Corrigendum: Salivary Gland Carcinoma: Novel Targets to Overcome Treatment Resistance in Advanced Disease
- 1Department of Medical Oncology, Hospital Israelita Albert Einstein, São Paulo, Brazil
- 2Department of Head and Neck Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
Salivary gland carcinomas (SGC) account for less than 5% of head and neck malignant neoplasms, further subcategorized in over 20 histological subtypes. For the most part, treatment for advanced disease is guided by morphology. SGC in general respond poorly to standard chemotherapy, with short durability and significant toxicity. More recently, next-generation sequencing provided significant input on the molecular characterization of each SGC subtype, not only improving diagnostic differentiation between morphologically similar tumor types, but also identifying novel driver pathways that determine tumor biology and may be amenable to targeted therapy. Amongst the most common histological subtype is adenoid cystic carcinoma, which often harbors a chromosome translocation resulting in a MYB-NFIB oncogene, with various degrees of Myb expression. In a smaller subset, NOTCH1 mutations occur, conferring a more aggressive disease and potential sensitivity to Notch inhibitors. Salivary duct carcinomas may overexpress Her-2 and androgen receptor, with promising clinical outcomes after exposure to targeted therapies approved for other indications. Secretory carcinoma, previously known as mammary analogue secretory carcinoma, is distinguished by an ETV6-NTRK3 fusion that can both help differentiate it from its morphologically similar acinar cell carcinoma and also make it susceptible to Trk inhibitors. In the present article, we discuss the molecular abnormalities, their impact on tumor biology, and therapeutic opportunities for the most common SGC subtypes and review published and ongoing clinical trials and future perspectives for this rare diseases.
Introduction
Salivary gland carcinoma (SGC) is a rare tumor and represents ~6% of head and neck cancers (1). Malignant tumors of the salivary glands constitute a heterogeneous group of neoplasms that vary depending on the histology and their anatomical location. According to the 2017 WHO Classification, there are 24 malignant histological subtypes (2). The most prevalent are mucoepidermoid carcinoma (MEC), representing around a third of SGC cases, followed by adenoid cystic carcinoma (ACC), with 23.8% (3). The parotid gland is the most frequent site of salivary gland tumors, although only 25% of such lesions are malignant. SGC can also originate in the submandibular glands (40–45% of the tumors are malignant), sublingual glands (70–90% are malignant), and minor salivary glands (50% are malignant) (4).
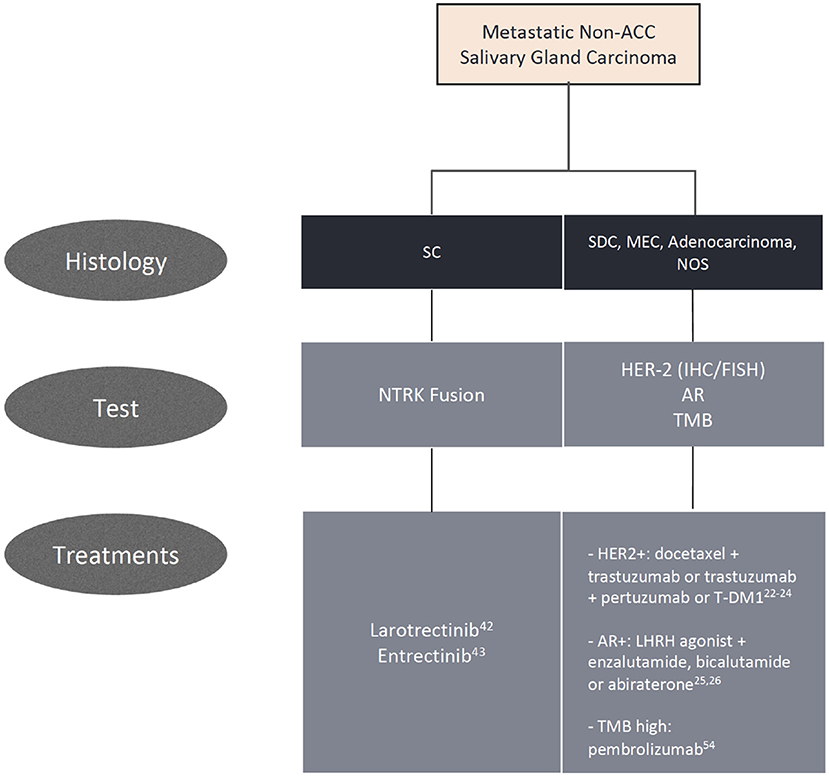
Treatment for metastatic disease is still mostly based on chemotherapy, despite low response, and survival rates (5). Currently, encouraging progress in immunohistochemical and molecular alterations, such as the presence of an NTRK fusion, overexpression of Her-2 and androgen receptor, has been made to improve outcomes with targeted therapy.
The aim of this article is to review the main molecular and immunohistochemical characteristics of the most common histological subtypes of SGC, in addition to reviewing current data on biomarker-driven targeted therapy and genomic findings that may be potentially actionable in the future.
Mucoepidermoid Carcinoma
MEC is the most common SGC (6). In addition to clinical staging, the grade of the tumor is also a prognostic factor in MEC and may guide treatment decision (7). Despite its prevalence, it is one of the subtypes with the least breakthroughs achieved so far.
A unique t (8, 9) translocation, leading to the CRTC1/MAML2 fusion, is present in 56–82% of all MECs (10, 11). This fusion protein induces aberrant activation of the Notch signaling pathway, inducing cell proliferation and, therefore, tumor progression (12). Data on how this abnormality impacts tumor biology and prognosis are conflicting. While some series indicate that fusion-positive MECs were diagnosed at an earlier stage, with lower grade, and a better prognosis (8, 12, 13), other studies do not suggest a prognostic role for the translocation (10, 14). CRTC1-MAML2-positive cells were sensitive to epidermal growth factor receptor (EGFR) tyrosine kinase inhibition pre-clinically, suggesting a potential role for such drugs (15).
The most common genomic abnormalities described in a study of 48 MEC patients were as follows: CDKN2A (41.6%), TP53 (39.6%), CDKN2B (29.2%), BAP1 (20.8%), PIK3CA (20.8%), HRAS (10.4%), BRCA (10.5%) mutations, and ERBB2 amplifications (8.3%) (16). The latter, though infrequent, may be amenable to Her-2 targeted therapy (17). TP53 mutation is one of the most common genomic alterations in MEC and is associated with the transformation of low-grade into high-grade tumors (12). In high-grade MEC, EGFR is overexpressed in 72.7% and was associated with a more aggressive behavior (18).
Salivary Duct Carcinoma
Salivary duct carcinoma (SDC) is an aggressive subtype of SGC that microscopically resembles high-grade ductal carcinoma of the breast. They can develop as de novo disease or arise from a pleomorphic adenoma (carcinoma ex-pleomorphic adenoma) (19). The first line of treatment is currently based on platinum chemotherapy, with low response rates and of short duration (9). Biomarkers of interest have been found within this subtype, showing promising results with targeted therapy.
Androgen receptor (AR) and Her-2 receptors are frequently overexpressed in SDC. In a study of 177 patients with SDC, AR was expressed in 96% of cases (20). Her-2 overexpression can be found in one third to two thirds of cases, by immunohistochemistry and/or fluorescent in situ hybridization (FISH) (20, 21). These markers were not associated with disease biology and prognosis.
As in breast cancer, patients with SDC, and Her-2 overexpression derive benefit from anti-Her-2 therapy. In a phase II study, 57 patients with advanced SDC received docetaxel and trastuzumab, with an objective response rate (ORR) of 70.2%. The median progression-free survival (PFS) was 8.9 months and overall survival (OS) was 39.7 months (22).
The use of double Her-2 blockade with trastuzumab and pertuzumab was also evaluated in a basket study, which included five patients with advanced, refractory SDC, all with Her-2 amplification/overexpression. Trastuzumab and pertuzumab, without chemotherapy, yielded a partial response in four out of five patients with Her-2-positive SDC (ORR of 80%) (23).
Ado-trastuzumab emtansine (T-DM1) was also studied in another basket trial, where 10 patients with a median of two previous systemic treatments and HER-2 amplification by next-generation sequencing (NGS) had an ORR of 90%, half of which were complete metabolic responses. Median duration of response and PFS had not been reached with a median follow-up of 12 months (24). In this same study, the amplification of HER-2 by NGS correlated well with HER2/CEP17 ≥2 by FISH or IHC 3+ (24).
Treatment with androgen deprivation therapy (ADT) has been proposed after progression to platinum-based chemotherapy when AR is present. In a phase II study, 36 patients with metastatic or locally advanced unresectable SGC, being 34 SDCs, received combined androgen blockade with the luteinizing hormone-releasing hormone (LHRH) analog leuprorelin associated with bicalutamide, with an ORR of 41.7%. The median PFS was 8.8 months and median OS was 30.5 months. The treatment was well-tolerated, with a low rate of toxicity (25). ADT was also studied in the adjuvant setting in a retrospective study. Stage IVA/B, AR-positive SDC patients who underwent a complete tumor resection received bicalutamide, an LHRH analog or a combination of both. The treatment was associated with a statistically significant increase in the 3-year disease-free survival when compared to a control group (48.2 vs. 27.7%) (26). A randomized phase II study comparing the efficacy and safety of ADT with platinum-based chemotherapy as first-line therapy for patients with metastatic SDC and AR expression is ongoing (NCT01969578).
