Treatment Strategies for Congenital Diaphragmatic Hernia: Change Sometimes Comes Bearing Gifts
- 1Department of Medical and Surgical Neonatology, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy
- 2Department of Pediatric Surgery, McGovern Medical School at the University of Texas Health Science Center at Houston, Children’s Memorial Hermann Hospital, Houston, TX, United States
Objective: To report treatment strategies’ evolution and its impact on congenital diaphragmatic hernia (CDH) outcome.
Design: Registry-based cohort study using the CDH Study Group database, 1995–2013.
Setting: International multicenter database.
Patients: CDH patients entered into the registry. Late presenters or patients with very incomplete data were excluded. Patients were divided into three Eras (1995–2000; 2001–2006; 2007–2013).
Main outcome measures: Treatment strategies and outcomes. One-way ANOVA, X2 test, and X2 test for trend were used. A Sydak-adjusted p < 0.0027 was considered significant. Prevalence or mean (SE) are reported.
Results: Patients: 8,603; included: 7,716; Era I: 2,146; Era II: 2,572; Era III: 2,998. From Era I to Era III, significant changes happened. Some severity indicators such as gestational age, prevalence of prenatal diagnosis, and inborn patients significantly worsened. Also, treatment strategies such as the use of prenatal steroids and inhaled nitric oxide, age at operation, prevalence of minimal access surgery, and the use of surfactant significantly changed. Finally, length of hospital stay became significantly longer and survival to discharge slightly but significantly improved, from 67.7 to 71.4% (p for trend 0.0019).
Conclusion: Treatment strategies for patients registered since 1995 in the CDH Study Group significantly changed. Survival to discharge slightly but significantly improved.
“Change always comes bearing gifts” Price Prichett.
Introduction
Congenital diaphragmatic hernia (CDH) was first described by Lazare Riviere in a postmortem examination of a 24-year-old male (1). In 1754, George Macaulay reported the first neonate with CDH, also in a necropsy finding in an infant who died from respiratory failure (2). Treatment successes for CDH appeared at the beginning of the 20th century (3), suggesting improved survival with early surgery (4). However, it was not until 1940, when Ladd and Gross reported 9 survivors out of 16 operated CDH patients (5), that surgery became an accepted treatment for CDH. In 1946, Gross also reported the first successful repair of a CDH in a newborn in the first 24 h of life (6). At that time, surgical correction of CDH was considered a surgical emergency and immediate repair was the mainstay of the treatment with 90–95% reported survival rates (7). Since then, the management of CDH has undergone many modifications, shifting from a surgical emergency to a physiologic one. Treatments such as extracorporeal membrane oxygenation (ECMO), high-frequency ventilation, inhaled nitric oxide (iNO), and minimal access surgery have been introduced and gained popularity. In the last 15–20 years, several single-institution studies have reported the impact of management changes on the outcome of CDH patients, with controversial results (8–13). The only study on treatment and outcomes changes in CDH, involving a large cohort of patients, was published in 2006 but was limited to patients with diaphragmatic agenesis and showed no significant improvements in the outcomes (14).
The aim of present study is to report the evolution of management strategies for all newborns with high-risk CDH over the last two decades and to evaluate any association with changes in outcome.
Materials and Methods
The CDH Study Group was formed in 1995 to compile data on live born infants with CDH at participating institutions to assess therapies and outcomes. The CDH Study Group consists of tertiary referral centers, distributed over four continents, which voluntarily provide data to a central registry (Appendix). Data on all infants with CDH who are born at or transferred to a participating center are entered into the database. Data are collected prospectively on all live born patients with CDH in participating hospitals and include information on delivery and subsequent hospital care until death or hospital discharge. The data from the registry forms are entered into a Microsoft (Redmond, WA, USA) Access database and are crosschecked against the original form. Patient demographics, birth information, treatment received, and outcome are recorded.
Patients with “high-risk” CDH (prenatal diagnosis and/or symptoms within the first 6 h of life) included in the Registry until December 2013 are the object of present study and are divided in three Eras (1995–2000, 2001–2006, 2007–2013). The definition of the Eras was made “a priori” and established to have three Eras of comparable length. Since 1995, there have been four versions of the data collection tool. With each update, the CDH Study Group has attempted to streamline information to provide an accurate reflection of the practice among individual centers. With each revision, data deemed unnecessary were eliminated and new data points were added as a result of lessons learned from previous versions. For this study, we included data that were collected throughout all the study period. Late presenters or patients with very incomplete data were excluded from the study, like outborn patients who were not referred to one of the participating centers.
Primary Outcome Measure
Survival to discharge (defined as discharge from the tertiary hospitals, either home or back to referring hospitals).
