- 1Department of Rheumatology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Department of Orthopaedics, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 3Department of Magnetic Resonance Imaging, Henan Key Laboratory of Functional Magnetic Resonance Imaging and Molecular Imaging, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Osteoporosis (OP) is a chronic bone disease characterized by aberrant microstructure and macrostructure of bone, leading to reduced bone mass and increased risk of fragile fractures. Anti-resorptive drugs, especially, bisphosphonates, are currently the treatment of choice in most developing countries. However, they do have limitations and adverse effects, which, to some extent, helped the development of anabolic drugs such as teriparatide and romosozumab. In patients with high or very high risk for fracture, sequential or combined therapies may be considered with the initial drugs being anabolic agents. Great endeavors have been made to find next generation drugs with maximal efficacy and minimal toxicity, and improved understanding of the role of different signaling pathways and their crosstalk in the pathogenesis of OP may help achieve this goal. Our review focused on recent progress with regards to the drug development by modification of Wnt pathway, while other pathways/molecules were also discussed briefly. In addition, new observations made in recent years in bone biology were summarized and discussed for the treatment of OP.
Introduction
The pathogenesis of osteoporosis (OP) may result from different factors such as aging, glucocorticoid use and heavy alcohol consumption. Aging is often associated with reduced bone mass, abnormal microstructure and fragile fracture, which poses a tremendous challenge to the medical communities (Compston et al., 2019; Tatangelo et al., 2019). Healthy bone has dynamic and balanced formation and resorption. Thus, two types of drugs, namely, anti-resorptive and pro-formative, are used to treat OP. Anti-resorptive drugs take their effect by interfering normal functions of osteoclasts. This type of drugs includes bisphosphonates (BPPs), estrogen, selective estrogen receptor modulators (SERMs), the antibodies against receptor activator of nuclear factor κB (NF-κB) ligand (RANKL), etc. While BPs can increase bone mineral density (BMD), they may decrease the flexibility of bone, increasing fracture risk (Russell et al., 2007). As such, pro-formative (anabolic) drugs have attracted wide attention in recent years (Langdahl, 2020). However, the concerns remain with regard to their cost-effectiveness, the efficacy in cortical bone, the potential adverse effects on endocrine and cardiovascular systems (Martin, 2016; Miller et al., 2016; Fuggle et al., 2020). Mounting data indicates a critical role of Wnt signaling pathway in bone formation, and novel therapeutics may be discovered through modifying inhibitors or activators of this pathway (Lerner and Ohlsson, 2015). Our review summarized the working mechanisms of both types of drugs and discussed the potential outcomes of some investigative drugs with the focus on Wnt pathway.
Methods
We searched PubMed for combinations of the following indexed subject headings (MeSH): Osteoporosis, antiresorptive drugs, anabolic drugs, Wnt signaling pathway, bone formation.
Skeletal Biology
Osteoclasts (OCs) are derived from hematopoietic stem cells and formed by the fusion of monocytes through complicated mechanisms. Multiple factors are involved in the differentiation, activation and survival of OCs including receptor activator of NF-κB ligand (RANKL), a molecule produced by different types of cells including osteoblasts (OBs), OCs, bone marrow stromal cells, lymphocytes, etc. In an acidic microenvironment formed by the sealing zone of OCs, cathepsin K is the most important enzyme to degrade non-mineral components of bone such as collagen type I (Col-I). The attachment of OCs on bone surface is mediated by integrins, mainly αvβ3 (Lewiecki, 2011). OBs are derived from mesenchymal stem cells (MSCs). Mature OBs produce osteoid consisting of Col-I and non-collagenous proteins. Mineralization of osteoid ensues and osteoblasts are embedded in bone, referred to as osteocytes (OCTs) (Lewiecki, 2011; Eastell et al., 2016). While OCTs were thought to be quiescent cells, several lines of evidence suggest they are active participants of bone metabolism. They can perceive mechanical loading signal and be regulated by hormones to coordinate coupling processes of formation and resorption mediated by OCs and OBs. In addition, OCTs are the major source of sclerostin, a potent inhibitor of Wnt pathway (Eastell et al., 2016) (Figure 1).
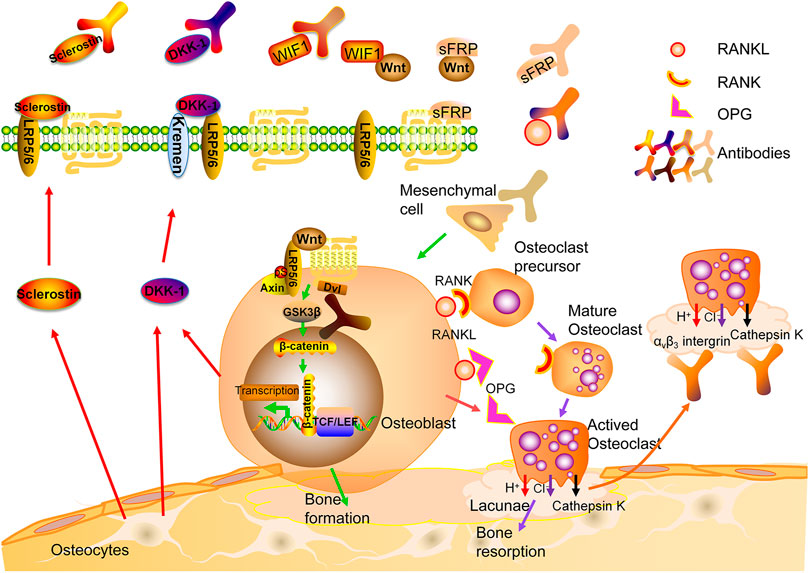
FIGURE 1. Bone remodeling and therapeutic targets for osteoporosis. RANK: Receptor activator of nuclear factor-kb; RANKL: RANK ligand; OPG: osteoprotegerin.
Anti-Resorptive Durgs
While some anti-resorptive agents such as BPPs, estrogen and denosumab, have been proven effective in some patients (Cheng et al., 2020), investigative agents targeting the molecules of resorption lacuna hold great promises.
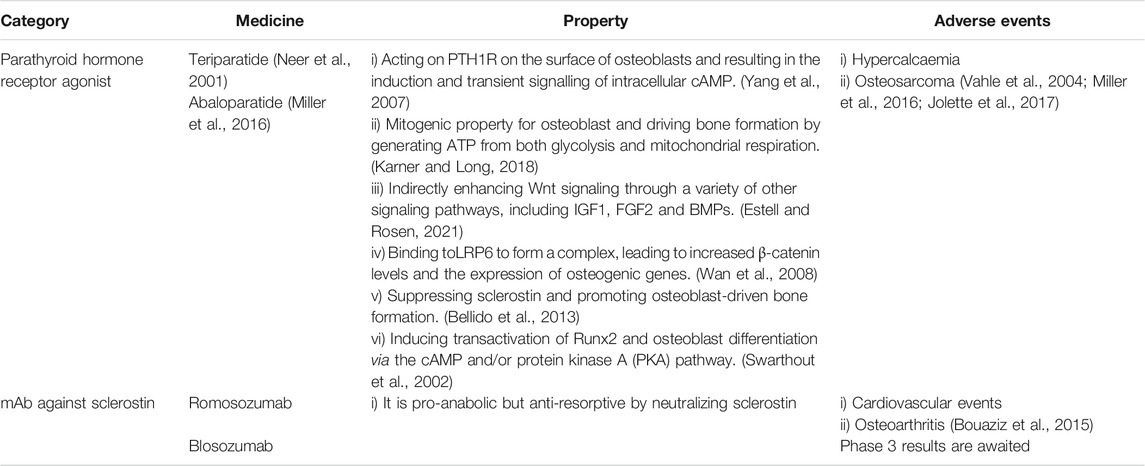
Currently Available Anti-resorptive Drugs
While BPPs are commonly used agents for primary and secondary osteoporosis to increase BMD, they do affect the flexibility of bone (Russell et al., 2007). They may cause atypical subtrochanteric fractures and are not recommended for young patients (Van den Wyngaert et al., 2006; Russell et al., 2007). Another concern is osteonecrosis of jaw, particularly, for those who will have dental procedures in the near future (Van den Wyngaert et al., 2006; Russell et al., 2007). Estrogen replacement therapy may increase cardiovascular events, venous thromboembolism and breast cancer (Rossouw et al., 2002; Almeida et al., 2017). While selective estrogen receptor modulators (SERMs) have a reduced risk of breast cancer (Cummings et al., 1999; Lindsay et al., 2009; Cummings et al., 2010), their efficacy is lower than estrogen (Ettinger et al., 1999; Silverman et al., 2008; Reid, 2015).
Denosumab is a fully human IgG2 monoclonal antibody (mAb) against the ligand of the RANK receptors on the surface of osteoclast precursors (RANKL) (Lacey et al., 2012). Binding of RANKL to RANK activates multiple signaling pathways. The binding of TNF receptor-associated factors (TRAFs) to specific sites in the cytoplasmic domain of RANK is crucial for differentiation and survival of OCs (Boyce and Xing, 2008). Osteoprotegerin (OPG), a decoy receptor, may compete with RANKL for the binding to RANK (Kearns et al., 2008; Infante et al., 2019). Bone mass was significantly reduced in OPG-knockout mice, while it is increased after overexpressing OPG (Nakamura et al., 2003) (Figure 1).
Previous studies have demonstrated that denosumab can improve the structure and thickness of cortical bone, and reduce the porosity of trabecular bone although it decelerates the turn-over of bone (Genant et al., 2013; Zebaze et al., 2016). Clinical trials have shown that in the first year, it may reduce the risk of vertebral and non-vertebral fractures (Cummings et al., 2009). While prolonged treatment leads to continuous increase of BMD, the risk of infection also increases. Besides, atypical femoral fractures and osteonecrosis may occur although the incidence is low (Bone et al., 2017). More studies are warranted to maximize its efficacy and minimize its adverse events. Of note, after withdrawal of denosumab, the BMD rapidly declines with subsequent increase in fracture risk. Thus, additional anti-resorptive drugs are required to maintain the treatment outcomes (Rizzoli et al., 2010; Collison, 2017).
Anti-resorptive Drugs Under development
Targeting the Molecules of Resorption Lacuna
Cathepsin K, the primary cysteine protease secreted by mature OCs, is involved in the degradation of Col-I and other bone matrix proteins (Costa et al., 2011). The observations made from different animal models have shown that inhibiting cathepsin K decreases osteoclastic bone resorption and increases bone formation (Gowen et al., 1999; Duong et al., 2016a; Duong et al., 2016b). The selective cathepsin K inhibitors, such as Odanacatib (Langdahl et al., 2012; Statham and Aspray, 2019), ONO-5334 (Engelke et al., 2014) and MIV-711 (Lindström et al., 2018; Conaghan et al., 2020), have been shown to reduce bone resorption and continuously increases BMD at multiple sites. Unfortunately, due to the adverse events, especially stroke, further development is restricted (Mullard, 2016; McClung et al., 2019). One explanation is that cathepsin K deficiency may disrupt the blood-brain barrier via AKT-mTOR-VEGF signaling, causing neurological deficits and neuron apoptosis (Zhao et al., 2019). Other concern is the rapid loss of functions after cessation of treatment (Eastell et al., 2014). Further, chloride channel-7 (ClC-7) and cathepsin K coexists and works synergistically in the ruffled border of OCs. The damage of ClC-7 results in severe OP, possibly due to the defect in bone degradation caused by the inability to acidify the sealing zone (Kornak et al., 2001). However, a CIC-7 inhibitor, N53736, showed a long-term anti-resorptive effect in ovariectomized (OVX) rats (Schaller et al., 2004), thus, more studies are needed.
As integrin αvβ3 mediates the attachment of OCs onto bone matrix proteins, it is reasonable to hypothesize that inhibiting the subunit of this integrin may prevent bone resorption. In different animal models of induced osteoporosis, αvβ3 integrin antagonists such as L-000845704 and HSA-ARLDDL significantly increase the BMD (Murphy et al., 2005; Lin et al., 2017). In addition, a dual-specific protein, macrophage colony-stimulating factor (M-CSFRGD), may bind to and inhibit both c-FMS and αvβ3 integrin. In vitro and in vivo studies shows that it inhibits OCs activity (Zur et al., 2018). These results indicate that targeting molecules adjacent to resorption lacuna may pave a new way to the treatment of OP.
All anti-resorptive agents mentioned above are listed in Table1.
