Corticosteroids in Pediatric Heart Surgery: Myth or Reality
- 1Department of Cardiac Surgery, Bristol Heart Institute, Bristol, United Kingdom
- 2Henry Welcome Laboratories for Integrative Neuroscience and Metabolism, School of Clinical Sciences, University of Bristol, Bristol, United Kingdom
- 3Cardiac Anesthesia and Intensive Care, Bristol Heart Institute - University Hospitals Bristol NHS Foundation Trust, University of Bristol, Bristol, United Kingdom
- 4Department of Congenital Cardiac Surgery, Bristol Royal Hospital for Children - University Hospitals Bristol NHS Foundation Trust, University of Bristol, Bristol, United Kingdom
Background: Corticosteroids have been administered prophylactically for more than 60 years in pediatric heart surgery, however, their use remains a matter of debate. There are three main indications for corticosteroid use in pediatric heart surgery with the use of cardiopulmonary bypass (CPB): (1) to blunt the systemic inflammatory response (SIRS) induced by the extracorporeal circuit; (2) to provide perioperative supplementation for presumed relative adrenal insufficiency; (3) for the presumed neuroprotective effect during deep hypothermic circulatory arrest operations. This review discusses the current evidence behind the use of corticosteroids in these three overlapping areas.
Materials and Methods: We conducted a structured research of the literature using PubMed and MEDLINE databases to November 2017 and additional articles were identified by cross-referencing.
Results: The evidence suggests that there is no correlation between the effect of corticosteroids on inflammation and their effect on clinical outcome. Due to the limitations of the available evidence, it remains unclear if corticosteroids have an impact on early post-operative outcomes or if there are any long-term effects. There is a limited understanding of the hypothalamic-pituitary-adrenal axis function during cardiac surgery in children. The neuroprotective effect of corticosteroids during deep hypothermic circulatory arrest surgery is controversial.
Conclusions: The utility of steroid administration for pediatric heart surgery with the use of CPB remains a matter of debate. The effect on early and late outcomes requires clarification with a large multicenter randomized trial. More research into the understanding of the adrenal response to surgery in children and the effect of corticosteroids on brain injury is warranted.
Introduction
The introduction of the cardiopulmonary bypass (CPB) circuit in the mid-50s made the surgical treatment of intracardiac lesions possible and led to a rapid progress in the field of cardiac surgery [1]. However, CPB is also known to provoke a systemic inflammatory response (SIRS) due to the contact of blood with the extracorporeal circuit, ischemic reperfusion injury of the heart or endotoxemia due to increased gut permeability. This systemic activation is potentially beneficial because it triggers an immune response that could prevent infection and promote healing, but can also prove detrimental and thus result in organ dysfunction and even death [2]. Therefore, since the introduction of the CPB, various strategies have been employed to modulate this SIRS with an aim to improve clinical outcomes. Such strategies include the use of glucocorticoids, aprotinin, antioxidants, and miniaturized or heparin-coated bypass circuits [3]. Furthermore, in pediatric heart surgery, the modulation of SIRS is of greater importance because it is believed that the inflammatory response is augmented by the surface of the extracorporeal circuit relative to the reduced circulating blood volume, the more frequent use of the deep hypothermic circulatory arrest (DHCA) and the more pronounced hemodilution [4] compared with procedures in adults. The use of corticosteroids in cardiac surgery began in the 1960s [5] and according to several current surveys of clinical practice, corticosteroids are still widely used in pediatric heart surgery that involves CPB [6, 7]. By contrast, in adult heart surgery, the use of prophylactic corticosteroids is no longer routine because of no clear evidence backing their administration. The DECS trial recruited 4,494 adult patients undergoing CPB surgery and found no impact of a single intraoperative dose of dexamethasone (1 mg/kg) on the composite end-point of death, myocardial infarction, stroke, renal failure and respiratory failure at 30 days. However, in the same study, dexamethasone was associated with reductions in postoperative infection, duration of mechanical ventilation and length of intensive care and hospital stays [8]. In the largest study of corticosteroids vs. placebo in adults to date, the SIRS trial, 7,507 patients were randomly assigned to methylprednisolone 250 mg at anesthetic induction and 250 mg at the initiation of CPB, or placebo [9]. Corticosteroids had no impact on the risk of death or major morbidity including infection, length of hospital, intensive care stay, respiratory, or renal failure.
The prophylactic use of corticosteroids in pediatric cardiac surgery population continues to be a matter of debate likely due to the lack of well-designed, large randomized controlled trials (RCTs) that can detect a treatment effect in the context of the current low perioperative mortality and morbidity. However, corticosteroids are also given in pediatric heart surgery to protect against the so-called relative adrenal insufficiency that can accompany the acute stress of surgery [10, 11]. Due to a lack of basic understanding of hypothalamic-pituitary-adrenal axis physiology during and after pediatric heart surgery, the evidence is limited in this area [12]. Finally, another potential use of corticosteroids in pediatric heart surgery is for their potential neuroprotective effect during DHCA procedures. In the current review, we will discuss the evidence and controversies around these three main indications of steroid use in pediatric heart surgery. We will discuss these topics separately, although their pathogenesis is interconnected.
Materials and Methods
We conducted a structured research of literature using PubMed and MEDLINE databases. The search strategy included a combination of the terms: “steroid,” “glucocorticoid,” “corticosteroid,” “dexamethasone,” “hydrocortisone,” “methylprednisolone,” “pediatric,” “pediatric,” “heart surgery,” “cardiac surgery,” “children,” “neonates,” “deep hypothermic circulatory arrest,” “adrenal.” The last search was conducted in November 2017. Additional articles were identified by cross-referencing from author reference lists and published review papers. We have included all articles assessing the effect of corticosteroids on inflammation, clinical outcomes, adrenal function and brain injury in children undergoing heart surgery with use of CPB.
Results
Corticosteroids, Inflammation, and Clinical Outcomes
Many studies have attempted to correlate markers of inflammation after glucocorticoid administration with clinical outcomes [13–26]. Firstly, it is well known that SIRS is a multifaceted, complex response that is challenging to characterize and modulate. Therefore, measuring only a few cytokines might not be accurate enough in view of the complex array of pro-inflammatory and anti-inflammatory mediators that are released during the SIRS process [27]. Secondly, while glucocorticoids can blunt inflammation, but this does not necessarily translate into improved short-term clinical outcomes [17, 25, 28]. Moreover, a study by Gessler et al. found no impact of glucocorticoid administration on markers of inflammation [18]. Graham et al. in an RCT of 68 children undergoing surgery with use of CPB found no effect single vs. 2-dose corticosteroids on inflammation [29]. Finally, there is some evidence that the host inflammatory response plays an important role and that clinicians should approach SIRS in a personalized rather than standardized manner. Huber et al. [30], in a study of 37 children undergoing heart surgery with use of CPB, demonstrated that the neutrophil phenotype signature could predict end-organ dysfunction associated with SIRS.