Secondary Outcome Measures
Prevalence of repair, prevalence of oxygen dependency at 30 days of life, length of mechanical ventilation, length of hospital stay.
In addition, the following variables were extracted.
Severity Indicators
Gestational age, birth weight, and prevalence of prenatal diagnosis, inborn status, chromosomal anomalies, and major associated cardiovascular malformations. Major cardiovascular anomalies included hypoplastic left heart syndrome, coarctation of the aorta, and tetralogy of Fallot. An isolated ventricular septal defect or atrial septal defect was considered minor. In addition, the probability of survival in the three Eras was calculated, using the CDH Study Group formula: probability of survival = 1 − 1/(1 + e−X) where −X = −5.024 + 0.9165(BW) + 0.4512(Apgar5) (15). BW is the birth weight in kilograms and Apgar5 is the 5-min Apgar score in the discrete ordinate range 0–10.
Treatment Strategies
Use of prenatal steroids, surfactant and iNO administration and ECMO, prevalence of repair, age at repair, use of minimal access surgery (either laparoscopy or thoracoscopy), type of open surgical approach (laparotomy vs thoracotomy) and minimal access surgical approach (laparoscopy vs thoracoscopy), patch repair, and of chest tube insertion.
Statistical Analysis
Data were analyzed using GraphPad Prism 5.0 Macintosh Version (GraphPad Software, San Diego, CA, USA; www.graphpad.com). One-way ANOVA was used to compare the three groups for continuous variables, Chi-squared test and Chi-squared test for trend were used for categorical variables. Results are given as mean (SE) or prevalence, as appropriate. As several null hypotheses were tested, a Sydak-adjusted significance level of 0.0027 was calculated to account for the increased possibility of type-I error. Two-sided p-values are reported.
The CDH Study Group registry was approved by the University of Texas-Houston Institutional Review Board (HSC-MS-03-223). Participating centers filed a Waiver of Consent for data submission or signed a Data Use Agreement for a Limited Dataset.
Results
In the study period, 8,603 newborns with CDH were prospectively enrolled in the CDH Study Group Registry. Late presenters (288 patients) and patients with very incomplete data (698 patients) were excluded. Therefore, 7,617 patients were included in the analysis: 2,146 in Era I; 2,572 in Era II; and 2,899 in Era III.
Primary Outcome Measures
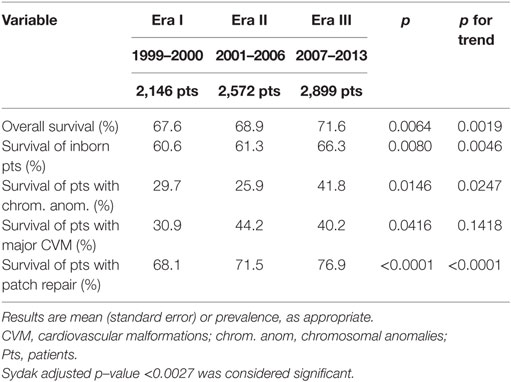
Overall survival to discharge slightly but significantly improved from Era I to Era III (Table 1). When each Era was compared with each other (Era I vs Era II: p = 0.3581; Era II vs Era III: p = 0.0274; Era I vs Era III: p = 0.0023), there was a significant improvement in survival from Era I to Era III. Patients died at 18.2 (1.3) days in Era I, at 27.0 (1.8) days in Era II, and at 25.0 (1.4) days in Era III (p = 0.0002; p for trend = 0.0017). The impact of patients’ severity on survival was also analyzed. In patients with some defined risk factors (inborn status, chromosomal anomalies, major CVM), survival rate changed but not reaching statistical significance. This was not true for patients requiring a patch repair, where we found a significant change in survival rate from 68.1 to 76.9% (p < 0.0001; p for trend < 0.0001), while those not requiring a patch had a non-significant change in survival (from 94.5 to 96.5%; p = 0.0597; p = 0.0269).
Secondary Outcome Measures
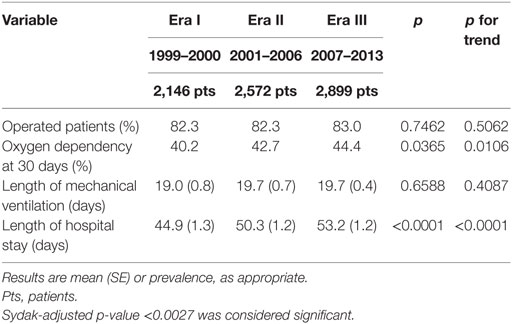
Among secondary outcomes, only length of hospital stay significantly changed from 44.9 (1.3) days in Era I to 53.2 (1.2) days in Era III (p < 0.0001, p for trend < 0.0001) (Table 2).
