l) in the Nac shell. Left panel (a,d,g,j) shows lower magnification. Scale bar: 50 μm. Middle (b,e,h,k) and right (c,f,I,l) panels show higher magnification of the white dashed box in the left panel (a,d,g,j). Scale bar: 2 μm. The right panel shows the VGLUT3 boutons co-labeled with SERT (c), 5-HT (f), and SERT + 5-HT (i). VGLUT3 puncta located within 5-HT varicosities were isolated using Imaris (l). Arrowheads show 5-HT varicosities collocated with SERT and VGLUT3 (c,f,i,l). (D: a,b) Quantification of the proportion of 5-HT varicosities not co-labeled (SERT-) and co-labeled (SERT+) with SERT in the NAc, showing a great majority of 5-HT varicosities co-labeled with SERT. (c,d) Quantification of the proportion of 5-HT+/VGLUT3+ not co-labeled (SERT–) and co-labeled (SERT+) with SERT in the NAc, showing a great majority of 5-HT+/VGLUT3+ varicosities co-labeled with SERT (t-test, ∗∗p < 0.01, ∗∗∗∗p < 0.0001).
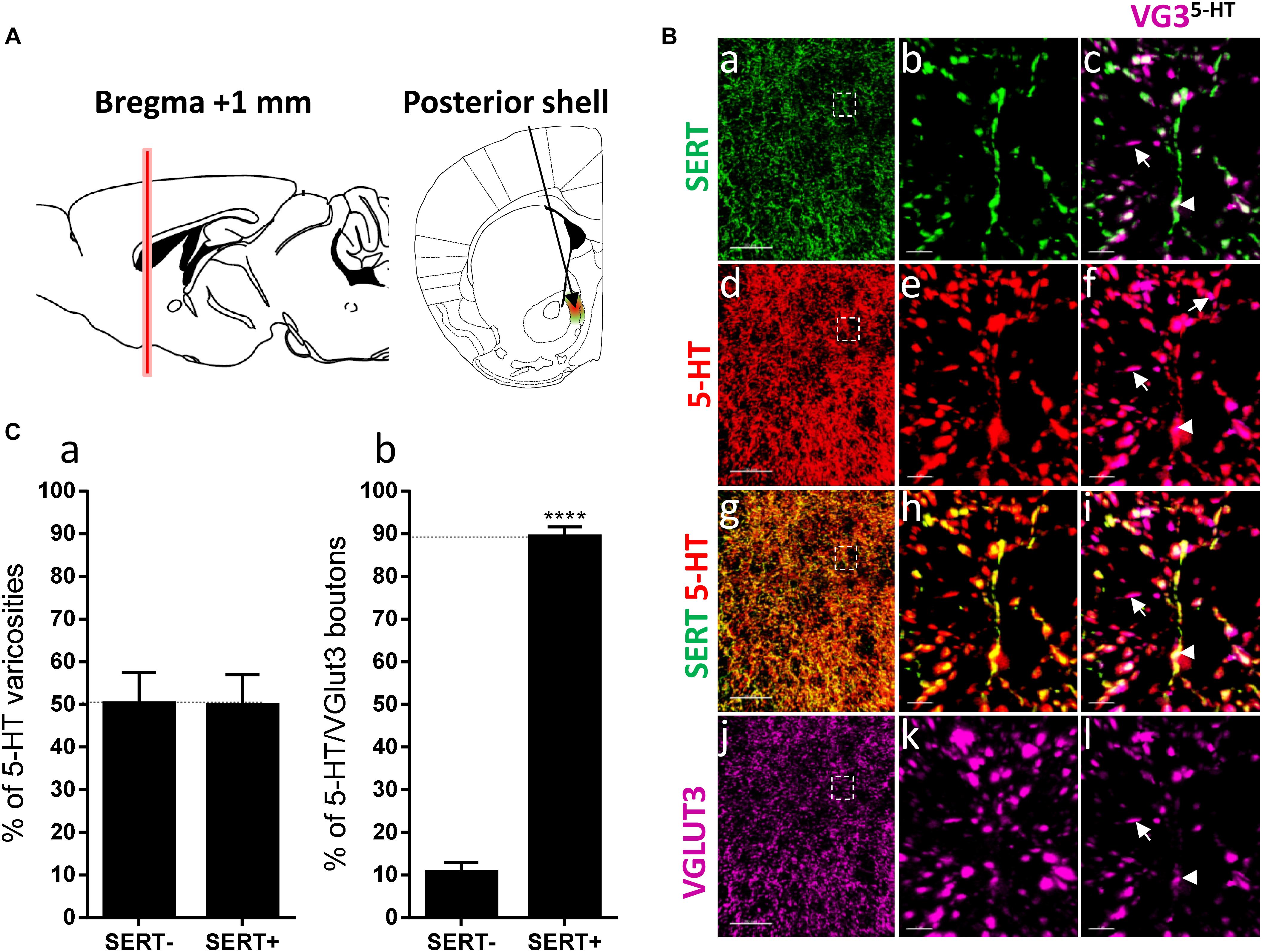
Figure 3. Distribution of VGLUT3+ boutons within 5-HT+ and SERT+ axons in the posterior shell of the nucleus accumbens. (A) Schematic drawing showing the location of the acquired micrographs. The posterior (caudal) shell of the nucleus accumbens (NAc) (green/red area) mosaic images were acquired at bregma +1 ± 0.2 mm (red vertical line). (B) Micrograph showing the distribution of the SERT (green, a–c), 5-HT (red, d–f), SERT (green) + 5-HT (red) (g–i), and VGLUT3 (magenta, j–l). Right panel (a,d,g,j) shows lower magnification. Scale bar: 50 μm. Middle (b,e,h,k) and right (c,f,i,l) panels show higher magnification of the white dashed box in the left panel (a,d,g,j). Scale bar: 2 μm. The right panel (c,f,i,l) shows the VGLUT3 boutons co-labeled with SERT (c), 5-HT (f) and SERT + 5-HT (i). VGLUT3 puncta located within 5-HT varicosities were isolated using Imaris (l). Arrowheads show 5-HT varicosities collocated with SERT and VGLUT3 (c,f,i,l). (C:a) Quantification of the proportion of 5-HT varicosities not co-labeled (SERT-) and co-labeled (SERT+) with SERT in the posterior shell of the NAc, showing a similar proportion of 5-HT varicosities co-labeled with SERT. (b) Quantification of the proportion of 5-HT+/VGLUT3+ not co-labeled (SERT-) and co-labeled (SERT+) with SERT in the posterior shell of the NAc, showing a great majority of 5-HT+/VGLUT3+ varicosities co-labeled with SERT (t-test, ∗∗∗∗p < 0.0001).
