- 1Department of Radiation Oncology, Cancer Hospital, The First Affiliated Hospital of Xiamen University, Teaching Hospital of Fujian Medical University, Xiamen, China
- 2State Key Laboratory of Oncology in South China, Department of Radiation Oncology, Sun Yat-sen University Cancer Center, Collaborative Innovation Center of Cancer Medicine, Guangzhou, China
- 3Department of Oncology, Dongguan Third People's Hospital, Affiliated Dongguan Shilong People's Hospital of Southern Medical University, Dongguan, China
Introduction: We conducted a non-inferiority analysis using real-world data to compare the survival outcomes of stage T1-2N2-3 (tumor size ≤5 cm and four or more node metastases) breast cancer after breast-conserving surgery (BCS) and mastectomy (MAST).
Methods: The study included patients with stage T1-2N2-3 invasive breast carcinoma from the Surveillance, Epidemiology, and End Results program, who underwent BCS or MAST between 2004 and 2012, along with both radiotherapy and chemotherapy. The statistical analyses used included the chi-squared test, multivariate Cox proportional hazards models, and propensity score matching (PSM).
Results: The study population comprised 13,263 patients, including 4,787 (36.1%) and 8,476 (63.9%) patients who were treated with BCS and MAST, respectively. Patients with younger age and advanced stage were more likely to have received MAST. The probability of receiving MAST increased over the years, while the probability of BCS decreased (p < 0.001). The 5-year breast cancer-specific survival (BCSS) was 86.1% in the BCS cohort compared to 83.1% in the MAST cohort (p < 0.001). Surgical procedure was an independent prognostic factor for BCSS. Patients who received MAST had worse BCSS than those treated with BCS (hazard ratio = 1.179, 95% confidence interval = 1.087–1.278, p < 0.001). These results remained significant after stratification by age, tumor grade, T stage, N stage as well as marital status. Similar results were obtained after PSM.
Conclusions: BCS resulted in noninferior outcome than MAST in patients with T1-2/N2-3 invasive breast carcinoma. BCS may therefore be an optimal treatment option when both treatment options are feasible and appropriate.
Background
Breast cancer is a major health concern in women, as approximately 2 million new breast cancer patients are diagnosed worldwide annually (1). As a result of all the advances in diagnostic and screening techniques, most cases of breast cancer are diagnosed in the early stages. Despite this, 10% of patients are diagnosed with high-risk breast cancer (four or more node metastases) in developed countries (2, 3), and this percentage is as high as 15–20% in developing countries (4, 5). Lymph node status is the main prognostic factor that affects the outcome of breast cancer, and patients with four or more node metastases (N2-3) have significantly worse survival than those who have no node metastases (N0) or one to three node metastases (N1) (6).
With regard to N0 and N1 breast cancer, studies from prospective and large-scale retrospective studies have confirmed that breast-conserving surgery (BCS) has similar and even superior outcomes to mastectomy (MAST) (9–15). In contrast, the current standard treatment strategies for patients with stage N2-3 breast cancer are local surgery plus chemotherapy and postoperative adjuvant radiotherapy (RT), endocrine therapy for hormone receptor (HoR)-positive patients, and targeted therapy for human epidermal growth factor receptor-2 (HER2)-positive tumors (7, 8). The usefulness of BCS in patients with N2-3 breast cancer remains unclear, especially for those in the N3 class, which is often considered as a contraindication for BCS (16). This is probably because in patients with N2-3 disease, the risk of local recurrence and distant metastasis is significantly higher than that in patients with N0 and N1 disease (6). No prospective studies or randomized controlled trials so far have compared the survival outcome of surgical treatment of N2-3 breast cancer. Further, although several retrospective studies have shown that BCS has superior outcomes to MAST in N2-3 breast cancer, the adjuvant treatment provided, including chemotherapy and RT, was found to be insufficient in most cases, which might have affected the evaluation of the results (2, 14, 17, 18). There is clearly a need to analyze these findings in a population of N2-3 breast cancer patients who underwent BCS and also received appropriate adjuvant treatment.