Enzalutamide, a more selective AR inhibitor, was given as monotherapy to patients with AR-positive SGC in a phase II trial (27). The majority (85%) of patients had SDC and 32.6% had prior AR-directed therapy. This study showed that 7 out of 46 patients (15%) had a partial response as best response, but only 4% (2/46) maintained the response until 8 weeks, thus failing to meet its primary endpoint. Therefore, we favor the administration of an antiandrogen agent in combination with an LHRH analog.
The experience of patients with prostate cancer can again be used in patients with SDC. Mechanisms of AR blockade resistance have been discovered in castration-resistant prostate cancer patients. The AR isoform splice variant 7 (AR-V7) results in a truncated receptor that lacks the binding site for androgen, activated even in the absence of ligands and stimulating tumor growth. Detection of AR-V7 in circulating tumor cells from patients with castrate-resistant metastatic prostate cancer was associated with worse PFS and OS in patients who received abiraterone or enzalutamide (28). In salivary duct carcinomas, the prevalence of AR-V7 is high, varying between 48 and 70% (29, 30). Interestingly, it is frequently detected in treatment-naive patients, as opposed to a mechanism of resistance to ADT as in prostate cancer. Therefore, its role in ADT sensitivity in SDC patients remains to be established, warranting further biomarker analysis in future trials. One case report of a patient with AR-positive SDC who expressed AR-V7 did not show response to second-line hormonal therapy with abiraterone (31).
Other potentially targetable pathways found in 28 SDC patients include TP53 (68%), HRAS (25%), PIK3CA (18%), NF1 (18%), PTEN (10%), BRAF (7%), and NOTCH1 (7%). In the same study, patients did not have common predictive biomarkers of response to immunotherapy: 82% were PD-L1 negative, 91% had a low tumor mutational burden, and no patients presented microsatellite instability (29). Tipifarnib, a potent inhibitor of farnesyltransferase, an enzyme required for downstream signaling in HRAS-mutated tumors, was evaluated in 12 patients with SGC, with 4 being SDC, none of whom achieved a response. A single patient with acinic cell carcinoma had a partial response lasting at least 14 months (32).
Secretory Carcinoma
Secretory carcinoma (SC), formerly known as mammary analog secretory carcinoma (MASC), was first described by Skálóva et al. a decade ago (33). It shows morphological, genetic, and immunohistochemical similarities with breast secretory carcinoma (34). One of the main differential diagnoses is acinic cell carcinoma (AcCC), which typically contains a basophilic cytoplasm with periodic acid-Schiff-positive zymogen granules and a more diverse cytologic profile compared to SC (35). SC has several architectural patterns (microcystic, solid, tubular, and cribriform), an abundant and eosinophilic cytoplasm, uniform proliferation and positivity for vimetin, mammaglobin, and S-100 protein in immunohistochemistry (36). The presence of a chromosomal translocation, t(12, 15), between the ETV6 gene on chromosome 12 with NTRK3 on chromosome 15, generates the fusion product ETV6–NTRK3. It can be detected with a high specificity by reverse-transcriptase polymerase chain reaction (RT-PCR), NGS, or FISH, being the gold standard methods for the diagnosis of this subtype (33, 34). Nuclear pattern of pan-Trk immunohistochemistry staining has a good sensitivity to detect an ETV6–NTRK3 fusion, thus aiding in differentiating SC from AcCC. However, it may miss other less frequent ETV6-X fusions, only detected by FISH or RT-PCR (37).
SC is more commonly found in men (55%), with a mean age of 44 years and mostly arising in the parotid gland, followed by several head and neck locations, including the oral cavity, submandibular glands, soft palate, buccal mucosa, base of tongue, and lips (38). It usually presents with an indolent clinical course and a good prognosis (39). Though a few cases of SC with high-grade histology and aggressive behavior have been described in association with ETV6-MET and ETV6-RET fusions, it has not yet been possible to correlate these recently described fusions with an overall behavioral pattern and disease prognosis (40, 41).
An NTRK fusion provides an actionable target for this disease by the Trk inhibitors larotrectinib and entrectinib. The benefit of larotrectinib was demonstrated by a phase II study including 12 cases of SC, with an objective response in 10 cases and an ORR of 80% by investigator's assessment (42). Entrectinib's activity was demonstrated by an integrated analysis of three phase I and II clinical trials (ALKA-372-001, STARTRK-1, and STARTRK-2), with the presence of seven (13%) cases of SC, which demonstrated an objective response in six of the seven cases (86%) (43). Both drugs received a tissue-agnostic FDA approval for tumors harboring an NTRK fusion.
Mechanisms of acquired resistance to larotrectinib have been described with an on-target mutation in the drug-binding site (42, 44). Selitrectinib (LOXO-195), a second-generation Trk inhibitor, was designed to overcome the acquired resistance to the first-line treatment. A phase I/II trial is ongoing (NCT03215511) and has evaluated 29 patients so far, with an ORR of 34% (45).
Adenocarcinoma, Not Otherwise Specified
Adenocarcinoma, not otherwise specified (NOS), presents as a particularly difficult diagnosis to establish. It is characterized by the presence of areas of glandular or ductal differentiation mixed with a variety of specific growth patterns (46). Therefore, it is an exclusion diagnosis. The literature is controversial regarding its incidence among SGCs, ranging from 5 to 25% (3, 47). They are highly malignant tumors, with an overall 15-year survival rate of 3%, associated with early development of distant metastases and limited treatment options (48). Since this entity can share some characteristics of other SGCs, it is important to test for actionable biomarkers, such as AR and HER-2. Despite at a lower prevalence, they may be present and predict responses to targeted therapy (26, 49).
Immunotherapy in Non-Adenoid Cystic Carcinoma
SGCs seem particularly resistant to immune checkpoint inhibitors. However, they represent a rather heterogeneous group of diseases that may behave differently in regard to the immune system. Linxweiler et al. demonstrated a distinct behavioral pattern in the different subtypes of SGCs. SDC exhibited higher levels of immune infiltration, T-cell dysfunction, and higher mutational load, whereas ACC presented with an overall lower mutational burden and an immune-excluded environment (50). PD-L1 expression was found to be associated with inferior disease-free survival (51).
Clinically, the KEYNOTE-028 study, a phase Ib basket trial, treated 26 patients with PD-L1-positive SGC with pembrolizumab at 10 mg/kg every 2 weeks. The low rate of PD-L1 positivity (<30%) limited patient accrual in the screening phase. Patients had adenocarcinoma, NOS (38%), mucoepidermoid (12%), undifferentiated (8%), squamous cell (8%), and ACC (8%). Despite being a PD-L1-enriched cohort, the results were overall disappointing, with an ORR of 12%. There were only three partial responses (two in adenocarcinoma, NOS and one in a high-grade serous carcinoma). The median PFS was 4 months (95% CI: 2 to 5 months) and median OS was 13 months (95% CI: 6 months to not reached) (52).
Another programmed-death 1 (PD-1) inhibitor is being evaluated in an ongoing phase II trial (NISCAHN trial). The use of nivolumab in 52 non-ACC patients demonstrated a 6-month non-progression rate (NPR6M) in 7 patients (14%, 90% CI: 6.8–24.7), with 2 partial responses (3.8%) and 22 patients with stable disease (42.3%). The median PFS was only 1.8 months (95% CI: 1.7–3.5) (53).
The role of tumor mutation burden (TMB) is unclear in SGCs. The subgroup analysis by TMB from the KEYNOTE-158 trial led to the approval of pembrolizumab for patients with TMB >10 mut/Mb as an agnostic treatment. There were three patients with salivary histologies and high TMB, one of whom achieved a partial response (54).
