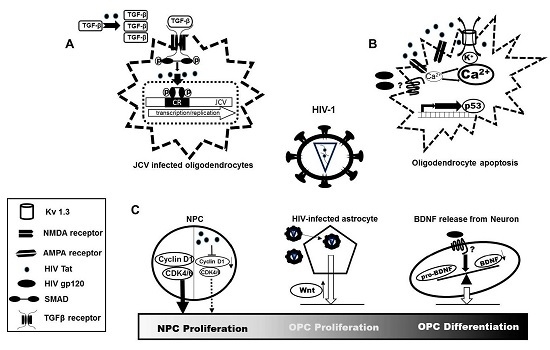
Oligodendrocyte Injury and Pathogenesis of HIV-1-Associated Neurocognitive Disorders
Abstract
:1. Introduction
2. Myelin/Oligodendrocyte Injury in HIV-1 Patients
3. Fate of Oligodendrocytes in HIV-1-Infected Brain
4. Association between Blood-Brain Barrier (BBB) Disruption and Myelin Injury
5. Cellular Mechanisms for Oligodendrocyte Injury in HIV-1-Infected Brain
6. Myelin Maintenance and Remyelination in HIV-1-Infected Brain
6.1. Alteration of OPC Proliferation and Differentiation in HIV-1-Infected Brains
6.2. Imbalance of OPC Differentiation and Remyelination in HIV-1-Infected Brain
7. Summary and Prospects
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Antinori, A.; Arendt, G.; Becker, J.T.; Brew, B.J.; Byrd, D.A.; Cherner, M.; Clifford, D.B.; Cinque, P.; Epstein, L.G.; Goodkin, K.; et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007, 69, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Heaton, R.K.; Franklin, D.R.; Ellis, R.J.; McCutchan, J.A.; Letendre, S.L.; LeBlanc, S.; Corkran, S.H.; Duarte, N.A.; Clifford, D.B.; Woods, S.P. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. J. Neurovirol. 2011, 17, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, V.; Balestra, P.; Bellagamba, R.; Corpolongo, A.; Salvatori, M.F.; Visco-Comandini, U.; Vlassi, C.; Giulianelli, M.; Galgani, S.; Antinori, A.; et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: Prevalence and risk factors. J. Acquir. Immune Defic. Syndr. 2007, 45, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; An, H.; Zhu, H.; Stone, T.; Smith, J.K.; Hall, C.; Bullitt, E.; Shen, D.; Lin, W. White matter abnormalities revealed by diffusion tensor imaging in non-demented and demented HIV+ patients. Neuroimage 2009, 47, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Gosztonyi, G.; Artigas, J.; Lamperth, L.; Webster, H.D. Human immunodeficiency virus (HIV) distribution in HIV encephalitis: Study of 19 cases with combined use of in situ hybridization and immunocytochemistry. J. Neuropathol. Exp. Neurol. 1994, 53, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Fuss, B.; Fitting, S.; Hahn, