- Centre of Clinical Neuroscience, Department of Neurology, University Hospital Carl Gustav Carus at the Technische Universität Dresden, Dresden, Germany
Background: Multiple sclerosis (MS) patients suffer very often from MS fatigue and sleep problems. Despite the detrimental impact on the activities of daily living, a short and objective quantification of fatigue and sleep problems is currently lacking.
Objective: The objective of the study was to systematically investigate tonic, intrinsic, and phasic alertness and the relationship of these performance-based measures with self-report measures of fatigue and quality of sleep.
Methods: Thirty-three MS patients without (MS−) and 26 with selected comorbid disorders (MS+) and 43 healthy controls (HCs) performed the pupillographic sleepiness test (measuring tonic alertness) and the alertness subtest of the Test of Attentional Performance (measuring intrinsic and phasic alertness).
Results: Self-reported and performance-based measures revealed poorer performance for both MS groups compared to HC. MS+ patients presented higher rates of MS fatigue, sleep problems and depressive symptoms but similar alertness scores compared to MS− patients. However, tonic alertness was only higher in MS− patients compared to HC. Intrinsic and phasic alertness correlated moderately with fatigue ratings.
Conclusion: In the diagnostic process of MS fatigue and quality of sleep comorbid disorders (depression, anemia, thyroid dysfunction) and performance-based measures such as alertness should be considered in daily clinical practice.
Introduction
Multiple sclerosis (MS) is an inflammatory disease of the central nervous system with a widespread demyelination and axonal loss (1). In addition to other neurological deficits, MS-related fatigue occurs in up to 90% of the patient population (2–4).
Multiple sclerosis-related fatigue is a heterogeneous clinical feature [see Ref. (5, 6) for an detailed multidimensional description] and is simply defined as a perceived subjective lack of (mental or physical) energy by the individual (6). New approaches describe MS-related fatigue as a complex symptom that includes three clinical different entities: asthenia (fatigue at rest), fatigability (fatigue with exercise), and worsening of symptoms with effort (5). Despite these entities, the pathophysiology underlying MS-related fatigue is still under investigation: Proinflammatory cytokines, overactivity of neural circuits, HPA axis involvement, and axonal injury are discussed [for an overview see Ref. (7)].
Multiple sclerosis-related fatigue negatively affects employment status as well as quality of life (8–11), and there is a strong need for a valid and reliable assessment (12). For the assessment of subjective perceived Fatigue, self-report measures are often used, because they are cheap and easy to administer. However, these measurements are vulnerable to a series of problems such as self-perception, social desirability, malingering, motives, memory processes, and overestimation (e.g., because of depression) or underestimation (e.g., because of anosognosia). Furthermore, a clear differentiation between primary (MS as cause for fatigue) and secondary fatigue (other conditions causing fatigue, such as depression, pain, e.g.) with these measures is not possible. That’s why the performance-based approach reflects an alternative way to objectively quantify MS-related fatigue (13). There have been some attempts to quantify the worsening of symptoms with effort by using sustained or repetitive muscle contractions or cognitive tests (14, 15). By using kinematic gait analysis (16) or hand dynamometer (14) correlations with physical but not with the cognitive dimension of subjective fatigue scales were found. In contrast, attentional functions and information processing speed, cognitive key deficit in MS (17–19), seem to correlate with the subjective perceived MS-related fatigue (14, 15).
Alertness—as the central nervous activation or the intensity dimension of attention—may serve as a potential interface between sleep on the one hand and more complex psychological functions such as fatigue on the other hand. Alertness is defined as achieving and maintaining a state of high sensitivity to incoming stimuli and represents a precondition for more complex cognitive functions (20–22) and is comprised of different subprocesses (23, 24).
The general wakefulness/arousal or tonic alertness—can be measured by pupillary hippus [pupillary unrest index (PUI)] in darkness as an index of sleepiness (25) [or asthenia in terms of Ref. (5)]. Tonic alertness is designated to a state of general wakefulness without doing any task. However, three different studies found no difference in pupillomotor instability between MS patients and healthy control (HC) (26–28), but moderate negative correlations with self-reported fatigue in MS patients (27, 28).
Different studies investigating a second sub-process—intrinsic alertness—by measuring the maintenance of an optimal level of arousal for a rather short time interval by expecting a stimulus in a specific task [or fatigability in terms of Ref. (5)] (20). By using a simple reaction time task, they replicate longer reaction times in MS patients compared to HC as an indicator for reduced intrinsic alertness (29–32). A reduced intrinsic alertness was especially pronounced in high fatigued MS patients compared to low fatigued MS patients (33). The intrinsic alertness in MS deteriorates over time (31) as well as after cognitive (and physical) load (30, 34).