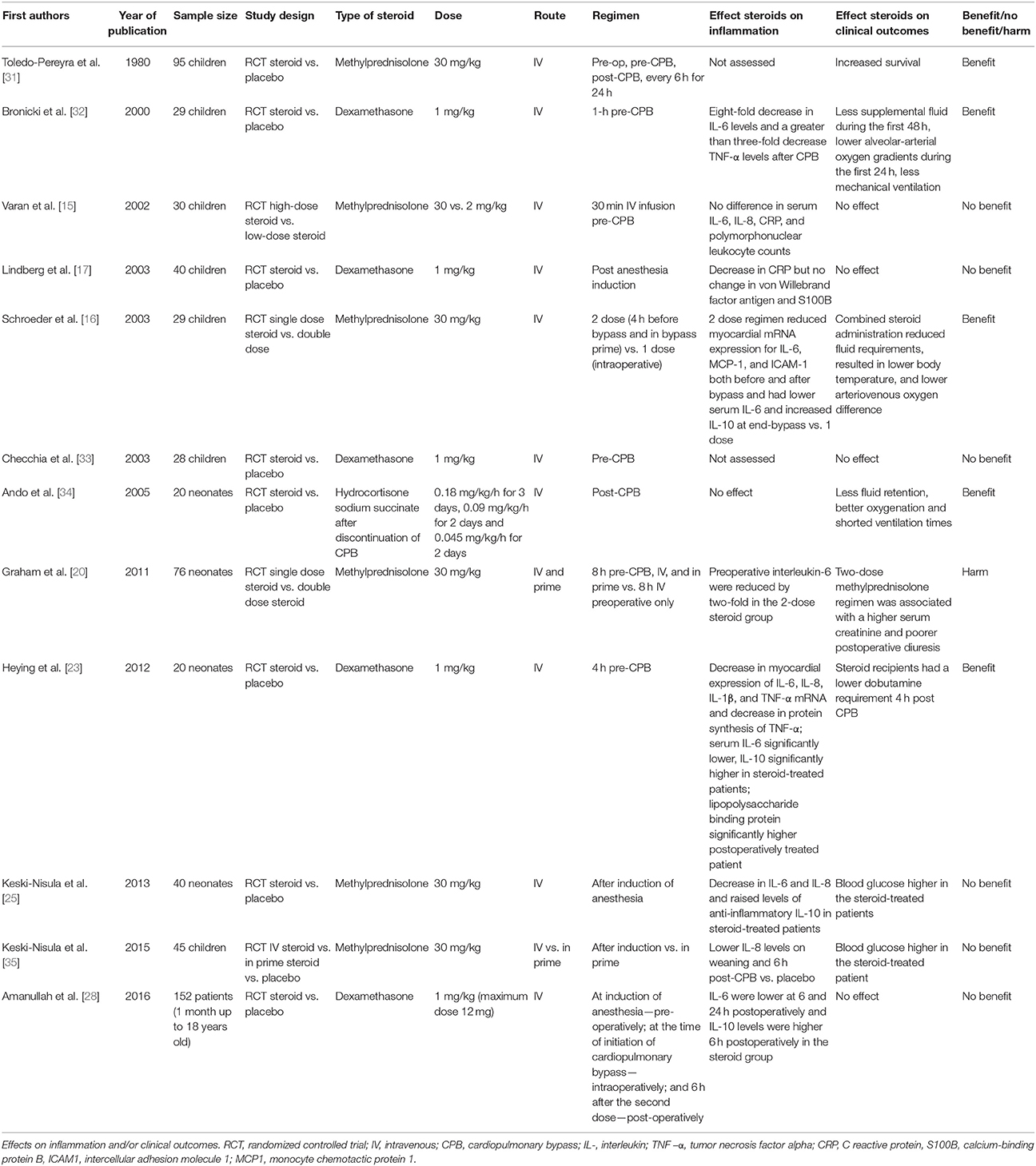
Several small-sized RCTs have focused on the effect of corticosteroids on clinical outcomes in the pediatric population (Table 1). Most of these trials measure inflammation parameters and the clinical data is measured either as a primary or secondary outcome. All have in common a small sample size and significant variability in the type and regimen of corticosteroid used. The first randomized controlled trial of corticosteroid (47 children) vs. placebo (48 children) was published by Toledo-Pereyra et al. in 1980. The authors reported improved survival in patients administered methylprednisolone 30 mg/kg: 1 h preoperatively, 5 min pre-CPB and every 6 h for 24 h; however, there was no correlation with other biochemical markers [31]. Bronicki et al. in an RCT of 29 children, found dexamethasone given 1 mg/kg, 1 h prior to CPB, was associated with lower alveolar-arterial gradients, lower fluid requirement and less mechanical ventilation [32]. In an RCT of 30 children, Varan et al. compared the dose-effect of methylprednisolone 30 mg/kg to methylprednisolone 2 mg/kg both given by intravenous infusion pre-CPB. There was no difference in clinical outcomes between the two groups (ventilation time, intensive care unit stay or oxygenation) [15]. Later, Lindberg et al. in an RCT of 40 children found no effect of dexamethasone 1 mg/kg after anesthesia induction on oxygenation, fluid balance, critical care stay, or ventilation time [17]. Schroeder et al in an RCT of 29 children, compared the effect of 30 mg/kg of methylprednisolone double-regimen (pre-operatively and intraoperatively) to a single dose (30 mg/kg) intraoperatively. The double-dose steroid recipients had better oxygenation, lower body temperature and reduced fluid requirements [16]. Checchia et al. reported in an RCT of 28 children no effect dexamethasone 1 mg/kg, given 1-h pre-CPB on sternal dehiscence rates, reoperation for bleeding, or gastrointestinal bleeding [33]. Ando et al. in small RCT of 20 patients found a benefit of hydrocortisone administration in terms of reduced body edema, improved oxygenation and reduced duration of ventilation [34]. Graham et al. randomized a total of 76 patients to either single (intraoperative) or double dose methylprednisolone (8-h pre-operative and intraoperative). The two-dose steroid group had a higher serum creatinine and poorer postoperative diuresis [20]. There was no difference in the incidence of low cardiac output syndrome, inotropic requirement, duration of mechanical ventilation, ICU, or hospital stay. Heying et al. evaluated the effect of dexamethasone 1 mg/kg 4 h pre-CPB in 20 neonates undergoing arterial switch and found no effect of corticosteroids in terms of postoperative cardiac parameters (heart rate, mean arterial pressure, central venous pressure), diuresis, or oxygenation. However, there was reduced dobutamine requirement 4 h post CPB [23]. Keski-Nisula et al. in an RCT of 40 neonates undergoing surgery with use of CPB, found no effect of 30 mg/kg of methylprednisolone given at induction of anesthesia on postoperative lactate, central venous saturation, inotropic score, duration of ventilation, or survival; however, the steroid arm had significantly increased blood glucose [25]. The same group investigated in a three-arm RCT of 45 children the effect of 30 mg/kg methylprednisolone after induction vs. 30 mg/kg methylprednisolone in prime vs. placebo. There were no differences between the three groups, in terms of lactate, inotropic score, duration of ventilation and intensive care stay but the steroid-treated arms had higher blood glucose compared to placebo [35]. Amanullah et al. in RCT of 152 children investigated the effect of dexamethasone 1 mg/kg given at three time-points (induction, pre-CPB and 6 h from the last dose) vs. placebo [28]. There was no difference between the two arms in terms of ventilation times, urine output, mean systolic and diastolic pressure, central venous pressure, inotrope score at 6 h and fluid requirement. An RCT of corticosteroid vs. placebo in 190 neonates undergoing heart surgery with use of CPB, has recently finalized recruitment and hopefully will inform us more on this matter and others [36]. Another larger RCT of 1,200 neonates is currently recruiting patients1.