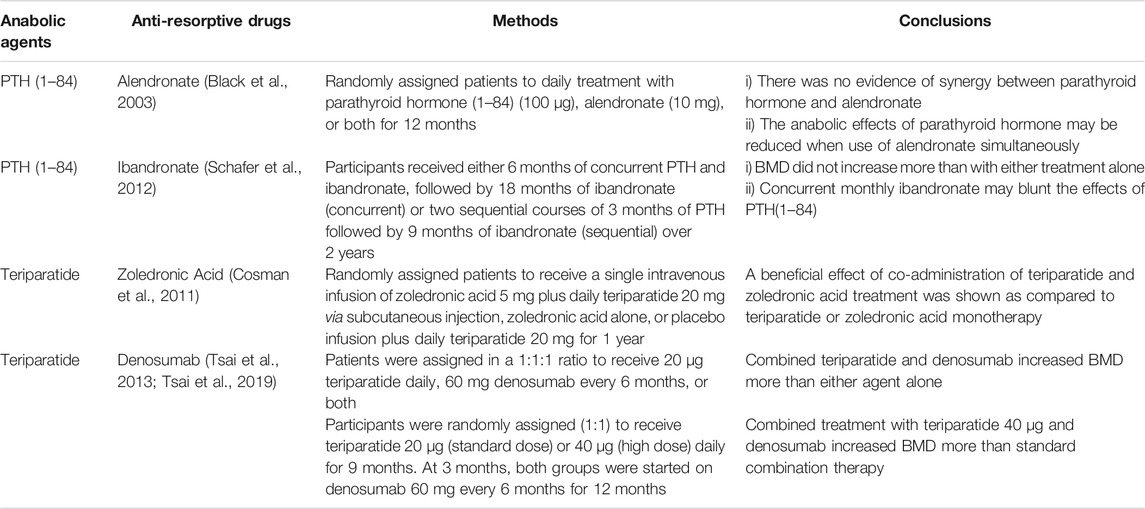
Anabolic Drugs
Non-wnt Related Anabolic Drugs
Teriparatide (a recombinant human PTH 1–34) may enhance bone formation by promoting osteoblast differentiation and functions. In the early stage of treatment, intermittent administration of teriparatide stimulates bone formation on cancellous, endosteal, and periosteal surfaces. Its effects on cortical bone vary at different sites (Martin, 2016). Randomised controlled trials (RCTs) show a higher efficacy of teriparatide than risedronate regarding the incidence of vertebral and non-vertebral fractures (Neer et al., 2001; Kendler et al., 2018). Similarly, Abaloparatide, a synthetic analogue of PTHrP, reduces the fracture risk in these sites. In addition, Abaloparatide has a higher efficacy in the increment of BMD and lower incidence of hypercalcaemia than Teriparatide (Leder et al., 2015a; Miller et al., 2016). Further, it is superior to Teriparatide and Alendronate with regard to the reduction of fracture risks (Miller et al., 2016; Reginster et al., 2019; Leder et al., 2020). Compared with Teriparatide, Abaloparatide has higher affinity to PTH1R and is able to specifically stimulate osteogenesis. Nevertheless, there is a controversy about whether these effects are due to decreased bone resorption or increased bone formation (Reginster et al., 2018). Although no increased risk of osteosarcoma is observed in patients, laboratory studies have shown a dose-dependent increase of osteosarcoma in rats treated with either Teriparatide or Abaloparatide (Vahle et al., 2004; Jolette et al., 2017). Therefore, it is recommended that the duration of Teriparatide treatment should be limited to 24 months (Andrews et al., 2012).
All currently available anabolic agents are in Table 2. In detailed discussion of romosozumab and blosozumab will be presented in the following section.
Wnt Signaling Pathways and Potential Agents and Targets
Wnt Signaling Pathway Activation
The Wnts are secreted, lipid-modified glycoproteins. After binding to their cell surface receptors, they can take effect via either canonical or non-canonical pathways. The canonical pathway is predominant in bone formation. The receptors of different Wnts in the canonical pathway consist of the low-density lipoprotein receptor related protein (LRP) single-pass transmembrane co-receptors 5/6 and the seven-transmembrane signaling receptor Frizzled (FZD) (Ng et al., 2019). In the downstream of this pathway, there is a destruction complex containing Axin, adenomatous polyposis coli (APC), casein kinase 1 (CK1) and glycogen synthase kinase 3β (GSK3β). In the absence of Wnt ligands, β-catenin is phosphorylated by GSK3β with subsequent ubiquitination and degradation (MacDonald et al., 2009; Clevers and Nusse, 2012). Upon Wnt binding, dishevelled (Dvl) disassembles the destruction complex, preventing phosphorylation of β-catenin. Non-phosphorylated β-catenin accumulates in the cytoplasm, and translocates to the nucleus whereby it forms a nuclear complex with T-cell specific transcription factor/lymphoid enhancing factor (TCF/LEF) transcription factor, which then causes the recruitment of co-activators and induction of gene transcription (Tolwinski and Wieschaus, 2004; MacDonald et al., 2009; Baron and Kneissel, 2013) (Figure 2).
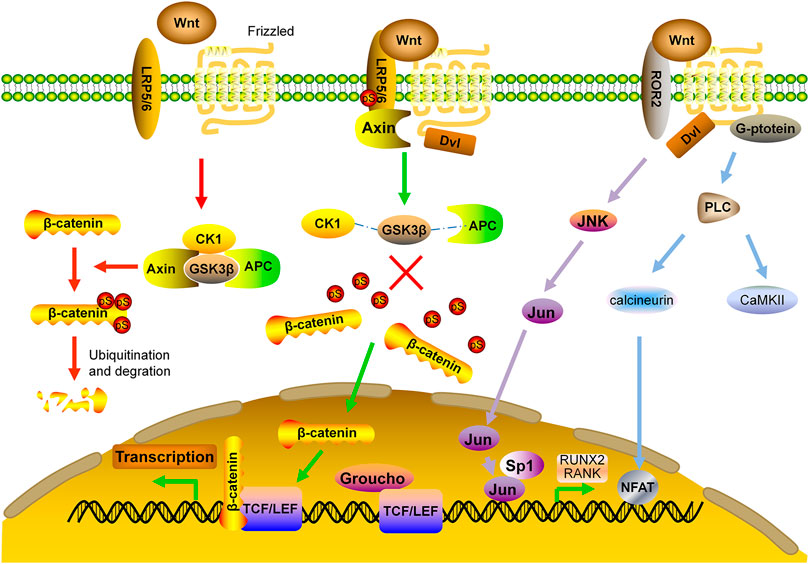
FIGURE 2. Wnt signaling pathway in bone formation. APC: adenomatous polyposis coli; CaMKII: calcium calmodulin-mediated kinase II; CK1: casein kinase one; Dvl: dishevelled; FZD: frizzled; GSK3β: glycogen synthase kinase 3β; JNK: c-Jun N-terminal kinase; LRP: low density lipoprotein receptor related protein; NFAT: nuclear factor of activated T cells; RUNX2: runt-related transcription factor 2; TCF/LEF: T-cell specific transcription factor/lymphoid enhancer binding factor.
Non-canonical Wnt signaling pathway is independent of β-catenin, instead, it takes effects by activating the heterotrimeric G-proteins and protein kinase C (PKC), which inhibits MSC differentiation toward adipocyte lineage and stimulates the nuclear factor of activated T cells (NFAT) to regulate bone formation and bone resorption (Kohn and Moon, 2005). Non-canonical Wnt signaling also induces Rho-or c-Jun N-terminal kinase (JNK)-dependent changes in the actin cytoskeleton, which facilitates Jun and Sp1 transcription factor to regulate the bone related molecules such as RANK and Runx2 (Veeman et al., 2003; Krishnan et al., 2006; Amjadi-Moheb and Akhavan-Niaki, 2019) (Figure 2).
Wnt Signaling and Bone Anabolism
Wnt signaling pathway enhances bone anabolism by inducing osteoblast differentiation, suppressing osteoclastogenesis and preventing adipogenesis. Expression of Wnt target genes such as Runx2, induces differentiation of MSC precursors to osteoblastic lineage, promoting bone formation (Gaur et al., 2005; Davis and Zur Nieden, 2008). Activation of Wnt pathway increases glycolysis in OBs, providing then the energy needed for collagen synthesis and matrix mineralization (Karner and Long, 2017). Remodeling on cortical bone is increased markedly due to activation of OBs on both the cortical and trabecular surface. In addition, canonical Wnt signaling inhibits bone resorption by increasing OPG production (Boyce et al., 2005). A study showed that bone formation was reduced in mice deficient with either FZD receptor or β-catenin although the production of OPG was not changed. It is postulated that Wnt signaling may repress osteoclastogenesis in a mechanism different from RANK/RANKL/OPG axis (Albers et al., 2013). Moreover, through enhancing phosphorylation of β-catenin, sclerostin facilitates adipogenesis (Fairfield et al., 2017). In a mouse model of myeloma, mAb against sclerostin increased bone mass and decreased the number of bone marrow adipocytes (McDonald et al., 2017).
Epigenetic Mechanisms Regulating Wnt Signaling
Epigenetic modification of some important molecules in Wnt pathway may affect bone metabolism. Bone biopsy from postmenopausal women with osteoporotic fractures shows a higher serum level of sclerostin. Increased CpGs methylation in the proximal region of the promoter of the Sost gene reduces the inhibitory effect of slcerostin on Wnt pathway, thereby enhancing bone formation (Reppe et al., 2015). Previous studies have shown that histone acetylation of Wnt gene promoter is reduced owing to the inhibition of lysine acetyltransferase 2A (GCN5) expression, resulting in suppression of Wnt signaling (Jing et al., 2018). In addition, a histone-lysine N-methyltransferase enzyme, an enhancer of zeste homolog 2 (EZH2), suppresses osteogenic differentiation of MSCs. Inhibition of EZH2 prevents bone loss (Dudakovic et al., 2015; Dudakovic et al., 2016). Overexpression of histone deacetylases 5 (HDAC5) downregulates the expression of sclerostin in osteocytes (Wein et al., 2015; Wein et al., 2016). miRNAs also play an important role in regulation of Wnt signaling (Amjadi-Moheb and Akhavan-Niaki, 2019). MiR-27a decreases OC differentiation and bone resorption through a binding site in the 3′-untranslational region of APC (Guo et al., 2018). During osteogenic differentiation of human stromal/stem cells, by inhibiting secreted frizzled-related proteins (sFRPs), dickkopf (DKK) and sclerostin, the signal amplification circuit between miR-218 and Wnt/β-catenin signals is established to drive Wnt-related transcription and OB differentiation (Hassan et al., 2012; Zhang et al., 2014). Other miRNAs, such as miR-29, miR-542-3p and miR-335-5p, can also regulate different molecules in Wnt pathway (Kapinas et al., 2010). Furthermore, miR-16-2*, by regulating the expression of Runx2, may be involved in OB differentiation, matrix mineralization and pathogenesis of OP (Duan et al., 2018).
Wnt Antagonists
Inhibition of canonical Wnt signaling pathway can be done by neutralizing Wnt ligands or blocking their binding to the receptor LRP/FZD. Wnt antagonists such as Wnt inhibitory factor 1 (WIF-1) and sFRPs prevent ligands binding to their cognate receptor. WIF-1 is structurally similar to the extracellular portion of the Derailed/Ryk class of transmembrane Wnt receptors. It may inhibit Wnt activity during OB differentiation and maturation (Vaes et al., 2005; Canalis, 2013). However, overexpression of WIF-1 activates canonical Wnt signaling and results in the loss of self-renewal potential of resident hematopoietic stem cells, suggesting it is not an optimal target for regulation of bone formation (Schaniel et al., 2011).
sFRPs block Wnt signaling by interacting with Wnts or FZD. Previous studies have demonstrated that sFRP1 is a negative regulator of cancellous bone formation and overexpression of sFRP4 in OBs reduces bone mass (Kawano and Kypta, 2003; Bodine et al., 2004; Nakanishi et al., 2008). Somewhat surprisingly, deletion of sFRP4 decreases the thickness of cortical bone, possibly by activating non-canonical signaling (Kiper et al., 2016; Chen et al., 2019), suggesting that fine-tuning the concentrations of sFRPs is needed before future trials.
Sclerostin and DKK1 block Wnt/β-catenin pathway by binding to LRP5/6. Sclerostin is mainly expressed by OCTs, and its binding to LRP5/6 inhibits bone formation and enhanced bone resorption (Li et al., 2005). Besides, osteocyte-produced sclerostin is transported to bone surface or adjacent OCTs, where it inhibits osteoblast-mediated bone formation, and increases bone resorption by OCs as well as osteocytic osteolysis by stimulating RANKL production and downregulating OPG expression (Ke et al., 2012; Appelman-Dijkstra and Papapoulos, 2018). Sclerostin may also play a role in other signaling pathways. An in vivo study has shown that mechanical stress activates Wnt pathway by down-regulating sclerostin expression, whereas upregulation of sclerostin expression in unloaded bone leads to bone loss (Robling et al., 2008). Of note, one underlying mechanism for anabolic effects of intermittent administration of PTH on bone is to inhibit sclerostin expression (Bellido et al., 2013).