Severity Indicators
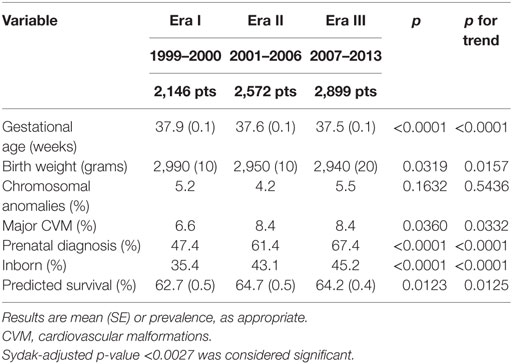
Gestational age significantly decreased, while prevalence of prenatal diagnosis and prevalence of inborn patients significantly increased from Era I to Era III (Table 3).
Treatment Strategies
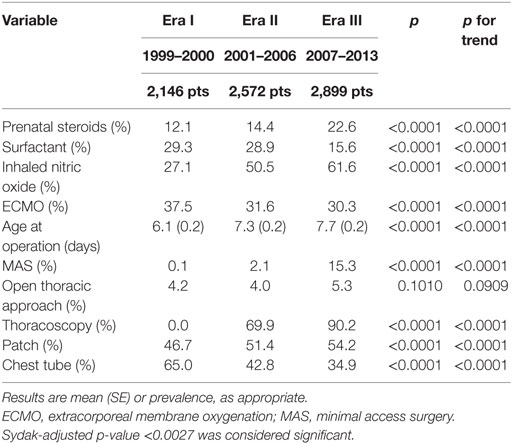
Prenatal steroids administration, iNO use, age at operation, patch closure, and use of minimal access surgery (that progressively shifted from laparoscopy to thoracoscopy) significantly rose (Table 4). Conversely, surfactant and ECMO use and chest tube placement significantly dropped.
Discussion
In an analysis of over 7,500 neonates with high-risk CDH prospectively enrolled from multiple Institutions worldwide during the last two decades, we found that treatment strategies significantly changed and survival to discharge improved, despite the severity remained stable.
In CDH patients, the use of prenatal steroids and surfactant is based on the opinion that their lung is immature. Both experimental and human studies showed conflicting results on surfactant components in CDH lungs (16–19). Clinically, some suggest benefits with surfactant administration, and propose its use as routine or rescue therapy (20, 21), while data published from the CDH Study Group show no significant advantage of surfactant use, both in term and preterm infants with CDH (22), conversely raising some concerns over its routine use (22). A potential alternative to postnatal surfactant administration is to induce lung maturation by prenatal steroids. The inverse trends of decreasing postnatal surfactant administration and increasing prenatal steroids use may be an attempt to enhance lung maturation without the risks of postnatal surfactant administration. Experimental studies suggest that prenatal steroids significantly improve both lung maturation and postnatal outcome in CDH (23, 24). However, clinical data do not confirm the benefit of prenatal steroids administration (11, 20) and a recent report from the CDH Study Group does not support their use in CDH (25). As prenatal steroids use is not devoid of complications (26), it is prudent to confine antenatal steroids treatment in infants between 24 and 34 weeks of gestation, if delivery is expected before 34 weeks (27, 28).
Extracorporeal membrane oxygenation use progressively dropped, especially between Era I and Era II. ECMO is a complex and expensive technique that allows the blood to be oxygenated outside the body obviating the need for gas exchange in the lungs and is a last resort therapy in patients not responding to maximal conventional therapy. Neonates with CDH represent a distinct subgroup that seems to benefit less from ECMO, with a disappointing 51% survival (29). A recent meta-analysis on both retrospective and randomized studies on ECMO use in CDH showed controversial results (30). The meta-analysis of retrospective studies suggested a significant benefit from ECMO, especially for the more severe patients. Only two randomized studies could be included in the meta-analysis, showing only short-term advantages. ECMO provides effective but short-term support for the respiratory failure associated with CDH. The use of ECMO allows time for reversing the persistent pulmonary hypertension, otherwise lethal, avoiding high volume and/or pressure ventilation, which is traumatic for the lungs. However, ECMO does not have an effect on pulmonary hypoplasia and will be ineffective in patients with truly lethal pulmonary hypoplasia. As the degree of pulmonary hypoplasia cannot be foreseen accurately, the problem remains of predicting appropriate use of ECMO.