Performance-based measures are often confounded by quality of sleep. In MS, there is a high prevalence of moderate to severe sleep problems (up to 52%) (35, 36) and a close conceptual proximity of fatigue and sleep has recently been discussed (36–38). Up to now the relation is not well understood, but quality of sleep and fatigue may act as relevant confounders to each other (37). There is some evidence that MS patients with relevant sleep disorders scored higher in fatigue scales compared to MS patients without relevant sleep disorders (36). In addition, sleep disorders are found to be the strongest predictor for MS fatigue (36), but the correlations between them are moderate (39, 40).
The aim of the study was twofold: first, to systematically measure the three sub-processes of alertness (tonic, intrinsic and phasic alertness) in MS patients and HCs as a performance-based measure of fatigue and sleep. Second, to investigate the relationship between these performance-based measures and self-report measures of fatigue and sleep in MS patients in comparison to HC.
Materials and Methods
Subjects
Fifty-nine outpatients (one was excluded because of technical problems with electronical questionaires) with clinically defined MS according to the McDonald criteria (41) were recruited at clinical visits at the local MS center and by local postings. Enclosed subtypes of MS were: relapsing-remitting MS (RRMS; n = 55) and secondary-progressive MS (SPMS; n = 4). MS patients were screened for anemia or thyroid dysfunction, relevant comedication (antidepressants, tretrahydrocannabinol, or modafinil) and depressive symptoms. A group of 33 MS patients without comorbidity (MS−) of anemia, thyroid dysfunction, depressive symptomsor antidepressants were analyzed in comparison to a group of 26 MS patients with at least one of the mentioned comorbid disorder (MS+). Four patients were diagnosed with anemia (hypochromic microcytic or normochromic normocytic), two patients with a thyroid dysfunction (thyroid stimulation hormone <0.27 or >4.20), and 12 were taking medicines (antidepressants, tretrahydrocannabinol, or modafinil; one of them had an anemia, too). Inclusion criteria for both MS groups comprised no relapses or steroid treatment within in the past 2 months. Exclusion criteria was a known treated comorbid disorder (six patients with anemia, thyroid dysfunction, or depression), pregnancy (one patient), and shift work (two patients). Twenty patients decline to participate the study. The MS groups did not differ between disease modifying drugs [χ2(4) = 2.20, p = 0.821; betaferon, copaxone, tysabri, fingolimod, and others].
Forty-three age- and sex-matched HC were recruited through local postings. They were also screened for selected comorbid disorders (anemia, hypothyroidism, depressive symptoms, relevant medication). Seven HC were excluded because of anemia (3 probands), thyroid dysfunction (1 proband), anemia and thyroid dysfunction (1 proband), or depressive symptoms (2 probands) measured with the Centre for Epidemiological Studies Depression Scale (CES-D) (42) and 16 HC decline to participate. Written informed consent was obtained from each participant prior to study entry. The protocol was reviewed and approved by the local ethics committee (TU-Dresden, Faculty of Medicine), and all participants gave their informed consent prior to their inclusion in the study. Main demographic and clinical data are compiled in Table 1.
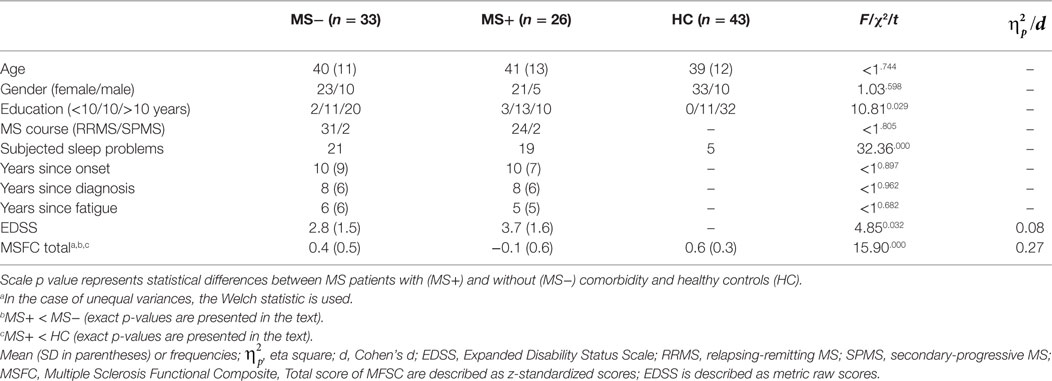
Table 1. Sample characteristics of MS patients (MS−) without and with comorbid (MS+) disorders in comparison to healthy controls (HC).
Assessment
Self-Report Measure
All participants were asked to rate fatigue by a standardized questionnaire [Modified Fatigue Impact Scale (MFIS) (43)] and by a visual analogue rating scale [on a scale from 0 to 10 how strong is fatigue at the moment; before and after the pupillographic sleepiness test (PST)]. An MFIS total score of ≥38 indicates MS-related fatigue (2).
Additionally, MS patients and HCs completed standardized questionnaires in order to control for different influences of depressive symptoms (29) [CES-D (42)], sleep [Pittsburgh quality of sleep index (PSQI) (44)], and daytime sleepiness [Epworth Sleepiness Scale (ESS) (45)] on the different fatigue measures. A CES-D total score of >23, an ESS total score of >10, and a PSQI total score of >5 indicates clinically relevant depression or daytime sleepiness and distinguishes between good and poor sleepers, respectively.
Performance-Based Measure
To objectify cognitive aspects of MS-related fatigue or sleep, the alertness subtest of the computerized Test of Attentional Performance [TAP (23)] was used. Alertness is designated as general wakefulness or arousal that enables a person to respond effectively to any given demand. It is essential and the basis of every attentional or cognitive performance. The test comprised a simple reaction time task lasting approximately 4.5 min. Information processing speed or mean reaction time for intrinsic (without warning signal) and phasic (with warning signal) alertness were the dependent measure. There were no significant differences between the two blocks of intrinsic and the two blocks of phasic alertness (Fs < 1.01, ps > 0.368 for effects of block or interactions of block and group), and the results of the two blocks were therefore collapsed, respectively.
The PST reflected the second performance-based measure. It was performed for 11 min in a quiet and dark room between 2:00 p.m. and 7:30 p.m. The pupil diameter was measured in order to quantify the typical pupillomotor hippus as a measure for tonic alertness or for central autonomic nervous activation (46). The PUI (in mm/min) was the dependent measure. The higher the PUI, the more pronounced the pupillomotor hippus and daytime sleepiness.
For MS patients, neurological disability was rated by Kurtzke’s Expanded Disability Status Scale (EDSS) using the neurostatus tool only by EDSS-certified Neurologists (47).
Statistical Analysis
Statistical analyses were performed with the Statistical Package for Social Sciences (SPSS®/IBM® Version 23.0 for Windows). Cross-sectional analysis was conducted using the Chi-square test (χ2) and analysis of variance or t-tests for independent samples (ANOVA; post hoc test: Tukey’s honestly significant difference) with respect to demographical, neurological, psychometric, and psychological variables. In the case of unequal variances between the groups, the Welch statistic is used. The Spearman correlation coefficient was calculated to measure the strength of correlations. Significance was accepted at a level of p ≤ 0.05.
Results
Sample Characteristics
MS+ patients differed from MS− patients with regard to clinical parameters although years since diagnosis and years with fatigue were comparable (see Table 1). MS+ patients presented smaller MSFC total scores (p < 0.01) and higher EDSS scores compared to MS− patients. MS− patients had almost similar MSFC scores compared to HC (p = 0.056) whereas MS + patients presented lower scores compared to HC (p < 0.001).
Self-Report Measures
An MFIS total score ≥38 was measured in 28 of 59 MS patients and by one of 43 HC (χ2 = 24.90, p < 0.001). MS patients presented higher daytime sleepiness and reduced quality of sleep compared to HC. Thirty-one of all interviewed MS patients and 14 HC had an ESS total score of >10 (χ2 = 4.03, p < 0.05), whereas 42 MS patients and 12 HC had a PSQI total score of >5 (χ2 = 18.70, p < 0.001).
Sixty-five percent of MS+ patients had a MFIS total score ≥38 in comparison to 33% of MS− patients (χ2 = 5.99, p < 0.014). The majority of MS patients (MS+ and MS−) mentioned a reduced quality of sleep with a PSQI total score >5 (61 and 85% of the MS− and MS+ patients, respectively; χ2 = 4.09, p < 0.043) and half of the MS patients in both groups had higher daytime sleepiness with an ESS total score of >10 (55 and 50% of the MS− and MS+ patients, respectively; p > 0.728). Only 6–8% of all MS patients met criteria for clinical fatigue with a MFIS total score ≥38 without having sleep problems (PSQI total score <5).
We found significant group effects in all self-report measures except in the daytime sleepiness (p = 0.139; see Table 2 for details). Post hoc analysis revealed higher mean scores in all fatigue rating scales and in the PSQI in MS− and MS+ patients compared to HC, respectively (ps < 0.01).
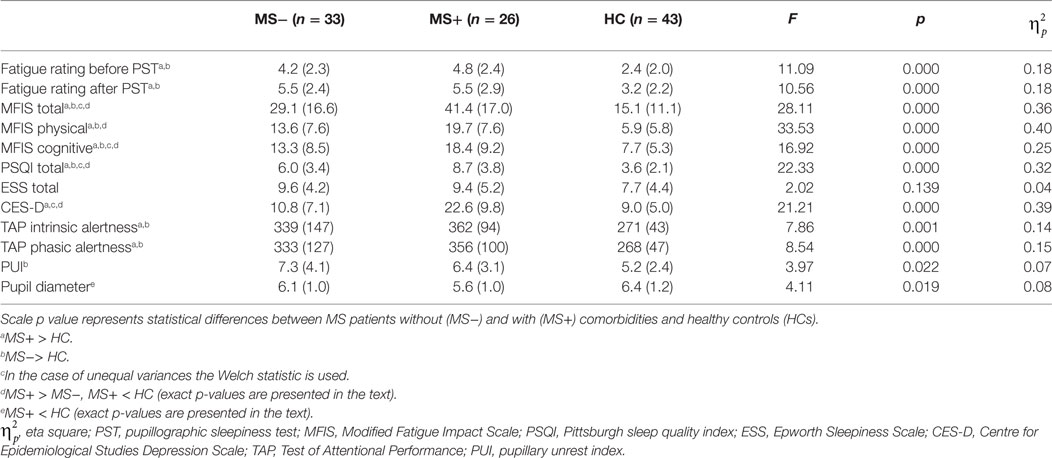
Table 2. Mean (SD) of self-report and performance-based measures for MS patients (MS−) without and with comorbid (MS+) disorders in comparison to healthy controls (HC).
Besides the fatigue rating before and after PST (ps > 0.563), MS+ patients had higher scores in comparison to MS− patients. The MFIS total (p < 0.01) and subscores (ps < 0.05) as well as the PSQI (p < 0.01) were higher in the subgroup of MS+ patients compared to the subgroup of MS− patients.
Performance-Based Measures
More MS patients (n = 27 of all 59) than HC (n = 8) showed abnormal PST results compared to the normative data (χ2 = 8.16, p < 0.05). The same pattern was found for the TAP. In the alertness subtest, significantly more MS patients (intrinsic alertness: 39; phasic alertness: 39) than HC (intrinsic alertness: 9; phasic alertness: 16) were below average compared to the normative sample of the TAP (intrinsic alertness: χ2 = 22.03, p < 0.001; phasic alertness: χ2 = 8.36, p < 0.01). MS− and MS + patients did not differ with respect to the frequency distribution of average or below average scores (ps > 0.104).
We found a significant group effect in all performance-based measures (see Table 2 for details). Post hoc analysis revealed a significant higher PUI in MS− patients compared to HC (p < 0.05) whereas the pupil diameter did not differ between these groups (p = 0.482). In contrast, MS + patients presented a smaller pupil diameter (p < 0.05) but a comparable PUI (p = 0.292) in comparison to HC. The MS groups did not differ from each other with respect to tonic alertness scores (ps > 0.212).
The ANOVA of the alertness subtest of the TAP with the two factors alertness (intrinsic vs. phasic) and group (MS−, MS + and HC) revealed a significant effect of group [F(2, 99) = 8.41, p < 0.001, ]. Subsequent analyses revealed that reaction times of MS− and MS+ patients were longer compared to HC, respectively (ps < 0.01). The MS groups did not differ from each other (ps > 0.628). There was no difference between intrinsic and phasic alertness and no interaction of alertness and group (ps > 0.169).
Correlation of Self-Reports and Performance-Based Measures
The PUI correlated positively with PSQI total score in MS+ patients (R2 = 0.156, see Table 3 for more details), but not in HC or in MS− patients (ps > 0.080). However, the PUI correlated with the ESS total score in HC (R2 = 0.147), but not in MS patients (ps > 0.244).
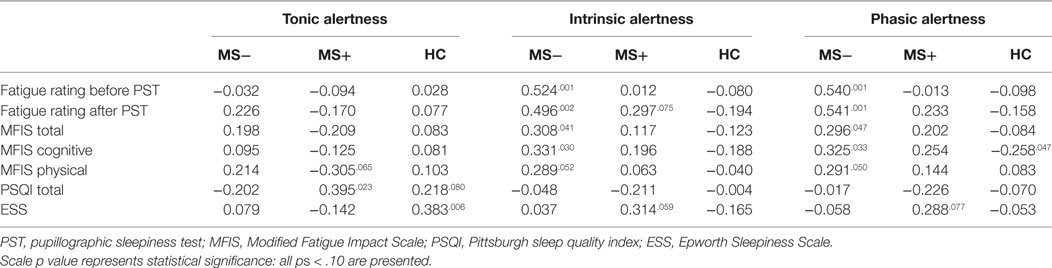
Table 3. Correlations of self-report and performance-based measures for MS patients without (MS−) and with (MS+) comorbid disorders in comparison to healthy controls (HC).