DKK1 is a secreted glycoprotein produced by OCTs and OBs, and it contains the cysteine-rich domains that can bind to LRP5/6. DKK1 coupled with transmembrane receptor Kremen may form a complex with LRP to inhibit Wnt signaling (Mao et al., 2002; Pinzone et al., 2009). Further, DKK1 antagonizes osteoblastogenesis from MSCs and Wnt-mediated OB differentiation. Increased production of RANKL and decreased production of OPG mediated by DKK1 causes net bone loss (Pinzone et al., 2009).
Drugs Related to the Wnt Signaling Pathway
Romosozumab, a humanized antibody that neutralizes sclerostin, has been approved by the FDA for OP treatment. Several trials have demonstrated that it significantly increases BMD and decreases new vertebral and non-vertebral fractures (McClung et al., 2014). However, romosozumab did not improve the fracture-healing-related outcomes of hip fractures (Schemitsch et al., 2020). A recent study showed that romosozumab induced a transient bone formation in the first 2 months and a sustained suppression of bone resorption for up to 12 months (Chavassieux et al., 2019). As the anabolic effects of anti-sclerostin therapy are short-lived, it is reasonable to hypothesize that intermittent and short-term treatment with romosozumab might be just as effective as the continuous treatment for 12 months (Cosman et al., 2016; Saag et al., 2017). Sustainable BMD gains can be achieved by sequential therapy with romosozumab followed by denosumab (McClung et al., 2018; Kendler et al., 2019; Lewiecki et al., 2019). The STRUCTURE trial has shown that romosozumab is superior to Teriparatide with regard to increase in bone mass and strength (Langdahl et al., 2017). Romosozumab is not recommended for patients with a previous myocardial infarction or other cardiovascular events because of potential adverse effects (Lewiecki et al., 2018). Two meta-analyses showed inconsistent results in terms of the increase in cardiovascular risk (Bovijn et al., 2020; Lv et al., 2020). One explanation is that sclerostin is expressed in aortic vascular smooth muscle and can inhibit angiotensin II-induced atherosclerosis. Systemic blockade of sclerostin may affect the remodeling process in the cardiovascular system (Krishna et al., 2017; Asadipooya and Weinstock, 2019). A study showed that the second course of treatment with romosozumab had similar effects as the treatment in the first year (McClung et al., 2020), however, the BMD increments were smaller than those observed during the first year (McClung et al., 2018; Kendler et al., 2019). The duration of romosozumab treatment remains a matter of debates. At the moment, it is well accepted that the treatment should be no longer than 12 months (Table 3).
Blosozumab, another mAb against sclerostin, has shown to be well-tolerated in completed phase 1 and phase 2 trials. It increased BMD in a dose-dependent manner. Phase 3 results are awaited with excitement (McColm et al., 2014; Recker et al., 2015). To the best of our knowledge, no clinical trials are conducted to compare the efficacy in BMD increment between blosozumab and romosozumab.
AbD09097, a new anti-sclerostin agent, was examined in vitro about its effect on bone formation (Boschert et al., 2016). Combination of mechanical loading and anti-sclerostin antibodies in mice caused higher bone formation than either anti-sclerostin antibodies or mechanical loading alone (Morse et al., 2018). This study suggests that a combination of pharmacotherapy and physiotherapy may achieve sustained improvement of bone quality and persistent reduction of fracture risk. The effectiveness of the available nanocarriers, mesoporous silica nanoparticles (MSNs) loading with osteostatin and SOST siRNA is evaluated, and its subcutaneous injection up-regulated the expression of osteogenic related genes, thus, improving bone microarchitecture. More studies are needed before clinical application of such delivery system (Mora-Raimundo et al., 2021).
Preclinical studies have been performed to test the effect of mAb to DKK1. It improved BMD improvement in OVX rodents, whereas only a minimal improvement was observed in OVX monkeys (Glantschnig et al., 2011; Li et al., 2011). Notably, a bispecific antibody directed at both sclerostin and DKK1 has been generated and shown a more significant BMD improvement than mono-antibody in OVX rats (Florio et al., 2016). Because of the concern of off-target effects of DKK1 inhibitors in non-skeletal tissues, no clinical trials are currently going on.
Lithium, a GSK3β inhibitor, can activate Wnt-β-catenin pathway. Mice treated with lithium chloride (LiCl) lowered fracture risk. It stimulated bone formation, but did not affect bone resorption (Clement-Lacroix et al., 2005; Vestergaard et al., 2005). A newly-developed GSK3β inhibitor rapidly increased the number of OBs and decreased the number of OCs, resulting in a significant increase in bone volume, trabecular number and trabecular thickness (Clement-Lacroix et al., 2005; Amirhosseini et al., 2018). LY294002, an inhibitor of phosphatidylinositol-3-kinase-protein kinase B (PI3K-AKT) signaling pathway, can inhibit OC differentiation. However, both LiCl and LY294002 are highly toxic at conventional doses (Huang et al., 2018). Low doses of combined LiCl and LY294002 not only promote bone formation and inhibit bone resorption, but also are more effective in the treatment of OP than either single compound (Bai et al., 2019). Additionally, ample phytochemicals, such as Baicalin, Aspp049, Wedelolactone, Ursolic acid, may enhance GSK3β phosphorylation, Runx2 expression, and nuclear translocation of β-catenin, thus, enhancing osteogenic differentiation and bone formation (Manandhar et al., 2020). Despite these results, lacking bone specificity and potential off-target effects hinder further development of GSK3β inhibitors for the treatment of OP (Hall et al., 2015).
Animal study was conducted to evaluate the effect of sFRP1 inhibitors on OP and these included imino-oxothiazolidines, diarylsulfone sulfonamides and N-substituted piperidinyl diphenylsulfonyl sulfonamides (WAY-316606). The results showed increased OB activation and bone formation (Claudel et al., 2019) Further, miR-542-3p and miR-1-3p inhibited sFRP1 expression and induced OB differentiation (Zhang et al., 2018; Gu et al., 2020). Based on these findings, miRNA-based therapies targeting sFRPs are likely to become novel approach to prevent and treat osteoporosis.
The possible therapeutic targets mentioned above have been identified in Figure 1.
Interaction of Wnt Pathway With Other Signaling Pathways
Bone morphogenetic proteins (BMPs) belong to the TGF-β superfamily. Among them, BMP-2 up-regulates the expression of Runx2 through Smad pathway, leading to enhanced bone formation. In addition, BMP-2 inhibits the activity of E3 ubiquitin ligase to prevent degradation of β-catenin and up-regulates the expression of WNT3A, WNT1, and LRP, which causes accumulation of β-catenin and activation of Wnt signaling pathway, thereby, increasing bone formation (Wu et al., 2016).
PI3K-AKT pathway can be activated in OBs by various growth factors. This pathway positively regulates Wnt signaling by stabilizing β-catenin and deactivating GSK3β. Previous studies have demonstrated that AKT may form a complex with BMP-2, and its related downstream signals are essential regulators for OB differentiation and endochondral ossification. AKT knockout mice had shorter bones and delayed bone ossification (Ulici et al., 2009). In addition, AKT phosphorylation by upstream kinase mTORC2 may cause accumulation of β-catenin both in cytoplasm and nucleus (Sarbassov et al., 2005; Rybchyn et al., 2011). One study shows that miR-483-5p mimic activates PI3K-AKT signaling pathway and affects cell viability, with significant down-regulation of the expressions of OPG, Runx2 and BMP2. Consistently, LY294002 and miR-483-5p inhibitor reverse these effects and increase BMD and biomechanical parameters for anabolism (Zhao et al., 2021). Moreover, interaction of MAPK pathway with Wnt signaling not only regulates survival and apoptosis of OCs, but also enhances BMP-2 expression and bone formation (Tang et al., 2008; Chen et al., 2014). A study demonstrates that miR-182-5p inhibits the expression of adenylyl cyclase isoform 6 (ADCY6) and activation of the Rap1/MAPK signaling pathway. Down-regulation of miR-182 promotes OB proliferation and differentiation (Pan et al., 2018).
Other pathways may also have cross-talks with Wnt pathway. For example, Adenosine Monophosphate Activated Protein kinase (AMPK) may activate canonical Wnt signaling pathway and up-regulate the expression of BMP-2 (Zhao et al., 2010). AMPK also phosphorylates HDAC5, resulting in the activation of Wnt signaling (Zhao et al., 2011).
Protein kinase C-binding protein NELL-1 is an osteoinductive growth factor that can bind to β1-integrin on the surface of bone cells. It not only activates canonical Wnt pathway and regulates the activity of Runx2, but also has a reciprocal impact on BMP-2 signaling by enhancing osteogenesis and inhibiting adipogenesis (Zhang et al., 2011; Shen et al., 2016; Pakvasa et al., 2017). In OVX mice, NELL-1 down-regulated RANKL expression and up-regulated OPG expression, leading to enhanced bone formation and decreased number of OCs (James et al., 2015). Delivering NELL-1 to vertebrae of osteoporotic sheep or femurs of OVX rats can improve the regeneration of cortical and trabecular bone (James et al., 2016; James et al., 2017). Additional studies are needed to determine the feasibility and efficacy of this protein as an anabolic agent (Figure 3).
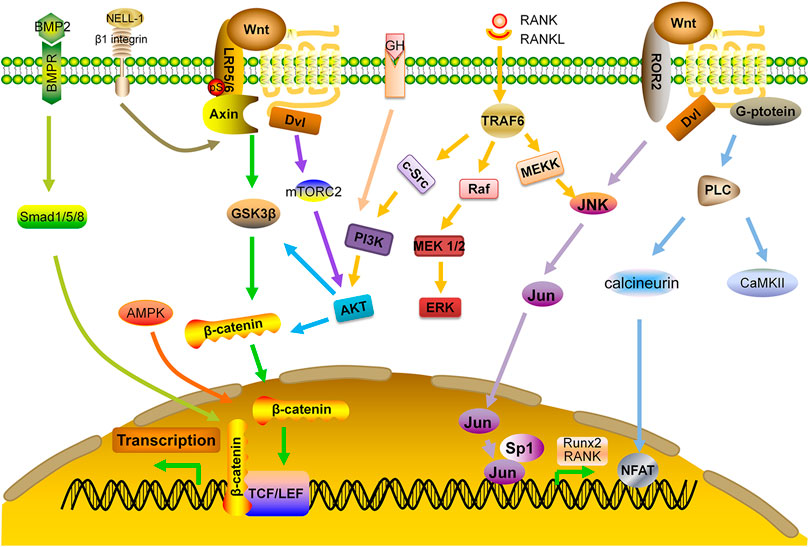
FIGURE 3. Interaction of Wnt signaling with other signaling pathways. AKT: protein kinase B; AMPK: adenosine monophosphate-activated protein kinase; BMP: bone morphogenetic protein; BMPR: BMP receptor; ERK: extracellular signal-regulated kinase; GF: growth factor; MEK: mitogen-activated protein kinase; NELL-1: NEL-like protein one; PI3K: phosphoinositide 3-kinase; Smad: small mothers against decapentaplegic; TRAF: TNF receptor-associated factors.
Combined and Sequential Therapies
Combined and Sequential Therapies
The effect of the most anti-osteoporotic drugs, except for BPs, is not sustainable on bone metabolism. In some cases, an overshooting response may occur when they are discontinued. In particular, withdrawal of anabolic drugs often causes rapid bone loss and increases risk of fractures. Further, anabolic treatment with Teriparatide or Abaloparatide may incite secondary stimulation of bone resorption. It is reasonable to postulate that the effects of bone-forming treatments may be improved and maintained with combined or sequential treatments. Ongoing clinical studies on combination and sequential therapies are summarized in Tables 4, 5. It is now unanimously accepted that the administration of bone-forming agents should be followed by an anti-resorptive agent. In addition, the evaluation of the effectiveness of combined therapies is still ongoing.
Discussion
Pathogenesis of OP, especially, in postmenopausal women, is multifaceted. Improved understanding of skeletal biology will help us identify new therapeutic targets with maximal efficacy and minimal adverse effects. Our review summarized recent progress in molecular mechanisms and major signaling pathways involved in bone homeostasis and OP pathogenesis. The approaches to prevent OP include anti-resorption by suppressing OC activity and pro-formation by enhancing OB functions. OCTs, used to be thought as the quiescent cells embedded in bone matrix, have been demonstrated to be critical in the regulation of OCs and OBs activities, warranting in-depth understanding of OCT biology. Taking cost-effectiveness into account, the mainstay of current treatments is still anti-resorptive drugs, particularly, BPPs, in most developing countries. However, as they can incorporate into bone and prevent bone resorption, normal dynamic remodeling process, especially in young adults, is interrupted, which may reduce the flexibility of bone (Russell et al., 2007).
