Corrigendum: Cerebrovascular reactivity measurement using magnetic resonance imaging: A systematic review
- 1Centre for Clinical Brain Sciences, University of Edinburgh, Edinburgh, United Kingdom
- 2UK Dementia Research Institute, Edinburgh, United Kingdom
Cerebrovascular reactivity (CVR) magnetic resonance imaging (MRI) probes cerebral haemodynamic changes in response to a vasodilatory stimulus. CVR closely relates to the health of the vasculature and is therefore a key parameter for studying cerebrovascular diseases such as stroke, small vessel disease and dementias. MRI allows in vivo measurement of CVR but several different methods have been presented in the literature, differing in pulse sequence, hardware requirements, stimulus and image processing technique. We systematically reviewed publications measuring CVR using MRI up to June 2020, identifying 235 relevant papers. We summarised the acquisition methods, experimental parameters, hardware and CVR quantification approaches used, clinical populations investigated, and corresponding summary CVR measures. CVR was investigated in many pathologies such as steno-occlusive diseases, dementia and small vessel disease and is generally lower in patients than in healthy controls. Blood oxygen level dependent (BOLD) acquisitions with fixed inspired CO2 gas or end-tidal CO2 forcing stimulus are the most commonly used methods. General linear modelling of the MRI signal with end-tidal CO2 as the regressor is the most frequently used method to compute CVR. Our survey of CVR measurement approaches and applications will help researchers to identify good practice and provide objective information to inform the development of future consensus recommendations.
Introduction
Cerebrovascular reactivity (CVR) reflects the ability of the blood vessels to dilate in order to match tissue blood supply to increased demand and can be investigated by measuring the change in cerebral blood flow (CBF) or cerebral blood volume (CBV) that vasodilation induces. It is a valuable tool for assessing vascular health in pathologies, including steno-occlusive diseases (Mandell et al., 2008b), while more subtle CVR impairments have been found in Alzheimer's disease (Chen, 2018) and cerebral small vessel disease (Wardlaw et al., 2019). The measurement of CVR relies on three key elements: the vasodilatory stimulus, the signal acquisition and the processing method.
Vasodilatory Stimulus
Vasodilation occurs naturally as a mechanism of CBF auto-regulation, but can also be triggered by exogenous stimuli inducing extracellular and intracellular acidosis. The resulting decrease in pH relaxes smooth muscle cells lining the arteries and arterioles, thereby increasing their diameter. Common stimuli include changes in arterial CO2 partial pressure (PaCO2) induced by voluntary modulations of the breathing pattern, including breath-holding, hyperventilation and paced breathing (Petersson and Glenny, 2014; Urback et al., 2017; Liu et al., 2019) or by inhalation of CO2-enriched gas (Fierstra et al., 2013; Liu et al., 2019). As PaCO2 cannot easily be measured in vivo, end-tidal CO2 (EtCO2), the most recent maximal exhaled CO2 partial pressure, is often used as a surrogate and can be measured by recording the CO2 level in the exhaled gas using a gas monitor. Several approaches exist to manipulate PaCO2: inhalation of gas with fixed CO2 concentration (e.g., CO2-enriched air or carbogen), rebreathing the exhaled gas, EtCO2 targeting manually or using a computer-controlled device (Fierstra et al., 2013). Vasodilation can be induced without modulating the composition of the inhaled gas or breathing pattern by injection of acetazolamide (ACZ), a carbonic anhydrase inhibitor that causes acidosis (Vagal et al., 2009).
Signal Acquisition
Several imaging methods can assess haemodynamic changes induced by the vasodilatory stimulus. Positron emission tomography (PET), single-photon emission computed tomography (SPECT) (Ogasawara et al., 2003) and computed tomography (CT) (Marion and Gerrit, 1991) have all been used to measure CVR, but involve ionising radiation and have low temporal resolution. Transcranial Doppler ultrasound is a practical alternative, but has a limited field of view that allows blood velocity measurements only in parts of single large vessels, which do not necessarily reflect local changes in tissue blood supply (Purkayastha and Farzaneh, 2012; McDonnell et al., 2013). Magnetic resonance imaging (MRI) is a non-invasive, non-ionising technique which allows CVR mapping using contrasts related to CBF and/or CBV. Arterial spin-labelling (ASL) and phase-contrast (PC) MRI measure CBF in tissue and large vessels, respectively (Valdueza et al., 1997; Noth et al., 2008), while vascular space occupancy (VASO) MRI measures CBV (Donahue et al., 2009). Dynamic susceptibility contrast (DSC)-MRI measures both CBF and CBV (Taneja et al., 2019) by monitoring the T2 or T2*-weighted signal following intravenous injection of a gadolinium-based contrast agent. Blood Oxygen Level Dependent (BOLD) imaging, using a T2 or T2*-weighted sequence, can also measure CVR due to its sensitivity to a combination of CBF and CBV.
Processing Method
The signal change due to the vasodilatory stimulus must be converted into a quantitative or semi-quantitative measurement of CVR using one of several methods. Pre-vs.-post-stimulus subtraction of the MRI signal relies on the computation of the absolute or relative signal difference before and after the stimulus has been applied (Donahue et al., 2013; Wu et al., 2017). Often, the pre- and post-values are calculated by taking the average of the MRI volumes acquired during each period respectively, discarding volumes that are acquired during the transition period. Linear regression is a method that investigates the linear relationship between the dependent variable (in this case the MRI signal or derived CBF) and independent variables (e.g., EtCO2, to reflect the vasodilatory stimulus; time, to model a linear signal drift) (Thrippleton et al., 2018; Liu et al., 2019), allowing the MRI time course to be modelled using multiple predictors simultaneously. Cross-correlation quantifies the similarity between two signals (e.g., the MRI signal and EtCO2) as a function of their relative time delay (Donahue et al., 2016) and has been used as a measure of CVR. Non-linear fitting involves modelling the MRI signal as a non-linear function (Ziyeh et al., 2005; Germuska et al., 2019). It requires some initial estimate of the CVR and other parameters such as CVR delay, and can be more challenging to implement than linear regression, but has the advantage that any models can be used to fit the MRI signal. Some models (e.g., calibrated fMRI models) also allow quantitative estimation of CVR and other parameters that can be of interest such as cerebral metabolic rate of oxygen (CMRO2). Frequency-based analysis includes transfer function (Duffin et al., 2015) and Fourier (Blockley et al., 2011) analyses. In both methods, the signals of interest (e.g., the MRI signal and EtCO2) are transformed into the frequency domain. The magnitude of the signal at the stimulus frequency is then defined as the CVR. Finally, the standard deviation of the MRI signal (Kannurpatti et al., 2014; Jahanian et al., 2017) can be computed as a metric of CBF change due to natural vasodilation and vasoconstriction.
Aims of the Review
Since many combinations of the above stimuli, imaging methods and analysis techniques are possible, there are potentially many different ways to measure CVR in-vivo, resulting in a high degree of methodological diversity in the literature. Previous reviews described common CVR-MRI experiments (Fierstra et al., 2013; Pillai and Mikulis, 2015; Moreton et al., 2016; Urback et al., 2017; Liu et al., 2019) or CVR data analysis (Fisher et al., 2018). However, as far as we are aware, there are no systematic reviews detailing the breadth of CVR-MRI acquisition techniques, processing methods and applications that have been presented and used in the literature.
We conducted a systematic literature review of papers reporting the use of CVR-MRI techniques. We present an overview of the different aspects of the CVR-MRI experiment reported and applied in the literature, describing the most common methods and clinical research applications. We classified and systematically analysed reports of the MRI techniques, vasodilatory stimuli, data processing methods and study populations. Based on these findings we identified recent practises, trends, technical findings and evidence from clinical studies to inform future application and standardisation of CVR-MRI protocols.
Materials and Methods
Search Strategy
We systematically reviewed the EMBASE and MEDLINE databases from 1980, until June 2020 using Ovid. The search strategy combined terms relating to: “Cerebrovascular reactivity,” “MRI,” “BOLD,” “ASL,” “PC,” “hypercapnia,” “acetazolamide,” and “CO2.” We manually added relevant articles from the authors' libraries. The search was not constrained to English-language literature. Full details of the search strategy are provided as Supplementary Information.
Eligibility Criteria
We included all studies that investigated changes in cerebral blood flow or cerebral blood volume using MRI due to vasodilation or vasoconstriction in humans. We excluded reviews, conference abstracts, editors' notes, and case reports (single-subject studies focussed on methodological aspects of CVR were included). We removed studies that did not investigate induced vasodilation in the brain or used another imaging modality (e.g., CT, PET) to measure CVR. Studies that measured the change in the BOLD signal in response to a functional task and hypercapnia but did not compute a CVR metric were also excluded.
Data Extraction
One author (E.S.) screened the titles and abstracts of all potentially eligible publications to exclude duplicates and assess eligibility against the inclusion criteria before reading the full text of the remaining articles to determine eligibility. Eligibility and data extraction were discussed with other authors where queries around inclusion or exclusion, or data extraction arose.
We extracted population characteristics, including pathology, sample size, age, and gender. We recorded MRI acquisition parameters including magnetic field strength, type of pulse sequence and sequence parameters (e.g., TR, TE, spatial resolution, field-of-view). We recorded the type of vasodilatory stimulus, measurement of EtCO2 and/or end-tidal O2 (EtO2), stimulus paradigm and, where available, information on tolerability, number and reason for any excluded or failed scans. Finally, we extracted information on the pre-processing steps, delay correction/computation methods and CVR processing methods applied, reported grey and white matter CVR values in healthy volunteers and relevant findings.
Results
Search Results
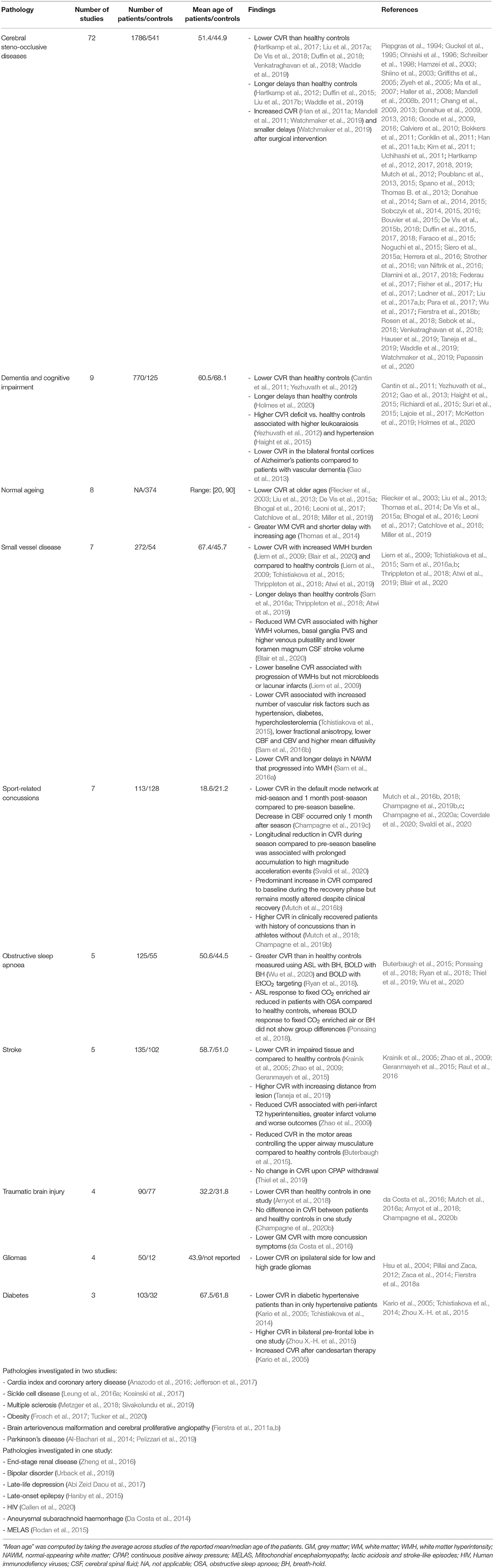
We identified 732 articles, 176 of which were removed as duplicates (Figure 1). Of the remaining 556 papers, 317 were excluded on review of the title and abstract due to a lack of analysable data or insufficient detail [n = 192: conference abstracts (n = 131), reviews (n = 34), and case reports and notes to the editor (n = 27)], inaccessibility (n = 1), only reporting rodent studies (n = 2), using other modalities (e.g., PET, TCD, CT, SPECT) (n = 71) and not measuring CVR (n = 51). After full text review an additional 14 papers were removed because they used other imaging modalities to measure CVR (n = 6) or did not measure CVR (n = 8). Additionally, 24 articles were added from the authors' libraries. We included 235 papers in the review. Summary data extracted from each study is included in the Supplementary Material.
Population Characteristics
The studies included 5,369 unique participants. 36 subjects were excluded before CVR due to contraindication to MRI (n = 6) or ACZ (n = 3), claustrophobia in the MRI scanner (n = 5), too large to fit in the MRI scanner (n = 1), anxiety during pre-testing of the stimulus (n = 1) and intolerance of the stimulus (n = 20). The remaining 5,333 unique participants who had a CVR scan comprised 2,394 patients and 2,939 healthy participants. All studies reported a sample size, with a mean sample size of 35 (median: 19, range: 1–536). Forty-five studies had fewer than 10 subjects whereas 9 included more than 100 subjects. Twelve papers did not report any age information, a further 18 papers reported only the age range. The mean age, computed as the mean of the mean or median ages, was 44.3 (1.4–92) years. The median gender distribution was 43% females and 57% males, excluding the 18 studies not reporting gender distribution.
The total number of scans including longitudinal scans was 7,437. The number of scans excluded from analyses was 518/7,437 (7%), not including scans that were selected from a database for being of good quality. Per study, the mean percentage of datasets excluded from analysis was 6% (range: 0–38%). Scans were excluded for one or more reasons: incomplete dataset (28/518, 5%), subject's discomfort (79/518, 15%), irregular breathing (3/518, 1%), non-compliance (38/518, 7%), technical issues (67/518, 13%), pre-processing issues (5/518, 1%), poor data quality (40/518, 8%), motion artefacts (183/518, 35%), outlier CVR values (13/518, 3%), non-CVR related (75/518, 14%, e.g., post-operative stroke, resolution of stenosis, hematoma, issue with therapeutic intervention) and no reasons reported (2/518, 0.3%).
Information on tolerability of the CVR experiment ranged from information regarding subject withdrawal to subjective rating of tolerability and was reported in 51/235 (22%) studies (1,162/5,333 unique subjects). Overall, the CVR experiment in these 51 studies was mostly described as well-tolerated. One article studied the tolerability of 434 CVR (294 subjects) scans acquired with EtCO2 targeting BOLD MRI and concluded that it was well-tolerated (Spano et al., 2013). Six studies reported subjective tolerability: the experiment was rated as tolerable to very tolerable with minimal discomfort on average in each study. Twenty-three studies detailed complaints of discomfort: 11 studies reported no complaints or adverse effects whereas 12 did. These 12 studies (618 subjects) reported 120 complaints transient to the CVR scan: respiratory symptoms due to gas inhalation such as breathing resistance and shortness of breath (n = 77), anxiety and/or claustrophobia (n = 16), dizziness and/or headache (n = 10), narrowness of head coil with gas apparatus (n = 4), tachycardia (n = 3), paraesthesia (n = 3), chest tightness (n = 1), conjunctive erythema (n = 1), tremor (n = 1), hand weakness (n = 1), nausea, confusion, and blurred vision (n = 1) and no details of the complaints (n = 2). No long-lasting symptoms were reported and no studies using acetazolamide injection detailed complaints or adverse effects. In 17 studies, 79 scans were defined as untolerable by the subject due to: anxiety (n = 21), claustrophobia (n = 16), discomfort related to gas apparatus in the scanner (n = 9), position in the head coil (n = 2) and no details (n = 31).