The present study sought to contribute to the knowledge about the efficiency of BCS for N2-3 breast cancer by conducting a non-inferiority analysis using real-world data to assess the long-term effects of BCS in patients with T1-2N2-3 (tumor size ≤5 cm and four or more node metastases) breast cancer who underwent BCT or MAST, along with chemotherapy and RT.
Materials and Methods
Study Population
In this study, patients with pathologically diagnosed T1-2N2-3M0 invasive breast cancer treated with BCS+postoperative beam RT+chemotherapy or MAST+postoperative beam RT+chemotherapy between 2004 and 2012 were included from the population-based Surveillance, Epidemiology, and End Results (SEER) program (19). The SEER program is headed by the National Cancer Institute, which maintains a database for de-identified cancer incidence, demographic and clinicopathological variables, first course of treatment as well as survival, through 18 cancer registries across the United States. Patients with insufficient data on race/ethnicity, tumor grade, HoR status, and marital status were excluded. As the SEER registries contain de-identified information about patients, this study was exempt from the approval process of the Institutional Review Board.
Procedures
Data on demographic, clinicopathological, and treatment-related variables as well as vital status were obtained from the SEER program. The variables included age (<50 years, ≥50 years), race/ethnicity (non-Hispanic white, Non-Hispanic clack, Hispanic, other), tumor grade (well differentiated, moderately differentiated, poorly differentiated/undifferentiated), T stage (T1, T2), N status (N2, N3), HoR status (estrogen receptor [ER]+ and progesterone receptor [PR]+, ER+, or PR+, ER- and PR-), marital status (unmarried, married), and surgical procedure (BCS, MAST). Data on HER2 status were not routinely registered before 2010, so HER2 status was not included in this study. The primary outcome of this study was breast cancer-specific survival (BCSS).
Statistical Analysis
The difference in categorical variables between the two treatment arms (MAST and BCS) were compared using the chi-squared test. A propensity score matching (PSM) method was used to reduce the potential confounding factors and selection bias between each group (20, 21). A 1:1 PSM cohort was created using the above 7 including variables, which could potentially be confounding factors. Long-term BCSS was assessed and compared by the Kaplan–Meier method and log-rank test. The variables that could influence BCSS were analyzed using the multivariate Cox proportional hazard model, and the risk for BCSS was assessed by calculating the hazard ratio (HR) and the corresponding 95% confidence interval (CI). Statistical analyses were conducted using IBM SPSS version 22.0 (IBM Corp., Armonk, NY), and a p < 0.05 was considered to indicate statistical significance.
Results
Demographic and Clinical Data
We included 13,263 patients who met the inclusion criteria (median age = 54 years). The patient characteristics are listed in Table 1. Two-thirds of the patients were aged ≥50 years (65.2%, n = 8,652), and the group comprised 8,936 (67.4%) non-Hispanic white patients, 9,075 (68.4%) patients with T2 stage cancer, 9,202 (69.4%) patients with N2 stage cancer, 8,161 (61.5%) ER+ and PR+ patients, and 8,316 (62.7%) married patients.