We focused on Wnt pathway because accumulating data indicated a pivotal role of this pathway in bone metabolism. Comparing with TGF-β and NF-kB pathways, Wnt signaling pathway is more complicated and more targets are available for modifications both extra- or intracellularly. Elegant studies from different animal models have laid a solid foundation for new drugs development by regulating Wnt pathway. In the canonical Wnt pathway, the modification of the destruction complex is under intensive studies. For example, manipulating the activity GSK3β may enhance anabolic property of OBs (Amirhosseini et al., 2018). Similarly, regulating the expression of Axin-2 and APC may cause constitutive activation of canonical pathway to promote bone formation (Nusse and Clevers, 2017; Huang et al., 2019). However, the specificity and their potential off-target risks of some newly developed agents for modifying Wnt pathways have been halted after phase 1 or phase 2 trials. Delivery systems using peptides or chemicals with high affinity to bone are expected to overcome these drawbacks (Guan et al., 2012; Zur et al., 2018; Rammal et al., 2019). Bi-specific Wnt mimetic targeting both FZD and LRP has demonstrated a rapid and robust effect on bone building and correction of bone mass deficiency (Fowler et al., 2021), however, more studies are needed before preclinical and clinical trials of this agent. Besides, a cell/gene therapy in combination with miRNA manipulation may become effective treatment for osteoporosis. For example, hybrid vector engineered OVX-BMSCs were used to lower miR-140*/miR-214 levels, promote osteogenesis and enhance bone quality (Li et al., 2016). Further, the utilization of nanocarriers-based therapies that interact Wnt pathway hold great promise as novel therapy for osteoporosis. In contrast, because of the complexity and multiple alternatives of non-canonical Wnt pathway, there is a scarcity of data regarding the role of non-canonical Wnt pathway in bone metabolism. New targets may be identified after extensive studies of non-canonical Wnt pathway (Lerner and Ohlsson, 2015).
Other research interests include the mechanisms and treatment of the loss of cortical bone as it is more closely related to osteoporotic fractures. Aging is also an important factor for OP. Targeting the senescent cells by modification of the aging-related genes or pharmacological methods, such as Janus kinase (JAK) inhibitor, have both anti-resorptive and pro-formative effects on bone (Farr et al., 2017). In addition, more investigations should be carried out to elucidate the mechanism for bone erosion in some autoimmune diseases, especially, in rheumatoid arthritis (Minisola et al., 2021). Of note, osteoporosis is common in patients with ankylosing spondyloarthritis (AS), even in young males (Sambrook and Geusens, 2012). A recent study showed that miR-96 may promote osteoblast differentiation and bone formation in AS mice via Wnt signaling activation by binding to sclerostin (Ma et al., 2019). Further, the major pathway mediating glucocorticoid induced bone loss need to be further dissected in order to preserve their anti-inflammatory activity, but avoid the harmful skeletal effect of this most commonly used drug in autoimmune rheumatic diseases (Hartmann et al., 2016).
Conclusion
Although significant progresses have been made in recent years, the prevention and treatment of osteoporosis and the related fractures remain an unmet medical need. In-depth understanding of molecular events in the pathogenesis of osteoporosis including epigenetic regulation of Wnt pathway may facilitate the development of new drugs with better efficacy and less side effects.
Author Contributions
S-SL, S-HH, and P-YX did literature retrieval and prepared the draft, WL, X-XZ and made the first revision of the manuscript, T-FL and D-FL finalized the manuscript.
Funding
This study was funded the grants from National Natural Science Foundation of China (Grant Nos. U1704177 and 81871811) and China Postdoctoral Science Foundation (Grant No. 2020TQ0281)
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Jonathan Holz for language polishing.
References
Albers, J., Keller, J., Baranowsky, A., Beil, F. T., Catala-Lehnen, P., Schulze, J., et al. (2013). Canonical Wnt Signaling Inhibits Osteoclastogenesis Independent of Osteoprotegerin. J. Cel Biol 200 (4), 537–549. doi:10.1083/jcb.201207142
Almeida, M., Laurent, M. R., Dubois, V., Claessens, F., O'Brien, C. A., Bouillon, R., et al. (2017). Estrogens and Androgens in Skeletal Physiology and Pathophysiology. Physiol. Rev. 97 (1), 135–187. doi:10.1152/physrev.00033.2015
Amirhosseini, M., Madsen, R. V., Escott, K. J., Bostrom, M. P., Ross, F. P., and Fahlgren, A. (2018). GSK-3β Inhibition Suppresses Instability-Induced Osteolysis by a Dual Action on Osteoblast and Osteoclast Differentiation. J. Cel Physiol 233 (3), 2398–2408. doi:10.1002/jcp.26111
Amjadi-Moheb, F., and Akhavan-Niaki, H. (2019). Wnt Signaling Pathway in Osteoporosis: Epigenetic Regulation, Interaction with Other Signaling Pathways, and Therapeutic Promises. J. Cel Physiol 234 (9), 14641–14650. doi:10.1002/jcp.28207
Andrews, E. B., Gilsenan, A. W., Midkiff, K., Sherrill, B., Wu, Y., Mann, B. H., et al. (2012). The US Postmarketing Surveillance Study of Adult Osteosarcoma and Teriparatide: Study Design and Findings from the First 7 Years. J. Bone Miner Res. 27 (12), 2429–2437. doi:10.1002/jbmr.1768
Appelman-Dijkstra, N. M., and Papapoulos, S. E. (2018). Clinical Advantages and Disadvantages of Anabolic Bone Therapies Targeting the WNT Pathway. Nat. Rev. Endocrinol. 14 (10), 605–623. doi:10.1038/s41574-018-0087-0
Asadipooya, K., and Weinstock, A. (2019). Cardiovascular Outcomes of Romosozumab and Protective Role of Alendronate. Atvb 39 (7), 1343–1350. doi:10.1161/ATVBAHA.119.312371
Bai, J., Xu, Y., Dieo, Y., and Sun, G. (2019). Combined Low-Dose LiCl and LY294002 for the Treatment of Osteoporosis in Ovariectomized Rats. J. Orthop. Surg. Res. 14 (1), 177. doi:10.1186/s13018-019-1210-1
Baron, R., and Kneissel, M. (2013). WNT Signaling in Bone Homeostasis and Disease: from Human Mutations to Treatments. Nat. Med. 19 (2), 179–192. doi:10.1038/nm.3074
Bellido, T., Saini, V., and Pajevic, P. D. (2013). Effects of PTH on Osteocyte Function. Bone 54 (2), 250–257. doi:10.1016/j.bone.2012.09.016
Black, D. M., Greenspan, S. L., Ensrud, K. E., Palermo, L., McGowan, J. A., Lang, T. F., et al. (2003). The Effects of Parathyroid Hormone and Alendronate Alone or in Combination in Postmenopausal Osteoporosis. N. Engl. J. Med. 349 (13), 1207–1215. doi:10.1056/NEJMoa031975
Bodine, P. V. N., Zhao, W., Kharode, Y. P., Bex, F. J., Lambert, A.-J., Goad, M. B., et al. (2004). The Wnt Antagonist Secreted Frizzled-Related Protein-1 Is a Negative Regulator of Trabecular Bone Formation in Adult Mice. Mol. Endocrinol. 18 (5), 1222–1237. doi:10.1210/me.2003-0498
Bone, H. G., Cosman, F., Miller, P. D., Williams, G. C., Hattersley, G., Hu, M.-y., et al. (2018). ACTIVExtend: 24 Months of Alendronate after 18 Months of Abaloparatide or Placebo for Postmenopausal Osteoporosis. J. Clin. Endocrinol. Metab. 103 (8), 2949–2957. doi:10.1210/jc.2018-00163
Bone, H. G., Wagman, R. B., Brandi, M. L., Brown, J. P., Chapurlat, R., Cummings, S. R., et al. (2017). 10 Years of Denosumab Treatment in Postmenopausal Women with Osteoporosis: Results from the Phase 3 Randomised FREEDOM Trial and Open-Label Extension. Lancet Diabetes Endocrinol. 5 (7), 513–523. doi:10.1016/s2213-8587(17)30138-9
Boschert, V., Frisch, C., Back, J. W., van Pee, K., Weidauer, S. E., Muth, E.-M., et al. (2016). The Sclerostin-Neutralizing Antibody AbD09097 Recognizes an Epitope Adjacent to Sclerostin's Binding Site for the Wnt Co-receptor LRP6. Open Biol. 6 (8), 160120. doi:10.1098/rsob.160120
Bouaziz, W., Funck-Brentano, T., Lin, H., Marty, C., Ea, H.-K., Hay, E., et al. (2015). Loss of Sclerostin Promotes Osteoarthritis in Mice via β-catenin-dependent and -independent Wnt Pathways. Arthritis Res. Ther. 17, 24. doi:10.1186/s13075-015-0540-6
Bovijn, J., Krebs, K., Chen, C. Y., Boxall, R., Censin, J. C., Ferreira, T., et al. (2020). Evaluating the Cardiovascular Safety of Sclerostin Inhibition Using Evidence from Meta-Analysis of Clinical Trials and Human Genetics. Sci. Translational Med. 12 (549), eaay6570. doi:10.1126/scitranslmed.aay6570
Boyce, B. F., Xing, L., and Chen, D. (2005). Osteoprotegerin, the Bone Protector, Is a Surprising Target for β-catenin Signaling. Cel Metab. 2 (6), 344–345. doi:10.1016/j.cmet.2005.11.011
Boyce, B. F., and Xing, L. (2008). Functions of RANKL/RANK/OPG in Bone Modeling and Remodeling. Arch. Biochem. Biophys. 473 (2), 139–146. doi:10.1016/j.abb.2008.03.018
Canalis, E. (2013). Wnt Signalling in Osteoporosis: Mechanisms and Novel Therapeutic Approaches. Nat. Rev. Endocrinol. 9 (10), 575–583. doi:10.1038/nrendo.2013.154
Chavassieux, P., Chapurlat, R., Portero‐Muzy, N., Roux, J. P., Garcia, P., Brown, J. P., et al. (2019). Bone‐Forming and Antiresorptive Effects of Romosozumab in Postmenopausal Women with Osteoporosis: Bone Histomorphometry and Microcomputed Tomography Analysis after 2 and 12 Months of Treatment. J. Bone Miner Res. 34 (9), 1597–1608. doi:10.1002/jbmr.3735
Chen, K., Ng, P. Y., Chen, R., Hu, D., Berry, S., Baron, R., et al. (2019). Sfrp4 Repression of the Ror2/Jnk cascade in Osteoclasts Protects Cortical Bone from Excessive Endosteal Resorption. Proc. Natl. Acad. Sci. USA 116 (28), 14138–14143. doi:10.1073/pnas.1900881116
Chen, M., Qiao, H., Su, Z., Li, H., Ping, Q., and Zong, L. (2014). Emerging Therapeutic Targets for Osteoporosis Treatment. Expert Opin. Ther. Targets 18 (7), 817–831. doi:10.1517/14728222.2014.912632
Cheng, C., Wentworth, K., and Shoback, D. M. (2020). New Frontiers in Osteoporosis Therapy. Annu. Rev. Med. 71, 277–288. doi:10.1146/annurev-med-052218-020620