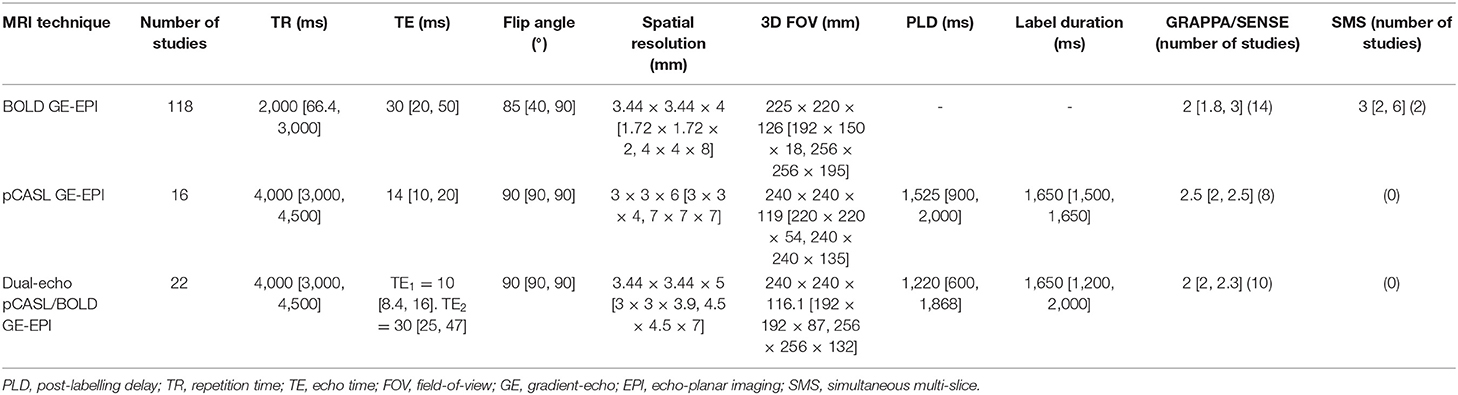
Pathologies
Cerebral steno-occlusive diseases (e.g,. Moyamoya disease, carotid stenosis/occlusion) were the most commonly investigated diseases (72/235 studies, 31%), followed by dementia and cognitive impairments (9/235, 4%), normal ageing (8/235, 3%), small vessel disease (7/235, 3%), sport-related concussions (7/235, 3%), obstructive sleep apnoea (5/235, 2%), stroke (5/235, 2%; one of which also investigated CVR in steno-occlusive disease), traumatic brain injury (4/235, 2%), tumours (4/235, 2%), diabetes with or without hypertension (3/235, 1%), and miscellaneous (18/235, 8%). Of the 142 articles reporting CVR measurements in pathology (referred to in Table 1), 70 studies assessed CVR to investigate pathophysiology, 48 studies explored the technical feasibility of a methodology to detect CVR impairment, 13 studies investigated the effect of a therapeutic intervention on CVR (surgical intervention for steno-occlusive diseases such as revascularisation, candesartan therapy for diabetes, bariatric surgery for obese subjects, haemodialysis for end-stage renal disease, therapeutic continuous positive airway pressure for obstructive sleep apnoea), six studies investigated the progression of pathologies, and five studies looked at the effect of CVR on fMRI BOLD activation. Relative to healthy controls, CVR was lower in patients in most of the pathologies (Krainik et al., 2005; Donahue et al., 2009; da Costa et al., 2016; Hartkamp et al., 2018; Thrippleton et al., 2018; McKetton et al., 2019) and CVR delays were longer in steno-occlusive diseases, small vessel disease and dementia (Hartkamp et al., 2012; Duffin et al., 2015; Thrippleton et al., 2018; Atwi et al., 2019; Holmes et al., 2020).
MRI Technique
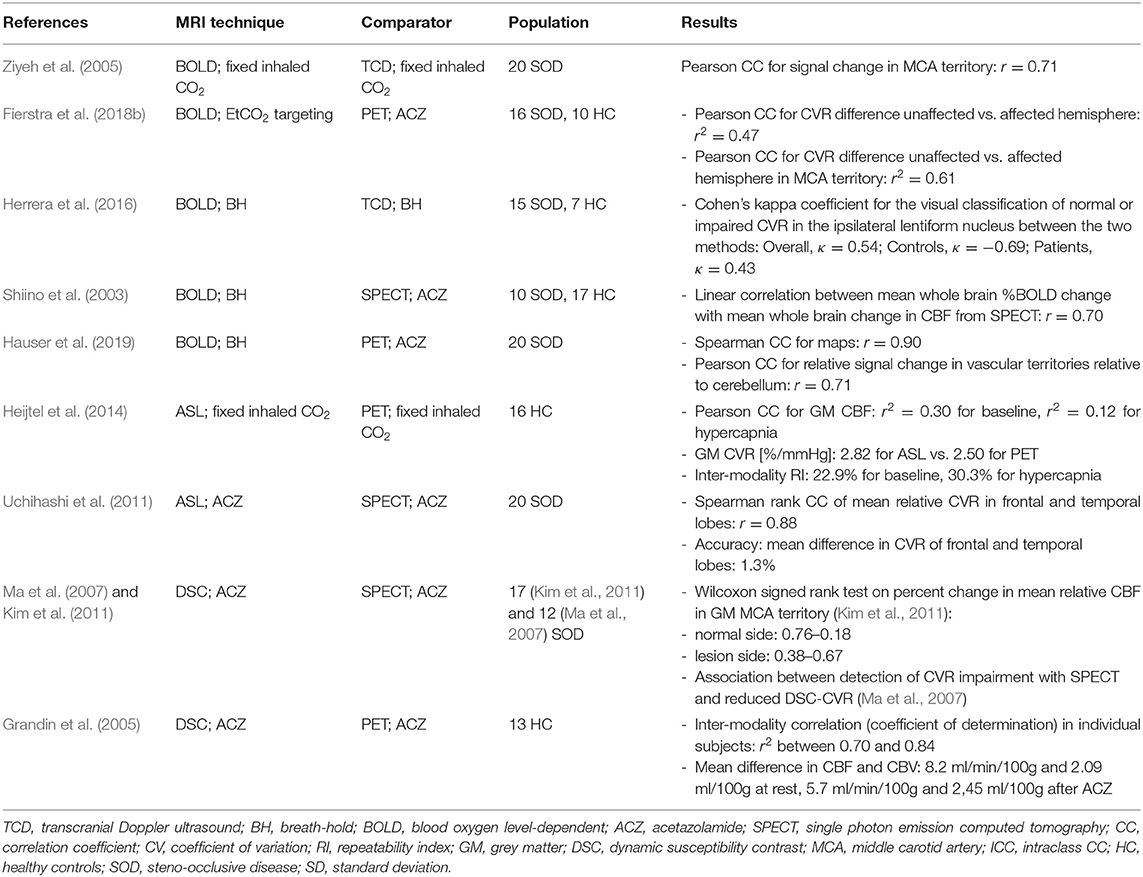
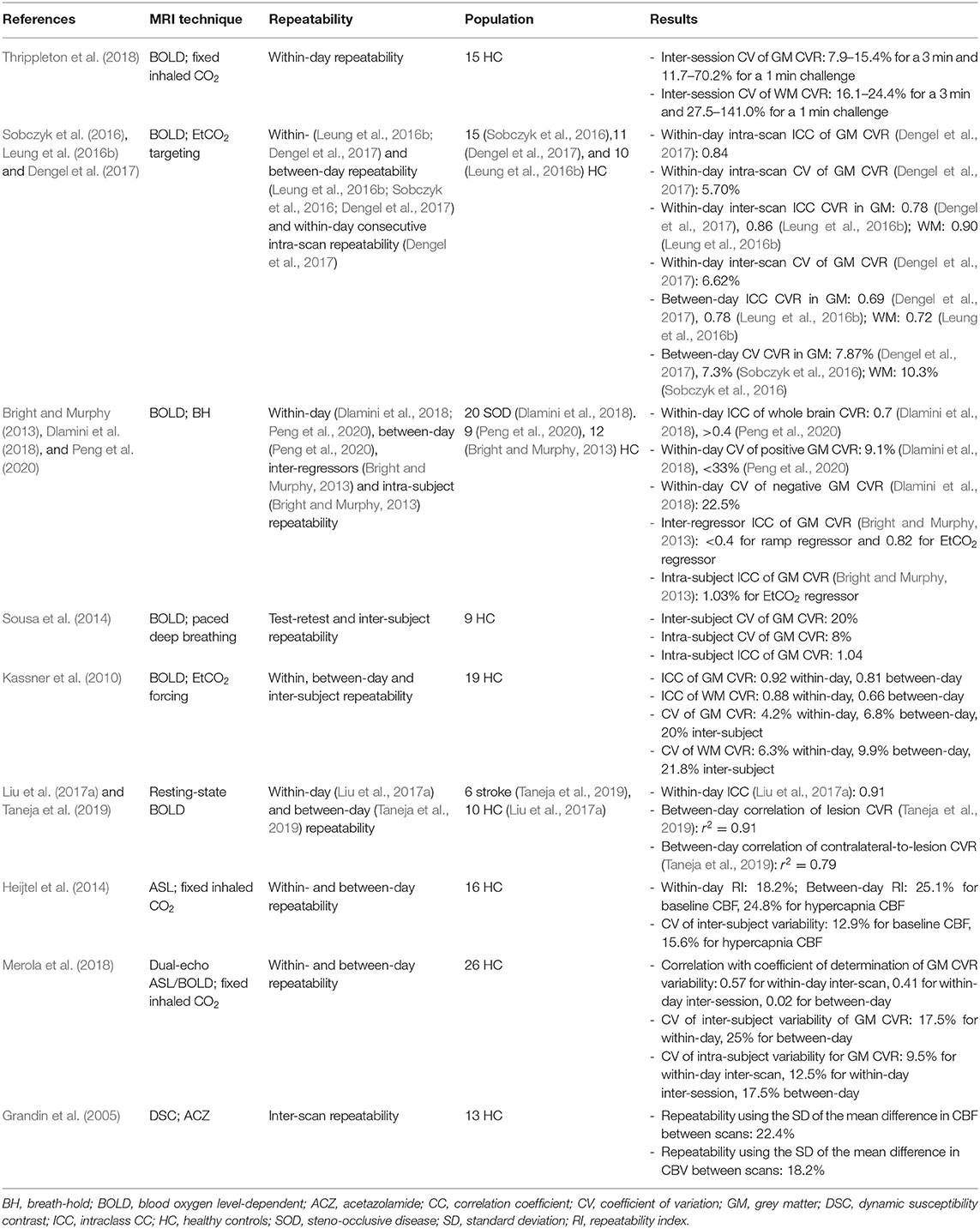
The number of CVR-MRI studies that were conducted at 3 T is 178/235 (74%), with the remainder acquired at: 1.5 T (47/235, 20%), 7 T (9/235, 4 %), 2 T (2/235, 1%) and a combination of 1.5 and 3 T (3/235, 1%). Studies used one or more MRI techniques to acquire CVR data (Figure 2): BOLD (155/235, 66%), ASL (41/235, 17%), dual-echo providing simultaneous ASL and BOLD data (27/235, 11%), PC (12/235, 5%), DSC (11/235, 5%), and VASO (3/235, 1%). In recent publications, BOLD, ASL and dual-echo ASL/BOLD are the most common MRI techniques. Summary MRI parameters for the BOLD gradient-echo echo-planar imaging (GE-EPI), pulsed continuous ASL (pCASL) and dual-echo ASL/BOLD GE-EPI techniques at 3 T are given in Table 2.
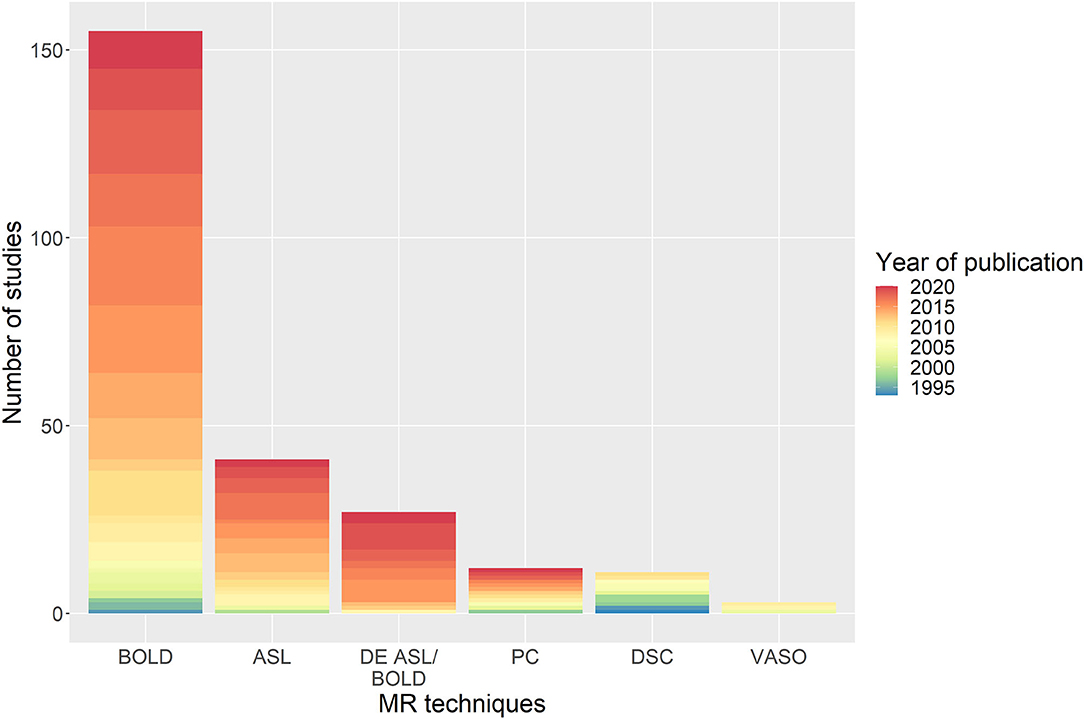
Figure 2. Distribution of the MRI sequences used in studies with the associated year of publication of the paper. BOLD, blood-oxygen-level-dependent; ASL, arterial spin-labelling; DE, dual-echo; PC, phase-contrast; DSC, dynamic susceptibility contrast; VASO, vascular space occupancy.
Three studies (n = 18) found BOLD-derived CVR values were lower at lower magnetic field strengths (Driver et al., 2010; Triantafyllou et al., 2011; Peng et al., 2020), two of which (n = 9) reported a linear relationship between BOLD-derived CVR and the field strength (Driver et al., 2010; Triantafyllou et al., 2011). In one study (n = 16), ASL-derived CVR did not differ at different field strengths (Noth et al., 2006). One study (n = 8) reported longer post-labelling delay results in lower baseline CBF and ASL-CVR measurements (Inoue et al., 2014). Use of EPI with parallel imaging compared to spiral imaging, reduced signal loss due to susceptibility-induced magnetic field gradients in BOLD-CVR measurements without affecting sensitivity, which was defined as the CVR t-statistic (n = 5) (Winter et al., 2009). Furthermore, one study (n = 5) showed that using simultaneous multi-slice acceleration of factor 2 and 3, can reduce scan duration by at least a half compared to conventional EPI while maintaining the CVR sensitivity (Ravi et al., 2016a). Compared to single-echo ASL or BOLD EPI, a multi-echo (four echoes) EPI acquisition followed by T2* fitting of the signal decay had higher inter-scan repeatability of breath-hold CVR analysed across voxels, CVR sensitivity and test-retest reliability analysed using the intra-class correlation coefficient (n = 14) (Cohen and Wang, 2019).
Vasodilatory Stimulus
To induce vasodilation, several stimuli were employed in the literature (Figure 3A): EtCO2 targeting manually or using a computer-controlled device such as RespirAct (Thornhill Research, Toronto, Canada) (81/235 studies, 34%), fixed inspired gas administration (69/235, 29%), breathing modulations (52/235, 22%), ACZ injection with median dose of 1 g (29/235, 12%), rebreathing (10/235, 4%), resting-state haemodynamic fluctuations (8/235, 3%) and not reported (1/235, 0.4%). Three different fixed inspired gases were identified: CO2-enriched (67%), O2-enriched (i.e. hyperoxia, 10%), and CO2- and O2-enriched air (i.e. carbogen, 23%). In some studies, these gas compositions were alternated during the same paradigm with or without intermittent normal air periods using the fixed inspired gas, EtCO2 targeting methods: alternating hypercapnia and hyperoxia (15/235, 6%), alternating CO2-enriched air and carbogen (1/235, 0.4%). For fixed inspired CO2 paradigms, the median percentage of inhaled CO2 was 5% (range: 2–10%). While the combination of MRI sequence and stimulus generally varied across studies DSC-MRI was used only with ACZ injection. Block design paradigms were most common (212/235 studies, 90%) with a median stimulus plateau duration of 1 min. The median total experiment duration was 9 min (Figure 3B). One study did not specify the type of paradigm, and 12 further studies did not report the duration of the CVR experiment.
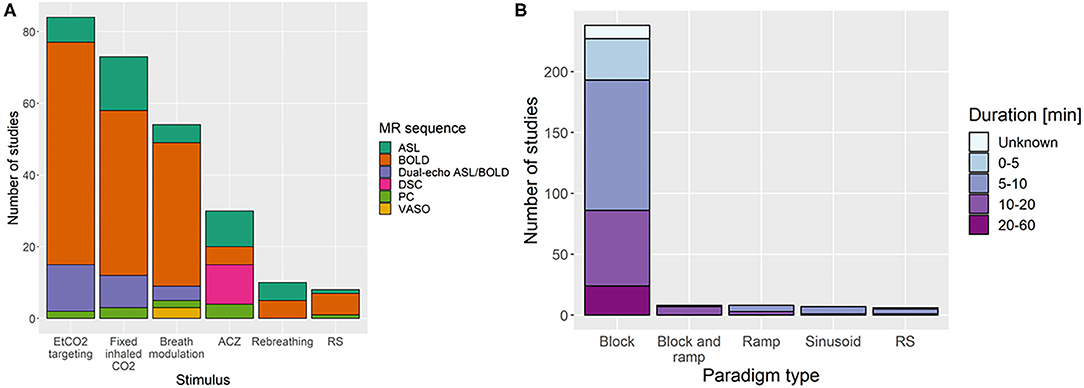
Figure 3. Distribution of the (A) stimuli with the associated MRI sequence and (B) paradigm types with associated total duration of the CVR experiment. In (A), the “breath modulation” stimulus includes breath-holding, paced breathing, and hyperventilation stimuli. ACZ, acetazolamide injection; RS, resting-state; BOLD, blood oxygen-level dependent; ASL, arterial spin-labelling; PC, phase contrast; DSC, dynamic susceptibility contrast; VASO, vascular space occupancy.
