- 1Department of Sleep Medicine and Metabolic Disorders, Medical University of Lodz, Lodz, Poland
- 2Department of Rheumatology, Medical University of Lodz, Lodz, Poland
Obstructive sleep apnea is a chronic condition characterized by recurrent episodes of apneas or hypopneas during sleep leading to intermittent hypoxemia and arousals. The prevalence of the sleep disordered breathing is estimated that almost 50% of men and 24% of women suffer from moderate to severe form of the disorder. Snoring, collapse of upper airways and intermittent hypoxia are main causes of smoldering systemic inflammation in patients suffering from obstructive sleep apnea. The systematic inflammation is considered one of the key mechanisms leading to significant cardiovascular complications. Blood platelets, formerly not even recognized as cells, are currently gaining attention as crucial players in the immune continuum. Platelet surface is endowed with receptors characteristic for cells classically belonging to the immune system, which enables them to recognize pathogens, immune complexes, and interact in a homo- and heterotypic aggregates. Platelets participate in the process of transcellular production of bioactive lipids by delivering both specific enzymes and substrate molecules. Despite their lack of nucleus, platelets synthetize proteins in a stimuli-dependent manner. Atherosclerosis and consequent cardiovascular complications result from disruption in homeostasis of both of the platelet roles: blood coagulation and inflammatory processes modulation. Platelet parameters, routinely evaluated as a part of complete blood count test, were proposed as markers of cardiovascular comorbidity in patients with obstructive sleep apnea. Platelets were found to be excessively activated in this group of patients, especially in obese subjects. Persistent activation results in enhanced spontaneous aggregability and change in cytokine production. Platelet-lymphocyte ratio was suggested as an independent marker for cardiovascular disease in obstructive sleep apnea syndrome and continuous positive air pressure therapy was found to have an impact on platelet parameters and phenotype. In this literature review we summarize the current knowledge on the subject of platelets involvement in obstructive sleep apnea syndrome and consider the possible pathways in which they contribute to cardiovascular comorbidity.
Introduction
Obstructive sleep apnea (OSA) is a chronic condition characterized by recurrent pauses in breathing during sleep, which lead to intermittent hypoxemia (IH), hypercapnia, arousals, reductions in intrathoracic pressure, and sleep fragmentation. The severity of the disorder is assessed by number of apneas and hypopneas occurring per hour of sleep (apnea-hypopnea index, AHI), where 5 ≥ AHI < 15 is defined as mild, 15 ≥ AHI < 30 as moderate and AHI ≥ 30 as severe disease (1). The prevalence of the sleep disordered breathing (SDB) is estimated that almost 50% of men and 24% of women suffer from moderate to severe form of the disorder (2). The gold standard method for diagnosis of OSA is nocturnal polysomnography (PSG). Typical symptoms of OSA include excessive daytime sleepiness, unrefreshing sleep (3), cognitive impairment (4) as well as snoring. Risk factors for OSA include obesity, male gender, older age, and higher neck circumference (5). Furthermore, it has been shown, that the patients diagnosed with OSA, more often suffer from immunological diseases that are associated with systemic inflammation (6–9) as well as metabolic disorders (10, 11). For over 30 years continuous positive air pressure is method of choice for OSA treatment, as through inhibition of airways collapse it reduces AHI and recurrent hypoxia (12).
OSA and Cardiovascular Diseases
OSA has been established as an independent risk factor for cardiovascular and cerebrovascular diseases (13–15). Through chronic recurrent IH and systemic inflammation, OSA contributes to cardiovascular complications such as arterial hypertension (16, 17), myocardial infraction (18, 19), and coronary artery disease (20, 21). OSA is associated with various conditions that increase the risk of cardiovascular diseases (CVD) themselves, such as atherosclerosis and hyperlipidemia (22). Usually, frequency of cardiovascular complications of OSA increase with severity of the disorder (19), while some cardiovascular OSA co-morbidities, such as hypertension, additionally show linear association with severity of hypoxia (23). It has been shown that OSA is not only an independent risk factor for developing systemic hypertension, but also is associated with increased morning diastolic blood pressure (24). Mechanisms leading to increased risk of developing cardiovascular complications in OSA are complex and intertwine with each other. Best established among them are: increased sympathetic activation, altered vascular regulation, endothelial dysfunction, arterial hypertension, oxidative stress, and chronic systemic inflammation (25). Number of studies have shown increased sympathetic activation following IH, both in animal and human models of OSA leading to hypertension (26, 27). OSA patients have increased sympathetic traffic to peripheral blood vessels and cardiac sympathetic drive (28, 29). It leads to upregulation of renin-angiotensin-aldosterone pathway and downregulation of nitric oxide synthesis (30). Furthermore, it has been shown that CPAP treatment reduces sympathetic activity as well as increases arterial baroreflex sensitivity (31, 32).
Endothelial dysfunction is frequently recognized in OSA patients, often before clinical manifestation of CVD (33). IH regulates the release of vasoactive substances. Nitric oxide, which is the most potent vasodilator is found to be decreased is OSA patients (34, 35). CPAP treatment normalizes the level of nitric oxide (36). Additionally, increased sympathetic activity in OSA has been associated with elevations of PAI-1 (plasminogen activator inhibitor 1) and with antifibrolitic activity (37). Furthermore, it has been shown in the OSA murine models that cardiovascular remodeling induced by IH can be reversed by normoxia (38). It has been also observed that IH and hypercapnia are responsible for progression of atherosclerosis that can be partially reversed through pharmacological treatment (39). Additionally, it has been found that sleep fragmentation independently from IH also contributes to endothelial dysfunction in murine model of OSA (40).
Recurrent hypoxia-reoxygenation caused by apneas promoted notable oxidative stress among OSA patients (41). This cycle is similar to ischemia-reoxygenation cardiac injury, during which increased generation of oxygen-derived free radical is observed. CPAP therapy decreases the levels of oxidative stress in OSA patients (42). Additionally, oxidative stress is involved in regulating cellular transcription trough activation of certain transcription factors. One of the transcription factors that is activated by hypoxia is HIF-1 (hypoxia inducible factor-1), which is responsible for activation of over 100 different genes (43), including vascular endothelial growth factor (VEGF) among many others (44). Kaczmarek et al. has shown increased expression of both, HIF-1α and VEGF, in skin biopsies of OSA patient following severe nocturnal hypoxemia (45). Interestingly, in experimental murine model of OSA it has been shown that short term IH can be a protective factor against further cardiovascular complications through a positive adaptation to hypoxia stimuli (46).
Local and systemic inflammation is widely present in OSA patients. Due to IH, numerous inflammation mediators, such as TNF-α and IL-6 are increased in OSA patients (47) and their levels normalize following CPAP treatment (48). However, there are discrepancies between the results of different studies focusing on inflammatory markers in OSA. McNicholas reviewed the literature regarding CRP levels in OSA, and found some inconsistence in levels and its response to CPAP treatment (49). More studies suggest heterogeneous origins of smoldering inflammation among OSA patients. Systemic inflammation has been shown to contribute to development of atherosclerosis (50), leading to CVD. As vast majority of patients suffering from OSA are obese (51), excessive amount of central adipose tissue also contributes to level of systemic inflammation among OSA patients. Detailed description of relationship between OSA and inflammation can be found in reviews that focus on this topic specifically (52).
Numerous mechanisms that participate in CVD in OSA patients intertwine with each other. Understanding molecular mechanisms leading from IH to complications associated with OSA became crucial to developing effective therapies. Platelets, known as one of the immune cells mediating atherosclerosis, are considered as a possible pathogenic link. However, knowledge about their contribution to CVD in OSA patients is limited.
Platelets in OSA
Research on the effect of OSA on hematological parameters is focused on its impact on blood platelets. Blood platelets play central role in hemostasis and thrombosis. They are a plausible middleman between systemic inflammation and the development of cardiovascular complications. Platelet reactivity affects blood viscosity, a dynamic parameter defined as inherent resistance of blood flow which is increased in patients with OSA in the morning (53).
Platelet indices, routinely measured as a part of a complete blood count test, were investigated as possible markers of OSA severity and certain comorbidities. Upon activation, platelets lose their regular, discoid shape for an increased surface area with lamellipodia and filopodia (54). Mean platelet volume (MPV), which reflects the average size of circulating platelets, could then serve as an indicator of platelet activity (55). Many works have shown strong correlation of MPV and thromboembolic complications, as well as worse outcome of cardiovascular events (56). Higher MPV values are associated with traditional cardiovascular disease risk factors, such as diabetes mellitus and hypertension (57). It was showed that MPV increases correspondingly to OSA severity and is associated with cardiovascular disease comorbidity (58). In two studies that excluded patients with any know cardiac disease, lung disease, diabetes mellitus, chronic renal, or hepatic disease, the only correlation found referred to patients with severe OSA. In this group of patients, MPV positively correlated with AHI (59, 60). A different research group established a correlation between MPV and both AHI and desaturation index, showing that both the number of nocturnal hypoxemia periods and the level of hypoxemia contribute to platelet activation (61). A similar study confirmed these results, showing additionally a positive correlation between MPV and high-sensitivity C-reactive protein (hs-CRP), by what it referred to the inflammatory component of OSA (62). In patients who had undergone uvulopalatal flap surgery, a procedure reducing permanent irritation due to repetitive nocturnal collapse of upper airways and snoring, MPV values were markedly decreased (63). CPAP treatment led to significant reduction of median MPV values in severe OSA patients (64, 65). However, the real usefulness of MPV as a marker of platelet activation, systemic inflammation, and predictor of thromboembolic events is far from being firmly established yet. Increased MPV could be both a possible cause and consequence of thrombosis or accelerated platelet turnover. Moreover, increased MPV is not specific and is influenced by pre-analytical conditions such as method of venipuncture or the type of anticoagulant used during blood collection, genetic polymorphisms, and lifestyle factors (66).
Platelet Distribution Width (PDW) reflects the variance in the size of circulating platelets. Formation of pseudopodia, occurring upon platelet activation, affects PDW (67). PDW is a parameter less influenced by pre-analytical conditions and thus better standardized than MPV. It was showed that increased PDW correlated with AHI in OSA patients and was significantly higher in severe OSA group (68, 69). CPAP therapy resulted in decrease of PDW (70).
Alongside with platelet shape change, changes in the composition of the phospholipid bilayer of the plasma membrane occur. Exposure of unsaturated acyl chains results in acceleration of platelet-dependent activation of serum coagulation factors. Surface receptors and adhesion molecules are upregulated what enables platelet interactions with other cells. The number of circulating platelet-lymphocyte complexes is indicative of platelet activation. It was found to reflect the severity of OSA and was independently associated with concomitant presence of hypertension (69). Not only was a platelet-lymphocyte ratio correlated with OSA severity, but regardless of OSA advancement it indicated cardiovascular complications (71). Platelets are capable of stimulating neutrophils, form aggregates with them or promote formation of neutrophil-lymphocyte complexes. Both platelet-lymphocyte ratio and neutrophil-lymphocyte ratio are increased in OSA patients, compared to a control group of snoring patients. They are parameters dependent on IH and inflammation (71).
Activated platelets release stored cytokines and chemokines in a stimuli-dependent manner. Analysis of blood serum can provide comprehensive information on the state of platelet activation and suggest a possible trigger (72). Examination of OSA patients serum showed an increased level of soluble markers of platelet activations, such as P-selectin (73) and sCD40L (74). Treatment with CPAP lowered the concentration of sCD40L by almost 50% (74). Flow cytometry analysis of blood enables to detect activated platelets upon their surface markers and specify the number of circulating platelet aggregates. Greater degree of hypoxia was predictive of platelet activation (75) and higher percentage of activated platelets was characteristic for OSA patients. Increased platelet activation was recorded during sleep and decreased over time from awakening (76). These results are consistent with another study that showed profound alteration in circadian rhythm of platelet activity in OSA patients in comparison to healthy controls. Whereas for healthy controls late night hours are the period of the lowermost platelet activity, OSA patients present the exactly reverse trend (77). It is a particularly interesting finding, as for OSA patients the peak of cardiac mortality occurs at sleeping hours (78). At the same time, not all patients respond do CPAP therapy with decrease in activated platelet percentage (79, 80).
Platelet-derived microparticles (PMP) are small fragments of platelet plasma membrane shed into the circulation. PMPs are the most abundant group of microparticles present in human blood and their number increases with platelet activation. PMPs are involved in intercellular communication and hemostasis (81). As highly procoagulant, PMPs were suggested as prognostic marker of atherosclerosis (82, 83). Even in minimally symptomatic OSA patients, PMP plasma levels are elevated (84). In more severe cases of OSA, PMP plasma levels correlate with AHI (85). Conversely, withdrawal of CPAP therapy resulted in increase of PMPs (86).
Finally, experimental stimulation of platelets with physiological stimuli (ADP, epinephrine, collagen), may assess platelet reactivity. Platelets collected from OSA patients are hyperaggregable, but do not differ significantly between patients with and without recognized cardiovascular comorbidity (87). While in healthy controls in vitro platelet aggregability weakened overnight, it was slightly increased in OSA patients. CPAP therapy reset platelet reactivity to level resembling that found in healthy controls (88).
Nevertheless, platelets are prone to activation upon numerous factors, including increased c-reactive protein or lipoprotein concentrations in blood (55), which commonly occur in OSA patients due to either systemic inflammation or frequent comorbidities. Due to limited availability of studies excluding confounding factors which may lead to platelet activation, it is difficult to attribute alterations of platelet parameters to IH beyond doubt. Platelet indices were mostly found to correlate with the severe form of SDB, which questions their utility in early stage of disease, when CPAP treatment is not always introduced and an antiplatelet pharmacological intervention could be beneficial.
Platelets as Immune Cells Mediating CVD
Platelets are the second most numerous population of human blood cells. They are responsible for initiating thrombosis at sites of blood vessel damage. Recent research describes platelets as immune cells involved in pathophysiology of various morbid states (89). In physiological conditions platelets are carried by the blood flow in close contact with the endothelial cell lining, but without adhering to it. Disruption of the vessel wall results in exposure of subendothelial structures–von Willebrand factor, type II collagen, laminin, thrombospondin, fibronectin, and vitronectin–ligands to platelets surface receptors (90). Endothelial damage by factors such as constantly elevated blood glucose (91), cigarette smoke chemicals (92), disrupted lipids homeostasis (93), turbulent blood flow, or inflammatory cytokines (94) causes persistent platelet activation and hyperaggregability. The same molecular pathways that enable blood coagulation and maintain physical barriers of the organism are the mechanisms sustaining chronic inflammation and promoting atherosclerosis. Existing atherosclerotic lesions potentiate turbulent blood flow and activate circulating blood platelets. Inflammatory milieu increases platelet response to shear stress, dyslipidemia and endothelial damage (95). A vicious loop perpetuating inflammation and pathological thrombosis is created. OSA patients often suffer from comorbidities which are established direct risk factors for endothelial damage, platelet activation, and atherosclerosis. These include: hypertension, diabetes mellitus, history of tobacco usage, and clinically present chronic ischemic heart disease. OSA with its main component intermittent hypoxia lead itself to platelet activation.