Several studies have attempted to quantify the effect of glucocorticoids on markers of myocardial injury [23, 25, 33, 35, 37, 38].However, measuring these markers around the time of cardiac surgery is both difficult to interpret and correlate with clinical outcomes (see Table 2). In the adult population, the SIRS trial demonstrated that the corticosteroid treated arm had an increase in myocardial injury as measured by elevation of the CK-MB enzyme compared with placebo [9].
In view of the limitations of the existing RCT evidence discussed above, the available meta-analyses should be interpreted with caution. The Cochrane meta-analysis by Robertson-Malt et al. concluded that corticosteroids do not significantly reduce post-operative complications as measured by length of stay in ICU, peak core temperature and duration of ventilation [39]. A more recent meta-analysis by Scrascia et al. concluded no significant effect of corticosteroids on mortality, mechanical ventilation time or ICU length of stay. Based only on 15 patients, the authors found a reduced prevalence of renal dysfunction associated with the use of steroid (13 vs. 2 patients) [40].
In addition to the number of small-sized randomized trials already discussed, several larger observational studies are worth discussing here (Table 3). The Pasquali et al. [41, 42] studies provide us with the largest sample despite their retrospective design. In a registry data-base study of 46,730 patients, of which 54% received perioperative corticosteroids, there was no difference in mortality or ventilation times, however steroid use was associated with increased length of stay, higher incidence of infection and greater use of insulin. In the analysis stratified by a congenital cardiac risk score (the RACHS-1 score), increased morbidity associated with steroid use was more evident in the lower risk categories (e.g., 1–3) [41]. Later, the same group published a multicenter database study focused on 3,180 neonates and found corticosteroids to be associated with increased infection across all regimens in the lower risk groups. [42]. An observational study by Mastropietro et al. on 76 children undergoing complex heart surgery found that a greater cumulative duration of steroid administration was associated with higher risk of postoperative infection [43].
Indeed, neonates have been the focus of multiple studies because they are considered to be the most vulnerable to CPB insult. This may be due not only to the immaturity of the HPA axis [11] but neonates also appear to display a distinct inflammatory response compared to older age groups [44]. However, a recent best evidence topic review by our group could not find a clear clinical benefit of corticosteroid in neonates mainly due to the limitations in the available evidence, as discussed earlier [45].
Dreher et al. [46] in a retrospective single-center database study, compared the effect of methylprednisolone administration in 303 children undergoing heart surgery during the 6 months prior to discontinuation of steroid use with a cohort of 222 children were no glucocorticoid was used. Overall, the steroid group had more wound infection and more respiratory failure requiring tracheostomy. There were no differences in the rest of the clinical outcomes (early mortality, ventilation time, renal failure etc.,). In the neonate subgroup analyses, no difference in clinical outcomes was detected. Using datasets from a clinical trial (the Pediatric Heart Network's Single Ventricle Reconstruction trial), on neonates that had the Norwood procedure, Elhoff et al. [47] compared the effect of intraoperative corticosteroid administration in 498 recipients with 51 non-recipients and found in the multivariate analysis no effect of corticosteroid on lengths of stay but a trend toward better hospital survival in non-recipients. This is one of the few studies where the authors have also looked at the neurodevelopment outcomes at 14 months and found no difference. A major limitation in the current studies of the impact of corticosteroids on the clinical outcomes of pediatric heart surgery is the lack of long-term follow-up of the effect of corticosteroid administration on late neurocognitive outcomes. In other patient groups, the early administration of corticosteroids had detrimental effects. In an RCT of 262 infants with severe respiratory distress syndrome requiring mechanical ventilation, dexamethasone 0.25 mg/kg given every 12 h for 1 week was associated with adverse effects on the neuromotor and cognitive function at school age [48].
In conclusion, most studies conclude that corticosteroids dampen the SIRS to surgery. The effect of corticosteroids on clinical outcomes has been studied in several small-sized RCTs with conflicting evidence. Some studies show a beneficial effect on clinical outcomes such as ventilation, oxygenation or renal function parameters while in other studies, the use of corticosteroids had no effect at all. Apart from being small-sample sized, these studies used various types of steroid and regimens, therefore, it is difficult to draw any valid conclusions. A few large registry studies have raised concerns about the association of corticosteroid administration with infection, however, their results remain limited by their retrospective design.
Relative Adrenal Insufficiency
Another justification for the use of corticosteroids is the so-called relative adrenal insufficiency or adrenal cortex exhaustion, originally described during critical illness or sepsis. According to this definition, the adrenal response and subsequent concentration of plasma cortisol is not adequate to cope with the stress of surgery [49, 50]. Therefore, it is thought that perioperative steroid supplementation could compensate for this potential deficit. However, defining relative adrenal insufficiency during critical illness remains a matter of debate. The most commonly used diagnostic criteria in the literature for adrenal insufficiency during critical illness or surgery is the ACTH stimulation test described by Anane et al. In this test, synthetic ACTH (Synacthen, cosyntropin, tetracosactrin) is given intravenously. Cortisol is measured before Synacthen injection and after, at 30 and 60 min respectively. In 189 consecutive adult patients with septic shock, the authors found that an incremental cortisol response to ACTH (defined as the difference between the basal cortisol and the highest value between cortisol measured at 30 and 60 min) of <9 μg/dL or a high baseline cortisol concentration (>34 μg/dL) were of good prognostic value for identifying patients at risk of death [50]. However, this test is based on a few time-point value measurements of cortisol. Therefore, this could prove inaccurate in the context of a dynamic, pulsatile cortisol secretion that was described in both healthy volunteers or adults undergoing heart surgery [51, 52]. The major limitation of the current literature aimed at understanding the HPA axis function during pediatric heart surgery is to try to correlate the findings of the ACTH test with the measures of clinical outcome. Other limitations include the measurement of only a few, random plasma cortisol levels, the variability in the dose used for Synacthen testing and most importantly the concomitant use of glucocorticoids at induction that obscures the assessment of the HPA axis [11, 12, 53–65]. Adequate assessment of the HPA axis during surgery and critical illness requires very frequent cortisol measurements [66].
Several studies have attempted to characterize dynamic HPA axis physiology during surgery or critical illness in children (Table 4). Kucera et al measured plasma cortisol at six time points in 24 children of various ages (ranging from 2 months to 15 years): the day before surgery, at the end of surface cooling, at the lowest temperature during CPB, 10 min after rewarming, at the end of CPB and on the 8th day, postoperatively [67]. They found the cortisol levels to be in the range of the normal laboratory values with a trend toward increase during rewarming. Anand et al measured cortisol levels at seven time points in 15 neonates: pre-operatively, pre-CPB, during DHCA, at 6, 12, and at 24 h. The peaks in cortisol secretion were recorded before CPB and at the end of the operation, then, the cortisol levels fell below preoperative values at 12 h [68]. Gajarski et al. measured cortisol at 10 time-points in 58 children: baseline—preoperative, time of surgery and every 6 h up to 48 h. They have stratified the cortisol profiles by various patient groups: control (non-bypass or non-cardiac surgery procedures), CPB only (no DHCA), DHCA (no steroid used), and DHCA with steroid administration. The cortisol and ACTH peaked within 2 h of surgery but without differences between the groups. In 9 patients, not from the DHCA-steroid group, they noted an elevated ACTH-cortisol ratio that correlated with an elevated inotropic score postoperatively [53]. In 21 neonates undergoing heart surgery with use of CPB, Garcia et al. assessed the HPA axis with a low dose ACTH stimulation test (1 μg) on day 1 postoperatively. The patients that had a significant serum cortisol increase after ACTH test (>50 mg/dL) also had a higher mean arterial blood pressure at 48 h postoperatively. All patients included in the study had dexamethasone 0.5 mg/kg midnight before surgery and at induction [54]. In 38 neonates undergoing complex heart surgery, Mackie et al. measured serum cortisol at 3 time-points: preoperatively, at 24 and 48 h postoperatively. They found the higher cortisol levels to be associated with greater atrial filling pressure and lower cardiac index. Again, all patients received methylprednisolone (30 mg/kg) at induction [55]. Wald et al. [56] measured Synacthen stimulated total cortisol and corticosteroid binding globulin levels preoperatively and postoperatively in 51 children undergoing surgery with use of CPB (subjects aged <2 years received 125 μg while subjects >2 years received 250 μg). Lower CBG and increased free cortisol (>6 μg/ml) correlated with worse clinical outcomes (longer length of stay, longer ventilation time, increased fluid requirement). The authors found that only 17.6% of the patient had a low baseline total cortisol and all had a “normal” response to Synacthen. They concluded that total cortisol is not a good measure of HPA axis function in this setting. All patient received 1 mg/kg of dexamethasone before CPB. Verweij et al. [64] in a retrospective analysis of 62 patients with low cardiac output post pediatric heart surgery, found no effect of hydrocortisone administration in patients with supposed adrenal insufficiency (defined as basal cortisol <100 nmol/l). Sasser et al. [65] in a retrospective analysis of 41 neonates found that a postoperative serum cortisol level >10 μg/dL was not associated with worse clinical outcomes (lactate measurements, inotropic score, fluid requirement, arteriovenous saturation difference, mean blood pressure, mean CV, mean heart rate or ventilation time). Furthermore, there was no difference in steroid responsiveness compared to the cortisol >10 μg/dL group, among patients that had hemodynamic compromise and were administered hydrocortisone, postoperatively. In a prospective analysis of 119 children undergoing heart surgery Schiller et al. measured cortisol levels pre-operatively and at 18 h after surgery. All patients were given methylprednisolone (30 mg/kg) at induction [63]. The authors defined adrenal insufficiency as a measured postoperative cortisol level of <18.1 μg/dL or a delta cortisol value of <9 μg/dL (e.g., postoperative cortisol - preoperative cortisol). There was no significant correlation between patients with adrenal insufficiency by this definition and the procedure complexity (low or high). Furthermore, the postoperative course (ICU stay ventilation time, lactate, urine output) of children with adrenal insufficiency was not different from the children with apparently normal adrenal function. A study by Bangalore et al. assessed postoperative serum cortisol levels at three time-points: immediately after surgery and in the first and second postoperative mornings. The cortisol fell significantly over the first 24 h. Higher postoperative cortisol measurements were associated with increased morbidity. All patients received methylprednisolone (30 mg/kg) at induction [62]. Crow et al measured serum cortisol and dexamethasone following 1 mg/kg of dexamethasone in 32 infants undergoing cardiac surgery with CPB. They noted significant variability in dexamethasone levels and grouped the patients into high dexamethasone (>15 mg/dL) or low dexamethasone (<15 mg/dL) on arrival to ICU. Although the patients that had a higher dexamethasone levels had more pronounced suppression of endogenous cortisol compared to basal levels this did not translate into any impact on clinical outcomes [61]. Teagarden et al. reviewed 24 patients that underwent surgery for congenital heart disease and found that lower pre-hydrocortisone cortisol concentrations were associated with improved hemodynamics after hydrocortisone administration [58]. Maeda et al. classified 32 neonates undergoing heart surgery into patients with adrenal insufficiency (baseline cortisol <15 μg/dL or incremental increase after testing of <9 μg/dL and baseline cortisol of 15–34 μg/dL) and a group with normal adrenal function, after ACTH test (3.5 μg/kg of tetracosactide acetate). All patients received perioperatively 1 mg/kg hydrocortisone every 6 h up to 18 h from first hydrocortisone dose. Only the patients diagnosed with adrenal insufficiency exhibited a significant increase in mean blood pressure and urine output in response to hydrocortisone administration [59]. A recent study by Crawford et al. [57] aimed to correlate relative adrenal insufficiency to clinical outcomes in 40 neonates undergoing complex heart surgery. Like the studies discussed above, all patients received preoperative methylprednisolone. The authors defined adrenal insufficiency as <9 μg/ml increase in cortisol at 30 min post ACTH test (cosyntropin 1 μg). Five percent of the patients had adrenal insufficiency post-CPB and this was significantly associated with increased serum lactate and higher inotrope requirement.