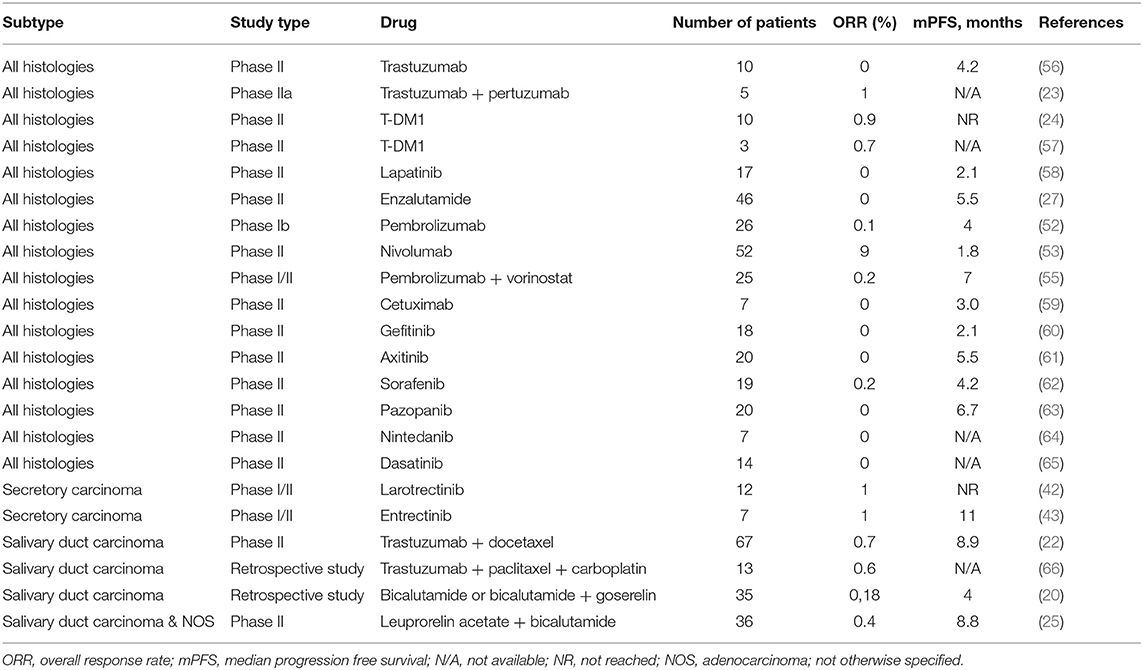
The addition of vorinostat, a histone deacetylase (HDAC) inhibitor, to pembrolizumab was evaluated in a phase I/II trial with 25 SGC patients. The association yielded a partial response in 4 patients (16%) and stable disease in 14 (56%), with a median PFS of 6.9 months and a median OS of 14 months (55). The combination of nivolumab and ipilimumab is being evaluated in an ongoing phase II study (NCT02834013). A summary of all relevant trials in non-ACC histologies is displayed in Table 1, and ongoing studies are shown in Table 2. We acknowledge the challenge in treating advanced SGC and propose practical alternatives to chemotherapy based on biomarkers in daily practice, displayed in Figure 1.
Adenoid Cystic Carcinoma
ACC is the second most common malignant salivary neoplasm, accounting for around one quarter of cases. It is more frequently diagnosed in females, affecting all age groups and often arising from the minor salivary glands (3, 67).
ACC usually has an indolent course, albeit difficult to eradicate due to its persistent nature and recurrent growth pattern, with predilection for perineural invasion. The literature demonstrates that 5-year disease-free survival in patients with ACC is only 30–40% (67). ACC commonly metastasizes to lungs, bones, and liver, with a median OS of 20–32 months in this setting (68).
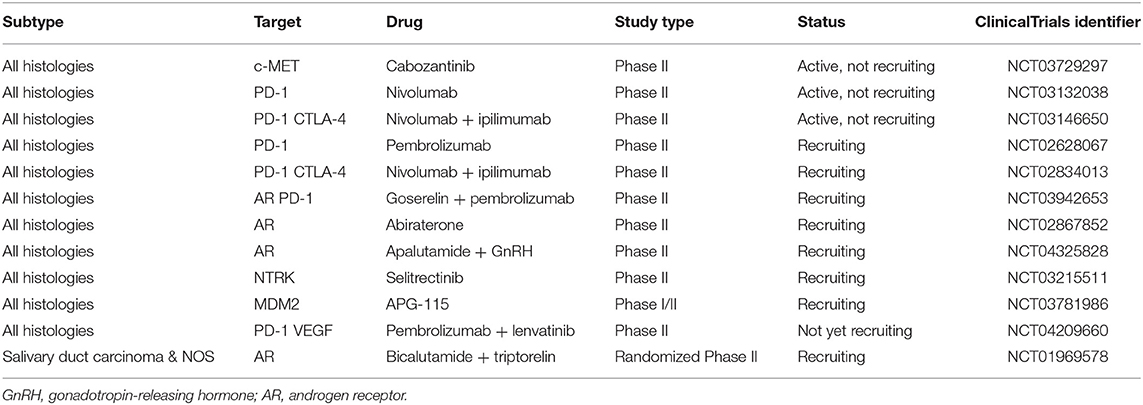
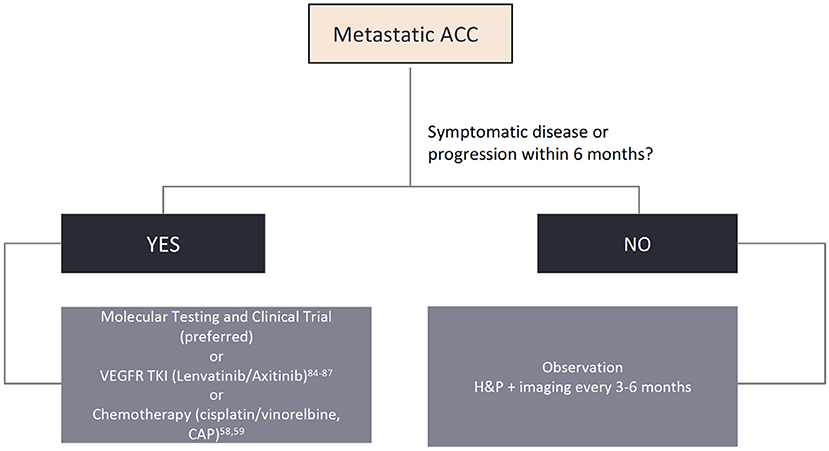
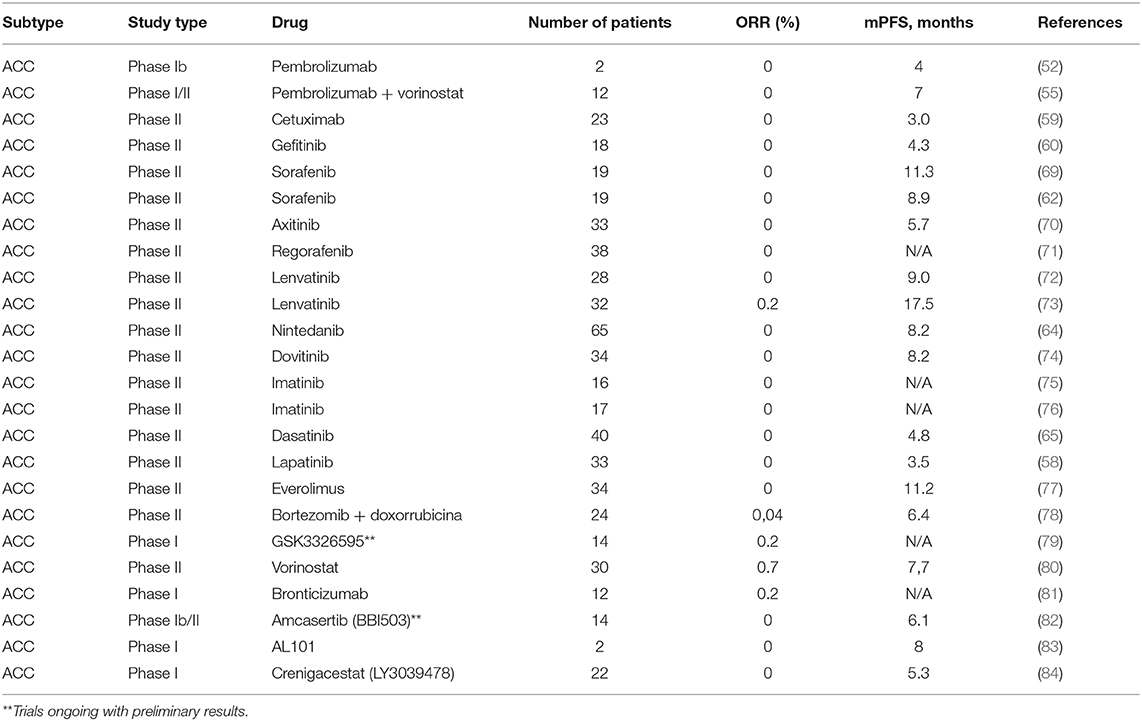
While surgery, with or without postoperative radiotherapy, is the mainstay treatment for localized disease, systemic therapy is reserved to the metastatic or unresectable locally advanced setting, with poor response rates and no consensus about the proper timing to be initiated. In this section, we will review proliferation pathways, molecular insights, and the development of new targeted drugs for patients with advanced disease. Though several actionable pathways are under scrutiny, limited evidence can aid in clinical practice. We propose a practical approach for newly diagnosed advanced ACC and options for later lines of therapy in Figure 2. Ongoing clinical trials are displayed in Table 3 and a summary of the main ACC studies conducted to date are displayed in Table 4.
Chemotherapy
Despite response rates of <30%, chemotherapy remains one of the most used treatments for this condition (85). The most consolidated regimen consists of cisplatin, doxorubicin and cyclophosphamide (CAP) (86). The best time to start treatment remains controversial, though it is commonly deferred until either symptomatic disease or a more accelerated growth pattern. Other cytotoxic agents have also been shown to be minimally active, such as mitoxantrone and vinorelbine, though other drugs such as paclitaxel should be avoided as single agents due to lack of proven efficacy (85).
MYB–NFIB Pathway
Myb, a nuclear transcription factor, is overexpressed in 60–80% of ACCs, usually correlated with a genetic translocation of the MYB gene to the transcription factor gene NFIB, resulting in the MYB-NFIB fusion, an important oncogene (t[6, 9]). This fusion has been postulated as the main driver of tumor proliferation in ACC (87, 88). The Myb protein has an N-terminal DNA-binding domain and a central transactivation domain that regulate genes involved in cell cycle control, such as NSR, MET, EGFR, IGF1R, and specifically IGF2 (89). The latter, by autocrine stimulation, controls the expression of the MYB-NFIB fusion in ACC cells, increasing proliferation and generating changes in the cell cycle and RNA processing (89–91). Other MYB-related fusions were described, however at lower frequencies than MYB-NIFB. Myb overexpression can also occur in the absence of detectable genetic alterations, implying that unknown pathways may be involved in its expression at the protein level (89).
Pre-clinical studies evaluated the role of targeted therapies, such as linsitinib (Igf1r inhibitor), gefitinib (EGFR inhibitor), and crizotinib (Alk and Met inhibitor) in vitro both as monotherapies and as a triplet regimen. Individually, none showed encouraging results, whereas a significant reduction of Myb expression was seen with the triplet regimen, suggesting a potential clinical benefit (92). In vivo studies are necessary to confirm activity in clinical practice with a tolerable toxicity profile, a major concern of combining these drugs.
More recently, the use of transretinoic acid (ATRA) showed interesting results in pre-clinical models. The drug reduced Myb binding in intensifying regions in MYB-translocated patient-derived xenograft models, thereby reducing the positive feedback for Myb overexpression cycle and thus reducing tumor proliferation (93). Two clinical trials are underway to address its role in treating patients with advanced ACC (NCT03999684; NCT04433169). Additionally, a study evaluating a Myb vaccine in combination with a novel anti-PD-1 is being conducted (NCT03287427).
NOTCH1, 2, 3
Notch are transmembrane proteins that bind to neighboring cells and activate a biochemical cascade that gives rise to the process of cell differentiation, in addition to acting in the process of lateral regulation, proliferation, and angiogenesis of cells through the MAPK pathways (87). Mutations in the NOTCH gene family, particularly NOTCH1, are present in around 20% of ACC patients and are potential oncogenic drivers. The presence of this mutation characterizes a population with more advanced disease, along with the presence of bone and liver metastases and worse outcomes compared to a wild-type population (94).
A phase I trial tested the efficacy of brontictuzumab (OMP-52M51), a humanized monoclonal antibody against the Notch1 protein in a basket trial for solid tumors. Twelve patients (25%) had a diagnosis of ACC, with two developing a partial response and three with stable disease as best response, with tolerable adverse events (81). Another phase Ib/II study is evaluating the role of amcasertib (BBI-553), a cancer stemness kinase inhibitor that impairs cancer stem cell survival, which is intimately related to deregulated Notch pathway activity (95). Preliminary results demonstrated a disease control rate of 86% and median overall survival of 28.3 months (82). AL101, a γ-secretase inhibitor, also works by inhibiting the Notch pathway during the cleavage process for Notch's protein action in the intracellular domain. A phase I basket trial revealed a partial response lasting 8 months in 1 of 2 patients with ACC accrued (83). The phase II trial ACCURACY (NCT03691207) for ACC patients bearing NOTCH activating mutations is ongoing. A trial with another Notch inhibitor, CB103, is also being conducted (NCT03422679).
Immunotherapy in ACC
The ACC cohort of the aforementioned KEYNOTE-028 represented only 8% of patients (N = 2), with none achieving a response. In terms of PFS and OS, results were poorer than with chemo or targeted therapy (52). Similarly, the combination of pembrolizumab in association with vorinostat was also disappointing in treatment of salivary gland tumors, including ACC, with low response rates (55). Nivolumab as a single agent was also evaluated in SGCs. In the ACC cohort, an ORR of 8.7% was observed (4/46 patients) (53). The combination of ipilimumab and nivolumab was initially thought to improve outcomes; however, only 2 out of 32 patients treated achieved a partial response, with a median PFS of 19.3 weeks in a prospective study (96). As previously stated, ACC appears to lack immune infiltration and harbors a lower mutation burden, being unlikely to benefit from immunotherapy (50).
EGFR Pathway
EGFR is commonly overexpressed in ACC, though its presence in normal salivary gland tissue precludes any conclusions in its role in cancer development. Mutations in genes related to the EGFR pathway, including EGFR, RAS family, PIK3CA, BRAF, and AKT1 are also present in ACC (97). Activating mutations in EGFR can be found in 10% of cases, though unlikely to be driver oncogenes in this setting (98). A phase I study tested gefitinib at 250 mg/day in 18 patients with ACC, and no responses were observed (60). Cetuximab was also evaluated in a single-arm, phase II study of EGFR-overexpressing patients, with disappointing results (59). Lapatinib has also been studied in patients who showed overexpression of EGFR and/or Her-2, again with unremarkable outcomes. Clinical benefit with stable disease was achieved in 36% of patients, with no objective responses (58).
PRMT-5
PRMT5 is an enzyme that methylates arginines in proteins important for tumor growth and development (99). The phase I basket trial METEOR-1 evaluated the role of GSK3326595, a potent and selective PRMT5 inhibitor. Of the selected patients, 14 (26%) had metastatic ACC. Clinical activity was observed in several tumor types, notably with partial responses observed in 3/14 ACC cases, with tolerable adverse events (79).
Histone Deacetylation
Epigenetic changes were found in most studies that carried out NGS. The acetylation of histone pathways, with mutations in chromatin remodeling genes, such as SMARCA2, CREBBP, and KDM6A, suggests aberrant epigenetic regulation in ACC oncogenesis (100). A pre-clinical study combining cisplatin and vorinostat found a remarkable efficacy in depleting CSCs and reducing tumor viability in all ACC primary cells (101). A phase II trial of vorinostat in ACC showed a partial response in 2/30 patients and stable disease in another 27 patients (80). However, a phase II trial combining vorinostat and pembrolizumab for recurrent or metastatic salivary gland cancer, as aforementioned, showed disappointing results, likely reflecting the immune-tolerant environment of ACC (55).
KIT/VEGFR
Other overexpressed potential target receptors in ACC are the vascular endothelial growth factor receptor (VEGFR) and fibroblast growth factor receptor 1 (FGFR1). These are well-established oncogenic pathways and can be inhibited by anti-VEGFR/FGFR drugs (102). Sorafenib, nintedanib, axitinib, regorafenib, dovitinib, and other multi-kinase inhibitors were tested and showed only a modest benefit, with few objective response rates (Table 4). Notably, lenvatinib was evaluated in a population with metastatic ACC, who had already received up to one line of chemotherapy. A total of 28 patients were enrolled in the study, and 11.5% showed a partial response (72). Additionally, 25 to 27% of patients with ACC had at least 20% reduction in target lesion size. The median PFS and OS were 9.1 and 27 months, respectively. Despite the encouraging results, 50% of the patients presented grade 3 toxicity and dose reductions were necessary in most of the study population. Similarly, Tchekmedyian et al. conducted another phase II study with lenvatinib, with a 15.6% ORR and a remarkable median PFS of 17.2 months (73). Axitinib is another multi-kinase inhibitor with interesting results in ACC, but with a lower ORR and median PFS (9.1% and 5.7 months, respectively) (70). More recently, the first randomized phase II trial of its kind showed a significant improvement in PFS with axitinib vs. observation (HR: 0.25; 95% CI: 0.14–0.42; P < 0.0001), but with no improvement in OS (HR: 0.6; 95% CI: 0.26–1.38; P = 0.23) (103). In this study, none of the 27 patients treated achieved a response, but all (100%) had stable disease. This rekindles the discussion of whether deferring treatment until a more symptomatic or aggressive course of disease remains acceptable. We favor the use of lenvatinib due to its numerical superiority in ORR and PFS compared to axitinib, but starting at a lower dose of 20 mg/day, with subsequent dose escalation if adequately tolerated.
Despite the high percentages (90%) of overexpression of c-Kit by IHC in ACC, targeted agents such as imatinib and dasatinib failed to show a meaningful activity in this disease (65, 75, 76, 104). The best response was stable disease in 50% of the patients treated with dasatinib (65). The disappointing outcomes likely result from the lack of an underlying gene amplification and/or a KIT activating mutation, such as seen in other malignancies (gastrointestinal stromal tumors and chronic myeloid leukemia).
