- 1Karl Landsteiner University of Health Sciences, Krems, Austria
- 2Department of Neurosurgery, University Hospital St. Poelten, St. Poelten, Austria
- 3Department of Neurosurgery, Medical University of Innsbruck, Innsbruck, Austria
Primary central nervous system lymphomas (PCNSL) are rare CNS tumors that harbor a conspicuously longer diagnostic delay compared to other malignant brain tumors. The gold standard for diagnosis is stereotactic biopsy to acquire tissue for histopathological analysis and therefore neurosurgery plays a central role when reducing the diagnostic period is mandated. However, histopathological diagnosis could be complicated if the patient was preoperatively exposed to corticosteroids. Besides the histopathological result, diagnosis of a PCNSL also requires full diagnostic workup to exclude cerebral metastatic disease of a systemic lymphoma. Most reviews of PCNSL discuss recent advancements in systemic treatment options from an (neuro-)oncologic viewpoint, whereas our intention was to discuss the optimization of the diagnostic period and therefore describe current standards of imaging, summarizing the diagnostic workup, discussing the surgical workup and future diagnostic prospects as well as the influence of preoperative corticosteroid therapy to reduce the diagnostic delay of PCNSL patients.
Introduction
Primary central nervous system lymphoma (PCNSL) is defined as extranodal malignant non-hodgkin lymphoma of the brain, spinal cord or the leptomeninges in absence of systemic involvement. Histologically, most PCNSL are diffuse large B-cell lymphomas, followed by Burkitt, lymphoblastic, marginal zone and T-cell lymphomas (1). It therefore constitutes a fairly rare CNS neoplasm with an incidence of 0.26 to 0.48 per 100.000 person-years, which accounts for approximately 3% of all primary brain tumors (2–4). Immunocompromised patients after transplantation or being affected by AIDS have a higher relative risk of development of PCNSL (5). Recently, most PCNSL patients are immunocompetent patients and the incidence within an elderly cohort is still increasing (4, 6, 7). Clinical symptoms can be concealed with cognitive impairment being the most frequent, followed by gait disturbances, focal neurologic deficits, symptoms of increased intracranial pressure and seizures (8). Treatment for PCNSL differs from systemic lymphomas and consists of different chemotherapy regimens, all containing systemic high-dose methotrexate (9, 10). Additionally, autologous stem cell transplantation is becoming more important, whereas radiotherapy is only rarely applied, e.g. in selected cases not suitable for aggressive systemic therapy (11, 12). The median community based overall survival has increased from 8.9 to 10 months to 25.3 months in recent studies, with a 5-year survival rate of 38% (7, 8, 13). Known favorable prognostic factors are high Karnofsky performance status and younger age at the time of diagnosis and treatment initiation (8). The primary role of neurosurgery is focused on safe and efficient planning and procedure of surgical biopsy to acquire tissue for histopathological diagnosis. Diagnostic biopsy represents the most time-critical step in the further course of the disease, resulting in a timespan between onset of symptoms to histopathological diagnosis described to range between 35 and 75 days, whereas more recent studies showed a decrease in this period (8, 13, 14). As a result, the diagnostic delay from the first imaging to definitive histopathological diagnosis is found to be significantly longer in PCNSL compared to, e.g., glioblastoma (15).
Most reviews of PCNSL encompass recent advances of systemic treatment from a neuro-oncological viewpoint. The aim of this particular review was to focus on the period between imaging and diagnosis, surgical planning and potential pitfalls in diagnosis, especially after corticosteroid therapy. Furthermore, we want to provide an overview of the diagnostic workup, which should be performed until histopathological confirmation.
Imaging
If PCNSL is suspected, early competent imaging analysis is crucial as it strongly influences further decision making and helps avoid hasty corticosteroid treatment (CST) before surgery. In clinical practice, unenhanced computed tomography (CT) is mostly used as first imaging resource after emergence of symptoms. Sometimes the classical location and appearance in CT imaging can already be indicative of PCNSL, radiographically, as CT shows an iso- to hyperdense lesion due to the hypercellularity and the relatively high ratio of the nucleus to the cytoplasm in PCNSL (Figure 1A) (16, 17). Though magnetic resonance imaging (MRI) is warranted for the imaging modality of choice for diagnosis and surgical planning, its accessibility during nights and weekends could be reduced. Upon imaging, typical regions where PCNSL can be found are periventricular, in the corpus callosum and deep gray matter (9, 18). In 30–48% of all cases PCNSL show multiple lesions (13, 17, 19). Classic findings in MRI are iso- to hypointense lesions on unenhanced T1-weighted MRI and iso- to hyperintense on T2-weighted MRI sequences (Figure 1C) (17, 20). In immunocompetent patients usually there is a strong to moderate homogenous contrast enhancement (Figure 1B), but rarely atypical enhancement patterns and cases with no enhancement have been described (17–19). Advanced imaging like diffusion-weighted imaging (DWI) and spectroscopy can help distinguish the lesion from other entities in atypical cases (21). DWI is usually restricted due to high cellularity, resulting in a hyperintense signal b-1000 and hypointense signal on ADC maps (Figures 1D, E) (17, 21, 22). Furthermore, low ADC values where shown to be a surrogate parameter for cellular density and potentially predict outcome (23). Spectroscopy usually shows a large choline peak, decreased N-acetylaspartate (NAA), a decrease of creatine and an increase in lactate and lipids (Figure 1F) (17, 19). Additional information can be obtained by performing cerebral 18F-Fluorodeoxyglucose-positrone-emission tomography (FDG-PET). FDG-PET analysis hereby focuses mainly on standardized uptake values (SUV), which are higher in PCNSL compared to glioblastomas (24).
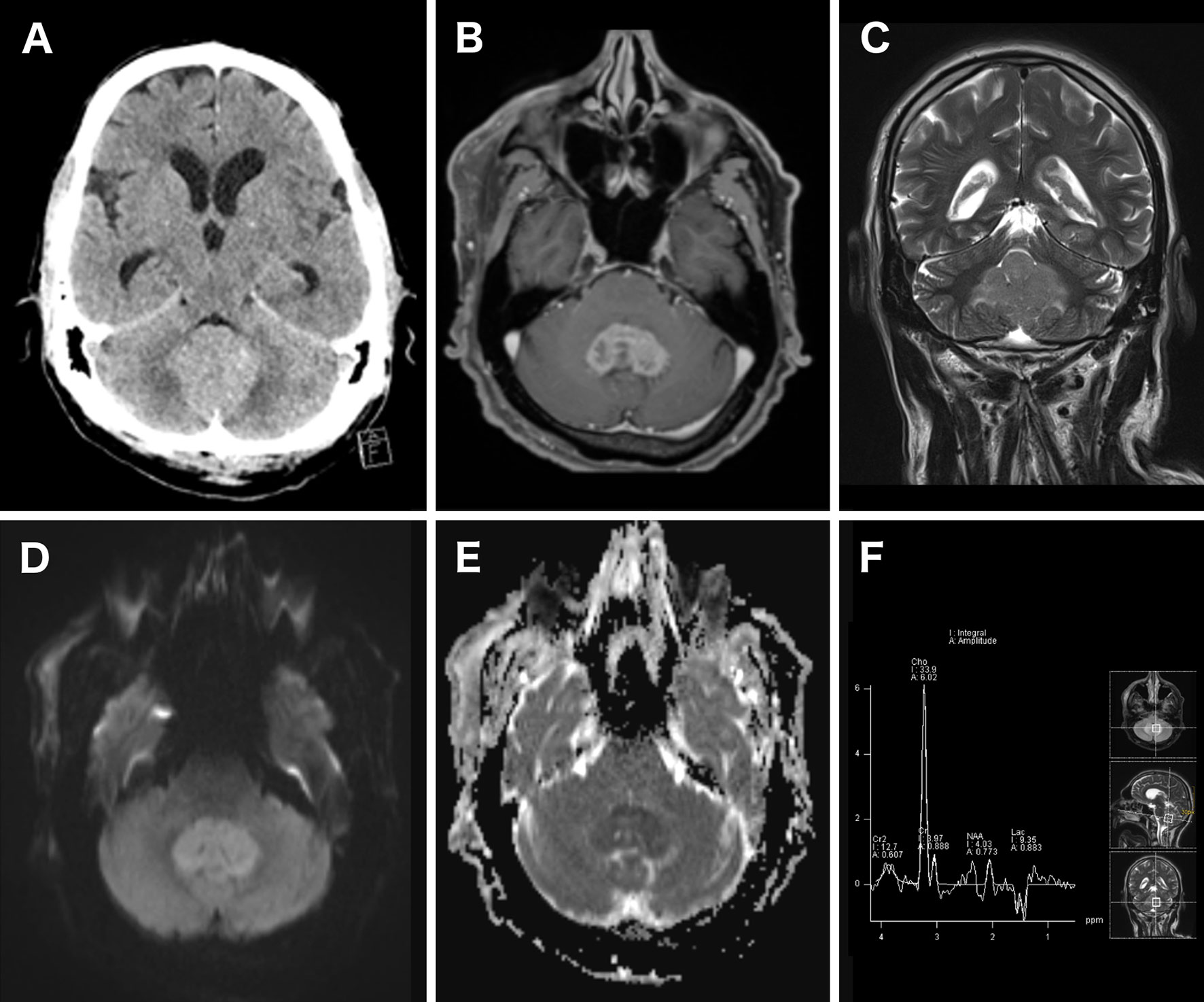
Figure 1 Imaging of a patient with a histopathological proven PCNSL. Unenhanced CT scan showed a hyperdense cerebellar lesion (A). Contrast-enhanced T1-weighted axial MRI showed a strong and homogenous contrast enhancement of the lesion (B). The lesion homogeneously appeared hyperintense in T2-weighted MRI (C). Diffusion restriction was detected as well, resulting in a bright DWI (b = 1,000) (D) and dark ADC map signal (E). 1H-MR spectroscopy showed an increased choline peak and decreased creatinine and N-acetylaspartat (F).
Recent studies on the use of radiomics, based on either MRI or FDG-PET, to differentiate PCNSL from other entities, particularly glioblastoma, have shown promising results (25–29) and wider use in practice is desirable, as quantitative imaging or the combined analysis of a radiologist and radiomics provide better diagnostic results than radiologists alone (26, 29). However, diagnostic models for PCNSL have been defined in retrospective studies with small patient cohorts and need to be validated in large data sets and in prospective multicenter studies. Solving other challenges of quantitative imaging techniques such as reproducibility, standardization, and different imaging protocols between different centers should only be a matter of time (30).
Diagnostic Workup
The diagnostic workup of PCNSL has the aim to rule out a systemic involvement and should either be performed while waiting for surgery or for the histopathological result. The preclusion of systemic disease is important as it influences the treatment regime and therefore time to initiate chemotherapy can be shortened. An interdisciplinary effort of neurology, radiology, neurosurgery, neuropathology and medical oncology is necessary to complete the accurate diagnosis of a PCNSL and to determine the extent and degree of the disease.
If spinal symptoms are suspected, MRI of the complete neuroaxis should be performed to rule out spinal or meningeal involvement (31).
Independent of visual symptoms, ocular examination including fundoscopy should be performed to determine potential ocular involvement (9), which can be found in 15 to 25% of all patients and needs further ophthalmological therapy (32).
Furthermore, staging should contain at least a contrast-enhanced CT scan of the chest, abdomen and pelvis, testicular ultrasound in older men and bone marrow biopsy (9, 31).
In patients without systemic involvement in contrast-enhanced CT, 18F-Fluorodeoxyglucose-PET revealed systemic PCNSL in 8% (33, 34) but it is not as easily available as contrast-enhanced CT and therefore not performed on a regular base.
Lumbar puncture should be performed after the preclusion of contraindication in imaging as CSF cytomorphology and flow cytometric analysis potentially allow a definitive diagnosis and can in some cases obviate the need for surgery (9). However, the diagnostic yield of cytomorphologic lumbar puncture is only 6–13.3%, which inevitably requires biopsy in most cases (35–38). Only if lumbar puncture successfully acquires the diagnosis, the inherent risk of brain biopsy can be avoided. However, prolonged time to therapy could decrease the outcome in PCNSL (15), and thus lumbar puncture should not delay surgery in clinical practice. As the period from imaging to histopathological diagnosis has been described to be as long as 28 days (14) and the time from imaging to biopsy 19 days (15), LP and CSF analysis could be performed without delay of surgery in many cases if performed early in the clinical course. Notably, recent research on additional analyses in liquid biopsy of CSF and serum showed promising results harboring great potential to possibly replace diagnostic brain biopsy in PCNSL. CSF analysis for CD79B and MYD88 or diagnostic markers like CXCL-13, B2M, and neopterin are promising prospects, yet there is currently not enough evidence for standardized clinical use (39–41) and therefore brain biopsy remains the current gold standard for diagnosis. However, digital PCR of cell-free DNA for mutations in the MYD88 gene showed a sensitivity and specificity of up to 100% in a small series by Yamagishi et al. (42). Moreover, the detection of mutations in genes such as MYD88 or CD79B in liquid biopsy could have additional clinical implications, as these mutations could enable targeted therapies (43). Because liquid biopsy has the advantage of being minimally invasive and does not require scheduling, it could also help reduce diagnostic delays once the findings allow for broad clinical application. The impact of CST on the diagnostic accuracy of liquid biopsy has not yet been studied.
Surgical Workup
Stereotactic or frameless biopsy is the standard neurosurgical procedure for acquiring tissue in PCNSL and achieves a diagnostic yield of more than 91% (13, 44). Overall, stereotactic biopsy is accompanied a periprocedural morbidity of 8.5% and mortality of 0.9% (45). Additional periprocedural techniques like frozen section (46) and 5-ALA fluorescence (47, 48) might help determine whether diagnostic tissue has been acquired (Figure 2). 5-ALA-Fluorescence in PCNSL is described in 79–83% with a high positive predictive value for diagnostic tissue (47, 49). Furthermore, positive 5-ALA fluorescence can help shorten surgical duration (50). Open surgery was historically without significance in PCNSL patients due to worse outcomes in older studies (51–53). However, this tenet has been challenged in a recent study by Weller et al. (54). The authors described an improvement of progression-free survival (PFS) and overall survival (OS) after resection compared to biopsy in their post hoc analysis. Yet, patients with single lesions more often underwent resection, and after further statistical adjustment for the number of lesions, only advantage for PFS remained. Other studies came to a similar conclusion reflecting that this might be due to a selection bias for patients with single lesions and patients without the involvement of deep structures (55–57). In contrast, a large retrospective study by Houillier et al. including 1002 patients did not find a difference in outcome regarding the type of surgery (8). As surgical procedures evolved, surgical resection appears to be safe nowadays in selected cases, but its clinical significance still must be determined in further studies (58).
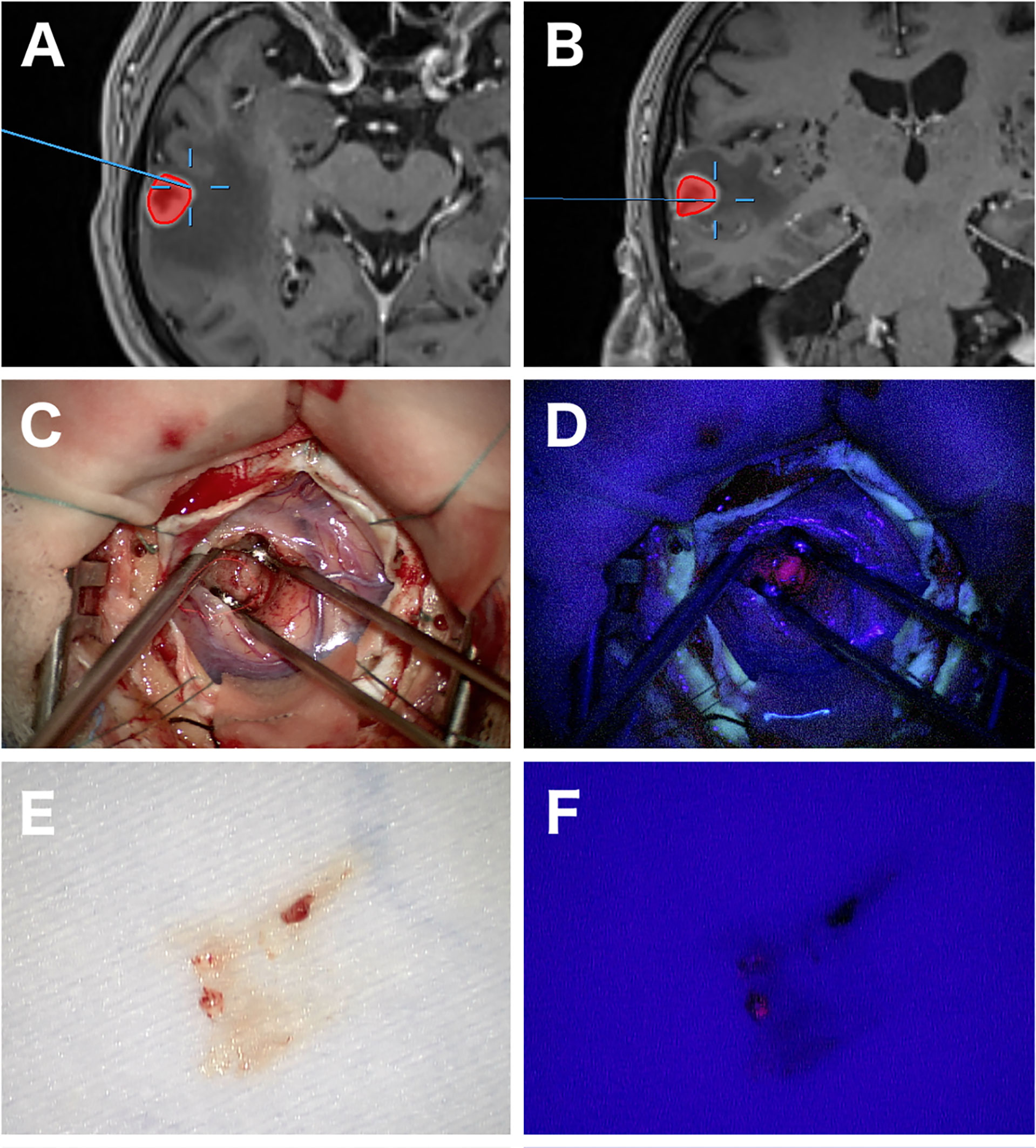
Figure 2 Images of open biopsy of a PCNSL with the aid of 5-ALA fluorescence. Axial and coronal navigational MRI showing a heterogeneous contrast enhancing lesion in the right temporal lobe and the exact location of the biopsy (A, B). Intraoperative images at the biopsy location with strong 5-ALA fluorescence (C, D). A tissue specimen later diagnosed as PCNSL showing positive fluorescence under 405 nm wavelength blue light in another patient (E, F).
Preoperative Corticosteroid Therapy
Preoperative corticosteroid therapy in PCSNL has been a point of debate for many years. As PCNSL cells may react with cell arrest and apoptosis to corticosteroid therapy (59–61), transient tumor shrinkage and morphological changes can be seen in up to 50%, potentially hindering histopathological diagnosis (62, 63). This phenomenon also gave PCNSL the name “ghost” or “vanishing” tumor (64). This was formerly described as diagnostic for PCNSL, but is obsolete nowadays, as also other tumor entities were identified to show transient regression after CST (65–68). Retrospective studies showed an increased rate of inconclusive biopsies after CST of 11–22% (13, 14, 69), while recent retrospective studies showed that there is not necessarily a decrease in the diagnostic yield after preoperative CST (44, 70–72). However, besides a potential selection bias, these studies lacked the statistical power to identify small differences in diagnostic rates. A recent combined analysis of the available studies showed an odds ratio of 3.3 for inconclusive biopsy after CST (44). Although absolute numbers of inconclusive biopsies decreased, the odds ratio for inconclusive biopsy after preoperative CST remained the same. Thus, CST should not be administered before surgery if PCNSL is suspected and tissue must be acquired (9). Yet, the clinical condition of patients sometimes require preoperative CST treatment, resulting in most PCNSL patients receiving CST preoperatively (13). A single dose of CST can already pose a challenge for histopathological diagnosis in some cases but prolonged CST led to a higher incidence of inconclusive biopsies in one study (69), therefore accurate evaluation of duration and dosage of CST is mandatory for further workup. The optimal management of PCNSL patients with preoperative CST remains controversial. The exact time of CST tapering that is necessary to overcome the influence of CST on diagnostic yield is not defined (13, 44). In practice, if a contrast-enhanced lesion shows distinct regression, surgery is usually delayed until new progression is evident in serial MRI (9, 73). In case of a PCNSL that only reacts with little or no regression, the risk of inconclusive biopsy must be weighed up against the significant delay to definitive therapy when biopsy is delayed.
Discussion
Diagnosis and therapy of PCNSL is a multidisciplinary task, with brain biopsy as performed by neurosurgery being at the center of it. These multiple intersections between different disciplines like neurology, radiology, neurosurgery, pathology and oncology harbor a risk of unnecessary delays and might account for the prolonged diagnostic period of PCNSL compared to other brain malignancies (15). At present, no clear evidence has been found that resection offers an outcome advantage for the patient. Therefore, and contrary to many other brain malignancies, neurosurgery cannot influence the outcome of the patient with resection itself. This highlights the potential benefit of non- or minimally-invasive diagnostic tools that have lower morbidity than surgery. Liquid biopsy, alone or along with quantitative imaging techniques, has great potential to replace stereotactic biopsy in diagnosis of PCNSL especially in radiologically typical cases. Although the available data do not allow a standard application, Yamagishi et al. described a case of pontine PCNSL that was successfully treated on the basis of diagnosis by imaging and MYD88 mutation analysis in CSF (42). In such cases where brain biopsy is expected to cause high morbidity and when PCSNL is highly suspected, diagnosis by liquid biopsy should be considered. However, based on the available evidence, brain biopsy remains the current gold standard and the goal for neurosurgery must be a most efficient management and safe diagnostic brain biopsy to facilitate adjuvant treatment. Even if biopsy is performed early in the clinical course, there is still a median period of 14 days to definitive adjuvant treatment (7). This time needs to be used efficiently to avoid further delay of definitive treatment and its potential negative influence on the outcome. A major clinical issue that may be responsible for distinct diagnostic delay is preoperative CST, especially if CST must be stopped before surgery due to regression (13). If clinically possible, CST should therefore strictly be avoided in potential PCNSL patients as it increases the rate of inconclusive biopsies (44). In patients with no history of malignancy or immunosuppression and periventricular tumors upon initial CT, we recommend withholding the patient from CST treatment until MRI provides further diagnostic information and subsequent steps of diagnosis should be executed shortly after. It remains unclear how long the pause of CST treatment should be carried out. Currently, many centers wait for another progression of PCNSL upon MRI, which leads to a significant delay. The ongoing debate on the clinical impact of preoperative CST must be clarified through future prospective studies. However, current evidence shows that the risk of inconclusive biopsies is significantly higher with preoperative CST treatment (44, 62). Even though absolute rates of inconclusive biopsies after CST are quite low in recent studies, it is recommendable to avoid this issue in the first place if possible.
To sum up, a potential PCNSL must be recognized as early as possible to avoid preoperative CST and schedule early surgery. Next, full diagnostic workup of PCNSL should be initiated while waiting on surgery or histopathological results to reduce delay in therapy (Figure 3).
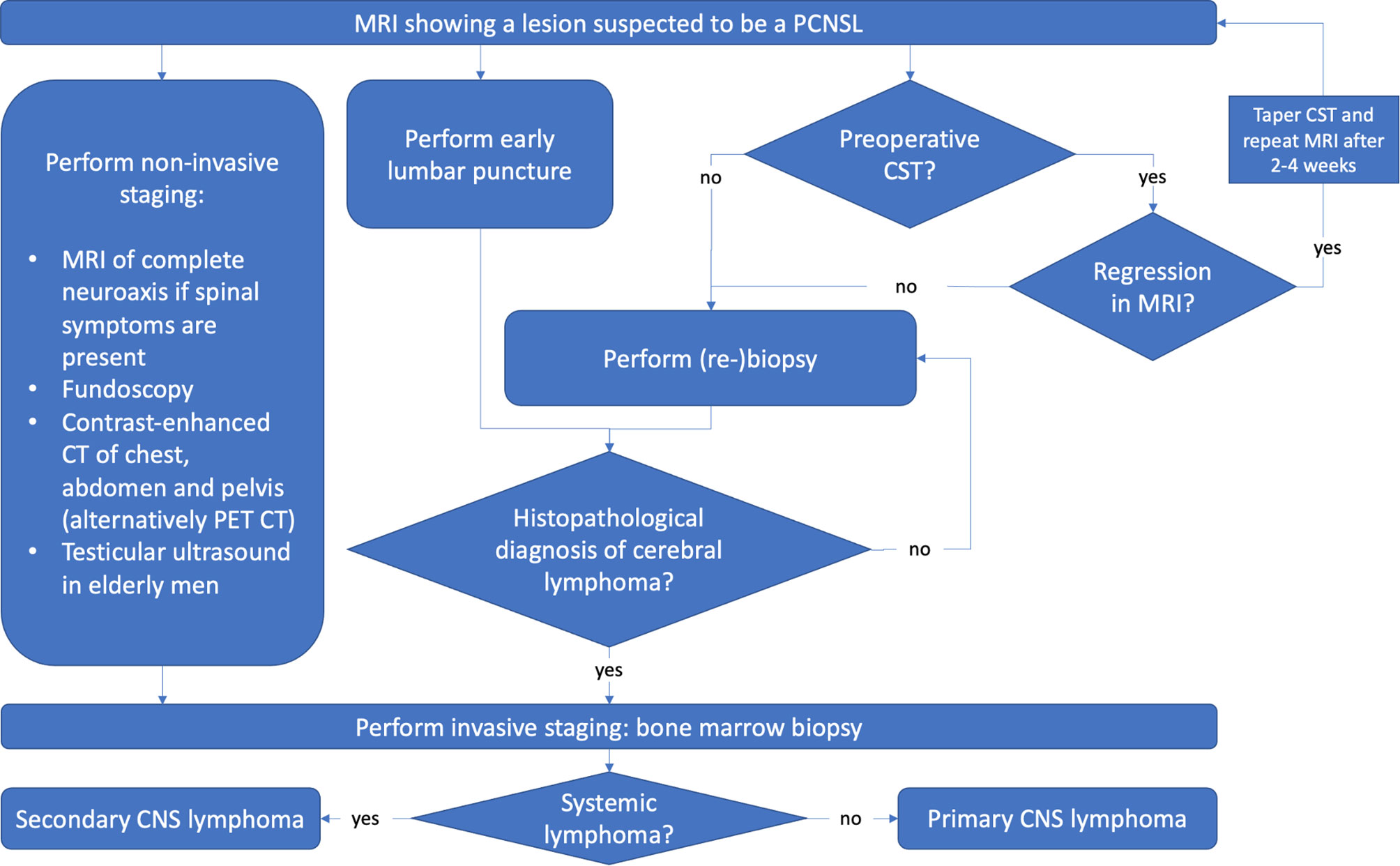
Figure 3 A systematic workflow for diagnosis of PCNSL. Ideally, lumbar puncture and CSF analysis should be performed early without delaying biopsy. Non-invasive staging should be performed while waiting for biopsy or histopathological results to reduce diagnostic delay.
Author Contributions
FS, DP, BP, FM, and CF contributed to conception and design of the study. FS wrote the first draft of the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The article processing charge was covered by the Open Access Publishing Fund of Karl Landsteiner University of Health Sciences, Krems, Austria.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors want to appreciate the contribution of NÖ Landesgesundheitsagentur, legal entity of University Hospitals in Lower Austria, for providing the organizational framework to conduct this research. The authors also acknowledge support by Open Access Publishing Fund of Karl Landsteiner University of Health Sciences, Krems, Austria.
References
1. Ferreri AJM, Marturano E. Primary CNS Lymphoma. Best Pract Res Clin Haematol (2012) 25:119–30. doi: 10.1016/j.beha.2011.12.001
2. Wöhrer A, Waldhör T, Heinzl H, Hackl M, Feichtinger J, Gruber-Mösenbacher U, et al. The Austrian Brain Tumour Registry: A Cooperative Way to Establish a Population-Based Brain Tumour Registry. J Neurooncol (2009) 95:401–11. doi: 10.1007/s11060-009-9938-9
3. Eloranta S, Brånvall E, Celsing F, Papworth K, Ljungqvist M, Enblad G, et al. Increasing Incidence of Primary Central Nervous System Lymphoma But No Improvement in Survival in Sweden 2000-2013. Eur J Haematol (2018) 100:61–8. doi: 10.1111/ejh.12980
4. Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ. Age, Gender, and Racial Differences in Incidence and Survival in Primary CNS Lymphoma. Br J Cancer (2011) 105:1414–8. doi: 10.1038/bjc.2011.357
5. Kadan-Lottick NS, Skluzacek MC, Gurney JG. Decreasing Incidence Rates of Primary Central Nervous System Lymphoma. Cancer (2002) 95:193–202. doi: 10.1002/cncr.10643
6. Bessell EM, Dickinson P, Dickinson S, Salmon J. Increasing Age at Diagnosis and Worsening Renal Function in Patients With Primary Central Nervous System Lymphoma. J Neurooncol (2011) 104:191–3. doi: 10.1007/s11060-010-0457-5
7. Neuhauser M, Roetzer T, Oberndorfer S, Kitzwoegerer M, Payer F, Unterluggauer JJ, et al. Increasing Use of Immunotherapy and Prolonged Survival Among Younger Patients With Primary CNS Lymphoma: A Population-Based Study. Acta Oncol (Madr) (2019) 58:967–76. doi: 10.1080/0284186X.2019.1599137
8. Houillier C, Soussain C, Ghesquières H, Soubeyran P, Chinot O, Taillandier L, et al. Management and Outcome of Primary CNS Lymphoma in the Modern Era: An LOC Network Study. Neurology (2020) 94:e1027–e1039. doi: 10.1212/WNL.0000000000008900
9. Hoang-Xuan K, Bessell E, Bromberg J, Hottinger AF, Preusser M, Rudà R, et al. Diagnosis and Treatment of Primary CNS Lymphoma in Immunocompetent Patients: Guidelines From the European Association for Neuro-Oncology. Lancet Oncol (2015) 16:e322–e332. doi: 10.1016/S1470-2045(15)00076-5
10. Ferreri AJM, Cwynarski K, Pulczynski E, Ponzoni M, Deckert M, Politi LS, et al. Chemoimmunotherapy With Methotrexate, Cytarabine, Thiotepa, and Rituximab (MATRix Regimen) in Patients With Primary CNS Lymphoma: Results of the First Randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) Phase 2 Trial. Lancet Haematol (2016) 3:e217–e227. doi: 10.1016/S2352-3026(16)00036-3
11. Seidel C, Viehweger C, Kortmann R-D. Is There an Indication for First Line Radiotherapy in Primary CNS Lymphoma? Cancers (Basel) (2021) 13:2580. doi: 10.3390/cancers13112580
12. Thiel E, Korfel A, Martus P, Kanz L, Griesinger F, Rauch M, et al. High-Dose Methotrexate With or Without Whole Brain Radiotherapy for Primary CNS Lymphoma (G-PCNSL-SG-1): A Phase 3, Randomised, non-Inferiority Trial. Lancet Oncol (2010) 11:1036–47. doi: 10.1016/S1470-2045(10)70229-1
13. Velasco R, Mercadal S, Vidal N, Alañá M, Barceló MI, Ibáñez-Juliá MJ, et al. Diagnostic Delay and Outcome in Immunocompetent Patients With Primary Central Nervous System Lymphoma in Spain: A Multicentric Study. J Neurooncol (2020) 148:545–54. doi: 10.1007/s11060-020-03547-z
14. Haldorsen IS, Espeland A, Larsen JL, Mella O. Diagnostic Delay in Primary Central Nervous System Lymphoma. Acta Oncol (Madr) (2005) 44:728–34. doi: 10.1080/02841860500256272
15. Cerqua R, Balestrini S, Perozzi C, Cameriere V, Renzi S, Lagalla G, et al. Diagnostic Delay and Prognosis in Primary Central Nervous System Lymphoma Compared With Glioblastoma Multiforme. Neurol Sci (2016) 37:23–9. doi: 10.1007/s10072-015-2353-4
16. Go JL, Lee SC, Kim PE. Imaging of Primary Central Nervous System Lymphoma. Neurosurg Focus (2006) 21:1–6. doi: 10.3171/foc.2006.21.5.5
17. Haldorsen IS, Espeland A, Larsson EM. Central Nervous System Lymphoma: Characteristic Findings on Traditional and Advanced Imaging. Am J Neuroradiol (2011) 32:984–92. doi: 10.3174/ajnr.A2171
18. Mansour A, Qandeel M, Abdel-Razeq H, Abu Ali HA. MR Imaging Features of Intracranial Primary CNS Lymphoma in Immune Competent Patients. Cancer Imaging (2014) 14:1–9. doi: 10.1186/1470-7330-14-22
19. Küker W, Nägele T, Korfel A, Heckl S, Thiel E, Bamberg M, et al. Primary Central Nervous System Lymphomas (PCNSL): MRI Features at Presentation in 100 Patients. J Neurooncol (2005) 72:169–77. doi: 10.1007/s11060-004-3390-7
20. Gliemroth J, Kehler U, Gaebel C, Arnold H, Missler U. Neuroradiological Findings in Primary Cerebral Lymphomas of non-AIDS Patients. Clin Neurol Neurosurg (2003) 105:78–86. doi: 10.1016/S0303-8467(02)00105-1
21. Calli C, Kitis O, Yunten N, Yurtseven T, Islekel S, Akalin T. Perfusion and Diffusion MR Imaging in Enhancing Malignant Cerebral Tumors. Eur J Radiol (2006) 58:394–403. doi: 10.1016/j.ejrad.2005.12.032
22. Zacharia TT, Law M, Naidich TP, Leeds NE. Central Nervous System Lymphoma Characterization by Diffusion-Weighted Imaging and MR Spectroscopy. J Neuroimaging (2008) 18:411–7. doi: 10.1111/j.1552-6569.2007.00231.x
23. Barajas RF, Chang JS, Sneed PK, Segal MR, McDermott MW, Cha S. Distinguishing Recurrent Intra-Axial Metastatic Tumor From Radiation Necrosis Following Gamma Knife Radiosurgery Using Dynamic Susceptibility-Weighted Contrast-Enhanced Perfusion MR Imaging. AJNR Am J Neuroradiol (2009) 30:367–72. doi: 10.3174/ajnr.A1362
24. Zhou W, Wen J, Hua F, Xu W, Lu X, Yin B, et al. 18 F-FDG PET/CT in Immunocompetent Patients With Primary Central Nervous System Lymphoma: Differentiation From Glioblastoma and Correlation With DWI. Eur J Radiol (2018) 104:26–32. doi: 10.1016/j.ejrad.2018.04.020
25. Kim Y, Cho H-H, Kim ST, Park H, Nam D, Kong DS. Radiomics Features to Distinguish Glioblastoma From Primary Central Nervous System Lymphoma on Multi-Parametric MRI. Neuroradiology (2018) 60:1297–305. doi: 10.1007/s00234-018-2091-4
26. Suh HB, Choi YS, Bae S, Ahn SS, Chang JH, Kang SG, et al. Primary Central Nervous System Lymphoma and Atypical Glioblastoma: Differentiation Using Radiomics Approach. Eur Radiol (2018) 28:3832–9. doi: 10.1007/s00330-018-5368-4
27. Chen C, Zheng A, Ou X, Wang J, Ma X. Comparison of Radiomics-Based Machine-Learning Classifiers in Diagnosis of Glioblastoma From Primary Central Nervous System Lymphoma. Front Oncol (2020) 10:1151. doi: 10.3389/fonc.2020.01151
28. Kong Z, Jiang C, Zhu R, Feng S, Wang Y, Li J, et al. 18f-FDG-PET-Based Radiomics Features to Distinguish Primary Central Nervous System Lymphoma From Glioblastoma. NeuroImage Clin (2019) 23:101912. doi: 10.1016/j.nicl.2019.101912
29. Xia W, Hu B, Li H, Geng C, Wu Q, Yang L, et al. Multiparametric-MRI-Based Radiomics Model for Differentiating Primary Central Nervous System Lymphoma From Glioblastoma: Development and Cross-Vendor Validation. J Magn Reson Imaging (2021) 53:242–50. doi: 10.1002/jmri.27344
30. Ibrahim A, Primakov S, Beuque M, Woodruff HC, Halilaj I, Wu G, et al. Radiomics for Precision Medicine: Current Challenges, Future Prospects, and the Proposal of a New Framework. Methods (2021) 188:20–9. doi: 10.1016/j.ymeth.2020.05.022
31. Abrey LE, Batchelor TT, Ferreri AJM, Gospodarowicz M, Pulczynski EJ, Zucca E, et al. Report of an International Workshop to Standardize Baseline Evaluation and Response Criteria for Primary CNS Lymphoma. J Clin Oncol (2005) 23:5034–43. doi: 10.1200/JCO.2005.13.524
32. Choi JY, Kafkala C, Foster CS. Primary Intraocular Lymphoma: A Review. Semin Ophthalmol (2006) 21:125–33. doi: 10.1080/08820530500350498
33. Mohile NA, Deangelis LM, Abrey LE. The Utility of Body FDG PET in Staging Primary Central Nervous System Lymphoma. Neuro Oncol (2008) 10:223–8. doi: 10.1215/15228517-2007-061
34. Bertaux M, Houillier C, Edeline V, Habert MO, Mokhtari K, Giron A, et al. Use of FDG-PET/CT for Systemic Assessment of Suspected Primary Central Nervous System Lymphoma: A LOC Study. J Neurooncol (2020) 148:343–52. doi: 10.1007/s11060-020-03525-5
35. Morell AA, Shah AH, Cavallo C, Eichberg DG, Sarkiss CA, Benveniste R, et al. Diagnosis of Primary Central Nervous System Lymphoma: A Systematic Review of the Utility of CSF Screening and the Role of Early Brain Biopsy. Neuro-Oncol Pract (2019) 6:415–23. doi: 10.1093/nop/npz015
36. Hegde U, Filie A, Little RF, Janik JE, Grant N, Steinberg SM, et al. High Incidence of Occult Leptomeningeal Disease Detected by Flow Cytometry in Newly Diagnosed Aggressive B-Cell Lymphomas at Risk for Central Nervous System Involvement: The Role of Flow Cytometry Versus Cytology. Blood (2005) 105:496–502. doi: 10.1182/blood-2004-05-1982
37. Orfao A, Quijano S, López A, Sancho JM, Panizo C, Debén G, et al. Identification of Leptomeningeal Disease in Aggressive B-Cell non-Hodgkin’s Lymphoma: Improved Sensitivity of Flow Cytometry. J Clin Oncol (2009) 27:1462–9. doi: 10.1200/JCO.2008.17.7089
