- 1Department of Allied Health Sciences, University of Connecticut, Storrs, CT, United States
- 2Institute for Collaboration on Health, Intervention, & Policy, University of Connecticut, Storrs, CT, United States
Introduction: Despite unequivocal evidence supporting the use of pre-exposure prophylaxis (PrEP), its scale-up has been gradual overall, and nearly absent among people who use drugs (PWUD). In the present study, we implemented the use of PrEP, as a part of an integrated HIV prevention approach, and explored the experiences and attitudes related to PrEP use among PWUD.
Methods: Between September 2016 and July 2017, we recruited 40 HIV-uninfected, methadone-maintained people, who reported HIV-risk behaviors, and were currently taking PrEP. We conducted both quantitative and in-depth semi-structured qualitative interviews that primarily focused on experiences, attitudes, acceptability, disclosure status, risk compensation-related attitudes, and barriers related to PrEP adherence.
Results: Results showed that participants were highly satisfied and perceived PrEP as valuable and acceptable for HIV prevention. Participants reported high adherence to PrEP. The most highly endorsed facilitators to PrEP adherence were use of memory aids, no out-of-pocket cost, perceived benefit, and support from social network. The barriers to adherence included side-effects, stigmatization, requirement of daily dosing, and accessibility of PrEP services. Additionally, participants expressed disagreement with the overall risk compensation-related attitudes (i.e., decreased personal concern about engaging in HIV risk behavior due to their perception that PrEP is now fully protecting them from contracting HIV) and indicated no increased engagement in risk behaviors while on PrEP.
Conclusions: The results from the current study provide preliminary evidence supporting the successful integration of PrEP within the substance abuse treatment setting, where high risk PWUD are concentrated.
Introduction
With the rise of illicit drug use in the United States (US), people who use drugs (PWUD) continue to remain a population at high-risk for HIV infection (1–3). The availability of pre-exposure prophylaxis (PrEP) is emerging as an important tool for curtailing the HIV epidemic and a recommended element of integrated HIV prevention approaches (4–6). Recent large-scale PrEP trials have shown PrEP to be safe, well tolerated, and highly efficacious in reducing the risk of HIV acquisition in individuals at increased risk for infection (7–11). Based on these trials, the Centers for Disease Control and Prevention (CDC) made recommendations and provided clinical practice guidelines on the use of PrEP for HIV prevention among most-at-risk populations, including PWUD (12).
In the US, the CDC estimates that PrEP could benefit 1 in 4 adult men who have sex with men (MSM), 1 in 5 people who inject drugs (PWID: who are subset of PWUD group), and 624,000 heterosexually active adults, three-quarters of whom are women (13). As such, PrEP implementation is underway around the world, particularly among MSM. However, the scale-up of PrEP among even very high-risk PWUD is nearly absent, despite being ideal candidates and expressing strong willingness to use PrEP (14–17). As indicated in the original PrEP trial among PWID (11), common drug treatment setting (e.g., methadone maintenance program: MMP) play a key role in the adoption and delivery of PrEP among PWUD (18). Particularly in the context of MMP, the requirement for routine counseling and coordination of care readily support the integration of PrEP and facilitate improvement of the PrEP continuum of care (cascade) among this underserved group (19). Furthermore, the requirement for daily contact with service providers for methadone administration may allow the counselors/nurses to monitor clients' adherence to medication through directly observed therapy (DOT).
Considering these factors, we implemented the use of PrEP as a part of an integrated primary HIV prevention approach among PWUD who exhibited high HIV transmission risk behaviors (both sex- and drug-related) in the context of an MMP (18, 20). In the present study, we explored the experiences, attitudes, acceptability, disclosure status, risk compensation-related attitudes, and barriers to adherence among PWUD using PrEP. Such information is crucial to optimize PrEP programs in addiction treatment, where high risk PWUD are concentrated, and are ideal settings to implement primary HIV prevention interventions among PWUD.
Methods
Study Setting and Participants
We used purposive sampling to recruit participants who met the eligibility criteria. A total of 40 participants were recruited from an inner-city MMP in New Haven, Connecticut if they (i) were age ≥18 years, (ii) were confirmed HIV-negative and on PrEP, (iii) reported drug- (e.g., sharing of injection equipment) or sex-related (e.g., condomless sex, multiple sex partners) HIV risk behaviors in the past 6 months, and (iv) enrolled in MMP.
Procedures
Between September 2016 and July 2017, we conducted both quantitative and in-depth, semi-structured qualitative interviews among PWUD who were using PrEP. Participants were recruited via flyers, peers, word-of-mouth, and direct referral from counselors at the MMP. Interested individuals were screened for HIV transmission risk behavior by the research assistant. Those who reported high-risk behavior and interest in taking PrEP in the screening form, but not already on PrEP (38 out of 40 participants), were referred for additional screening (i.e., PrEP eligibility) (12) by an onsite primary care physician for PrEP prescription. Those who chose to initiate PrEP were then enrolled in the study and provided written consent prior to completing the assessments. Participants were reimbursed for their time ($45). The study protocol was approved by the Institutional Review Board at the University of Connecticut and received board approval from the methadone clinic.