177Lu-PSMA
ACC cells can express prostate-specific membrane antigen (PSMA) in over 90% of cases, with significant uptake in PSMA-PET/CT (105). Such as in prostate cancer, this can be useful not only for staging and surveillance but also as an opportunity for PSMA-directed therapy. Lutetium-177 (177Lu)-PSMA is a radiolabeled small molecule that binds with high affinity to PSMA, enabling beta particle therapy targeted to metastatic castration-resistant prostate cancer, with promising results in this tumor type (106). A single case report so far has been reported in ACC, with a transient pain relief after one dose. However, the patient died within 6 weeks due to a highly refractory and advanced tumor (107). An ongoing clinical trial is prospectively evaluating the role of 177Lu-PSMA in advanced ACC (NCT04291300).
Conclusions
In conclusion, SGCs may be challenging to treat due to its several histological subtypes. Molecular diagnostics are able to aid in diagnosis and guide discovery for subtype-specific targeted therapy. Currently, significant efforts are being undertaken to improve outcomes for advanced disease with biomarker-driven research. Given the limited efficacy with chemotherapy, a more personalized approach is of utmost importance to move forward in the management of this infrequent entity.
Author Contributions
LD, IS, FT, RF, and GS participated in the concept design, writing, review, and approval of the final manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Carvalho AL, Nishimoto IN, Califano JA, Kowalski LP. Trends in incidence and prognosis for head and neck cancer in the United States: a site-specific analysis of the SEER database. Int J Cancer. (2005) 114:806–16. doi: 10.1002/ijc.20740
2. El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ, et al. World Health Organization Classification of Tumours of Head and Neck. Lyon: IARC (2017).
3. Jones AV, Craig GT, Speight PM, Franklin CD. The range and demographics of salivary gland tumours diagnosed in a UK population. Oral Oncol. (2008) 44:407–17. doi: 10.1016/j.oraloncology.2007.05.010
4. Guzzo M, Locati LD, Prott FJ, Gatta G, McGurk M, Licitra L, et al. Major and minor salivary gland tumors. Crit Rev Oncol Hematol. (2010) 74:134–48. doi: 10.1016/j.critrevonc.2009.10.004
5. Alfieri S, Granata R, Bergamini C, Resteghini C, Bossi P, Licitra LF, et al. Systemic therapy in metastatic salivary gland carcinomas: a pathology-driven paradigm? Oral Oncol. (2017) 66:58–63. doi: 10.1016/j.oraloncology.2016.12.016
6. Coca-Pelaz A, Rodrigo JP, Triantafyllou A, Hunt JL, Rinaldo A, Strojan P, et al. Salivary mucoepidermoid carcinoma revisited. Eur Arch Otorhinolaryngol. (2015) 272:799–819. doi: 10.1007/s00405-014-3053-z
7. Chen MM, Roman SA, Sosa JA, Judson BL. Histologic grade as prognostic indicator for mucoepidermoid carcinoma: a population-level analysis of 2400 patients. Head Neck. (2014) 36:158–63. doi: 10.1002/hed.23256
8. Morita M, Murase T, Okumura Y, Ueda K, Sakamoto Y, Masaki A, et al. Clinicopathological significance of EGFR pathway gene mutations and CRTC1/3-MAML2 fusions in salivary gland mucoepidermoid carcinoma. Histopathology. (2020) 76:1013–22. doi: 10.1111/his.14100
9. Nakano K, Sato Y, Sasaki T, Shimbashi W, Fukushima H, Yonekawa H, et al. Combination chemotherapy of carboplatin and paclitaxel for advanced/metastatic salivary gland carcinoma patients: differences in responses by different pathological diagnoses. Acta Otolaryngol. (2016) 136:948–51. doi: 10.3109/00016489.2016.1170876
10. Saade RE, Bell D, Garcia J, Roberts D, Weber R. Role of CRTC1/MAML2 translocation in the prognosis and clinical outcomes of mucoepidermoid carcinoma. JAMA Otolaryngol Head Neck Surg. (2016) 142:234–40. doi: 10.1001/jamaoto.2015.3270
11. Luk PP, Wykes J, Selinger CI, Ekmejian R, Tay J, Gao T, et al. Diagnostic and prognostic utility of mastermind-like 2 (MAML2) gene rearrangement detection by fluorescent in situ hybridization (FISH) in mucoepidermoid carcinoma of the salivary glands. Oral Surg Oral Med Oral Pathol Oral Radiol. (2016) 121:530–41. doi: 10.1016/j.oooo.2016.01.003
12. Nachtsheim L, Arolt C, Dreyer T, Meyer MF, Brobeil A, Gamerdinger U, et al. Mucoepidermoidcarcinoma – importance in molecular pathology. Laryngo Rhino Otol. (2020) 99:144–8. doi: 10.1055/a-1083-6805
13. Anzick SL, Chen WD, Park Y, Meltzer P, Bell D, El-Naggar AK, et al. Unfavorable prognosis of CRTC1- MAML2 positive mucoepidermoid tumors with CDKN2A deletions. Genes Chromosomes Cancer. (2010) 49:59–69. doi: 10.1002/gcc.20719
14. Birkeland AC, Foltin SK, Michmerhuizen NL, Hoesli RC, Rosko AJ, Byrd S, et al. Correlation of Crtc1/3-Maml2 fusion status, grade and survival in mucoepidermoid carcinoma. Oral Oncol. (2017) 68:5–8. doi: 10.1016/j.oraloncology.2017.02.025
15. Chen Z, Chen J, Gu Y, Hu C, Li JL, Lin S, et al. Aberrantly activated AREG–EGFR signaling is required for the growth and survival of CRTC1–MAML2 fusion-positive mucoepidermoid carcinoma cells. Oncogene. (2014) 33:3869–77. doi: 10.1038/onc.2013.348
16. Wang K, McDermott JD, Schrock AB, Elvin JA, Gay L, Karam SD, et al. Comprehensive genomic profiling of salivary mucoepidermoid carcinomas reveals frequent BAP1, PIK3CA, and other actionable genomic alterations. Ann Oncol. (2017) 28:748–53. doi: 10.1093/annonc/mdw689
17. De Block K, Vander Poorten V, Dormaar T, Nuyts S, Hauben E, Floris G, et al. Metastatic HER-2-positive salivary gland carcinoma treated with trastuzumab and a taxane: a series of six patients. Acta Clin Belg. (2016) 71:383–8. doi: 10.1080/17843286.2016.1173940
18. Lujan B, Hakim S, Moyano S, Nadal A, Caballero M, Diaz A, et al. Activation of the EGFR/ERK pathway in high-grade mucoepidermoid carcinomas of the salivary glands. Br J Cancer. (2010) 103:510–6. doi: 10.1038/sj.bjc.6605788
19. Simpson RH. Salivary duct carcinoma: new developments–morphological variants including pure in situ high grade lesions; proposed molecular classification. Head Neck Pathol. (2013) 7 (Suppl. 1):S48–58. doi: 10.1007/s12105-013-0456-x
20. Boon E, Bel M, van Boxtel W, van der Graaf WTA, van RJJ, Eerenstein Es SEJ, et al. A clinicopathological study and prognostic factor analysis of 177 salivary duct carcinoma patients from the Netherlands. Int J Cancer. (2018) 143:758–66. doi: 10.1002/ijc.31353
21. McHugh JB, Visscher DW, Barnes EL. Update on selected salivary gland neoplasms. Arch Pathol Lab Med. (2009) 133:1763–74. doi: 10.1043/1543-2165-133.11.1763
22. Takahashi H, Tada Y, Saotome T, Akazawa K, Ojiri H, Fushimi C, et al. Phase II trial of trastuzumab and docetaxel in patients with human epidermal growth factor receptor 2-positive salivary duct carcinoma. J Clin Oncol. (2019) 37:125–34. doi: 10.1200/JCO.18.00545
23. Hainsworth JD, Meric-Bernstam F, Swanton C, Hurwitz H, Spigel DR, Sweeney C, et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from MyPathway, an open-label, phase IIa multiple basket study. J Clin Oncol. (2018) 36:536–42. doi: 10.1200/JCO.2017.75.3780
24. Li BT, Shen R, Offin M, Buonocore DJ, Myers ML, Venkatesh A, et al. Ado-trastuzumab emtansine in patients with HER2 amplified salivary gland cancers (SGCs): results from a phase II basket trial. J Clin Oncol. (2019) 37:6001. doi: 10.1200/JCO.2019.37.15_suppl.6001