The alertness subtests of the TAP correlated moderately with the fatigue rating before (R2 = 0.275 for intrinsic and R2 = 0.292 for phasic alertness) and after PST (R2 = 0.246 for intrinsic and R2 = 0.293 for phasic alertness) in MS− patients, but not in MS+ patients (ps > 0.075) or in HC (ps > 0.109). The same pattern was found for the correlation of alertness with the cognition subscale of the MFIS (R2 = 0.110 for intrinsic and R2 = 0.106 for phasic alertness) and with the MFIS total score in MS− patients (R2 = 0.095 for intrinsic and R2 = 0.088 for phasic alertness). In HC, a negative correlation of phasic alertness and the cognition subscale of the MFIS were found (R2 = 0.067). Neither a correlation with the physical subscale of the MFIS (ps > 0.050) nor any other correlation was significant.
Discussion
We investigated the relationship between MS fatigue and sleep problems by systematically measuring tonic, intrinsic, and phasic alertness in MS patients and HC as a potential performance-based measure of fatigue and sleep.
Alertness—as the central nervous activation or the intensity dimension of attention—may serve as a potential interface between sleep on the one hand and more complex psychological functions such as fatigue on the other hand. We systematically analyzed the three subprocesses of alertness in our study.
First, tonic alertness or the central autonomic nervous activation was measured by the PUI. MS− patients (controlled for anemia, thyroid dysfunction, and medication) presented a higher PUI compared to HC. This is in contrast to previous studies, which did not found any differences between MS patients and HC (26–28). In contrast to previous studies, we did control for anemia, thyroid dysfunction as well as relapse at the time of testing. In accordance with earlier studies (26–28), we found no difference between MS patients with comorbidity and HC. Our data provide first evidence that comorbidities such as anemia, thyroid dysfunction, or depressive symptoms have significant influence on measures of tonic alertness. In order to further elucidate the exact nature of this interaction, comorbidities should be taken into account in future studies and clinical testing of tonic alertness.
Second, intrinsic and phasic alertness, a measure of general response readiness and the ability to increase response readiness for a short period was measured by simple reaction time tasks. Although the MS groups did not differ from each other, all MS patients (without and with comorbidity) showed a reduced intrinsic and phasic alertness compared to HC. Our data are in accordance with earlier findings and support the conclusion of reduced information processing speed as a cognitive key deficit in MS (29–32).
The second goal of this study was to investigate the relationship of performance-based measures and self-report measures of MS-related fatigue and quality of sleep in MS patients and HC. Although MS− patients had poorer performance in tonic alertness than HC, we did not detect a significant correlation with fatigue or with sleep measures in this subgroup. This is not in accordance with two earlier studies, which found moderate negative correlations with self-reported fatigue and tonic alertness in MS patients. However, both studies did not control for anemia or thyroid dysfunction or for depressive symptoms/antidepressant medication (27, 28). So it is unclear whether fatigue in these studies is potentially confounded with other fatigue causing conditions. In our study, MS+ patients showed higher self-reported fatigue and more sleep problems compared to MS− patients, and a correlation between tonic alertness and quality of sleep (measured by the PSQI) was observed. This is a new finding because no known study investigated sleep-associated problems besides daytime sleepiness. In contrast to a previous study, we found no correlation with daytime sleepiness (26).
Furthermore, intrinsic and phasic alertness (measured by the TAP) seems to be moderately associated with fatigue in MS− patients. This finding is in accordance with earlier studies, which found moderate correlations (29, 30, 32–34, 48).
However, the correlations are only moderate and the explained variance is small. That’s why future studies should investigate further confounding variables such as neurological disability (EDSS score differed between MS− and MS+ patients in our study), cognitive dysfunction, medication (of neuropathic pain or of other disease/symptoms modifying drugs) or MS-related symptoms (spasticity, urinary problems, neuropathic pain) as a potential cause of secondary fatigue. We focused on alertness as a potential psychological explanation of fatigue. Future investigations could concentrate more on the different entities of fatigue [e.g., asthenia, fatigability, worsening of symptoms cf. (5)] in order to clarify the moderate correlations with MS-related fatigue measured with conventional questionnaires and to clarify a potential relationship of the different entities between alertness on the one side and fatigue on the other side.