Claudel, M., Jouzeau, J. Y., and Cailotto, F. (2019). Secreted Frizzled‐related Proteins (sFRPs) in Osteo‐articular Diseases: Much More Than Simple Antagonists of Wnt Signaling? FEBS J. 286 (24), 4832–4851. doi:10.1111/febs.15119
Clement-Lacroix, P., Ai, M., Morvan, F., Roman-Roman, S., Vayssiere, B., Belleville, C., et al. (2005). Lrp5-independent Activation of Wnt Signaling by Lithium Chloride Increases Bone Formation and Bone Mass in Mice. Proc. Natl. Acad. Sci. 102 (48), 17406–17411. doi:10.1073/pnas.0505259102
Clevers, H., and Nusse, R. (2012). Wnt/β-Catenin Signaling and Disease. Cell 149 (6), 1192–1205. doi:10.1016/j.cell.2012.05.012
Collison, J. (2017). Discontinuing Denosumab Discouraged. Nat. Rev. Rheumatol. 13 (10), 571. doi:10.1038/nrrheum.2017.144
Compston, J. E., McClung, M. R., and Leslie, W. D. (2019). Osteoporosis. The Lancet 393 (10169), 364–376. doi:10.1016/s0140-6736(18)32112-3
Conaghan, P. G., Bowes, M. A., Kingsbury, S. R., Brett, A., Guillard, G., Rizoska, B., et al. (2020). Disease-Modifying Effects of a Novel Cathepsin K Inhibitor in Osteoarthritis. Ann. Intern. Med. 172 (2), 86–95. doi:10.7326/M19-0675
Cosman, F., Crittenden, D. B., Adachi, J. D., Binkley, N., Czerwinski, E., Ferrari, S., et al. (2016). Romosozumab Treatment in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 375 (16), 1532–1543. doi:10.1056/NEJMoa1607948
Cosman, F., Eriksen, E. F., Recknor, C., Miller, P. D., Guañabens, N., Kasperk, C., et al. (2011). Effects of Intravenous Zoledronic Acid Plus Subcutaneous Teriparatide [rhPTH(1-34)] in Postmenopausal Osteoporosis. J. Bone Miner Res. 26 (3), 503–511. doi:10.1002/jbmr.238
Costa, A. G., Cusano, N. E., Silva, B. C., Cremers, S., and Bilezikian, J. P. (2011). Cathepsin K: its Skeletal Actions and Role as a Therapeutic Target in Osteoporosis. Nat. Rev. Rheumatol. 7 (8), 447–456. doi:10.1038/nrrheum.2011.77
Cummings, S. R., Eckert, S., Krueger, K. A., Grady, D., Powles, T. J., Cauley, J. A., et al. (1999). The Effect of Raloxifene on Risk of Breast Cancer in Postmenopausal Women. JAMA 281 (23), 2189–2197. doi:10.1001/jama.281.23.2189
Cummings, S. R., Ensrud, K., Delmas, P. D., LaCroix, A. Z., Vukicevic, S., Reid, D. M., et al. (2010). Lasofoxifene in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 362 (8), 686–696. doi:10.1056/NEJMoa0808692
Cummings, S. R., Martin, J. S., McClung, M. R., Siris, E. S., Eastell, R., Reid, I. R., et al. (2009). Denosumab for Prevention of Fractures in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 361 (8), 756–765. doi:10.1056/NEJMoa0809493
Davis, L. A., and Zur Nieden, N. I. (2008). Mesodermal Fate Decisions of a Stem Cell: the Wnt Switch. Cell. Mol. Life Sci. 65 (17), 2658–2674. doi:10.1007/s00018-008-8042-1
Duan, L., Zhao, H., Xiong, Y., Tang, X., Yang, Y., Hu, Z., et al. (2018). miR-16-2* Interferes with WNT5A to Regulate Osteogenesis of Mesenchymal Stem Cells. Cell Physiol Biochem 51 (3), 1087–1102. doi:10.1159/000495489
Dudakovic, A., Camilleri, E. T., Riester, S. M., Paradise, C. R., Gluscevic, M., O'Toole, T. M., et al. (2016). Enhancer of Zeste Homolog 2 Inhibition Stimulates Bone Formation and Mitigates Bone Loss Caused by Ovariectomy in Skeletally Mature Mice. J. Biol. Chem. 291 (47), 24594–24606. doi:10.1074/jbc.M116.740571
Dudakovic, A., Camilleri, E. T., Xu, F., Riester, S. M., McGee-Lawrence, M. E., Bradley, E. W., et al. (2015). Epigenetic Control of Skeletal Development by the Histone Methyltransferase Ezh2. J. Biol. Chem. 290 (46), 27604–27617. doi:10.1074/jbc.M115.672345
Duong, L. T., Crawford, R., Scott, K., Winkelmann, C. T., Wu, G., Szczerba, P., et al. (2016a). Odanacatib, Effects of 16-month Treatment and Discontinuation of Therapy on Bone Mass, Turnover and Strength in the Ovariectomized Rabbit Model of Osteopenia. Bone 93, 86–96. doi:10.1016/j.bone.2016.09.012
Duong, L. T., Pickarski, M., Cusick, T., Chen, C. M., Zhuo, Y., Scott, K., et al. (2016b). Effects of Long Term Treatment with High Doses of Odanacatib on Bone Mass, Bone Strength, and Remodeling/modeling in Newly Ovariectomized Monkeys. Bone 88, 113–124. doi:10.1016/j.bone.2016.04.024
Eastell, R., Nagase, S., Small, M., Boonen, S., Spector, T., Ohyama, M., et al. (2014). Effect of ONO-5334 on Bone mineral Density and Biochemical Markers of Bone Turnover in Postmenopausal Osteoporosis: 2-year Results from the OCEAN Study. J. Bone Miner Res. 29 (2), 458–466. doi:10.1002/jbmr.2047
Eastell, R., O'Neill, T. W., Hofbauer, L. C., Langdahl, B., Reid, I. R., Gold, D. T., et al. (2016). Postmenopausal Osteoporosis. Nat. Rev. Dis. Primers 2, 16069. doi:10.1038/nrdp.2016.69
Engelke, K., Nagase, S., Fuerst, T., Small, M., Kuwayama, T., Deacon, S., et al. (2014). The Effect of the Cathepsin K Inhibitor ONO-5334 on Trabecular and Cortical Bone in Postmenopausal Osteoporosis: the OCEAN Study. J. Bone Miner Res. 29 (3), 629–638. doi:10.1002/jbmr.2080
Estell, E. G., and Rosen, C. J. (2021). Emerging Insights into the Comparative Effectiveness of Anabolic Therapies for Osteoporosis. Nat. Rev. Endocrinol. 17 (1), 31–46. doi:10.1038/s41574-020-00426-5
Ettinger, B., Black, D. M., Mitlak, B. H., Knickerbocker, R. K., Nickelsen, T., Genant, H. K., et al. (1999). Reduction of Vertebral Fracture Risk in Postmenopausal Women with Osteoporosis Treated with RaloxifeneResults from a 3-Year Randomized Clinical Trial. JAMA 282 (7), 637–645. doi:10.1001/jama.282.7.637
Fairfield, H., Rosen, C. J., and Reagan, M. R. (2017). Connecting Bone and Fat: The Potential Role for Sclerostin. Curr. Mol. Bio Rep. 3 (2), 114–121. doi:10.1007/s40610-017-0057-7
Farr, J. N., Xu, M., Weivoda, M. M., Monroe, D. G., Fraser, D. G., Onken, J. L., et al. (2017). Targeting Cellular Senescence Prevents Age-Related Bone Loss in Mice. Nat. Med. 23 (9), 1072–1079. doi:10.1038/nm.4385
Fleisch, H. (1998). Bisphosphonates: Mechanisms of Action. Endocr. Rev. 19 (1), 80–100. doi:10.1210/edrv.19.1.0325
Florio, M., Gunasekaran, K., Stolina, M., Li, X., Liu, L., Tipton, B., et al. (2016). A Bispecific Antibody Targeting Sclerostin and DKK-1 Promotes Bone Mass Accrual and Fracture Repair. Nat. Commun. 7, 11505. doi:10.1038/ncomms11505
Fowler, T. W., Mitchell, T. L., Janda, C. Y., Xie, L., Tu, S., Chen, H., et al. (2021). Development of Selective Bispecific Wnt Mimetics for Bone Loss and Repair. Nat. Commun. 12 (1), 3247. doi:10.1038/s41467-021-23374-8
Fuggle, N. R., Cooper, C., Harvey, N. C., Al-Daghri, N., Brandi, M.-L., Bruyere, O., et al. (2020). Assessment of Cardiovascular Safety of Anti-osteoporosis Drugs. Drugs 80 (15), 1537–1552. doi:10.1007/s40265-020-01364-2
Gaur, T., Lengner, C. J., Hovhannisyan, H., Bhat, R. A., Bodine, P. V. N., Komm, B. S., et al. (2005). Canonical WNT Signaling Promotes Osteogenesis by Directly Stimulating Runx2 Gene Expression. J. Biol. Chem. 280 (39), 33132–33140. doi:10.1074/jbc.M500608200
Genant, H. K., Libanati, C., Engelke, K., Zanchetta, J. R., Høiseth, A., Yuen, C. K., et al. (2013). Improvements in Hip Trabecular, Subcortical, and Cortical Density and Mass in Postmenopausal Women with Osteoporosis Treated with Denosumab. Bone 56 (2), 482–488. doi:10.1016/j.bone.2013.07.011
Glantschnig, H., Scott, K., Hampton, R., Wei, N., McCracken, P., Nantermet, P., et al. (2011). A Rate-Limiting Role for Dickkopf-1 in Bone Formation and the Remediation of Bone Loss in Mouse and Primate Models of Postmenopausal Osteoporosis by an Experimental Therapeutic Antibody. J. Pharmacol. Exp. Ther. 338 (2), 568–578. doi:10.1124/jpet.111.181404
Gowen, M., Lazner, F., Dodds, R., Kapadia, R., Feild, J., Tavaria, M., et al. (1999). Cathepsin K Knockout Mice Develop Osteopetrosis Due to a Deficit in Matrix Degradation but Not Demineralization. J. Bone Miner Res. 14 (10), 1654–1663. doi:10.1359/jbmr.1999.14.10.1654
Gu, H., Shi, S., Xiao, F., Huang, Z., Xu, J., Chen, G., et al. (2020). MiR-1-3p Regulates the Differentiation of Mesenchymal Stem Cells to Prevent Osteoporosis by Targeting Secreted Frizzled-Related Protein 1. Bone 137, 115444. doi:10.1016/j.bone.2020.115444
Guan, M., Yao, W., Liu, R., Lam, K. S., Nolta, J., Jia, J., et al. (2012). Directing Mesenchymal Stem Cells to Bone to Augment Bone Formation and Increase Bone Mass. Nat. Med. 18 (3), 456–462. doi:10.1038/nm.2665
Guo, L., Chen, K., Yuan, J., Huang, P., Xu, X., Li, C., et al. (2018). Estrogen Inhibits Osteoclasts Formation and Bone Resorption via microRNA-27a Targeting PPARγ and APC. J. Cel Physiol 234 (1), 581–594. doi:10.1002/jcp.26788
Hall, A. P., Escott, K. J., Sanganee, H., and Hickling, K. C. (2015). Preclinical Toxicity of AZD7969. Toxicol. Pathol. 43 (3), 384–399. doi:10.1177/0192623314544468
Hartmann, K., Koenen, M., Schauer, S., Wittig-Blaich, S., Ahmad, M., Baschant, U., et al. (2016). Molecular Actions of Glucocorticoids in Cartilage and Bone during Health, Disease, and Steroid Therapy. Physiol. Rev. 96 (2), 409–447. doi:10.1152/physrev.00011.2015
Hassan, M. Q., Maeda, Y., Taipaleenmaki, H., Zhang, W., Jafferji, M., Gordon, J. A. R., et al. (2012). miR-218 Directs a Wnt Signaling Circuit to Promote Differentiation of Osteoblasts and Osteomimicry of Metastatic Cancer Cells. J. Biol. Chem. 287 (50), 42084–42092. doi:10.1074/jbc.M112.377515
Hofbauer, L. C., and Schoppet, M. (2004). Clinical Implications of the osteoprotegerin/RANKL/RANK System for Bone and Vascular Diseases. JAMA 292 (4), 490–495. doi:10.1001/jama.292.4.490
Huang, H.-J., Chen, S.-L., Chang, Y.-T., Chyuan, J.-H., and Hsieh-Li, H. (2018). Administration of Momordica Charantia Enhances the Neuroprotection and Reduces the Side Effects of LiCl in the Treatment of Alzheimer's Disease. Nutrients 10 (12), 1888. doi:10.3390/nu10121888
Huang, P., Yan, R., Zhang, X., Wang, L., Ke, X., and Qu, Y. (2019). Activating Wnt/β-Catenin Signaling Pathway for Disease Therapy: Challenges and Opportunities. Pharmacol. Ther. 196, 79–90. doi:10.1016/j.pharmthera.2018.11.008
Infante, M., Fabi, A., Cognetti, F., Gorini, S., Caprio, M., and Fabbri, A. (2019). RANKL/RANK/OPG System beyond Bone Remodeling: Involvement in Breast Cancer and Clinical Perspectives. J. Exp. Clin. Cancer Res. 38 (1), 12. doi:10.1186/s13046-018-1001-2