During the study period, the use of iNO progressively increased despite three different randomized controlled trials showed no benefit in patients with CDH (31–33). Nitric oxide is an endothelial-derived vasodilator that, inhaled, has a selective effect on the pulmonary arteries, and bronchodilator and anti-inflammatory effects. A recent Cochrane systematic review showed that iNO use resulted in prompt improvement of oxygenation and reduced the need for ECMO in neonates with persistent pulmonary hypertension, excepting CDH patients (34). It was concluded that term or near term neonates with hypoxic respiratory failure unresponsive to other therapy should have a trial of iNO, excluding infants with CDH. The increase seen during the study period, despite the available evidence, cannot be due to lack of awareness of the evidence. Most probably, physicians’ motivation to normalize physiology prevails when faced with a hypoxic ventilated CDH baby. As this treatment is hugely expensive and potentially harmful (35), further studies should aim to evaluate its efficacy and standardize its application in order to optimize patient outcomes and ensure cost-effective practices.
During the study period, age at operation and minimal access surgery use progressively rose. Although no clear evidence exists favoring delayed surgery in CDH (36), it is common belief that preoperative stabilization may improve the patients’ outcome. The progressive increase of age at operation may relate to a longer time required for patients’ stabilization and suggests increased severity. The progressive increase of the proportion of prenatal diagnosis, inborn patients, and patch repair, which correlate with severity and outcome (37, 38), are in agreement with this interpretation. One reason for this increase in severity may be the increased accuracy of prenatal diagnosis. Outborn severely ill CDH patients may die before transport to a tertiary referral center and hence constitute a hidden mortality. More accurate/sensitive prenatal diagnosis results in a higher detection rate of larger defects, with stomach and/or liver in the chest, and in an increase of “in utero” transfer of severely affected patients to tertiary referral centers. This hypothesis fits well with the increasing prevalence of patch repair, a proxy of defect size, which is a robust indicator of severity as it correlates with both mortality and morbidity at discharge (37, 39, 40). In theory, the increase in prenatal ultrasound accuracy may also lead to an increase in pregnancy termination. However, the stability of the proportion of patients with major cardiovascular anomalies and chromosomal anomalies, which are major causes inducing to termination of pregnancy, suggests that patterns of termination of pregnancy did not change over the study period. The progressive and corresponding increase in inborn patients we found over the three Eras rather corroborates the hypothesis of growing “in utero” referral of patients with prenatal diagnosis. The gradual increase of length of stay also suggests a worsening population, probably due to more demanding associated morbidities, as length of mechanical ventilation did not change over the study period. On the other hand, the disease severity, as predicted by the CDH Study Group formula (15), seemed stable over the study period.
Minimal access surgical approach progressively gained popularity, with a complete shift from laparoscopy to thoracoscopy. With its progression in the pediatric population, this approach was extended also to CDH patients. The comparison of minimal access surgery with open surgery for CDH shows controversial results, and the former was associated with higher recurrence rates (41) and intraoperative hypercapnea and acidosis (42), calling into question its safety. Consequently, its use should be cautious in patients with high-risk CDH and be limited to the less severe cases.
The Survival rate slightly but significantly improved over the study period, particularly between Era I and Era III, with the trend being statistically significant, suggesting an improvement in CDH management. It is possible that the concomitant increase seen in the severity of patients enrolled in the CDHSG registry may have led to the limited improvement in survival recorded in this study.
However, bordering the analysis to those groups of patients recognized as more severe, the increase in survival rate is more “clinical” than “statistical.” Survival increase from 61 to 66% in inborn patients, from 30 to 42% in those with chromosomal anomalies, from 31 to 40% in patients with major CVM, and from 68 to 77% in those requiring a patch. In all except those requiring a patch repair, these differences (approximating 10% improvement) did not reach statistical significance, possibly due to the design of the statistical analysis where we set the significance level well below the commonly accepted p < 0.05. These data suggest that changes in CDH management may have had an impact especially in the more severely affected patients. The finding regarding the change in survival in patients requiring patch repair is in contrast with the previous report from the CDH Study Group on patients with diaphragmatic agenesis, which showed no improvements in terms of mortality, hospital stay, and morbidity (14). It is possible that surgeon’s indication for patch repair became more liberal with time, with its use also in patients who would have undergone primary repair in the earlier years. On the other hand, the previous study was “limited” to 10 years while present study has a longer time span, allowing more time for treatment changes to influence the outcomes. The history of CDH outcome reporting shows bipolar courses with cycles of enthusiasm and nihilism. The majority of the reports derive from single tertiary referral institutions. This may lead to case selection bias and the existence of a “hidden mortality,” which is difficult to quantify (43). Outcomes analyzed at a supra-Institution level show a slight but significant improvement in the long run. The improvements in outcomes have been in turn attributed to the introduction of new treatment modalities. However, randomized studies, failed to show any true impact of their use, singularly, on the outcomes of CDH patients (31–33, 44–46). Most of these studies were not specifically designed for CDH patients, analyzed only as relatively small subgroups. Therefore, it is possible that they were not powered enough to detect the effects of the investigated treatments, if present. An alternative explanation is that the single intervention examined was not able to cause significant changes in the outcome by itself. In our cohort, a combined effect of several interventions cannot be excluded. Additionally, it is possible that other strategies that we were not able to assess, because not recorded in the registry, may have had an impact on survival evolution. The several changes in treatment strategies that happened during the last two decades combined together may have led to the improvement in survival rate seen in high-risk CDH patients enrolled in the CDH Study Group registry.
The CDH Registry was formed to allow collection and analysis of data on treatment and outcomes of infants with CDH from a large number of centers. While registries have proven to be a good way to collect information on various disorders, care should be taken in interpreting the data. In addition, they allow the analysis only on collected data, and we have limited the analysis only to data collected all along the study period. For example, data on the use of gentle ventilation, which was an important change introduced in the 1990s (47), are not recorded in the Registry and some of the data used to assess severity such as observed to expected lung to head ratio, the presence of the liver in the chest, or data used in the Brindle’s clinical prediction rule (48), were recorded only during the third Era. This may cause some uncertainity in the interpretation of the severity of patients groups in the three Eras. On the other hand, despite its limitations, present study allows the description of changes over a very long period, on a huge number of patients, which would have been impossible without a tool such as the Registry. The database is observational and conclusions about therapies should be made cautiously. The data are collected from institutions that differ significantly in their patient referral base and their criteria for accepting an infant with CDH for admission. Criteria for “non-salvageable” patients vary between centers and some patients in the repaired group in one center may not have undergone operation in another. Many infants with severe cardiac and chromosomal anomalies did not undergo repair making the true impact of these factors difficult to determine. There are also variations in surgical practice that may determine differences in timing of surgery and whether a patch is used. Similarly, the definition of agenesis is likely to vary somewhat between centers. Finally, the definition of the Eras was not based on the commencement or change in frequency use of each single treatment analyzed. However, this study is not designed to define the impact of one single treatment on the outcome of CDH. Rather, this study describes how treatment strategies used in CDH patients changed in the last decades and if this change was accompanied by a change in their outcome. While the characteristics of the registry does not allow to define the effects of a single treatment, its international and supra-Institutional nature is ideal to describe the changes in treatment strategies and overall trends of the outcomes. The observations of such a registry can be considered valid because they are free from single Institutions biases and policies are not driven by superimposed protocols. In addition, the huge number of patients enrolled gives further strength to the study leading to reliable interpretations and meaningful conclusions.
Conclusion
The analysis of over 7,500 high-risk CDH patients shows several changes in the treatment strategies over the study period. During the same period, survival rate slightly but significantly improved, despite the patients’ population remained stable. Most of these changes were adopted based on common opinion rather than on evidence. Despite none of the treatments introduced represents per se the quested “magic bullet” in high-risk CDH management, a combined effect may be responsible for the improvements recorded. Data on long-term outcomes are currently missing from the registry. However, they would deserve attention to define if changes in early management of CDH patients have an impact on their long-term prognosis.
Author Contributions
FM conceptualized and designed the study, carried out the initial analyses, drafted, reviewed and revised the manuscript, and approved the final manuscript as submitted. KL designed the data collection instruments, conceptualized and designed the study, critically reviewed the manuscript, and approved the final manuscript as submitted. PL designed the data collection instruments, coordinated and supervised data collection, reviewed and revised the manuscript, and approved the final manuscript as submitted. RC and IC reviewed and revised the manuscript, critically reviewed the manuscript, and approved the final manuscript as submitted. PB conceptualized and designed the study, critically reviewed the manuscript, and approved the final manuscript as submitted. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to acknowledge the ongoing contributions of highly committed Congenital Diaphragmatic Hernia Study Group members who voluntarily participate in the study of congenital diaphragmatic hernia.
References
1. Riverius L. Observatio LXVII: Situs ventriculi monstruosus. In: Riverius L, Jacoz S, editors. Observationum Medicarum & Curationum Insignum. Centuria Quarta. Hagae Comitum: Apud Adrianum Vlacq (1662). 39 p.
2. Macaulay G. An account of a child whose abdominal viscera were chiefly found within the cavity of the thorax. Med Obs Inquiries (1757) 1:26–30.