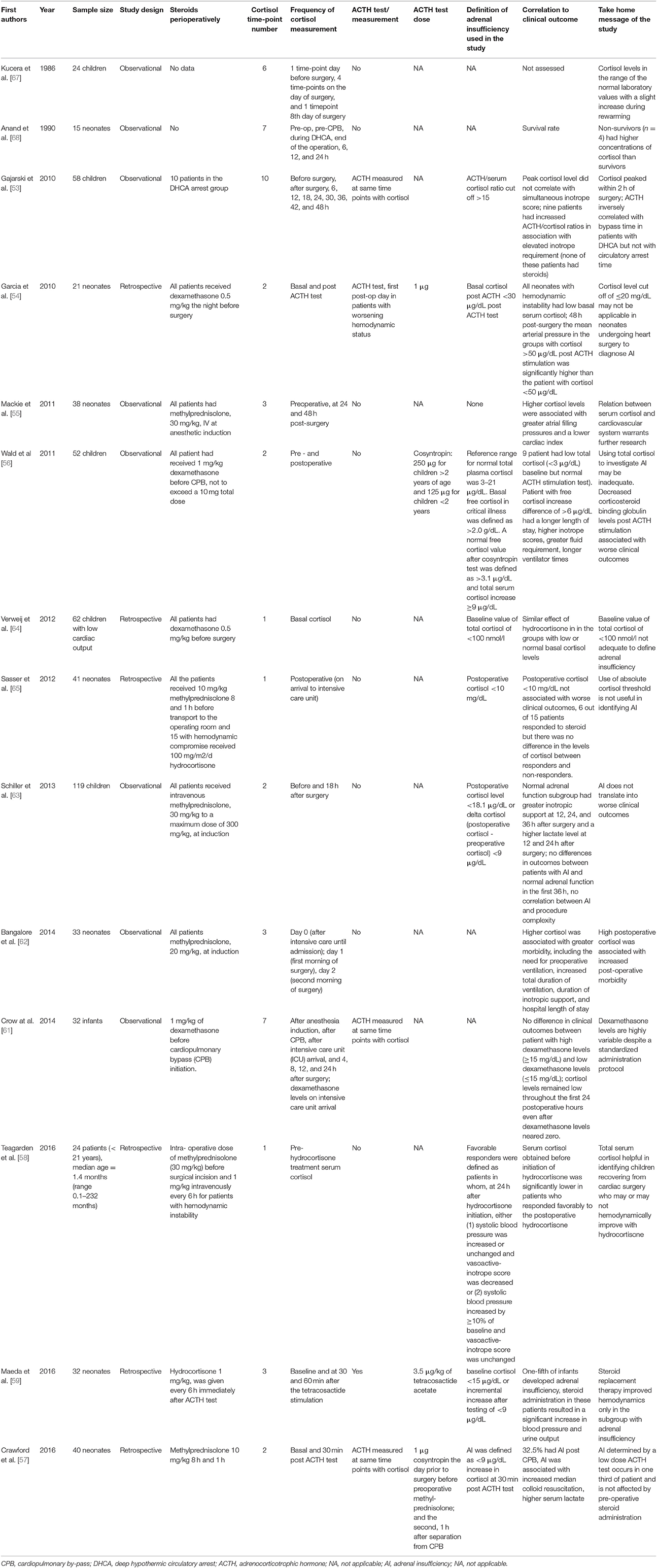
Table 4. Studies aimed at understanding the hypothalamic pituitary adrenal axis function in children.
We still do not have an accepted definition of adrenal insufficiency in children undergoing heart surgery, hence it is difficult to draw conclusions about the effect of corticosteroids on adrenal function and clinical outcomes. Most of the studies so far have used very few time-point measurements, with conflicting results and were undertaken in children that received corticosteroids at induction, therefore, making an accurate assessment of the HPA axis almost impossible.
Corticosteroids and Cerebral Protection
Cardiac surgery with use of deep hypothermic circulatory arrest is known to be associated with impaired cerebral oxygen metabolism and cerebral edema [69, 70]. Corticosteroids are used to treat cerebral edema secondary to brain tumors [71] but are also used in the context of head trauma [72]. Therefore, another justification for the use of corticosteroids is the use for DHCA surgery. However, the CRASH trial showed an increase in death at 2 weeks and 6 months with the use of corticosteroids [72, 73]. The evidence of the impact of corticosteroids on brain protection during the use of DHCA has not been studied extensively and is limited to in vitro studies [74] and a few in vivo experiments on piglets [69, 75] with conflicting results.
Schmitt et al. investigated the effect of deep hypothermic circulatory arrest on an in vitro model of mouse neonatal astrocytes, neurons, and BV-2 microglia cells. The effect of methylprednisolone (100 nM) was tested in cells that were incubated according to a protocol that mimics the temperature changes during pediatric deep hypothermic circulatory arrest: deep hypothermia, slow rewarming and normothermia. The authors measured in all cell lines the cytotoxicity and the production of IL-6 as a marker for neuroprotection and regeneration. While steroid administration had no effect in the normothermic treated cells, in the deep hypothermia-treated cells methylprednisolone increased cell survival but decreased the protective IL-6 [74].
Langley et al. randomized two groups of 8 piglets to placebo and intramuscular methylprednisolone 30 mg/kg, given 8- and 2 h before induction. All piglets underwent cooling to 18°C, 60 min of circulatory arrest and 60 min of reperfusion and rewarming. The steroid-treated arm had a significantly higher recovery of cerebral blood flow and cerebral oxygen metabolism [69]. In a later study, Schubert et al. randomized two groups of seven neonatal piglets to intravenous methylprednisolone (30 mg/kg), given 24 h before surgery vs. placebo. The piglets were then cooled to 15°C for a longer period of DHCA-−120 min–before rewarming for 40 min. The authors conducted quantitative histological studies in the hippocampus, cortex, cerebellum and caudate nucleus. Piglets in the steroid-treated arm had increased neuronal cell death and apoptosis in the dentate gyrus of the hippocampus.
The neuroprotective potential of corticosteroids in cases of pediatric heart surgery with use of deep hypothermic circulatory arrest has therefore been the least studied. Current evidence is contradictory and the results from the available animal studies highlight the need for robust human trials aimed at this high-risk patient subgroup.
Conclusions
We found no clear evidence that the anti-inflammatory effect of corticosteroids translates into better clinical outcomes. Most randomized studies in the literature report too few patients, different endpoints and vary widely in the steroid type, doses, and regimens. Although some registry studies examined the effect of corticosteroids on larger patient populations, they are limited by their retrospective design. The effect of corticosteroids on clinical outcomes will need to be clarified by a large, multicenter, randomized controlled trial with clear agreed methodology and aims. Our knowledge about the basic physiology of the hypothalamic-pituitary-adrenal axis during surgery remains limited and it is unclear how to define relative or absolute adrenal insufficiency in the context of pediatric heart surgery. To comprehend this will require understanding of the dynamic, pulsatile secretion of adrenal cortisol which can only be gained from studies that use frequent time-point measurements in patients not receiving exogenous corticosteroids. Finally, the neuroprotective effect of corticosteroids during deep hypothermic circulatory arrest remains even more controversial and warrants further research.
Author Contributions
DF: literature review, writing, design, supervision; BG, TU, SS, MC, and SL: writing of manuscript sections, revision; GA: writing, design, supervision.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer EA and handling Editor declared their shared affiliation.
Acknowledgments
This study was supported by the British Heart Foundation, the NIHR Biomedical Centre at the University Hospitals Bristol NHS Foundation Trust and the University of Bristol. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health or the British Heart Foundation.
Footnotes
1. ^STeroids to REduce Systemic Inflammation After Neonatal Heart Surgery (STRESS). Available online at: https://clinicaltrials.gov/ct2/show/NCT03229538
References
1. Cooley DA, Frazier OH. The past 50 years of cardiovascular surgery. Circulation (2000) 102:IV87–93. doi: 10.1161/01.CIR.102.suppl_4.IV-87
2. Laffey JG, Boylan JF, Cheng DCH. The systemic inflammatory response to cardiac surgery: implications for the anesthesiologist. Anesthesiology (2002) 97:215–52. doi: 10.1097/00000542-200207000-00030
3. Fudulu D, Angelini G. Oxidative Stress after surgery on the immature heart. Oxid Med Cell Longev. (2016) 2016:1971452. doi: 10.1155/2016/1971452
4. Kouchoukos NT, Blackstone EH, Hanley FL, Kirklin JK (eds.). Myocardial management during cardiac surgery with cardiopulmonary bypass. In: Kirklin/Barratt-Boyes Cardiac Surgery. Philadelphia, PA: Elsevier (2012). p. 150–2.
5. Replogle RL, Gazzaniga AB, Gross RE. Use of corticosteroids during cardiopulmonary bypass: possible lysosome stabilization. Circulation (1966) 33:I86–92. doi: 10.1161/01.CIR.33.4S1.I-86
6. Checchia PA, Bronicki RA, Costello JM, Nelson DP. Steroid use before pediatric cardiac operations using cardiopulmonary bypass: an international survey of 36 centers. Pediatr Crit Care Med. (2005) 6:441–4. doi: 10.1097/01.PCC.0000163678.20704.C5
7. Allen M, Sundararajan S, Pathan N, Burmester M, Macrae D. Anti-inflammatory modalities: their current use in pediatric cardiac surgery in the United Kingdom and Ireland. Pediatr Crit Care Med. (2009) 10:341–5. doi: 10.1097/PCC.0b013e3181a3105d
8. Dieleman JM, Nierich AP, Rosseel PM, van der Maaten JM, Hofland J, Diephuis JC, et al. Intraoperative high-dose dexamethasone for cardiac surgery. JAMA (2012) 308:1761–7. doi: 10.1001/jama.2012.14144
9. Whitlock RP, Devereaux PJ, Teoh KH, Lamy A, Vincent J, Pogue J, et al. Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): a randomised, double-blind, placebo-controlled trial. Lancet (2015) 386:1243–53. doi: 10.1016/S0140-6736(15)00273-1
10. Boonen E, Bornstein SR, Van den Berghe G. New insights into the controversy of adrenal function during critical illness. Lancet Diabetes Endocrinol. (2015) 3:805–15. doi: 10.1016/S2213-8587(15)00224-7
11. Green ML, Koch J. Adrenocortical function in the postoperative pediatric cardiac surgical patient. Curr Opin Pediatr. (2012) 24:285–90. doi: 10.1097/MOP.0b013e3283532d12
12. Graham EM, Bradley SM. First nights, the adrenal axis, and steroids. J Thorac Cardiovasc Surg. (2017) 153:1164–6. doi: 10.1016/j.jtcvs.2016.12.013
13. Tennenberg SD, Bailey WW, Cotta LA, Brodt JK, Solomkin JS. The effects of methylprednisolone on complement-mediated neutrophil activation during cardiopulmonary bypass. Surgery (1986) 100:134–42.
14. Bronicki RA, Checchia PA, Stuart-Killion RB, Dixon DJ, Backer CL. The effects of multiple doses of glucocorticoids on the inflammatory response to cardiopulmonary bypass in children. World J Pediatr Congenit Heart Surg. (2012) 3:439–45. doi: 10.1177/2150135112447544
15. Varan B, Tokel K, Mercan S, Dönmez A, Aslamaci S. Systemic inflammatory response related to cardiopulmonary bypass and its modification by methyl prednisolone: high dose versus low dose. Pediatr Cardiol. (2002) 23:437–41. doi: 10.1007/s00246-002-0118-3
16. Schroeder VA. Combined steroid treatment for congenital heart surgery improves oxygen delivery and reduces postbypass inflammatory mediator expression. Circulation (2003) 107:2823–8. doi: 10.1161/01.CIR.0000070955.55636.25
17. Lindberg L, Forsell C, Jogi P, Olsson AK. Effects of dexamethasone on clinical course, C-reactive protein, S100B protein and von Willebrand factor antigen after paediatric cardiac surgery. Br J Anaesth. (2003) 90:728–32. doi: 10.1093/bja/aeg125
18. Gessler P, Hohl V, Carrel T, Pfenninger J, Schmid ER, Baenziger O, et al. Administration of steroids in pediatric cardiac surgery: impact on clinical outcome and systemic inflammatory response. Pediatr Cardiol. (2005) 26:595–600. doi: 10.1007/s00246-004-0827-x
19. Grosek S, Ihan A, Wraber B, Gabrijelcic T, Kosin M, Osredkar J, et al. Methylprednisolone, cortisol and the cell-mediated immune response in children after ventricular septal defect repair. Clin Chem Lab Med. (2007) 45:1366–72. doi: 10.1515/CCLM.2007.278
20. Graham EM, Atz AM, Butts RJ, Baker NL, Zyblewski SC, Deardorff RL, et al. Standardized preoperative corticosteroid treatment in neonates undergoing cardiac surgery: results from a randomized trial. J Thorac Cardiovasc Surg. (2011) 142:1523–9. doi: 10.1016/j.jtcvs.2011.04.019
21. Bocsi J, Hänzka M-C, Osmancik P, Hambsch J, Dähnert I, Sack U, et al. Modulation of the cellular and humoral immune response to pediatric open heart surgery by methylprednisolone. Cytometry B Clin Cytom. (2011) 80:212–20. doi: 10.1002/cyto.b.20587
22. Lerzo F, Peri G, Doni A, Bocca P, Morandi F, Pistorio A, et al. Dexamethasone prophylaxis in pediatric open heart surgery is associated with increased blood long pentraxin PTX3: potential clinical implications. Clin Dev Immunol. (2011) 2011:1–6. doi: 10.1155/2011/730828
23. Heying R, Wehage E, Schumacher K, Tassani P, Haas F, Lange R, et al. Dexamethasone pretreatment provides antiinflammatory and myocardial protection in neonatal arterial switch operation. Ann Thorac Surg. (2012) 93:869–76. doi: 10.1016/j.athoracsur.2011.11.059
24. Abbasi Tashnizi M, Soltani G, Moeinipour AA, Ayatollahi H, Tanha AS, Jarahi L, et al. Comparison between preoperative administration of methylprednisolone with its administration before and during congenital heart surgery on serum levels of IL-6 and IL-10. Iran Red Crescent Med J. (2013) 15:147–51. doi: 10.5812/ircmj.8039
25. Keski-Nisula J, Pesonen E, Olkkola KT, Peltola K, Neuvonen PJ, Tuominen N, et al. Methylprednisolone in neonatal cardiac surgery: reduced inflammation without improved clinical outcome. Ann Thorac Surg. (2013) 95:2126–32. doi: 10.1016/j.athoracsur.2013.02.013
26. Byrnes JW, Bhutta AT, Rettiganti MR, Gomez A, Garcia X, Dyamenahalli U, et al. Steroid therapy attenuates acute phase reactant response among children on ventricular assist device support. Ann Thorac Surg. (2015) 99:1392–8. doi: 10.1016/j.athoracsur.2014.11.046
27. Jaffer U, Wade RG, Gourlay T. Cytokines in the systemic inflammatory response syndrome: a review. HSR Proc Intensive Care Cardiovasc Anesth. (2010) 2:161–75.
28. Amanullah MM, Hamid M, Hanif HM, Muzaffar M, Siddiqui MT, Adhi F, et al. Effect of steroids on inflammatory markers and clinical parameters in congenital open heart surgery: a randomised controlled trial. Cardiol Young (2016) 26:506–15. doi: 10.1017/S1047951115000566
29. Graham EM, Atz AM, McHugh KE, Butts RJ, Baker NL, Stroud RE, et al. Preoperative steroid treatment does not improve markers of inflammation after cardiac surgery in neonates: results from a randomized trial. J Thorac Cardiovasc Surg. (2014) 147:902–8. doi: 10.1016/j.jtcvs.2013.06.010
30. Huber JN, Hilkin BM, Hook JS, Brophy PD, Davenport TL, Davis JE, et al. Neutrophil phenotype correlates with postoperative inflammatory outcomes in infants undergoing cardiopulmonary bypass. Pediatr Crit Care Med. (2017) 18:1145–52. doi: 10.1097/PCC.0000000000001361
31. Toledo-Pereyra LH, Lin CY, Kundler H, Replogle RL. Steroids in heart surgery: a clinical double-blind and randomized study. Am Surg. (1980) 46:155–60.
32. Bronicki RA, Backer CL, Baden HP, Mavroudis C, Crawford SE, Green TP. Dexamethasone reduces the inflammatory response to cardiopulmonary bypass in children. Ann Thorac Surg. (2000) 69:1490–5. doi: 10.1016/S0003-4975(00)01082-1
33. Checchia PA, Backer CL, Bronicki RA, Baden HP, Crawford SE, Green TP, et al. Dexamethasone reduces postoperative troponin levels in children undergoing cardiopulmonary bypass. Crit Care Med. (2003) 31:1742–5. doi: 10.1097/01.CCM.0000063443.32874.60
34. Ando M, Park IS, Wada N, Takahashi Y. Steroid supplementation: a legitimate pharmacotherapy after neonatal open heart surgery. Ann Thorac Surg. (2005) 80:1672–8. doi: 10.1016/j.athoracsur.2005.04.035
35. Keski-Nisula J, Suominen PK, Olkkola KT, Peltola K, Neuvonen PJ, Tynkkynen P, et al. Effect of timing and route of methylprednisolone administration during pediatric cardiac surgical procedures. Ann Thorac Surg. (2015) 99:180–5. doi: 10.1016/j.athoracsur.2014.08.042
36. Graham EM. Corticosteroid Therapy in Neonates Undergoing Cardiopulmonary Bypass. Available online at: https://clinicaltrials.gov/ct2/show/NCT01579513?term=steroid+neonates&rank=3
37. Malagon I, Hogenbirk K, van Pelt J, Hazekamp MG, Bovill JG. Effect of dexamethasone on postoperative cardiac troponin T production in pediatric cardiac surgery. Intensive Care Med. (2005) 31:1420–6. doi: 10.1007/s00134-005-2788-9
38. Pesonen E, Keski-Nisula J, Passov A, Vähätalo R, Puntila J, Andersson S, et al. Heart-type fatty acid binding protein and high-dose methylprednisolone in pediatric cardiac surgery. J Cardiothorac Vasc Anesth. (2017) 31:1952–6. doi: 10.1053/j.jvca.2017.05.013
39. Robertson-Malt S, El Barbary M. Prophylactic steroids for paediatric open-heart surgery: a systematic review. Int J Evid Based Health (2008) 6:391–5. doi: 10.1097/01258363-200812000-00003
40. Scrascia G, Rotunno C, Guida P, Amorese L, Polieri D, Codazzi D, et al. Perioperative steroids administration in pediatric cardiac surgery: a meta-analysis of randomized controlled trials. Pediatr Crit Care Med. (2014) 15:435–42. doi: 10.1097/PCC.0000000000000128
41. Pasquali SK, Hall M, Li JS, Peterson ED, Jaggers J, Lodge AJ, et al. Corticosteroids and outcome in children undergoing congenital heart surgery: analysis of the pediatric health information systems database. Circulation (2010) 122:2123–30. doi: 10.1161/CIRCULATIONAHA.110.948737
42. Pasquali SK, Li JS, He X, Jacobs ML, O'Brien SM, Hall M, et al. Perioperative methylprednisolone and outcome in neonates undergoing heart surgery. Pediatrics (2012) 129:e385–91.
43. Mastropietro CW, Barrett R, Davalos MC, Zidan M, Valentine KM, Delius RE, et al. Cumulative corticosteroid exposure and infection risk after complex pediatric cardiac surgery. Ann Thorac Surg. (2013) 95:2133–9. doi: 10.1016/j.athoracsur.2013.02.026
44. Alcaraz AJ, Sancho L, Manzano L, Esquivel F, Carrillo A, Prieto A, et al. Newborn patients exhibit an unusual pattern of interleukin 10 and interferon γ serum levels in response to cardiac surgery. J Thorac Cardiovasc Surg. (2002) 123:451–8. doi: 10.1067/mtc.2002.120006
45. Fudulu D, Schadenberg A, Angelini G, Stoica S. Perioperative use of steroids in neonatal heart surgery: evidence based practice or tradition? Ann Med Surg. (2016) 9:67–71. doi: 10.1016/j.amsu.2016.07.003
46. Dreher M, Glatz AC, Kennedy A, Rosenthal T, Gaynor JW. A single-center analysis of methylprednisolone use during pediatric cardiopulmonary bypass. J Extra Corpor Technol. (2015) 47:155–9.
47. Elhoff JJ, Chowdhury SM, Zyblewski SC, Atz AM, Bradley SM, Graham EM. Intraoperative steroid use and outcomes following the norwood procedure: an analysis of the pediatric heart network's public database. Pediatr Crit Care Med. (2016) 17:30–5. doi: 10.1097/PCC.0000000000000541
48. Yeh TF, Lin YJ, Lin HC, Huang CC, Hsieh WS, Lin CH, et al. Outcomes at school age after postnatal dexamethasone therapy for lung disease of prematurity. N Engl J Med. (2004) 350:1304–13. doi: 10.1056/NEJMoa032089
49. Nair A, Bonneau RH. Stress-induced elevation of glucocorticoids increases microglia proliferation through NMDA receptor activation. J Neuroimmunol. (2006) 171:72–85. doi: 10.1016/j.jneuroim.2005.09.012
50. Annane D. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA (2000) 283:1038–45. doi: 10.1001/jama.283.8.1038
51. Gibbison B, Spiga F. Europe PMC funders group dynamic pituitary-adrenal interactions in response to cardiac surgery. Crit Care Med. (2014) 43:791–800. doi: 10.1097/CCM.0000000000000773
52. Bhake RC, Leendertz JA, Linthorst ACE, Lightman SL. Automated 24-hours sampling of subcutaneous tissue free cortisol in humans. J Med Eng Technol. (2013) 37:180–4. doi: 10.3109/03091902.2013.773096
53. Gajarski RJ, Stefanelli CB, Graziano JN, Kaciroti N, Charpie JR, Vazquez D. Adrenocortical response in infants undergoing cardiac surgery with cardiopulmonary bypass and circulatory arrest. Pediatr Crit Care Med. (2010) 11:44–51. doi: 10.1097/PCC.0b013e3181a64743
54. Garcia X, Bhutta AT, Dyamenahalli U, Imamura M, Jaquiss RDB, Prodhan P. Adrenal insufficiency in hemodynamically unstable neonates after open-heart surgery. Congenit Heart Dis. (2010) 5:422–9. doi: 10.1111/j.1747-0803.2010.00447.x
55. Mackie AS, Gauvreau K, Booth KL, Newburger JW, Laussen PC, Roth SJ. Hemodynamic correlates of serum cortisol in neonates after cardiopulmonary bypass. Pediatr Crit Care Med. (2011) 12:297–303. doi: 10.1097/PCC.0b013e3181f36929
56. Wald EL, Preze E, Eickhoff JC, Backer CL. The effect of cardiopulmonary bypass on the hypothalamic-pituitary-adrenal axis in children. Pediatr Crit Care Med. (2011) 12:190–6. doi: 10.1097/PCC.0b013e3181f36d17