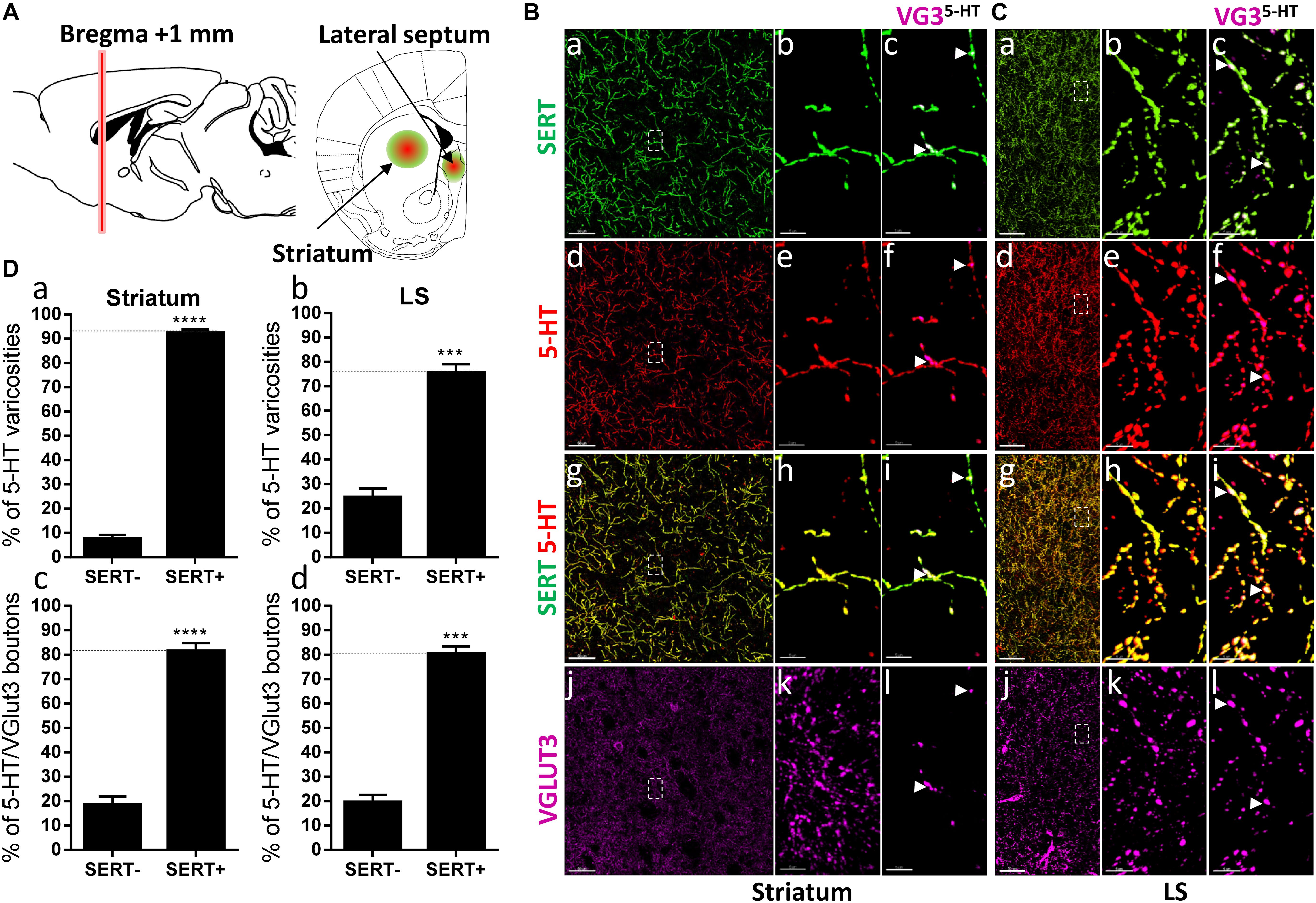
Figure 4. Distribution of VGLUT3+ boutons within 5-HT+ and SERT+ axons in the striatum and lateral septum. (A) Schematic drawing showing the location of the acquired micrographs. Mediolateral dorsal striatum and lateral septum (LS) mosaic images were acquired at bregma +1 ± 0.2 mm (red vertical line) (green/red circles). (B) Micrograph showing the distribution of the SERT (green, a–c), 5-HT (red, d–f), SERT (green) + 5-HT (red) (g–i), and VGLUT3 (magenta, j–l) in the striatum. Left panel shows lower magnification. Scale bar: 50 μm. Middle (b,e,h,k) and right (c,f,i,l) panels show higher magnification of the white dashed box in the left panel (a,d,g,j). Scale bar: 2 μm. The right panel shows the VGLUT3 boutons co-labeled with SERT (c), 5-HT (f) and SERT + 5-HT (i). VGLUT3 puncta located within 5-HT varicosities were isolated using Imaris (l). Arrowheads show 5-HT varicosities collocated with SERT and VGLUT3 (c,f,i,l). (C) Micrograph showing the distribution of the SERT (green, a–c), 5-HT (red, d–f), SERT (green) + 5-HT (red) (g–i), and VGLUT3 (magenta, j–l) in the LS. Left panel shows lower magnification. Scale bar: 50 μm. Middle (b,e,h,k) and right (c,f,i,l) panels show higher magnification of the white dashed box in the left panel (a,d,g,j). Scale bar: 2 μm. The right panel (c,f,i,l) shows the VGLUT3 boutons co-labeled with SERT (c), 5-HT (f) and SERT + 5-HT (i). VGLUT3 puncta located within 5-HT varicosities (l). Arrowheads show 5-HT varicosities collocated with SERT and VGLUT3 (c,f,i,l). (D: a,b) Quantification of the proportion of 5-HT varicosities not co-labeled (SERT-) and co-labeled (SERT+) with SERT in the striatum (a) and LS (b), showing a great majority of 5-HT varicosities co-labeled with SERT. (c,d) Quantification of the proportion of 5-HT+/VGLUT3+ not co-labeled (SERT-) and co-labeled (SERT+) with SERT in the striatum (c) and LS (d), showing a great majority of 5-HT+/VGLUT3+ varicosities co-labeled with SERT (t-test, ∗∗∗∗p < 0.0001; ∗∗∗p < 0.001).
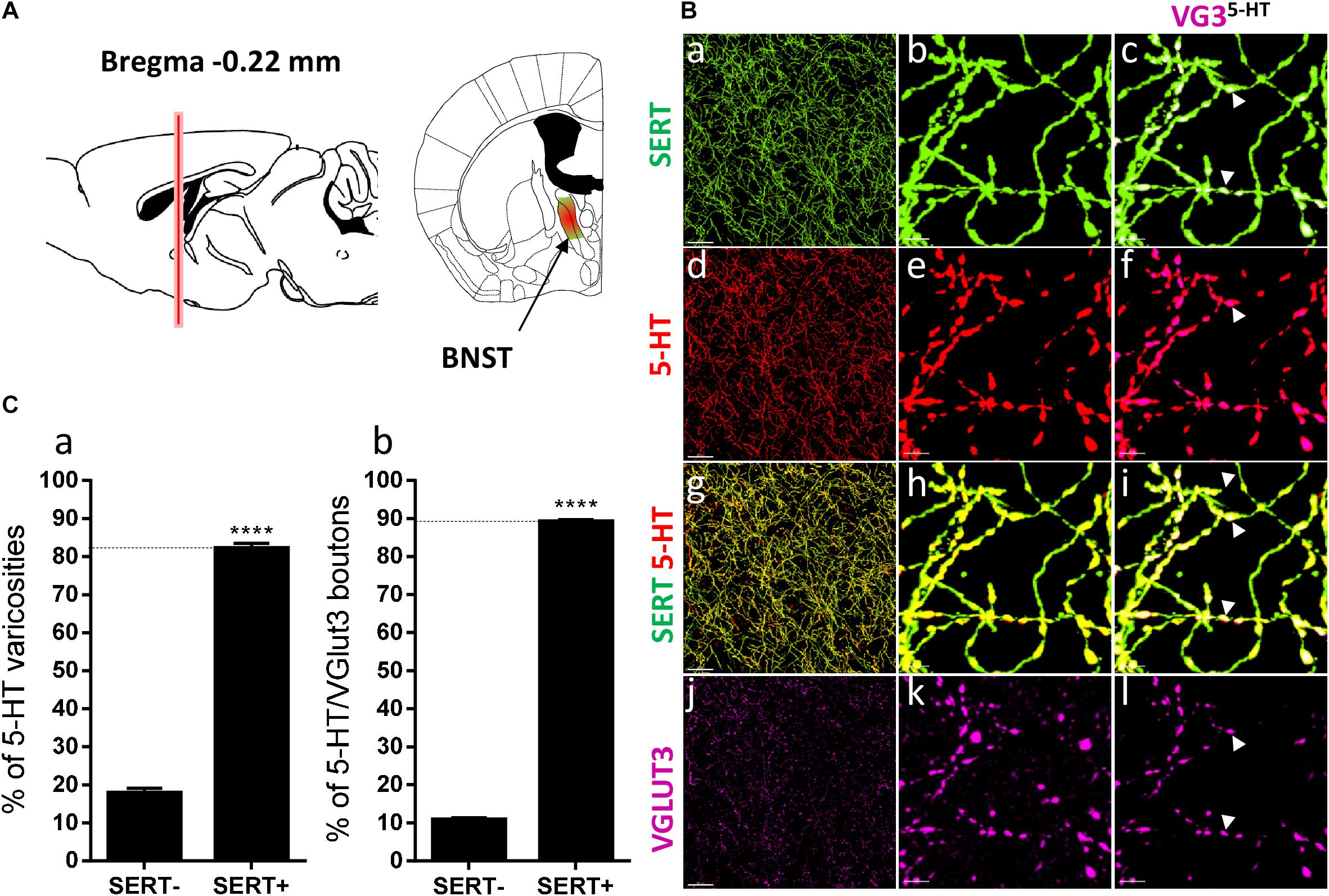
Figure 5. Distribution of VGLUT3+ boutons within 5-HT+ and SERT+ axons in the bed nucleus of the stria terminalis. (A) Schematic drawing showing the location of the acquired micrographs. The bed nucleus of the stria terminalis (BNST) mosaic images were acquired at bregma –0.22 ± 0.3 mm (red vertical line) in the medial posteromedial BNST (BSTMPM) and postero-intermediate part of the BNST (BSTMPI) (green/red area). (B) Micrograph showing the distribution of the SERT (green, a–c), 5-HT (red, d–f), SERT (green) + 5-HT (red) (g–i), and VGLUT3 (magenta, j–l) in the BNST. Left panel (a,d,g,j) shows lower magnification. Scale bar: 50 μm. Middle (b,e,h,k) and right (c,f,i,l) panels show higher magnification of the white dashed box in the left panel (a,d,g,j). Scale bar: 2 μm. The right panel (c,f,i,l) shows the VGLUT3 boutons co-labeled with SERT (c), 5-HT (f), and SERT + 5-HT (i). VGLUT3 puncta located within 5-HT varicosities were isolated using Imaris (l). Arrowheads show 5-HT varicosities collocated with SERT and VGLUT3 (c,f,i,l). (C:a) Quantification of the proportion of 5-HT varicosities not co-labeled (SERT-) and co-labeled (SERT+) with SERT in the BNST, showing a great majority of 5-HT varicosities co-labeled with SERT. (b) Quantification of the proportion of 5-HT+/VGLUT3+ not co-labeled (SERT-) and co-labeled (SERT+) with SERT in the BNST, showing a great majority of 5-HT+/VGLUT3+ varicosities co-labeled with SERT (t-test, ∗∗∗∗p < 0.0001).