Activated platelets adhere to the site of vascular lesion and initiate thrombus formation. Adherent platelets release cytokines and chemokines that support inflammatory recruitment of immune cells executive in atherosclerotic plaques formations. Platelets secrete chemoattractant RANTES that binds to activated endothelium and triggers monocyte arrest, enabling the initial stages of atherogenesis (96). PMP, circulating in increased numbers during persistent platelet activation, are a source of endothelium-deposited RANTES as well (97). RANTES is the key chemokine in atherogenesis and its expression is increased in vascular wall subjected to intermittent hypoxia. Moreover, its inhibition prevents vascular remodeling induced by intermittent hypoxia (98). P-selectin, the key adhesion molecule in leukocyte recruitment, is necessary for the process of RANTES deposition (90). Soluble platelet P-selectin is a potent prothrombotic mediator stimulating fibrin deposition (99) and membrane-bound platelet P-selectin enables formation of platelet-leukocyte aggregates (100). Other cell types, including human arterial endothelial cells, also express P-selectin. The expression of P-selectin, as well as proatherogenic cytokines characteristic for endothelium, increased after stimulation with OSA patients' serum. These molecules activate platelets in a positive feedback mechanism (101). Platelet P-selectin is involved in atherosclerotic lesions formation and their further maturation and progression (100). Another platelet-derived cytokine, Platelet Factor 4 (PF4) is deposited in atherosclerotic lesions. Through interaction with low density lipoprotein (LDL) receptor PF4 interferes with LDL binding and thus promotes LDL oxidation (102). It increases binding of oxidized LDL to endothelium and contributes to fatty streaks formation within atherosclerotic lesion (103). The extent of PF4 deposition correlates with clinical symptoms of atherosclerosis and with lesion severity (104). CD40 ligand (CD40L) is expressed on the surface and secreted by activated platelets. It launches inflammatory response of the vessel wall, thrombus formation, accelerates sclerotic plaque development (105) (Figure 1). Persistent platelet activation leads to a characteristic thromboembolic end point of myocardial infarction.
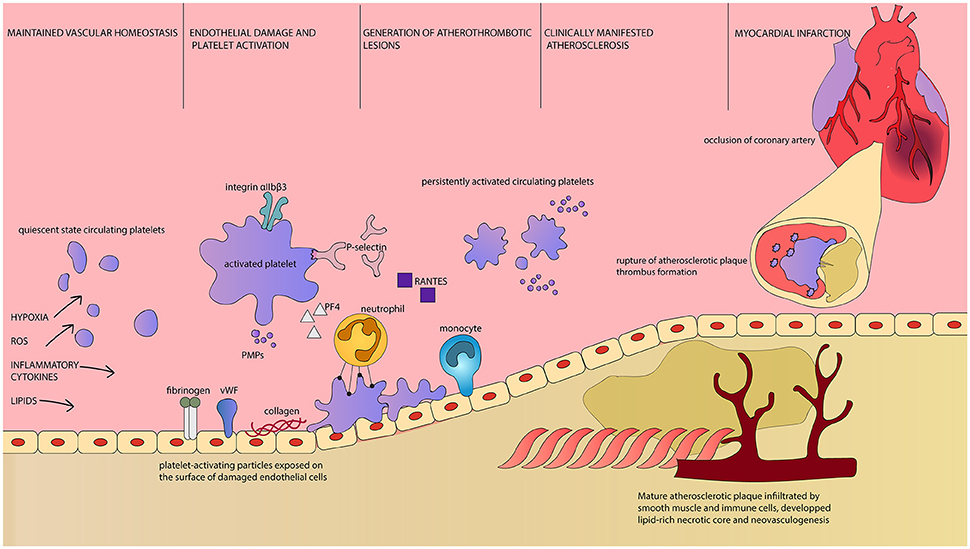
Figure 1. Platelets as first and final effector cells in atherogenesis and its complications. Intermittent hypoxia, oxidative stress, elevated concentration of circulating inflammatory cytokines, and lipids are factors leading to disruption of vascular homeostasis in OSA patients. Parallel to endothelial damage and demonstration of otherwise unexposed molecules, platelet activation occurs. Activated platelets are characterized by increased surface area, upregulation of surface receptors and release of PMPs as well as plethora of bioactive substances (106). Reorganization of platelet integrin αIIbβ3 conformation enables fibrinogen binding and platelet aggregation (107). P-selectin is both exposed on the surface of platelets and released. It is essential for leukocyte recruitment and enables adhesion between platelets and neutrophils, as well as platelet aggregation. Platelet-derived RANTES is deposited on endothelial cells and allows monocyte arrest and infiltration. PF4 attracts neutrophils, but also interacts with endothelial LDL receptor and promotes lipid peroxidation. PMPs are highly coagulant carriers of platelet-derived molecules, including regulatory miRNA. Platelet-neutrophil interaction is dependent on the aforementioned P-selectin and PF4 as well as CD40L and GP1βα along with their ligands expressed on the surface of neutrophil, respectively PSGL-1, CD40, Mac1 and CCL5 (89). Platelets enhance neutrophil extracellular traps formation and neutrophil oxidative burst, which can lead to tissue damage. Persistent platelet activation results in constant presence of proinflammatory and proatherogenic substances, immune cells infiltration of the endothelium and consequently, to the development of atherosclerotic plaques. Clinical manifestation of atherosclerosis indicates the presence of mature plaques with rich in lipids necrotic core, neovascularization and fragile fibrous cap. At this time, circulating platelets display prothrombotic phenotype and are easily activated. Rupture of such atherosclerotic plaque results in rapid thrombus formation. It can lead to occlusion of critical arteries, including coronary vessels, and dramatical/major consequences. ROS, Reactive Oxygen Species; vWF, von Willebrandt Factor; PMPs, Platelet-derived Microparticles; PF4, Platelet Factor 4; Integrin αIIbβ3, glycoprotein IIb/IIIa; GP1βα, glycoprotein Ib alpha chain; RANTES, regulated on activation, normal T-cell expressed and secreted, CCL5; CD40L, CD40 Ligand, CD154; PSGL-1, P-selectin glycoprotein ligand-1; CD40, Cluster of differentiation 40; Mac1, Macrophage-1 antigen, integrin αMβ2.
Increased sympathetic activity is thought to be the main factor promoting persistent platelet activation in OSA patients. Recurrent arousals from sleep overlap with repetitive surges of sympathetic neural activity, as well as increases in concentrations of vasoconstrictive peptides and circulating catecholamines, which directly activate platelets (108). Effectiveness of CPAP therapy in reducing platelet activation confirms this interdependence (109).
Inflammatory and pro-atherogenic cytokines, circulating in elevated levels in OSA patients, may directly and indirectly activate platelets (110–112). Obesity, so tightly associated with collapse of upper airways and shift toward inflammatory phenotype, was considered to be the factor responsible for enhanced platelet activation in OSA patients (113). However, in most studies the control groups for OSA patients were age, sex- and BMI- matching subjects with excluded OSA. The level of hemoglobin deoxygenation was the variable correlating with platelet hyperreactivity (75) and CPAP therapy reduced platelet aggregability (114). At the same time increased native platelet reactivity was shown in a study of obese cohort (115). So, it seems plausible that both, OSA and obesity are independent but usually concurrent risk factors for persistent platelet activity. The issue of pathophysiological pathways in multimorbidity needs interdisciplinary research (116).