Assessments
Quantitative Assessment
All quantitative measures were assessed using an audio computer-assisted self-interview (ACASI). In addition to demographic and social characteristics, we assessed homeless status, current methadone dose, perceived HIV risk, length of PrEP use, PrEP prescription venue, and PrEP disclosure status. Other measures are described as follows:
HIV risk behaviors
The HIV risk assessment, adapted from National Institute on Drug Abuse (NIDA)'s Risk Behavior Assessment (21) was used to measure several aspects of HIV risk behaviors, including a measurement of “any” high risk behavior (sexual or drug-related) as well as measurements of event-level (i.e., partner-by-partner) behaviors. For example, “Have you ever used illicit drugs?”; “Have you injected illicit drugs in the last 30 days?” Responses were reported using a “yes” or “no”.
PrEP adherence
PrEP adherence was assessed using a self-reported, validated 3-item scale developed by Wilson et al. (22) Items included: “In the last 30 days, on how many days did you miss at least one dose of your PrEP medication?”; “In the last 30 days, how often did you take your PrEP medication in the way you were supposed to?”; and “In the last 30 days, how good a job did you do at taking your PrEP medication in the way you were supposed to?” Summary scales were calculated as the mean of the three individual items with higher score indicating better adherence (0–100) (22).
Acceptability of PrEP use
Acceptability of PrEP use was assessed using a series of 8-item acceptability rating profile (23). Participants used a five-point Likert scale (0 = strongly disagree to 4 = strongly agree) to rate the extent to which they agreed with each acceptability statement. For example, “PrEP is useful for individuals who engage in high risk behaviors (e.g., unsafe sex, needle sharing),” “I would be willing to recommend PrEP to my friends who may benefit from it.” An intervention acceptability score was calculated by first adding the scores and then converting into a score on the 0–100 scale with higher values indicating greater acceptability (α = 0.797).
Risk compensation related attitudes
Risk Compensation related attitudes was assessed using the Treatment-Related Reduced HIV Risk measure (24), an 8-item scale adapted for PrEP use that assesses decreased personal concern about engaging in unsafe sex and the potential for infecting others because of PrEP use. For example, “Because I am taking PrEP, I am less concerned about becoming HIV positive,” “I am more willing to take a chance of getting infected now that I am taking PrEP.” Responses were reported using a “yes” or “no.” The scale was internally reliable in this sample (α = 0.752).
Qualitative Assessment
All qualitative interviews were semi-structured around a set of carefully predetermined interview guides (Table 1). These guides were brief, semi-structured to encourage free-flowing discussion between interviewer and participant. Interviews explored overall experiences taking PrEP to prevent HIV, facilitators and barriers to PrEP use, and engagement in risk behaviors while on PrEP. All qualitative interviews were audiotaped with participants' permission and transcribed verbatim.
Data Analyses
Analyses of quantitative data were performed in SPSS. Descriptive statistics were performed to provide a demographic, sexual and substance use profile of study participants. Means and standard deviations (continuous variables) and frequencies and percentages (categorical variables) were derived to assess participants' adherence, acceptability and risk compensation regarding the use of PrEP for HIV prevention. Analysis of qualitative data followed a thematic analysis approach, applying several qualitative data analysis procedures (e.g., inductive analysis, cross-case analysis). In the initial inductive analysis, emergent themes and patterns were identified directly in the transcripts. The coding process was completed for each transcript and a master list of themes was compiled to reflect overarching themes. Two research team members met regularly to become acquainted with participant narratives, to contextualize differences, to build consensus, and to cross-case analysis decisions of emerging themes.
Results
Participant Characteristics
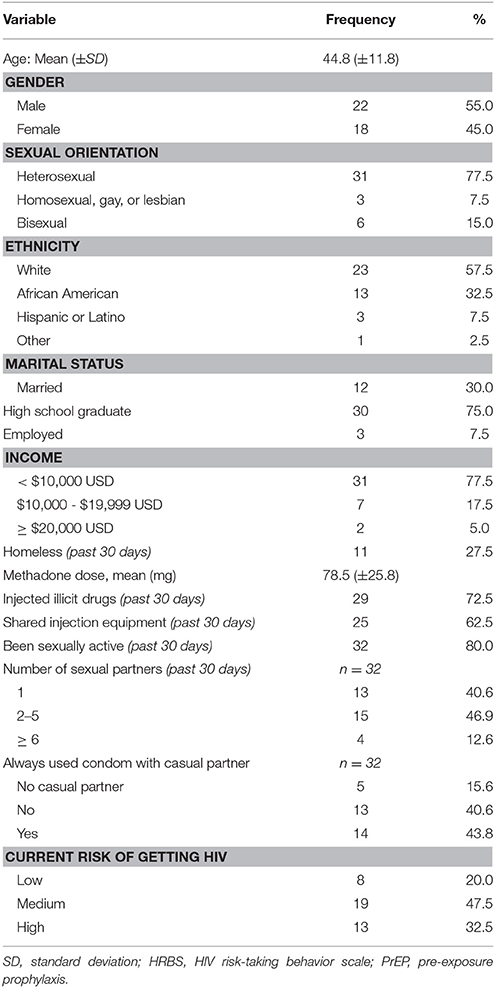
Participants were mostly in their mid-40s (mean = 44.8), male (55.0%), White (57.5%), and single (47.5%). Most participants identified as heterosexual (77.5%) and graduated from high school (75.0%). Only 7.5% of the participants reported being currently employed and 77.5% were earning less than $10,000 per year. Almost one-third of the participants (27.5%) reported to have been homeless in the past 30 days. All participants were enrolled in an inner-city MMP and were maintained on a stable dose of methadone (mean = 78.5 mg) (Table 2).
Quantitative Results
Sexual Risk and Substance Use Behavior
Majority of the participants (72.5%) reported current drug injection in the past 30 days. Of those, almost two-thirds reported having shared injection equipment. Of those who were sexually active (80.0%), 59.5% reported having sex with more than one sexual partner and 43.8% reported always using condoms with casual sexual partners. Most participants perceived themselves to be at medium to high risk of contracting HIV (80.0%).
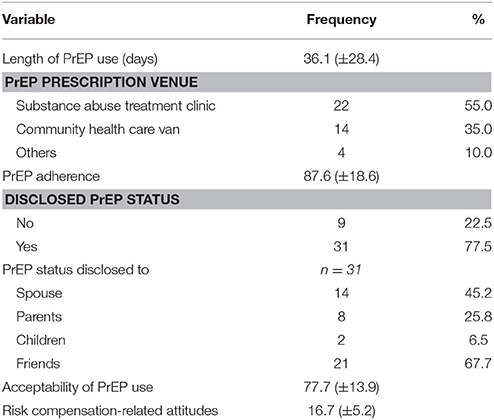
PrEP Adherence and Disclosure Status
The mean length of participants on PrEP was 36.1 days. Primary care physicians at the substance abuse treatment clinic were noted as the top source of PrEP prescriptions. Participants reported having taken PrEP in the past 30 days with a mean adherence score of 87.6 (±18.6). A considerable proportion of the participants reported to have disclosed their PrEP status to other people (77.5%), and out of these, 67% disclosed their status to their friends, 45.2% to their spouse, followed by their parents (25.8%) and children (6.5%) (Table 3).
Acceptability of PrEP Use
Our results showed that participants were satisfied and perceived the use of PrEP for HIV prevention as valuable and acceptable. Mean acceptability (range: 0–100) for the PrEP use was 77.7 (±13.9), which conveys strong agreement among participants about the acceptability of the PrEP use. Additionally, the majority of participants (90.0%) viewed the use of PrEP as beneficial for individuals who engage in high risk behaviors (e.g., condomless sex, sharing of injection equipment). Similarly, the majority of participants indicated that individuals in substance abuse treatment clinics have high risk behaviors that justifies their use of PrEP (92.5%) and expressed a desire to recommend PrEP to others who may benefit from it (92.5%).
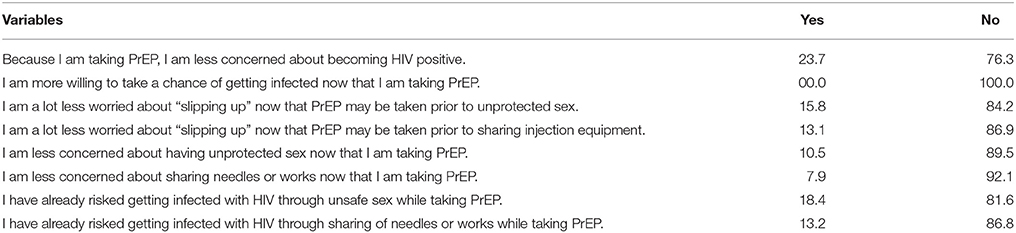
Risk Compensation-Related Attitudes
In terms of risk compensation-related attitudes (i.e., decreased personal concern about engaging in HIV risk behavior due to their perception that PrEP is now fully protecting them from contracting HIV), a minority of participants expressed agreement on the overall risk compensation-related attitudes scale (16.7 ± 5.2) (Range: 8–40). For example, none of the participants reported that they were willing to take a chance of getting infected because they were taking PrEP. Similarly, very few of the participants expressed concern about having unprotected sex (10.5%) and sharing of injected equipment (7.9%) while on PrEP. When asked if they have already risked getting infected with HIV through sex (8.16%) and unsafe sharing of injection equipment (86.8%) while on PrEP, most participants responded negatively (Table 4).
Qualitative Results
Overall, the results from the qualitative interviews identified the following key themes and subthemes regarding participants' attitudes and experiences surrounding PrEP use (Table 5):
Adherence to PrEP
When asked about participants' use of PrEP as a HIV prevention method, most indicated that they had positive experiences with PrEP use and only minor adverse clinical outcomes. The majority of the participants reported consistent adherence patterns to reduce their chances of contracting HIV infection:
“I've been taking it [PrEP] regularly… I have it right next to my bed and take it every night before I go to sleep, and it's that easy… To be honest, I've only missed a couple of days since I started taking it.”
Overall, participants expressed favorable attitudes toward the use of PrEP for HIV prevention. A few expressed concerns about the complexities related to its use, whereas, others indicated a few factors to facilitate adherence to this medication.
PrEP Adherence Barriers
Side-effects
Participants reported several side-effects when using PrEP, including nausea, headache, stomach-area pain, diarrhea, dizziness, tiredness, problems sleeping, loss of appetite, being short of breath or fast breathing, joint or muscle aches, sore throat, and abnormal dreams. Participants described side-effects that emerged shortly after initiating PrEP, but these diminished over time and did not significantly deter them from taking the pills:
“So far, I haven't had any issues but I'm a little worried about the long-term side-effects… I used to take it in the mornings. I realized that I was feeling dizzy every time after taking it. Then I started taking it before bedtime and I've had no problem since then.”
Stigma
A few participants noted the potential social risks (e.g., discrimination, exclusion, loss of trust) of taking PrEP, and pointed to instances of both experienced stigma and potential situations where stigmatization could occur. Participants reflected on the social consequences of taking PrEP, which included relationship challenges, rumors, and experienced and perceived stigma, due to PrEP users being perceived as having HIV or being illicit drug users:
“My roommate asked me if I was taking it for HIV. That threw me off… Because many people out there have no idea what it [PrEP] is for, they would not understand, they will probably think I am infected, and tell others.”
Daily dosing
As participants were asked about the challenges or concerns of taking PrEP as prescribed, some brought up the requirement of daily dosing as one of the barriers. They thought that it was hard for them to adhere to it every day, especially when they were actively using drugs. Few participants expressed a preference for intermittent use as it would be a less-burdensome schedule of pill taking:
“It'd have been so much easier if we could take it [PrEP] whenever we needed them.”
Access
Some participants pointed out concerns about getting refills and having to make regular follow-up visits as barriers to their adherence to PrEP regimen:
“Well, refills were a problem. There were only 7 days of medication. Getting there [to the clinic] once a week was a bit of a hassle. I missed a day or two because of that. They should've given it [PrEP] for the whole 30 days.”
PrEP Adherence Facilitators
Use of memory aids
Most participants indicated that they had been using memory aids, such as cell phone, pill organizer, post-it notes to help them remember take PrEP medication:
“I put an alarm in my phone. That's the only way I remember…I get a text message, which has been a big help for me to remember to take it [PrEP].”
Cost
Participants overwhelmingly reported that the cost for PrEP regimen, including co-payments, related clinical, and laboratory services, were fully covered by their insurance, which facilitated adherence to PrEP medication:
“The insurance I currently have covered the doctor visit, blood work they did on me, and for this [PrEP] med…I heard it's really expensive. Glad I don't have to pay.”
Perceived benefit
Participants frequently indicated that knowing the high efficacy of PrEP in terms of reducing the risk of HIV infection motivated them to continue taking it:
“If there's a medication out there which is 90% effective in reducing HIV, then why not take it [PrEP]…When I'm using, I don't think I'll worry about condoms, so it's good that to have PrEP.”
Social support
Some participants reported that good support from their family and friends has been influential in facilitating adherence to PrEP:
“Actually, it was my wife who asked me to get on the meds [PrEP]. She has HIV, you know, and she reminds me to take it every single day.”
Risk Compensation
Participants overwhelmingly reported no increases in engagement in HIV risk behaviors. Surprisingly, the majority of participants reported an increased use of condoms while on PrEP:
“It [use of PrEP] makes me feel I need to be more cautious. Actually, I used a condom last week, that was first time in a while…It makes me think and I've talked to my boyfriend about using condoms. And, it's been working so far.”