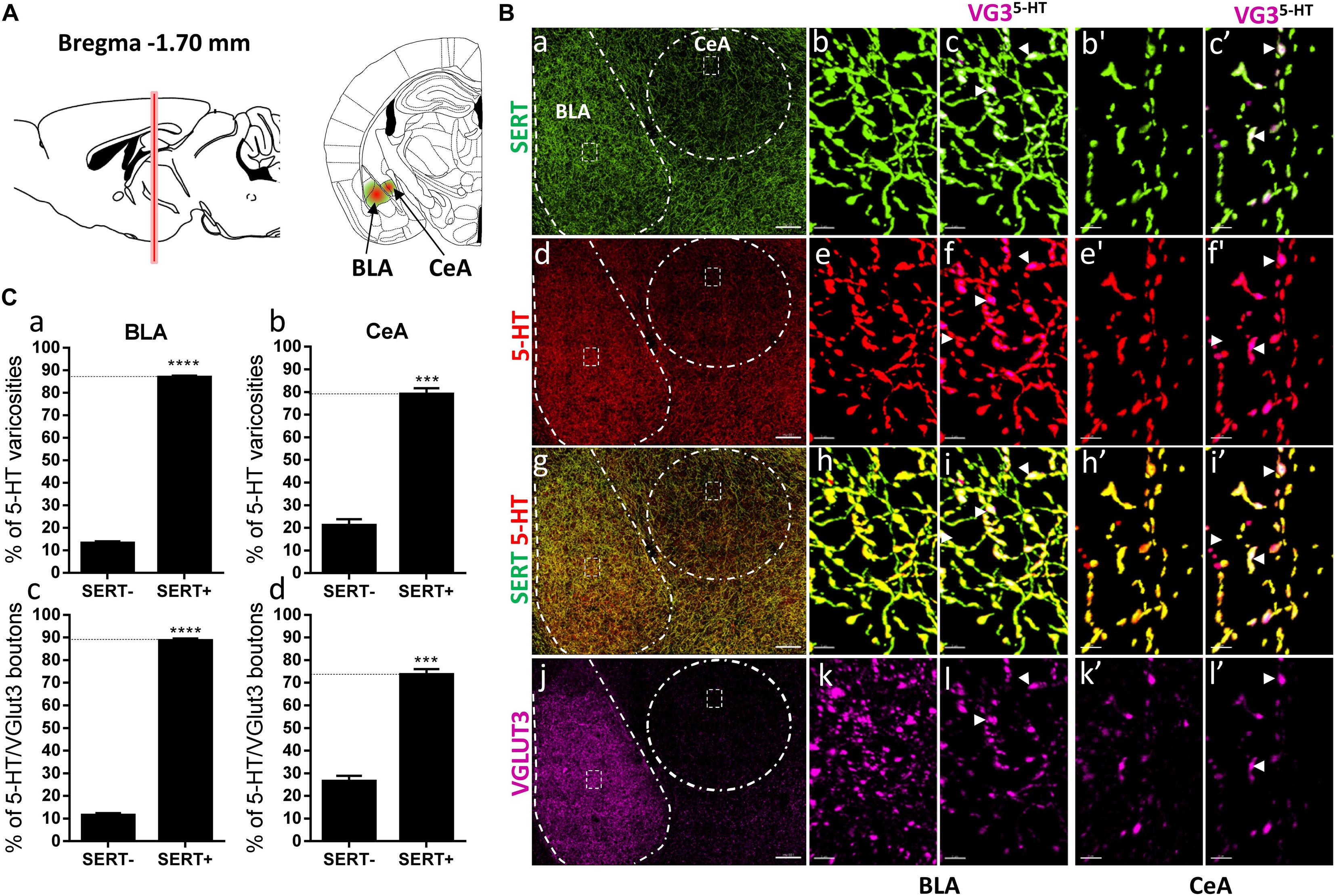
Figure 6. Distribution of VGLUT3+ boutons within 5-HT+ and SERT+ axons in the basolateral amygdala and central nucleus of the amygdala. (A) Schematic drawing showing the location of the acquired micrographs. The basolateral amygdala (BLA) and central nucleus of the amygdala (CeA) mosaic images were acquired at bregma –1.70 ± 0.2 mm (red vertical line) in the anterior part of the basolateral amygdala and the lateral CeA (green/red areas). (B) Micrograph showing the distribution of the SERT (green, a–c,b’,c’), 5-HT (red, d–f,e’,f’), SERT (green) + 5-HT (red) (g–i,h’,i’), and VGLUT3 (magenta, j–l,k’,l’) in the CeA and BLA. Left panel (a,d,g,j) shows lower magnification. Scale bar: 150 μm. Middle (b,e,h,k and b’,e’,h’,k’) and right (c,f,i,l and c’,i’,f’,l’) panels show higher magnification of the white dashed areas in the left panel (a,d,g,j). Scale bar: 2 μm. The right panels (c,f,i,l and c’,f’,i’,l’) shows the VGLUT3 boutons co-labeled with SERT (c,c’), 5-HT (f,f’), and SERT + 5-HT (i,i’) in the BLA and CeA. VGLUT3 puncta located within 5-HT varicosities were isolated using Imaris (l,l’). Arrowheads show 5-HT varicosities collocated with SERT and VGLUT3 (c,f,i,l and c’,f’,i’,l’). (C: a,b) Quantification of the proportion of 5-HT varicosities not co-labeled (SERT-) and co-labeled (SERT+) with SERT in the BLA (a) and CeA (b), showing a great majority of 5-HT varicosities co-labeled with SERT. (c,d) Quantification of the proportion of 5-HT+/VGLUT3+ not co-labeled (SERT–) and co-labeled (SERT+) with SERT in the BLA (c) and CeA (d), showing a great majority of 5-HT+/VGLUT3+ varicosities co-labeled with SERT (t-test, ∗∗∗∗p < 0.0001; ∗∗∗p < 0.001).
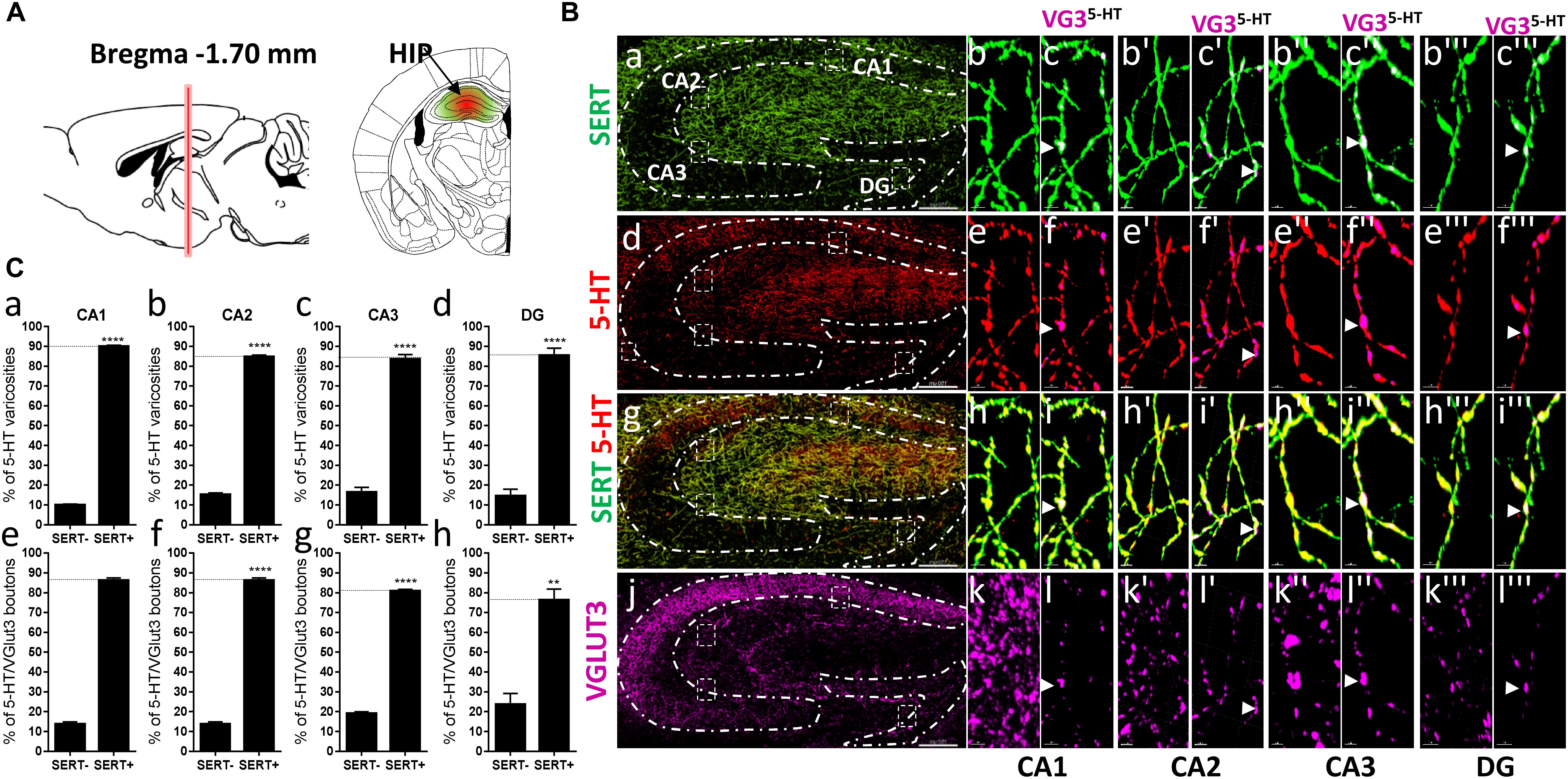
Figure 7. Distribution of VGLUT3+ boutons within 5-HT+ and SERT+ axons in the CA1, CA2, CA3, dentate gyrus of the hippocampus. (A) Schematic drawing showing the location of the acquired micrographs. The hippocampus (HIP) mosaic images were acquired at bregma –1.70 ± 0.3 mm (red vertical line) in the dorsal hippocampus (green/red area). (B) Micrograph showing the distribution of the SERT (green, a–c”’), 5-HT (red, d–f”’), SERT (green) + 5-HT (red) (g–i”’), and VGLUT3 (magenta, j–l”’) in the CA1, CA2, CA3, and DG. Left panel (a,d,g,j) shows lower magnification. Scale bar: 150 μm. Right panels show higher magnification of the CA1 (b–l), CA2 (b’–l’), CA3 (b”–l”), and DG (b”’–l”’) in the white dashed box in the left panel (a,d,g,j). Scale bar: 2 μm. The right panel shows the VGLUT3 boutons co-labeled with SERT (c–c”’), 5-HT (f–f”’), and SERT + 5-HT (i–i”’). VGLUT3 puncta located within 5-HT varicosities were isolated using Imaris in the CA1 (l–l”’). Arrowheads show 5-HT varicosities collocated with SERT and VGLUT3 in the CA1 (c–c”’, f–f”’, i–i”’, and l–l”’). (C:a–d) Quantification of the proportion of 5-HT varicosities not co-labeled (SERT-) and co-labeled (SERT+) with SERT in the CA1 (a), CA2 (b), CA3 (c), and DG (d) of the HIP, showing a great majority of 5-HT varicosities co-labeled with SERT. (e–h) Quantification of the proportion of 5-HT + /VGLUT3+ not co-labeled (SERT-) and co-labeled (SERT+) with SERT in the CA1 (e), CA2 (f), CA3 (g), and DG (h) of the HIP, showing a great majority of 5-HT+/VGLUT3+ varicosities co-labeled with SERT (t-test, ∗∗∗∗p < 0.0001; ∗∗p < 0.01).