Conclusion
In sum, we found significant differences between MS patients compared to HC in self-report measures (MS-related fatigue, quality of sleep) and performance-based measures (tonic, intrinsic, and phasic alertness). Tonic alertness measured by PST was higher in MS patients without comorbid disorders (MS−) compared to HC, whereas intrinsic and phasic alertness measured with the TAP differed in MS patients without and with comorbidity in comparison to HC. Intrinsic and phasic alertness measures correlated moderately with conventional fatigue ratings in MS patients.
Finally, our results add new insights into the understanding of fatigue and sleep problems in that way that somatic conditions such as anemia, endocrine dysfunction (e.g., hypothyroidism) should be considered more often in clinical practice in order to exclude primary organic dysfunctions as a reason for fatigue and sleep problems.
Ethics Statement
The protocol was reviewed and approved by the local ethics committee (TU-Dresden, Faculty of Medicine) and all participants gave their informed consent in accordance with the Declaration of Helsinki prior to their inclusion in the study.
Author Contributions
SK and MP had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: SK and MP. Acquisition of data, statistical analysis, interpretation of the data, and drafting of the manuscript: MP. Critical revision of the manuscript for important intellectual content and study supervision: TZ, SK, and MP.
Conflict of Interest Statement
SK reports grants from Novartis Pharma GmbH, Germany, during the conduct of the study; personal fees from Biogen-Idec, personal fees from Teva Pharma, outside the submitted work. MP reports grants from Novartis Pharma GmbH, Germany, during the conduct of the study. TZ has received compensation for consulting services from Almirall, Biogen Idec, Bayer, Genzyme, GlaxoSmithKline, MSD, Merck Serono, Novartis, Sanofi, Teva, and Synthon and has received research support from Bayer, Biogen Idec, the Hertie Foundation, the Roland Ernst Foundation, the German Diabetes Foundation, Merck Serono, Novartis, Teva, and Sanofi Aventis.
Acknowledgments
We thank Alina Kästner, Eric Legler, and Karin Bernaciak for their assistance in data collection.
Funding
This study was supported by the Novartis Pharma GmbH. The sponsor did not participate in any aspect of the design or performance of the study, including data collection, management, analysis, and interpretation or the writing of the manuscript.
References
1. Compston A, Coles A. Multiple sclerosis. Lancet (2002) 359(9313):1221. doi:10.1016/S0140-6736(02)08220-X
2. Flachenecker P, Kümpfel T, Kallmann B, Gottschalk M, Grauer O, Rieckmann P, et al. Fatigue in multiple sclerosis: a comparison of different rating scales and correlation to clinical parameters. Mult Scler (2002) 8(6):523–6. doi:10.1191/1352458502ms839oa
3. Wood B, van der Mei IA, Ponsonby AL, Pittas F, Quinn S, Dwyer T, et al. Prevalence and concurrence of anxiety, depression and fatigue over time in multiple sclerosis. Mult Scler (2013) 19(2):217–24. doi:10.1177/1352458512450351
4. Ziemssen T. Multiple sclerosis beyond EDSS: depression and fatigue. J Neurol Sci (2009) 277:S37–41. doi:10.1016/S0022-510X(09)70011-5
5. Iriarte J, Subirá ML, de Castro P. Modalities of fatigue in multiple sclerosis: correlation with clinical and biological factors. Mult Scler (2000) 6(2):124–30. doi:10.1177/135245850000600212
6. Multiple Sclerosis Council for Clinical Practice Guidelines. Fatigue and Multiple Sclerosis: Evidence-Based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America (1998).