Discussion
Several important findings were gleaned from this exploratory study that have promising implications for expanding and improving the PrEP continuum of care in settings such as MMPs (19), where PrEP use among PWUD was originally examined (11). The overwhelmingly positive response regarding PrEP use from study participants was encouraging given the prevalence of high HIV risk behaviors among our sample. Such preferential use of PrEP among our sample of PWUD, 80% of whom perceived themselves to be at moderate to high risk of acquiring HIV, shows these individuals' capacity to recognize and respond appropriately to health risks when given appropriate options. In addition to the biomedical prevention benefits of PrEP, the structured nature of MMP context including the requirement for routine behavioral counseling suggests that MMP settings could readily support the integration of PrEP into existing evidenced-based behavioral risk reduction strategies (18). Our findings thus provide reason for optimism about the potential implementation of PrEP services within the substance abuse treatment settings, where high risk PWUD are easily reached, and thus, has a potential to have profound impact on HIV prevention efforts among PWUD.
Some prescribers and clinicians have raised concerns related to potential low adherence rates leading to low effectiveness of PrEP use and drug resistance among PWUD (18, 25). Participants in the current study, however, reported high adherence to daily PrEP use, as in the original PrEP trial in PWID in MMP (11). As suggested in prior research, adherence might have been related to participants' self-assessed risk of HIV infection (26). It is encouraging to see such patterns among our sample as high adherence to PrEP is critical to its efficacy (7–11). This result also supports our continued efforts to pursue this innovative biomedical approach to address significant risk of acquiring HIV as experienced by many PWUD in treatment. It should be noted that prior research has documented disengagement from PrEP services to be substantial after a brief period of participation in PrEP trials and HIV infection rates during gaps in PrEP use is found to be high (27). These results thus suggest the need to monitor and provide support for long-term adherence given that participants in the current study have been on PrEP for just over a month. Furthermore, these results should be interpreted with caution, given that self-reported adherence to PrEP may not always correlate strongly with actual drug levels (28).
Our qualitative data further suggest that higher PrEP adherence in our sample may have been due to various facilitators of medication adherence. For example, participants reported the use of memory aids (e.g., alarms, text messaging) as a potential tool to improve adherence. This is consistent with prior findings, where the use of mobile technologies (mHealth) has been shown to significantly improve medication adherence in vulnerable population (29–31). In contrary to prior findings (32, 33), all participants in the current study reported no out-of-pocket costs related to PrEP (e.g., medication, clinic visits), thus facilitating their PrEP uptake and adherence. It is encouraging that most private and public insurance plans in the US currently cover the cost of PrEP. Similarly, many participants indicated the perceived benefit of PrEP use as a reason for adhering to PrEP use. Prior studies reported similar findings, where individuals were willing to use PrEP with higher efficacy in preventing HIV (34, 35); no such studies exist for PWUD. Furthermore, support from social networks, for example family and friends, tended to positively impact participants' adherence to PrEP, and this is consistent with prior studies (36, 37). Interestingly, with PrEP use, partner support in discordant relationship was significant. This is important given that sero-discordant couples are considered to be at high-risk of HIV transmission, and could significantly benefit from increased uptake and adherence to PrEP (12).
Not surprisingly, our findings lend support to previous research highlighting the side-effects as one of the potential barriers for PrEP use. Previous studies have shown potential side effects from PrEP medications as being one of the major barriers to uptake (32, 35, 38, 39), yet numerous studies suggest that currently approved PrEP medications have few or no perceived side effects (7–11). Similarly, participants' concerns about social stigma corroborate previous research identifying PrEP-related stigma as limiting the individual and public health benefits of PrEP among at-risk individuals, including PWUD (40, 41). Many participants expressed concern that taking PrEP medication could lead others to believe they are HIV-infected and on an ART regimen, which negatively influenced their adherence to PrEP. Furthermore, participants perceived the need for daily use of PrEP as a potential barrier to adherence. Contrary to national recommendations (12), participants in this study preferred if PrEP could be taken on an as needed basis. This finding aligns well with a recent finding which has documented on-demand PrEP to be effective in reducing HIV transmission (42, 43). Additionally, developing methods of delivery other than daily oral dosing, such as long-acting injectable PrEP (44), may minimize adherence concerns in the future.
Researchers, clinicians, and community members have often raised concerns that PrEP could lead to an increase in high-risk behavior because of a reduced perception of HIV risk (25). Interestingly, participants in the current study overwhelmingly reported no increases in risky behaviors while on PrEP. It should be noted, however, that almost two-thirds of participants shared injection equipment and less than half of participants reported consistent condom use and, overall, they tended to maintain this pattern while taking PrEP. Given that a significant proportion of our sample exhibited risky behaviors, continuation of such behaviors may put these individuals at ongoing risk for HIV infection despite being on PrEP. These data suggest that although PrEP use alone is not associated with risk compensation, PrEP users are at high risk for HIV infection and require complementary behavioral HIV risk reduction strategies.