Statistics
Statistical analyses were carried out using GraphPad Prism 7.0. The proportion of 5-HT or 5-HT/VGLUT3 varicosities immunopositive for SERT were compared to SERT immunonegative boutons within each brain region using a two-tailed unpaired t-test. The densities and relative densities of 5-HT/VGLUT3 boutons within all the analyzed brain regions were compared using one-way ANOVA with Bonferroni correction for multiple comparison. Correlation analysis of the density of 5-HT+/VGLUT3+ varicosities and density of 5-HT was performed using the Pearson correlation test. A p-value <0.05 was considered significant, with all values expressed as the mean ± SEM.
Results
Previous studies have suggested that VGLUT3 always segregates with SERT within the varicosities of the 5-HT axons projecting to the prelimbic region of the prefrontal cortex (Amilhon et al., 2010). Therefore, we first examined the distribution of VGLUT3 and SERT immunoreactive 5-HT varicosities within the different layers of the prelimbic cortex (PrL), at bregma +2.46 mm (Figure 1A). Co-labeling of the SERT and 5-HT revealed that most of the varicosities reconstructed were co-labeled for the two markers (Figure 1B: a–i’). Labeling of VGLUT3 showed the typical scattered punctate fluorescence (Figure 1B: j) and by combining the 3D-reconstruction and the masking functions of Imaris, we isolated the VGLUT3 labeling that was only contained in 3D-reconstructed 5-HT varicosities (Figure 1B: k,k’). We observed that almost all the 5-HT/VGLUT3 boutons were co-labeled with SERT (arrowheads, Figure 1B: c,f,i,l and c’,f’,i’,l’). Indeed, the absolute quantifications of the proportion of 5-HT varicosities that were co-labeled with SERT confirmed that 85 and 91% of 5-HT varicosities were co-labeled with SERT in the layers 4–5 (t = 17.64, df = 8, p < 0.0001) and 1–3 (t = 32.01, df = 8, p < 0.0001), respectively (Figure 1C: a,b). Similar proportions of 5-HT/VGLUT3 boutons (82%, t = 11.12, df = 8, p < 0.0001 and 91%, t = 29.19, df = 8, p < 0.0001) were also co-labeled with SERT (Figure 1C: c,d). These results suggest that only a small proportion of 5-HT boutons (9–18%) do not express detectable levels of SERT in the PrL and, that VGLUT3 and SERT do not predominantly segregate within the 5-HT axonal varicosities in this brain region.
The co-release of 5-HT and glutamate by 5-HT neurons from the dorsal raphe has been proposed to play an important role in the regulation of the neurotransmission in the ventral striatum and the modulation of reward-related behaviors (Liu et al., 2014). Hence, we next investigated the distribution of SERT and VGLUT3 immunolabeled varicosities within the NAc core and shell, at bregma +1.42 mm (Figure 2A). Both in the core (94%, t = 18.99, df = 9, p < 0.0001) and the shell (95%, t = 19.52, df = 2, p < 0.01) regions of the NAc, the great majority of 5-HT boutons were also immunoreactive for SERT (Figures 2B,C: a–i, D: a,b). Hence, a great proportion of 5-HT+/VGLUT3+ boutons were co-labeled with the SERT, both in the core (92%, t = 11.88, df = 8, p < 0.0001) and the shell (89%, t = 17.1, df = 10, p < 0.0001) (arrowheads, Figures 2B,C: c,f,i,l, D: c,d). These results support a high degree of overlap between 5-HT and SERT immunoreactive axons in the core and the shell of the rostral NAc (Brown and Molliver, 2000), and further highlight the absence of any particular segregation between SERT and VGLUT3 within 5-HT varicosities.
Brown and Molliver (2000) also observed that a subset of 5-HT axons lack SERT in the caudal part of the rat NAc shell. Therefore, we investigated the distribution of SERT and VGLUT3 within the 5-HT varicosities in the posterior NAc shell, at bregma +1.00 mm, at a similar level to the aforementioned study in rat (i.e., “septal pole” or “cone region”) (Figure 3A). Interestingly, we found the 5-HT varicosities either co-labeled (arrowheads) or not labeled (or only weakly/partially labeled, arrows) for the SERT in the posterior NAc shell (Figure 3B: a–i) and both SERT+ and SERT- serotonergic varicosities were co-labeled for VGLUT3 (Figure 3B: c,f,i–l). The quantification revealed that half of the 5-HT varicosities do not express the SERT in the posterior shell of the mouse NAc (t = 0.03096, df = 6; p = 0.97; Figure 3C: a) as previously described in rats. Although VGLUT3 was observed in some SERT-varicosities, this subtype of serotonergic boutons only represents a minority (11%), as the great majority (89%, t = 17.1, df = 10, Figure 3C: b) of the 5-HT+/VGLUT3+ boutons were also co-labeled for SERT. These results further suggest that VGLUT3 are rather colocalized than segregated with the SERT in 5-HT varicosities.
The segregation of SERT and VGLUT3 was also reported in the dorsal striatum and the LS (Voisin et al., 2016). Hence, we investigated their distribution at bregma +1.00 mm (Figure 4A). Again, the great majority of the 5-HT varicosities express the SERT in both the dorsal striatum (92%, t = 28.02, df = 11; Figures 4B: a–i, D: a) and LS (75%, t = 10.11, df = 5, Figures 4C: a–i, D: b). Therefore, no axonal segregation could be observed, indeed more than 80% of the VGLUT3 immunoreactive 5-HT boutons were also co-labeled for the SERT, in both the striatum and LS (Figures 4B,C: c,f,i,l, arrowheads; Figure 4D: c,d). These results further evidence that SERT and VGLUT3 cannot be used as markers to classify different 5-HTergic axonal subtypes.
DR 5-HT neurons that send VGLUT3 immunoreactive axons to the NAc also send collaterals to different brain regions, including the BNST, CeA, and BLA. We therefore investigated the distribution of SERT and VGLUT3 within 5-HT varicosities in those brain regions (Figures 5, 6). In the anterior BNST at bregma -0.22 mm (Figure 5A), we again observed a high degree of overlapping between SERT and 5-HT immunoreactivity (82%, t = 23.17, df = 17, p < 0.0001; Figures 5B: a–i, C: a), with a high proportion (89%, t = 71.51, df = 17, p < 0.0001) of 5-HT/VGLUT3 varicosities that were co-labeled for SERT (Figures 5B: a,f,i,l, arrowheads, C: b). Similarly, in the amygdala at bregma -1.70 mm (Figure 6A), the great majority of 5-HT boutons were co-labeled for SERT in both the BLA (87%, t = 27.6, df = 11, p < 0.0001) and CeA (79%, t = 16.58, df = 5, p < 0.0001; Figures 6B: a–i’, C: a,b). Consequently, a great proportion of 5-HT+/VGLUT3+ boutons were co-labeled with the SERT within the BLA (89%, t = 38.6 df = 11, p < 0.0001) and CeA (73%, t = 9.46, df = 5, p = 0.0002) (arrowheads, Figures 6B: i–l’, C: c,d).