25. Fushimi C, Tada Y, Takahashi H, Nagao T, Ojiri H, Masubuchi T, et al. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann Oncol. (2018) 29:979–84. doi: 10.1093/annonc/mdx771
26. Van Boxtel W, Locati LD, van Engen-van Grunsven ACH, Bergamini C, Jonker MA, Fiets E, et al. Adjuvant androgen deprivation therapy for poor-risk, androgen receptor-positive salivary duct carcinoma. Eur J Cancer. (2019) 110:62–70. doi: 10.1016/j.ejca.2018.12.035
27. Ho AL, Foster NR, Zoroufy AJ, Worden FP, Price KA, Adkins D, et al. Alliance A091404: a phase II study of enzalutamide (NSC# 766085) for patients with androgen receptor-positive salivary cancers. J Clin Oncol. (2019) 37 (15 Suppl):6020. doi: 10.1200/JCO.2019.37.15_suppl.6020
28. Armstrong AJ, Halabi S, Luo J, Nanus DM, Giannakakou P, Szmulewitz RZ, et al. Prospective multicenter validation of androgen receptor splice variant 7 and hormone therapy resistance in high-risk castration-resistant prostate cancer: the PROPHECY study. J Clin Oncol. (2019) 37:1120–9. doi: 10.1200/JCO.18.01731
29. Gargano SM, Senarathne W, Feldman R, Florento E, Stafford P, Swensen J, et al. Novel therapeutic targets in salivary duct carcinoma uncovered by comprehensive molecular profiling. Cancer Med. (2019) 8:7322–9. doi: 10.1002/cam4.2602
30. Yang RK, Zhao P, Lu C, Luo J, Hu R. Expression pattern of androgen receptor and AR-V7 in androgen deprivation therapy naive salivary duct carcinomas. Hum Pathol. (2018) 84:173–82. doi: 10.1016/j.humpath.2018.09.009
31. Cappelletti V, Miodini P, Reduzzi C, Alfieri S, Daidone MG, Licitra L, et al. Tailoring treatment of salivary duct carcinoma by liquid biopsy: ARv7 expression in circulating tumor cells. Ann Oncol. (2018) 29:1598–600. doi: 10.1093/annonc/mdy141
32. Ho AL, Hanna GJ, Scholz CR, Gualberto A, Park SH. Preliminary activity of tipifarnib in tumors of the head and neck, salivary gland and urothelial tract with HRAS mutations. J Clin Oncol. (2020) 38:6504. doi: 10.1200/JCO.2020.38.15_suppl.6504
33. Skálová A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. (2010) 34:599–608. doi: 10.1097/PAS.0b013e3181d9efcc
34. Balanzá R, Arrangoiz R, Cordera F, Muñoz M, Luque-de-León E, Moreno M, et al. Mammary analog secretory carcinoma of the parotid gland: a case report and literature review. Int J Surg Case Rep. (2015) 16:187–91. doi: 10.1016/j.ijscr.2015.09.031
35. Parekh V, Stevens TM. Mammary analogue secretory carcinoma. Arch Pathol Lab Med. (2016) 140:997–1001. doi: 10.5858/arpa.2015-0075-RS
36. Fehr A, Löning T, Stenman G. Mammary analogue secretory carcinoma of the salivary glands with ETV6-NTRK3 gene fusion. Am J Surg Pathol. (2011) 35:1600–2. doi: 10.1097/PAS.0b013e31822832c7
37. Hung YP, Jo VY, Hornick JL. Immunohistochemistry with a pan-TRK antibody distinguishes secretory carcinoma of the salivary gland from acinic cell carcinoma. Histopathology. (2019) 75:54–62. doi: 10.1111/his.13845
38. Sethi R, Kozin E, Remenschneider A, VanderLaan P, Faquin W, et al. Mammary analogue secretory carcinoma: update on a new diagnosis of salivary gland malignancy. Laryngoscope. (2014) 124:188–95. doi: 10.1002/lary.24254
39. Montalvo N, Galarza D, Redrobán L. Secretory carcinoma of the parotid: making the correct diagnosis of a rare salivary gland carcinoma when molecular biology testing is not available. Case Rep Pathol. (2019) 2019:5103496. doi: 10.1155/2019/5103496
40. Rooper LM, Karantanos T, Ning Y, Bishop JA, Gordon SW, Kang H. Salivary secretory carcinoma with a novel ETV6-MET fusion expanding the molecular spectrum of a recently described entity. Am J Surg Pathol. (2018) 42:1121–6. doi: 10.1097/PAS.0000000000001065
41. Skalova A, Vanecek T, Martinek P, Weinreb I, Stevens TM, Simpson RHW, et al. Molecular profiling of mammary analog secretory carcinoma revealed a subset of tumors harboring a novel ETV6-RET translocation report of 10 cases. Am J Surg Pathol. (2018) 42:234–46. doi: 10.1097/PAS.0000000000000972
42. Drilon A, Laetsch T, Kummar S, DuBois S, Lassen U, Demetri G, et al. Efficacy of larotrectinib in TRK fusion– positive cancers in adults and children. N Engl J Med. (2018) 378:731–9. doi: 10.1056/NEJMoa1714448
43. Doebele R, Drilon A, Paz-Ares L, Siena S, Shaw A, Farago A, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet. (2020) 21:271–82. doi: 10.1016/s1470-2045(19)30691-6
44. Hemming M, Nathenson M, Lin J, Shaolin M, Du Z, Malik K, et al. Response and mechanisms of resistance to larotrectinib and selitrectinib in metastatic undifferentiated sarcoma harboring oncogenic fusion of NTRK. JCO Precis Oncol. (2020) 4:79–90. doi: 10.1200/PO.19.00287
45. Hyman D, Kummar S, Farago A, Geoerger B, Mau-Sorensen M, Taylor M, et al. CT-127 - phase I and expanded access experience of LOXO-195 (BAY 2731954), a selective next-generation TRK inhibitor (TRKi) [abstract]. In: Proceedings of the 10th Annual Meeting of the American Association for Cancer Research; 2019. Philadelphia, PA: AACR (2019). p. 127.
46. Li J, Wang BY, Nelson M, Li L, Hu Y, Urken ML, et al. Salivary adenocarcinoma, not otherwise specified: a collection of orphans. Arch Pathol Lab Med. (2004) 128:1385–94. doi: 10.1043/1543-2165(2004)128<1385:SANOSA>2.0.CO;2
47. Reinheimer A, Vieira DS, Cordeiro MM, Rivero ER. Retrospective study of 124 cases of salivary gland tumors and literature review. J Clin Exp Dent. (2019) 11:e1025–32. doi: 10.4317/jced.55685
48. Psarris A, Koufopoulos N, Grivas A, Papatheodorou DC, Khaldi L. Tumor to tumor metastasis from adenocarcinoma not otherwise specified of the parotid gland to uterine leiomyoma: presentation of a unique case. Cureus. (2020) 12:e6789. doi: 10.7759/cureus.6789
49. Wang K, Russell JS, McDermott JD, Elvin JA, Khaira D, Johnson A, et al. Profiling of 149 salivary duct carcinomas, carcinoma ex pleomorphic adenomas, and adenocarcinomas, not otherwise specified reveals actionable genomic alterations. Clin Cancer Res. (2016) 22:6061–8. doi: 10.1158/1078-0432.CCR-15-2568
50. Linxweiler M, Kuo F, Katabi N, Lee M, Nadeem Z, Dalin MG, et al. The immune microenvironment and neoantigen landscape of aggressive salivary gland carcinomas differ by subtype. Clin Cancer Res. (2020) 26:2859–70. doi: 10.1158/1078-0432.CCR-19-3758
51. Mukaigawa T, Hayashi R, Hashimoto K, Ugumori T, Hato N, Fujii S. Programmed death ligand-1 expression is associated with poor disease free survival in salivary gland carcinomas. J Surg Oncol. (2016) 114:36–43. doi: 10.1002/jso.24266
52. Cohen RB, Delord JP, Doi T, Piha-Paul SA, Liu SV, Gilbert J, et al. Pembrolizumab for the treatment of advanced salivary gland carcinoma: findings of the phase 1b KEYNOTE-028 study. Am J Clin Oncol. (2018) 41:1083–8. doi: 10.1097/COC.0000000000000429
53. Fayette J, Even C, Digue L, Geoffrois L, Rolland F, Cupissol D, et al. NISCAHN: a phase II, multicenter nonrandomized trial aiming at evaluating nivolumab (N) in two cohorts of patients (pts) with recurrent/metastatic (R/M) salivary gland carcinoma of the head and neck (SGCHN), on behalf of the unicancer head and neck group. J Clin Oncol. (2019) 37:6083. doi: 10.1200/JCO.2019.37.15_suppl.6083