Finally, hypoxia itself damages endothelial lining of blood vessels and contributes to the development of endothelial dysfunction and its consequences (117). Hypoxia initiates p53-dependent pathway of endothelial cells apoptosis (118). Circulating platelets come into contact with damaged endothelium and undergo activation. Figure 2 summarizes mechanisms of increased platelet activation in OSA.
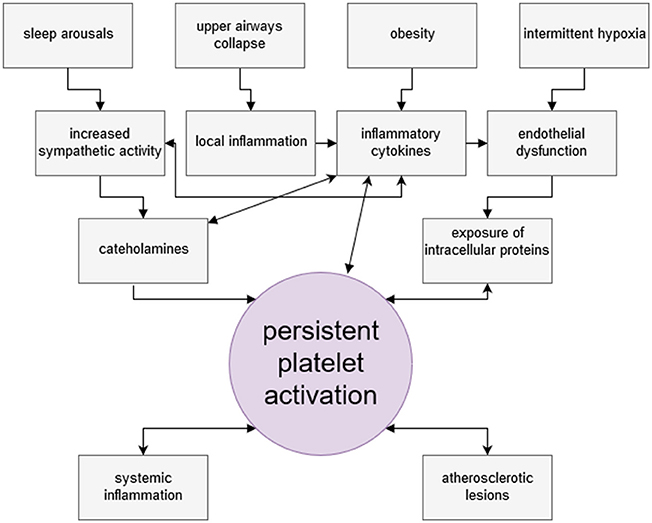
Figure 2. Factors leading to persistent platelet activation among obstructive sleep apnea patients. Platelets of OSA patients present thrombogenic phenotype and are hyperreactive. Among various intertwining factors leading to persistent platelet activation, four are considered as most important. Deprivation of sleep rhythm and nocturnal arousals result in increased sympathetic activity in OSA patients, manifested by elevated levels of circulating catecholamines. Noradrenaline is a potent direct platelet activator. Recurrent hypoxia events cause endothelial damage and presentation of molecules otherwise unexposed to platelets. OSA patients are characteristically obese and obesity entails the increase of the level inflammatory mediators and PAI-1 in circulation. Obesity is linked with susceptibility to upper airway collapse, which by causing mechanical irritation, contributes to sustaining inflammatory milieu. Circulating inflammatory cytokines may activate platelets, and PAI-1 inhibits antithrombotic serum activity. In turn, activated platelet secrete a plethora of bioactive, proinflammatory substances themselves. Through interaction with LDL receptor, activated platelets contribute to lipid peroxidation and, consequently, oxidative stress. Platelets are early effector cells of atherosclerosis. Persistent platelet activation may lead to serious thrombotic complications.
Myocardial infarction and other serious thromboembolic complications are an end point effects of persistent excessive platelet activation. OSA is a risk factor of these adverse events independently of age, BMI, hypertension, or diabetes mellitus (119). Among patients undergoing percutaneous coronary intervention, OSA was an independent predictor of lower effectiveness of antiplatelet therapy (120). Statin treatment in OSA patients lowered blood pressure and corrected lipid panel, but did not affect early atherosclerosis (121). This observation leads to questioning whether platelets should be considered a therapeutic target in OSA or rather a component of pathogenetic pathways. Studies on platelet role in OSA repetitively conclude that the gold standard treatment–CPAP therapy–affects platelet activities and reduces cardiovascular risk. CPAP also significantly improved platelet response to therapy with aspirin (122). An early manifestation of hyperreactivity of these immune cells sustaining systemic inflammation, is atherosclerosis. The presence of atherosclerotic lesions promotes platelet activation itself (95). However, platelet indices failed to predict the presence of atherosclerosis among OSA patients and were found to be a less useful marker than a non-invasive measurement of carotid intima-media thickness (123). There is paucity of studies assessing the role antiplatelet therapy in OSA patients. Current research provides evidence that in the group suffering from SDB, CPAP treatment is an intervention that effectively lowers the risk of thromboembolic events and reduces platelet activation. There is necessity of establishing guidelines of cardiovascular management in OSA patients.
Conclusions
Available literature provides support to the fact that blood platelets in OSA patients are a viable therapeutic target to decrease CVD risk. Especially, that it has been shown that CPAP influences function of blood (87). Available anti-platelet therapies as well as other commonly administered drugs with pleiotropic effect could be beneficial to OSA patients, as treatment parallel to CPAP. Nevertheless, the molecular mechanisms behind platelet involvement in OSA and its complications still remain not fully understood. The unanswered questions comprise molecular pathways of platelet activation by hypoxia, platelet micro-RNA patterns in OSA. Since CPAP therapy adherence is limited, such studies could highlight target points of future pharmacological treatment. Additionally, more evidence for the use of platelet indices, which are routinely measured in blood count test, in monitoring OSA patients, needs to be presented to firmly establish their role in everyday clinical practice. An important issue that should be addressed by future studies is the influence of most common OSA comorbidities on platelet status and determining independent modifiers. Raising awareness of cardiovascular risk attributed to OSA among both physicians and patients is a crucial step in disease management.
Author Contributions
AG and ZMŁ created the concept of the paper, conducted literature research and wrote the manuscript. PB and JSM revised the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
AG is supported by grant no. 564/1-000-00/564-20-021 from Medical University of Lodz.
Abbreviations
AHI, apnea–hypopnea index; BMI, body mass index; CPAP, continuous positive air pressure; CVD, cardiovascular disease; EDS, excessive daily sleepiness; HIF-1, hypoxia inducible factor-1; hs-CRP, high-sensitivity C-reactive protein; IH, intermittent hypoxemia; IL-6, interleukin 6; LDL, low density lipoprotein; MPV, mean platelet volume; OSA, obstructive sleep apnea syndrome; PAI-1, plasminogen activator inhibitor 1; PDW, platelet distribution width; PF4, Platelet Factor 4; PMP, Platelet-derived microparticles; PSG, polysomnography; SDB, sleep disordered breathing; TNF-α, tumor nectosis factor α; VEGF, vascular endothelial growth factor.
References
1. Kapur VK, Auckley DH, Chowdhuri S, Kuhlmann DC, Mehra R, Ramar K, et al. Clinical practice guideline for diagnostic testing for adultobstructive sleep apnea: an American academy of sleep medicine clinical practice guideline. J Clin Sleep Med. (2017) 13:479–504. doi: 10.5664/jcsm.6506
2. Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Resp Med. (2015) 3:310–8. doi: 10.1016/S2213-2600(15)00043-0
3. Mediano O, Barceló A, la Peña de M, Gozal D, Agustí A, Barbé F. Daytime sleepiness and polysomnographic variables in sleep apnoea patients. Eur Respir J. (2007) 30:110–3. doi: 10.1183/09031936.00009506
4. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. (2008) 5:136–43. doi: 10.1513/pats.200709-155MG
5. Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA (2000) 284:3015–21. doi: 10.1001/jama.284.23.3015
6. Gabryelska A, Sochal M, Wasik B, Białasiewicz P. Patients with obstructive sleep apnea are over four times more likely to suffer from psoriasis than the general population. J Clin Sleep Med. (2018) 14:153. doi: 10.5664/jcsm.6908
7. Puthalapattu S, Ioachimescu OC. Asthma and obstructive sleep apnea: clinical and pathogenic interactions. J Investig Med. (2014) 62:665–75. doi: 10.2310/JIM.0000000000000065
8. Kuczynski W, Gabryelska A, Mokros Ł, Białasiewicz P. Obstructive sleep apnea syndrome and hypothyroidism - merely concurrence or causal association? Pneumonol Alergol Pol. (2016) 84:302–6. doi: 10.5603/PiAP.2016.0038
9. Mirrakhimov AE. Obstructive sleep apnea and autoimmune rheumatic disease: is there any link? Inflamm Allergy Drug Targets (2013) 12:362–367. doi: 10.2174/18715281113129990051
10. Seetho IW, Wilding JPH. Sleep-disordered breathing, type 2 diabetes and the metabolic syndrome. Chron Respir Dis. (2014) 11:257–75. doi: 10.1177/1479972314552806
11. Lin QC, Chen LD, Yu YH, Liu KX, Gao SY. Obstructive sleep apnea syndrome is associated with metabolic syndrome and inflammation. Eur Arch Otorhinolaryngol. (2014) 271:825–31. doi: 10.1007/s00405-013-2669-8
12. Morgenthaler TI, Kapen S, Lee-Chiong T, Alessi C, Boehlecke B, Brown T, et al. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep (2006) 29:1031–5.