Treatment Data
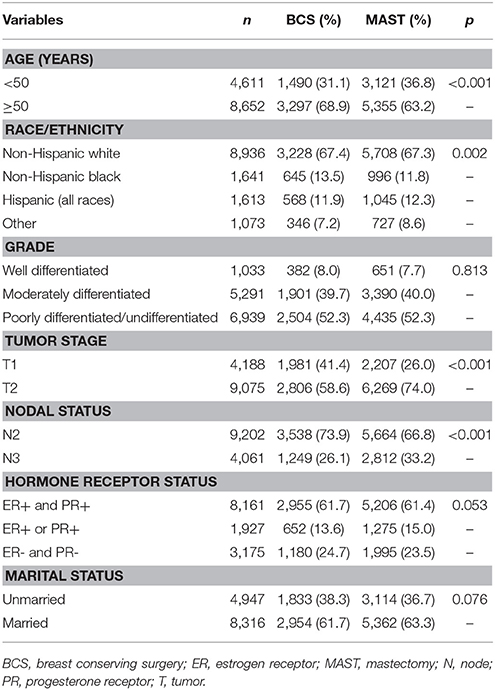
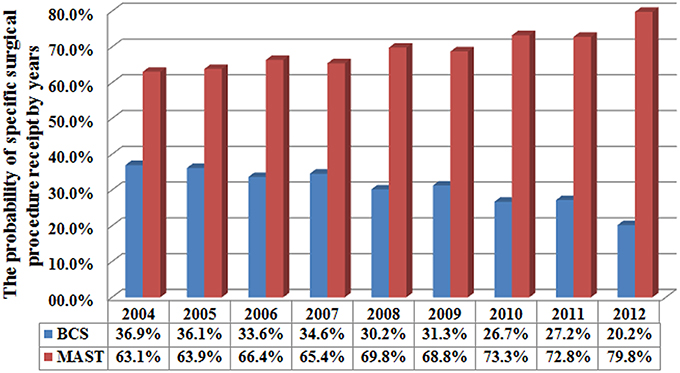
A total of 4,787 (36.1%) and 8,476 (63.9%) patients were treated with BCS and MAST, respectively. Patients with younger age, T2, and N3 stage disease were more likely to have undergone MAST. Figure 1 lists the temporal trends in surgical procedure from 2004 to 2012: notably, the probability of undergoing MAST increased over the years, while the probability of BCS decreased over the years (p < 0.001). Data about specific treatment methods for 8,036 patients who underwent MAST were available: 5,322 (66.2%), 950 (11.8%), and 1,764 (22.0%) patients underwent MAST without removal of the contralateral breast, MAST along with removal of the contralateral breast, and MAST along with tissue and/or implant reconstruction, respectively. Figure 2 lists the temporal trends in the specific surgical procedures used for MAST from 2004 to 2012: notably, the probability of MAST without removal of the contralateral breast significantly decreased over the years, while the probability of MAST along with removal of the contralateral breast and reconstruction significantly increased over the years (p < 0.001).
Treatment Outcomes
A total of 2,728 breast cancer-related deaths were observed during a median follow-up duration of 71 months (range, 3–143 months). The 5-year and 10-year BCSS was 84.2 and 72.0%, respectively. Kaplan-Meier analysis and the log-rank test showed significantly higher crude 5-year BCSS for BCS than for MAST (86.1 vs. 83.1%, p < 0.001) (Figure 3A).
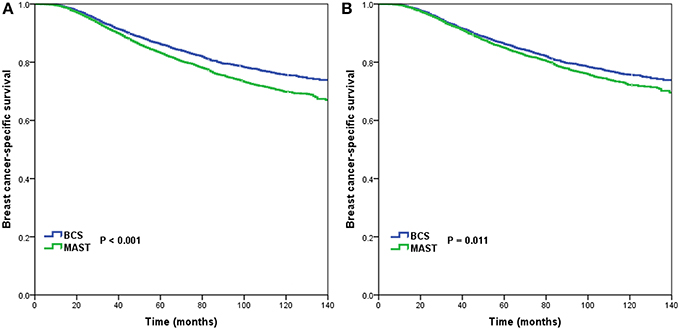
Figure 3. Breast cancer-specific survival after breast-conserving surgery and mastectomy before (A) and after (B) propensity score matching.
Multivariate Analysis Findings Before PSM
After the data were adjusted for age, race/ethnicity, grade, T stage, N stage, and HoR status as well as marital status, surgical procedure was found to be an independent prognostic factor associated with BCSS. Further, patients who received MAST had a worse BCSS than those treated with BCS (HR = 1.179, 95% CI = 1.087–1.278, p < 0.001). Multivariate analysis also showed that race/ethnicity, tumor grade, T stage, N stage, HoR status and marital status were independently associated with BCSS (Table 2). These results remained significant after stratification by age, tumor grade, T stage, N stage as well as marital status. After stratification for race/ethnicity, MAST showed worse BCSS than BCS in non-Hispanic white patients, but it showed similar BCSS to BCS in non-Hispanic black, Hispanic (all races), and other races/ethnicities. Moreover, for ER+ and PR+ tumors, MAST had a worse BCSS than BCS, but no significant difference was found between MAST and BCS in ER+ or PR+ as well as ER- and PR- patients (Figure 4).