3. Heidenhain L. Geschichte eines falles von chronischer incarceration des Magens in einer angeborenen zwerchfellhernie welcher durch laparotomie geheilt wurde mit anschliessende bemerkungen tiber die moglichkeit das kardiacarcinom der speiseröhre zu resesziren. Deutsch Z Chir (1905) 76:394–403. doi:10.1007/BF02791475
4. Hedblom CA. Diaphragmatic hernia. A study of three hundred and seventy-eight cases in which operation was performed. JAMA (1925) 85:947–53. doi:10.1001/jama.1925.02670130009003
5. Ladd WE, Gross RE. Congenital diaphragmatic hernia. N Engl J Med (1940) 223:917–25. doi:10.1056/NEJM194012052232301
6. Gross RE. Congenital hernia of the diaphragm. Am J Dis Child (1946) 71:579–92. doi:10.1001/archpedi.1946.02020290002001
7. Gross RE. Congenital hernia of the diaphragm. In: Gross RE, editor. The Surgery of Infancy and Childhood: Its Principles and Techniques. Philadelphia, PA: WB Saunders (1953). p. 428–44.
8. Azarow K, Messineo A, Pearl R, Filler R, Barker G, Bohn D. Congenital diaphragmatic hernia – a tale of two cities: the Toronto experience. J Pediatr Surg (1997) 32:395–400. doi:10.1016/S0022-3468(97)90589-3
9. Wilson JM, Lund DP, Lillehei CW, Vacanti JP. Congenital diaphragmatic hernia – a tale of two cities: the Boston experience. J Pediatr Surg (1997) 32:401–5. doi:10.1016/S0022-3468(97)90590-X
10. Weber TR, Kountzman B, Dillon PA, Silon ML. Improved survival in congenital diaphragmatic hernia with evolving therapeutic strategies. Arch Surg (1998) 133:498–502. doi:10.1001/archsurg.133.5.498
11. Kays DW, Langham MR Jr, Ledbetter DJ, Talbert JL. Detrimental effects of standard medical therapy in congenital diaphragmatic hernia. Ann Surg (1999) 230:340–8. doi:10.1097/00000658-199909000-00007
12. Stege G, Fenton A, Jaffray B. Nihilism in the 1990s: the true mortality of congenital diaphragmatic hernia. Pediatrics (2003) 112:532–5. doi:10.1542/peds.112.3.532
13. Downard CD, Jaksic T, Garza JJ, Dzakovic A, Nemes L, Jennings RW, et al. Analysis of an improved survival rate for congenital diaphragmatic hernia. J Pediatr Surg (2003) 38:729–32. doi:10.1016/jpsu.2003.50194
14. Lally KP, Lally PA, Van Meurs KP, Bohn DJ, Davis CF, Rodgers B, et al. Treatment evolution in high-risk congenital diaphragmatic hernia. Ten years’ experience with diaphragmatic agenesis. Ann Surg (2006) 244:505–13. doi:10.1097/01.sla.0000239027.61651.fa
15. Lally KP, Jaksic T, Wilson JM, Clark RH, Hardin WD Jr, Ronald Hirschl RB, et al. Estimating disease severity of congenital diaphragmatic hernia in the first 5 minutes of life. J Pediatr Surg (2001) 36:141–5. doi:10.1053/jpsu.2001.20032
16. Van Tuyl M, Blommaart PJ, Keijzer R, Wert SE, Ruijter J, Lamers WH, et al. Pulmonary surfactant protein A, B, and C mRNA and protein expression in the nitrofen-induced congenital diaphragmatic hernia rat model. Pediatr Res (2003) 54:641–52. doi:10.1203/01.PDR.0000086906.19683.42
17. Brandsma AE, Tibboel D, Vulto IM, Egberts J, Ten Have-Opbroek AA. Ultrastructural features of alveolar epithelial cells in the late fetal pulmonary acinus: a comparison between normal and hypoplastic lungs using a rat model of pulmonary hypoplasia and congenital diaphragmatic hernia. Microsc Res Tech (1993) 26:389–99. doi:10.1002/jemt.1070260507
18. Asabe K, Tsuji K, Handa N, Kurosaka N, Kajiwara M. Immunohistochemical distribution of surfactant apoprotein-A in congenital diaphragmatic hernia. J Pediatr Surg (1997) 32:667–72. doi:10.1016/S0022-3468(97)90001-4
19. Ijsselstijn H, Zimmermann LJ, Bunt JE, de Jongste JC, Tibboel D. Prospective evaluation of surfactant composition in bronchoalveolar lavage fluid of infants with congenital diaphragmatic hernia and of age-matched controls. Crit Care Med (1998) 26:573–80. doi:10.1097/00003246-199803000-00035
