- 1Department of Pharmacy, Peking University Third Hospital, Beijing, China
- 2Department of Pharmacy Administration and Clinical Pharmacy, School of Pharmaceutical Science, Peking University Health Science Center, Beijing, China
- 3Institute for Drug Evaluation, Peking University Health Science Center, Beijing, China
- 4Department of Pharmacy, Children's Hospital of Capital Institute of Pediatrics, Beijing, China
- 5Department of Pharmacy, Shengjing Hospital of China Medical University, Shenyang, China
- 6Department of Health Research Methods, Evidence and Impact, McMaster University, Hamilton, ON, Canada
Objective: To evaluate efficacy, safety, and economics profiles of intravenous levetiracetam (LEV) for status epilepticus (SE).
Methods: We searched PubMed, Embase, the Cochrane Library, Clinicaltrials.gov, and OpenGrey.eu for eligible studies published from inception to June 12th 2019. Meta-analyses were conducted using random-effect model to calculate odds ratio (OR) of included randomized controlled trials (RCTs) with RevMan 5.3 software.
Results: A total of 478 studies were obtained. Five systematic reviews (SRs)/meta-analyses, 9 RCTs, 1 non-randomized trial, and 27 case series/reports and 1 economic study met the inclusion criteria. Five SRs indicated no statistically significant difference in rates of seizure cessation when LEV was compared with lorazepam (LOR), phenytoin (PHT), or valproate (VPA). Pooled results of included RCTs indicated no statistically significant difference in seizure cessation when LEV was compared with LOR [OR = 1.04, 95% confidence interval (CI) 0.37 to 2.92], PHT (OR = 0.90, 95% CI 0.64 to 1.27), and VPA (OR = 1.47, 95% CI 0.81 to 2.67); and no statistically significant difference in seizure freedom within 24 h compared with LOR [OR = 1.83, 95% CI 0.57 to 5.90] and PHT (OR = 1.08, 95% CI 0.63 to 1.87). Meanwhile, LEV did not increase the risk of mortality during hospitalization compared with LOR (OR = 1.03, 95% CI 0.31 to 3.39), PHT (OR = 0.89, 95% CI 0.37 to 2.10), VPA (OR = 1.28, 95% CI 0.32 to 5.07), and placebo (plus clonazepam, OR = 0.73, 95% CI 0.16 to 3.38). LEV had lower need for artificial ventilation (OR = 0.23, 95% CI 0.06 to 0.92) and a lower risk of hypotension (OR = 0.15, 95% CI 0.03 to 0.84) compared to LOR. A trend of lower risk of hypotension and higher risk of agitation was found when LEV was compared with PHT. Case series and case report studies indicated psychiatric and behavioral adverse events of LEV. Cost-effectiveness evaluations indicated LEV as the most cost-effective non-benzodiazepines anti-epileptic drug (AED).
Conclusions: LEV has a similar efficacy as LOR, PHT, and VPA for SE, but a lower need for ventilator assistance and risk of hypotension, thus can be used as a second-line treatment for SE. However, more well-conducted studies to confirm the role of intravenous LEV for SE are still needed.
Introduction
Status epilepticus (SE) is a relatively common and life-threatening neurological emergency with an estimated incidence of up to 61 episodes per 100,000 population per year (Betjemann and Lowenstein, 2015; Trinka and Kalviainen, 2017). The overall mortality rate of refractory SE is 17% to 27%, and up to 36% in super-refractory SE (Brophy et al., 2012; Kantanen et al., 2015). Predictably, its substantial morbidity and mortality could incur high health care costs. The total direct and indirect costs for epilepsy in Europe would add up to €13.8 billion per year, of which SE was an important source of direct costs (Olesen et al., 2012). In Germany, the average cost of hospitalization for SE was €14,946 per patient per admission with a mean length of stay was 19 days, and super-refractory SE (SRSE) was the main cost-drive with the estimated cost up to €50,488 (Kortland et al., 2016). The total cost of direct hospitalization in the United States (US) caused by SE each year was even as high as $4 billion (Penberthy et al., 2005). The International League against Epilepsy (ILAE)'s updated definition of SE in 2015 highlights the long-term effects of prolonged seizure activity on the nervous system, including neuronal death and neuronal injury (Trinka et al., 2015a). Therefore, the key of SE treatment strategy is to identify and terminate seizure activities as early as possible before irreversible neuronal damage occurs (Trinka and Kalviainen, 2017).
Currently, there is high level evidence only for the first-line medications of SE including intravenous (IV) benzodiapines (preferably lorazepam, LOR) (Shorvon et al., 2008; Meierkord et al., 2010; Capovilla et al., 2013; Glauser et al., 2016). But first-line therapy may fail to control at least 30%–40% of the time and LOR could not be obtained in some countries or areas, alternative treatment is necessary (Alldredge et al., 2001; Trinka et al., 2015b). Some of the conventional agents including phenytoin (PHT), phenobarbital (PB), and valproate (VPA) were available as second-line treatment. However, toxicity limited the applications, such as hypotension and respiratory suppression in PB, and hepatic injury in VPA (Sanchez Fernandez and Loddenkemper, 2015; Trinka et al., 2016). Therefore, newer, more effective and less toxic drugs for management of SE were needed. More recently, some new anti-epileptic drugs (AEDs) with IV dosage form, such as levetiracetam (LEV) and laconamide, have been used as alternative AEDs for the treatment of SE (Prasad et al., 2005; Trinka, 2011).
In 1999, LEV was approved as adjunctive therapy for adults with focal epilepsy in the US. In 2006, the US Food and Drug Administration approved IV LEV for patients above 16 years old when oral treatment was not feasible. Since then, efficacy of IV LEV has been reported in many open-label case-series in adults and children with SE. And some retrospective studies that have suggested LEV as an effective treatment for various forms of SE as a first- or second-, or third-line treatment (Berning et al., 2009; Moddel et al., 2009; Trinka and Dobesberger, 2009; Tripathi et al., 2010; Khan et al., 2011). Current researches suggested that LEV may bind to the synaptic vesicle protein 2A, and thus depressed the epilepsy discharge through participating in the exocytosis of synaptic vesicles and regulating the release of neurotransmitters (especially the excitatory amino acids) (Rigo et al., 2002; Matagne et al., 2008; Deshpande and Delorenzo, 2014). LEV has a favorable pharmacokinetic profile. No clinically significant interactions with other AEDs were found since LEV is mainly renal excreted unchanged independent of liver cytochrome P450 (Emswiler and Cumpston, 2017) Compared to many other AEDs, LEV had fewer reported adverse events (AEs), but this may be because of its relatively short period of clinical use (Trinka and Dobesberger, 2009; Trinka, 2011). In recent years, apart from psychiatric and behavioral side effects (Yi et al., 2018), some rare AEs of LEV including rhabdomyolysis, thrombocytopenia and anaphylaxis were reported (Koklu et al., 2014; Di Lorenzo and Li, 2017; Kim and Shin, 2017).
Thus, we conducted a systematic review (SR) and meta-analysis to evaluate the evidence on the efficacy, safety and economic benefits of IV LEV compared with all other AEDs for SE.
Materials and Methods
Search Strategy
We searched PubMed, Embase, Cochrane Library, ClinicalTrials.gov, and OpenGrey.eu from inception to July 19th 2018 and updated the search results till June 12th, 2019. The following keywords were used in search terms for relevant literatures of LEV: “status epilepticus,” “epilepsy,” “seizures,” and “epileptic*” for the disease, and terms “Keppra,” “levetiracetam,” “Desitrend,” “Spritam,” “Kepcet,” “Kevtam,” “Levitam,” “injection,” and “intravenous” for the medication. We used the Boolean logic “AND” to combine the two sets of terms. The study was registered on PROSPERO (No. CRD 42017069367).
Study Selection and Outcome Measures
Two independent authors (X-LZ and M-LW) screened all retrieved records for potentially eligible studies manually by title and abstract screening in the first stage, and the full-text screening secondly. During the title and abstract screening, studies that may meet the inclusion criteria, or need further information for a clear judgment were included for a full-text screening process. Studies were included if: (1) enrolled SE patients, (2) compared the efficacy, safety or economic profiles of IV LEV against all other anticonvulsants for SE, with no restriction to dosage and duration, and (3) SR, meta-analysis, randomized controlled trials (RCTs), observational studies (cohort and case–control studies, case reports, and case series) and economic studies were considered. Studies were excluded if: (1) original data was not presented (for example, comments and expert opinions), (2) published in non-English languages without required data translated into English, and (3) data extraction/interpretation was not possible. Disagreements was resolved through discussion, and a third party was consulted and discussed (Z-MY) if necessary.
The primary efficacy outcomes focused on SE cessation. The secondary efficacy outcomes included seizures freedom within 24 h, neurological outcome at discharge, need for ventilator assistance, and mortality during hospitalization, discontinuation due to AEs, serious AEs, total AEs, single AEs, and cost-effectiveness.
Data Extraction and Quality Assessment
Two independent investigators (X-LZ and M-LW) performed data extraction according to a pre-designed data collection form. Information including authors, publication year, number of LEV trials, participant characteristics (participation-eligibility criteria, gender and age), dosage, treatment duration, outcomes of interest, and drop-out rate were extracted.
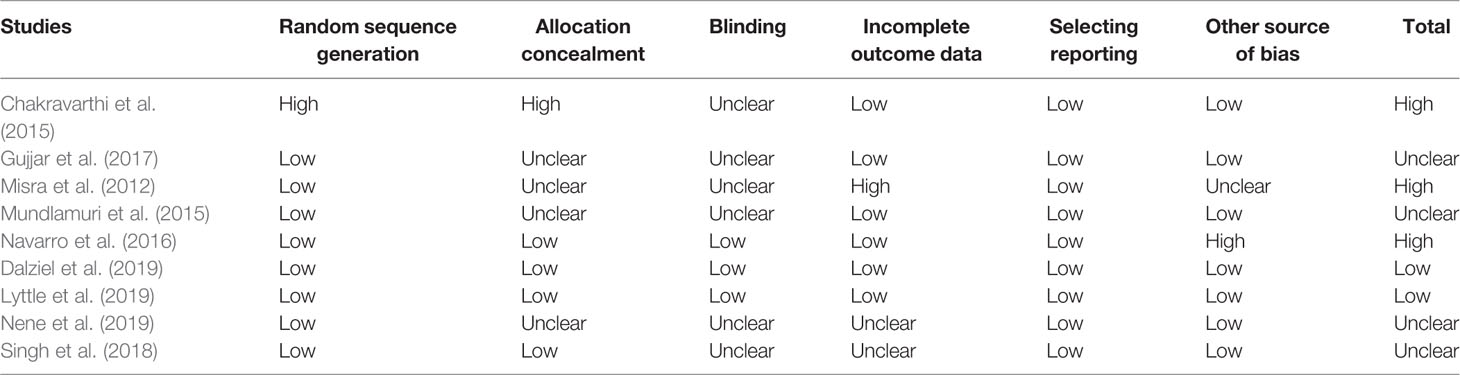
Methodological quality of included studies was independently assessed by the two investigators. The quality of included SRs was assessed with the Assessment of Multiple Systematic Reviews tool (range, 0~11) (Shea et al., 2007). The risk of bias in the eligible RCTs was assessed with the Cochrane risk of bias assessment tool (Higgins et al., 2011). The quality of the eligible economic study was assessed with consolidated health economic evaluation reporting standard (CHEERS) (Husereau et al., 2013) We did not conduct a quality assessment of the included observational studies. The authors of eligible studies were contacted for clarifications if there was missing data. We resolved all disagreements about data extraction and quality assessment through discussion among all authors.
Statistical Analysis
Treatment effect of RCTs evaluating similar interventions in similar participants was pooled using random-effect model through meta-analyses in an intention to treat manner (following the allocation of participants in studies) with RevMan 5.3 software. The odds ratio (OR) for categorical outcomes was calculated. P < 0.05 was considered statistically significant. Safety outcomes of observational studies were described and numbers of case reports as well as case-series was pooled by classification of AEs. Statistical heterogeneity was assessed with the Mantel-Haenszel chi-square test and quantified with the I2 test.
Results
Study Selection
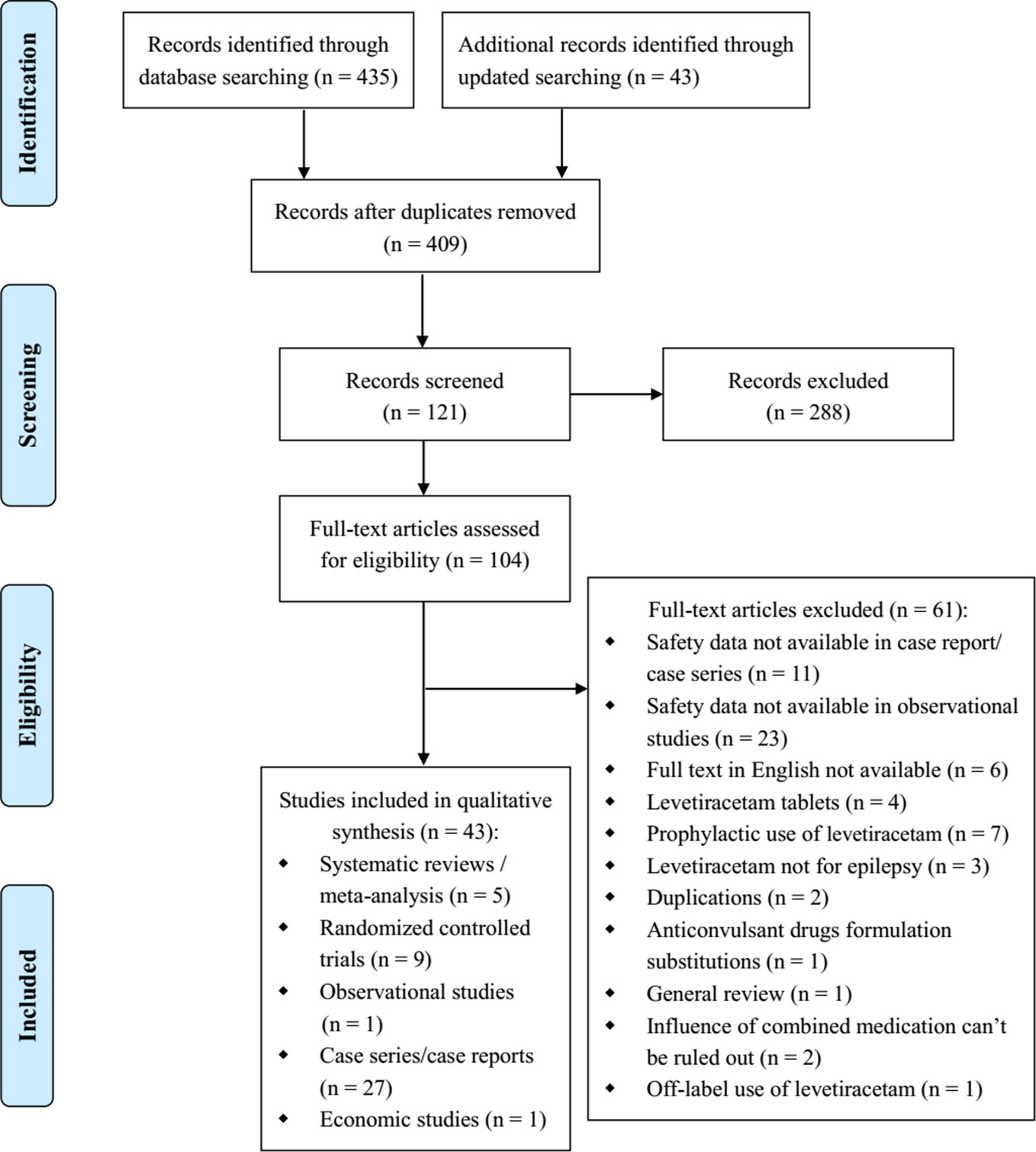
There were 435 relevant studies identified in the initial search, and 43 studies were identified in the updated search. After duplicates removed, we screened 409 title and abstract records. Of these, 288 studies were excluded after title/abstract screening, and 104 studies were selected for full-text review. And 43 reports met the inclusion criteria after full-text review: 5 SRs/meta-analyses (Liu et al., 2012; Zelano and Kumlien, 2012; Prasad et al., 2014; Yasiry and Shorvon, 2014; Brigo et al., 2016), 9 RCTs (Misra et al., 2012; Chakravarthi et al., 2015; Mundlamuri et al., 2015; Navarro et al., 2016; Gujjar et al., 2017; Singh et al., 2018; Dalziel et al., 2019; Lyttle et al., 2019; Nene et al., 2019), 1 non randomized trial (Tripathi et al., 2010), 27 case series/reports (Falip et al., 2006; Baulac et al., 2007; Michaelides et al., 2008; Ruegg et al., 2008; Abend et al., 2009; Berning et al., 2009; Beyenburg et al., 2009; Kirmani et al., 2009; Moddel et al., 2009; Wheless et al., 2009; Depositario-Cabacar et al., 2010; Ng et al., 2010; Reiter et al., 2010; Usery et al., 2010; Khan et al., 2011; Standish et al., 2011; Bahr et al., 2012; McTague et al., 2012; Rakshasbhuvankar et al., 2013; Isguder et al., 2014; Koklu et al., 2014; Atmaca et al., 2015; Lang et al., 2015; Bernatowicz and Li, 2017; Kim and Shin, 2017; May et al., 2017; Shahbaz et al., 2017), and 1 economic study (Sánchez Fernández et al., 2019) (Figure 1).
Study Characteristics and Quality Assessment
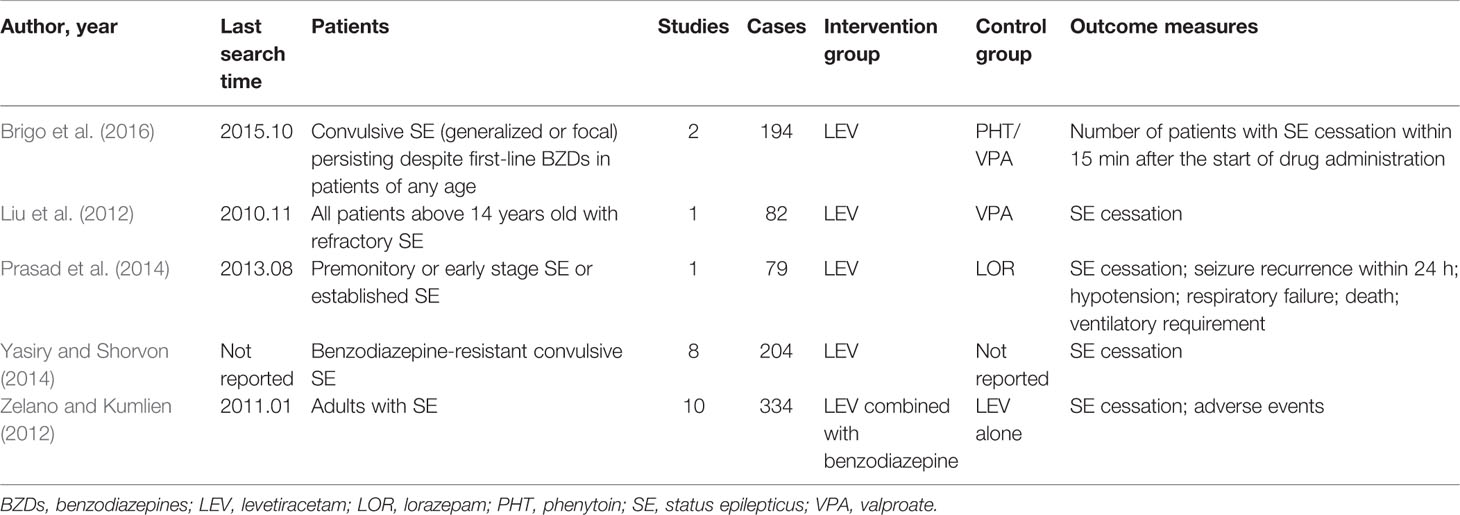
The included SRs published between 2012 and 2016, enrolling patients with convulsive SE, non-convulsive SE and refractory SE. Two SRs compared LEV with VPA (Liu et al., 2012; Brigo et al., 2016), 1 SR compared LEV with PHT (Brigo et al., 2016), 1 SR compared LEV with LOR (Prasad et al., 2014) and 2 SRs evaluated the efficacy of LEV as first or second-line therapy in SE (Zelano and Kumlien, 2012; Yasiry and Shorvon, 2014) Among the two SRs, one SR evaluated the efficacy of five AEDs (included LEV) as a second-line therapy in benzodiazepine-resistant SE (Yasiry and Shorvon, 2014), another one evaluated LEV as a first-line AED, as single treatment or combined with benzodiazepines (Zelano and Kumlien, 2012). Characteristics of included SRs were showed in Table 1.
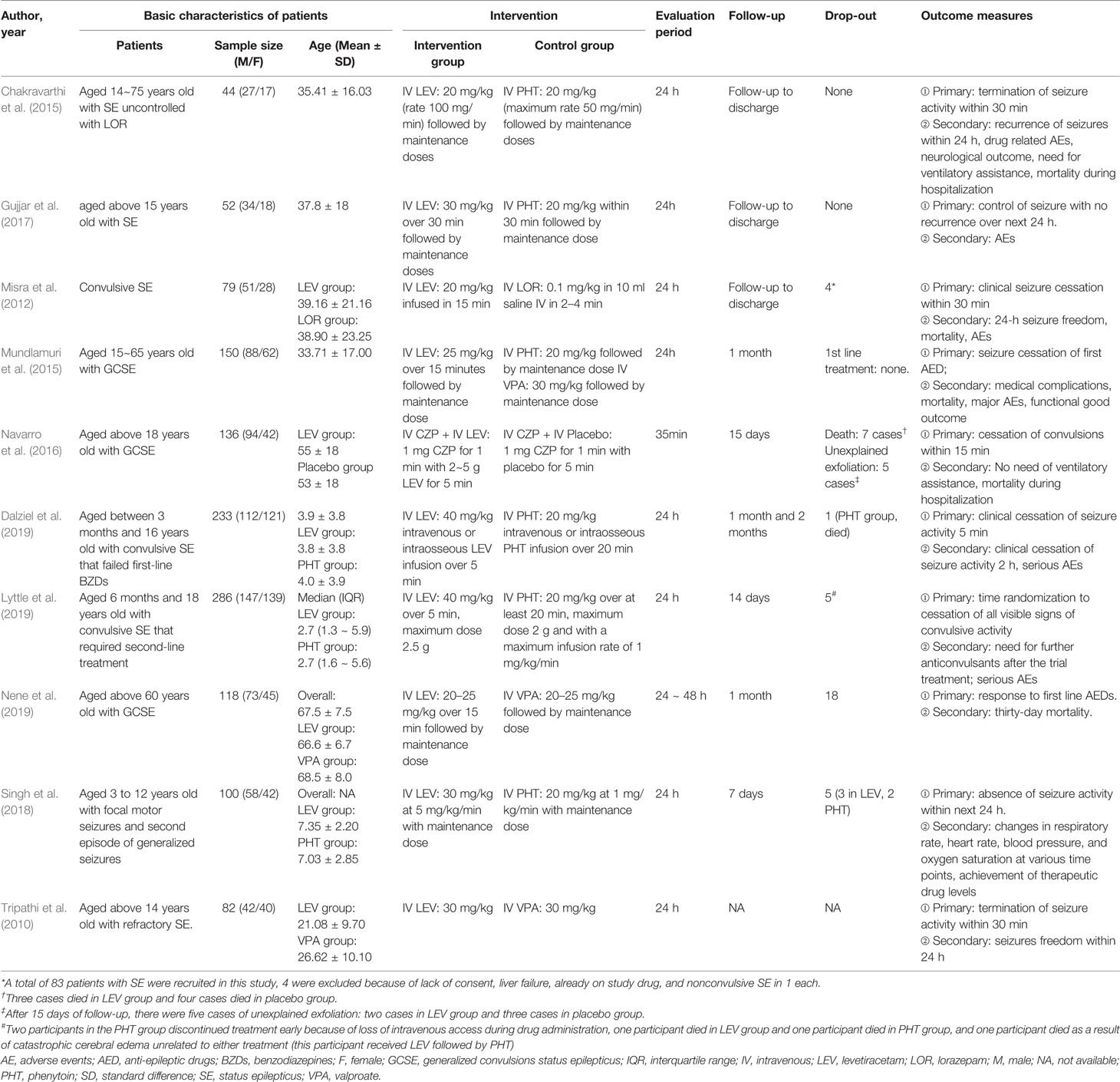
Among the included RCTs, 6 compared LEV with PHT for second-line treatment (Chakravarthi et al., 2015; Mundlamuri et al., 2015; Gujjar et al., 2017; Singh et al., 2018; Dalziel et al., 2019; Lyttle et al., 2019), 2 compared LEV with VPA (Mundlamuri et al., 2015; Nene et al., 2019), 1 compared LEV with LOR (Misra et al., 2012), and 1 compared LEV plus clonazepam with placebo plus clonazepam (Navarro et al., 2016), One non randomized trial compared LEV with VPA was included (Tripathi et al., 2010). Study characteristics of included RCTs and the non randomized trial were showed in Table 2.
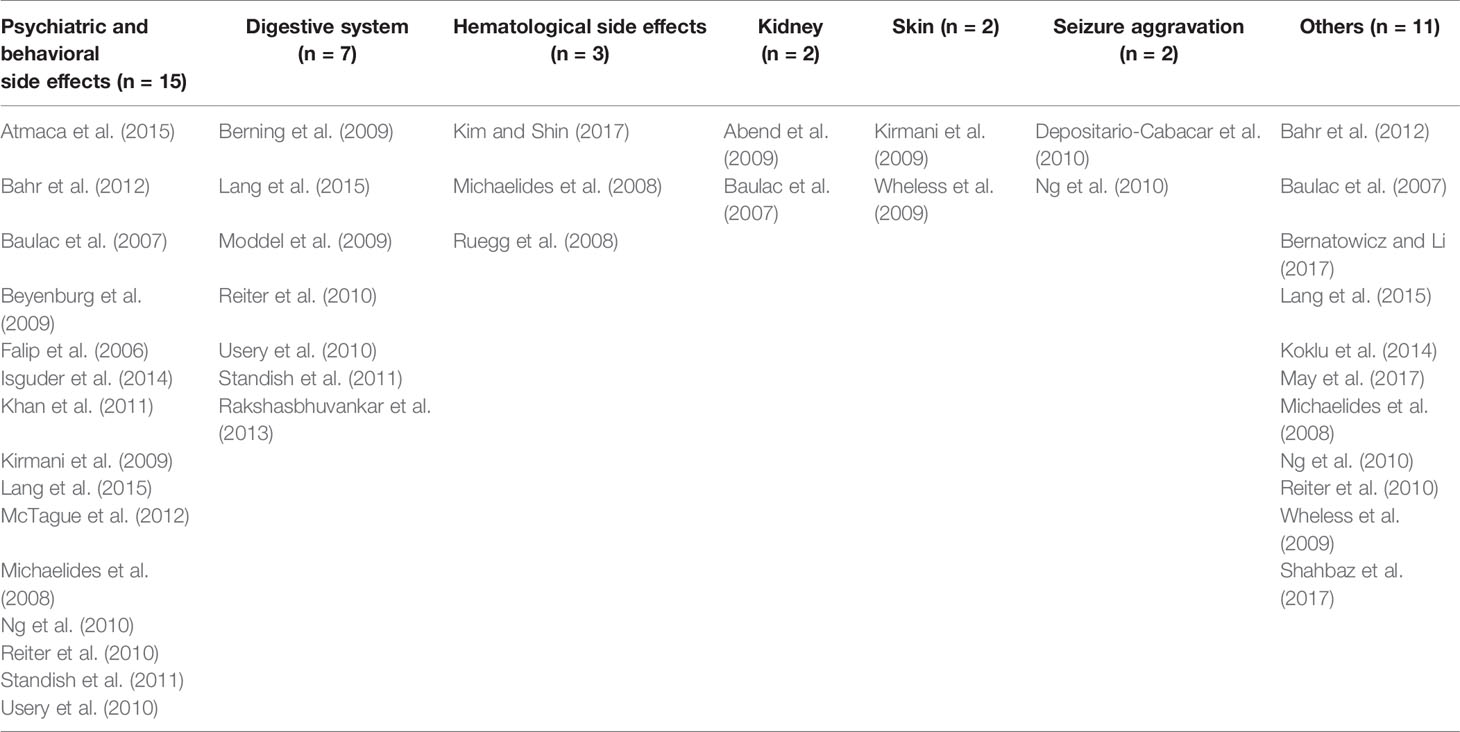
Study characteristics of included 27 case reports/series were showed in Table 3.
A total of one economic study was from the U.S. evaluating the cost-effectiveness of non-benzodiazepine AEDs (non-BZD AEDs) for treatment of BZD-resistant convulsive SE was included (Sánchez Fernández et al., 2019). Decision analysis model populated with effectiveness data from a SR and meta-analysis of literature, and cost data from publicly available prices. The primary outcome was cost per seizure stopped.
Generally, included SRs and economic study was of good quality, but RCTs were of unclear or high risk of bias. Of the five included SRs, two SRs were of high quality (Prasad et al., 2014; Brigo et al., 2016), two SRs were of moderate quality due to unclear information in 3 items and unreported information in 2 items in Liu et al. (2012) while unclear information in 6 items and unreported information in 2 items in Yasiry 2014 (Yasiry and Shorvon, 2014), and one SR was of low quality due to unclear or unreported information in 10 of 11 items (Zelano and Kumlien, 2012). The included nine RCTs were generally of unclear or high risk of bias mainly due to unclear risk of bias in allocation concealment and blinding domains, only two RCTs were of low risk of bias (Dalziel et al., 2019; Lyttle et al., 2019). The included RCTs were generally open-label randomized clinical trials, only one RCT used the double-blind design (Navarro et al., 2016) and one RCT used the single-blind design (Nene et al., 2019). All included nine RCTs analyzed data with the intention-to-treat principle (Table 4). The included economic study was of high quality, as it covered most of the items specified by the CHEERS statement except descriptions of study perspective and discount rate (Sánchez Fernández et al., 2019).
SE Cessation
Five SRs evaluated rates of SE cessation (Liu et al., 2012; Zelano and Kumlien, 2012; Prasad et al., 2014; Yasiry and Shorvon, 2014; Brigo et al., 2016). One SR indicated no statistically significant difference when LEV was compared with LOR (Prasad et al., 2014) as first-line treatment (risk ratio, RR= 0.97, 95% CI 0.44 to 2.13) or PHT (Brigo et al., 2016) as second-line treatment (OR = 1.18, 95% CI 0.50 to 2.79). Two SRs (Liu et al., 2012; Brigo et al., 2016) indicated no statistically significant difference when LEV was compared with VPA as second-line treatment (OR = 1.16, 95% CI 0.45 to 2.97, indirect comparisons; (Brigo et al., 2016) and OR = 0.80, 95% CI 0.32 to 2.01) (Liu et al., 2012). One SR indicated that the efficacy of LEV ranged from 44% to 94% (Zelano and Kumlien, 2012).
Meta-analyses of newly included RCTs showed no statistically significant difference in SE cessation when LEV was compared with LOR (Misra et al., 2012) (first-line treatment, OR = 1.04, 95% CI 0.37 to 2.92), PHT (Chakravarthi et al., 2015; Mundlamuri et al., 2015; Gujjar et al., 2017; Singh et al., 2018; Dalziel et al., 2019; Lyttle et al., 2019) (second-line treatment, OR = 0.90, 95% CI 0.64 to 1.27), and VPA (Mundlamuri et al., 2015; Nene et al., 2019) (second-line treatment, OR = 1.47, 95% CI 0.81 to 2.67). And there was no statistically significant difference when LEV plus clonazepam was compared with placebo plus clonazepam (Misra et al., 2012) (OR = 1.00, 95% CI 0.43 to 2.35) (Figure 2).
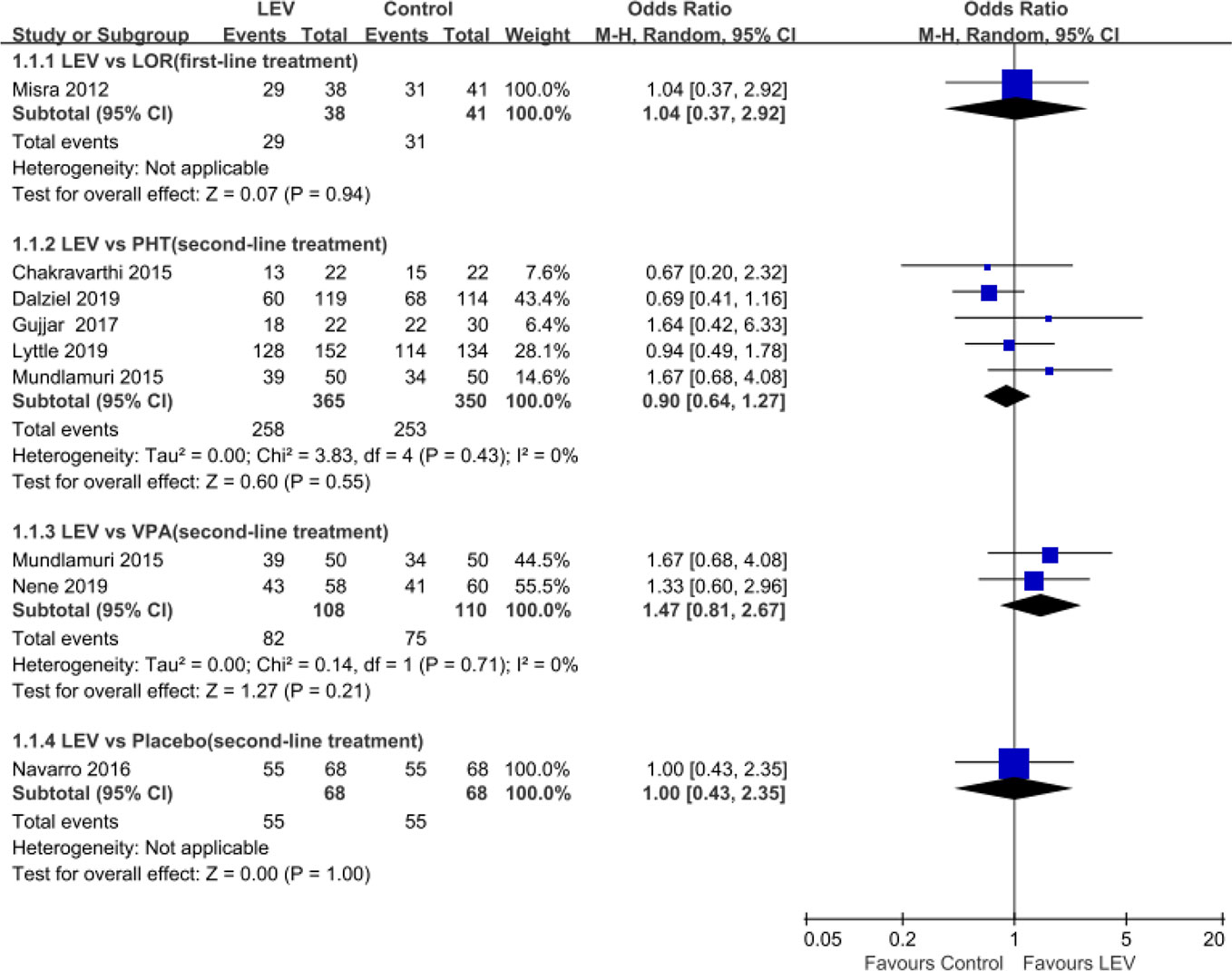
Figure 2 Rates of seizure cessation of included randomized controlled trials. Abbreviations: CLO, clonazepam; LEV, levetiracetam; LOR, lorazepam; PHT, phenytoin; VPA, valproate.
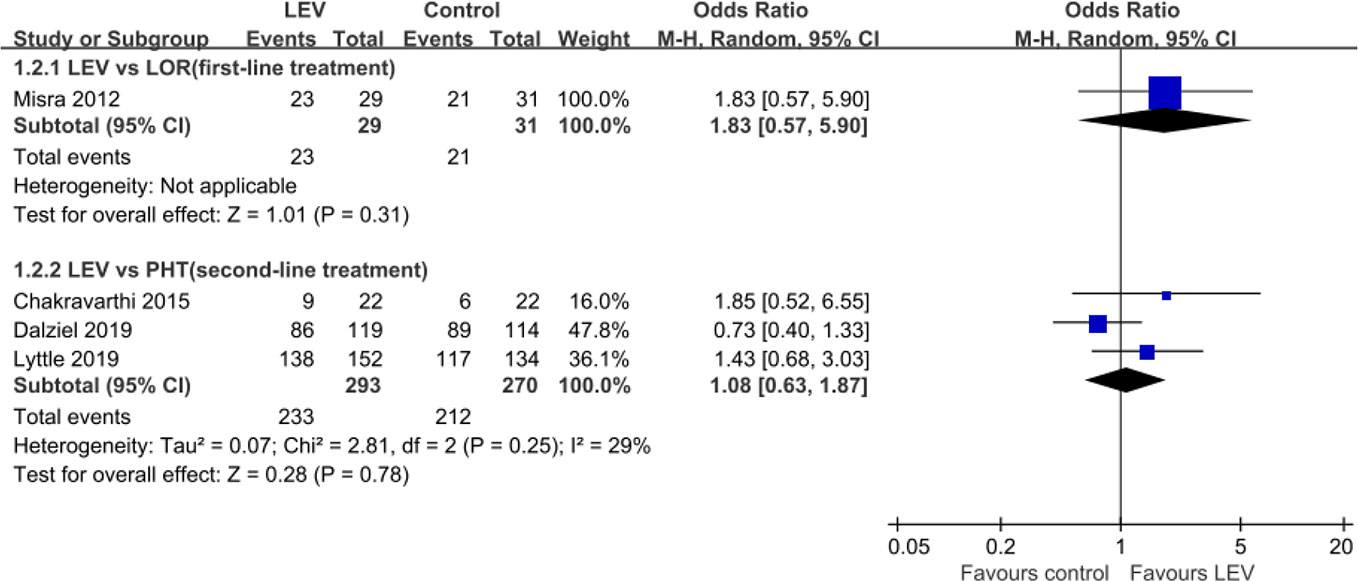
Seizure Freedom Within 24 h
Four RCTs (Misra et al., 2012; Chakravarthi et al., 2015; Dalziel et al., 2019; Lyttle et al., 2019) evaluated the rates of seizure freedom within 24 h and indicated no statistically significant difference in rates of seizure freedom within 24 h when LEV was compared with LOR (Misra et al., 2012) [first-line treatment, OR = 1.83, 95% confidence interval (CI) 0.57 to 5.90], PHT (Chakravarthi et al., 2015; Dalziel et al., 2019; Lyttle et al., 2019) (second-line treatment, OR = 1.08, 95% CI 0.63 to 1.87) (Figure 3).
Neurological Outcome at Discharge
Three RCTs (Chakravarthi et al., 2015; Mundlamuri et al., 2015; Gujjar et al., 2017) reported the neurological outcome at discharge. The definition of good functional outcome in one RCT is 5~7 points in functional independence measure (Chakravarthi et al., 2015) and 0~3 points in the modified Rankin scale in two RCTs (Mundlamuri et al., 2015; Gujjar et al., 2017). Pooled results showed no statistically significant difference between LEV and PHT in the number of good functional outcomes at discharge (OR = 1.87, 95% CI 0.95 to 3.70) (Figure 4).
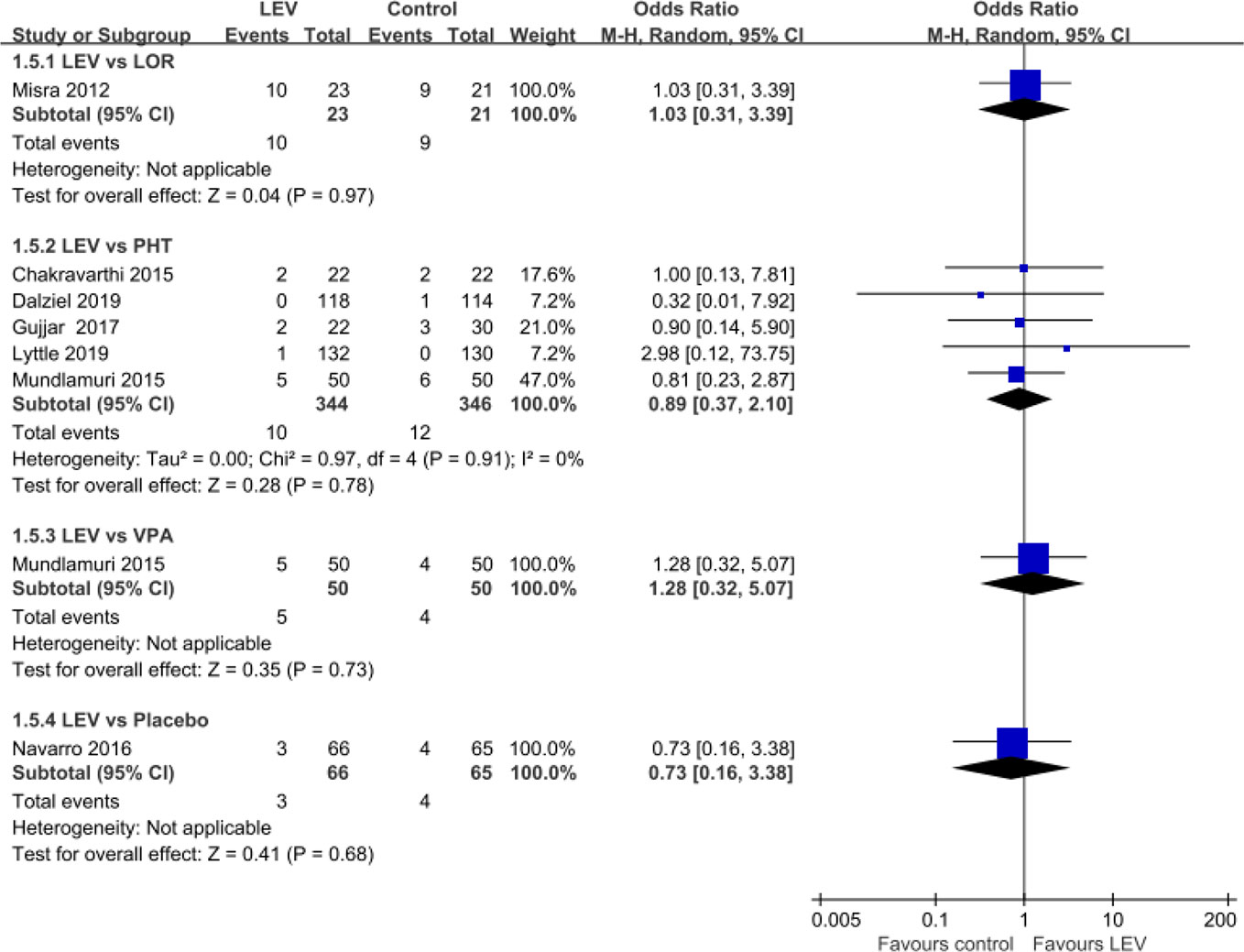
Mortality During Hospitalization
Seven RCTs (Misra et al., 2012; Chakravarthi et al., 2015; Mundlamuri et al., 2015; Navarro et al., 2016; Gujjar et al., 2017; Dalziel et al., 2019; Lyttle et al., 2019) reported mortality during hospitalization. Pooled results showed no statistically significant difference in rates of mortality during hospitalization when LEV was compared with LOR (first-line treatment, OR = 1.03, 95% CI 0.31 to 3.39), PHT (second-line treatment, OR = 0.89, 95% CI 0.37 to 2.10), and VPA (second-line treatment, OR = 1.28, 95% CI 0.32 to 5.07), placebo (plus clonazepam, OR = 0.73, 95% CI 0.16 to 3.38) (Figure 5).
Discontinuation Due to Adverse Events and Serious Adverse Events
The newly included RCTs showed no cases of discontinuation due to AEs.
What's more, the meta-analysis of newly included RCTs indicated that there was no statistically significant difference between LEV and PHT (OR = 0.65, 95% CI 0.11 to 3.96) in the rates of serious AEs (Dalziel et al., 2019; Lyttle et al., 2019), only one RCT reported serious AEs and indicated no statistically significant difference when LEV plus clonazepam was compared with placebo plus clonazepam (19/47 versus 28/47) (Navarro et al., 2016).
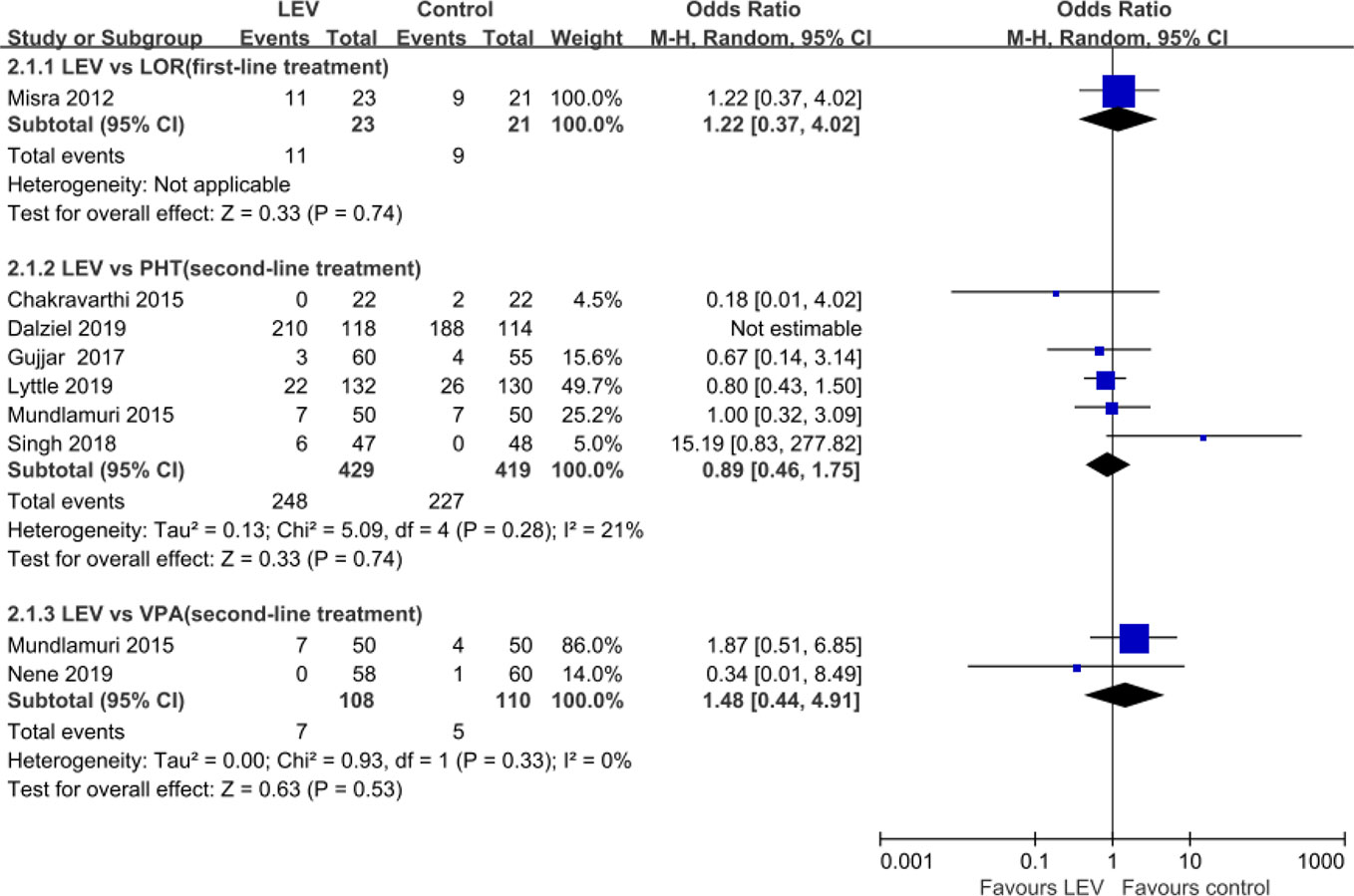
Total Adverse Events
Among the five included SRs, only one SR (Zelano and Kumlien, 2012) reported AEs and indicated sedation/somnolence as the most common AEs with rates ranging from 12.5% to 40% with LEV.
Nine RCTs (Misra et al., 2012; Chakravarthi et al., 2015; Mundlamuri et al., 2015; Navarro et al., 2016; Gujjar et al., 2017; Singh et al., 2018; Dalziel et al., 2019; Lyttle et al., 2019; Nene et al., 2019) reported total AEs. Meta-analysis of eight included RCTs (Misra et al., 2012; Chakravarthi et al., 2015; Mundlamuri et al., 2015; Gujjar et al., 2017; Singh et al., 2018; Dalziel et al., 2019; Lyttle et al., 2019; Nene et al., 2019) showed no statistically significant difference when LEV was compared with LOR (OR = 1.22, 95% CI 0.37 to 4.02) (Misra et al., 2012), PHT (OR = 0.89, 95% CI 0.46 to 1.75) (Chakravarthi et al., 2015; Mundlamuri et al., 2015; Gujjar et al., 2017; Singh et al., 2018; Dalziel et al., 2019; Lyttle et al., 2019), and VPA (OR = 1.48, 95% CI 0.44 to 4.91) (Mundlamuri et al., 2015; Nene et al., 2019) in rates of total AEs (Figure 6). Another RCT reported lower but no statistically significant difference total non-serious AEs of LEV plus clonazepam compared with placebo plus clonazepam (90/197 versus 107/197) (Navarro et al., 2016). None of AEs led to withdrawal from drug treatment.
Single AEs
One RCT compared LEV plus clonazepam and placebo plus clonazepam study reported the most common AEs as respiratory disorders (32%), general disorders (19%), musculoskeletal and connective tissue disorders (12%), psychiatric disorders (12%), infections (10%), and nervous system disorders (9%) in LEV plus clonazepam group, while the most common AEs as respiratory disorders (32%), musculoskeletal and connective tissue disorders (19%), general disorders (18%), infections (16%), gastrointestinal disorders (15%), and vascular disorders (12%) in LEV plus placebo group (Navarro et al., 2016).
One RCT study indicated a lower need for artificial ventilation (4/23 vs 10/21, OR = 0.23, 95% CI 0.06~0.92) and a lower risk of hypotension (2/23 vs 8/21, OR = 0.15, 95% CI 0.03~0.84) when LEV compared with LOR. Other AEs in LEV and LOR groups were pneumonia (8/7), thrombocytopenia (4/1), agitation (4/0), liver dysfunction (0/2), rash (1/0), and urinary infection (0/1), with no statistic difference (Misra et al., 2012).
Six included RCTs (Chakravarthi et al., 2015; Mundlamuri et al., 2015; Gujjar et al., 2017; Singh et al., 2018; Dalziel et al., 2019; Lyttle et al., 2019) compared the incidence of single AEs for LEV and PHT. Four of these studies (Chakravarthi et al., 2015; Mundlamuri et al., 2015; Gujjar et al., 2017; Lyttle et al., 2019) indicated that LEV had a trend of lower risk of hypotension compared with PHT (OR = 0.34, 95% CI 0.10~1.21); while two included RCTs (Gujjar et al., 2017; Lyttle et al., 2019) indicated that LEV had a trend of higher risk of agitation (OR = 2.86, 95% CI 0.95~8.59).
One RCT indicated that LEV had a trend of higher risk of hallucinations compared with VPA (3/50 vs 0/50) (Mundlamuri et al., 2015). Another RCT indicated that LEV had a lower risk of hepatic dysfunction (0/58 vs 1/60) compared with VPA, but no difference was found (Nene et al., 2019). One non randomized trial showed that no AEs such as hepatic dysfunction, hypotension, abnormal behavior, depressed respiratory, or thrombocytopenia occurred in any group (Tripathi et al., 2010).
Among the 27 case reports/series, 15 reported psychiatric and behavioral AEs and 7 reported gastrointestinal AEs. Rates of behavioral AEs (such as aggression and irritability) varied from 1.1% to 15% and nausea/vomiting varied from 3.1% to 5.6% (Table 2).
Cost-Effectiveness
One cost-effectiveness evaluation for BZD-resistant convulsive SE with decision analysis model was conducted in the US (Sánchez Fernández et al., 2019). The economic study showed the most cost-effective non-BZD AED was LEV (incremental cost-effectiveness ratio, incremental cost-effectiveness ratio, ICER: $18.55/seizure-stop), followed by VPA (ICER: $94.44/seizure-stop), and lastly PB (ICER: $847.22/seizure-stop). PHT and lacosamide were not cost-effective compared to the other options. Probabilistic sensitivity analysis indicated that LEV was the most cost-effective strategy in most second-order Monte Carlo simulations for a willingness to pay between approximately $25/seizure-stop and $100/seizure-stop.
Discussion
The present study indicated that LEV had similar efficacy in SE cessation and mortality during hospitalization compared with LOR, PHT and VPA; no statistically significant difference in rates of seizure freedom within 24 h compared with LOR and PHT, as well as number of good functional outcome at discharge compared with PHT. Concerning safety, no statistically significant difference among LEV, LOR, PHT, and VPA in rates of total AEs was found. However, LEV had a lower risk of hypotension and requirement for ventilator assistance compared with LOR and PHT.
Available IV AEDs are limited in clinical practice. Considering AEs of AEDs, one medication may be preferable for patients. Hong Kong Epilepsy Guideline suggested that IV LEV can be considered as an alternative to PHT in benzodiazepine-resistant SE, such as established SE (Kellinghaus and Stogbauer, 2012; Fung and Fung, 2017; Lyttle et al., 2017). The National Institute for Health and Care Excellence guideline suggested that LEV are potentially as effective as PB and safer for SE (Lyttle et al., 2017). Compared with VPA, PHT and lacosamide, LEV was the first choice drug for IV administration of SE, according to a Germany chart review (Kellinghaus and Stogbauer, 2012).
In this study, we collected the most comprehensive evidence of IV LEV, while safety profiles were often missed in the previous studies (Zelano and Kumlien, 2012; Prasad et al., 2014; Yasiry and Shorvon, 2014; Chakravarthi et al., 2015). Second, types of literature included in the study include SRs, RCTs, and observational studies. The study can provide reliable evidence for physicians or policymakers.
There are still some limitations to this study. First, only English-language studies were included. Moreover, the included RCTs were of unclear or high risk of bias. SE needs rapid termination of seizure activity to minimize neurological injury and systemic dysfunction, so it is difficult to achieve strict and consistent randomized control. However, although most of the included RCTs were not double-blinded, the judgment of some outcomes, such as SE cessation was not influenced. Third, no subgroup analysis of different types of SE such as convulsive SE, non-convulsive forms of SE were conducted due to limited studies included.
Considering the complexity of SE and the relatively high incidence of complications, the management of SE and its pharmacological treatment still need more high quality evidence to inform clinical practice and decision making.
Conclusions
IV LEV can be used as a second-line treatment for SE. For patients with SE, LEV has similar efficacy with LOR, PHT, and VPA, and a lower risk of hypotension and requirement for ventilator assistance compared with LOR and PHT. Regarding tolerability, LEV also showed good qualities for it does not increase risks of serious AEs or discontinuation from studies due to AEs in current researches. However, there is still a lack of evidence to support its cost-effectiveness, and more studies are needed to confirm its role for SE.
Author Contributions
Z-MY conceived this review. X-LZ and M-LW identified reports of trials and extracted data. YZ provided statistical advice. Z-MY, X-LZ, and M-LW did all statistical analyses, checked for statistical inconsistency, and interpreted data. S-DZ contributed to data interpretation. Z-MY drafted the report and all other authors (X-LZ, M-LW, YZ, and S-DZ) critically reviewed the article. All authors read and approved the final manuscript.
Funding
The authors declare that this study received funding from Beijing Pharmaceutical Association and UCB China Inc. The funder was not involved in the study design; collection, analysis, and interpretation of data; the writing of this article; or the decision to submit it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
References
Abend, N. S., Monk, H. M., Licht, D. J., Dlugos, D. J. (2009). Intravenous levetiracetam in critically ill children with status epilepticus or acute repetitive seizures. Pediatr. Crit. Care Med. 10 (4), 505–510. doi: 10.1097/PCC.0b013e3181a0e1cf
Alldredge, B. K., Gelb, A. M., Isaacs, S. M., Corry, M. D., Allen, F., Ulrich, S., et al. (2001). A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N. Engl. J. Med. 345 (9), 631–637. doi: 10.1056/NEJMoa002141
Atmaca, M. M., Orhan, E. K., Bebek, N., Gurses, C. (2015). Intravenous levetiracetam treatment in status epilepticus: A prospective study. Epilepsy Res. 114, 13–22. doi: 10.1016/j.eplepsyres.2015.04.003
Bahr, O., Hermisson, M., Rona, S., Rieger, J., Nussbaum, S., Kortvelyessy, P., et al. (2012). Intravenous and oral levetiracetam in patients with a suspected primary brain tumor and symptomatic seizures undergoing neurosurgery: the HELLO trial. Acta Neurochir. (Wien) 154 (2), 229–235; discussion 235. doi: 10.1007/s00701-011-1144-9
Baulac, M., Brodie, M. J., Elger, C. E., Krakow, K., Stockis, A., Meyvisch, P., et al. (2007). Levetiracetam intravenous infusion as an alternative to oral dosing in patients with partial-onset seizures. Epilepsia 48 (3), 589–592. doi: 10.1111/j.1528-1167.2006.00959.x
Bernatowicz, R., Li, Y. (2017). Rhabdomyolysis-an unrecognized adverse effect of Levetiracetam. Neurology 88 (16 Supplement), P6.226.