Figure 4. Hazard ratios for breast-conserving surgery compared to mastectomy with regard to breast cancer-specific survival in relation to predefined prognostic factors.
PSM Findings
Using PSM, 4,518 completely matched pairs were obtained. Kaplan-Meier analysis and the log-rank test showed a significantly higher crude BCSS for BCS than for MAST (75.7% vs. 72.2%, p = 0.011) (Figure 3B). After the data were adjusted for age, race/ethnicity, grade, T stage, N stage, HoR status as well as marital status, surgical procedure was found to be an independent prognostic factor related to BCSS. Further, patients who had undergone MAST had a worse BCSS than those who had undergone BCS (HR = 1.124, 95% CI = 1.023–1.236, p = 0.015) (Table 2).
Discussion
In this study, we used a population-based analysis to assess the treatment outcome of BCS and MAST in patients with stage T1-2N2-3 breast cancer. Our results indicate that patients who underwent BCS had a better BCSS than those who underwent MAST before and after PSM was applied.
As expected, in the present study, most breast cancer patients with N2-3 disease were treated with MAST, similar to the findings of previous studies (2, 14, 17, 18). In addition, the rate at which MAST is performed gradually increased over the years—from 63.1% in 2004 to 79.8% in 2012; the increase can be mainly attributed to contralateral and bilateral mastectomy and breast reconstruction. Our results were similar to those of a study by the National Cancer Data Base (22). Although postoperative RT has a potential impact on breast reconstruction (23–25), in the present study, the number of patients who underwent reconstruction increased from 16.2% in 2004 to 28.2% in 2012. A prior SEER study also showed an increasing trend in breast reconstruction in patients who are suitable for postoperative RT (26). In China, although BCS and breast reconstructive surgery for N2-3 stage cancer have been increasing, only 5.4 and 1.9% of patients receive BCS and breast reconstruction, respectively (4). Fear of tumor recurrence may be an important factor that influences the preference for MAST, as it is perceived to reduce the need for repetitive surgery (27).
A population-based study from the Netherlands found better survival outcomes with BCS than with MAST in T1-2N2 patients diagnosed in 1999–2005, but such superior survival outcomes were not found in a 2006–2012 cohort of the same study (14). However, in their study, 16.2 and 20.8% of patients in the BCS and MAST group did not receive adjuvant systemic therapy, respectively, and 15% of the patients were not treated with postoperative RT (14). Another study from Canada found that the 5-year BCSS was 72.9 and 89.8% in patients with stage III disease who received MAST and BCS, respectively (2). However, nearly 50% of the patients did not receive adjuvant chemotherapy, and only one-third of the MAST patients were treated with adjuvant RT (2). Insufficient adjuvant treatment was also an observation in another Dutch study of the Netherlands Cancer Registry, which showed better overall survival for BCS than for MAST in patients with T2N2 disease, but not in patients with T1N2 disease (14). To ascertain the efficacy of BCS in patients with T1-2N2-3 breast cancer, our study assessed the non-inferiority of survival outcomes of BCS+chemotherapy+RT compared to MAST+chemotherapy+RT in a national cancer registry. The findings of our study also show that patients who received BCS had better BCSS than those who underwent MAST, but in contrast to all the previous studies, the patients included in our study received adjuvant treatment in accordance with the current clinical chemotherapy and radiotherapy practices. Our study therefore makes a valuable contribution to the current clinical practice of surgical treatment for N2-3 breast cancer patients.
Our subgroup analysis suggested that the BCSS was better with BCS than with MAST, regardless of age, grade, T stage, N stage as well as marital status. N3 stage disease is often considered a contraindication for BCS (16). However, we found that even in patients who have a high risk of locoregional and distant recurrence, BCSS is significantly better with BCS than with MAST. In addition, we found that ER+ or PR+ patients as well as ER- and PR- patients had poorer BCSS than ER+ and PR+ patients. Further, in ER+ or PR+ and ER- and PR- patients, BCSS was similar in the BCS and MAST groups. A prior study also showed that single HoR-positive breast cancer had similar outcomes to triple-negative breast cancer (28). These findings indicate that contemporary chemotherapy regimens may not maintain the survival advantage of BCS over MAST in these subgroups.