20. Finer NN, Tierney A, Etches PC, Peliowski A, Ainsworth W. Congenital diaphragmatic hernia: developing a protocolized approach. J Pediatr Surg (1998) 33:1331–7. doi:10.1016/S0022-3468(98)90001-X
21. Somaschini M, Locatelli G, Salvoni L, Bellan C, Colombo A. Impact of new treatments for respiratory failure on outcome of infants with congenital diaphragmatic hernia. Eur J Pediatr (1999) 158:780–4. doi:10.1007/s004310051203
22. Lally KP, Lally PA, Langham MR, Hirschl R, Moya FR, Tibboel D, et al. Surfactant does not improve survival rate in preterm infants with congenital diaphragmatic hernia. J Pediatr Surg (2004) 39:829–33. doi:10.1016/j.jpedsurg.2004.02.011
23. Suen HC, Bloch KD, Donahoe PK. Antenatal glucocorticoid corrects pulmonay immaturity in experimentally induced congenital diaphragmatic hernia in rats. Pediatr Res (1994) 35:523–9. doi:10.1203/00006450-199405000-00002
24. Schnitzer JJ, Hedrick HL, Pacheco BA, Losty PD, Ryan DP, Doody DP, et al. Prenatal glucocorticoid therapy reverses pulmonary immaturity in congenital diaphragmatic hernia in fetal sheep. Ann Surg (1996) 224:430–7. doi:10.1097/00000658-199610000-00002
25. Lally KP, Bagolan P, Hosie S, Lally PA, Stewart M, Cotten CM, et al. Corticosteroids for fetuses with congenital diaphragmatic hernia: can we show benefit? J Pediatr Surg (2006) 41:668–74. doi:10.1016/j.jpedsurg.2005.12.007
26. McLaughlin KJ, Crowther CA, Walker N, Harding JE. Effects of a single course of corticosteroids given more than 7 days before birth: a systematic review. Aust N Z J Obstet Gynaecol (2003) 43:101–6. doi:10.1046/j.0004-8666.2003.00052.x
27. NIH consensus development panel on the effect of corticosteroids for fetal maturation on perinatal outcomes. Effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA (1995) 273:413–8. doi:10.1001/jama.1995.03520290065031
28. National Institutes of Health Consensus Development Panel. Antenatal corticosteroids revisited: repeat courses – National Institutes of Health Consensus Development Conference Statement, August 17–18, 2000. Obstet Gynecol (2001) 98:144–50. doi:10.1097/00006250-200107000-00027
29. Paden ML, Conrad SA, Rycus PT, Thiagarajan RR; ELSO Registry. Extracorporeal Life Support Organization Registry Report 2012. ASAIO J (2013) 59:202–10. doi:10.1097/MAT.0b013e3182904a52
30. Morini F, Goldman A, Pierro A. Extracorporeal membrane oxygenation in infants with congenital diaphragmatic hernia: a systematic review of the evidence. Eur J Pediatr Surg (2006) 16:385–91. doi:10.1055/s-2006-924751
31. The Neonatal Inhaled Nitric Oxide Study Group (NINOS). Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia. Pediatrics (1997) 99:838–45. doi:10.1542/peds.99.6.838
32. Kinsella JP, Truog WE, Walsh WF, Goldberg RN, Bancalari E, Mayock DE, et al. Randomized, multicenter trial of inhaled nitric oxide and high-frequency oscillatory ventilation in severe, persistent pulmonary hypertension of the newborn. J Pediatr (1997) 131:55–62. doi:10.1016/S0022-3476(97)70124-0
33. Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, et al. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med (2000) 342:469–74. doi:10.1056/NEJM200002173420704
34. Finer N, Barrington KJ. Nitric oxide for respiratory failure in infants born at or near term. Cochrane Database Syst Rev (2006) 4:CD000399. doi:10.1002/14651858.CD000399.pub2
35. Putnam LR, Tsao K, Morini F, Lally PA, Miller CC, Lally KP, et al. Variability in inhaled nitric oxide use and pulmonary hypertension in patients with congenital diaphragmatic hernia. JAMA Pediatr (2016) 170:1188–94. doi:10.1001/jamapediatrics.2016.2023
36. Moyer V, Moya F, Tibboel R, Losty P, Nagaya M, Lally KP. Late versus early surgical correction for congenital diaphragmatic hernia in newborn infants. Cochrane Database Syst Rev (2002) 3:CD001695. doi:10.1002/14651858.CD001695