Berning, S., Boesebeck, F., van Baalen, A., Kellinghaus, C. (2009). Intravenous levetiracetam as treatment for status epilepticus. J. Neurol. 256 (10), 1634–1642. doi: 10.1007/s00415-009-5166-7
Betjemann, J. P., Lowenstein, D. H. (2015). Status epilepticus in adults. Lancet Neurol. 14 (6), 615–624. doi: 10.1016/S1474-4422(15)00042-3
Beyenburg, S., Reuber, M., Maraite, N. (2009). Intravenous levetiracetam for epileptic seizure emergencies in older people. Gerontology 55 (1), 27–31. doi: 10.1159/000166610
Brigo, F., Bragazzi, N., Nardone, R., Trinka, E. (2016). Direct and indirect comparison meta-analysis of levetiracetam versus phenytoin or valproate for convulsive status epilepticus. Epilepsy Behav. 64, 110–115. doi: 10.1016/j.yebeh.2016.09.030
Brophy, G. M., Bell, R., Claassen, J., Alldredge, B., Bleck, T. P., Glauser, T., et al. (2012). Guidelines for the evaluation and management of status epilepticus. Neurocrit. Care 17 (1), 3–23. doi: 10.1007/s12028-012-9695-z
Capovilla, G., Beccaria, F., Beghi, E., Minicucci, F., Sartori, S., Vecchi, M. (2013). Treatment of convulsive status epilepticus in childhood: recommendations of the Italian League Against Epilepsy. Epilepsia 54 (Suppl 7), 23–34. doi: 10.1111/epi.12307
Chakravarthi, S., Goyal, M. K., Modi, M., Bhalla, A., Singh, P. (2015). Levetiracetam versus phenytoin in management of status epilepticus. J. Clin. Neurosci. 22 (6), 959–963. doi: 10.1016/j.jocn.2014.12.013
Dalziel, S. R., Borland, M. L., Furyk, J., Bonisch, M., Neutze, J., Donath, S., et al. (2019). Levetiracetam versus phenytoin for second-line treatment of convulsive status epilepticus in children (ConSEPT): an open-label, multicentre, randomised controlled trial. Lancet 393 (10186), 2135–2145. doi: 10.1016/S0140-6736(19)30722-6
Depositario-Cabacar, D. T., Peters, J. M., Pong, A. W., Roth, J., Rotenberg, A., Riviello, J. J. Jr., et al. (2010). High-dose intravenous levetiracetam for acute seizure exacerbation in children with intractable epilepsy. Epilepsia 51 (7), 1319–1322. doi: 10.1111/j.1528-1167.2010.02519.x
Deshpande, L. S., Delorenzo, R. J. (2014). Mechanisms of levetiracetam in the control of status epilepticus and epilepsy. Front. Neurol. 5, 11. doi: 10.3389/fneur.2014.00011
Di Lorenzo, R., Li, Y. (2017). Rhabdomyolysis associated with levetiracetam administration. Muscle Nerve 56 (1), e1–e2. doi: 10.1002/mus.25548
Emswiler, M. P., Cumpston, K. L. (2017). Second Generation Anticonvulsants: Gabapentin, Lamotrigine, Levetiracetam, and Topiramate. In: Brent, J., et al. (eds) Critical Care Toxicology. Cham: Springer. Available at: https://link.springer.com/referenceworkentry/10.1007/978-3-319-17900-1_41#citeas
Falip, M., Carreno, M., Amaro, S., Donaire, A., Delgado, R., Toledo, M., et al. (2006). Use of levetiracetam in hospitalized patients. Epilepsia 47 (12), 2186–2188. doi: 10.1111/j.1528-1167.2006.00850.x
Fung, E. L., Fung, B. B. (2017). Review and update of the Hong Kong Epilepsy Guideline on status epilepticus. Hong Kong Med. J. 23 (1), 67–73. doi: 10.12809/hkmj166025
Glauser, T., Shinnar, S., Gloss, D., Alldredge, B., Arya, R., Bainbridge, J., et al. (2016). Evidence-Based Guideline: Treatment of Convulsive Status Epilepticus in Children and Adults: Report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr. 16 (1), 48–61. doi: 10.5698/1535-7597-16.1.48
Gujjar, A. R., Nandhagopal, R., Jacob, P. C., Al-Hashim, A., Al-Amrani, K., Ganguly, S. S., et al. (2017). Intravenous levetiracetam vs phenytoin for status epilepticus and cluster seizures: A prospective, randomized study. Seizure 49, 8–12. doi: 10.1016/j.seizure.2017.05.001
Higgins, J. P., Altman, D. G., Gotzsche, P. C., Juni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi: 10.1136/bmj.d5928
Husereau, D., Drummond, M., Petrou, S., Carswell, C., Moher, D., Greenberg, D., et al. (2013). Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 346, f1049. doi: 10.1136/bmj.f1049
Isguder, R., Guzel, O., Agin, H., Yilmaz, U., Akarcan, S. E., Celik, T., et al. (2014). Efficacy and safety of IV levetiracetam in children with acute repetitive seizures. Pediatr. Neurol. 51 (5), 688–695. doi: 10.1016/j.pediatrneurol.2014.07.021
Kantanen, A. M., Reinikainen, M., Parviainen, I., Ruokonen, E., Ala-Peijari, M., Backlund, T., et al. (2015). Incidence and mortality of super-refractory status epilepticus in adults. Epilepsy Behav. 49, 131–134. doi: 10.1016/j.yebeh.2015.04.065
Kellinghaus, C., Stogbauer, F. (2012). Treatment of status epilepticus in a large community hospital. Epilepsy Behav. 23 (3), 235–240. doi: 10.1016/j.yebeh.2011.12.020
Khan, O., Chang, E., Cipriani, C., Wright, C., Crisp, E., Kirmani, B. (2011). Use of intravenous levetiracetam for management of acute seizures in neonates. Pediatr. Neurol. 44 (4), 265–269. doi: 10.1016/j.pediatrneurol.2010.11.005
Kim, J., Shin, J. W. (2017). Levetiracetam-induced thrombocytopenia in a patient with status epilepticus. Epileptic Disord. 19 (1), 104–108. doi: 10.1684/epd.2017.0889
Kirmani, B. F., Crisp, E. D., Kayani, S., Rajab, H. (2009). Role of intravenous levetiracetam in acute seizure management of children. Pediatr. Neurol. 41 (1), 37–39. doi: 10.1016/j.pediatrneurol.2009.02.016
Koklu, E., Ariguloglu, E. A., Koklu, S. (2014). Levetiracetam-induced anaphylaxis in a neonate. Pediatr. Neurol. 50 (2), 192–194. doi: 10.1016/j.pediatrneurol.2013.09.006
Kortland, L. M., Alfter, A., Bahr, O., Carl, B., Dodel, R., Freiman, T. M., et al. (2016). Costs and cost-driving factors for acute treatment of adults with status epilepticus: A multicenter cohort study from Germany. Epilepsia 57 (12), 2056–2066. doi: 10.1111/epi.13584
Lang, N., Esser, W., Evers, S., Kellinghaus, C., Nguento, A., Schlegel, U., et al. (2015). Intravenous levetiracetam in clinical practice–Results from an independent registry. Seizure 29, 109–113. doi: 10.1016/j.seizure.2015.03.018
Liu, X., Wu, Y., Chen, Z., Ma, M., Su, L. (2012). A systematic review of randomized controlled trials on the theraputic effect of intravenous sodium valproate in status epilepticus. Int. J. Neurosci. 122 (6), 277–283. doi: 10.3109/00207454.2012.657376
Lyttle, M. D., Gamble, C., Messahel, S., Hickey, H., Iyer, A., Woolfall, K., et al. (2017). Emergency treatment with levetiracetam or phenytoin in status epilepticus in children-the EcLiPSE study: study protocol for a randomised controlled trial. Trials 18 (1), 283. doi: 10.1186/s13063-017-2010-8
Lyttle, M. D., Rainford, N. E. A., Gamble, C., Messahel, S., Humphreys, A., Hickey, H., et al. (2019). Levetiracetam versus phenytoin for second-line treatment of paediatric convulsive status epilepticus (EcLiPSE): a multicentre, open-label, randomised trial. Lancet 393 (10186), 2125–2134. doi: 10.1016/S0140-6736(19)30724-X
Matagne, A., Margineanu, D. G., Kenda, B., Michel, P., Klitgaard, H. (2008). Anti-convulsive and anti-epileptic properties of brivaracetam (ucb 34714), a high-affinity ligand for the synaptic vesicle protein, SV2A. Br. J. Pharmacol. 154 (8), 1662–1671. doi: 10.1038/bjp.2008.198
May, C. C., Taylor, J., Erwin, B., Black, J. R. (2017). Shapshak AH. A case of rhabdomyolysis exacerbated by levetiracetam administration. Neurocrit. Care 27 (2), S456. doi: 10.1007/s12028-017-0465-9
McTague, A., Kneen, R., Kumar, R., Spinty, S., Appleton, R. (2012). Intravenous levetiracetam in acute repetitive seizures and status epilepticus in children: Experience from a children's hospital. Seizure 21 (7), 529–534. doi: 10.1016/j.seizure.2012.05.010
Meierkord, H., Boon, P., Engelsen, B., Gocke, K., Shorvon, S., Tinuper, P., et al. (2010). EFNS guideline on the management of status epilepticus in adults. Eur. J. Neurol. 17 (3), 348–355. doi: 10.1111/j.1468-1331.2009.02917.x
Michaelides, C., Thibert, R. L., Shapiro, M. J., Kinirons, P., John, T., Manchharam, D., et al. (2008). Tolerability and dosing experience of intravenous levetiracetam in children and infants. Epilepsy Res. 81 (2-3), 143–147. doi: 10.1016/j.eplepsyres.2008.05.004
Misra, U. K., Kalita, J., Maurya, P. K. (2012). Levetiracetam versus lorazepam in status epilepticus: a randomized, open labeled pilot study. J. Neurol. 259 (4), 645–648. doi: 10.1007/s00415-011-6227-2