The conclusions drawn from this study are not without limitations. As these results are specific to high-risk PWUD in a substance abuse treatment setting, our findings may not be generalizable to other risk populations or PWUD in other settings. In addition, we relied on self-reported measures to assess participants' experiences, perceptions, and behaviors, which may have resulted in them under- or over-reporting certain behaviors (e.g., drug- and sex-related risk behaviors, PrEP adherence). Furthermore, the sample size limited our ability to observe potential differential patterns among sub-groups, specifically by age, sexual orientation, or race/ethnicity. Regardless of these limitations, we believe that our findings carry important implications for efforts to improve the PrEP continuum of care among high-risk PWUD in the US, where PrEP for primary HIV prevention still remains understudied and underutilized.
Conclusion
To our knowledge, this is the first study to explore the experiences and attitudes related to PrEP uptake among PWUD in a substance abuse treatment setting. The results from the current study provide preliminary evidence supporting the successful integration of PrEP within a MMP—a common type of drug treatment setting—where high risk PWUD can be readily reached. The findings support our continued efforts to offer PrEP to high-risk PWUD as an important part of a combined HIV prevention package (4–6). Overall, we believe our findings have encouraging implications for future public health research and for health promotion interventions, practices, and policies for PWUD, particularly in real world treatment settings.
Author Contributions
RS and MC conceived the study design. RS led data collection. Both RS and MC conducted statistical analyses and supported interpretation of results. RS wrote the first draft of the manuscript. Both the authors provided considerable editing, revisions, and content review of the initial manuscript draft and approved the final draft of the manuscript.
Funding
This work was supported by grants from the National Institute on Drug Abuse for research (R01 DA032290) and for career development (K02 DA033139) to MC. NIDA was not involved in the collection, analysis or interpretation of data and was also not involved in writing this manuscript or the decision to submit it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the study participants for the time and effort they have dedicated to this research. We also want to acknowledge Brian Sibilio, Pramila Karki, and Jennifer Boyd for their support with the project.
References
1. Marshall BDL, Friedman SR, Monteiro JFG, Paczkowski M, Tempalski B, Pouget ER, et al. Prevention And Treatment Produced Large Decreases In HIV Incidence In A Model Of People Who Inject Drugs. Health Affairs (2014) 33:401–9. doi: 10.1377/hlthaff.2013.0824
2. Strathdee SA, Hallett TB, Bobrova N, Rhodes T, Booth R, Abdool R, et al. HIV and risk environment for injecting drug users: the past, present, and future. Lancet (2010) 376:268–84. doi: 10.1016/S0140-6736(10)60743-X
3. CDC. HIV Surveillance Report: Diagnoses of HIV Infection in the United States and Dependent Areas, 2015. Atlanta, GA (2016).
4. Bekker L-G, Beyrer C, Quinn TC. Behavioral and biomedical combination strategies for HIV prevention. Cold Spring Harbor Perspect Med. (2012) 2:8. doi: 10.1101/cshperspect.a007435
5. Brown JL, Sales JM, DiClemente RJ. Combination HIV prevention interventions: the potential of integrated behavioral and biomedical approaches. Curr HIV/AIDS Rep. (2014) 11:363–75. doi: 10.1007/s11904-014-0228-6
6. Buchbinder SP, Liu A. Pre-exposure prophylaxis and the promise of combination prevention approaches. AIDS Behav. (2011) 15:72–9. doi: 10.1007/s10461-011-9894-1
7. Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. (2010) 363:2587–99. doi: 10.1056/NEJMoa1011205
8. Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. (2012) 367:399–410. doi: 10.1056/NEJMoa1108524
9. Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. (2012) 367:423–34. doi: 10.1056/NEJMoa1110711
10. Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. (2012) 367:411–22. doi: 10.1056/NEJMoa1202614
11. Choopanya K, Martin M, Suntharasamai P, Sangkum U, Mock PA, Leethochawalit M, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet (2013) 381:2083–90. doi: 10.1016/S0140-6736(13)61127-7
12. CDC. Preexposure Prophylaxis for the Prevention of HIV Infection in the United States-−2014: A Clinical Practice Guideline. Washington, DC: Department of Health and Human Services USA—Centers for Disease Control and Prevention. (2014).