Removing studies that used ACZ stimulus, 160/207 studies measured EtCO2 (77%) of which 21 did not report the targeted or achieved EtCO2 variation (14%), 80 studies also measured EtO2 (39%). The median EtCO2 change induced by the stimulus was 9 mmHg (range: 2.2–28 mmHg). Seventy-five studies reported mean baseline EtCO2 at rest (47%), with a median value of 39 mmHg (range: 31.2–43.4 mmHg). 21% of the studies that used EtCO2 targeting controlled the baseline EtCO2 (40 mmHg for all studies) instead of using the individual EtCO2 value when breathing normal air.
One study (n = 4) found BOLD response to EtCO2 is 60 times higher than to EtO2, but demonstrated that during hypercapnic CVR-BOLD experiments, EtO2 should be controlled if the change in EtCO2 is small compared to the change in EtO2 (Prisman et al., 2008). One study (n = 9) demonstrated that carbogen should not be used with BOLD or ASL to measure CVR due to a lack of correlation between both MRI techniques as opposed to CVR measurements using CO2-enriched air with BOLD or ASL (Hare et al., 2013). Another study (n = 20) found that, for a gas challenge, an effect of at least 2 mmHg EtCO2 change is required to detect haemodynamic impairment using BOLD at 3 T (De Vis et al., 2018). RS-BOLD was found to give CVR results that were associated with fixed-inspired CO2 BOLD (n = 48, Liu et al., 2017a) and RespirAct BOLD (n = 13, Golestani et al., 2016) measurements. One study (n = 8) reported differences in response amplitude and onset time depending on whether BH was performed before and after expiration (Leoni et al., 2008). For BOLD-BH, one study (n = 6) demonstrated that the fraction activation volume saturated for breath-hold durations of 20 s and above; thus recommended using breath-hold durations of 20 s to give sufficient sensitivity to BOLD signal changes to detect impaired CVR (Liu et al., 2002).
CVR Data Processing Methods
Common pre-processing steps that were reported (Figure 4) were sequence-dependent and included motion correction (167/235 studies, 71%), spatial smoothing (107/235, 46%), registration of functional volumes to MNI or subject space (96/235, 41%), region-of-interest or whole brain delay correction (93/235, 40%), drift removal/modelling (79/235, 34%), voxel-wise delay correction (62/235, 26%), and discarding transient MRI volumes to consider only those where steady-state signal was reached (42/235, 18%). Only 3% of papers corrected for sampling line delay. Slice-time correction was used in 51 of 180 BOLD/DE-BOLD studies. Eroding the edges of the regions of interest can reduce vascular contamination of CVR due to larger responses to CO2 in blood vessels than in tissues (Thrippleton et al., 2018). T1 correction was recommended for CVR-ASL data involving the use of carbogen or other hyperoxic gas because of changes in the longitudinal relaxation time during hyperoxia (n = 24, Siero et al., 2015b). The most common software packages used for pre-processing and/or CVR analysis were Statistical Parametric Mapping (SPM, 89/235 studies, 38%), in-house Matlab (The Mathworks, Natick, MA, United States) software (90/235, 38%), FMRIB Software Library (FSL, 65/235, 28%), and Analysis of Functional NeuroImages (AFNI, 54, 23%) (some studies used more than one package in combination). Only one in-house Matlab script (for pre-processing BOLD and EtCO2 data) reported to be publicly available (Lu et al., 2014).
Figure 4. Bar chart showing the number of studies that apply different pre-processing steps. ROI, region of interest; WB, whole brain; HRF, haemodynamic response function.
Of the six classes of CVR calculation methods identified, linear regression is the most common method overall (149/235 studies, 63%) and in recent publications. However, several newer methods are under development including frequency-based analysis (Duffin et al., 2015). The main reference signal used to compute linear regression or cross-correlation is the EtCO2 (89/235 studies, 38%). An HRF was incorporated in the MRI signal model in 14% of the studies (32/235), with the single or double gamma function being the most common choice (22/235 studies, 9%). A relatively new method to find an appropriate regressor is RIPTiDe (Regressor Interpolation at Progressive Time Delays), which derives the reference signal from the MRI data by iteratively applying principal component analysis on aligned MRI time courses until convergence of the regressor (Tong et al., 2011; Donahue et al., 2016). Twenty one studies did not clearly describe the CVR processing method, of which two included no information, these were excluded from the summary of CVR processing method (Figure 5A).
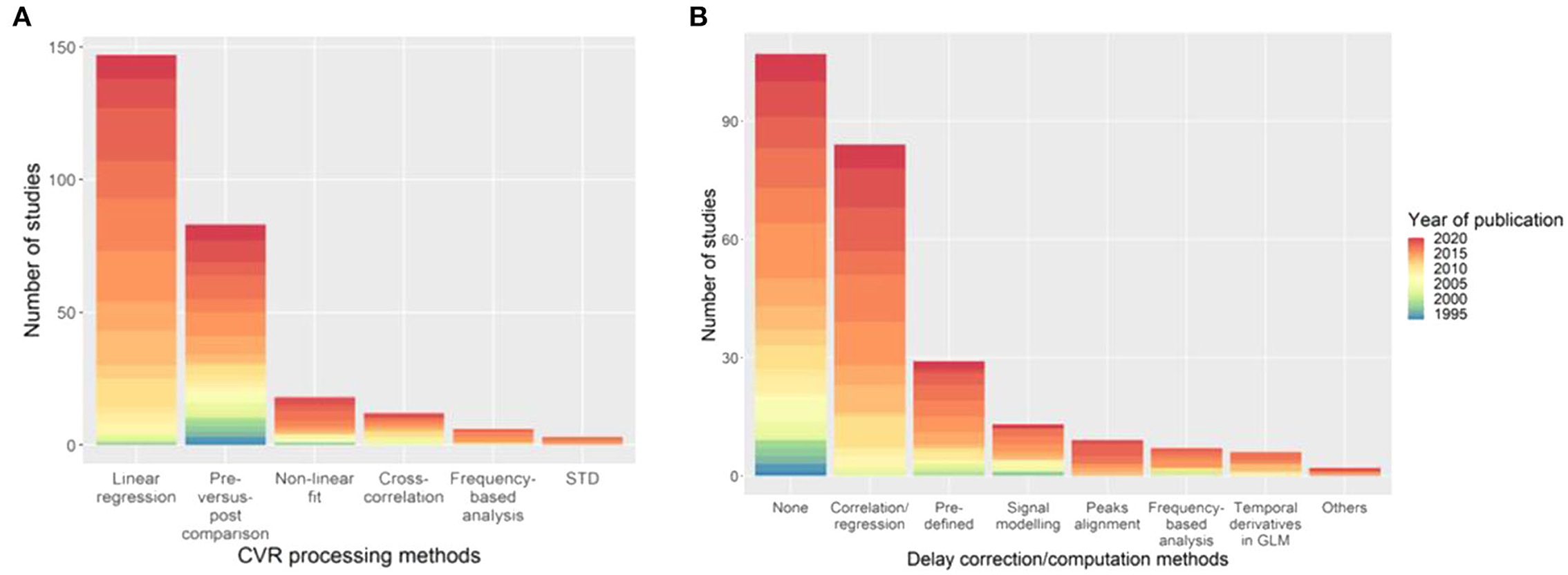
Figure 5. Distribution of the (A) CVR processing and (B) delay computation methods with the associated year of publication of the paper. The category “Others” in (B) includes deconvolution to find the HRF between the EtCO2 and the MRI signal, and GLM with two (“fast” and “slow”) regressors. STD, standard deviation of MRI signal; HRF, haemodynamic response function; GLM, general linear model.
Dynamic aspects of CVR (e.g., lung-to-brain delay, response time) were computed in 128/235 studies (54%) using different methods (Figure 5B), however some studies used different MRI techniques and multiple associated delay processing methods. Fourteen of the delay computation methods were not clearly described and 8 of which could not be included in Figure 5B. Cross-correlation- and linear regression-based methods can be used to compute CVR delay by determining the time shift that gives the best correlation between the BOLD signal and a reference signal (e.g., EtCO2). The most common delay computation methods are cross-correlation- or, equivalently, linear regression-based approaches (84/128 studies, 66%) and pre-defined delay, e.g., from literature, voxel examination, (29/128, 23%). The delay between two signals can be found using linear regression or cross-correlation by determining the time shift giving the best correlation between these two signals. As with CVR computation, delay computation is an evolving area and new methods are arising including obtaining the delay directly from the HRF between the BOLD signal and EtCO2 (Atwi et al., 2019). One study corrected the hypercapnic delay for delay due to the vasculature (i.e., the delay it takes for the blood and CO2 to travel from the lungs to the brain tissues) by using the BOLD delay from a hyperoxia challenge as a surrogate of vasculature delay and assuming no vasodilation due to hyperoxia (Champagne et al., 2019a). This correction can distinguish between delay due to vasculature and delay due to vasodilation.
CVR values in whole brain, grey and white matter of healthy volunteers are summarised in Table 3. The associated processing methods were linear regression (72/104, 69%), pre-vs.-post stimulus value comparison (17/104 values, 16%), non-linear signal modelling (13/104, 13%) and frequency-based analysis (2/104, 2%). CVR in grey matter was higher than CVR in white matter. Moreover, measuring white matter CVR using ASL is not common, probably due to the fact that ASL suffers from low contrast-to-noise ratio (CNR) (Liu et al., 2019).
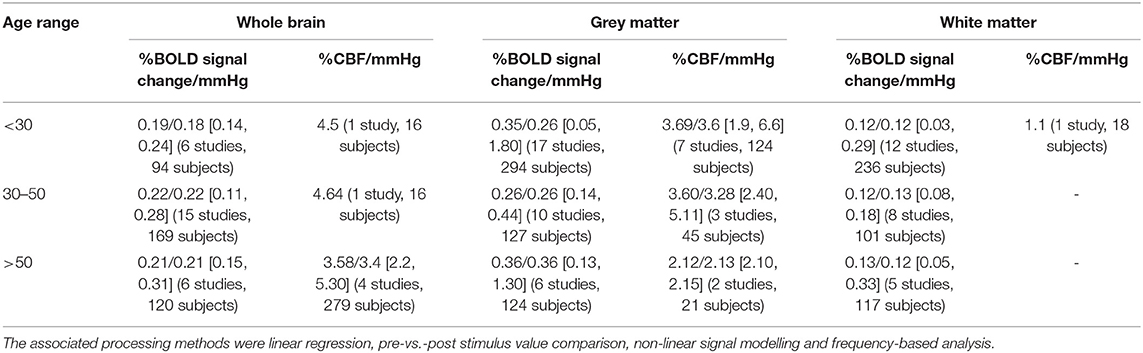
Table 3. Mean/median CVR values at 3 T in healthy volunteers as a function of the age range (in the square brackets are the minimum and maximum values and in the round brackets is the number of values and number of subjects used to compute the mean).
Repeatability, Reproducibility, and Accuracy of CVR Measurements
CVR values determined using MRI were generally found to be similar or well-correlated with those obtained using other imaging modalities such as PET, SPECT or TCD (Table 4: 10 studies, 193 subjects). Within- and between-day repeatability of MRI was studied mostly in healthy participants and in some stroke and steno-occlusive patients (Table 5: 14 studies, 191 subjects). The reported coefficients of variation show that CVR measurements are less repeatable between- than within-days (Kassner et al., 2010; Dengel et al., 2017) and less repeatable in white matter than in grey matter due to lower CNR in white matter (Kassner et al., 2010; Thrippleton et al., 2018).
CVR-MRI measurements were also compared between MRI techniques. CVR-BOLD and CVR-ASL were well-correlated using fixed CO2 concentration (n = 127) (Hare et al., 2013; Donahue et al., 2014; Zhou Y. et al., 2015) and computer-controlled EtCO2 using RespirAct (n = 13) (Zande et al., 2005). One study found no correlation between CVR-BOLD and CVR-ASL using carbogen (n = 9) (Hare et al., 2013). Using acetazolamide CVR-DSC correlated well with CVR-BOLD (n = 16) (Wu et al., 2017), but there was a lack of agreement between CVR-DSC and PC-MRI (n = 8) (Spilt et al., 2002).
The Relationship Between BOLD Response and PaCO2
The healthy BOLD signal response to CO2 was found to be sigmoidal in two studies (n = 18) (Tancredi and Hoge, 2013; Bhogal et al., 2014). The sigmoid model of the BOLD response to CO2 was used in a further three studies (n = 65) (Bhogal et al., 2015, 2016; De Vis et al., 2018). In four studies, vasodilatory resistance to CO2 was modelled using the BOLD response (Sobczyk et al., 2014; Duffin et al., 2017, 2018; McKetton et al., 2019). The relationship between resistance and CO2 was assumed sigmoidal due to the limited ability of the blood vessels to constrict and dilate (n = 141). One study (n = 32) suggested that steal phenomenon associated with some pathologies could alter the sigmoid relationship between CO2 and vasodilatory resistance (Sobczyk et al., 2014).
Potential Confounders of CVR Analysis
When analysing CVR measurements, baseline MRI signal or EtCO2 values (Bhogal et al., 2016) can lead to misinterpretation of CVR data (Mandell et al., 2008a; Blockley et al., 2011). Higher baseline EtCO2 was associated with lower CVR (n = 291) (Halani et al., 2015; van Niftrik et al., 2018; Hou et al., 2019). Baseline CBF and CBV were lower with age (n = 81) (Petrella et al., 1998; Leung et al., 2016a; Leoni et al., 2017), by contrast one study suggested age-related differences in baseline CBF may result from differences in baseline EtCO2 (n = 46) (De Vis et al., 2015a).
Negative CVR clusters correspond to MRI responses anti-correlated to the stimulus. In some cases this might simply reflect long CVR delays if they are not appropriately modelled. Negative CVR could also reflect the steal phenomenon, where tissues with high CVR “steal” blood flow from other regions due to flow redistribution (Shiino et al., 2003; Mandell et al., 2008a; Han et al., 2011a,b; Sobczyk et al., 2014; Poublanc et al., 2015; Fisher et al., 2017; Para et al., 2017; McKetton et al., 2018; Venkatraghavan et al., 2018; Hartkamp et al., 2019). However, they usually appear in the deep white matter (Mandell et al., 2008a), near and in the ventricles (Blockley et al., 2011). Therefore, others have suggested that they may result from low CNR in the white matter tissues leading to spurious CVR values (Blockley et al., 2011), or from reduction in cerebrospinal fluid (CSF) partial volume due to vasodilation (Thomas B. P. et al., 2013; Bright et al., 2014; Ravi et al., 2016b). The latter effect can be diminished by shortening TE (Ravi et al., 2016b).
CVR Definition and Units
CVR was defined differently across studies and was reported in several units: relative change in BOLD signal divided by absolute change in EtCO2 with %/mmHg units (110/235, 47%), relative change in CBF divided by absolute change in EtCO2 with %/mmHg units (36/235, 15%), relative change in BOLD signal with % units (50/235, 21%), relative change in BOLD signal divided by relative change in total haemoglobin concentration ([Hb]) with %/[Hb] units (1/235, 0.4%), relative change in BOLD signal divided by breath-by-breath O2-CO2 exchange ratio with % units (1/235, 0.4%), relative change in BOLD signal divided by relative change in EtCO2 with % units (1/235, 0.4%), relative change in BOLD signal during one period of breath-hold with %/s units (1/235, 0.4%), relative change in CBF with units % (27/235, 11%), relative change in CBF during one period of breath-hold with %/s units (1/235, 0.4%), absolute change in CBF with ml.100 g−1.min−1 units (5/235, 2%), absolute change in CBF divided by the change in EtCO2 with ml.100 g−1.min−1mmHg−1 units (2/235, 1%), absolute change in CBF divided by mean arterial pressure divided by change in EtCO2 with ml/min/mmHg2 (1/235, 0.4%), mean arterial pressure divided by change in CBF with mmHg.ml−1.min.100 g units (1/235, 0.4%), relative change in CBV with % units (n = 13), absolute change in CBV with ml.100 g−1 units (1/235, 0.4%), absolute change in BOLD signal divided by change in EtCO2 a.u./mmHg (2/235, 1%), relative change in T2* with % units (1/235, 0.4%), absolute change in T2* divided by change in EtCO2 with ms/mmHg units (1/235, 0.4%), absolute change in R2* divided by change in EtCO2 with s−1/mmHg (1/235, 0.4%). A further nine CVR definitions had no units because CVR was defined as the correlation coefficient between two time courses (7/235, 3%) and two were defined as the absolute change in BOLD signal divided by the standard deviation of the baseline BOLD signal (1/235, 0.4%) or by the absolute change in mean cerebellum BOLD signal (1/235, 0.4%). One article described different resistance sigmoid parameters associated with CVR such as resting reserve or amplitude, i.e., extend of vascular resistance from resting EtCO2 state to maximum vasodilation and extend of vascular resistance from maximum vasoconstriction to maximum vasodilation, respectively. Both resting reserve and amplitude are resistance parameters in mmHg/nL/s.