It is not entirely clear why BCS had a better outcome than MAST in the current cohort, but here are a few possible reasons. First, patients treated with BCS may have better self-image and sense of sexuality, which was related to better psycho-social wellbeing, better psychological health, and a higher level of satisfaction with life than those treated with MAST (29, 30). A prior study has indicated that a better quality of life was associated with better outcome after breast cancer treatment (31). This might explain why although the patient-reported cosmetic satisfaction is similar between BCS and breast reconstruction after MAST, the breast reconstruction cohort has a worse patient-reported outcome when postoperative RT is administered (23–25). Second, patients who receive BCS are more likely to be treated by experienced surgeons with academic affiliations (32), and the decision to perform BCS and associated surgical procedures may be run through a decision board group (33). Yet another reason could be that patients who receive BCS have better treatment compliance as a result of better health, and they may also have greater access to post-treatment medical surveillance. Finally, MAST is a more extensive and aggressive surgical method that may result in more adverse effects, including a more pronounced inflammatory response and tissue damage, which could negatively affect the immune system and promote residual tumor cell growth (34, 35).
The primary strengths of our study are its population-based characteristics including the large cohort of patients and stratification for demographic and clinicopathological characteristics. However, we need to acknowledge several limitations of this study too. First, our study was a retrospective study, which means that several confounding factors and potential selection biases cannot be ruled out, even though we used propensity score analysis. However, since there are no randomized controlled trials that compare the outcomes of BCT and MAST in patients with high-risk breast cancer, our study reported the outcome from real-world data. We believe that the findings are important in light of daily treatment practices for breast cancer surgery. Second, the SEER dataset does not include detailed information regarding the chemotherapy regimen, endocrine therapy, anti-HER2 targeted therapies, or sequential surgery and chemotherapy data, which may limit the generalizability of our findings. Third, the details of RT regarding RT technique, target volume, RT dose, and tumor bed boost between the two groups were also lacking in the SEER database. Finally, the patterns of disease recurrence and treatment history after tumor recurrence were not captured in the SEER program.
Conclusion
In conclusion, the findings of our study indicate that patients with T1-2N2-3 invasive breast cancer who undergo BCS have a noninferior outcome than those who receive MAST. Given that MAST is a more invasive surgical procedure that has more sequelae than BCS, we believe that BCS may be an optimal treatment option when both treatment options are feasible and appropriate.
Ethics Statement
The study was exempt from the approval processes of the Institutional Review Boards because the SEER database patient information is de-identified.
Author Contributions
S-GW aided in data collection. JW, J-PD, YD, J-YS, and W-WZ are authors who participated in manuscript drafting, table/figure creation, and manuscript revision. S-GW and Z-YH are the corresponding authors who initially developed the concept and drafted and revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was partly supported by the Natural Science Foundation of China (No. 81872459, 81803050), Natural Science Foundation of Fujian Province (No. 2016J01635), the Social Science and Technology Development Major Project of Dongguan (No. 2018507150241630), and the Science and Technology Planning Projects of Xiamen Science and Technology Bureau (No. 3502Z20174070).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
2. Fisher S, Gao H, Yasui Y, Dabbs K, Winget M. Survival in stage I-III breast cancer patients by surgical treatment in a publicly funded health care system. Ann Oncol. (2015) 26:1161–9. doi: 10.1093/annonc/mdv107
3. van Maaren MC, de Munck L, Strobbe LJA, Sonke GS, Westenend PJ, Smidt ML, et al. Ten-year recurrence rates for breast cancer subtypes in the Netherlands: a large population-based study. Int J Cancer. (2019) 144:263–72. doi: 10.1002/ijc.31914
4. Huang NS, Liu MY, Chen JJ, Yang BL, Xue JY, Quan CL, et al. Surgical management of breast cancer in China: a 15-year single-center retrospective study of 18,502 patients. Medicine. (2016) 95:e4201. doi: 10.1097/MD.0000000000004201
5. Peng Z, Wei J, Lu X, Zheng H, Zhong X, Gao W, et al. Treatment and survival patterns of Chinese patients diagnosed with breast cancer between 2005 and 2009 in Southwest China: an observational, population-based cohort study. Medicine. (2016) 95:e3865. doi: 10.1097/MD.0000000000003865
6. Wu SG, Wang Y, Zhou J, Sun JY, Li FY, Lin HX, et al. Number of negative lymph nodes should be considered for incorporation into staging for breast cancer. Am J Cancer Res. (2015) 5:844–53.