13. Drager LF, Li J, Reinke C, Bevans-Fonti S, Jun JC, Polotsky VY. Intermittent hypoxia exacerbates metabolic effects of diet-induced obesity. Obesity (2011) 19:2167–74. doi: 10.1038/oby.2011.240
14. Drager LF, Togeiro SM, Polotsky VY, Lorenzi-Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. (2013) 62:569–76. doi: 10.1016/j.jacc.2013.05.045
15. Levy P, Tamisier R, Minville C, Launois S, Pepin JL. Sleep apnoea syndrome in 2011: current concepts and future directions. Eur Respir Rev. (2011) 20:134–46. doi: 10.1183/09059180.00003111
16. Hedner J, Bengtsson-Boström K, Peker Y, Grote L, Råstam L, Lindblad U. Hypertension prevalence in obstructive sleep apnoea and sex: a population-based case-control study. Eur Respir J. (2006) 27:564–70. doi: 10.1183/09031936.06.00042105
17. Robinson GV, Stradling JR, Davies RJO. Sleep 6: obstructive sleep apnoea/hypopnoea syndrome and hypertension. Thorax (2004) 59:1089–94. doi: 10.1136/thx.2003.015875
18. Sforza E, Roche F. Chronic intermittent hypoxia and obstructive sleep apnea: an experimental and clinical approach. Hypoxia (2016) 4:99–108. doi: 10.2147/HP.S103091
19. Marin JM, Carrizo SJ, Vicente E, Agusti AGN. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet (2005) 365:1046–53. doi: 10.1016/S0140-6736(05)71141-7
20. Gottlieb DJ, Yenokyan G, Newman AB, O'Connor GT, Punjabi NM, Quan SF, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation (2010) 122:352–60. doi: 10.1161/CIRCULATIONAHA.109.901801
21. Kent BD, Garvey JF, Ryan S, Nolan G, Dodd JD, McNicholas WT. Severity of obstructive sleep apnoea predicts coronary artery plaque burden: a coronary computed tomographic angiography study. Eur Respir J. (2013) 42:1263–70. doi: 10.1183/09031936.00094812
22. Drager LF, Jun J, Polotsky VY. Obstructive sleep apnea and dyslipidemia: implications for atherosclerosis. Curr Opin Endocrinol Diabetes Obes. (2010) 17:161–5. doi: 10.1097/MED.0b013e3283373624
23. McNicholas WT, Bonsigore MR, Bonsignore MR, Management Committee of EU COST ACTION B26. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. (2007) 29:156–78. doi: 10.1183/09031936.00027406
24. Mokros Ł, Kuczynski W, Franczak Ł, Białasiewicz P. Morning diastolic blood pressure may be independently associated with severity of obstructive sleep apnea in non-hypertensive patients: a cross-sectional study. J Clin Sleep Med. (2017) 13:905–10. doi: 10.5664/jcsm.6664
25. Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, et al. Sleep apnea and cardiovascular disease: an American Heart Association/american College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation (2008) 118:1080–111. doi: 10.1161/CIRCULATIONAHA.107.189375
26. Beaudin AE, Waltz X, Hanly PJ, Poulin MJ. Impact of obstructive sleep apnoea and intermittent hypoxia on cardiovascular and cerebrovascular regulation. Experiment Physiol. (2017) 102:743–63. doi: 10.1113/EP086051
27. Foster GE, Poulin MJ, Hanly PJ. Intermittent hypoxia and vascular function: implications for obstructive sleep apnoea. Experiment Physiol. (2007) 92:51–65. doi: 10.1113/expphysiol.2006.035204
28. Narkiewicz K, Montano N, Cogliati C, van de Borne PJ, Dyken ME, Somers VK. Altered cardiovascular variability in obstructive sleep apnea. Circulation (1998) 98:1071–7.
29. Narkiewicz K, van de Borne PJ, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation (1998) 98:772–6.
30. Prabhakar NR, Fields RD, Baker T, Fletcher EC. Intermittent hypoxia: cell to system. Am J Physiol Lung Cell Mol Physiol. (2001) 281:L524–8. doi: 10.1152/ajplung.2001.281.3.L524
31. Maser RE, Lenhard MJ, Rizzo AA, Vasile AA. Continuous positive airway pressure therapy improves cardiovascular autonomic function for persons with sleep-disordered breathing. Chest (2008) 133:86–91. doi: 10.1378/chest.07-1580
32. Marrone O, Salvaggio A, Bue AL, Bonanno A, Riccobono L, Insalaco G, Bonsignore MR. Blood pressure changes after automatic and fixed CPAP in obstructive sleep apnea: relationship with nocturnal sympathetic activity. Clin Exp Hypertens. (2011) 33:373–80. doi: 10.3109/10641963.2010.531853
33. la Peña de M, Barceló A, Barbé F, Piérola J, Pons J, Rimbau E, et al. Endothelial function and circulating endothelial progenitor cells in patients with sleep apnea syndrome. Respiration (2008) 76:28–32. doi: 10.1159/000109643
34. Kohler M, Craig S, Pepperell JCT, Nicoll D, Bratton DJ, Nunn AJ, et al. CPAP improves endothelial function in patients with minimally symptomatic OSA. Chest (2013) 144:896–902. doi: 10.1378/chest.13-0179
35. Haight JSJ, Djupesland PG. Nitric oxide (NO) and obstructive sleep apnea (OSA). Sleep Breath (2003) 7:53–62. doi: 10.1007/s11325-003-0053-4
36. Oyama J-I, Yamamoto H, Maeda T, Ito A, Node K, Makino N. Continuous positive airway pressure therapy improves vascular dysfunction and decreases oxidative stress in patients with the metabolic syndrome and obstructive sleep apnea syndrome. Clin Cardiol. (2012) 35:231–6. doi: 10.1002/clc.21010
37. Känel von R, Dimsdale JE. Hemostatic alterations in patients with obstructive sleep apnea and the implications for cardiovascular disease. Chest (2003) 124:1956–67. doi: 10.1378/chest.124.5.1956
38. Castro-Grattoni AL, Alvarez-Buvé R, Torres M, Farré R, Montserrat JM, Dalmases M, et al. Intermittent hypoxia-induced cardiovascular remodeling is reversed by normoxia in a mouse model of sleep apnea. Chest (2016) 149:1400–8. doi: 10.1016/j.chest.2015.11.010
39. Xue J, Zhou D, Poulsen O, Imamura T, Hsiao Y-H, Smith TH, et al. Intermittent hypoxia and hypercapnia accelerate atherosclerosis, partially via trimethylamine-oxide. Am J Respir Cell Mol Biol. (2017) 57:581–8. doi: 10.1165/rcmb.2017-0086OC
40. Carreras A, Zhang SX, Peris E, Qiao Z, Gileles-Hillel A, Li RC, et al. Chronic sleep fragmentation induces endothelial dysfunction and structural vascular changes in mice. Sleep (2014) 37:1817–24. doi: 10.5665/sleep.4178
41. Lavie L. Oxidative stress-a unifying paradigm in obstructive sleep apnea and comorbidities. Prog Cardiovasc Dis. (2009) 51:303–12. doi: 10.1016/j.pcad.2008.08.003
42. Celec P, Hodosy J, Behuliak M, Pálffy R, Gardlík R, Halčák L, et al. Oxidative and carbonyl stress in patients with obstructive sleep apnea treated with continuous positive airway pressure. Sleep Breath (2011) 16:393–8. doi: 10.1007/s11325-011-0510-4
43. Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, et al. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood (2005) 105:659–69. doi: 10.1182/blood-2004-07-2958
44. Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ (2008) 15:621–7. doi: 10.1038/cdd.2008.12
45. Kaczmarek E, Bakker JP, Clarke DN, Csizmadia E, Kocher O, Veves A, et al. Molecular biomarkers of vascular dysfunction in obstructive sleep apnea. PLoS ONE (2013) 8:e70559. doi: 10.1371/journal.pone.0070559
46. Almendros I, Wang Y, Gozal D. The polymorphic and contradictory aspects of intermittent hypoxia. Am J Physiol Lung Cell Mol Physiol. (2014) 307:L129–40. doi: 10.1152/ajplung.00089.2014
47. Imagawa S, Yamaguchi Y, Ogawa K, Obara N, Suzuki N, Yamamoto M, et al. Interleukin-6 and tumor necrosis factor-alpha in patients with obstructive sleep apnea-hypopnea syndrome. Respiration (2004) 71:24–9. doi: 10.1159/000075645
48. Steiropoulos P, Kotsianidis I, Nena E, Tsara V, Gounari E, Hatzizisi O, et al. Long-term effect of continuous positive airway pressure therapy on inflammation markers of patients with obstructive sleep apnea syndrome. Sleep (2009) 32:537–43. doi: 10.1007/s11325-011-0510-4
49. McNicholas WT. Obstructive sleep apnea and inflammation. Prog Cardiovasc Dis. (2009) 51:392–9. doi: 10.1016/j.pcad.2008.10.005
50. Ross R. Atherosclerosis — an inflammatory disease. N Engl J Med. (1999) 340:115–26. doi: 10.1056/NEJM199901143400207
51. Mokros Ł, Kuczynski W, Gabryelska A, Franczak Ł, Spałka J, Białasiewicz P. High negative predictive value of normal body mass index for obstructive sleep apnea in the lateral sleeping position. J Clin Sleep Med. (2018) 14:985–90. doi: 10.5664/jcsm.7166
52. Unnikrishnan D, Jun J, Polotsky V. Inflammation in sleep apnea: an update. Rev Endocr Metab Disord. (2015) 16:25–34. doi: 10.1007/s11154-014-9304-x
53. Toraldo DM, Peverini F, De Benedetto M, De Nuccio F. Obstructive sleep apnea syndrome: blood viscosity, blood coagulation abnormalities, and early atherosclerosis. Lung (2012) 191:1–7. doi: 10.1007/s00408-012-9427-3
54. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-−2015 update: a report from the american heart association. Circulation (2015) 131:e29–322. doi: 10.1161/CIR.0000000000000152
55. Tsiara S, Elisaf M, Jagroop IA, Mikhailidis DP. Platelets as predictors of vascular risk: is there a practical index of platelet activity? Clin Appl Thromb Hemost. (2003) 9:177–90. doi: 10.1177/107602960300900301
56. Slavka G, Perkmann T, Haslacher H, Greisenegger S, Marsik C, Wagner OF, et al. Mean platelet volume may represent a predictive parameter for overall vascular mortality and ischemic heart disease. Arterioscler Thromb Vasc Biol. (2011) 31:1215–8. doi: 10.1161/ATVBAHA.110.221788
57. Kodiatte T, Rao S, Manikyam U, Reddy M, Lakshmaiah V, Jagadish T, et al. Mean platelet volume in type 2 diabetes mellitus. J Lab Physicians (2012) 4:5. doi: 10.4103/0974-2727.98662
58. Kanbay A, Tutar N, Kaya E, Buyukoglan H, Ozdogan N, Oymak FS, et al. Mean platelet volume in patients with obstructive sleep apnea syndrome and its relationship with cardiovascular diseases. Blood Coagul Fibr. (2013) 24:532–6. doi: 10.1097/MBC.0b013e32835e98da
59. Sökücü SN, Özdemir C, Dalar L, Karasulu L, Aydin S, Altin S. Is mean platelet volume really a severity marker for obstructive sleep apnea syndrome without comorbidities? Pulmon Med. (2014) 2014:1–7. doi: 10.1155/2014/754839
60. Karakaş MS, Altekin RE, Baktir AO, Küçük M, Cilli A, Yalçinkaya S. Association between mean platelet volume and severity of disease in patients with obstructive sleep apnea syndrome without risk factors for cardiovascular disease. Turk Kardiyol Dern Ars. (2013) 41:14–20. doi: 10.5543/tkda.2013.42948
61. Varol E, Ozturk O, Gonca T, Has M, Ozaydin M, Erdogan D, et al. Mean platelet volume is increased in patients with severe obstructive sleep apnea. Scand J Clin Lab Invest. (2010) 70:497–502. doi: 10.3109/00365513.2010.520733
62. Akyol S, Cortuk M, Baykan AO, Kiraz K, Börekçi A, Seker T, et al. Mean platelet volume is associated with disease severity in patients with obstructive sleep apnea syndrome. Clinics (2015) 70:481–5. doi: 10.6061/clinics/2015(07)04
63. Simsek G, Haytoglu S, Muluk NB, Arikan OK, Cortuk M, Kiraz K. Mean platelet volume decreases in adult patients with obstructive sleep apnea after uvulopalatal flap surgery. J Craniofac Surg. (2015) 26:2152–4. doi: 10.1097/SCS.0000000000001954
64. Varol E, Ozturk O, Yucel H, Gonca T, Has M, Dogan A, et al. The effects of continuous positive airway pressure therapy on mean platelet volume in patients with obstructive sleep apnea. Platelets (2011) 22:552–6. doi: 10.3109/09537104.2011.578182
65. Sökücü SN, Özdemir C, Dalar L, Karasulu L, Aydin S, Altin S. Complete blood count alterations after six months of continuous positive airway pressure treatment in patients with severe obstructive sleep apnea. J Clin Sleep Med. (2014) 10:873–8. doi: 10.5664/jcsm.3958
66. Harrison P, Goodall AH. Studies on mean platelet volume (MPV) - new editorial policy. Platelets (2016) 27:605–6. doi: 10.1080/09537104.2016.1225467
67. Vagdatli E, Gounari E, Lazaridou E, Katsibourlia E, Tsikopoulou F, Labrianou I. Platelet distribution width: a simple, practical and specific marker of activation of coagulation. Hippokratia (2010) 14:28–32.
68. Bülbül Y, Aydin Özgür E, Örem A. Platelet indices in obstructive sleep apnea: the role of mean platelet volume, platelet distribution widht and plateletcrit. Tuberk Toraks (2016) 64:206–10.