Moddel, G., Bunten, S., Dobis, C., Kovac, S., Dogan, M., Fischera, M., et al. (2009). Intravenous levetiracetam: a new treatment alternative for refractory status epilepticus. J. Neurol. Neurosurg. Psychiatry 80 (6), 689–692. doi: 10.1136/jnnp.2008.145458
Mundlamuri, R. C., Sinha, S., Subbakrishna, D. K., Prathyusha, P. V., Nagappa, M., Bindu, P. S., et al. (2015). Management of generalised convulsive status epilepticus (SE): a prospective randomised controlled study of combined treatment with intravenous lorazepam with either phenytoin, sodium valproate or levetiracetam–Pilot study. Epilepsy Res. 114, 52–58. doi: 10.1016/j.eplepsyres.2015.04.013
Navarro, V., Dagron, C., Elie, C., Lamhaut, L., Demeret, S., Urien, S., et al. (2016). Prehospital treatment with levetiracetam plus clonazepam or placebo plus clonazepam in status epilepticus (SAMUKeppra): a randomised, double-blind, phase 3 trial. Lancet Neurol. 15 (1), 47–55. doi: 10.1016/S1474-4422(15)00296-3
Nene, D., Mundlamuri, R. C., Satishchandra, P., Prathyusha, P. V., Nagappa, M., Bindu, P. S., et al. (2019). Comparing the efficacy of sodium valproate and levetiracetam following initial lorazepam in elderly patients with generalized convulsive status epilepticus (GCSE): A prospective randomized controlled pilot study. Seizure 65, 111–117. doi: 10.1016/j.seizure.2019.01.015
Ng, Y. T., Hastriter, E. V., Cardenas, J. F., Khoury, E. M., Chapman, K. E. (2010). Intravenous levetiracetam in children with seizures: a prospective safety study. J. Child Neurol. 25 (5), 551–555. doi: 10.1177/0883073809348795
Olesen, J., Gustavsson, A., Svensson, M., Wittchen, H. U., Jonsson, B. (2012). The economic cost of brain disorders in Europe. Eur. J. Neurol. 19 (1), 155–162. doi: 10.1111/j.1468-1331.2011.03590.x
Penberthy, L. T., Towne, A., Garnett, L. K., Perlin, J. B., DeLorenzo, R. J. (2005). Estimating the economic burden of status epilepticus to the health care system. Seizure 14 (1), 46–51. doi: 10.1016/j.seizure.2004.06.001
Prasad, K., Al-Roomi, K., Krishnan, P. R., Sequeira, R. (2005). Anticonvulsant therapy for status epilepticus. Cochrane Database Syst. Rev. (4), CD003723. doi: 10.1002/14651858.CD003723.pub2
Prasad, M., Krishnan, P. R., Sequeira, R., Al-Roomi, K. (2014). Anticonvulsant therapy for status epilepticus. Cochrane Database Syst. Rev. 2014 (9), CD003723. doi: 10.1002/14651858.CD003723.pub3
Rakshasbhuvankar, A., Rao, S., Kohan, R., Simmer, K., Nagarajan, L. (2013). Intravenous levetiracetam for treatment of neonatal seizures. J. Clin. Neurosci. 20 (8), 1165–1167. doi: 10.1016/j.jocn.2012.08.014
Reiter, P. D., Huff, A. D., Knupp, K. G., Valuck, R. J. (2010). Intravenous levetiracetam in the management of acute seizures in children. Pediatr. Neurol. 43 (2), 117–121. doi: 10.1016/j.pediatrneurol.2010.03.017
Rigo, J. M., Hans, G., Nguyen, L., Rocher, V., Belachew, S., Malgrange, B., et al. (2002). The anti-epileptic drug levetiracetam reverses the inhibition by negative allosteric modulators of neuronal GABA- and glycine-gated currents. Br. J. Pharmacol. 136 (5), 659–672. doi: 10.1038/sj.bjp.0704766
Ruegg, S., Naegelin, Y., Hardmeier, M., Winkler, D. T., Marsch, S., Fuhr, P. (2008). Intravenous levetiracetam: treatment experience with the first 50 critically ill patients. Epilepsy Behav. 12 (3), 477–480. doi: 10.1016/j.yebeh.2008.01.004
Sánchez Fernández, I., Gaínza-Lein, M., Lamb, N., Loddenkemper, T. (2019). Meta-analysis and cost-effectiveness of second-line antiepileptic drugs for status epilepticus. Neurology 92 (20), e2339–e2348. doi: 10.1212/WNL.0000000000007503
Sanchez Fernandez, I., Loddenkemper, T. (2015). Therapeutic choices in convulsive status epilepticus. Expert Opin. Pharmacother. 16 (4), 487–500. doi: 10.1517/14656566.2015.997212
Shahbaz, N., Younus, S. M., Khan, S. A., Ain, Q., Khan, M. A., Memon, M. H. (2017). Levetiracetam Induced Increase in Creatine Phosphokinase Levels. J. Coll. Physicians Surg. Pak. 27 (3), S63–S64.
Shea, B. J., Grimshaw, J. M., Wells, G. A., Boers, M., Andersson, N., Hamel, C., et al. (2007). Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med. Res. Methodol. 7, 10. doi: 10.1186/1471-2288-7-10
Shorvon, S., Baulac, M., Cross, H., Trinka, E., Walker, M. (2008). The drug treatment of status epilepticus in Europe: consensus document from a workshop at the first London Colloquium on Status Epilepticus. Epilepsia 49 (7), 1277–1285. doi: 10.1111/j.1528-1167.2008.01706_3.x
Singh, K., Aggarwal, A., Faridi, M. M. A., Sharma, S. (2018). IV Levetiracetam versus IV Phenytoin in childhood seizures: A randomized controlled trial. J. Pediatr. Neurosci. 13 (2), 158–164. doi: 10.4103/JPN.JPN_126_17
Standish, J. C., Hilmas, E., Falchek, S. J. (2011). Levetiracetam for the treatment of pediatric status epilepticus: A case series. J. Pediatr. Neurol. 9 (2), 195–201. doi: 10.3233/JPN-2011-0458
Trinka, E., Dobesberger, J. (2009). New treatment options in status epilepticus: a critical review on intravenous levetiracetam. Ther. Adv. Neurol. Disord. 2 (2), 79–91. doi: 10.1177/1756285608100460
Trinka, E., Kalviainen, R. (2017). 25 years of advances in the definition, classification and treatment of status epilepticus. Seizure 44, 65–73. doi: 10.1016/j.seizure.2016.11.001
Trinka, E., Cock, H., Hesdorffer, D., Rossetti, A. O., Scheffer, I. E., Shinnar, S., et al. (2015a). A definition and classification of status epilepticus–Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 56 (10), 1515–1523. doi: 10.1111/epi.13121
Trinka, E., Hofler, J., Leitinger, M., Brigo, F. (2015b). Pharmacotherapy for Status Epilepticus. Drugs 75 (13), 1499–1521. doi: 10.1007/s40265-015-0454-2
Trinka, E., Hofler, J., Leitinger, M., Rohracher, A., Kalss, G., Brigo, F. (2016). Pharmacologic treatment of status epilepticus. Expert Opin. Pharmacother. 17 (4), 513–534. doi: 10.1517/14656566.2016.1127354
Trinka, E. (2011). What is the evidence to use new intravenous AEDs in status epilepticus? Epilepsia 52 (Suppl 8), 35–38. doi: 10.1111/j.1528-1167.2011.03232.x
Tripathi, M., Vibha, D., Choudhary, N., Prasad, K., Srivastava, M. V., Bhatia, R., et al. (2010). Management of refractory status epilepticus at a tertiary care centre in a developing country. Seizure 19 (2), 109–111. doi: 10.1016/j.seizure.2009.11.007
Usery, J. B., Michael, L. M., 2nd, Sills, A. K., Finch, C. K. (2010). A prospective evaluation and literature review of levetiracetam use in patients with brain tumors and seizures. J. Neurooncol. 99 (2), 251–260. doi: 10.1007/s11060-010-0126-8
Wheless, J. W., Clarke, D., Hovinga, C. A., Ellis, M., Durmeier, M., McGregor, A., et al. (2009). Rapid infusion of a loading dose of intravenous levetiracetam with minimal dilution: a safety study. J. Child Neurol. 24 (8), 946–951. doi: 10.1177/0883073808331351
Yasiry, Z., Shorvon, S. D. (2014). The relative effectiveness of five antiepileptic drugs in treatment of benzodiazepine-resistant convulsive status epilepticus: A meta-analysis of published studies. Seizure 23 (3), 167–174. doi: 10.1016/j.seizure.2013.12.007
Yi, Z. M., Wen, C., Cai, T., Xu, L., Zhong, X. L., Zhai, S. D., et al. (2018). Levetiracetam for epilepsy: an evidence map of efficacy, safety and economic profiles. Neuropsychiatr. Dis. Treat. 15, 1–19. doi: 10.2147/NDT.S181886
Keywords: seizure cessation, seizure freedom, mortality, artificial ventilation, agitation, hypotension, cost-effectiveness
Citation: Yi Z-M, Zhong X-L, Wang M-L, Zhang Y and Zhai S-D (2020) Efficacy, Safety, and Economics of Intravenous Levetiracetam for Status Epilepticus: A Systematic Review and Meta-Analysis. Front. Pharmacol. 11:751. doi: 10.3389/fphar.2020.00751
Received: 13 February 2020; Accepted: 06 May 2020;
Published: 21 May 2020.
Edited by:
Shahzad Hussain, NIH Clinical Center (CC), United StatesReviewed by:
Honoré Mitonga Kabwebwe, University of Namibia, NamibiaRobert L. Lins, Independent Researcher, Antwerp, Belgium
Copyright © 2020 Yi, Zhong, Wang, Zhang and Zhai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Suo-Di Zhai, zhaisuodi@163.com
†These authors have contributed equally to this work
 Zhan-Miao Yi
Zhan-Miao Yi Xu-Li Zhong
Xu-Li Zhong Ming-Lu Wang1,5
Ming-Lu Wang1,5