38. Schroers R, Baraniskin A, Heute C, Vorgerd M, Brunn A, Kuhnhenn J, et al. Diagnosis of Leptomeningeal Disease in Diffuse Large B-Cell Lymphomas of the Central Nervous System by Flow Cytometry and Cytopathology. Eur J Haematol (2010) 85:520–8. doi: 10.1111/j.1600-0609.2010.01516.x
39. Hiemcke-jiwa LS, Leguit RJ, Snijders TJ, Jiwa NM, Kuiper JJW, Weger RA, et al. Critical Reviews in Oncology / Hematology Molecular Analysis in Liquid Biopsies for Diagnostics of Primary Central Nervous System Lymphoma: Review of Literature and Future Opportunities. Crit Rev Oncol / Hematol (2018) 127:56–65. doi: 10.1016/j.critrevonc.2018.05.010
40. van Westrhenen A, Smidt LCA, Seute T, Nierkens S, Stork ACJ, Minnema MC, et al. Diagnostic Markers for CNS Lymphoma in Blood and Cerebrospinal Fluid: A Systematic Review. Br J Haematol (2018) 182:384–403. doi: 10.1111/bjh.15410
41. Cirillo M, Craig AFM, Borchmann S, Kurtz DM. Liquid Biopsy in Lymphoma: Molecular Methods and Clinical Applications. Cancer Treat Rev (2020) 91:1–22. doi: 10.1016/j.ctrv.2020.102106
42. Yamagishi Y, Sasaki N, Nakano Y, Matushita Y, Omura T, Shimizu S, et al. Liquid Biopsy of Cerebrospinal Fluid for MYD88 L265P Mutation is Useful for Diagnosis of Central Nervous System Lymphoma. Cancer Sci (2021) 112:4702–10. doi: 10.1111/cas.15133
43. Lionakis MS, Dunleavy K, Roschewski M, Widemann BC, Butman JA, Schmitz R, et al. Inhibition of B Cell Receptor Signaling by Ibrutinib in Primary CNS Lymphoma. Cancer Cell (2017) 31:833–843.e5. doi: 10.1016/j.ccell.2017.04.012
44. Scheichel F, Marhold F, Pinggera D, Kiesel B, Rossmann T, Popadic B, et al. Influence of Preoperative Corticosteroid Treatment on Rate of Diagnostic Surgeries in Primary Central Nervous System Lymphoma: A Multicenter Retrospective Study. BMC Cancer (2021) 21:754. doi: 10.1186/s12885-021-08515-y
45. Khatab S, Spliet W, Woerdeman PA. Frameless Image-Guided Stereotactic Brain Biopsies: Emphasis on Diagnostic Yield. Acta Neurochir (Wien) (2014) 156:1441–50. doi: 10.1007/s00701-014-2145-2
46. Brainard JA, Prayson RA, Barnett GH. Frozen Section Evaluation of Stereotactic Brain Biopsies: Diagnostic Yield at the Stereotactic Target Position in 188 Cases. Arch Pathol Lab Med (1997) 121:481–4.
47. Kiesel B, Millesi M, Woehrer A, Furtner J, Bavand A, Roetzer T, et al. 5-ALA–induced Fluorescence as a Marker for Diagnostic Tissue in Stereotactic Biopsies of Intracranial Lymphomas: Experience in 41 Patients. Neurosurg Focus (2018) 44:E7. doi: 10.3171/2018.3.FOCUS1859
48. Grossman R, Nossek E, Shimony N, Raz M, Ram Z. Intraoperative 5-Aminolevulinic Acid-Induced Fluorescence in Primary Central Nervous System Lymphoma. Evidence-Based Hematol (2014) 120:317–24. doi: 10.3171/2013.9.JNS131076
49. Yamamoto J, Kitagawa T, Akiba D, Nishizawa S. 5-Aminolevulinic Acid-Induced Fluorescence in Cerebellar Primary Central Nervous System Lymphoma: A Case Report and Literature Review. Turk Neurosurg (2015) 25:796–800. doi: 10.5137/1019-5149.JTN.10594-14.1
50. Shofty B, Richetta C, Haim O, Kashanian A, Gurevich A, Grossman R. 5-ALA-Assisted Stereotactic Brain Tumor Biopsy Improve Diagnostic Yield. Eur J Surg Oncol (2019) 45:2375–8. doi: 10.1016/j.ejso.2019.07.001
51. Bataille B, Delwail V, Menet E, Vandermarcq P, Ingrand P, Wager M, et al. Primary Intracerebral Malignant Lymphoma: Report of 248 Cases. J Neurosurg (2000) 92:261–6. doi: 10.3171/jns.2000.92.2.0261
52. Henry JM, Heffner RR, Dillard SH, Earle KM, Davis RL. Primary Malignant Lymphomas of the Central Nervous System. Cancer (1974) 34:1293–302. doi: 10.1002/1097-0142(197410)34:4<1293::aid-cncr2820340441>3.0.co;2-p
53. DeAngelis LM, Yahalom J, Heinemann MH, Cirrincione C, Thaler HT, Krol G. Primary CNS Lymphoma: Combined Treatment With Chemotherapy and Radiotherapy. Neurology (1990) 40:80–6. doi: 10.1212/wnl.40.1.80
54. Weller M, Martus P, Roth P, Thiel E, Korfel A. Surgery for Primary CNS Lymphoma? Challenging a Paradigm. Neuro Oncol (2012) 14:1481–4. doi: 10.1093/neuonc/nos159
55. Wu S, Wang J, Liu W, Hu F, Zhao K, Jiang W, et al. The Role of Surgical Resection in Primary Central Nervous System Lymphoma: A Single-Center Retrospective Analysis of 70 Patients. BMC Neurol (2021) 21:1–9. doi: 10.1186/s12883-021-02227-3
56. Rae AI, Mehta A, Cloney M, Kinslow CJ, Wang TJC, Bhagat G, et al. Craniotomy and Survival for Primary Central Nervous System Lymphoma. Clin Neurosurg (2019) 84:935–44. doi: 10.1093/neuros/nyy096
57. Schellekes N, Barbotti A, Abramov Y, Sitt R, Di Meco F, Ram Z, et al. Resection of Primary Central Nervous System Lymphoma: Impact of Patient Selection on Overall Survival. J Neurosurg (2021) 135:1016–25. doi: 10.3171/2020.9.JNS201980