James, A. W., Chiang, M., Asatrian, G., Shen, J., Goyal, R., Chung, C. G., et al. (2016). Vertebral Implantation of NELL-1 Enhances Bone Formation in an Osteoporotic Sheep Model. Tissue Eng. A 22 (11-12), 840–849. doi:10.1089/ten.TEA.2015.0230
James, A. W., Shen, J., Tsuei, R., Nguyen, A., Khadarian, K., Meyers, C. A., et al. (2017). NELL-1 Induces Sca-1+ Mesenchymal Progenitor Cell Expansion in Models of Bone Maintenance and Repair. JCI Insight 2 (12). doi:10.1172/jci.insight.92573
James, A. W., Shen, J., Zhang, X., Asatrian, G., Goyal, R., Kwak, J. H., et al. (2015). NELL-1 in the Treatment of Osteoporotic Bone Loss. Nat. Commun. 6, 7362. doi:10.1038/ncomms8362
Jing, H., Su, X., Gao, B., Shuai, Y., Chen, J., Deng, Z., et al. (2018). Epigenetic Inhibition of Wnt Pathway Suppresses Osteogenic Differentiation of BMSCs during Osteoporosis. Cell Death Dis 9 (2), 176. doi:10.1038/s41419-017-0231-0
Jolette, J., Attalla, B., Varela, A., Long, G. G., Mellal, N., Trimm, S., et al. (2017). Comparing the Incidence of Bone Tumors in Rats Chronically Exposed to the Selective PTH Type 1 Receptor Agonist Abaloparatide or PTH(1-34). Regul. Toxicol. Pharmacol. 86, 356–365. doi:10.1016/j.yrtph.2017.04.001
Kapinas, K., Kessler, C., Ricks, T., Gronowicz, G., and Delany, A. M. (2010). miR-29 Modulates Wnt Signaling in Human Osteoblasts through a Positive Feedback Loop. J. Biol. Chem. 285 (33), 25221–25231. doi:10.1074/jbc.M110.116137
Karner, C. M., and Long, F. (2018). Glucose Metabolism in Bone. Bone 115, 2–7. doi:10.1016/j.bone.2017.08.008
Karner, C. M., and Long, F. (2017). Wnt Signaling and Cellular Metabolism in Osteoblasts. Cel. Mol. Life Sci. 74 (9), 1649–1657. doi:10.1007/s00018-016-2425-5
Kawano, Y., and Kypta, R. (2003). Secreted Antagonists of the Wnt Signalling Pathway. J. Cel Sci 116 (Pt 13), 2627–2634. doi:10.1242/jcs.00623
Ke, H. Z., Richards, W. G., Li, X., and Ominsky, M. S. (2012). Sclerostin and Dickkopf-1 as Therapeutic Targets in Bone Diseases. Endocr. Rev. 33 (5), 747–783. doi:10.1210/er.2011-1060
Kearns, A. E., Khosla, S., and Kostenuik, P. J. (2008). Receptor Activator of Nuclear Factor κB Ligand and Osteoprotegerin Regulation of Bone Remodeling in Health and Disease. Endocr. Rev. 29 (2), 155–192. doi:10.1210/er.2007-0014
Kendler, D. L., Bone, H. G., Massari, F., Gielen, E., Palacios, S., Maddox, J., et al. (2019). Bone mineral Density Gains with a Second 12-month Course of Romosozumab Therapy Following Placebo or Denosumab. Osteoporos. Int. 30 (12), 2437–2448. doi:10.1007/s00198-019-05146-9
Kendler, D. L., Marin, F., Zerbini, C. A. F., Russo, L. A., Greenspan, S. L., Zikan, V., et al. (2018). Effects of Teriparatide and Risedronate on New Fractures in post-menopausal Women with Severe Osteoporosis (VERO): a Multicentre, Double-Blind, Double-Dummy, Randomised Controlled Trial. The Lancet 391 (10117), 230–240. doi:10.1016/s0140-6736(17)32137-2
Khosla, S., Bilezikian, J. P., Dempster, D. W., Lewiecki, E. M., Miller, P. D., Neer, R. M., et al. (2012). Benefits and Risks of Bisphosphonate Therapy for Osteoporosis. J. Clin. Endocrinol. Metab. 97 (7), 2272–2282. doi:10.1210/jc.2012-1027
Kiper, P. O. S., Saito, H., Gori, F., Unger, S., Hesse, E., Yamana, K., et al. (2016). Cortical-Bone Fragility--Insights from sFRP4 Deficiency in Pyle's Disease. N. Engl. J. Med. 374 (26), 2553–2562. doi:10.1056/NEJMoa1509342
Kohn, A. D., and Moon, R. T. (2005). Wnt and Calcium Signaling: β-Catenin-independent Pathways. Cell Calcium 38 (3-4), 439–446. doi:10.1016/j.ceca.2005.06.022
Kornak, U., Kasper, D., Bösl, M. R., Kaiser, E., Schweizer, M., Schulz, A., et al. (2001). Loss of the ClC-7 Chloride Channel Leads to Osteopetrosis in Mice and Man. Cell 104 (2), 205–215. doi:10.1016/s0092-8674(01)00206-9
Krishna, S. M., Seto, S.-W., Jose, R. J., Li, J., Morton, S. K., Biros, E., et al. (2017). Wnt Signaling Pathway Inhibitor Sclerostin Inhibits Angiotensin II-Induced Aortic Aneurysm and Atherosclerosis. Arterioscler Thromb. Vasc. Biol. 37 (3), 553–566. doi:10.1161/ATVBAHA.116.308723
Krishnan, V., Bryant, H. U., and Macdougald, O. A. (2006). Regulation of Bone Mass by Wnt Signaling. J. Clin. Invest. 116 (5), 1202–1209. doi:10.1172/JCI28551
Lacey, D. L., Boyle, W. J., Simonet, W. S., Kostenuik, P. J., Dougall, W. C., Sullivan, J. K., et al. (2012). Bench to Bedside: Elucidation of the OPG-RANK-RANKL Pathway and the Development of Denosumab. Nat. Rev. Drug Discov. 11 (5), 401–419. doi:10.1038/nrd3705
Langdahl, B., Binkley, N., Bone, H., Gilchrist, N., Resch, H., Rodriguez Portales, J., et al. (2012). Odanacatib in the Treatment of Postmenopausal Women with Low Bone mineral Density: Five Years of Continued Therapy in a Phase 2 Study. J. Bone Miner Res. 27 (11), 2251–2258. doi:10.1002/jbmr.1695
Langdahl, B. L., Libanati, C., Crittenden, D. B., Bolognese, M. A., Brown, J. P., Daizadeh, N. S., et al. (2017). Romosozumab (Sclerostin Monoclonal Antibody) versus Teriparatide in Postmenopausal Women with Osteoporosis Transitioning from Oral Bisphosphonate Therapy: a Randomised, Open-Label, Phase 3 Trial. The Lancet 390 (10102), 1585–1594. doi:10.1016/s0140-6736(17)31613-6
Langdahl, B. L. (2020). Overview of Treatment Approaches to Osteoporosis. Br. J. Pharmacol. 178, 1891–1906. doi:10.1111/bph.15024
Leder, B. Z., Mitlak, B., Hu, M.-y., Hattersley, G., and Bockman, R. S. (2020). Effect of Abaloparatide vs Alendronate on Fracture Risk Reduction in Postmenopausal Women with Osteoporosis. J. Clin. Endocrinol. Metab. 105 (3), 938–943. doi:10.1210/clinem/dgz162
Leder, B. Z., O'Dea, L. S. L., Zanchetta, J. R., Kumar, P., Banks, K., McKay, K., et al. (2015a). Effects of Abaloparatide, a Human Parathyroid Hormone-Related Peptide Analog, on Bone mineral Density in Postmenopausal Women with Osteoporosis. J. Clin. Endocrinol. Metab. 100 (2), 697–706. doi:10.1210/jc.2014-3718
Leder, B. Z., Tsai, J. N., Uihlein, A. V., Wallace, P. M., Lee, H., Neer, R. M., et al. (2015b). Denosumab and Teriparatide Transitions in Postmenopausal Osteoporosis (The DATA-Switch Study): Extension of a Randomised Controlled Trial. The Lancet 386 (9999), 1147–1155. doi:10.1016/s0140-6736(15)61120-5
Lee, K., Jessop, H., Suswillo, R., Zaman, G., and Lanyon, L. (2003). Bone Adaptation Requires Oestrogen Receptor-α. Nature 424 (6947), 389. doi:10.1038/424389a
Lerner, U. H., and Ohlsson, C. (2015). The WNT System: Background and its Role in Bone. J. Intern. Med. 277 (6), 630–649. doi:10.1111/joim.12368
Lewiecki, E. M., Blicharski, T., Goemaere, S., Lippuner, K., Meisner, P. D., Miller, P. D., et al. (2018). A Phase III Randomized Placebo-Controlled Trial to Evaluate Efficacy and Safety of Romosozumab in Men with Osteoporosis. J. Clin. Endocrinol. Metab. 103 (9), 3183–3193. doi:10.1210/jc.2017-02163
Lewiecki, E. M., Dinavahi, R. V., Lazaretti‐Castro, M., Ebeling, P. R., Adachi, J. D., Miyauchi, A., et al. (2019). One Year of Romosozumab Followed by Two Years of Denosumab Maintains Fracture Risk Reductions: Results of the FRAME Extension Study. J. Bone Miner Res. 34 (3), 419–428. doi:10.1002/jbmr.3622
Lewiecki, E. M. (2011). New Targets for Intervention in the Treatment of Postmenopausal Osteoporosis. Nat. Rev. Rheumatol. 7 (11), 631–638. doi:10.1038/nrrheum.2011.130
Li, K.-C., Chang, Y.-H., Yeh, C.-L., and Hu, Y.-C. (2016). Healing of Osteoporotic Bone Defects by Baculovirus-Engineered Bone Marrow-Derived MSCs Expressing MicroRNA Sponges. Biomaterials 74, 155–166. doi:10.1016/j.biomaterials.2015.09.046
Li, X., Grisanti, M., Fan, W., Asuncion, F. J., Tan, H.-L., Dwyer, D., et al. (2011). Dickkopf-1 Regulates Bone Formation in Young Growing Rodents and upon Traumatic Injury. J. Bone Miner Res. 26 (11), 2610–2621. doi:10.1002/jbmr.472
Li, X., Zhang, Y., Kang, H., Liu, W., Liu, P., Zhang, J., et al. (2005). Sclerostin Binds to LRP5/6 and Antagonizes Canonical Wnt Signaling. J. Biol. Chem. 280 (20), 19883–19887. doi:10.1074/jbc.M413274200
Lin, T.-H., Yang, R.-S., Tu, H.-J., Liou, H.-C., Lin, Y.-M., Chuang, W.-J., et al. (2017). Inhibition of Osteoporosis by the αvβ3 Integrin Antagonist of Rhodostomin Variants. Eur. J. Pharmacol. 804, 94–101. doi:10.1016/j.ejphar.2017.03.019
Lindsay, R., Gallagher, J. C., Kagan, R., Pickar, J. H., and Constantine, G. (2009). Efficacy of Tissue-Selective Estrogen Complex of Bazedoxifene/conjugated Estrogens for Osteoporosis Prevention in At-Risk Postmenopausal Women. Fertil. Sterility 92 (3), 1045–1052. doi:10.1016/j.fertnstert.2009.02.093
Lindström, E., Rizoska, B., Henderson, I., Terelius, Y., Jerling, M., Edenius, C., et al. (2018). Nonclinical and Clinical Pharmacological Characterization of the Potent and Selective Cathepsin K Inhibitor MIV-711. J. Transl Med. 16 (1). doi:10.1186/s12967-018-1497-4
Lv, F., Cai, X., Yang, W., Gao, L., Chen, L., Wu, J., et al. (2020). Denosumab or Romosozumab Therapy and Risk of Cardiovascular Events in Patients with Primary Osteoporosis: Systematic Review and Meta- Analysis. Bone 130, 115121. doi:10.1016/j.bone.2019.115121
Ma, S., Wang, D. D., Ma, C. Y., and Zhang, Y. D. (2019). microRNA‐96 Promotes Osteoblast Differentiation and Bone Formation in Ankylosing Spondylitis Mice through Activating the Wnt Signaling Pathway by Binding to SOST. J. Cel Biochem 120 (9), 15429–15442. doi:10.1002/jcb.28810
MacDonald, B. T., Tamai, K., and He, X. (2009). Wnt/β-Catenin Signaling: Components, Mechanisms, and Diseases. Develop. Cel 17 (1), 9–26. doi:10.1016/j.devcel.2009.06.016
Manandhar, S., Kabekkodu, S. P., and Pai, K. S. R. (2020). Aberrant Canonical Wnt Signaling: Phytochemical Based Modulation. Phytomedicine 76, 153243. doi:10.1016/j.phymed.2020.153243
Mao, B., Wu, W., Davidson, G., Marhold, J., Li, M., Mechler, B. M., et al. (2002). Kremen Proteins Are Dickkopf Receptors that Regulate Wnt/β-Catenin Signalling. Nature 417 (6889), 664–667. doi:10.1038/nature756