In the hippocampus, VGLUT3 was shown to modulate 5-HTergic tone, and to stimulate VMAT2-dependent accumulation of 5-HT in synaptic vesicles (Amilhon et al., 2010), which further suggests that VGLUT3/5-HT synaptic cross-talk may play an important role in hippocampal-mediated behaviors such as anxiety and depression. Since these behaviors are also dependent upon SERT activity/blockade by serotonergic antidepressant, whether VGLUT3 and SERT segregate or colocalize within 5-HT varicosities in the hippocampus is question of great interest and could further help in developing improved therapeutics for the treatment of anxiety- or depression-related disorders. Therefore, we rigorously investigated the distribution of SERT and VGLUT3 in 5-HT axonal varicosities within the different subregions of the hippocampus, at bregma -1.70 ± 0.3 mm (Figure 7A). Most of 5-HT boutons were also immunoreactive for SERT in all four regions of the hippocampus, CA1 (90%, t = 87.01, df = 3, p < 0.0001), CA2 (85%, t = 45.56, df = 3, p < 0.0001), CA3 (84%, t = 14.07, df = 3, p = 0.0008), and DG (86%, t = 10.17, df = 3, p = 0.002) (Figures 7B: g–i”’, C: a–d). A similar proportion of 5-HT+/VGLUT3+ boutons were co-labeled with SERT in the CA1 (87%, t = 32.76, df = 3, p < 0.0001), CA2 (87%, t = 32.76, df = 3, p < 0.0001), CA3 (82%, t = 37.55, df = 3, p < 0.0001), and DG (78%, t = 4.766 df = 3, p = 0.0175) (arrowheads, Figures 7B: c–c”’,f–f”’,i–i”’,l–l”’, C: e–h).
The absence of segregation we observed was further confirmed by quantification of the degree of colocalization between the different fluorophores/markers in selected brain regions (DLS, LS, and dorsal HIP) using Mander’s overlap coefficients (MOC; Supplementary Figure S1).
We have reconstructed a total of 106 serotonergic varicosities from various brain regions, including the prelimbic cortex (5 × 104), NAc (10 × 104), LS (15 × 104), BNST (25 × 104), amygdala (27 × 104), hippocampus (15 × 104), and dorsal striatum (5 × 104). Our quantification of the volumetric density of 5-HT+/VGLUT3+ boutons revealed a high heterogeneity along the different brain regions. The, CA1–3, LS, amygdala (BLA and CeA) and NAc shell show the highest density, and the NAc core, striatum, DG and prelimbic cortex show the lowest density of 5-HT+/VGLUT3+ varicosities [Figure 8A, One-way ANOVA, F(12,94) = 10.55, p < 0.0001 see Supplementary Table 1 for Bonferroni’s multiple comparisons]. The relative density of 5-HT+/VGLUT3+ boutons, calculated as a percentage of total 5-HT varicosities within each brain region, shows the largest proportion is located in CA1–3 (24–32%), followed by the PrL (20–23%) > DG (19%) > CeA (16%) > LS (14%) > BLA (13%) > NAc shell (10%) > BNST (7%) > striatum (6%) > NAc core (4.2%) [Figure 8B, F(12,94) = 46.37, p < 0.0001, see Supplementary Table 2 for Bonferroni’s multiple comparisons, and Figure 9]. Correlation analysis shows that the density of 5-HT+/VGLUT3+ varicosities is totally independent of the density of 5-HT boutons within each brain region (Pearson’s coefficients r = 0.32 and R2 = 0.10, p = 0.28). This data suggests that the heterogeneous distribution of 5-HT+/VGLUT3+ varicosities in the forebrain represents a functionally relevant feature of 5-HT neurons complex topology, rather than a biased detection of those varicosities in our methodological approach.
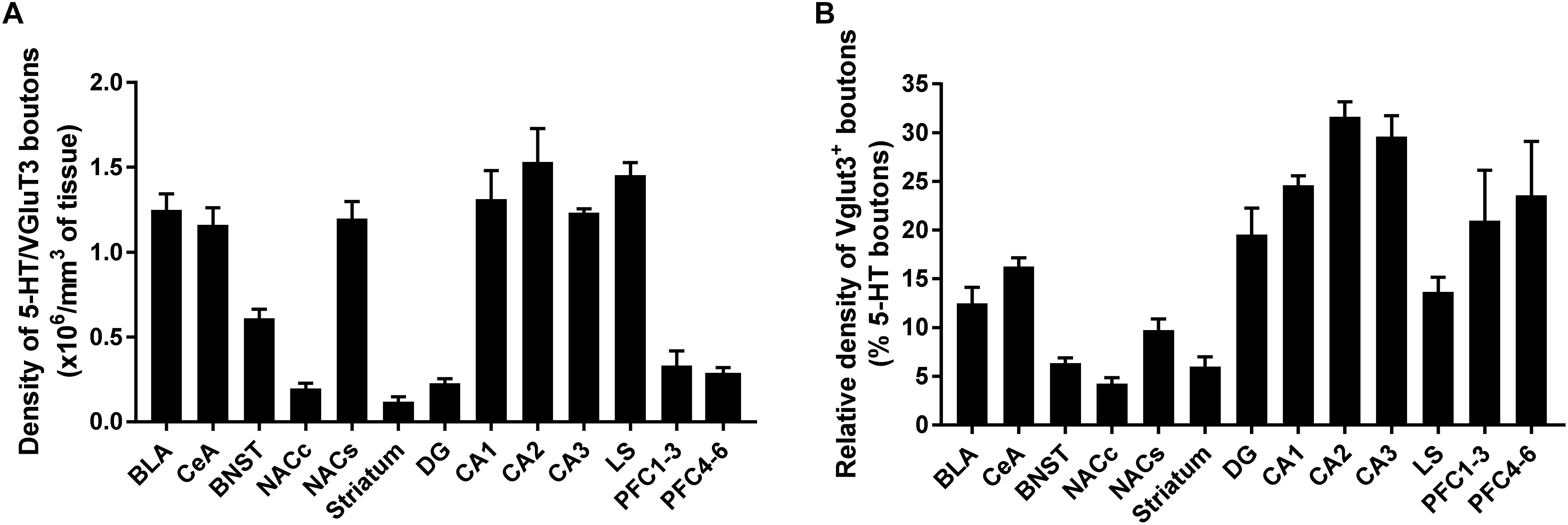
Figure 8. Quantification of 5-HT neurons expressing vesicular glutamate transporter (VGLUT3) in various regions of the mouse forebrain. Volumetric quantification of the density of VGLUT3 immunoreactive boutons within 5-HT-labeled fibers in each brain region. The results are expressed as density of boutons per 106mm3 of 5-HT+ fiber (A), or percent of boutons in 5-HT+ fiber (B) and represented as the mean ± SEM.
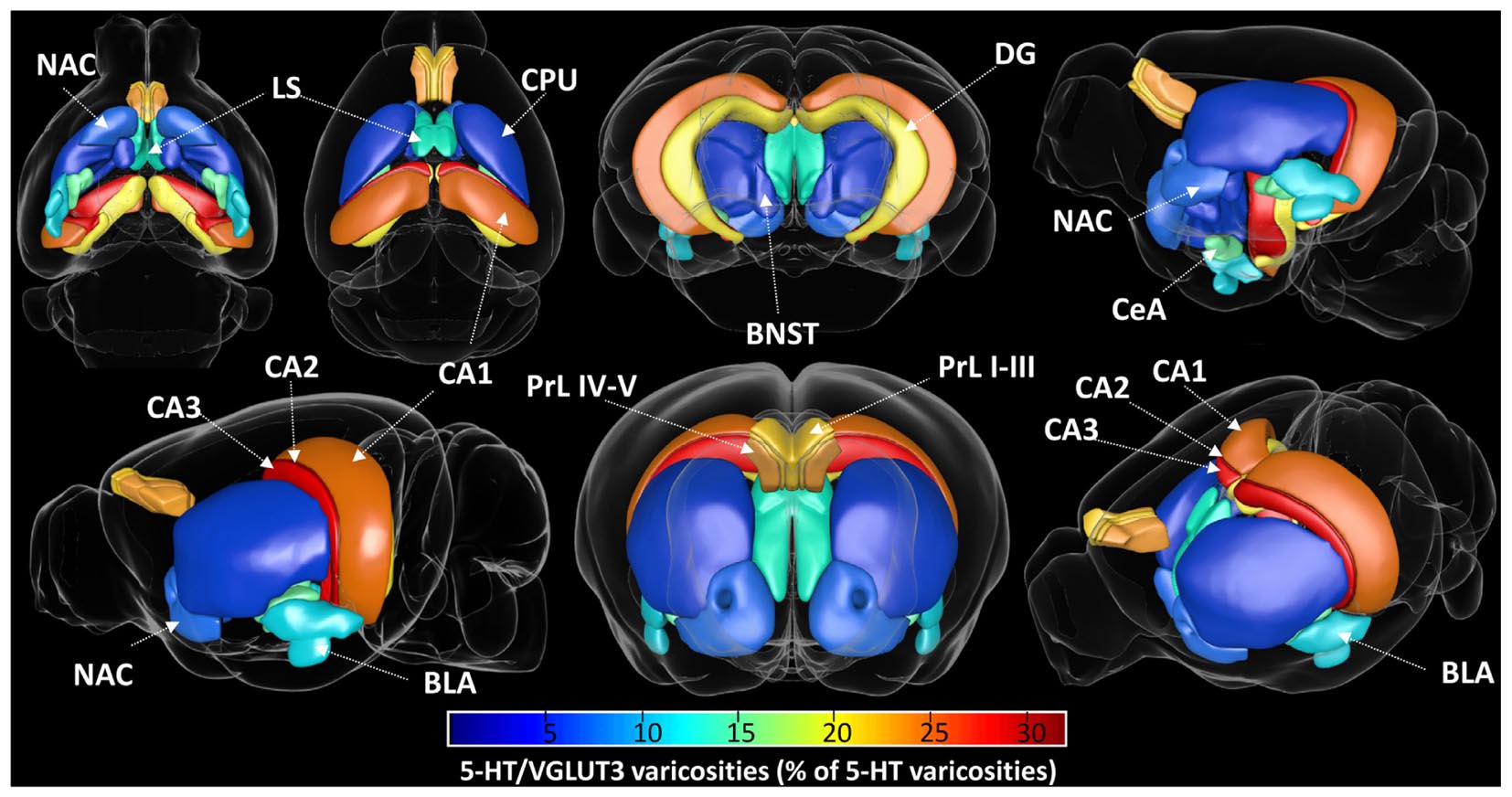
Figure 9. Visual representation of the proportion of 5-HT varicosities expressing the vesicular glutamate transporter VGLUT3 in various regions of the mouse forebrain. The percentage of total 5-HT varicosities that are co-labeled with VGLUT3 is represented with a color coding from 0 (dark blue) to 32% (dark red) within each analyzed brain region, from various viewing angle. The highest proportion of VGLUT3 was found in CA2, followed by CA3, CA1, PrL IV-V, PrL I-III, DG, CeA, BLA, NAc shell, BNST, and NAc core.
Discussion
The main finding of the present study is that SERT and VGLUT3 rarely segregate within 5-HT varicosities, but rather preferentially colocalize in most of the mouse forebrain regions we analyzed. These results are in agreement with a previous observation in rats (Gagnon and Parent, 2014), but surprisingly differ from two previous studies in WT littermates (vglut3+/+) of a transgenic mouse line, obtained by the breeding of vglut3+/- mice (Amilhon et al., 2010; Voisin et al., 2016). In these mice with mixed C57Bl/6 and 129/Sv backgrounds, a clear segregation of SERT and VGLUT3 was reported in the varicosities of the 5-HTergic axons projecting to the prelimbic cortex, the ventral and dorsal CA3 field of the hippocampus, the dorsal striatum and the LS (Amilhon et al., 2010; Voisin et al., 2016). In our study in pure wild-type C57Bl6/j background male and female mice of similar age (around P60), we did not observe this segregation between SERT and VGLUT3 in any brain region, suggesting that this difference may originate from mouse strain or gender variations. These discrepancies could also arise from differences in the methodology, probably related to antibody specificity or sensitivity. Indeed, the goat-anti SERT antibody used by Voisin et al. (2016; Santa Cruz; C-20) in their quantification was shown to yield non-specific binding on brain extracts from SERT knock-out mice, as opposed to the rabbit anti-SERT antibody from Calbiochem (PC177) used in the present study which was shown to be highly specific (Biezonski and Meyer, 2010). Further, Voisin et al. (2016) performed their immunohistochemistry experiments on 100 μm-thick sections while we used 30 μm sections in the present study, which could also produce variations in antibody penetration and immunodetection. Furthermore, in their immunogold staining of SERT and VGLUT3, they reported the use of normal goat serum for blocking, in combination with anti-goat secondary antibodies to label the SERT. This could result in anti-goat antibodies binding non-specifically to goat serum IgGs bound to the sections, and lead to underestimation of SERT immunoreactivity. A comparison between the two antibodies would indeed be of high interest to identify the proposed discrepancies, however, the C-20 antibody from Santa Cruz has now been discontinued.
Further studies using comprehensive titrations of different commercially available antibodies, or using conditional co-expression of tagged/fluorescent VGLUT3 and SERT proteins selectively in 5-HT neurons such as TPH2-CRE mice, are therefore needed to confirm the colocalization or segregation of SERT and VGLUT3 within 5-HT axonal varicosities in the mouse brain.
Axonal segregation between SERT and VGLUT3 within 5-HT varicosities would imply that a significant subset of 5-HT varicosities do not express the SERT. However, our results demonstrate that the great majority of 5-HT boutons (about 90%) are immunoreactive for the SERT, in almost all the brain regions studied. This absence of segregation of SERT and VGLUT3 therefore argues against the existence of a subset of 5-HT terminals with enhanced extracellular 5-HT levels after release due to weak 5-HT reuptake (Voisin et al., 2016). The only brain region with an equivalent proportion of SERT-positive and SERT-negative 5-HT boutons was the posterior part of the NAc shell. This is in line with previous reports in rats showing that only subtle differences could be detected between 5-HT and of SERT immunostaining in particular brain regions, with only a subset of 5-HT axonal projections that lack the SERT in the posterior part of the NAc (Brown and Molliver, 2000). While we observed that only half of 5-HT varicosities express the SERT in this brain region, we found VGLUT3 preferentially localized within SERT immunoreactive varicosities with only a small proportion (10%) of VGLUT3 localized in boutons lacking the SERT. This further suggests that the ability to co-release 5-HT and glutamate from the same vesicles relies on the presence of effective 5-HT reuptake machinery in the varicosities.
While the existence of 5-HT immunoreactive axons lacking the SERT has been evidenced almost 20 years ago (Brown and Molliver, 2000), only little is known regarding the origin or physiology of those SERT-/5-HT+ axons. Since 5-HT could also be stored in dopaminergic neurons of the substantia nigra or the ventral tegmental area (Zhou et al., 2005), we assessed the potential dopaminergic nature of the SERT-/5-HT+ varicosities. The absence of immunoreactivity for the tyrosine hydroxylase confirmed the non-catecholaminergic phenotype of these varicosities (data not shown), however, we cannot rule out the possibility that these 5-HT+/SERT- axons are not truly serotonergic. Retrograde tracing studies are currently performed in our laboratory to determine the nature and origin of this particular subset of SERT-/5-HT+ axons, i.e., whether they expressed all these projections originate from the dorsal, median, or caudal raphe, whether this particular subtype of 5-HT neurons are restricted to specific raphe subnuclei.
Our results also show that 5-HT axons differentially express VGLUT3 in various brain regions, with a relative density of 5-HT varicosities expressing VGLUT3 ranging from 5 to 10% (dorsal and ventral striatum, BNST) to 20–30% (hippocampus and prefrontal cortex) of total 5-HT varicosities. This suggests that glutamate co-release could be differentially involved in 5-HT signaling across brain regions. The high density of 5-HT/glutamate co-release sites that we identified in particular brain regions known to be involved in the control of emotion, reward and decision-making such as the amygdala, NAc shell, hippocampus and prefrontal cortex, evokes new potential mechanisms for the control of neuroplasticity in these brain regions, and its dysregulation in the development of addictive disorders (Kauer and Malenka, 2007). In line with this, genetic ablation of vglut3 in mice was shown to predispose to cocaine abuse (Sakae et al., 2015), however, the authors reported that this mechanism is likely independent of 5-HT signaling but rather linked to an increased dopamine and glutamate signaling in the NAc, from VTA dopaminergic and cortical glutamatergic inputs, respectively. Since our results highlight a high density of 5-HT/VGLUT3 boutons in the hippocampus and NAC, where 5-HT is known to play an important role in the regulation of glutamate and dopamine release at the level of axon terminals (Fink and Göthert, 2007), further studies are needed to identify the contribution of serotonin/glutamate co-signaling in addictive behaviors.
Indeed, recent evidence supports a significant role of 5-HT/glutamate co-transmission in both reward and emotion (Amilhon et al., 2010; Liu et al., 2014). Further, the activation of dopamine- and cAMP-regulated phosphoprotein of M(r) 32,000 (DARPP-32) by dopamine/glutamate co-transmission has been postulated to act as a molecular switch that control the reward pathway plasticity that mediates the behavioral sensitization to various drugs of abuse (Nairn et al., 2004; Valjent et al., 2005). DARPP-32 also appears to be involved in some biochemical and behavioral actions of 5-HT (Svenningsson et al., 2002), however, there is no evidence to date that serotonin-induced DARPP-32 activation is mediated by 5-HT/glutamate co-transmission. Although our results suggest that 5-HT and glutamate are co-released in the ventral and dorsal striatum where DARPP-32 is likely recruited following exposure to drugs of abuse, further studies are required to determine the precise role played by 5-HT and/or glutamate and their different receptors in DARPP-32 activation, the subsequent neuroplastic changes and the cognitive/emotional deficits produced by drugs of abuse. Furthermore, genetic ablation of VGLUT3 in mice (vglut3-/-) produces increased anxiety (Amilhon et al., 2010; Sakae et al., 2018), enhanced fear and altered stress axis regulation (Balázsfi et al., 2018), concomitant to the desensitization of 5-HT1A autoreceptors (Amilhon et al., 2010), suggesting a potential cross-regulation between 5-HT1A receptors and glutamate co-release. In line with this, unpublished evidence from our laboratory suggests that chronic treatment with drugs targeting the 5-HT1A receptors alter the density of 5-HT/VGLUT3 varicosities in specific brain region, including the hippocampus. Since the desensitization of 5-HT1A autoreceptors is essential for the antidepressant effect of selective serotonin reuptake inhibitors (SSRIs) (Popa et al., 2010), notably in the hippocampus, this further suggests that selective pharmacological ablation of VGLUT3 might represent a potential adjunct for antidepressant therapy.