57. Crawford JH, Hull MS, Borasino S, Steenwyk BL, Hock KM, Wall K, et al. Adrenal insufficiency in neonates after cardiac surgery with cardiopulmonary bypass. Paediatr Anaesth. (2017) 27:77–84. doi: 10.1111/pan.13013
58. Teagarden AM, Mastropietro CW. Clinical significance of serum cortisol levels following surgery for congenital heart disease. Cardiol Young (2016) 27:318–24. doi: 10.1017/S104795111600055X
59. Maeda T, Takeuchi M, Tachibana K, Nishida T, Kagisaki K, Imanaka H. Steroids improve hemodynamics in infants with adrenal insufficiency after cardiac surgery. J Cardiothorac Vasc Anesth. (2016) 30:936–41. doi: 10.1053/j.jvca.2015.11.025
60. Mastropietro CW, Miletic K, Chen H, Rossi NF. Effect of corticosteroids on arginine vasopressin after pediatric cardiac surgery. J Crit Care (2014) 29:982–6. doi: 10.1016/j.jcrc.2014.07.007
61. Crow SS, Oliver WCJ, Kiefer JA, Snyder MR, Dearani JA, Li Z, et al. Dexamethasone levels predict cortisol response after infant cardiopulmonary bypass. J Thorac Cardiovasc Surg. (2014) 147:475–81. doi: 10.1016/j.jtcvs.2013.09.023
62. Bangalore H, Ocampo EC, Rodriguez LM, Minard CG, Checchia PA, Heinle JS, et al. Serum cortisol and early postoperative outcome after stage-1 palliation for hypoplastic left heart syndrome. Pediatr Crit Care Med. (2014) 15:211–8. doi: 10.1097/PCC.0000000000000050
63. Schiller O, Dagan O, Birk E, Bitan S, Amir G, Frenkel G, et al. Adrenal insufficiency in children undergoing heart surgery does not correlate with more complex postoperative course. Pediatr Cardiol. (2013) 34:1860–7. doi: 10.1007/s00246-013-0728-y
64. Verweij EJ, Hogenbirk K, Roest AAW, van Brempt R, Hazekamp MG, de Jonge E. Serum cortisol concentration with exploratory cut-off values do not predict the effects of hydrocortisone administration in children with low cardiac output after cardiac surgery. Interact Cardiovasc Thorac Surg. (2012) 15:685–9. doi: 10.1093/icvts/ivs292
65. Sasser WC, Robert SM, Carlo WF, Borasino S, Dabal RJ, Kirklin JK, et al. Postoperative serum cortisol concentration and adrenal insufficiency in neonates undergoing open-heart surgery. World J Pediatr Congenit Heart Surg. (2012) 3:214–20. doi: 10.1177/2150135111431268
66. Powell B, Nason GP, Angelini GD, Lightman SL, Gibbison B. Optimal sampling frequency of serum cortisol concentrations after cardiac surgery. Crit Care Med. (2017) 45:e1103–4. doi: 10.1097/CCM.0000000000002534
67. Kucera V, Hampl R, Stárka L. Corticoids during hypothermic open-heart operations in children. Horm Metab Res. (1986) 18:577–8. doi: 10.1055/s-2007-1012378
68. Anand KJ, Hansen DD, Hickey PR. Hormonal-metabolic stress responses in neonates undergoing cardiac surgery. Anesthesiology (1990) 73:661–70. doi: 10.1097/00000542-199010000-00012
69. Langley SM, Chai PJ, Jaggers JJ, Ungerleider RM. Preoperative high dose methylprednisolone attenuates the cerebral response to deep hypothermic circulatory arrest. Eur J Cardiothorac Surg. (2000) 17:279–86. doi: 10.1016/S1010-7940(00)00336-5
70. Greeley WJ, Bracey VA, Ungerleider RM, Greibel JA, Kern FH, Boyd JL, et al. Recovery of cerebral metabolism and mitochondrial oxidation state is delayed after hypothermic circulatory arrest. Circulation (1991) 84:III400–6.
71. Koehler PJ. Use of corticosteroids in neuro-oncology. Anticancer Drugs (1995) 6:19–33. doi: 10.1097/00001813-199502000-00002
72. Olldashi F, Muzha I, Filipi N, Lede R, Copertari P, Traverso C, et al. Effect of intravenous corticosteroids on death within 14 days in 10008 adults with clinically significant head injury (MRC CRASH trial): randomised placebo-controlled trial. Lancet (2004) 364:1321–8. doi: 10.1016/S0140-6736(04)17188-2
73. Baigent C, Bracken M, Chadwick D, Curley K, Duley L, Farrell B, et al. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury - outcomes at 6 months. Lancet (2005) 365:1957–9. doi: 10.1016/S0140-6736(05)66552-X
74. Schmitt KRL, Kern C, Berger F, Ullrich O, Hendrix S, Abdul-Khaliq H. Methylprednisolone attenuates hypothermia- and rewarming-induced cytotoxicity and IL-6 release in isolated primary astrocytes, neurons and BV-2 microglia cells. Neurosci Lett. (2006) 404:309–14. doi: 10.1016/j.neulet.2006.05.064
75. Schubert S, Stoltenburg-Didinger G, Wehsack A, Troitzsch D, Boettcher W, Huebler M, et al. Large-dose pretreatment with methylprednisolone fails to attenuate neuronal injury after deep hypothermic circulatory arrest in a neonatal piglet model. Anesth Analg. (2005) 101:1311–18. doi: 10.1213/01.ANE.0000180206.95542.76
Keywords: corticosteroids, clinical outcomes, relative adrenal insufficiency, deep hypothermic circulatory arrest, pediatric heart surgery, cardiopulmonary by-pass
Citation: Fudulu DP, Gibbison B, Upton T, Stoica SC, Caputo M, Lightman S and Angelini GD (2018) Corticosteroids in Pediatric Heart Surgery: Myth or Reality. Front. Pediatr. 6:112. doi: 10.3389/fped.2018.00112
Received: 24 January 2018; Accepted: 04 April 2018;
Published: 20 April 2018.
Edited by:
Paul Checchia, Baylor College of Medicine, United StatesReviewed by:
Christopher W. Mastropietro, Riley Hospital for Children, United StatesElumalai Appachi, Baylor College of Medicine, United States
Jacqueline Ong, National University Healthcare System, Singapore
Copyright © 2018 Fudulu, Gibbison, Upton, Stoica, Caputo, Lightman and Angelini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel P. Fudulu, daniel.fudulu@bristol.ac.uk
 Daniel P. Fudulu
Daniel P. Fudulu Ben Gibbison
Ben Gibbison Thomas Upton2
Thomas Upton2  Massimo Caputo
Massimo Caputo Gianni D. Angelini
Gianni D. Angelini