7. Induruwa I, Constantinescu CS, Gran B. Fatigue in multiple sclerosis – a brief review. J Neurol Sci (2012) 323(1–2):9–15. doi:10.1016/j.jns.2012.08.007
8. Janardhan V, Bakshi R. Quality of life in patients with multiple sclerosis. J Neurol Sci (2002) 205(1):51–8. doi:10.1016/S0022-510X(02)00312-X
9. Krause I, Kern S, Horntrich A, Ziemssen T. Employment status in multiple sclerosis: impact of disease-specific and non-disease-specific factors. Mult Scler (2013) 19(13):1792–9. doi:10.1177/1352458513485655
10. Simmons RD, Tribe KL, McDonald EA. Living with multiple sclerosis: longitudinal changes in employment and the importance of symptom management. J Neurol (2010) 257(6):926–36. doi:10.1007/s00415-009-5441-7
11. Ziemssen T, Hoffman J, Apfel R, Kern S. Effects of glatiramer acetate on fatigue and days of absence from work in first-time treated relapsing-remitting multiple sclerosis. Health Qual Life Outcomes (2008) 6:67. doi:10.1186/1477-7525-6-67
12. Ziemssen T. Symptom management in patients with multiple sclerosis. J Neurol Sci (2011) 311(Suppl 1):S48–52. doi:10.1016/S0022-510X(11)70009-0
13. Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology (2013) 80(4):409–16. doi:10.1212/WNL.0b013e31827f07be
14. Greim B, Benecke R, Zettl UK. Qualitative and quantitative assessment of fatigue in multiple sclerosis (MS). J Neurol (2007) 254(S2):II58–64. doi:10.1007/s00415-007-2014-5
15. Andreasen AK, Spliid PE, Andersen H, Jakobsen J. Fatigue and processing speed are related in multiple sclerosis. Eur J Neurol (2010) 17(2):212–8. doi:10.1111/j.1468-1331.2009.02776.x
16. Sehle A, Mündermann A, Starrost K, Sailer S, Becher I, Dettmers C, et al. Objective assessment of motor fatigue in multiple sclerosis using kinematic gait analysis: a pilot study. J Neuroeng Rehabil (2011) 8:59. doi:10.1186/1743-0003-8-59
17. Langdon DW. Cognition in multiple sclerosis. Curr Opin Neurol (2011) 24(3):244–9. doi:10.1097/WCO.0b013e328346a43b
18. Lembach Y, Adler G. Kognitive Beeinträchtigungen bei Multipler Sklerose. Akt Neurol (2013) 40(03):147–65. doi:10.1055/s-0032-1333014
19. Paul RH, Beatty WW, Schneider R, Blanco CR, Hames KA. Cognitive and physical fatigue in multiple sclerosis: relations between self-report and objective performance. Appl Neuropsychol (1998) 5(3):143–8. doi:10.1207/s15324826an0503_5
20. Sturm W, Willmes K. On the functional neuroanatomy of intrinsic and phasic alertness. Neuroimage (2001) 14(1):S76–84. doi:10.1006/nimg.2001.0839
21. Clemens B, Zvyagintsev M, Sack AT, Heinecke A, Willmes K, Sturm W. Revealing the functional neuroanatomy of intrinsic alertness using fMRI: methodological peculiarities. PLoS One (2011) 6(9):e25453. doi:10.1371/journal.pone.0025453
22. Posner MI, Rothbart MK. Research on attention networks as a model for the integration of psychological science. Annu Rev Psychol (2007) 58(1):1–23. doi:10.1146/annurev.psych.58.110405.085516
23. Zimmermann P, Fimm B. TAP Testbatterie zur Aufmerksamkeitsprüfung: Version 2.3. Herzogenrath: Psytest (2012).
24. Sturm W, de Simone A, Krause BJ, Specht K, Hesselmann V, Radermacher I, et al. Functional anatomy of intrinsic alertness: evidence for a fronto-parietal-thalamic-brainstem network in the right hemisphere. Neuropsychologia (1999) 37(7):797–805. doi:10.1016/S0028-3932(98)00141-9
25. Reimann M, Manz R, Prieur S, Reichmann H, Ziemssen T. Education research: cognitive performance is preserved in sleep-deprived neurology residents. Neurology (2009) 73(21):e99–103. doi:10.1212/WNL.0b013e3181c34a93
26. Frauscher B, Egg R, Brandauer E, Ulmer H, Berger T, Poewe W, et al. Daytime sleepiness is not increased in mild to moderate multiple sclerosis: a pupillographic study. Sleep Med (2005) 6(6):543–7. doi:10.1016/j.sleep.2005.05.001
27. Egg R, Högl B, Glatzl S, Beer R, Berger T. Autonomic instability, as measured by pupillary unrest, is not associated with multiple sclerosis fatigue severity. Mult Scler (2002) 8(3):256–60. doi:10.1191/1352458502ms793oa
28. Groß Rüdiger. Fatigue bei Multipler Sklerose: Eine pupillometrische Studie [Dissertation] University of Hamburg (2006).