In conclusion, despite an evident absence of segregation between VGLUT3 and SERT in 5-HT varicosities, our study confirms previous observations that 5-HT neurons and their axonal projections are heterogeneous in the rodent brain. The differences observed in the topological expression of VGLUT3 in 5-HT varicosities within various brain regions support the idea that 5-HT/glutamate co-release is a mechanism that is tightly regulated. Whether the co-release of glutamate and serotonin is involved in a particular type of neuroplasticity, mediates distinct behaviors or is implicated in specific neuropsychiatric disorders still need to be determined.
Ethics Statement
This study was carried out in accordance with the recommendations of National Health and Medical Research Council (NHMRC) guidelines to promote the well-being of animals used for scientific purposes and the Australian code for the care and use of animals for scientific purposes. The protocol was approved by the Queensland University of Technology Animal Ethics Committee and the University of Queensland Animal Ethics Committee.
Author Contributions
AB performed the IHC experiments. AB and KB acquired the confocal images. KB analyzed the images. AB, KB, and SB designed the experiments and analyzed the results. AJ, OP, and FS assisted with the data collection and figure formatting. AB and KB drafted the manuscript. AB, KB, AJ, OP, and SB revised the manuscript. All authors approved the final version of the manuscript.
Funding
This work was supported by National Health and Medical Research Council (NHMRC) (GNT104942, GNT1061979, and GNT1146417) to SB and Australian Research Council (ARC) (FT1110884) to SB.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are thankful to PACE animal facility manager Lisa Foster and her staff for the good care of our animals. We are grateful to the imaging facility of the Translational Research Institute, the facility manager Dr. Sandrine Roy and the microscopy officer Ali Ju for the extensive use of resources. We thank Mike Grohuk and Ded Kibagami for their technical advice.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fncel.2019.00193/full#supplementary-material
Abbreviations
5-HT, 5-hydroxytryptamine; AMG, amygdala; BLA, basolateral amygdala; BNST, bed nucleus of the stria terminalis; CA1, 2, or 3, cornu ammonis 1, 2 or 3; CeA, central amygdala; CPU, caudate putamen; DG, dentate gyrus; HIP, hippocampus; LS, lateral septum; NAc, nucleus accumbens; NAc core, nucleus accumbens core; NAc shell, nucleus accumbens shell; PrL I–III, prelimbic cortex layer I to III; PrL IV–V, prelimbic cortex layer IV and V; SERT, serotonin transporter; VGLUT3/VG3, vesicular glutamate transporter 3.
References
Amilhon, B., Lepicard, E., Renoir, T., Mongeau, R., Popa, D., Poirel, O., et al. (2010). VGLUT3 (vesicular glutamate transporter type 3) contribution to the regulation of serotonergic transmission and anxiety. J. Neurosci. Off. J. Soc. Neurosci. 30, 2198–2210. doi: 10.1523/JNEUROSCI.5196-09.2010
Aznar, S., Kostova, V., Christiansen, S. H., and Knudsen, G. M. (2005). Alpha 7 nicotinic receptor subunit is present on serotonin neurons projecting to hippocampus and septum. Synapse 55, 196–200. doi: 10.1002/syn.20108
Bakker, R., Tiesinga, P., and Kötter, R. (2015). The scalable brain atlas: instant web-based access to public brain atlases and related content. Neuroinformatics 13, 353–366. doi: 10.1007/s12021-014-9258-x
Balázsfi, D., Fodor, A., Török, B., Ferenczi, S., Kovács, K. J., Haller, J., et al. (2018). Enhanced innate fear and altered stress axis regulation in VGluT3 knockout mice. Stress Amst. Neth. 21, 151–161. doi: 10.1080/10253890.2017.1423053
Belmer, A., Klenowski, P. M., Patkar, O. L., and Bartlett, S. E. (2016). Mapping the connectivity of serotonin transporter immunoreactive axons to excitatory and inhibitory neurochemical synapses in the mouse limbic brain. Brain Struct. Funct. 222, 1297–1314. doi: 10.1007/s00429-016-1278-x
Biezonski, D. K., and Meyer, J. S. (2010). Effects of 3,4-methylenedioxymethamphetamine (MDMA) on serotonin transporter and vesicular monoamine transporter 2 protein and gene expression in rats: implications for MDMA neurotoxicity. J. Neurochem. 112, 951–962. doi: 10.1111/j.1471-4159.2009.06515.x
Bonnavion, P., Bernard, J.-F., Hamon, M., Adrien, J., and Fabre, V. (2010). Heterogeneous distribution of the serotonin 5-HT(1A) receptor mRNA in chemically identified neurons of the mouse rostral brainstem: Implications for the role of serotonin in the regulation of wakefulness and REM sleep. J. Comp. Neurol. 518, 2744–2770. doi: 10.1002/cne.22331
Brown, P., and Molliver, M. E. (2000). Dual serotonin (5-HT) projections to the nucleus accumbens core and shell: relation of the 5-HT transporter to amphetamine-induced neurotoxicity. J. Neurosci. Off. J. Soc. Neurosci. 20, 1952–1963.
Calizo, L. H., Akanwa, A., Ma, X., Pan, Y., Lemos, J. C., Craige, C., et al. (2011). Raphe serotonin neurons are not homogenous: electrophysiological, morphological and neurochemical evidence. Neuropharmacology 61, 524–543. doi: 10.1016/j.neuropharm.2011.04.008
Descarries, L., Riad, M., and Parent, M. (2010). “Chapter 1.4 - Ultrastructure of the serotonin innervation in the mammalian central nervous system,” in Handbook of Behavioral Neuroscience Handbook of the Behavioral Neurobiology of Serotonin, eds C. Muller and B. Jacobs (Berlin: Elsevier), 65–101.
Fasano, C., Rocchetti, J., Pietrajtis, K., Zander, J.-F., Manseau, F., Sakae, D. Y., et al. (2017). Regulation of the hippocampal network by VGLUT3-positive CCK- GAB aergic basket cells. Front. Cell. Neurosci. 11:140. doi: 10.3389/fncel.2017.00140
Fernandez, S. P., Cauli, B., Cabezas, C., Muzerelle, A., Poncer, J.-C., and Gaspar, P. (2016). Multiscale single-cell analysis reveals unique phenotypes of raphe 5-HT neurons projecting to the forebrain. Brain Struct. Funct. 221, 4007–4025. doi: 10.1007/s00429-015-1142-4
Fink, K. B., and Göthert, M. (2007). 5-HT receptor regulation of neurotransmitter release. Pharmacol. Rev. 59, 360–417. doi: 10.1124/pr.59.07103
Fu, W., Le Maître, E., Fabre, V., Bernard, J.-F., David Xu, Z.-Q., and Hökfelt, T. (2010). Chemical neuroanatomy of the dorsal raphe nucleus and adjacent structures of the mouse brain. J. Comp. Neurol. 518, 3464–3494. doi: 10.1002/cne.22407
Gagnon, D., and Parent, M. (2014). Distribution of VGLUT3 in highly collateralized axons from the rat dorsal raphe nucleus as revealed by single-neuron reconstructions. PLoS One 9:e87709. doi: 10.1371/journal.pone.0087709
Gaspar, P., and Lillesaar, C. (2012). Probing the diversity of serotonin neurons. Philos. Trans. R. Soc. B Biol. Sci. 367, 2382–2394. doi: 10.1098/rstb.2011.0378
Gras, C., Herzog, E., Bellenchi, G. C., Bernard, V., Ravassard, P., Pohl, M., et al. (2002). A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J. Neurosci. Off. J. Soc. Neurosci. 22, 5442–5451.
Hajós, M., Allers, K. A., Jennings, K., Sharp, T., Charette, G., Sík, A., et al. (2007). Neurochemical identification of stereotypic burst-firing neurons in the rat dorsal raphe nucleus using juxtacellular labelling methods. Eur. J. Neurosci. 25, 119–126. doi: 10.1111/j.1460-9568.2006.05276.x
Hioki, H., Nakamura, H., Ma, Y.-F., Konno, M., Hayakawa, T., Nakamura, K. C., et al. (2010). Vesicular glutamate transporter 3-expressing nonserotonergic projection neurons constitute a subregion in the rat midbrain raphe nuclei. J. Comp. Neurol. 518, 668–686. doi: 10.1002/cne.22237
Hornung, J. P., Fritschy, J. M., and Törk, I. (1990). Distribution of two morphologically distinct subsets of serotoninergic axons in the cerebral cortex of the marmoset. J. Comp. Neurol. 297, 165–181. doi: 10.1002/cne.902970202
Kast, R. J., Wu, H.-H., Williams, P., Gaspar, P., and Levitt, P. (2017). Specific connectivity and unique molecular identity of MET receptor tyrosine kinase expressing serotonergic neurons in the caudal dorsal raphe nuclei. ACS Chem. Neurosci. 8, 1053–1064. doi: 10.1021/acschemneuro.7b00020
Kauer, J. A., and Malenka, R. C. (2007). Synaptic plasticity and addiction. Nat. Rev. Neurosci. 8, 844–858. doi: 10.1038/nrn2234
Kirby, L. G., Pernar, L., Valentino, R. J., and Beck, S. G. (2003). Distinguishing characteristics of serotonin and non-serotonin-containing cells in the dorsal raphe nucleus: electrophysiological and immunohistochemical studies. Neuroscience 116, 669–683.