37. Congenital Diaphragmatic Hernia Study Group; Lally KP, Lally PA, Lasky RE, Tibboel D, Jaksic T, et al. Defect size determines survival in infants with congenital diaphragmatic hernia. Pediatrics (2007) 120:e651–7. doi:10.1542/peds.2006-3040
38. Wynn J, Krishnan U, Aspelund G, Zhang Y, Duong J, Stolar CJ, et al. Outcomes of congenital diaphragmatic hernia in the modern era of management. J Pediatr (2013) 163:114–9. doi:10.1016/j.jpeds.2012.12.036
39. Valfre L, Braguglia A, Conforti A, Morini F, Trucchi A, Iacobelli BD, et al. Long term follow-up in high-risk congenital diaphragmatic hernia survivors: patching the diaphragm affects the outcome. J Pediatr Surg (2011) 46:52–6. doi:10.1016/j.jpedsurg.2010.09.061
40. Peetsold MG, Kneepkens CM, Heij HA, IJsselstijn H, Tibboel D, Gemke RJ. Congenital diaphragmatic hernia: long-term risk of gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr (2010) 51:448–53. doi:10.1097/MPG.0b013e3181d1b149
41. Tsao K, Lally PA, Lally KP, Congenital Diaphragmatic Hernia Study Group. Minimally invasive repair of congenital diaphragmatic hernia. J Pediatr Surg (2011) 46:1158–64. doi:10.1016/j.jpedsurg.2011.03.050
42. Bishay M, Giacomello L, Retrosi G, Thyoka M, Garriboli M, Brierley J, et al. Hypercapnia and acidosis during open and thoracoscopic repair of congenital diaphragmatic hernia and esophageal atresia: results of a pilot randomized controlled trial. Ann Surg (2013) 258:895–900. doi:10.1097/SLA.0b013e31828fab55
43. Harrison MR, Adzick NS, Estes JM, Howell LJ. A prospective study of the outcome for fetuses with diaphragmatic hernia. JAMA (1994) 271:382–4. doi:10.1001/jama.1994.03510290064038
44. Bennett CC, Johnson A, Field DJ, Elbourne D, UK Collaborative ECMO Trial Group. UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation: follow-up to age 4 years. Lancet (2001) 357:1094–6. doi:10.1016/S0140-6736(00)04310-5
45. Harrison MR, Keller RL, Hawgood SB, Kitterman JA, Sandberg PL, Farmer DL, et al. A randomized trial of fetal endoscopic tracheal occlusion for severe fetal congenital diaphragmatic hernia. N Engl J Med (2003) 349:1916–24. doi:10.1056/NEJMoa035005
46. Snoek KG, Capolupo I, van Rosmalen J, Hout L de J, Vijfhuize S, Greenough A, et al. Conventional mechanical ventilation versus high-frequency oscillatory ventilation for congenital diaphragmatic hernia: a randomized clinical trial (the VICI-trial). Ann Surg (2016) 263:867–74. doi:10.1097/SLA.0000000000001533
47. Wung JT, Sahni R, Moffitt ST, Lipsitz E, Stolar CJ. Congenital diaphragmatic hernia: survival treated with very delayed surgery, spontaneous respiration, and no chest tube. J Pediatr Surg (1995) 30:406–9. doi:10.1016/0022-3468(95)90042-X
48. Brindle ME, Cook EF, Tibboel D, Lally PA, Lally KP, Congenital Diaphragmatic Hernia Study Group. A clinical prediction rule for the severity of congenital diaphragmatic hernias in newborns. Pediatrics (2014) 134:e413–9. doi:10.1542/peds.2013-3367
Appendix
Keywords: congenital diaphragmatic hernia, extracorporeal membrane oxygenation, inhaled nitric oxide, intensive care, minimal access surgery, outcome, surgery
Citation: Morini F, Lally KP, Lally PA, Crisafulli RM, Capolupo I and Bagolan P (2017) Treatment Strategies for Congenital Diaphragmatic Hernia: Change Sometimes Comes Bearing Gifts. Front. Pediatr. 5:195. doi: 10.3389/fped.2017.00195
Received: 26 July 2017; Accepted: 23 August 2017;
Published: 14 September 2017
Edited by:
Kenneth Wong, University of Hong Kong, Hong KongReviewed by:
Jorge Correia-Pinto, University of Minho, PortugalGo Miyano, Juntendo University, Japan
Copyright: © 2017 Morini, Lally, Lally, Crisafulli, Capolupo and Bagolan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Morini, francesco.morini@opbg.net
 Francesco Morini
Francesco Morini Kevin P. Lally2
Kevin P. Lally2
 Pietro Bagolan
Pietro Bagolan