13. Smith DK, Van Handel M, Wolitski RJ, Stryker JE, Hall HI, Prejean J, et al. Vital signs: estimated percentages and numbers of adults with indications for preexposure prophylaxis to prevent HIV acquisition—United States, 2015. MMWR Morb Mortal Wkly Rep. (2015) 64:1291–5. doi: 10.15585/mmwr.mm6446a4
14. Shrestha R, Karki P, Altice FL, Huedo-Medina TB, Meyer JP, Madden L, et al. Correlates of willingness to initiate pre-exposure prophylaxis and anticipation of practicing safer drug- and sex-related behaviors among high-risk drug users on methadone treatment. Drug Alcohol Depend. (2017) 173:107–16. doi: 10.1016/j.drugalcdep.2016.12.023
15. Stein M, Thurmond P, Bailey G. Willingness to use HIV pre-exposure prophylaxis among opiate users. AIDS Behav. (2014) 18:1694–700. doi: 10.1007/s10461-014-0778-z
16. Kuo I, Olsen H, Patrick R, Phillips G II, Magnus M, Opoku J, et al. Willingness to use HIV pre-exposure prophylaxis among community-recruited, older people who inject drugs in Washington, DC. Drug Alcohol Depend. (2016) 164:8–13. doi: 10.1016/j.drugalcdep.2016.02.044
17. Shrestha R, Karki P, Altice F, Dubov O, Fraenkel L, Huedo-Medina T, et al. Measuring acceptability and preferences for implementation of pre-exposure prophylaxis (PrEP) using conjoint analysis: an application to primary HIV prevention among high risk drug users. AIDS Behav. (2017) 22:1228–38. doi: 10.1007/s10461-017-1851-1
18. Shrestha R, Altice F, Karki P, Copenhaver M. Developing an integrated, brief bio-behavioral HIV prevention intervention for high risk drug users in treatment: the process and outcome of formative research. Front Immunol. (2017) 8:561. doi: 10.3389/fimmu.2017.00561
19. Liu A, Colfax G, Cohen S, Bacon O. The spectrum of engagement in HIV prevention: proposal for a Pre-Exposure Prophylaxis (PrEP) cascade. In: 7th International Conference on HIV Treatment and Prevention Adherence. Miami (2012).
20. Shrestha R, Altice F, Karki P, Copenhaver M. Integrated biobehavioral approach to improve adherence to pre-exposure prophylaxis and reduce HIV risk in people who use drugs. Drug Alcohol Depend. (2018) doi: 10.1007/s10461-018-2099-0. [Epub ahead of print].
21. Dowling-Guyer S, Johnson ME, Fisher DG, Needle R, Watters J, Andersen M, et al. Reliability of drug users' self-reported HIV risk behaviors and validity of self-reported recent drug use. Assessment (1994) 1:383–92. doi: 10.1177/107319119400100407
22. Wilson IB, Lee Y, Michaud J, Fowler FJ, Rogers WH. Validation of a new three-item self-report measure for medication adherence. AIDS Behav. (2016) 20:2700–8. doi: 10.1007/s10461-016-1406-x
23. Tarnowski KJ, Simonian SJ. Assessing treatment acceptance: the abbreviated acceptability rating profile. J Behav Ther Exp Psychiatry (1992) 23:101–6. doi: 10.1016/0005-7916(92)90007-6
24. Vanable PA, Ostrow DG, McKirnan DJ. Viral load and HIV treatment attitudes as correlates of sexual risk behavior among HIV-positive gay men. J Psychosomat Res. (2003) 54:263–9. doi: 10.1016/S0022-3999(02)00483-X
25. Spector AY, Remien RH, Tross S. PrEP in substance abuse treatment: a qualitative study of treatment provider perspectives. Subst Abuse Treat Prev Policy (2015) 10:1. doi: 10.1186/1747-597X-10-1
26. Martin M, Vanichseni S, Suntharasamai P, Sangkum U, Mock PA, Chaipung B, et al. Factors associated with the uptake of and adherence to HIV pre-exposure prophylaxis in people who have injected drugs: an observational, open-label extension of the Bangkok Tenofovir Study. Lancet HIV (2017) 4:e59–66. doi: 10.1016/S2352-3018(16)30207-7
27. Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. (2014) 14:820–9. doi: 10.1016/S1473-3099(14)70847-3
28. Agot K, Taylor D, Corneli AL, Wang M, Ambia J, Kashuba ADM, et al. Accuracy of self-report and pill-count measures of adherence in the FEM-PrEP clinical trial: implications for future HIV-prevention trials. AIDS Behav. (2015) 19:743–51. doi: 10.1007/s10461-014-0859-z
29. Pop-Eleches C, Thirumurthy H, Habyarimana JP, Zivin JG, Goldstein MP, De Walque D, et al. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: a randomized controlled trial of text message reminders. AIDS (2011) 25:825. doi: 10.1097/QAD.0b013e32834380c1
30. Finitsis DJ, Pellowski JA, Johnson BT. Text message intervention designs to promote adherence to antiretroviral therapy (ART): a meta-analysis of randomized controlled trials. PLoS ONE (2014) 9:e88166. doi: 10.1371/journal.pone.0088166