Discussion
We identified 235 papers using MRI to measure CVR including 5,333 subjects, which covered several different acquisition and analysis methods. Stimuli, paradigm and duration, sequences used for acquisition and processing methods varied considerably. We found several papers, which investigated specific aspects of the CVR-MRI experiment such as processing methods or reproducibility of CVR measurement, but sample sizes were often low, and validation studies remain limited. Reporting was also inconsistent.
Reporting Standards
Most papers included sufficient detail on the acquisition of CVR data (222/235, 94%). Only 22% of studies (51/235) reported CVR tolerability, less than half of which (23/235, 10%) reported presence or absence of discomfort complaints which may affect suitability for some patient populations. Processing (214/235, 91%) including delay computation methods (114/128, 89%) were well-reported, though only 54% (128/235) accounted for delay.
Clinical Populations
CVR was measured in several pathologies including steno-occlusive diseases, stroke, small vessel disease, brain injuries, and dementia. Patients generally had lower CVR compared to healthy participants (Krainik et al., 2005; Donahue et al., 2009; da Costa et al., 2016; Hartkamp et al., 2018; Thrippleton et al., 2018; McKetton et al., 2019), though in obstructive sleep apnoea findings were mixed. Delays were longer in steno-occlusive, small vessel disease and dementia patients than in healthy controls, but were not reported in other pathologies. CVR metrics have been associated with cerebrovascular dysfunction, disease severity, and response to interventions (including revascularisation surgery for steno-occlusive diseases). CVR is therefore a promising biomarker of haemodynamic impairment and changes with broad applicability.
Acquisition
Most CVR studies used a 3 T scanner (178/235, 74%) and 2D GE-EPI BOLD sequence (118/235, 50%) for acquisition. While several different sequences can measure CVR, all have limitations. BOLD signal results from a complex interaction between CBF, CBV, haemoglobin concentration, oxygen extraction fraction, cerebral metabolic rate of oxygen consumption and arterial O2 partial pressure (Liu et al., 2019). Changes in any of these parameters can alter the BOLD signal; however, there is evidence that CBV and CBF change together during hypercapnia (Chen and Pike, 2010; Hoge, 2012) and that CVR-BOLD is well-correlated with CVR-ASL (Mandell et al., 2008b; Hare et al., 2013; Zhou Y. et al., 2015). Cerebral metabolic rate of oxygen consumption might change during hypercapnia; however it is thought that these changes are small for low levels of CO2 stimulus (Hoge, 2012). ASL allows direct measurement of CBF and is also widely used (41/235, 17%), but suffers from low CNR (Liu et al., 2019); differences in labelling duration and efficiency, and bolus arrival time can also potentially affect CVR estimation. Calibrated fMRI (9/235, 4%) using dual-echo BOLD/ASL allows simultaneous quantification of CVR and cerebral metabolism parameters (e.g., rate of oxygen consumption and oxygen extraction fraction) (Germuska et al., 2016, 2019; Merola et al., 2017, 2018). However, calibrated fMRI models depend on the initialisation values of model parameters, model assumptions such as the oxygen metabolism not being altered during hypercapnia and hyperoxia stimuli (Germuska and Wise, 2019), and are more complex to implement. PC-MRI (12/235, 5%) measures CVR at the large-vessel level and generally provides limited spatial coverage; although 4D phase-contrast flow imaging (Miller et al., 2019; Morgan et al., 2020) is developing rapidly, the long scan duration currently limits applicability for measuring CVR in patients. Several different paradigms were used, which varied in duration and number of repetitions. EtCO2 targeting (81/235, 34%) and fixed CO2-inhalation (69/235, 29%) are the most widely used vasodilatory stimuli with a block paradigm (212/235, 90%) with a median paradigm duration of 9 min. Fixed CO2-inhalation is easier to set up than EtCO2 targeting but the change in EtCO2 associated with a specific inspired CO2 concentration may vary between subjects. EtCO2 targeting allows precise control over the EtCO2 and paradigm but requires expensive, specialist equipment. 75% of respiratory challenge studies (160/207) measured ETCO2. However, in patients with lung diseases, using EtCO2 is not a direct surrogate for PaCO2 (Petersson and Glenny, 2014).
Processing Methods
CVR was mainly computed using linear regression (149/235, 63%). Few studies described why a particular processing method or regressor was used, and comparisons between different methods are lacking (Bright et al., 2017). 40% of the studies (93/235) calculated a whole brain or single region-of-interest delay that was applied to all voxels. While this method may be relatively robust against noise, delay is known to vary between and within tissue types (Thrippleton et al., 2018; Atwi et al., 2019). However, only 26% of studies (62/235) accounted for voxel-wise delays. An HRF between the stimulus and MRI signal was used in only 14% of the studies (32/235). This might be because the CVR HRF is unknown and may vary between stimuli, paradigms and pathologies (Poublanc et al., 2015; Sam et al., 2016a). Assuming a non-delta-function HRF allowed delay and steady-state CVR to be investigated in parallel (Poublanc et al., 2015; Donahue et al., 2016), but can be more complex to implement and computationally demanding.
Validation
CVR measurements and detection of CVR impairment using MRI and other imaging modalities [e.g., BOLD-CVR to TCD-CVR (Ziyeh et al., 2005), BOLD-CVR to SPECT-CVR (Shiino et al., 2003), DSC-CVR to PET-CVR (Grandin et al., 2005)] were well-correlated, validating the CVR-MRI experiment. Furthermore, biological validation such as results from studies comparing CVR in patients with steno-occlusive diseases and healthy controls, also supports use of CVR as a biomarker (Ziyeh et al., 2005; Bokkers et al., 2011; Uchihashi et al., 2011; Thomas B. et al., 2013; De Vis et al., 2015b). Preclinical CVR imaging is also a fast-growing field which has been applied in preclinical models of stroke, cancer and Alzheimer's disease (Wells et al., 2015; Lake et al., 2016; Gonçalves et al., 2017). Preclinical CVR studies predominantly follow similar approaches to human studies but involve additional considerations such as the effect of anaesthetic agents on resting CBF (Stringer et al., 2021). Isolated vessel preparations (Seitz et al., 2004; Joutel et al., 2010), laser speckle imaging (Lynch et al., 2020), and multiphoton microscopy (Joo et al., 2017; Kisler et al., 2017) can also assess CVR preclinically and may help improve understanding of how impaired vasoreactivity develops and further direct validation of CVR-MRI as a clinical biomarker of cerebrovascular health (Stringer et al., 2021). CVR measurements using MRI techniques showed lower repeatability between-days than within-days (Dengel et al., 2017; Merola et al., 2018). CVR measurements were also less repeatable in white matter than in grey matter due to a lower CNR (Kassner et al., 2010; Thrippleton et al., 2018). Studies with higher sample sizes and investigating reproducibility in different pathologies would be helpful to further validate the CVR-MRI experiment.
Definition and Interpretation of CVR
The definition and units of CVR vary across studies depending on choice of sequence, stimulus, paradigm and analysis methods. However, CVR is most commonly reported as the relative change in BOLD signal (110/235, 47%) or CBF (36/235, 15%) per unit change in EtCO2 as %/mmHg.
Several aspects influence CVR values beyond the vasodilatory capacity of vessels, which must be considered in interpreting the results. The CVR steal phenomenon has been proposed as a systemic mechanism governing the cerebrovascular system by prioritising blood supply to specific regions and potentially leading to local deficits elsewhere. Low or negative CVR values may also result from low CNR or blood vessel dilation near the ventricles shrinking the CSF space and artificially decreasing the BOLD signal due to differences in CSF and blood signal intensities (Thomas B. P. et al., 2013; Bright et al., 2014; Ravi et al., 2016b). Excluding voxels that contain CSF or using a shorter TE (e.g., 21 ms for a TR of 1,500 ms at 3T) can reduce negative artefacts in CVR data (Ravi et al., 2016b). Other physiological factors can affect the MR signal, including resting CBF and oxygen extraction fraction. Finally, as blood vessels have a limited vasodilation capacity, the linearity of the MRI response to the vasodilatory stimulus has a restricted range. Indeed, the shape of the MRI response to the stimulus and baseline parameters including resting CBF and EtCO2 can influence CVR values (Bhogal et al., 2014, 2016; van Niftrik et al., 2018; Hou et al., 2019). Despite some gaps in current knowledge, CVR has a proven validity and utility in several diseases as described above.
Definition and Interpretation of CVR Delay
Delay in the MRI response to a stimulus can lead to inaccurate CVR values if it is not accounted for, and could give further information on vascular health. Voxel-wise or ROI delay should be favoured as opposed to whole brain delay to better account for differences in tissue response and distance from blood vessels. Artificially high or low delay values can be obtained when the noise level is high, i.e., low CNR. Definitions of delay were inconsistent in distinguishing between lung-to-brain delay and duration of the vasodilation process (Thomas et al., 2014). For example, one study computed the lung-to-brain delay, assuming instantaneous MRI response, as the shift in the EtCO2 that gives the lowest residual when regressed against the MRI time course: the delay in grey and white matter were approximately 15 and 35 s, respectively (Thomas et al., 2014). Another study computed the response time using a mono-exponential fit of the MRI signal: they found response time constants between a few seconds in grey matter up to 100 s in white matter (Poublanc et al., 2015).
Implications for Future Research
Harmonisation of the CVR-MRI Experiment
Variability in the implementation of CVR experiments, including the choice of sequence and MRI parameters, such as TR and TE for BOLD MRI and post-labelling delay for ASL MRI (Inoue et al., 2014), causes heterogeneity in the CVR values, making it challenging to interpret findings across studies and conduct meta-analyses. CVR measurements are highly dependent on MRI sequence (e.g., BOLD, ASL, PC, and DSC), since each measures a different quantity as an estimate or surrogate of CBF, which are not directly comparable (Zhou Y. et al., 2015).
Harmonisation of acquisition and processing methods would allow more uniform definitions of CVR, delay and HRF, enhancing inter-study comparability, although specific techniques may be better suited to some pathologies and patient groups. Such efforts may also find consensus on the optimal paradigm duration to ensure that CO2 reaches the region of interest and the MRI response reaches the steady state. As many groups have developed in-house software to process CVR data, making these publicly available, as a step towards development of validated, community-driven open-source software, would also promote reproducibility and harmonisation.
While little consensus currently exists, our review reveals evidence of convergence in some aspects of the CVR-MRI experiment: the use of BOLD at 3T with a block paradigm for the acquisition and definition of CVR as the relative change in BOLD signal per unit change in mmHg (%/mmHg). Early attempts to establish a framework for reaching consensus have recently been initiated (Bright et al., 2017). Further work is needed to reach consensus regarding signal processing and CO2 delivery methods. CVR is also highly dependent on the image analysis methods used, including the erosion of regions of interest to avoid signal contamination from neighbouring tissues, or region of interest vs. voxel-wise analysis.
Considerations for Future Studies
Detailed reporting of methods and results is essential for interpretation and inter-study comparison of CVR data. Future publications should give sufficient detail to allow processing to be reproduced and, where possible, authors should release their software in version controlled open-source repositories. Results should preferably be reported in relative signal units to allow inter-study comparisons. Accurate recording and reporting of tolerability and reasons for excluding CVR scans is also important to facilitate clinical translation.
Non-linearity due to the limited vasodilation capacity of the blood vessels, is a consideration when interpreting CVR values. In this case, CVR reflects both the maximum response as well as the sensitivity to CO2. Research is needed to identify the aspects of the CVR response (e.g., maximum response, MRI response vs. EtCO2 slope) that are most sensitive and specific in key pathologies. Accounting for voxel-wise lung-to-brain delay would allow direct comparison of the BOLD signal and EtCO2 and should improve the accuracy of CVR values. Drift in the MRI signal can be significant and should be controlled for during signal processing (Liu et al., 2019).
Finally, there are age-related changes in CVR values: CVR is lower with increasing age in grey and white matter (Thomas et al., 2014; Leung et al., 2016c; Leoni et al., 2017). Statistical analyses should account for such key covariates, which requires larger sample sizes or matched study design. CVR is also associated with vascular risk factors including hypertension, diabetes, hypercholesterolemia and smoking (Haight et al., 2015; Tchistiakova et al., 2015; Sam et al., 2016b; Blair et al., 2020).
Strengths and Weaknesses
This review included foreign language papers (5/236), though one such paper was inaccessible. Most but not all of the required information was obtained during the data extraction. This might have added a bias to the results of this review: for example, description of the CVR processing and delay computation methods were not clear in 9% and 11% of the studies, respectively. Furthermore, the sample size of many studies was low (mean sample size: 35, 45/235 studies ≤ 10 participants), particularly in studies investigating repeatability and reproducibility of CVR values (mean: 16). This review was also restricted to human studies; therefore it does not provide a detailed description of preclinical CVR methods, although the main processing techniques are similar.
Conclusion
To our knowledge, this is the first systematic review to summarise and describe the diverse acquisition and analysis techniques used to measure CVR using MRI, and their applications in health and disease. While CVR-MRI is a relatively new and evolving technique we identified applications in several clinical populations including steno-occlusive and small vessel disease, highlighting the value of CVR measurements in medical research. However, acquisition techniques, analysis methods and definitions of CVR all varied substantially. Future work should target consensus recommendations to facilitate more reliable and harmonised CVR measurement for use in clinical research and trials of new therapies.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
ES performed the search, analysed the data, and prepared the manuscript. MT, JW, MS, and IM contributed to the work by discussing the eligibility and data extraction of some papers and by reviewing the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the Medical Research Council (MRC) and UK Dementia Research Institute (UK DRI) which receives its funding from DRI Ltd, funded by the UK Medical Research Council, Alzheimer's Society and Alzheimer's Research UK, the European Union Horizon 2020, PHC-03-15, project No 666881 SVDs@Target, the Fondation Leducq Transatlantic Network of Excellence for the Study of Perivascular Spaces in Small Vessel Disease, ref no. 16 CVD 05, and Scottish Chief Scientist Office through the NHS Lothian Research and Development Department. MT acknowledges financial support from the NHS Lothian Research and Development Office.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Xiaodi (Dillys) Liu, Tetiana Poliakova, and Manuel Deckart for their help in extracting the relevant information from foreign language papers.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphys.2021.643468/full#supplementary-material
References
Abi Zeid Daou, M., Boyd, B. D., Donahue, M. J., Albert, K., and Taylor, W. D. (2017). Frontocingulate cerebral blood flow and cerebrovascular reactivity associated with antidepressant response in late-life depression. J. Affect. Disord. 215, 103–110. doi: 10.1016/j.jad.2017.03.027
Al-Bachari, S., Parkes, L. M., Vidyasagar, R., Hanby, M. F., Tharaken, V., Leroi, I., et al. (2014). Arterial spin labelling reveals prolonged arterial arrival time in idiopathic parkinsons disease. NeuroImage: Clinical 6, 1–8. doi: 10.1016/j.nicl.2014.07.014
Amyot, F., Kenney, K., Moore, C., Haber, M., Turtzo, L. C., Shenouda, C., et al. (2018). Imaging of cerebrovascular function in chronic traumatic brain injury. J. Neurotrauma 35, 1116–1123. doi: 10.1089/neu.2017.5114
Anazodo, U. C., Shoemaker, J. K., Suskin, N., Ssali, T., Wang, D. J. J., and St. Lawrence, K. S. (2016). Impaired cerebrovascular function in coronary artery disease patients and recovery following cardiac rehabilitation. Front. Aging Neurosci. 7:224. doi: 10.3389/fnagi.2015.00224
Atwi, S., Shao, H., Crane, D. E., da Costa, L., Aviv, R. I., Mikulis, D. J., et al. (2019). BOLD-based cerebrovascular reactivity vascular transfer function isolates amplitude and timing responses to better characterize cerebral small vessel disease. NMR Biomed. 32:e4064. doi: 10.1002/nbm.4064
Bhogal, A. A., De Vis, J. B., Siero, J. C. W., Petersen, E. T., Luijten, P. R., Hendrikse, J., et al. (2016). The BOLD cerebrovascular reactivity response to progressive hypercapnia in young and elderly. NeuroImage 139, 94–102. doi: 10.1016/j.neuroimage.2016.06.010
Bhogal, A. A., Philippens, M. E. P., Siero, J. C. W., Fisher, J. A., Petersen, E. T., Luijten, P. R., et al. (2015). Examining the regional and cerebral depth-dependent bold cerebrovascular reactivity response at 7T. NeuroImage 114, 239–48. doi: 10.1016/j.neuroimage.2015.04.014
Bhogal, A. A., Siero, J. C. W., Fisher, J. A., Froeling, M., Luijten, P., Philippens, M., et al. (2014). Investigating the Non-linearity of the BOLD cerebrovascular reactivity response to targeted hypo/hypercapnia at 7T. NeuroImage 98, 296–305. doi: 10.1016/j.neuroimage.2014.05.006
Blair, G. W., Thrippleton, M. J., Shi, Y., Hamilton, I., Stringer, M., Chappell, F., et al. (2020). Intracranial hemodynamic relationships in patients with cerebral small vessel disease. Neurology 94, 2258–2269. doi: 10.1212/WNL.0000000000009483
Blockley, N. P., Driver, I. D., Francis, S. T., Fisher, J. A., and Gowland, P. A. (2011). An improved method for acquiring cerebrovascular reactivity maps. Magn. Reson. Med. 65, 1278–1286. doi: 10.1002/mrm.22719
Bokkers, R. P. H., Van Osch, M. J. P., Klijn, C. J. M., Kappelle, L. J., and Hendrikse, J. (2011). Cerebrovascular reactivity within perfusion territories in patients with an internal carotid artery occlusion. J. Neurol. Neurosurg. Psychiatry 82, 1011–1016. doi: 10.1136/jnnp.2010.233338
Bouvier, J., Detante, O., Tahon, F., Attye, A., Perret, T., Chechin, D., et al. (2015). Reduced CMRO2 and cerebrovascular reserve in patients with severe intracranial arterial stenosis: a combined multiparametric QBOLD oxygenation and BOLD FMRI study. Hum. Brain Mapp. 36, 695–706. doi: 10.1002/hbm.22657
Bright, M. G., Bianciardi, M., de Zwart, J. A., Murphy, K., and Duyn, J. H. (2014). Early anti-correlated BOLD signal changes of physiologic origin. NeuroImage 87, 287–296. doi: 10.1016/j.neuroimage.2013.10.055
Bright, M. G., Mazerolle, E. L., Sobczyk, O., Fan, A. P., Matthias, J. P., van Osch, C. I., et al. (2017). Clinical mapping of cerebrovascular reactivity using MRI: a framework for reaching consensus. Abstract retrieved from International Society for Mangetic Resonance in Medicine (abstract number 1666).