69. Song Y-J, Kwon JH, Kim JY, Kim BY, Cho KI. The platelet-to-lymphocyte ratio reflects the severity of obstructive sleep apnea syndrome and concurrent hypertension. Clin Hypertens (2015) 22:1. doi: 10.1186/s40885-015-0036-3
70. Cortuk M, Simsek G, Kiraz K, Haytoglu S, Zitouni B, Bayar Muluk N, et al. Effect of continuous positive airway pressure treatment on mean platelet volume and platelet distribution width in patients with sleep apnea syndrome. Eurasian J Pulmonol. (2016) 18:85–9. doi: 10.5152/ejp.2016.84803
71. Koseoglu S, Ozcan KM, Ikinciogullari A, Cetin MA, Yildirim E, Dere H. Relationship between neutrophil to lymphocyte ratio, platelet to lymphocyte ratio and obstructive sleep apnea syndrome. Adv Clin Exp Med. (2015) 24:623–7. doi: 10.17219/acem/47735
72. Yun S-H, Sim E-H, Goh R-Y, Park J-I, Han J-Y. Platelet activation: the mechanisms and potential biomarkers. Biomed Res Int. (2016) 2016:9060143. doi: 10.1155/2016/9060143
73. Cofta S, Wysocka E, Dziegielewska-Gesiak S, Michalak S, Piorunek T, Batura-Gabryel H, Torlinski L. Plasma selectins in patients with obstructive sleep apnea. Adv Exp Med Biol. (2013) 756:113–9. doi: 10.1007/978-94-007-4549-0_15
74. Akinnusi ME, Paasch LL, Szarpa KR, Wallace PK, Solh El AA. Impact of nasal continuous positive airway pressure therapy on markers of platelet activation in patients with obstructive sleep apnea. Respiration (2009) 77:25–31. doi: 10.1159/000158488
75. Rahangdale S, Yeh SY, Novack V, Stevenson K, Barnard MR, Furman MI, et al. The influence of intermittent hypoxemia on platelet activation in obese patients with obstructive sleep apnea. J Clin Sleep Med. (2011) 7:172–8.
76. Geiser T, Buck F, Meyer BJ, Bassetti C, Haeberli A, Gugger M. In vivo platelet activation is increased during sleep in patients with obstructive sleep apnea syndrome. Respiration (2002) 69:229–34. doi: 10.1159/000063625
77. Barceló A, Piérola J, la Peña de M, Frontera G, Yañez A, Alonso-Fernández A, et al. Impaired circadian variation of platelet activity in patients with sleep apnea. Sleep Breath (2012) 16:355–60. doi: 10.1007/s11325-011-0501-5
78. Gami AS, Howard DE, Olson EJ, Somers VK. Day–night pattern of sudden death in obstructive sleep apnea. N Engl J Med. (2005) 352:1206–14. doi: 10.1056/NEJMoa041832
79. Bokinsky G, Miller M, Ault K, Husband P, Mitchell J. Spontaneous platelet activation and aggregation during obstructive sleep apnea and its response to therapy with nasal continuous positive airway pressure: a preliminary investigation. Chest (1995) 108:625–30. doi: 10.1378/chest.108.3.625
80. Shimizu M, Kamio K, Haida M, Ono Y, Miyachi H, Yamamoto M, et al. Platelet activation in patients with obstructive sleep apnea syndrome and effects of nasal-continuous positive airway pressure. Tokai J Exp Clin Med. (2002) 27:107–12.
81. Italiano JE, Mairuhu ATA, Flaumenhaft R. Clinical relevance of microparticles from platelets and megakaryocytes. Curr Opin Hematol. (2010) 17:578–84. doi: 10.1097/MOH.0b013e32833e77ee
82. Sinauridze EI, Kireev DA, Popenko NY, Pichugin AV, Panteleev MA, Krymskaya OV, et al. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost. (2007) 97:425–434.
83. Boulanger CM, Amabile N, Tedgui A. Circulating microparticles: a potential prognostic marker for atherosclerotic vascular disease. Hypertension (2006) 48:180–6. doi: 10.1161/01.HYP.0000231507.00962.b5
84. Ayers L, Ferry B, Craig S, Nicoll D, Stradling JR, Kohler M. Circulating cell-derived microparticles in patients with minimally symptomatic obstructive sleep apnoea. Eur Respir J. (2009) 33:574–80. doi: 10.1183/09031936.00107408
85. Maruyama K, Morishita E, Sekiya A, Omote M, Kadono T, Asakura H, et al. Plasma levels of platelet-derived microparticles in patients with obstructive sleep apnea syndrome. JAT (2012) 19:98–104. doi: 10.5551/jat.8565
86. Ayers L, Turnbull C, Petousi N, Ferry B, Kohler M, Stradling J. Withdrawal of continuous positive airway pressure therapy for 2 weeks in obstructive sleep apnoea patients results in increased circulating platelet and leucocyte-derived microvesicles. Respiration (2016) 91:412–3. doi: 10.1159/000446077
87. Oga T, Chin K, Tabuchi A, Kawato M, Morimoto T, Takahashi K, et al. Effects of obstructive sleep apnea with intermittent hypoxia on platelet aggregability. JAT (2010) 16:862–9. doi: 10.5551/jat.2188
88. Sanner BM, Konermann M, Tepel M, Groetz J, Mummenhoff C, Zidek W. Platelet function in patients with obstructive sleep apnoea syndrome. Eur Respir J. (2000) 16:648–52.
89. Łukasik ZM, Makowski M, Makowska JS. From blood coagulation to innate and adaptive immunity: the role of platelets in the physiology and pathology of autoimmune disorders. Rheumatol Int. (2018) 38:959–74. doi: 10.1007/s00296-018-4001-9
90. Chen J, Lopez JA. Interactions of platelets with subendothelium and endothelium. Microcirculation (2005) 12:235–46. doi: 10.1080/10739680590925484
91. De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. (2000) 130:963–74. doi: 10.1038/sj.bjp.0703393
92. Messner B, Bernhard D. Smoking and cardiovascular diseasesignificance: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol. (2014) 34:509–15. doi: 10.1161/ATVBAHA.113.300156
93. Carvalho ACA, Colman RW, Lees RS. Platelet function in hyperlipoproteinemia. N Engl J Med. (1974) 290:434–8. doi: 10.1056/NEJM197402212900805
94. Davizon-Castillo P, Ashworth K, Higa K, Hernandez G, Acharya S, Campbell RA, et al. Pro-inflammatory cytokines drive the development of a prothrombotic platelet phenotype in inflammation and aging. Blood (2016) 128:3747.