Kiyasova, V., Bonnavion, P., Scotto-Lomassese, S., Fabre, V., Sahly, I., Tronche, F., et al. (2013). A subpopulation of serotonergic neurons that do not express the 5-HT1A autoreceptor. ACS Chem. Neurosci. 4, 89–95. doi: 10.1021/cn300157s
Kiyasova, V., Fernandez, S. P., Laine, J., Stankovski, L., Muzerelle, A., Doly, S., et al. (2011). A genetically defined morphologically and functionally unique subset of 5-HT neurons in the mouse raphe nuclei. J. Neurosci. Off. J. Soc. Neurosci. 31, 2756–2768. doi: 10.1523/JNEUROSCI.4080-10.2011
Kosofsky, B. E., and Molliver, M. E. (1987). The serotoninergic innervation of cerebral cortex: different classes of axon terminals arise from dorsal and median raphe nuclei. Synapse 1, 153–168. doi: 10.1002/syn.890010204
Lacoste, B., Riad, M., and Descarries, L. (2006). Immunocytochemical evidence for the existence of substance P receptor (NK1) in serotonin neurons of rat and mouse dorsal raphe nucleus. Eur. J. Neurosci. 23, 2947–2958. doi: 10.1111/j.1460-9568.2006.04833.x
Landmann, L., and Marbet, P. (2004). Colocalization analysis yields superior results after image restoration. Microsc. Res. Tech. 64, 103–112. doi: 10.1002/jemt.20066
Larm, J. A., Shen, P.-J., and Gundlach, A. L. (2003). Differential galanin receptor-1 and galanin expression by 5-HT neurons in dorsal raphé nucleus of rat and mouse: evidence for species-dependent modulation of serotonin transmission. Eur. J. Neurosci. 17, 481–493.
Liu, Z., Zhou, J., Li, Y., Hu, F., Lu, Y., Ma, M., et al. (2014). Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron 81, 1360–1374. doi: 10.1016/j.neuron.2014.02.010
Mintz, E. M., and Scott, T. J. (2006). Colocalization of serotonin and vesicular glutamate transporter 3-like immunoreactivity in the midbrain raphe of Syrian hamsters (Mesocricetus auratus). Neurosci. Lett. 394, 97–100. doi: 10.1016/j.neulet.2005.10.033
Nairn, A. C., Svenningsson, P., Nishi, A., Fisone, G., Girault, J.-A., and Greengard, P. (2004). The role of DARPP-32 in the actions of drugs of abuse. Neuropharmacology 47(Suppl. 1), 14–23. doi: 10.1016/j.neuropharm.2004.05.010
Popa, D., Cerdan, J., Repérant, C., Guiard, B. P., Guilloux, J.-P., David, D. J., et al. (2010). A longitudinal study of 5-HT outflow during chronic fluoxetine treatment using a new technique of chronic microdialysis in a highly emotional mouse strain. Eur. J. Pharmacol. 628, 83–90. doi: 10.1016/j.ejphar.2009.11.037
Prouty, E. W., Chandler, D. J., and Waterhouse, B. D. (2017). Neurochemical differences between target-specific populations of rat dorsal raphe projection neurons. Brain Res. 1675, 28–40. doi: 10.1016/j.brainres.2017.08.031
Puighermanal, E., Cutando, L., Boubaker-Vitre, J., Honoré, E., Longueville, S., Hervé, D., et al. (2017). Anatomical and molecular characterization of dopamine D1 receptor-expressing neurons of the mouse CA1 dorsal hippocampus. Brain Struct. Funct. 222, 1897–1911. doi: 10.1007/s00429-016-1314-x
Ren, J., Friedmann, D., Xiong, J., Liu, C. D., Ferguson, B. R., Weerakkody, T., et al. (2018). Anatomically defined and functionally distinct dorsal raphe serotonin sub-systems. Cell 175, 472–487.e20. doi: 10.1016/j.cell.2018.07.043
Sakae, D. Y., Marti, F., Lecca, S., Vorspan, F., Martín-García, E., Morel, L. J., et al. (2015). The absence of VGLUT3 predisposes to cocaine abuse by increasing dopamine and glutamate signaling in the nucleus accumbens. Mol. Psychiatry 20, 1448–1459. doi: 10.1038/mp.2015.104
Sakae, D. Y., Ramet, L., Henrion, A., Poirel, O., Jamain, S., El Mestikawy, S., et al. (2018). Differential expression of VGLUT3 in laboratory mouse strains: impact on drug-induced hyperlocomotion and anxiety-related behaviors. Genes Brain Behav. 18:e12528. doi: 10.1111/gbb.12528
Schäfer, M. K.-H., Varoqui, H., Defamie, N., Weihe, E., and Erickson, J. D. (2002). Molecular cloning and functional identification of mouse vesicular glutamate transporter 3 and its expression in subsets of novel excitatory neurons. J. Biol. Chem. 277, 50734–50748. doi: 10.1074/jbc.M206738200
Sengupta, A., Bocchio, M., Bannerman, D. M., Sharp, T., and Capogna, M. (2017). Control of amygdala circuits by 5-HT neurons via 5-HT and glutamate cotransmission. J. Neurosci. 37, 1785–1796. doi: 10.1523/JNEUROSCI.2238-16.2016
Shutoh, F., Ina, A., Yoshida, S., Konno, J., and Hisano, S. (2008). Two distinct subtypes of serotonergic fibers classified by co-expression with vesicular glutamate transporter 3 in rat forebrain. Neurosci. Lett. 432, 132–136. doi: 10.1016/j.neulet.2007.12.050
Sotelo, C., Cholley, B., El Mestikawy, S., Gozlan, H., and Hamon, M. (1990). Direct immunohistochemical evidence of the existence of 5-HT1A autoreceptors on serotoninergic neurons in the midbrain raphe nuclei. Eur. J. Neurosci. 2, 1144–1154.
Svenningsson, P., Tzavara, E. T., Liu, F., Fienberg, A. A., Nomikos, G. G., and Greengard, P. (2002). DARPP-32 mediates serotonergic neurotransmission in the forebrain. Proc. Natl. Acad. Sci. U.S.A. 99, 3188–3193. doi: 10.1073/pnas.052712699
Tarren, J. R., Lester, H. A., Belmer, A., and Bartlett, S. E. (2017). Acute ethanol administration upregulates synaptic α4-subunit of neuronal nicotinic acetylcholine receptors within the nucleus accumbens and amygdala. Front. Mol. Neurosci. 10:338. doi: 10.3389/fnmol.2017.00338
Valjent, E., Pascoli, V., Svenningsson, P., Paul, S., Enslen, H., Corvol, J.-C., et al. (2005). Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc. Natl. Acad. Sci. U.S.A. 102, 491–496. doi: 10.1073/pnas.0408305102
Voisin, A. N., Mnie-Filali, O., Giguère, N., Fortin, G. M., Vigneault, E., El Mestikawy, S., et al. (2016). Axonal segregation and role of the vesicular glutamate transporter VGLUT3 in serotonin neurons. Front. Neuroanat. 10:39. doi: 10.3389/fnana.2016.00039
Xu, Z. Q., and Hökfelt, T. (1997). Expression of galanin and nitric oxide synthase in subpopulations of serotonin neurons of the rat dorsal raphe nucleus. J. Chem. Neuroanat. 13, 169–187.
Keywords: serotonin, serotonin transporter, SERT, VGLUT3, 5-HT varicosity
Citation: Belmer A, Beecher K, Jacques A, Patkar OL, Sicherre F and Bartlett SE (2019) Axonal Non-segregation of the Vesicular Glutamate Transporter VGLUT3 Within Serotonergic Projections in the Mouse Forebrain. Front. Cell. Neurosci. 13:193. doi: 10.3389/fncel.2019.00193
Received: 11 January 2019; Accepted: 17 April 2019;
Published: 10 May 2019.
Edited by:
Andrew L. Gundlach, The Florey Institute of Neuroscience and Mental Health, AustraliaReviewed by:
Massimo Pierucci, University of Malta, MaltaMario Valentino, University of Malta, Malta
Copyright © 2019 Belmer, Beecher, Jacques, Patkar, Sicherre and Bartlett. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arnauld Belmer, arnauld.belmer@qut.edu.au Selena E. Bartlett, selena.bartlett@qut.edu.au
†These authors have contributed equally to this work
 Arnauld Belmer
Arnauld Belmer Kate Beecher
Kate Beecher Angela Jacques
Angela Jacques Omkar L. Patkar
Omkar L. Patkar Florian Sicherre
Florian Sicherre Selena E. Bartlett
Selena E. Bartlett