54. Marabelle A, Fakih MG, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. 1192OAssociation of tumour mutational burden with outcomes in patients with select advanced solid tumours treated with pembrolizumab in KEYNOTE-158. Ann Oncol. (2019) 30:v477–8. doi: 10.1093/annonc/mdz253.018
55. Rodriguez CP, Wu QV, Voutsinas JM, Fromm JP, Jiang X, Pillarisetty VG, et al. A phase II trial of pembrolizumab and vorinostat in recurrent metastatic head and neck squamous cell carcinomas and salivary gland cancer. Clin Cancer Res. (2020) 26:837–45. doi: 10.1158/1078-0432.CCR-19-2214
56. Haddad R, Colevas AD, Krane JF, Cooper D, Glisson B, Amrein PC, et al. Herceptin in patients with advanced or metastatic salivary gland carcinomas. A phase II study. Oral Oncol. (2003) 39:724–7. doi: 10.1016/S1368-8375(03)00097-6
57. Jhaveri KL, Wang XV, Makker V, Luoh SW, Mitchell EP, Zwiebel JA, et al. Ado-trastuzumab emtansine (T-DM1) in patients with HER2-amplified tumors excluding breast and gastric/gastroesophageal junction (GEJ) adenocarcinomas: results from the NCI-MATCH trial (EAY131) subprotocol Q. Ann Oncol. (2019) 30:1821–30. doi: 10.1093/annonc/mdz291
58. Agulnik M, Cohen EW, Cohen RB, Chen EX, Vokes EE, Hotte SJ, et al. Phase II study of lapatinib in recurrent or metastatic epidermal growth factor receptor and/or erbB2 expressing adenoid cystic carcinoma and non adenoid cystic carcinoma malignant tumors of the salivary glands. J Clin Oncol. (2007) 25:3978–84. doi: 10.1200/JCO.2007.11.8612
59. Locati LD, Bossi P, Perrone F, Potepan P, Crippa F, Mariani L, et al. Cetuximab in recurrent and/or metastatic salivary gland carcinomas: a phase II study. Oral Oncol. (2009) 45:574–8. doi: 10.1016/j.oraloncology.2008.07.010
60. Jakob JA, Kies MS, Glisson BS, Kupferman ME, Liu DD, Lee JJ, et al. Phase II study of gefitinib in patients with advanced salivary gland cancers. Head Neck. (2015) 37:644–9. doi: 10.1002/hed.23647
61. Locati LD, Cavalieri S, Bergamini CI, Resteghini CI, Alfieri S, Calareso G, et al. Phase II trial with axitinib in recurrent and/or metastatic salivary gland cancers of the upper aerodigestive tract. Head Neck. (2019) 41:3670–76. doi: 10.1002/hed.25891
62. Locati LD, Perrone F, Cortelazzi B, Bergamini C, Bossi P, Civelli E, et al. A phase II study of sorafenib in recurrent and/or metastatic salivary gland carcinomas: translational analyses and clinical impact. Eur J Cancer. (2016) 69:158–65. doi: 10.1016/j.ejca.2016.09.022
63. Guigay J, Fayette J, Even C, Cupissol D, Rolland F, Peyrade F, et al. PACSA: phase II study of pazopanib in patients with progressive recurrent or metastatic (R/M) salivary gland carcinoma (SGC). J Clin Oncol. (2016) 4:6086. doi: 10.1200/JCO.2016.34.15_suppl.6086
64. Kim Y, Lee SJ, Lee JY, Lee SH, Sun JM, Park K, et al. Clinical trial of nintedanib in patients with recurrent or metastatic salivary gland cancer of the head and neck: a multicenter phase 2 study (Korean cancer study group HN14-01). Cancer. (2017) 123:1958–64. doi: 10.1002/cncr.30537
65. Wong SJ, Karrison T, Hayes DN, Kies MS, Cullen KJ, Tanvetyanon T, et al. Phase II trial of dasatinib for recurrent or metastatic c-KIT expressing adenoid cystic carcinoma and for nonadenoid cystic malignant salivary tumors. Ann Oncol. (2016) 27:318–23. doi: 10.1093/annonc/mdv537
66. Limaye SA, Posner MR, Krane JF, Fonfria M, Lorch JH, Dillon DA, et al. Trastuzumab for the treatment of salivary duct carcinoma. Oncologist. (2013) 18:294–300. doi: 10.1634/theoncologist.2012-0369
67. Nascimento AG, Amaral Amaral ALP. Adenoid cystic carcinoma of salivary glands. A study of 61 cases with clinicopathologic correlation. Cancer. (1986) 57:312–19. doi: 10.1002/1097-0142(19860115)57:2<312::aid-cncr2820570220>3.0.co;2-a
68. van der Wal JE, Becking AG, Snow GB, van der Waal I. Distant metastases of adenoid cystic carcinoma of the salivary glands and the value of diagnostic examinations during follow-up. Head Neck. (2002) 24:779–83. doi: 10.1002/hed.10126
69. Thomson DJ, Silva P, Denton K, Bonington S, Mak SK, Swindell R, et al. Phase II trial of sorafenib in advanced salivary adenoid cystic carcinoma of the head and neck. Head Neck. (2015) 37:182–7. doi: 10.1002/hed.23577
70. Ho AL, Dunn L, Sherman EJ, Fury MG, Baxi SS, Chandramohan R, et al. A phase II study of axitinib (AG-013736) in patients with incurable adenoid cystic carcinoma. Ann Oncol. (2016) 27:1902–8. doi: 10.1093/annonc/mdw287
71. Ho AL, Sherman EJ, Baxi SS, Haque S, Ni A, Antonescu CR, et al. Phase II study of regorafenib in progressive, recurrent/metastatic adenoid cystic carcinoma. J Clin Oncol. (2016) 34:6–20. doi: 10.1200/JCO.2016.34.15_suppl.6096
72. Locati LD, Galbiati D, Calareso G, Alfieri S, Singer S, Cavalieri S, et al. Patients with adenoid cystic carcinomas of the salivary glands treated with lenvatinib: activity and quality of life. Cancer. (2020) 126:1888–94. doi: 10.1002/cncr.32754
73. Tchekmedyian V, Sherman EJ, Dunn L, Tran C, Baxi S, Katabi N, et al. A phase II study of lenvatinib in patients with progressive, recurrent/metastatic adenoid cystic carcinoma. J Clin Oncol. (2018) 36:6022. doi: 10.1200/JCO.2018.36.15_suppl.6022
74. Dillon PM, Petroni GR, Horton BJ, Moskaluk CA, Fracasso PM, Douvas MG, et al. A phase II study of dovitinib in patients with recurrent or metastatic adenoid cystic carcinoma. Clin Cancer Res. (2017) 23:4138–45. doi: 10.1158/1078-0432.CCR-16-2942
75. Hotte SJ, Winquist EW, Lamont E, MacKenzie M, Vokes E, Chen EX, et al. Imatinib mesylate in patients with adenoid cystic cancers of the salivary glands expressing c-kit: a princess margaret hospital phase II consortium study. J Clin Oncol. (2005) 23:585–90. doi: 10.1200/JCO.2005.06.125
76. Guigay MJ, Bidault F, Temam S, Janot F, Raymond E, Faivre S. Antitumor activity of imatinib in progressive, highly expressing KIT adenoid cystic carcinoma of the salivary glands: a phase II study. J Clin Oncol. (2007) 25:6086. doi: 10.1200/jco.2007.25.18_suppl.6086
77. Kim DW, Oh DY, Shin SH, Kang JH, Cho BC, Chung JS, et al. A multicenter phase II study of everolimus in patients with progressive unresectable adenoid cystic carcinoma. BMC Cancer. (2014) 14:795. doi: 10.1186/1471-2407-14-795
78. Argiris A, Ghebremichael M, Burtness B, Axelrod RS, Deconti RC, Forastiere AA. A phase 2 trial of bortezomib followed by the addition of doxorubicin at progression in patients with recurrent or metastatic adenoid cystic carcinoma of the head and neck: a trial of the eastern cooperative oncology group (E1303). Cancer. (2011) 117:3374–82. doi: 10.1002/cncr.25852
79. Siu LL, Rasco DW, Vinay SP, Romano PM, Menis J, Heinhuis KM, et al. Meteor-1: a phase I study of GSK3326595, a first-in-class proteins arginine methyltransferase 5 (PRMT5) inhibitor, in advanced solid tumors. Ann Oncol. (2019) 30:59–193. doi: 10.1093/annonc/mdz244
80. Gonçalves PH, Heilbrun LK, Barrett MT, Kummar S, Hansen AR, Siu LL, et al. A phase 2 study of vorinostat in locally advanced, recurrent, or metastatic adenoid cystic carcinoma. Oncotarget. (2017) 8:32918–29. doi: 10.18632/oncotarget.16464
81. Ferrarotto R, Eckhardt G, Patnaik A, LoRusso P, Faoro L, Heymach JV, et al. A phase I dose-escalation and dose-expansion study of brontictuzumab in subjects with selected solid tumors. Ann Oncol. (2018) 29:1561–8. doi: 10.1093/annonc/mdy171