95. Ma Y, Ashraf MZ, Podrez EA. Scavenger receptor BI modulates platelet reactivity and thrombosis in dyslipidemia. Blood (2010) 116:1932–41. doi: 10.1182/blood-2010-02-268508
96. Hundelshausen von P, Weber KSC, Huo Y, Proudfoot AEI, Nelson PJ, Ley K, et al. RANTES Deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation (2001) 103:1772–7. doi: 10.1161/01.CIR.103.13.1772
97. Mause SF, Hundelshausen von P, Zernecke A, Koenen RR, Weber C. Platelet microparticles: a transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler Thromb Vasc Biol. (2005) 25:1512–8. doi: 10.1161/01.ATV.0000170133.43608.37
98. Arnaud C, Beguin PC, Lantuejoul S, Pepin J-L, Guillermet C, Pelli G, et al. The inflammatory preatherosclerotic remodeling induced by intermittent hypoxia is attenuated by RANTES/CCL5 inhibition. Am J Respir Crit Care Med. (2011) 184:724–31. doi: 10.1164/rccm.201012-2033OC
99. Andre P, Hartwell D, Hrachovinova I, Saffaripour S, Wagner DD. Pro-coagulant state resulting from high levels of soluble P-selectin in blood. Proc Natl Acad Sci USA. (2000) 97:13835–40. doi: 10.1073/pnas.250475997
100. Burger PC, Wagner DD. Platelet P-selectin facilitates atherosclerotic lesion development. Blood (2003) 101:2661–6. doi: 10.1182/blood-2002-07-2209
101. Zychowski KE, Sanchez B, Pedrosa RP, Lorenzi-Filho G, Drager LF, Polotsky VY, et al. Serum from obstructive sleep apnea patients induces inflammatory responses in coronary artery endothelial cells. Atherosclerosis (2016) 254:59–66. doi: 10.1016/j.atherosclerosis.2016.09.017
102. Sachais BS, Kuo A, Nassar T, Morgan J, Kariko K, Williams KJ, et al. Platelet factor 4 binds to low-density lipoprotein receptors and disrupts the endocytic machinery, resulting in retention of low-density lipoprotein on the cell surface. Blood (2002) 99:3613–22. doi: 10.1182/blood.V99.10.3613
103. Nassar T, Sachais BS, Akkawi S, Kowalska MA, Bdeir K, Leitersdorf E, et al. Platelet factor 4 enhances the binding of oxidized low-density lipoprotein to vascular wall cells. J Biol Chem. (2003) 278:6187–93. doi: 10.1074/jbc.M208894200
104. Pitsilos S, Hunt J, Mohler ER, Prabhakar AM, Poncz M, Dawicki J, et al. Platelet factor 4 localization in carotid atherosclerotic plaques: correlation with clinical parameters. Thromb Haemost. (2003) 90:1112–20. doi: 10.1160/TH03-02-0069
105. Lievens D, Zernecke A, Seijkens T, Soehnlein O, Beckers L, Munnix ICA, et al. Platelet CD40L mediates thrombotic and inflammatory processes in atherosclerosis. Blood (2010) 116:4317–27. doi: 10.1182/blood-2010-01-261206
106. Coppinger JA, Cagney G, Toomey S, Kislinger T, Belton O, McRedmond JP, et al. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood (2004) 103:2096–104. doi: 10.1182/blood-2003-08-2804
107. Bennett JS, Berger BW, Billings PC. The structure and function of platelet integrins. J Thromb Haemost. (2009) 7:200–5. doi: 10.1111/j.1538-7836.2009.03378.x
108. Olson LJ, Olson EJ, Somers VK. Obstructive sleep apnea and platelet activation. Chest (2004) 126:339–41. doi: 10.1378/chest.126.2.339
109. Hui DS, Ko FW, Fok JP, Chan MC, Li TS, Tomlinson B, et al. The effects of nasal continuous positive airway pressure on platelet activation in obstructive sleep apnea syndrome. Chest (2004) 125:1768–75. doi: 10.1378/chest.125.5.1768
110. Lawati Al N, Mulgrew A, Cheema R, vanEeden S, Butt A, Fleetham J, et al. Pro-atherogenic cytokine profile of patients with suspected obstructive sleep apnea. Sleep Breath (2009) 13:391–5. doi: 10.1007/s11325-009-0259-1
111. Shamsuzzaman ASM, Winnicki M, Lanfranchi P, Wolk R, Kara T, Accurso V, et al. Elevated C-reactive protein in patients with obstructive sleep apnea. Circulation (2002) 105:2462–4. doi: 10.1161/01.CIR.0000018948.95175.03
112. Yokoe T, Minoguchi K, Matsuo H, Oda N, Minoguchi H, Yoshino G, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation (2003) 107:1129–34. doi: 10.1161/01.CIR.0000052627.99976.18
113. Samocha-Bonet D, Justo D, Rogowski O, Saar N, Abu-Abeid S, Shenkerman G, et al. Platelet counts and platelet activation markers in obese subjects. Mediators Inflamm (2008) 2008:834153–6. doi: 10.1155/2008/834153
114. Bokinsky G, Miller M, Ault K, Husband P, Mitchell J. Spontaneous platelet activation and aggregation during obstructive sleep apnea and its response to therapy with nasal continuous positive airway pressure. Chest (1995) 108:625–30. doi: 10.1378/chest.108.3.625
115. Bordeaux BC, Qayyum R, Yanek LR, Vaidya D, Becker LC, Faraday N, et al. Effect of obesity on platelet reactivity and response to low-dose aspirin. Prev Cardiol. (2010) 13:56–62. doi: 10.1111/j.1751-7141.2009.00058.x
116. Peker Y. Platelet activity and cardiovascular risk in obesity and obstructive sleep apnea: compelling need for interdisciplinary research? J Clin Sleep Med. (2011) 7:179–80.
117. Sawatari H, Chishaki A, Nishizaka M, Tokunou T, Adachi S, Yoshimura C, et al. Cumulative hypoxemia during sleep predicts vascular endothelial dysfunction in patients with sleep-disordered breathing. Am J Hypertens. (2016) 29:458–63. doi: 10.1093/ajh/hpv135
118. Stempien-Otero A, Karsan A, Cornejo CJ, Xiang H, Eunson T, Morrison RS, et al. Mechanisms of hypoxia-induced endothelial cell death. Role of p53 in apoptosis. J Biol Chem. (1999) 274:8039–45. doi: 10.1074/jbc.274.12.8039
119. Lee C-H, Sethi R, Li R, Ho H-H, Hein T, Jim M-H, et al. Obstructive sleep apnea and cardiovascular events after percutaneous coronary intervention. Circulation (2016) 133:2008–17. doi: 10.1161/CIRCULATIONAHA.115.019392
120. Jiang X-M, Qian X-S, Gao X-F, Ge Z, Tian N-L, Kan J, et al. Obstructive sleep apnea affecting platelet reactivity in patients undergoing percutaneous coronary intervention. Chin Med J. (2018) 131:1023–9. doi: 10.4103/0366-6999.230732
121. Joyeux-Faure M, Tamisier R, Baguet J-P, Dias-Domingos S, Perrig S, Leftheriotis G, et al. Response to statin therapy in obstructive sleep apnea syndrome: a multicenter randomized controlled trial. Mediators Inflamm. (2014) 2014:423120. doi: 10.1155/2014/423120
122. Artiles CF, Siqueiros A, Butler A, Cantu J, McNerney J, Petrini J, et al. Platelet response to aspirin in patients with moderate to severe obstructive sleep apnea before and after positive airway pressure [abstract]. In: American Thoracic Society 2013 International Conference. Philadelphia, PA: ATS 2013. (2013). p. A109.
Keywords: osa, blood platelets, cardiovascular diseases, hypoxia, sleep apnea
Citation: Gabryelska A, Łukasik ZM, Makowska JS and Białasiewicz P (2018) Obstructive Sleep Apnea: From Intermittent Hypoxia to Cardiovascular Complications via Blood Platelets. Front. Neurol. 9:635. doi: 10.3389/fneur.2018.00635
Received: 21 April 2018; Accepted: 16 July 2018;
Published: 03 August 2018.
Edited by:
Vsevolod Polotsky, Johns Hopkins University, United StatesReviewed by:
Isaac Almendros, University of Barcelona, SpainLeszek Kubin, University of Pennsylvania, United States
Luciano Drager, Instituto do Coração, Universidade de São Paulo, Brazil
Copyright © 2018 Gabryelska, Łukasik, Makowska and Białasiewicz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Agata Gabryelska, agata.gabryelska@gmail.com
 Agata Gabryelska
Agata Gabryelska Zuzanna M. Łukasik
Zuzanna M. Łukasik Joanna S. Makowska2
Joanna S. Makowska2