Martin, T. J. (2016). Parathyroid Hormone-Related Protein, its Regulation of Cartilage and Bone Development, and Role in Treating Bone Diseases. Physiol. Rev. 96 (3), 831–871. doi:10.1152/physrev.00031.2015
McClung, M., Harris, S. T., Miller, P. D., Bauer, D. C., Davison, K. S., Dian, L., et al. (2013). Bisphosphonate Therapy for Osteoporosis: Benefits, Risks, and Drug holiday. Am. J. Med. 126 (1), 13–20. doi:10.1016/j.amjmed.2012.06.023
McClung, M. R., O'Donoghue, M. L., Papapoulos, S. E., Bone, H., Langdahl, B., Saag, K. G., et al. (2019). Odanacatib for the Treatment of Postmenopausal Osteoporosis: Results of the LOFT Multicentre, Randomised, Double-Blind, Placebo-Controlled Trial and LOFT Extension Study. Lancet Diabetes Endocrinol. 7 (12), 899–911. doi:10.1016/S2213-8587(19)30346-8
McClung, M. R., Bolognese, M. A., Brown, J. P., Reginster, J.-Y., Langdahl, B. L., Maddox, J., et al. (2020). A Single Dose of Zoledronate Preserves Bone mineral Density for up to 2 Years after a Second Course of Romosozumab. Osteoporos. Int. 31 (11), 2231–2241. doi:10.1007/s00198-020-05502-0
McClung, M. R., Brown, J. P., Diez-Perez, A., Resch, H., Caminis, J., Meisner, P., et al. (2018). Effects of 24 Months of Treatment with Romosozumab Followed by 12 Months of Denosumab or Placebo in Postmenopausal Women with Low Bone Mineral Density: A Randomized, Double-Blind, Phase 2, Parallel Group Study. J. Bone Miner Res. 33 (8), 1397–1406. doi:10.1002/jbmr.3452
McClung, M. R., Grauer, A., Boonen, S., Bolognese, M. A., Brown, J. P., Diez-Perez, A., et al. (2014). Romosozumab in Postmenopausal Women with Low Bone mineral Density. N. Engl. J. Med. 370 (5), 412–420. doi:10.1056/NEJMoa1305224
McColm, J., Hu, L., Womack, T., Tang, C. C., and Chiang, A. Y. (2014). Single- and Multiple-Dose Randomized Studies of Blosozumab, a Monoclonal Antibody against Sclerostin, in Healthy Postmenopausal Women. J. Bone Miner Res. 29 (4), 935–943. doi:10.1002/jbmr.2092
McDonald, M. M., Reagan, M. R., Youlten, S. E., Mohanty, S. T., Seckinger, A., Terry, R. L., et al. (2017). Inhibiting the Osteocyte-specific Protein Sclerostin Increases Bone Mass and Fracture Resistance in Multiple Myeloma. Blood 129 (26), 3452–3464. doi:10.1182/blood-2017-03-773341
Miller, P. D., Hattersley, G., Riis, B. J., Williams, G. C., Lau, E., Russo, L. A., et al. (2016). Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women with Osteoporosis. JAMA 316 (7), 722–733. doi:10.1001/jama.2016.11136
Minisola, S., Pepe, J., and Cipriani, C. (2021). Rheumatoid Arthritis, Bone and Drugs: a Dangerous Interweave. Ann. Rheum. Dis. 80, 409–410. doi:10.1136/annrheumdis-2020-219545
Mödder, U. I., Clowes, J. A., Hoey, K., Peterson, J. M., McCready, L., Oursler, M. J., et al. (2011). Regulation of Circulating Sclerostin Levels by Sex Steroids in Women and in Men. J. Bone Miner Res. 26 (1), 27–34. doi:10.1002/jbmr.128
Mora-Raimundo, P., Lozano, D., Benito, M., Mulero, F., Manzano, M., and Vallet-Regi, M. (2021). Osteoporosis Remission and New Bone Formation with Mesoporous Silica Nanoparticles. Adv. Sci. (Weinh), e2101107. doi:10.1002/advs.202101107
Morse, A., Schindeler, A., McDonald, M. M., Kneissel, M., Kramer, I., and Little, D. G. (2018). Sclerostin Antibody Augments the Anabolic Bone Formation Response in a Mouse Model of Mechanical Tibial Loading. J. Bone Miner Res. 33 (3), 486–498. doi:10.1002/jbmr.3330
Mullard, A. (2016). Merck & Co. Drops Osteoporosis Drug Odanacatib. Nat. Rev. Drug Discov. 15 (10), 669. doi:10.1038/nrd.2016.207
Murphy, M. G., Cerchio, K., Stoch, S. A., Gottesdiener, K., Wu, M., Recker, R., et al. (2005). Effect of L-000845704, an αVβ3 Integrin Antagonist, on Markers of Bone Turnover and Bone Mineral Density in Postmenopausal Osteoporotic Women. J. Clin. Endocrinol. Metab. 90 (4), 2022–2028. doi:10.1210/jc.2004-2126
Nakamura, M., Udagawa, N., Matsuura, S., Mogi, M., Nakamura, H., Horiuchi, H., et al. (2003). Osteoprotegerin Regulates Bone Formation through a Coupling Mechanism with Bone Resorption. Endocrinology 144 (12), 5441–5449. doi:10.1210/en.2003-0717
Nakanishi, R., Akiyama, H., Kimura, H., Otsuki, B., Shimizu, M., Tsuboyama, T., et al. (2008). Osteoblast-targeted Expression of Sfrp4 in Mice Results in Low Bone Mass. J. Bone Miner Res. 23 (2), 271–277. doi:10.1359/jbmr.071007
Neer, R. M., Arnaud, C. D., Zanchetta, J. R., Prince, R., Gaich, G. A., Reginster, J.-Y., et al. (2001). Effect of Parathyroid Hormone (1-34) on Fractures and Bone mineral Density in Postmenopausal Women with Osteoporosis. N. Engl. J. Med. 344 (19), 1434–1441. doi:10.1056/NEJM200105103441904
Ng, L., Kaur, P., Bunnag, N., Suresh, J., Sung, I., Tan, Q., et al. (2019). WNT Signaling in Disease. Cells 8 (8), 826. doi:10.3390/cells8080826
Nusse, R., and Clevers, H. (2017). Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 169 (6), 985–999. doi:10.1016/j.cell.2017.05.016
Pakvasa, M., Alverdy, A., Mostafa, S., Wang, E., Fu, L., Li, A., et al. (2017). Neural EGF-like Protein 1 (NELL-1): Signaling Crosstalk in Mesenchymal Stem Cells and Applications in Regenerative Medicine. Genes Dis. 4 (3), 127–137. doi:10.1016/j.gendis.2017.07.006
Pan, B.-L., Tong, Z.-W., Li, S.-D., Wu, L., Liao, J.-L., Yang, Y.-X., et al. (2018). Decreased microRNA-182-5p Helps Alendronate Promote Osteoblast Proliferation and Differentiation in Osteoporosis via the Rap1/MAPK Pathway. Biosci. Rep. 38 (6), BSR20180696. doi:10.1042/BSR20180696
Pinzone, J. J., Hall, B. M., Thudi, N. K., Vonau, M., Qiang, Y.-W., Rosol, T. J., et al. (2009). The Role of Dickkopf-1 in Bone Development, Homeostasis, and Disease. Blood 113 (3), 517–525. doi:10.1182/blood-2008-03-145169
Rammal, H., Entz, L., Dubus, M., Moniot, A., Bercu, N. B., Sergheraert, J., et al. (2019). Osteoinductive Material to Fine-Tune Paracrine Crosstalk of Mesenchymal Stem Cells with Endothelial Cells and Osteoblasts. Front. Bioeng. Biotechnol. 7, 256. doi:10.3389/fbioe.2019.00256
Recker, R. R., Benson, C. T., Matsumoto, T., Bolognese, M. A., Robins, D. A., Alam, J., et al. (2015). A Randomized, Double‐Blind Phase 2 Clinical Trial of Blosozumab, a Sclerostin Antibody, in Postmenopausal Women with Low Bone Mineral Density. J. Bone Miner Res. 30 (2), 216–224. doi:10.1002/jbmr.2351
Reginster, J.-Y., Bianic, F., Campbell, R., Martin, M., Williams, S. A., and Fitzpatrick, L. A. (2019). Abaloparatide for Risk Reduction of Nonvertebral and Vertebral Fractures in Postmenopausal Women with Osteoporosis: a Network Meta-Analysis. Osteoporos. Int. 30 (7), 1465–1473. doi:10.1007/s00198-019-04947-2
Reginster, J.-Y., Hattersley, G., Williams, G. C., Hu, M.-y., Fitzpatrick, L. A., and Lewiecki, E. M. (2018). Abaloparatide Is an Effective Treatment Option for Postmenopausal Osteoporosis: Review of the Number Needed to Treat Compared with Teriparatide. Calcif Tissue Int. 103 (5), 540–545. doi:10.1007/s00223-018-0450-0
Reid, I. R. (2015). Short-term and Long-Term Effects of Osteoporosis Therapies. Nat. Rev. Endocrinol. 11 (7), 418–428. doi:10.1038/nrendo.2015.71
Reppe, S., Noer, A., Grimholt, R. M., Halldórsson, B. V., Medina-Gomez, C., Gautvik, V. T., et al. (2015). Methylation of BoneSOST, its mRNA, and Serum Sclerostin Levels Correlate Strongly with Fracture Risk in Postmenopausal Women. J. Bone Miner Res. 30 (2), 249–256. doi:10.1002/jbmr.2342
Rizzoli, R., Benhamou, C.-L., Halse, J., Miller, P. D., Reid, I. R., Rodríguez Portales, J. A., et al. (2016). Continuous Treatment with Odanacatib for up to 8 Years in Postmenopausal Women with Low Bone mineral Density: a Phase 2 Study. Osteoporos. Int. 27 (6), 2099–2107. doi:10.1007/s00198-016-3503-0
Rizzoli, R., Yasothan, U., and Kirkpatrick, P. (2010). Denosumab. Nat. Rev. Drug Discov. 9 (8), 591–592. doi:10.1038/nrd3244
Robling, A. G., Niziolek, P. J., Baldridge, L. A., Condon, K. W., Allen, M. R., Alam, I., et al. (2008). Mechanical Stimulation of Bone In Vivo Reduces Osteocyte Expression of Sost/sclerostin. J. Biol. Chem. 283 (9), 5866–5875. doi:10.1074/jbc.M705092200
Rossouw, J. E., Anderson, G. L., Prentice, R. L., LaCroix, A. Z., Kooperberg, C., Stefanick, M. L., et al. (2002). Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal Women: Principal Results from the Women's Health Initiative Randomized Controlled Trial. JAMA 288 (3), 321–333. doi:10.1001/jama.288.3.321
Russell, R. G. G., Xia, Z., Dunford, J. E., Oppermann, U., Kwaasi, A., Hulley, P. A., et al. (2007). Bisphosphonates: an Update on Mechanisms of Action and How These Relate to Clinical Efficacy. Ann. N. Y Acad. Sci. 1117, 209–257. doi:10.1196/annals.1402.089
Rybchyn, M. S., Slater, M., Conigrave, A. D., and Mason, R. S. (2011). An Akt-dependent Increase in Canonical Wnt Signaling and a Decrease in Sclerostin Protein Levels Are Involved in Strontium Ranelate-Induced Osteogenic Effects in Human Osteoblasts. J. Biol. Chem. 286 (27), 23771–23779. doi:10.1074/jbc.M111.251116
Saag, K. G., Petersen, J., Brandi, M. L., Karaplis, A. C., Lorentzon, M., Thomas, T., et al. (2017). Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N. Engl. J. Med. 377 (15), 1417–1427. doi:10.1056/NEJMoa1708322
Sambrook, P. N., and Geusens, P. (2012). The Epidemiology of Osteoporosis and Fractures in Ankylosing Spondylitis. Ther. Adv. Musculoskelet. 4 (4), 287–292. doi:10.1177/1759720X12441276
Sarbassov, D. D., Guertin, D. A., Ali, S. M., and Sabatini, D. M. (2005). Phosphorylation and Regulation of Akt/PKB by the Rictor-mTOR Complex. Science 307 (5712), 1098–1101. doi:10.1126/science.1106148
Schafer, A. L., Sellmeyer, D. E., Palermo, L., Hietpas, J., Eastell, R., Shoback, D. M., et al. (2012). Six Months of Parathyroid Hormone (1-84) Administered ConcurrentlyVersusSequentially with Monthly Ibandronate over Two Years: The PTH and Ibandronate Combination Study (PICS) Randomized Trial. J. Clin. Endocrinol. Metab. 97 (10), 3522–3529. doi:10.1210/jc.2012-1844