31. Mbuagbaw L, van der Kop ML, Lester RT, Thirumurthy H, Pop-Eleches C, Ye C, et al. Mobile phone text messages for improving adherence to antiretroviral therapy (ART): an individual patient data meta-analysis of randomised trials. BMJ Open (2013) 3:e002954. doi: 10.1136/bmjopen-2013-003950
32. Galea JT, Kinsler JJ, Salazar X, Lee S-J, Giron M, Sayles JN, et al. Acceptability of pre-exposure prophylaxis as an HIV prevention strategy: barriers and facilitators to pre-exposure prophylaxis uptake among at-risk Peruvian populations. Int J STD AIDS (2011) 22:256–62. doi: 10.1258/ijsa.2009.009255
33. Brooks RA, Kaplan RL, Lieber E, Landovitz RJ, Lee S-J, Leibowitz AA. Motivators, concerns, and barriers to adoption of preexposure prophylaxis for HIV prevention among gay and bisexual men in HIV-serodiscordant male relationships. AIDS Care (2011) 23:1136–45. doi: 10.1080/09540121.2011.554528
34. Golub SA, Kowalczyk W, Weinberger CL, Parsons JT. Preexposure prophylaxis and predicted condom use among high-risk men who have sex with men. J Acquir Immune Defic Syndr. (2010) 54:548–55. doi: 10.1097/QAI.0b013e3181e19a54
35. Mustanski B, Johnson AK, Garofalo R, Ryan D, Birkett M. Perceived likelihood of using HIV pre-exposure prophylaxis medications among young men who have sex with men. AIDS Behav. (2013) 17:2173–9. doi: 10.1007/s10461-012-0359-y
36. Ware NC, Wyatt MA, Haberer JE, Baeten JM, Kintu A, Psaros C, et al. What's love got to do with it? explaining adherence to oral antiretroviral pre-exposure prophylaxis for HIV-serodiscordant couples. J Acquir Immune Defic Syndr. (2012) 59:463–8. doi: 10.1097/QAI.0b013e31824a060b
37. Gilmore HJ, Liu A, Koester KA, Amico KR, McMahan V, Goicochea P, et al. Participant experiences and facilitators and barriers to pill use among men who have sex with men in the iPrEx pre-exposure prophylaxis trial in San Francisco. AIDS Patient Care STDs (2013) 27:560–6. doi: 10.1089/apc.2013.0116
38. Mack N, Odhiambo J, Wong CM, Agot K. Barriers and facilitators to pre-exposure prophylaxis (PrEP) eligibility screening and ongoing HIV testing among target populations in Bondo and Rarieda, Kenya: results of a consultation with community stakeholders. BMC Health Services Res. (2014) 14:231. doi: 10.1186/1472-6963-14-231
39. Gersh JK, Fiorillo SP, Burghardt L, Nichol AC, Thrun M, Campbell TB. Attitudes and barriers towards pre-exposure prophylaxis (Prep) among high-risk HIV-seronegative men who have sex with men. J AIDS Clin Res. (2014) doi: 10.4172/2155-6113.1000335
40. Biello KB, Oldenburg CE, Mitty JA, Closson EF, Mayer KH, Safren SA, et al. The “Safe Sex” conundrum: anticipated stigma from sexual partners as a barrier to PrEP use among substance using MSM engaging in transactional sex. AIDS Behav. (2017) 21:300–6. doi: 10.1007/s10461-016-1466-y
41. Eaton LA, Kalichman SC, Price D, Finneran S, Allen A, Maksut J. Stigma and conspiracy beliefs related to pre-exposure prophylaxis (PrEP) and interest in using PrEP among black and white men and transgender women who have sex with men. AIDS Behav. (2017) 21:1236–46. doi: 10.1007/s10461-017-1690-0
42. McCormack S, Dunn DT, Desai M, Dolling DI, Gafos M, Gilson R, et al. Pre-exposure prophylaxis to prevent acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet (2016) 387:53–60. doi: 10.1016/S0140-6736(15)00056-2
43. Molina JM, Capitant C, Spire B, Pialoux G, Cotte L, Charreau I, et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. (2015) 373:2237–46. doi: 10.1056/NEJMoa1506273
Keywords: HIV prevention, pre-exposure prophylaxis, people who use drugs, substance abuse, methadone maintenance program
Citation: Shrestha R and Copenhaver M (2018) Exploring the Use of Pre-exposure Prophylaxis (PrEP) for HIV Prevention Among High-Risk People Who Use Drugs in Treatment. Front. Public Health 6:195. doi: 10.3389/fpubh.2018.00195
Received: 06 January 2018; Accepted: 26 June 2018;
Published: 13 July 2018.
Edited by:
John B. F. de Wit, Utrecht University, NetherlandsReviewed by:
Frits Van Griensven, Thai Red Cross Society, ThailandAnthony J. Santella, Hofstra University, United States
Copyright © 2018 Shrestha and Copenhaver. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roman Shrestha, roman.shrestha@uconn.edu
 Roman Shrestha
Roman Shrestha Michael Copenhaver1,2
Michael Copenhaver1,2