58. Cloney MB, Sonabend AM, Yun J, Yang J, Iwamoto F, Singh S, et al. The Safety of Resection for Primary Central Nervous System Lymphoma: A Single Institution Retrospective Analysis. J Neurooncol (2017) 132:189–97. doi: 10.1007/s11060-016-2358-8
59. Roth P, Happold C, Weller M. Corticosteroid Use in Neuro-Oncology: An Update. Neuro-Oncol Pract (2015) 2:6–12. doi: 10.1093/nop/npu029
60. Sionov RV, Spokoini R, Kfir-Erenfeld S, Cohen O, Yefenof E. Mechanisms Regulating the Susceptibility of Hematopoietic Malignancies to Glucocorticoid-Induced Apoptosis. Adv Cancer Res (2008) 101:127–248. doi: 10.1016/S0065-230X(08)00406-5
61. Miller AL, Webb MS, Copik AJ, Wang Y, Johnson BH, Kumar R, et al. P38 Mitogen-Activated Protein Kinase (MAPK) is a Key Mediator in Glucocorticoid-Induced Apoptosis of Lymphoid Cells: Correlation Between P38 MAPK Activation and Site-Specific Phosphorylation of the Human Glucocorticoid Receptor at Serine 211. Mol Endocrinol (2005) 19:1569–83. doi: 10.1210/me.2004-0528
62. Brück W, Brunn A, Klapper W, Kuhlmann T, Metz I, Paulus W, et al. Differenzialdiagnose Lymphoider Infiltrate Im Zentralnervensystem: Erfahrungen Des Netzwerks Lymphome Und Lymphomatoide Läsionen Des Nervensystems. Pathologe (2013) 34:186–97. doi: 10.1007/s00292-013-1742-9
63. Önder E, Arikök AT, Önder S, Han Ü, Sorar M, Kertmen H, et al. Corticosteroid Pre-Treated Primary CNS Lymphoma: A Detailed Analysis of Stereotactic Biopsy Findings and Consideration of Interobserver Variability. Int J Clin Exp Pathol (2015) 8:7798–808.
64. Pirotte B, Levivier M, Goldman S, Brucher JM, Brotchi J, Hildebrand J. Glucocorticoid-Induced Long-Term Remission in Primary Cerebral Lymphoma: Case Report and Review of the Literature. J Neurooncol (1997) 32:63–9. doi: 10.1023/a:1005733416571
65. Hasegawa H, Pal D, Ramirez R, Ismail A, Marks P. Glioblastoma Multiforme Fades on CT Imaging After Dexamethasone Therapy. J Clin Neurosci (2009) 16:1707–8. doi: 10.1016/j.jocn.2009.02.024
66. Bromberg JEC, Siemers MD, Taphoorn MJB. Is a “Vanishing Tumor” Always a Lymphoma? Neurology (2002) 59:762–4. doi: 10.1212/WNL.59.5.762
67. Goh JJ, See SJ, Ang E, Ng WH. Vanishing Glioblastoma After Corticosteroid Therapy. J Clin Neurosci (2009) 16:1226–8. doi: 10.1016/j.jocn.2008.10.029
68. Zaki HS, Jenkinson MD, Du Plessis DG, Smith T, Rainov NG. Vanishing Contrast Enhancement in Malignant Glioma After Corticosteroid Treatment. Acta Neurochir (Wien) (2004) 146:841–5. doi: 10.1007/s00701-004-0282-8
69. Manoj N, Arivazhagan A, Mahadevan A, Bhat DI, Arvinda HR, Devi BI, et al. Central Nervous System Lymphoma: Patterns of Incidence in Indian Population and Effect of Steroids on Stereotactic Biopsy Yield. Neurol India (2014) 62:19–25. doi: 10.4103/0028-3886.128272
70. Porter AB, Giannini C, Kaufmann T, Lucchinetti CF, Wu W, Decker PA, et al. Primary Central Nervous System Lymphoma can be Histologically Diagnosed After Previous Corticosteroid Use: A Pilot Study to Determine Whether Corticosteroids Prevent the Diagnosis of Primary Central Nervous System Lymphoma. Ann Neurol (2008) 63:662–7. doi: 10.1002/ana.21366
71. Bullis CL, Maldonado-Perez A, Bowden SG, Yaghi N, Munger D, Wood MD, et al. Diagnostic Impact of Preoperative Corticosteroids in Primary Central Nervous System Lymphoma. J Clin Neurosci (2020) 72:287–91. doi: 10.1016/j.jocn.2019.10.010
72. Shaw A, Iyer V, Rooney N, Wragg R, Waits P, Roberts E, et al. Diagnosis of Primary Cerebral Lymphomas: Possible Value of PCR Testing in Equivocal Cases Requiring Rebiopsy. Br J Neurosurg (2014) 28:214–9. doi: 10.3109/02688697.2013.817531
Keywords: Primary central nervous system lymphoma (PCNSL), corticosteroid therapy, diagnostic workup, diagnostic delay, diagnostic yield
Citation: Scheichel F, Pinggera D, Popadic B, Sherif C, Marhold F and Freyschlag CF (2022) An Update on Neurosurgical Management of Primary CNS Lymphoma in Immunocompetent Patients. Front. Oncol. 12:884724. doi: 10.3389/fonc.2022.884724
Received: 26 February 2022; Accepted: 18 March 2022;
Published: 20 April 2022.
Edited by:
Luca Ricciardi, Sapienza University of Rome, ItalyReviewed by:
Antonella Mangraviti, Sant’Andrea Hospital, ItalyGiovanni Raffa, University of Messina, Italy
Copyright © 2022 Scheichel, Pinggera, Popadic, Sherif, Marhold and Freyschlag. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Franz Marhold, Franz.marhold@stpoelten.lknoe.at
 Florian Scheichel
Florian Scheichel Daniel Pinggera
Daniel Pinggera Branko Popadic
Branko Popadic Camillo Sherif
Camillo Sherif Franz Marhold
Franz Marhold Christian Franz Freyschlag
Christian Franz Freyschlag