Schaller, S., Henriksen, K., Sveigaard, C., Heegaard, A.-M., Hélix, N., Stahlhut, M., et al. (2004). The Chloride Channel Inhibitor NS3736 Prevents Bone Resorption in Ovariectomized Rats without Changing Bone Formation. J. Bone Miner Res. 19 (7), 1144–1153. doi:10.1359/JBMR.040302
Schaniel, C., Sirabella, D., Qiu, J., Niu, X., Lemischka, I. R., and Moore, K. A. (2011). Wnt-inhibitory Factor 1 Dysregulation of the Bone Marrow Niche Exhausts Hematopoietic Stem Cells. Blood 118 (9), 2420–2429. doi:10.1182/blood-2010-09-305664
Schemitsch, E. H., Miclau, T., Karachalios, T., Nowak, L. L., Sancheti, P., Poolman, R. W., et al. (2020). A Randomized, Placebo-Controlled Study of Romosozumab for the Treatment of Hip Fractures. J. Bone Jt. Surg Am 102 (8), 693–702. doi:10.2106/JBJS.19.00790
Shen, J., James, A. W., Zhang, X., Pang, S., Zara, J. N., Asatrian, G., et al. (2016). Novel Wnt Regulator NEL-like Molecule-1 Antagonizes Adipogenesis and Augments Osteogenesis Induced by Bone Morphogenetic Protein 2. Am. J. Pathol. 186 (2), 419–434. doi:10.1016/j.ajpath.2015.10.011
Silverman, S. L., Christiansen, C., Genant, H. K., Vukicevic, S., Zanchetta, J. R., de Villiers, T. J., et al. (2008). Efficacy of Bazedoxifene in Reducing New Vertebral Fracture Risk in Postmenopausal Women with Osteoporosis: Results from a 3-Year, Randomized, Placebo-, and Active-Controlled Clinical Trial*. J. Bone Mineral Res. 23 (12), 1923–1934. doi:10.1359/jbmr.080710
Statham, L. A., and Aspray, T. J. (2019). Odanacatib: the Best Osteoporosis Treatment We Never Had? Lancet Diabetes Endocrinol. 7 (12), 888–889. doi:10.1016/s2213-8587(19)30348-1
Swarthout, J. T., D'Alonzo, R. C., Selvamurugan, N., and Partridge, N. C. (2002). Parathyroid Hormone-dependent Signaling Pathways Regulating Genes in Bone Cells. Gene 282 (1-2), 1–17. doi:10.1016/s0378-1119(01)00798-3
Tang, C.-H., Yang, R.-S., Chien, M.-Y., Chen, C.-C., and Fu, W.-M. (2008). Enhancement of Bone Morphogenetic Protein-2 Expression and Bone Formation by Coumarin Derivatives via P38 and ERK-dependent Pathway in Osteoblasts. Eur. J. Pharmacol. 579 (1-3), 40–49. doi:10.1016/j.ejphar.2007.10.013
Tatangelo, G., Watts, J., Lim, K., Connaughton, C., Abimanyi-Ochom, J., Borgström, F., et al. (2019). The Cost of Osteoporosis, Osteopenia, and Associated Fractures in Australia in 2017. J. Bone Miner Res. 34 (4), 616–625. doi:10.1002/jbmr.3640
Tolwinski, N. S., and Wieschaus, E. (2004). A Nuclear Escort for β-catenin. Nat. Cel Biol 6 (7), 579–580. doi:10.1038/ncb0704-579
Tsai, J. N., Lee, H., David, N. L., Eastell, R., and Leder, B. Z. (2019). Combination Denosumab and High Dose Teriparatide for Postmenopausal Osteoporosis (DATA-HD): a Randomised, Controlled Phase 4 Trial. Lancet Diabetes Endocrinol. 7 (10), 767–775. doi:10.1016/s2213-8587(19)30255-4
Tsai, J. N., Uihlein, A. V., Lee, H., Kumbhani, R., Siwila-Sackman, E., McKay, E. A., et al. (2013). Teriparatide and Denosumab, Alone or Combined, in Women with Postmenopausal Osteoporosis: the DATA Study Randomised Trial. The Lancet 382 (9886), 50–56. doi:10.1016/s0140-6736(13)60856-9
Ulici, V., Hoenselaar, K. D., Agoston, H., McErlain, D. D., Umoh, J., Chakrabarti, S., et al. (2009). The Role of Akt1 in Terminal Stages of Endochondral Bone Formation: Angiogenesis and Ossification. Bone 45 (6), 1133–1145. doi:10.1016/j.bone.2009.08.003
Vaes, B., Dechering, K., Vansomeren, E., Hendriks, J., Vandeven, C., Feijen, A., et al. (2005). Microarray Analysis Reveals Expression Regulation of Wnt Antagonists in Differentiating Osteoblasts. Bone 36 (5), 803–811. doi:10.1016/j.bone.2005.02.001
Vahle, J. L., Long, G. G., Sandusky, G., Westmore, M., Ma, Y. L., and Sato, M. (2004). Bone Neoplasms in F344 Rats Given Teriparatide [rhPTH(1-34)] Are Dependent on Duration of Treatment and Dose. Toxicol. Pathol. 32 (4), 426–438. doi:10.1080/01926230490462138
Van den Wyngaert, T., Huizing, M. T., and Vermorken, J. B. (2006). Bisphosphonates and Osteonecrosis of the Jaw: Cause and Effect or a Post Hoc Fallacy? Ann. Oncol. 17 (8), 1197–1204. doi:10.1093/annonc/mdl294
Veeman, M. T., Axelrod, J. D., and Moon, R. T. (2003). A Second Canon. Develop. Cel 5 (3), 367–377. doi:10.1016/s1534-5807(03)00266-1
Vestergaard, P., Rejnmark, L., and Mosekilde, L. (2005). Reduced Relative Risk of Fractures Among Users of Lithium. Calcif Tissue Int. 77 (1), 1–8. doi:10.1007/s00223-004-0258-y
Wan, M., Yang, C., Li, J., Wu, X., Yuan, H., Ma, H., et al. (2008). Parathyroid Hormone Signaling through Low-Density Lipoprotein-Related Protein 6. Genes Develop. 22 (21), 2968–2979. doi:10.1101/gad.1702708
Wein, M. N., Liang, Y., Goransson, O., Sundberg, T. B., Wang, J., Williams, E. A., et al. (2016). SIKs Control Osteocyte Responses to Parathyroid Hormone. Nat. Commun. 7, 13176. doi:10.1038/ncomms13176
Wein, M. N., Spatz, J., Nishimori, S., Doench, J., Root, D., Babij, P., et al. (2015). HDAC5 Controls MEF2C-Driven Sclerostin Expression in Osteocytes. J. Bone Miner Res. 30 (3), 400–411. doi:10.1002/jbmr.2381
Weitzmann, M. N., and Pacifici, R. (2006). Estrogen Deficiency and Bone Loss: an Inflammatory Tale. J. Clin. Invest. 116 (5), 1186–1194. doi:10.1172/JCI28550
Wu, M., Chen, G., and Li, Y.-P. (2016). TGF-β and BMP Signaling in Osteoblast, Skeletal Development, and Bone Formation, Homeostasis and Disease. Bone Res. 4, 16009. doi:10.1038/boneres.2016.9
Yang, D., Singh, R., Divieti, P., Guo, J., Bouxsein, M. L., and Bringhurst, F. R. (2007). Contributions of Parathyroid Hormone (PTH)/PTH-related Peptide Receptor Signaling Pathways to the Anabolic Effect of PTH on Bone. Bone 40 (6), 1453–1461. doi:10.1016/j.bone.2007.02.001
Zebaze, R., Libanati, C., McClung, M. R., Zanchetta, J. R., Kendler, D. L., Høiseth, A., et al. (2016). Denosumab Reduces Cortical Porosity of the Proximal Femoral Shaft in Postmenopausal Women with Osteoporosis. J. Bone Miner Res. 31 (10), 1827–1834. doi:10.1002/jbmr.2855
Zhang, W.-B., Zhong, W.-J., and Wang, L. (2014). A Signal-Amplification Circuit between miR-218 and Wnt/β-Catenin Signal Promotes Human Adipose Tissue-Derived Stem Cells Osteogenic Differentiation. Bone 58, 59–66. doi:10.1016/j.bone.2013.09.015
Zhang, X., Ting, K., Bessette, C. M., Culiat, C. T., Sung, S. J., Lee, H., et al. (2011). Nell-1, a Key Functional Mediator of Runx2, Partially Rescues Calvarial Defects in Runx2+/− Mice. J. Bone Miner Res. 26 (4), 777–791. doi:10.1002/jbmr.267
Zhang, X., Zhu, Y., Zhang, C., Liu, J., Sun, T., Li, D., et al. (2018). miR‐542‐3p Prevents Ovariectomy‐induced Osteoporosis in Rats via Targeting SFRP1. J. Cel Physiol 233 (9), 6798–6806. doi:10.1002/jcp.26430
Zhao, F., Xu, Y., Ouyang, Y., Wen, Z., Zheng, G., Wan, T., et al. (2021). Silencing of miR-483-5p Alleviates Postmenopausal Osteoporosis by Targeting SATB2 and PI3K/AKT Pathway. Aging 13 (5), 6945–6956. doi:10.18632/aging.202552
Zhao, J.-X., Yue, W.-F., Zhu, M.-J., and Du, M. (2011). AMP-activated Protein Kinase Regulates β-Catenin Transcription via Histone Deacetylase 5. J. Biol. Chem. 286 (18), 16426–16434. doi:10.1074/jbc.M110.199372
Zhao, J., Yue, W., Zhu, M. J., Sreejayan, N., and Du, M. (2010). AMP-activated Protein Kinase (AMPK) Cross-Talks with Canonical Wnt Signaling via Phosphorylation of β-catenin at Ser 552. Biochem. Biophys. Res. Commun. 395 (1), 146–151. doi:10.1016/j.bbrc.2010.03.161
Zhao, R., He, X.-W., Shi, Y.-H., Liu, Y.-S., Liu, F.-D., Hu, Y., et al. (2019). Cathepsin K Knockout Exacerbates Haemorrhagic Transformation Induced by Recombinant Tissue Plasminogen Activator after Focal Cerebral Ischaemia in Mice. Cell Mol Neurobiol 39 (6), 823–831. doi:10.1007/s10571-019-00682-8
Zur, Y., Rosenfeld, L., Keshelman, C. A., Dalal, N., Guterman-Ram, G., Orenbuch, A., et al. (2018). A Dual-specific Macrophage colony-stimulating Factor Antagonist of C-FMS and αvβ3 Integrin for Osteoporosis Therapy. Plos Biol. 16 (8), e2002979. doi:10.1371/journal.pbio.2002979
Glossary
AMPK Adenosine Monophosphate Activated Protein kinase
APC adenomatous polyposis coli
AS ankylosing spondyloarthritis
BMD bone mineral density
BMP Bone morphogenetic protein
BMSCs bone marrow stem cells
BPPs bisphosphonates
CIC-7 Chloride channel-7
CK1 casein kinase 1
Col-I collagen type I
DKK1 Dickkopf 1
EZH2 enhancer of zeste homolog 2
FZD Frizzled;
GCN5 lysine acetyltransferase 2A
GSK3β phosphorylating enzyme glycogen synthase kinase 3β
HDAC5 histone deacetylases 5
JAK Janus kinase
JNK c-Jun N-terminal kinase
LiCl lithium chloride
LRP low density lipoprotein receptor related protein
M-CSF macrophage colony-stimulating factor
MSCs mesenchymal stem cells
NELL-1 NEL-Like molecule-1;
NFAT nuclear factor of activated T cells
OBs osteoblasts
OCs osteoclasts
OP osteoporosis
OPG osteoprotegerin
OCTs osteocytes
OVX ovariectomized
PI3K-AKT phosphatidylinositol-3-kinase-protein kinase B
PKC protein kinase C
RANKL receptor activator of nuclear factor κB (NF-κB) ligand
Runx2 runt-related transcription factor 2
SERMs selective estrogen receptor modulators
sFRPs secreted frizzled-related proteins
TCF/LEF T-cell specific transcription factor/lymphoid enhancing factor
TRAFs TNF receptor-associated factors
WIF-1 Wnt inhibitory factor 1
Keywords: osteoporosis, antiresorptive drugs, anabolic drugs, wnt signaling pathway, bone formation
Citation: Li S-S, He S-H, Xie P-Y, Li W, Zhang X-X, Li T-F and Li D-F (2021) Recent Progresses in the Treatment of Osteoporosis. Front. Pharmacol. 12:717065. doi: 10.3389/fphar.2021.717065
Received: 30 May 2021; Accepted: 12 July 2021;
Published: 22 July 2021.
Edited by:
Liangliang Xu, Guangzhou University of Chinese Medicine, ChinaReviewed by:
Bin Wang, Guangzhou Regenerative Medicine and Health Guangdong Laboratory, ChinaDaohua Xu, Guangdong Medical University, China
Xianzuo Zhang, Anhui Provincial Hospital, China
Copyright © 2021 Li, He, Xie, Li, Zhang, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tian-Fang Li, tfli@zzu.edu.cn; Dai-Feng Li, lidaifeng_ldf@163.com
 Shan-Shan Li
Shan-Shan Li Shi-Hao He1
Shi-Hao He1 Xin-Xin Zhang
Xin-Xin Zhang Tian-Fang Li
Tian-Fang Li Dai-Feng Li
Dai-Feng Li