7. NCCN. NCCN Clinical Practice Guidelines in Oncology V.2.2018. Breast Cancer. (2018). Available online at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (Accessed Oct 21, 2018).
8. Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2015) 26 (Suppl. 5):v8–30. doi: 10.1093/annonc/mdv298
9. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. (2002) 347:1233–41. doi: 10.1056/NEJMoa022152
10. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. (2002) 347:1227–32. doi: 10.1056/NEJMoa020989
11. Litière S, Werutsky G, Fentiman IS, Rutgers E, Christiaens MR, Van Limbergen E, et al. Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol. (2012) 13:412–9. doi: 10.1016/S1470-2045(12)70042-6
12. van Maaren MC, de Munck L, de Bock GH, Jobsen JJ, van Dalen T, Linn SC, et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol. (2016) 17:1158–70. doi: 10.1016/S1470-2045(16)30067-5
13. de Boniface J, Frisell J, Bergkvist L, Andersson Y. Breast-conserving surgery followed by whole-breast irradiation offers survival benefits over mastectomy without irradiation. Br J Surg. (2018) 105:1607–14. doi: 10.1002/bjs.10889
14. Lagendijk M, van Maaren MC, Saadatmand S, Strobbe LJA, Poortmans PMP, Koppert LB, et al. Breast conserving therapy and mastectomy revisited: breast cancer-specific survival and the influence of prognostic factors in 129,692 patients. Int J Cancer. (2018) 142:165–75. doi: 10.1002/ijc.31034
15. Thöne K, Rudolph A, Obi N, Chang-Claude J, Flesch-Janys D. Prognostic impact of surgery for early-stage invasive breast cancer on breast cancer-specific survival, overall survival, and recurrence risk: a population-based analysis. Breast Cancer Res Treat. (2018) 170:381–90. doi: 10.1007/s10549-018-4754-6
16. NABON. Dutch guideline breast cancer (Landelijke richtlijn mammacarcinoom) (2016). Available online at: http://www.oncoline.nl/mamma carcinoom (Accessed May 19, 2016).
17. van Maaren MC, de Munck L, Jobsen JJ, Poortmans P, de Bock GH, Siesling S, et al. Breast-conserving therapy versus mastectomy in T1–2N2 stage breast cancer: a population-based study on 10-year overall, relative, and distant metastasis-free survival in 3071 patients. Breast Cancer Res Treat. (2016) 160:511–21. doi: 10.1007/s10549-016-4012-8
18. Chen K, Liu J, Zhu L, Su F, Song E, Jacobs LK. Comparative effectiveness study of breast-conserving surgery and mastectomy in the general population: a NCDB analysis. Oncotarget. (2015) 6:40127–40. doi: 10.18632/oncotarget.5394
19. Surveillance, Epidemiology and End Results Program Database: Incidence - SEER 18 Regs Custom Data (with additional treatment fields), Nov 2017 Sub (1973–2015 varying) - Linked To County Attributes - Total U.S., 1969–2016 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2018, based on the November 2017 submission.
20. Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. (1985) 39:33–8.
21. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. multivariate. Behav Res. (2011) 46:399–424. doi: 10.1080/00273171.2011.568786
22. Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA. Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg. (2015) 150:9–16. doi: 10.1001/jamasurg.2014.2895
23. Tsoi B, Ziolkowski NI, Thoma A, Campbell K, O'Reilly D, Goeree R. Safety of tissue expander/implant versus autologous abdominal tissue breast reconstruction in postmastectomy breast cancer patients: a systematic review and meta-analysis. Plast Reconstr Surg. (2014) 133:234–49. doi: 10.1097/01.prs.0000436847.94408.11
24. El-Sabawi B, Sosin M, Carey JN, Nahabedian MY, Patel KM. Breast reconstruction and adjuvant therapy: a systematic review of surgical outcomes. J Surg Oncol. (2015) 112:458–64. doi: 10.1002/jso.24028
25. Barry M, Kell MR. Radiotherapy and breast reconstruction: a meta-analysis. Breast Cancer Res Treat. (2011) 127:15–22. doi: 10.1007/s10549-011-1401-x
26. Frasier LL, Holden S, Holden T, Schumacher JR, Leverson G, Anderson B, et al. Temporal trends in postmastectomy radiation therapy and breast reconstruction associated with changes in national comprehensive cancer network guidelines. JAMA Oncol. (2016) 2:95–101. doi: 10.1001/jamaoncol.2015.3717
27. Cao JQ, Olson RA, Tyldesley SK. Comparison of recurrence and survival rates after breast-conserving therapy and mastectomy in young women with breast cancer. Curr Oncol. (2013) 20:e593–601. doi: 10.3747/co.20.1543
28. Bae SY, Kim S, Lee JH, Lee HC, Lee SK, Kil WH, et al. Poor prognosis of single hormone receptor- positive breast cancer: similar outcome as triple-negative breast cancer. BMC Cancer. (2015) 15:138. doi: 10.1186/s12885-015-1121-4
29. Cipora E, Konieczny M, Karwat ID, Roczniak W, Babuśka-Roczniak M. Surgical method of treatment and level of satisfaction with life among women diagnosed with breast cancer, according to time elapsed since performance of surgery. Ann Agric Environ Med. (2018) 25:453–9. doi: 10.26444/aaem/91586
30. Klassen AF, Pusic AL, Scott A, Klok J, Cano SJ. Satisfaction and quality of life in women who undergo breast surgery: a qualitative study. BMC Womens Health. (2009) 9:11. doi: 10.1186/1472-6874-9-11
31. De Aguiar SS, Bergmann A, Mattos IE. Quality of life as a predictor of overall survival after breast cancer treatment. Qual Life Res. (2014) 23:627–37. doi: 10.1007/s11136-013-0476-8
32. Gu J, Groot G, Boden C, Busch A, Holtslander L, Lim H. Review of factors influencing women's choice of mastectomy versus breast conserving therapy in early stage breast cancer: a systematic review. Clin Breast Cancer. (2018) 18:e539–54. doi: 10.1016/j.clbc.2017.12.013
33. Whelan T, Levine M, Gafni A, Sanders K, Willan A, Mirsky D, et al. Mastectomy or lumpectomy? Helping women make informed choices. J Clin Oncol. (1999) 17:1727–35. doi: 10.1200/JCO.1999.17.6.1727
34. Jawa RS, Anillo S, Huntoon K, Baumann H, Kulaylat M. Interleukin-6 in surgery, trauma, and critical care part II: clinical implications. J Intensive Care Med. (2011) 26:73–87. doi: 10.1177/0885066610384188
Keywords: invasive breast cancer, breast conserving treatment, mastectomy, radiotherapy, node positive
Citation: Wang J, Deng J-P, Sun J-Y, Dong Y, Zhang W-W, He Z-Y and Wu S-G (2019) Noninferior Outcome After Breast-Conserving Treatment Compared to Mastectomy in Breast Cancer Patients With Four or More Positive Lymph Nodes. Front. Oncol. 9:143. doi: 10.3389/fonc.2019.00143
Received: 28 December 2018; Accepted: 19 February 2019;
Published: 12 March 2019.
Edited by:
José Bines, Brazilian National Cancer Institute (INCA), BrazilReviewed by:
Alexandra Resch, Medical University of Vienna, AustriaTamara Louise Kalir, Icahn School of Medicine at Mount Sinai, United States
Copyright © 2019 Wang, Deng, Sun, Dong, Zhang, He and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen-Yu He, hezhy@sysucc.org.cn
San-Gang Wu, unowu12345@hotmail.com
†These authors have contributed equally to this work
 Jun Wang
Jun Wang Jia-Peng Deng2†
Jia-Peng Deng2† Wen-Wen Zhang
Wen-Wen Zhang Zhen-Yu He
Zhen-Yu He San-Gang Wu
San-Gang Wu