Bright, M. G., and Murphy, K. (2013). Reliable quantification of BOLD FMRI cerebrovascular reactivity despite poor breath-hold performance. NeuroImage 83, 559–68. doi: 10.1016/j.neuroimage.2013.07.007
Buterbaugh, J., Charles, W., Natalie, P., Daniel, C., Michael, G., and Sairam, P. (2015). Cerebrovascular reactivity in young subjects with sleep apnea. Sleep 38, 241–250. doi: 10.5665/sleep.4406
Callen, A. L., Dupont, S. M., Pyne, J., Talbott, J., Tien, P., Calabrese, E., et al. (2020). The regional pattern of abnormal cerebrovascular reactivity in HIV-infected, virally suppressed women. J. Neurovirol. 26, 734–742. doi: 10.1007/s13365-020-00859-8
Calviere, L., Catalaa, I., Marlats, F., Viguier, A., Bonneville, F., Cognard, C., et al. (2010). Correlation between cognitive impairment and cerebral hemodynamic disturbances on perfusion magnetic resonance imaging in european adults with moyamoya disease: clinical article. J. Neurosurg. 113, 753–759. doi: 10.3171/2010.4.JNS091808
Cantin, S. M., Villien, O., Moreaud, I., Tropres, S., Keignart, E., Chipon, J.-F., et al. (2011). Impaired cerebral vasoreactivity to CO2 in Alzheimers disease using BOLD FMRI. Neuroimage 58, 579–587. doi: 10.1016/j.neuroimage.2011.06.070
Catchlove, S. J., Parrish, T. B., Chen, Y., Macpherson, H., Hughes, M. E., and Pipingas, A. (2018). Regional cerebrovascular reactivity and cognitive performance in healthy aging. J. Exp. Neurosci. 12:1179069518785151. doi: 10.1177/1179069518785151
Champagne, A. A., Bhogal, A. A., Coverdale, N. S., Mark, C. I., and Cook, D. J. (2019a). A novel perspective to calibrate temporal delays in cerebrovascular reactivity using hypercapnic and hyperoxic respiratory challenges. NeuroImage 187, 154–165. doi: 10.1016/j.neuroimage.2017.11.044
Champagne, A. A., Coverdale, N. S., Fernandez-Ruiz, J., Mark, C. I., and Cook, D. J. (2020a). Compromised resting cerebral metabolism after sport-related concussion: a calibrated MRI study. Brain Imaging Behav. 15, 133–146. doi: 10.1007/s11682-019-00240-2
Champagne, A. A., Coverdale, N. S., Germuska, M., and Cook, D. J. (2019b). Multi-parametric analysis reveals metabolic and vascular effects driving differences in BOLD-based cerebrovascular reactivity associated with a history of sport concussion. Brain Injury 33, 1479–1489. doi: 10.1080/02699052.2019.1644375
Champagne, A. A., Coverdale, N. S., Nashed, J. Y., Fernandez-Ruiz, J., and Cook, D. J. (2019c). Resting CMRO2 fluctuations show persistent network hyper-connectivity following exposure to sub-concussive collisions. NeuroImage Clin. 22:101753. doi: 10.1016/j.nicl.2019.101753
Champagne, A. A., Coverdale, N. S., Ross, A., Chen, Y., Murray, C. I., Dubowitz, D., et al. (2020b). Multi-modal normalization of resting-state using local physiology reduces changes in functional connectivity patterns observed in mTBI patients. Neuroimage Clin. 26:102204. doi: 10.1016/j.nicl.2020.102204
Chang, T. Y., Kuan, W. C., Huang, K. L., Chang, C. H., Chang, Y. J., Wong, H. F., et al. (2013). Heterogeneous cerebral vasoreactivity dynamics in patients with carotid stenosis. PLoS ONE 8:e76072. doi: 10.1371/journal.pone.0076072
Chang, T. Y., Liu, H. L., Lee, T. H., Kuan, W. C., Chang, C. H., Wu, H. C., et al. (2009). Change in cerebral perfusion after carotid angioplasty with stenting is related to cerebral vasoreactivity: a study using dynamic susceptibility-weighted contrast-enhanced MR imaging and functional MR imaging with a breath-holding paradigm. Am. J. Neuroradiol. 30, 1330–1336. doi: 10.3174/ajnr.A1589
Chen, J. J. (2018). Cerebrovascular-reactivity mapping using MRI: considerations for Alzheimers disease. Front. Aging Neurosci. 10:170. doi: 10.3389/fnagi.2018.00170
Chen, J. J., and Pike, G. B. (2010). MRI measurement of the BOLD-specific flow–volume relationship during hypercapnia and hypocapnia in humans. Neuroimage 53, 383–391. doi: 10.1016/j.neuroimage.2010.07.003
Cohen, A. D., and Wang, Y. (2019). Improving the assessment of breath-holding induced cerebral vascular reactivity using a multiband multi-echo ASL/BOLD sequence. Sci. Rep. 9:5079. doi: 10.1038/s41598-019-41199-w
Conklin, J., Fierstra, J., Crawley, A. P., Han, J. S., Poublanc, J., Silver, F. L., et al. (2011). Mapping white matter diffusion and cerebrovascular reactivity in carotid occlusive disease. Neurology 77, 431–438. doi: 10.1212/WNL.0b013e318227b1e7
Coverdale, N. S., Fernandez-Ruiz, J., Champagne, A. A., Mark, C. I., and Cook, D. J. (2020). Co-localized impaired regional cerebrovascular reactivity in chronic concussion is associated with BOLD activation differences during a working memory task. Brain Imaging Behav. 14, 2438–2449. doi: 10.1007/s11682-019-00194-5
Da Costa, L., Fierstra, J., Fisher, J. A., Mikulis, D. J., Han, J. S., and Tymianski, M. (2014). BOLD MRI and early impairment of cerebrovascular reserve after aneurysmal subarachnoid hemorrhage. J. Magn. Reson. Imaging 40, 972–979. doi: 10.1002/jmri.24474
da Costa, L., van Niftrik, C. B., Crane, D., Fierstra, J., and Bethune, A. (2016). Temporal profile of cerebrovascular reactivity impairment, gray matter volumes, and persistent symptoms after mild traumatic head injury. Front. Neurol. 7:70. doi: 10.3389/fneur.2016.00070
De Vis, J. B., Bhogal, A. A., Hendrikse, J., Petersen, E. T., and Siero, J. C. W. (2018). Effect sizes of BOLD CVR, resting-state signal fluctuations and time delay measures for the assessment of hemodynamic impairment in carotid occlusion patients. NeuroImage 179, 530–539. doi: 10.1016/j.neuroimage.2018.06.017
De Vis, J. B., Hendrikse, J., Bhogal, A., Adams, A., Kappelle, L. J., and Petersen, E. T. (2015a). Age-related changes in brain hemodynamics; a calibrated MRI study. Hum. Brain Mapp. 36, 3973–3987. doi: 10.1002/hbm.22891
De Vis, J. B., Petersen, E. T., Bhogal, A., Hartkamp, N. S., Klijn, C. J. M., Kappelle, L. J., et al. (2015b). Calibrated MRI to evaluate cerebral hemodynamics in patients with an internal carotid artery occlusion. J. Cereb. Blood Flow Metab. 35, 1015–1023. doi: 10.1038/jcbfm.2015.14
Dengel, D. R., Evanoff, N. G., Marlatt, K. L., Geijer, J. R., Mueller, B. A., and Lim, K. O. (2017). Reproducibility of blood oxygen level-dependent signal changes with end-tidal carbon dioxide alterations. Clin. Physiol. Funct. Imaging 37, 794–798. doi: 10.1111/cpf.12358
Dlamini, N., Shah-Basak, P., Leung, J., Kirkham, F., Shroff, M., Kassner, A., et al. (2018). Breath-hold blood oxygen level-dependent MRI: a tool for the assessment of cerebrovascular reserve in children with Moyamoya disease. Am. J. Neuroradiol. 39, 1717–1723. doi: 10.3174/ajnr.A5739
Dlamini, N., Yau, I., Westmacott, R., Shroff, M., Armstrong, D., Logan, W., et al. (2017). Cerebrovascular reactivity and intellectual outcome in childhood stroke with transient cerebral arteriopathy. Pediat. Neurol. 69, 71–78. doi: 10.1016/j.pediatrneurol.2017.01.001
Donahue, M. J., Ayad, M., Moore, R., van Osch, M., Singer, R., Clemmons, P., et al. (2013). Relationships between hypercarbic reactivity, cerebral blood flow, and arterial circulation times in patients with moyamoya disease. J. Magn. Reson. Imaging 38, 1129–1139. doi: 10.1002/jmri.24070
Donahue, M. J., Dethrage, L. M., Faraco, C. C., Jordan, L. C., Clemmons, P., Singer, R., et al. (2014). Routine clinical evaluation of cerebrovascular reserve capacity using carbogen in patients with intracranial stenosis. Stroke 45, 2335–2341. doi: 10.1161/STROKEAHA.114.005975
Donahue, M. J., Strother, M. K., Lindsey, K. P., Hocke, L. M., Tong, Y., and Frederick, B. D. B. (2016). Time delay processing of hypercapnic FMRI allows quantitative parameterization of cerebrovascular reactivity and blood flow delays. J. Cereb. Blood Flow Metab. 36, 1767–1779. doi: 10.1177/0271678X15608643
Donahue, M. J., van Laar, P. J., van Zijl, P. C., Stevens, R. D., and Hendrikse, J. (2009). Vascular space occupancy (VASO) cerebral blood volume-weighted MRI identifies hemodynamic impairment in patients with carotid artery disease. J. Magn. Reson. Imag. 29, 718–724. doi: 10.1002/jmri.21667
Driver, I., Blockley, N., Fisher, J., Francis, S., and Gowland, P. (2010). The change in cerebrovascular reactivity between 3 T and 7 T measured using graded hypercapnia. Neuroimage 51, 274–279. doi: 10.1016/j.neuroimage.2009.12.113
Duffin, J., Sobczyk, O., Crawley, A., Poublanc, J., Venkatraghavan, L., Sam, K., et al. (2017). The role of vascular resistance in BOLD responses to progressive hypercapnia. Hum. Brain Mapp. 38, 5590–5602. doi: 10.1002/hbm.23751
Duffin, J., Sobczyk, O., Crawley, A. P., Poublanc, J., Mikulis, D. J., and Fisher, J. A. (2015). The dynamics of cerebrovascular reactivity shown with transfer function analysis. NeuroImage 114, 207–216. doi: 10.1016/j.neuroimage.2015.04.029
Duffin, J., Sobczyk, O., McKetton, L., Crawley, A., Poublanc, J., Venkatraghavan, L., et al. (2018). Cerebrovascular resistance: the basis of cerebrovascular reactivity. Front. Neurosci. 12:409. doi: 10.3389/fnins.2018.00409
Faraco, C. C., Strother, M. K., Dethrage, L. M., Jordan, L., Singer, R., Clemmons, P. F., et al. (2015). Dual echo vessel-encoded ASL for simultaneous BOLD and CBF reactivity assessment in patients with ischemic cerebrovascular disease. Magn. Reson. Med. 73, 1579–1592. doi: 10.1002/mrm.25268
Federau, C., Christensen, S., Zun, Z., Park, S.-W., Ni, W., Moseley, M., et al. (2017). Cerebral blood flow, transit time, and apparent diffusion coefficient in moyamoya disease before and after acetazolamide. Neuroradiology 59, 5–12. doi: 10.1007/s00234-016-1766-y
Fierstra, J., Conklin, J., Krings, T., Slessarev, M., Han, J. S., Fisher, J. A., et al. (2011a). Impaired peri-nidal cerebrovascular reserve in seizure patients with brain arteriovenous malformations. Brain 134, 100–109. doi: 10.1093/brain/awq286
Fierstra, J., Spieth, S., Tran, L., Conklin, J., Tymianski, M., ter Brugge, K. G., et al. (2011b). Severely impaired cerebrovascular reserve in patients with cerebral proliferative angiopathy. J. Neurosurg. Pediat. 8, 310–315. doi: 10.3171/2011.6.PEDS1170
Fierstra, J., van Niftrik, C., Piccirelli, M., Bozinov, O., Pangalu, A., Krayenbuhl, N., et al. (2018a). Diffuse Gliomas Exhibit Whole Brain Impaired Cerebrovascular Reactivity. Magn. Reson. Imaging 45, 78–83. doi: 10.1016/j.mri.2017.09.017
Fierstra, J., van Niftrik, C., Warnock, G., Wegener, S., Piccirelli, M., Pangalu, A., et al. (2018b). Staging hemodynamic failure with blood oxygen-level-dependent functional magnetic resonance imaging cerebrovascular reactivity: a comparison versus gold standard (15O-)H2O-positron emission tomography. Stroke 49, 621–629. doi: 10.1161/STROKEAHA.117.020010
Fierstra, J. O., Sobczyk, A., Battisti-Charbonney, D. M., Mandell, J., Poublanc, A. P., Crawley, D. J., et al. (2013). Measuring cerebrovascular reactivity: what stimulus to use? J. Physiol. 591, 5809–5821. doi: 10.1113/jphysiol.2013.259150
Fisher, J. A., Sobczyk, O., Crawley, A., Poublanc, J., Dufort, P., Venkatraghavan, L., et al. (2017). Assessing cerebrovascular reactivity by the pattern of response to progressive hypercapnia. Hum. Brain Mapp. 38, 3415–3427. doi: 10.1002/hbm.23598
Fisher, J. A., Venkatraghavan, L., and Mikulis, D. J. (2018). Magnetic resonance imaging–based cerebrovascular reactivity and hemodynamic reserve. Stroke 49, 2011–2018. doi: 10.1161/STROKEAHA.118.021012
Frosch, O. H., Yau, P. L., Osorio, R. S., Rusinek, H., Storey, P., and Convit, A. (2017). Insulin resistance among obese middle-aged is associated with decreased cerebrovascular reactivity. Neurology 89, 249–255. doi: 10.1212/WNL.0000000000004110
Gao, Y. Z., Zhang, J. J., Liu, H., Wu, G. Y., Xiong, L., and Shu, M. (2013). Regional cerebral blood flow and cerebrovascular reactivity in alzheimers disease and vascular dementia assessed by arterial spinlabeling magnetic resonance imaging. Curr. Neurovasc. Res. 10, 49–53. doi: 10.2174/156720213804806016
Geranmayeh, F., Wise, R. J. S., Leech, R., and Murphy, K. (2015). Measuring vascular reactivity with breath-holds after stroke: a method to aid interpretation of group-level BOLD signal changes in longitudinal FMRI studies. Hum. Brain Mapp. 36, 1755–1771. doi: 10.1002/hbm.22735
Germuska, M., Chandler, H. L., Stickland, R. C., Foster, C., Fasano, F., Okell, T. W., et al. (2019). Dual-calibrated FMRI measurement of absolute cerebral metabolic rate of oxygen consumption and effective oxygen diffusivity. NeuroImage 184, 717–728. doi: 10.1016/j.neuroimage.2018.09.035
Germuska, M., Merola, A., Murphy, K., Babic, A., Richmond, L., Khot, S., et al. (2016). A forward modelling approach for the estimation of oxygen extraction fraction by calibrated FMRI. Neuroimage 139, 313–323. doi: 10.1016/j.neuroimage.2016.06.004
Germuska, M., and Wise, R. G. (2019). Calibrated FMRI for mapping absolute CMRO2: practicalities and prospects. NeuroImage Physiol. Quant. MRI 187, 145–153. doi: 10.1016/j.neuroimage.2018.03.068
Golestani, A. M., Wei, L. L., and Chen, J. J. (2016). Quantitative mapping of cerebrovascular reactivity using resting-state BOLD FMRI: validation in healthy adults. NeuroImage 138, 147–163. doi: 10.1016/j.neuroimage.2016.05.025
Gonçalves, M. R., Sean Peter, J., Rajiv, R., Mark, F., Lythgoe, R., Barbara, P., et al. (2017). The effect of imatinib therapy on tumour cycling hypoxia, tissue oxygenation and vascular reactivity. Wellcome Open Research 2:38. doi: 10.12688/wellcomeopenres.11715.1
Goode, S. D., Altaf, N., Auer, D. P., and MacSweeney, S. T. R. (2009). Carotid endarterectomy improves cerebrovascular reserve capacity preferentially in patients with preoperative impairment as indicated by asymmetric BOLD response to hypercapnia. Eur. J. Vasc. Endovasc. Surg. 38, 546–551. doi: 10.1016/j.ejvs.2009.06.010
Goode, S. D., Altaf, N., Munshi, S., MacSweeney, S. T. R., and Auer, D. P. (2016). Impaired cerebrovascular reactivity predicts recurrent symptoms in patients with carotid artery occlusion: a hypercapnia BOLD FMRI study. Am. J. Neuroradiol. 37, 904–909. doi: 10.3174/ajnr.A4739
Grandin, C. B., Bol, A., Smith, A. M., Michel, C., and Cosnard, G. (2005). Absolute CBF and CBV measurements by MRI bolus tracking before and after acetazolamide challenge: repeatabilily and comparison with PET in humans. Neuroimage 26, 525–535. doi: 10.1016/j.neuroimage.2005.02.028
Griffiths, P. D., Gaines, P., Cleveland, T., Beard, J., Venables, G., and Wilkinson, I. D. (2005). Assessment of cerebral haemodynamics and vascular reserve in patients with symptomatic carotid artery occlusion: an integrated MR method. Neuroradiology 47, 175–182. doi: 10.1007/s00234-005-1362-z
Guckel, F., Brix, G., Schmiedek, P., Piepgras, A., Rempp, K., Kopke, J., et al. (1995). Noninvasive quantification of regional cerebral blood flow and blood volume with dynamic MR-imaging. Preliminary results in volunteers and patients with cerebrovascular disorders. Radiologe 35, 791–800.