82. Cote GM, Edenfield WJ, Laurie SA, Chau NG, Becerra C, Spira AI, et al. A phase 1b/2 study of amcasertib, a first-in-class cancer stemness kinase inhibitor, in advanced adenoid cystic carcinoma. J Clin Oncol. (2017) 35:6036. doi: 10.1200/JCO.2017.35.15_suppl.6036
83. El-Khoueiry AB, Desai J, Iyer SP, Gadgeel SM, Ramalingam SS, Horn L, et al. A phase I study of AL101, a pan-NOTCH inhibitor, in patients (pts) with locally advanced or metastatic solid tumors. J Clin Oncol. (2018) 36:2515. doi: 10.1200/JCO.2018.36.15_suppl.2515
84. Even C, Lassen UN, Merchan JR, Torneau CL, Soria JC, Ferte C, et al. Notch pathway inhibition with LY3039478 in adenoid cystic carcinoma (ACC). J Clin Oncol. (2017) 35:6024. doi: 10.1200/JCO.2017.35.15_suppl.6024
85. Laurie SA, Ho AL, Fury MG, Sherman E, Pfister DG. Systemic therapy in the management of metastatic or locally recurrent adenoid cystic carcinoma of the salivary glands: a systematic review. Lancet Oncol. (2011) 12:815–24. doi: 10.1016/S1470-2045(10)70245-X
86. Licitra L, Cavina R, Grandi C, Palma SD, Guzzo M, Demicheli R, et al. Cisplatin, doxorubicin and cyclophosphamide in advanced salivary gland carcinoma. A phase II trial of 22 patients. Ann Oncol. (1996) 7:640–2. doi: 10.1093/oxfordjournals.annonc.a010684
87. Allen S, Ho Ochoa A, Jayakumaran G, Zehir A, Mayor CV, Tepe J, et al. Genetic hallmarks of recurrent/metastatic adenoid cystic carcinoma. J Clin Invest. (2019) 129:4276–89. doi: 10.1172/JCI128227
88. Xu LH, Zhao F, Yang WW, Chen CW, Du ZH, Fu M, et al. MYB promotes the growth and metastasis of salivary adenoid cystic carcinoma. Int J Oncol. (2019) 54:1579–90. doi: 10.3892/ijo.2019.4754
89. Andersson MK, Åman P, Stenman G. IGF2/IGF1R signaling as a therapeutic target in MYB-positive adenoid cystic carcinomas and other fusion gene-driven tumors. Cells. (2019) 8:913. doi: 10.3390/cells8080913
90. Wang Y, Zhang CY, Xia RH, Han J, Sun B, Sun SY, et al. The MYB/miR-130a/NDRG2 axis modulates tumor proliferation and metastatic potential in salivary adenoid cystic carcinoma. Cell Death Dis. (2018) 9:917. doi: 10.1038/s41419-018-0966-2
91. Almeida-Pinto YD, Costa SFDS, de Andrade BAB, Altemani A, Vargas PA, Abreu LG, et al. t(6;9)(MYB-NFIB) in head and neck adenoid cystic carcinoma: a systematic review with meta-analysis. Oral Dis. (2019) 25:1277–82. doi: 10.1111/odi.12984
92. Andersson MK, Afshari MK, Andrén Y, Wick MJ, Stenman G. Targeting the oncogenic transcriptional regulator MYB in adenoid cystic carcinoma by inhibition of IGF1R/AKT signaling. J Natl Cancer Inst. (2017) 109:djx017. doi: 10.1093/jnci/djx017
93. Mandelbaum J, Shestopalov IA, Henderson RE, Chau NG, Knoechel B, Wick MG, et al. Zebrafish blastomere screen identifies retinoic acid suppression of MYB in adenoid cystic carcinoma. J Exp Med. (2018) 10:2673–85. doi: 10.1084/jem.20180939
94. Ferrarotto R, Mitani Y, Diao L, Guijarro I, Wang J, Zweidler-McKay P, et al. Activating NOTCH1 mutations define a distinct subgroup of patients with adenoid cystic carcinoma who have poor prognosis, propensity to bone and liver metastasis, and potential responsiveness to notch1 inhibitors. J Clin Oncol. (2017) 35:352–60. doi: 10.1200/JCO.2016.67.5264
95. Saygin C, Matei D, Majeti R, Reizes O, Lathia JD. Targeting cancer stemness in the clinic: from hype to hope. Cell Stem Cell. (2019) 24:25–40. doi: 10.1016/j.stem.2018.11.017
96. Tchekmedyian V, Sherman EJ, Dunn L, Fetten JV, Michel LS, Kriplani A, et al. A phase II trial cohort of nivolumab plus ipilimumab in patients (Pts) with recurrent/metastatic adenoid cystic carcinoma (R/M ACC). J Clin Oncol. (2019) 37:6084. doi: 10.1200/JCO.2019.37.15_suppl.6084
97. Saida K, Murase T, Ito M, Fujii K, Takino H, Masaki A, et al. Mutation analysis of the EGFR pathway genes, EGFR, RAS, PIK3CA, BRAF, and AKT1, in salivary gland adenoid cystic carcinoma. Oncotarget. (2018) 9:17043–55. doi: 10.18632/oncotarget.24818
98. Williams MD, Roberts DB, Kies MS, Mao L, Weber RS, El-Naggar AK. Genetic and expression analysis of HER-2 and EGFR genes in salivary duct carcinoma: empirical and therapeutic significance. Clin Cancer Res. (2010) 16:2266–74. doi: 10.1158/1078-0432.CCR-09-0238
99. Tada Y, Kokabu S, Sugiyama G, Nakatomi C, Aoki K, Fukushima H, et al. The novel IκB kinase β inhibitor IMD-0560 prevents bone invasion by oral squamous cell carcinoma. Oncotarget. (2014) 5:12317–30. doi: 10.18632/oncotarget.2640
100. Stephens PJ, Davies HR, Mitani Y, Loo PV, Shlien A, Tarpey PS, et al. Whole exome sequencing of adenoid cystic carcinoma. J Clin Invest. (2013) 123:2965–8. doi: 10.1172/JCI67201
101. Almeida LO, Guimarães DM, Martins MD, Martins MAT, Warner KA, Nör JE, et al. Unlocking the chromatin of adenoid cystic carcinomas using HDAC inhibitors sensitize cancer stem cells to cisplatin and induces tumor senescence. Stem Cell Res. (2017) 21:94–105. doi: 10.1016/j.scr.2017.04.003
102. Lim JJ, Kang S, Lee MR, Pai HK, Yoon HJ, Lee JI, et al. Expression of vascular endothelial growth factor in salivary gland carcinomas and its relation to p53, Ki-67 and prognosis. J Oral Pathol Med. (2003) 32:552–61. doi: 10.1034/j.1600-0714.2003.00073.x-i1
103. Keam B, Kang EJ, Ahn MJ, Ock CY, Lee KW, Kwon JH, et al. Randomized phase II study of axitinib vs. observation in patients with recurred or metastatic adenoid cystic carcinoma. J Clin Oncol. (2020) 38:6503. doi: 10.1200/JCO.2020.38.15_suppl.6503
104. Pfeffer MR, Talmi Y, Catane R, Symon Z, Yosepovitch A, Levitt M. A phase II study of imatinib for advanced adenoid cystic carcinoma of head and neck salivary glands. Oral Oncol. (2007) 43:33–6. doi: 10.1016/j.oraloncology.2005.12.026
105. van Boxtel W, Lütje S, van Engen-van Grunsven ICH, Verhaegh GW, Schalken JA, Jonker MA, et al. 68 Ga-PSMA-HBED-CC PET/CT imaging for adenoid cystic carcinoma and salivary duct carcinoma: a phase 2 imaging study. Theranostics. (2020) 10:2273–83. doi: 10.7150/thno.38501
106. Hofman MS, Violet J, Hicks RJ, Ferdinandus J, Thang SP, Akhurst T, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. (2018) 19:825–33. doi: 10.1016/S1470-2045(18)30198-0
Keywords: salivary gland cancer, molecular targeted therapy, androgen receptor, immunotherapy, ERBB-2 receptor, gene fusion, drug resistance
Citation: Di Villeneuve L, Souza IL, Tolentino FDS, Ferrarotto R and Schvartsman G (2020) Salivary Gland Carcinoma: Novel Targets to Overcome Treatment Resistance in Advanced Disease. Front. Oncol. 10:580141. doi: 10.3389/fonc.2020.580141
Received: 04 July 2020; Accepted: 09 September 2020;
Published: 22 October 2020.
Edited by:
Floriana Morgillo, Second University of Naples, ItalyReviewed by:
Susumu Okano, National Cancer Center Hospital East, JapanSandro J. Stoeckli, Kantonsspital St. Gallen, Switzerland
Copyright © 2020 Di Villeneuve, Souza, Tolentino, Ferrarotto and Schvartsman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gustavo Schvartsman, gustavo.schvartsman@einstein.br
†These authors share senior authorship
 Larissa Di Villeneuve
Larissa Di Villeneuve Ive Lima Souza
Ive Lima Souza Fernanda Davila Sampaio Tolentino
Fernanda Davila Sampaio Tolentino Renata Ferrarotto
Renata Ferrarotto Gustavo Schvartsman
Gustavo Schvartsman