29. Rotstein D, O’Connor P, Lee L, Murray BJ. Multiple sclerosis fatigue is associated with reduced psychomotor vigilance. Can J Neurol Sci (2012) 39(2):180–4. doi:10.1017/S0317167100013196
30. Claros-Salinas D, Dittmer N, Neumann M, Sehle A, Spiteri S, Willmes K, et al. Induction of cognitive fatigue in MS patients through cognitive and physical load. Neuropsychol Rehabil (2012) 23(2):182–201. doi:10.1080/09602011.2012.726925
31. Claros-Salinas D, Bratzke D, Greitemann G, Nickisch N, Ochs L, Schröter H. Fatigue-related diurnal variations of cognitive performance in multiple sclerosis and stroke patients. J Neurol Sci (2010) 295(1–2):75–81. doi:10.1016/j.jns.2010.04.018
32. Neumann M, Sterr A, Claros-Salinas D, Gütler R, Ulrich R, Dettmers C. Modulation of alertness by sustained cognitive demand in 5MS6 as surrogate measure of fatigue and fatigability. J Neurol Sci (2014) 15(340):178–82. doi:10.1016/j.jns.2014.03.024
33. Weinges-Evers N, Brandt AU, Bock M, Pfueller CF, Dörr J, Bellmann-Strobl J, et al. Correlation of self-assessed fatigue and alertness in multiple sclerosis. Mult Scler (2010) 16(9):1134–40. doi:10.1177/1352458510374202
34. Meissner H, Volkert J, König H, Alpers G, Flachenecker P. Fatigue in multiple sclerosis: subjective complaints and intensity of attention. Abstract of the 23rd Congress of the European Committee for Treatment and Research in Multiple Sclerosis and the 12th Annual Conference of Rehabilitation in Multiple Sclerosis. Prague, Czech Republic (2007).
35. Bamer AM, Johnson KL, Amtmann D, Kraft GH. Prevalence of sleep problems in individuals with multiple sclerosis. Mult Scler (2008) 14(8):1127–30. doi:10.1177/1352458508092807
36. Veauthier C, Radbruch H, Gaede G, Pfueller CF, Dörr J, Bellmann-Strobl J, et al. Fatigue in multiple sclerosis is closely related to sleep disorders: a polysomnographic cross-sectional study. Mult Scler (2011) 17(5):613–22. doi:10.1177/1352458510393772
37. Veauthier C, Paul F. Sleep disorders in multiple sclerosis and their relationship to fatigue. Sleep Med (2014) 15(1):5–14. doi:10.1016/j.sleep.2013.08.791
38. Veauthier C, Gaede G, Radbruch H, Gottschalk S, Wernecke KD, Paul F. Treatment of sleep disorders may improve fatigue in multiple sclerosis. Clin Neurol Neurosurg (2013) 115(9):1826–30. doi:10.1016/j.clineuro.2013.05.018
39. Stanton BR, Barnes F, Silber E. Sleep and fatigue in multiple sclerosis. Mult Scler (2006) 12(4):481–6. doi:10.1191/135248506ms1320oa
40. Pokryszko-Dragan A, Bilińska M, Gruszka E, Biel Ł, Kamińska K, Konieczna K. Sleep disturbances in patients with multiple sclerosis. Neurol Sci (2012) 34(8):1291–6. doi:10.1007/s10072-012-1229-0
41. McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol (2001) 50(1):121–7. doi:10.1002/ana.1032
43. Multiple Sclerosis Council for Clinical Practice Guidelines. Modified Fatigue Impact Scale. Fatigue and Multiple Sclerosis: Evidence-based Management Strategies for Fatigue in Multiple Sclerosis. Washington, DC: Paralyzed Veterans of America (1998).
44. Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res (1989) 28(2):193–213. doi:10.1016/0165-1781(89)90047-4
45. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep (1991) 14(6):540–5. doi:10.1093/sleep/14.6.540
46. Wilhelm B. Pupillographischer Schläfrigkeitstest. In: Peter H, Penzel T, Peter JH, editors. Enzyklopädie der Schlafmedizin: Mit 137 Tabellen. Heidelberg: Springer-Medizin-Verl (2007). p. 977–80.
47. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology (1983) 33(11):1444. doi:10.1212/WNL.33.11.1444
Keywords: multiple sclerosis-related fatigue, sleep, tonic/intrinsic/phasic alertness, pupillographic sleepiness test, multiple sclerosis
Citation: Paucke M, Kern S and Ziemssen T (2018) Fatigue and Sleep in Multiple Sclerosis Patients: A Comparison of Self-Report and Performance-Based Measures. Front. Neurol. 8:703. doi: 10.3389/fneur.2017.00703
Received: 11 August 2017; Accepted: 06 December 2017;
Published: 04 January 2018
Edited by:
Zsolt Illes, University of Southern Denmark Odense, DenmarkReviewed by:
Melinda Magyari, European Committee for Treatment and Research in Multiple Sclerosis, SwitzerlandHenrik Boye Jensen, Sygehus Lillebælt, Denmark
Copyright: © 2018 Paucke, Kern and Ziemssen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tjalf Ziemssen, tjalf.ziemssen@uniklinikum-dresden.de
 Madlen Paucke
Madlen Paucke Simone Kern
Simone Kern Tjalf Ziemssen
Tjalf Ziemssen