Haight, T. J., Nick Bryan, R., Erus, G., Davatzikos, C., Jacobs, D. R., DEsposito, M., et al. (2015). Vascular risk factors, cerebrovascular reactivity, and the default-mode brain network. Neuroimage 115, 7–16. doi: 10.1016/j.neuroimage.2015.04.039
Halani, S., Kwinta, J. B., Golestani, A. M., Khatamian, Y. B., and Chen, J. J. (2015). Comparing cerebrovascular reactivity measured using BOLD and cerebral blood flow MRI: the effect of basal vascular tension on vasodilatory and vasoconstrictive reactivity. NeuroImage 110, 110–23. doi: 10.1016/j.neuroimage.2015.01.050
Haller, S., Bonati, L. H., Rick, J., Klarhoafer, M., Speck, O., Lyrer, P. A., et al. (2008). Reduced cerebrovascular reserve at CO2 BOLD MR imaging is associated with increased risk of periinterventional ischemic lesions during carotid endarterectomy or stent placement: preliminary results. Radiology 249, 251–258. doi: 10.1148/radiol.2491071644
Hamzei, F., Knab, R., Weiller, C., and Rother, J. (2003). The influence of extra- and intracranial artery disease on the BOLD signal in FMRI. Neuroimage 20, 1393–1399. doi: 10.1016/S1053-8119(03)00384-7
Han, J. S., Abou-Hamden, A., Mandell, D. M., Poublanc, J., Crawley, A. P., Fisher, J. A., et al. (2011a). Impact of extracranial-intracranial bypass on cerebrovascular reactivity and clinical outcome in patients with symptomatic moyamoya vasculopathy. Stroke 42, 3047–3054. doi: 10.1161/STROKEAHA.111.615955
Han, J. S., Mikulis, D. J., Mardimae, A., Kassner, A., Poublanc, J., Crawley, A. P., et al. (2011b). Measurement of cerebrovascular reactivity in pediatric patients with cerebral vasculopathy using blood oxygen level-dependent MRI. Stroke 42, 1261–1269. doi: 10.1161/STROKEAHA.110.603225
Hanby, M. F., Al-Bachari, S., Makin, F., Vidyasagar, R., Parkes, L. M., and Emsley, H. C. (2015). Structural and physiological MRI correlates of occult cerebrovascular disease in late-onset epilepsy. NeuroImage Clin. 9, 128–133. doi: 10.1016/j.nicl.2015.07.016
Hare, H. V., Germuska, M., Kelly, M. E., and Bulte, D. P. (2013). Comparison of CO2 in air versus carbogen for the measurement of cerebrovascular reactivity with magnetic resonance imaging. J. Cereb. Blood Flow Metab. 33, 1799–1805. doi: 10.1038/jcbfm.2013.131
Hartkamp, N. S., Bokkers, R. P. H., van Osch, M. J. P., de Borst, G. J., and Hendrikse, J. (2017). Cerebrovascular reactivity in the caudate nucleus, lentiform nucleus and thalamus in patients with carotid artery disease. J. Neuroradiol. 44, 143–150. doi: 10.1016/j.neurad.2016.07.003
Hartkamp, N. S., Hendrikse, J., de Borst, G. J., Kappelle, L. J., and Bokkers, R. P. H. (2019). Intracerebral steal phenomenon in symptomatic carotid artery disease. J. Neuroradiol. 46, 173–178. doi: 10.1016/j.neurad.2018.09.008
Hartkamp, N. S., Hendrikse, J., van der Worp, H. B., de Borst, G. J., and Bokkers, R. P. (2012). Time course of vascular reactivity using repeated phase-contrast mr angiography in patients with carotid artery stenosis. Stroke 43, 553–556. doi: 10.1161/STROKEAHA.111.637314
Hartkamp, N. S., Petersen, E. T., Chappell, M. A., Okell, T. W., Uyttenboogaart, M., Zeebregts, C. J., et al. (2018). Relationship between haemodynamic impairment and collateral blood flow in carotid artery disease. J. Cereb. Blood Flow Metabo. 38, 2021–2032. doi: 10.1177/0271678X17724027
Hauser, T. K., Seeger, A., Bender, B., Klose, U., Thurow, J., Ernemann, U., et al. (2019). Hypercapnic BOLD MRI compared to H215O PET/CT for the hemodynamic evaluation of patients with moyamoya disease. NeuroImage Clin. 22:101713. doi: 10.1016/j.nicl.2019.101713
Heijtel, D. F., Mutsaerts, H. J., Bakker, E., Schober, P., Stevens, M. F., Petersen, E. T., et al. (2014). Accuracy and precision of pseudo-continuous arterial spin labeling perfusion during baseline and hypercapnia: a head-to-head comparison with 15O H2O positron emission tomography. NeuroImage 92, 182–192. doi: 10.1016/j.neuroimage.2014.02.011
Herrera, C. R., Beltramini, G. C., Avelar, W. M., Lima, F. O., and Li, L. M. (2016). Cerebral vasomotor reactivity assessment using transcranial doppler and MRI with apnea test. Braz. J. Med. Biol. Res. 49:e5437. doi: 10.1590/1414-431x20165437
Holmes, K. R., Tang-Wai, D., Sam, K., Mcketton, L., Poublanc, J., Crawley, A. P., et al. (2020). Slowed temporal and parietal cerebrovascular response in patients with Alzheimers disease. Can. J. Neurol. Sci. 47, 366–373. doi: 10.1017/cjn.2020.30
Hou, X., Liu, P., Li, Y., Jiang, D., De Vis, J. B., Lin, Z., et al. (2019). The association between BOLD-based cerebrovascular reactivity (CVR) and end-tidal CO2 in healthy subjects. Neuroimage. 207:116365. doi: 10.1016/j.neuroimage.2019.116365
Hsu, Y.-Y., Chang, C.-N., Jung, S.-M., Lim, K.-E., Huang, J.-C., Fang, S.-Y., et al. (2004). Blood oxygenation level-dependent MRI of cerebral gliomas during breath holding. J. Magn. Reson. Imaging 19, 160–167. doi: 10.1002/jmri.10447
Hu, H. H., Li, Z., Pokorney, A. L., Chia, J. M., Stefani, N., Pipe, J. G., et al. (2017). Assessment of cerebral blood perfusion reserve with acetazolamide using 3D spiral ASL MRI: preliminary experience in pediatric patients. Magn. Reson. Imaging 35, 132–140. doi: 10.1016/j.mri.2016.08.019
Inoue, Y., Tanaka, Y., Hata, H., and Hara, T. (2014). Arterial spin-labeling evaluation of cerebrovascular reactivity to acetazolamide in healthy subjects. Am. J. Neuroradiol. 35, 1111–1116. doi: 10.3174/ajnr.A3815
Jahanian, H., Christen, T., Moseley, M. E., Pajewski, N. M., Wright, C. B., Tamura, M. K., et al. (2017). Measuring vascular reactivity with resting-state Blood Oxygenation Level-Dependent (BOLD) signal fluctuations: a potential alternative to the breath-holding challenge?. J. Cereb. Blood Flow Metabo. 37, 2526–2538. doi: 10.1177/0271678X16670921
Jefferson, A. L., Liu, D., Gupta, D. K., Pechman, K. R., Watchmaker, J. M., Gordon, E. A., et al. (2017). Lower cardiac index levels relate to lower cerebral blood flow in older adults. Neurology 89, 2327–2334. doi: 10.1212/WNL.0000000000004707
Joo, I. L., Lai, A. Y., Bazzigaluppi, P., Koletar, M. M., Dorr, A., Brown, M. E., et al. (2017). Early neurovascular dysfunction in a transgenic rat model of Alzheimers disease. Sci. Rep. 7:46427. doi: 10.1038/srep46427
Joutel, A., Monet-Leprêtre, M., Gosele, C., Baron-Menguy, C., Hammes, A., Schmidt, S., et al. (2010). Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J. Clin. Invest. 120, 433–445. doi: 10.1172/JCI39733
Kannurpatti, S. S., Motes, M. A., Biswal, B. B., and Rypma, B. (2014). Assessment of unconstrained cerebrovascular reactivity marker for large age-range FMRI studies. PLoS ONE 9:e88751. doi: 10.1371/journal.pone.0088751
Kario, K., Ishikawa, J., Hoshide, S., Matsui, Y., Morinari, M., Eguchi, K., et al. (2005). Diabetic brain damage in hypertension: role of renin-angiotensin system. Hypertension 45, 887–893. doi: 10.1161/01.HYP.0000163460.07639.3f
Kassner, A., Winter, J. D., Poublanc, J., Mikulis, D. J., and Crawley, A. P. (2010). Blood-oxygen level dependent mri measures of cerebrovascular reactivity using a controlled respiratory challenge: reproducibility and gender differences. J. Magn. Reson. Imaging 31, 298–304. doi: 10.1002/jmri.22044
Kim, H. J., Kim, T. W., Ryu, S.-Y., Yang, P. S., Kwon, M. J., Kim, J. C., et al. (2011). Acetazolamide-challenged perfusion magnetic resonance imaging for assessment of cerebrovascular reserve capacity in patients with symptomatic middle cerebral artery stenosis: comparison with technetium-99m-hexamethylpropyleneamine oxime single-photon emission computed tomography. Clin. Imaging 35, 413–420. doi: 10.1016/j.clinimag.2011.03.001
Kisler, K., Nelson, A. R., Rege, S. V., Ramanathan, A., Wang, Y., Ahuja, A., et al. (2017). Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat. Neurosci. 20, 406–416. doi: 10.1038/nn.4489
Kosinski, P. D., Croal, P. L., Leung, J., Williams, S., Odame, I., Hare, G. M., et al. (2017). The severity of anaemia depletes cerebrovascular dilatory reserve in children with sickle cell disease: a quantitative magnetic resonance imaging study. Br. J. Haematol. 176, 280–287. doi: 10.1111/bjh.14424
Krainik, A., Hund-Georgiadis, M., Zysset, S., and Von Cramon, D. Y. (2005). Regional impairment of cerebrovascular reactivity and BOLD signal in adults after stroke. Stroke 36, 1146–1152. doi: 10.1161/01.STR.0000166178.40973.a7
Ladner, T. R., Donahue, M. J., Arteaga, D. F., Faraco, C. C., Roach, B. A., Davis, L. T., et al. (2017). Prior Infarcts, Reactivity, and Angiography in Moyamoya Disease (PIRAMD): a scoring system for moyamoya severity based on multimodal hemodynamic imaging. J. Neurosurg. 126, 495–503. doi: 10.3171/2015.11.JNS15562
Lajoie, I., Nugent, S., Debacker, C., Dyson, K., Tancredi, F. B., Badhwar, A., et al. (2017). Application of calibrated FMRI in Alzheimers disease. NeuroImage Clin. 15, 348–358. doi: 10.1016/j.nicl.2017.05.009
Lake, E. M., Bazzigaluppi, P., and Stefanovic, B. (2016). Functional magnetic resonance imaging in chronic ischaemic stroke. Philos. Trans. R Soc. Lond. B Biol. Sci. 371:20150353. doi: 10.1098/rstb.2015.0353
Leoni, R. F., Mazzeto-Betti, K. C., Andrade, K. C., and de Araujo, D. B. (2008). Quantitative evaluation of hemodynamic response after hypercapnia among different brain territories by FMRI. Neuroimage 41, 1192–1198. doi: 10.1016/j.neuroimage.2008.03.035
Leoni, R. F., Oliveira, I. A. F., Pontes-Neto, O. M., Santos, A. C., and Leite, J. P. (2017). Cerebral blood flow and vasoreactivity in aging: an arterial spin labeling study. Braz. J. Med. Biol. Res. 50:e5670. doi: 10.1590/1414-431x20175670
Leung, J., Duffin, J., Fisher, J. A., and Kassner, A. (2016a). MRI-based cerebrovascular reactivity using transfer function analysis reveals temporal group differences between patients with sickle cell disease and healthy controls. NeuroImage Clin. 12, 624–630. doi: 10.1016/j.nicl.2016.09.009
Leung, J., Kim, J. A., and Kassner, A. (2016b). Reproducibility of cerebrovascular reactivity measures in children using BOLD MRI. J. Magn. Reson. Imaging 43, 1191–1195. doi: 10.1002/jmri.25063
Leung, J., Kosinski, P. D., Croal, P. L., and Kassner, A. (2016c). Developmental trajectories of cerebrovascular reactivity in healthy children and young adults assessed with magnetic resonance imaging. J. Physiol. 594, 2681–2689. doi: 10.1113/JP271056
Liem, M. K., Lesnik Oberstein, S. A. J., Haan, J., Boom, R. V. D., Ferrari, M. D., Buchem, M. A. V., et al. (2009). Cerebrovascular reactivity is a main determinant of white matter hyperintensity progression in CADASIL. Am. J. Neuroradiol. 30, 1244–1247. doi: 10.3174/ajnr.A1533
Liu, H.-L., Huang, J.-C., Wu, C.-T., and Hsu, Y.-Y. (2002). Detectability of blood oxygenation level-dependent signal changes during short breath hold duration. Magn. Reson. Imaging 20, 643–648. doi: 10.1016/S0730-725X(02)00595-7
Liu, P., De Vis, J. B., and Lu, H. (2019). Cerebrovascular reactivity (CVR) MRI with CO2 challenge: a technical review. Neuroimage 187, 104–115. doi: 10.1016/j.neuroimage.2018.03.047
Liu, P., Hebrank, A. C., Rodrigue, K. M., Kennedy, K. M., Section, J., Park, D. C., et al. (2013). Age-related differences in memory-encoding fmri responses after accounting for decline in vascular reactivity. Neuroimage 78, 415–425. doi: 10.1016/j.neuroimage.2013.04.053
Liu, P., Li, Y., Pinho, M., Park, D. C., Welch, B. G., and Lu, H. (2017a). Cerebrovascular reactivity mapping without gas challenges. NeuroImage 146, 320–26. doi: 10.1016/j.neuroimage.2016.11.054
Liu, P., Welch, B. G., Li, Y., Gu, H., King, D., Yang, Y., et al. (2017b). Multiparametric imaging of brain hemodynamics and function using gas-inhalation MRI. NeuroImage 146, 715–723. doi: 10.1016/j.neuroimage.2016.09.063
Lu, H., Liu, P., Yezhuvath, U., Cheng, Y., Marshall, O., and Ge, Y. (2014). MRI mapping of cerebrovascular reactivity via gas inhalation challenges. J. Visual. Exp. 17:52306. doi: 10.3791/52306
Lynch, C. E., Maxwell, E., Moustafa, A., Matilde, B., Scott, F., Benoit, M., Nicole, S., et al. (2020). Impairment of cerebrovascular reactivity in response to hypercapnic challenge in a mouse model of repetitive mild traumatic brain injury. J. Cereb. Blood Flow Metab. 13:271678X20954015. doi: 10.1177/0271678X20954015
Ma, J., Mehrkens, J. H., Holtmannspoetter, M., Linke, R., Schmid-Elsaesser, R., Steiger, H.-J., et al. (2007). Perfusion MRI before and after acetazolamide administration for assessment of cerebrovascular reserve capacity in patients with symptomatic internal carotid artery (ICA) occlusion: comparison with 99mTc-ECD SPECT. Neuroradiology 49, 317–326. doi: 10.1007/s00234-006-0193-x
Mandell, D. M., Han, J. S., Poublanc, J., Crawley, A. P., Fierstra, J., and Tymianski, M. (2011). Quantitative measurement of cerebrovascular reactivity by blood oxygen level-dependent mr imaging in patients with intracranial stenosis: preoperative cerebrovascular reactivity predicts the effect of extracranial-intracranial bypass surgery. AJNR Am. J. Neuroradiol. 32, 721–727. doi: 10.3174/ajnr.A2365
Mandell, D. M., Han, J. S., Poublanc, J., Crawley, A. P., Kassner, A., Fisher, J. A., et al. (2008a). Selective reduction of blood flow to white matter during hypercapnia corresponds with leukoaraiosis. Stroke 39, 1993–1998. doi: 10.1161/STROKEAHA.107.501692
Mandell, D. M., Han, J. S., Poublanc, J., Crawley, A. P., Stainsby, J. A., Fisher, J. A., et al. (2008b). Mapping cerebrovascular reactivity using blood oxygen level-dependent MRI in patients with arterial steno-occlusive disease: comparison with arterial spin labeling MRI. Stroke 39, 2021–2028. doi: 10.1161/STROKEAHA.107.506709
Marion, D., and Gerrit, B. (1991). The use of stable xenon-enhanced computed tomographic studies of cerebral blood flow to define changes in cerebral carbon dioxide vasoresponsivity caused by a severe head injury. Neurosurgery 29, 869–873. doi: 10.1227/00006123-199112000-00011
McDonnell, M. N., Berry, N. M., Cutting, M. A., Keage, H. A., Buckley, J. D., and Howe, P. R. (2013). Transcranial Doppler ultrasound to assess cerebrovascular reactivity: reliability, reproducibility and effect of posture. PeerJ 1:e65. doi: 10.7717/peerj.65
McKetton, L., Cohn, M., Tang-Wai, D. F., Sobczyk, O., Duffin, J., Holmes, K. R., et al. (2019). Cerebrovascular resistance in healthy aging and mild cognitive impairment. Front. Aging Neurosci. 11:79. doi: 10.3389/fnagi.2019.00079
McKetton, L., Sobczyk, O., Duffin, J., Poublanc, J., Sam, K., Crawley, A. P., et al. (2018). The aging brain and cerebrovascular reactivity. NeuroImage 181, 132–41. doi: 10.1016/j.neuroimage.2018.07.007
Merola, A., Germuska, M. A., Murphy, K., and Wise, R. G. (2018). Assessing the repeatability of absolute CMRO2, OEF and haemodynamic measurements from calibrated FMRI. NeuroImage 173, 113–26. doi: 10.1016/j.neuroimage.2018.02.020
Merola, A., Germuska, M. A., Warnert, E. A., Richmond, L., Helme, D., Khot, S., et al. (2017). Mapping the pharmacological modulation of brain oxygen metabolism: the effects of caffeine on absolute CMRO2 measured using dual calibrated FMRI. Neuroimage 155, 331–343. doi: 10.1016/j.neuroimage.2017.03.028
Metzger, A., Le Bars, E., Deverdun, J., Molino, F., Maréchal, B., Picot, M. C., et al. (2018). Is impaired cerebral vasoreactivity an early marker of cognitive decline in multiple sclerosis patients?. Eur. Radiol. 28, 1204–1214. doi: 10.1007/s00330-017-5068-5
Miller, K. B., Howery, A. J., Rivera-Rivera, L. A., Johnson, S. C., Rowley, H. A., Wieben, O., et al. (2019). Age-related reductions in cerebrovascular reactivity using 4D flow MRI. Front. Aging Neurosci. 11:281. doi: 10.3389/fnagi.2019.00281
Moreton, F. C., Dani, K. A., Goutcher, C., O'Hare, K., and Muir, K. W. (2016). Respiratory challenge MRI: practical aspects. NeuroImage Clin. 11, 667–677. doi: 10.1016/j.nicl.2016.05.003
Morgan, A. G., Thrippleton, M. J., Wardlaw, J. M., and Marshall, I. (2020). 4D flow MRI for non-invasive measurement of blood flow in the brain: a systematic review. J. Cereb. Blood Flow Metab. 41, 206–218. doi: 10.1177/0271678X20952014
Mutch, W. A., Ellis, M. J., Ryner, L. N., Graham, M. R., Dufault, B., Gregson, B., et al. (2016a). Brain magnetic resonance imaging CO2 stress testing in adolescent postconcussion syndrome. J. Neurosurg. 125, 648–660. doi: 10.3171/2015.6.JNS15972
Mutch, W. A. C., Ellis, M. J., Ryner, L. N., McDonald, P. J., Morissette, M. P., Pries, P., et al. (2018). Patient-specific alterations in CO2 cerebrovascular responsiveness in acute and sub-acute sports-related concussion. Front. Neurol. 9:23. doi: 10.3389/fneur.2018.00023
Mutch, W. A. C., Ellis, M. J., Ryner, L. N., Morissette, M. P., Pries, P. J., Dufault, B., et al. (2016b). Longitudinal brain magnetic resonance imaging CO2 stress testing in individual adolescent sports-related concussion patients: a pilot study. Front. Neurol. 7:107. doi: 10.3389/fneur.2016.00107
Mutch, W. A. C., Mandell, D. M., Fisher, J. A., Mikulis, D. J., Crawley, A. P., Pucci, O., et al. (2012). Approaches to brain stress testing: BOLD magnetic resonance imaging with computer-controlled delivery of carbon dioxide. PLoS ONE 7:e47443. doi: 10.1371/journal.pone.0047443
Noguchi, T., Kawashima, M., Nishihara, M., Egashira, Y., Azama, S., and Irie, H. (2015). noninvasive method for mapping CVR in moyamoya disease using ASL-MRI. Eur. J. Radiol. 84, 1137–1143. doi: 10.1016/j.ejrad.2015.03.011
Noth, U., Futoshi, K., Ralf, D., Robert, T., and Douglas, R. C. (2008). Mapping of the cerebral vascular response to hypoxia and hypercapnia using quantitative perfusion MRI at 3 T. NMR Biomed. 21, 464–472. doi: 10.1002/nbm.1210
Noth, U., Meadows, G. E., Kotajima, F., Deichmann, R., Corfield, D. R., and Turner, R. (2006). Cerebral vascular response to hypercapnia: determination with perfusion MRI at 1.5 and 3.0 tesla using a pulsed arterial spin labeling technique. J. Magn. Reson. Imaging 24, 1229–1235. doi: 10.1002/jmri.20761
Ogasawara, K., Ito, H., Sasoh, M., Okuguchi, T., Kobayashi, M., Yukawa, H., et al. (2003). Quantitative measurement of regional cerebrovascular reactivity to acetazolamide using 123I-N-Isopropyl-p-Iodoamphetamine autoradiography with SPECT: validation study using H215O with PET. J. Nucl. Med. 44, 520–525. Available online at: https://jnm.snmjournals.org/content/44/4/520
Ohnishi, T., Nakano, S., Yano, T., Hoshi, H., Jinnouchi, S., Nagamachi, S., et al. (1996). Susceptibility-weighted MR for evaluation of vasodilatory capacity with acetazolamide challenge. Am. J. Neuroradiol. 17, 631–637.
Papassin, J., Heck, O., Condamine, E., Pietras, J., Detante, O., and Krainik, A. (2020). Impaired cerebrovascular reactivity is associated with recurrent stroke in patients with severe intracranial arterial stenosis: a C02 BOLD FMRI study. J. Neuroradiol. doi: 10.1016/j.neurad.2020.04.005. [Epub ahead of print].
Para, A. E., Sam, K., Poublanc, J., Fisher, J. A., Crawley, A. P., and Mikulis, D. J. (2017). Invalidation of FMRI experiments secondary to neurovascular uncoupling in patients with cerebrovascular disease. J. Magn. Reson. Imaging 46, 1448–1455. doi: 10.1002/jmri.25639
Pelizzari, L., Lagana, M. M., Rossetto, F., Bergsland, N., Galli, M., Baselli, G., et al. (2019). Cerebral blood flow and cerebrovascular reactivity correlate with severity of motor symptoms in Parkinsons disease. Therap. Adv. Neurol. Disord. 12:1756286419838354. doi: 10.1177/1756286419838354
Peng, S.-L., Yang, H.-C., Chen, C.-M., and Shih, C.-T. (2020). Short- and long-term reproducibility of BOLD signal change induced by breath-holding at 1.5 and 3 T. NMR Biomed. 33:e4195. doi: 10.1002/nbm.4195
Petersson, J., and Glenny, R. W. (2014). Gas exchange and ventilation–perfusion relationships in the lung. Eur. Resp. J. 44, 1023–1041. doi: 10.1183/09031936.00037014
Petrella, J. R., DeCarli, C., Dagli, M., Grandin, C. B., Duyn, J. H., Frank, J. A., et al. (1998). Age-related vasodilatory response to acetazolamide challenge in healthy adults: a dynamic contrast-enhanced MR study. Am. J. Neuroradiol. 19:39–44.
Piepgras, A., Gückel, F., Lämmler, B., Weigel, R., and Schmiedek, P. (1994). [Noninvasive diagnosis of cerebral ischemia with nuclear magnetic resonance tomography and near infrared spectroscopy]. Nichtinvasive Diagnostik Zerebraler Ischamie Mit Kernspintomographie Und Nahe-Infrarotspektroskopie 34, 627–631.
Pillai, J. J., and Mikulis, D. J. (2015). Cerebrovascular reactivity mapping: an evolving standard for clinical functional imaging. Am. J. Neuroradiol. 36, 7–13. doi: 10.3174/ajnr.A3941
Pillai, J. J., and Zaca, D. (2012). Comparison of BOLD cerebrovascular reactivity mapping and DSC MR perfusion imaging for prediction of neurovascular uncoupling potential in brain tumors. Technol. Cancer Res. Treat. 11, 361–374. doi: 10.7785/tcrt.2012.500284
Ponsaing, L. B., Lindberg, U., Rostrup, E., Iversen, H. K., Larsson, H. B. W., and Jennum, P. (2018). Impaired cerebrovascular reactivity in obstructive sleep apnea: a case-control study. Sleep Med. 43, 7–13. doi: 10.1016/j.sleep.2017.10.010
Poublanc, J., Crawley, A. P., Sobczyk, O., Montandon, G., Sam, K., Mandell, D. M., et al. (2015). Measuring cerebrovascular reactivity: the dynamic response to a step hypercapnic stimulus. J. Cereb. Blood Flow Metab. 35, 1746–1756. doi: 10.1038/jcbfm.2015.114
Poublanc, J., Han, J. S., Mandell, D. M., Conklin, J., Stainsby, J. A., Fisher, J. A., et al. (2013). Vascular steal explains early paradoxical blood oxygen level-dependent cerebrovascular response in brain regions with delayed arterial transit times. Cerebrovasc. Dis. Extra. 3, 55–64. doi: 10.1159/000348841
Prisman, E., Slessarev, M., Han, J., Poublanc, J., Mardimae, A., Crawley, A., et al. (2008). Comparison of the effects of independently-controlled end-tidal PCO2 and PO2 on Blood Oxygen Level–Dependent (BOLD) MRI. J. Magn. Reson. Imaging 27, 185–191. doi: 10.1002/jmri.21102
Purkayastha, S., and Farzaneh, S. (2012). Transcranial doppler ultrasound: technique and application. Semin. Neurol. 32, 411–420. doi: 10.1055/s-0032-1331812
Raut, R. V., Nair, V. A., Sattin, J. A., and Prabhakaran, V. (2016). Hypercapnic evaluation of vascular reactivity in healthy aging and acute stroke via functional MRI. NeuroImage Clin. 12, 173–179. doi: 10.1016/j.nicl.2016.06.016
Ravi, H., Liu, P., Peng, S.-L., Liu, H., and Lu, H. (2016a). Simultaneous Multi-Slice (SMS) acquisition enhances the sensitivity of hemodynamic mapping using gas challenges. NMR Biomed. 29, 1511–1518. doi: 10.1002/nbm.3600
Ravi, H., Thomas, B. P., Peng, S. L, Hanli, L., and Hanzhang, L. (2016b). On the optimization of imaging protocol for the mapping of cerebrovascular reactivity. J. Magn. Reson. Imaging 43, 661–668. doi: 10.1002/jmri.25028
Richiardi, J., Monsch, A. U., Haas, T., Barkhof, F., Van de Ville, D., Radu, E. W., et al. (2015). Altered cerebrovascular reactivity velocity in mild cognitive impairment and Alzheimers disease. Neurobiol. Aging 36, 33–41. doi: 10.1016/j.neurobiolaging.2014.07.020
Riecker, A., Grodd, W., Klose, U., Schulz, J. B., Groschel, K., Erb, M., et al. (2003). Relation between regional functional MRI activation and vascular reactivity to carbon dioxide during normal aging. J. Cereb. Blood Flow Metab. 23, 565–573. doi: 10.1097/01.WCB.0000056063.25434.04
Rodan, L. H., Poublanc, J., Fisher, J. A., Sobczyk, O., Wong, T., Hlasny, E., et al. (2015). Cerebral hyperperfusion and decreased cerebrovascular reactivity correlate with neurologic disease severity in MELAS. Mitochondrion 22, 66–74. doi: 10.1016/j.mito.2015.03.002
Rosen, C., McKetton, L., Russell, J., Sam, K., Poublanc, J., Crawley, A., et al. (2018). Long-term changes in cerebrovascular reactivity following EC-IC bypass for intracranial steno-occlusive disease. J. Clin. Neurosci. 54, 77–82. doi: 10.1016/j.jocn.2018.06.009
Ryan, C. M., Battisti-Charbonney, A., Sobczyk, O., Mikulis, D. J., Duffin, J., Fisher, J. A., et al. (2018). Evaluation of cerebrovascular reactivity in subjects with and without obstructive sleep apnea. J. Stroke Cerebrovasc. Dis. 27, 162–168. doi: 10.1016/j.jstrokecerebrovasdis.2017.08.015
Sam, K., Conklin, J., Holmes, K. R., Sobczyk, O., Poublanc, J., Crawley, A. P., et al. (2016a). Impaired dynamic cerebrovascular response to hypercapnia predicts development of white matter hyperintensities. NeuroImage Clin. 11, 796–801. doi: 10.1016/j.nicl.2016.05.008
Sam, K., Peltenburg, B., Conklin, J., Sobczyk, O., Poublanc, J., Crawley, A. P., et al. (2016b). Cerebrovascular reactivity and white matter integrity. Neurology 87, 2333–2339. doi: 10.1212/WNL.0000000000003373
Sam, K., Poublanc, J., Sobczyk, O., Han, J. S., Battisti-Charbonney, A., Mandell, D. M., et al. (2015). Assessing the effect of unilateral cerebral revascularisation on the vascular reactivity of the non-intervened hemisphere: a retrospective observational study. BMJ Open 5:e006014. doi: 10.1136/bmjopen-2014-006014
Sam, K., Small, E., Poublanc, J., Han, J. S., Mandell, D. M., Fisher, J. A., et al. (2014). Reduced contralateral cerebrovascular reserve in patients with unilateral steno-occlusive disease. Cerebrovasc. Dis. 38, 94–100. doi: 10.1159/000362084
Schreiber, W. G., Friedemann, G, Stritzke, P., Schmiedek, A., and Schwartz, G. (1998). Cerebral blood flow and cerebrovascular reserve capacity: estimation by dynamic magnetic resonance imaging. J. Cereb. Blood Flow Metab. 18, 1143–1156. doi: 10.1097/00004647-199810000-00011
Sebok, M., Van Niftrik, C. H. B., Piccirelli, M., Bozinov, O., Wegener, S., Esposito, G., et al. (2018). Bold cerebrovascular reactivity as a novel marker for crossed cerebellar diaschisis. Neurology 91, E1328–E1337. doi: 10.1212/WNL.0000000000006287
Seitz, I., Ulrich, D., and Ute, L. (2004). Impaired vascular reactivity of isolated rat middle cerebral artery after cortical spreading depression in vivo. J. Cereb. Blood Flow Metab. 24, 526–530. doi: 10.1097/00004647-200405000-00006
Shiino, A., Morita, Y., Tsuji, A., Maeda, K., Ito, R., Furukawa, A., et al. (2003). Estimation of cerebral perfusion reserve by blood oxygenation level-dependent imaging: comparison with single-photon emission computed tomography. J. Cereb. Blood Flow Metab. 23, 121–135. doi: 10.1097/01.WCB.0000037546.46809.CA
Siero, J. C. W., Hartkamp, N. S., Donahue, M. J., Harteveld, A. A., Compter, A., Petersen, E. T., et al. (2015a). Neuronal activation induced BOLD and CBF responses upon acetazolamide administration in patients with steno-occlusive artery disease. NeuroImage 105, 276–285. doi: 10.1016/j.neuroimage.2014.09.033
Siero, J. C. W., Strother, M. K., Faraco, C. C., Hoogduin, H., Hendrikse, J., and Donahue, M. J. (2015b). In vivo quantification of hyperoxic arterial blood water T1. NMR Biomed. 28, 1518–1525. doi: 10.1002/nbm.3411
Sivakolundu, D. K., West, K. L., Maruthy, G. B., Zuppichini, M., Turner, M. P., Abdelkarim, D., et al. (2019). Reduced arterial compliance along the cerebrovascular tree predicts cognitive slowing in multiple sclerosis: evidence for a neurovascular uncoupling hypothesis. Mult. Scler. J. 26, 1486–1496. doi: 10.1177/1352458519866605
Sobczyk, O., Battisti-Charbonney, A., Fierstra, J., Mandell, D. M., Poublanc, J., Crawley, A. P., et al. (2014). A conceptual model for CO2-induced redistribution of cerebral blood flow with experimental confirmation using BOLD MRI. NeuroImage 92, 56–68. doi: 10.1016/j.neuroimage.2014.01.051
Sobczyk, O., Battisti-Charbonney, A., Poublanc, J., Crawley, A. P., Sam, K., Fierstra, J., et al. (2015). Assessing cerebrovascular reactivity abnormality by comparison to a reference atlas. J. Cereb. Blood Flow Metab. 35, 213–220. doi: 10.1038/jcbfm.2014.184
Sobczyk, O., Crawley, A. P., Poublanc, J., Sam, K., Mandell, D. M., Mikulis, D. J., et al. (2016). Identifying significant changes in cerebrovascular reactivity to carbon dioxide. Am. J. Neuroradiol. 37, 818–824. doi: 10.3174/ajnr.A4679
Sousa, I., Vilela, P., and Figueiredo, P. (2014). Reproducibility of hypocapnic cerebrovascular reactivity measurements using BOLD FMRI in combination with a paced deep breathing task. NeuroImage 98, 31–41. doi: 10.1016/j.neuroimage.2014.04.049
Spano, V. R., Mandell, D. M., Poublanc, J., Sam, K., Battisti-Charbonney, A., Pucci, O., et al. (2013). CO2 blood oxygen level-dependent mr mapping of cerebrovascular reserve in a clinical population: safety, tolerability, and technical feasibility. Radiology 266, 592–598. doi: 10.1148/radiol.12112795
Spilt, A., Van Den Boom, R., Kamper, A. M., Blauw, G. J., Bollen, E. L. E. M., and Van Buchem, M. A. (2002). MR assessment of cerebral vascular response: a comparison of two methods. J. Magn. Reson. Imag. 16, 610–616. doi: 10.1002/jmri.10188
Stringer, M. S., Lee, H., Huuskonen, M. T., MacIntosh, B. J., Brown, R., Montagne, A., et al. (2021). A review of translational magnetic resonance imaging in human and rodent experimental models of small vessel disease. Transl. Stroke Res. 12, 15–30. doi: 10.1007/s12975-020-00843-8
Strother, M. K., Buckingham, C., Faraco, C. C., Arteaga, D. F., Lu, P., Xu, Y., et al. (2016). Crossed cerebellar diaschisis after stroke identified noninvasively with cerebral blood flow-weighted arterial spin labeling MRI. Eur. J. Radiol. 85, 136–142. doi: 10.1016/j.ejrad.2015.11.003
Suri, S., Clare, E. M., Michael, E. K., Michael, G., Elizabeth, M. T., Giovanni, B., et al. (2015). Reduced cerebrovascular reactivity in young adults carrying the APOE Epsilon4 allele. Alzheimers Dement. 11, 648–657. doi: 10.1016/j.jalz.2014.05.1755
Svaldi, D. O., Joshi, C., McCuen, E. C., Music, J. P., Hannemann, R., Leverenz, L. J., et al. (2020). Accumulation of high magnitude acceleration events predicts cerebrovascular reactivity changes in female high school soccer athletes. Brain Imaging Behav. 14, 164–174. doi: 10.1007/s11682-018-9983-0
Tancredi, F. B., and Hoge, R. D. (2013). Comparison of cerebral vascular reactivity measures obtained using breath-holding and CO2 inhalation. J. Cereb. Blood Flow Metab. 33, 1066–1074. doi: 10.1038/jcbfm.2013.48
Taneja, K., Lu, H., Welch, B. G., Thomas, B. P., Pinho, M., Lin, D., et al. (2019). Evaluation of cerebrovascular reserve in patients with cerebrovascular diseases using resting-state MRI: a feasibility study. Magn. Reson. Imaging 59, 46–52. doi: 10.1016/j.mri.2019.03.003
Tchistiakova, E., Anderson, N. D., Greenwood, C. E., and MacIntosh, B. J. (2014). Combined effects of type 2 diabetes and hypertension associated with cortical thinning and impaired cerebrovascular reactivity relative to hypertension alone in older adults. NeuroImage Clin. 5, 36–41. doi: 10.1016/j.nicl.2014.05.020
Tchistiakova, E., Crane, D. E., Mikulis, D. J., Anderson, N. D., Greenwood, C. E., Black, S. E., et al. (2015). Vascular risk factor burden correlates with cerebrovascular reactivity but not resting state coactivation in the default mode network. J. Magn. Reson. Imaging 42, 1369–1376. doi: 10.1002/jmri.24917
Thiel, S., Lettau, F., Rejmer, P., Rossi, C., Haile, S. R., Schwarz, E. I., et al. (2019). Effects of short-term continuous positive airway pressure withdrawal on cerebral vascular reactivity measured by blood oxygen level-dependent magnetic resonance imaging in obstructive sleep apnoea: a randomised controlled trial. Eur. Respir. J. 53:1801854. doi: 10.1183/13993003.01854-2018
Thomas, B., William, L., Elizabeth, J. D., and Manohar, S. (2013). Assessment of cerebrovascular reactivity using real-time BOLD FMRI in children with moyamoya disease: a pilot study. Childs Nerv. Syst. 29, 457–463. doi: 10.1007/s00381-012-1952-0
Thomas, B. P., Liu, P., Aslan, S., King, K. S., van Osch, M. J. P., and Lu, H. (2013). Physiologic underpinnings of negative BOLD cerebrovascular reactivity in brain ventricles. NeuroImage 83, 505–512. doi: 10.1016/j.neuroimage.2013.07.005
Thomas, B. P., Liu, P., Park, D. C., Van Osch, M. J. P., and Lu, H. (2014). Cerebrovascular reactivity in the brain white matter: magnitude, temporal characteristics, and age effects. J. Cereb. Blood Flow Metab. 34, 242–247. doi: 10.1038/jcbfm.2013.194
Thrippleton, M. J., Shi, Y., Blair, G., Hamilton, I., Waiter, G., Schwarzbauer, C., et al. (2018). Cerebrovascular reactivity measurement in cerebral small vessel disease: rationale and reproducibility of a protocol for MRI acquisition and image processing. Int. J. Stroke 13, 195–206. doi: 10.1177/1747493017730740
Tong, Y., Bergethon, P. R., and Frederick, B. D. (2011). An improved method for mapping cerebrovascular reserve using concurrent FMRI and near-infrared spectroscopy with Regressor Interpolation at Progressive Time Delays (RIPTiDe). Neuroimage 56, 2047–2057. doi: 10.1016/j.neuroimage.2011.03.071
Triantafyllou, C., Wald, L. L., and Hoge, R. D. (2011). Echo-time and field strength dependence of BOLD reactivity in veins and parenchyma using flow-normalized hypercapnic manipulation. PLoS ONE 6:e24519. doi: 10.1371/journal.pone.0024519
Tucker, W. J., Thomas, B. P., Puzziferri, N., Samuel, T. J., Zaha, V. G., Lingvay, I., et al. (2020). Impact of bariatric surgery on cerebral vascular reactivity and cognitive function: a non-randomized pilot study. Pilot Feasibility Stud. 6:21. doi: 10.1186/s40814-020-00569-2
Uchihashi, Y., Hosoda, K., Zimine, I., Fujita, A., Fujii, M., Sugimura, K., et al. (2011). Clinical application of arterial spin-labeling MR imaging in patients with carotid stenosis: quantitative comparative study with single-photon emission CT. Am. J. Neuroradiol. 32, 1545–1551. doi: 10.3174/ajnr.A2525
Urback, A. L., MacIntosh, B. J., and Goldstein, B. I. (2017). Cerebrovascular reactivity measured by functional magnetic resonance imaging during breath-hold challenge: a systematic review. Neurosci. Biobehav. Rev. 79, 27–47. doi: 10.1016/j.neubiorev.2017.05.003
Urback, A. L., Metcalfe, A. W., Korczak, D. J., MacIntosh, B. J., and Goldstein, B. I. (2019). Reduced cerebrovascular reactivity among adolescents with bipolar disorder. Bipolar Disord. 21, 124–131. doi: 10.1111/bdi.12719
Vagal, A. S., Leach, J. L., Fernandez-Ulloa, M., and Zuccarello, M. (2009). The acetazolamide challenge: techniques and applications in the evaluation of chronic cerebral ischemia. Am. J. Neuroradiol. 30, 876–884. doi: 10.3174/ajnr.A1538
Valdueza, J. M., Balzer, J. O., Villringer, A., Vogl, T. J., Kutter, R., and Einhäupl, K. M. (1997). Changes in blood flow velocity and diameter of the middle cerebral artery during hyperventilation: assessment with mr and transcranial doppler sonography. AJNR. Am. J. Neuroradiol. 18, 1929–1934.
van Niftrik, C. H., Piccirelli, M., Bozinov, O., Pangalu, A., Valavanis, A., Regli, L., et al. (2016). Fine tuning breath-hold-based cerebrovascular reactivity analysis models. Brain Behav. 6:e00426. doi: 10.1002/brb3.426
van Niftrik, C. H. B., Piccirelli, M., Bozinov, O., Maldaner, N., Strittmatter, C., Pangalu, A., et al. (2018). Impact of baseline CO2 on blood-oxygenation-level-dependent mri measurements of cerebrovascular reactivity and task-evoked signal activation. Magn. Reson. Imaging 49, 123–130. doi: 10.1016/j.mri.2018.02.002
Venkatraghavan, L., Poublanc, J., Han, J. S., Sobczyk, O., Rozen, C., Sam, K., et al. (2018). Measurement of cerebrovascular reactivity as blood oxygen level-dependent magnetic resonance imaging signal response to a hypercapnic stimulus in mechanically ventilated patients. J. Stroke Cerebrovasc. Dis. 27, 301–308. doi: 10.1016/j.jstrokecerebrovasdis.2017.08.035
Waddle, S. L., Juttukonda, M. R., Lants, S. K., Davis, L. T., Chitale, R., Fusco, M. R., et al. (2019). Classifying intracranial stenosis disease severity from functional MRI data using machine learning. J. Cereb. Blood Flow Metab. 40, 705–719. doi: 10.1177/0271678X19848098
Wardlaw, J. M., Colin, S., and Martin, D. (2019). Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 18, 684–696. doi: 10.1016/S1474-4422(19)30079-1
Watchmaker, J. M., Frederick, B. D., Fusco, M. R., Davis, L. T., Juttukonda, M. R., Lants, S. K., et al. (2019). Clinical use of cerebrovascular compliance imaging to evaluate revascularization in patients with moyamoya. Clin. Neurosurg. 84, 261–271. doi: 10.1093/neuros/nyx635
Wells, J. A., Holmes, H. E., O'Callaghan, J. M., Colgan, N., Ismail, O., Fisher, E. M., et al. (2015). Increased cerebral vascular reactivity in the tau expressing rTg4510 mouse: evidence against the role of tau pathology to impair vascular health in Alzheimer's disease. J. Cereb. Blood Flow Metab. 35, 359–362. doi: 10.1038/jcbfm.2014.224
Winter, J. D., Poublanc, J., Crawley, A. P., and Kassner, A. (2009). Comparison of spiral imaging and SENSE-EPI at 1.5 and 3.0 T using a controlled cerebrovascular challenge. J. Magn. Reson. Imaging 29, 1206–1210. doi: 10.1002/jmri.21745
Wu, J., Dehkharghani, S., Nahab, F., and Qiu, D. (2017). Acetazolamide-Augmented Dynamic BOLD (AczBOLD) imaging for assessing cerebrovascular reactivity in chronic steno-occlusive disease of the anterior circulation: an initial experience. NeuroImage Clin. 13, 116–22. doi: 10.1016/j.nicl.2016.11.018
Wu, P.-H., Rodriguez-Soto, A. E., Rodgers, Z. B., Englund, E. K., Wiemken, A., Langham, M. C., et al. (2020). MRI evaluation of cerebrovascular reactivity in obstructive sleep apnea. J. Cereb. Blood Flow Metab. 40, 1328–1337. doi: 10.1177/0271678X19862182
Yezhuvath, U. S., Uh, J., Cheng, Y., Martin-Cook, K., Weiner, M., Diaz-Arrastia, R., et al. (2012). Forebrain-dominant deficit in cerebrovascular reactivity in Alzheimers disease. Neurobiol. Aging 33, 75–82. doi: 10.1016/j.neurobiolaging.2010.02.005
Zaca, D., Jovicich, J., Nadar, S. R., Voyvodic, J. T., and Pillai, J. J. (2014). Cerebrovascular reactivity mapping in patients with low grade gliomas undergoing presurgical sensorimotor mapping with BOLD FMRI. J. Magn. Reson. Imag. 40, 383–390. doi: 10.1002/jmri.24406
Zande, F. H., Hofman, P. A., and Backes, W. H. (2005). Mapping hypercapnia-induced cerebrovascular reactivity using BOLD MRI. Neuroradiology 47, 114–120. doi: 10.1007/s00234-004-1274-3
Zhao, P., Alsop, D. C., Abduljalil, A., Selim, M., Lipsitz, L., Novak, P., et al. (2009). Vasoreactivity and peri-infarct hyperintensities in stroke. Neurology 72, 643–649. doi: 10.1212/01.wnl.0000342473.65373.80
Zheng, G., Wen, J., Yu, W., Li, X., Chen, H., Kong, X., et al. (2016). Anemia rather than hypertension contributes to cerebral hyperperfusion in young adults undergoing hemodialysis: a phase contrast MRI study. Sci. Rep. 6:22346. doi: 10.1038/srep22346
Zhou, X.-H., Li, W., Zhang, Y., Lu, S., Ni, C.-L., Song, W.-Y., et al. (2015). Breath holding fMRI evaluation of cerebrovascular reactivity in patients with type 2 diabetes mellitus. Chin. J. Med. Imag. Technol. 31, 688–692. doi: 10.13929/j.1003-3289.2015.05.01
Zhou, Y., Rodgers, Z. B., and Kuo, A. H. (2015). Cerebrovascular reactivity measured with arterial spin labeling and blood oxygen level dependent techniques. Magn. Reson. Imaging 33, 566–576. doi: 10.1016/j.mri.2015.02.018
Keywords: cerebrovascular reactivity, magnetic resonance imaging, blood oxygen-level dependent, arterial spin labelling MRI, Hypercapnia (CO(2)) inhalation, systematic review
Citation: Sleight E, Stringer MS, Marshall I, Wardlaw JM and Thrippleton MJ (2021) Cerebrovascular Reactivity Measurement Using Magnetic Resonance Imaging: A Systematic Review. Front. Physiol. 12:643468. doi: 10.3389/fphys.2021.643468
Received: 18 December 2020; Accepted: 01 February 2021;
Published: 25 February 2021.
Edited by:
Nicholas P. Blockley, University of Nottingham, United KingdomReviewed by:
Erik Nicolaas Theodorus Petrus Bakker, University of Amsterdam, NetherlandsDaniela Carnevale, Sapienza University of Rome, Italy
Copyright © 2021 Sleight, Stringer, Marshall, Wardlaw and Thrippleton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael S. Stringer, m.stringer@ed.ac.uk
 Emilie Sleight
Emilie Sleight Michael S. Stringer1,2*
Michael S. Stringer1,2* Ian Marshall
Ian Marshall Joanna M. Wardlaw
Joanna M. Wardlaw Michael J. Thrippleton
Michael J. Thrippleton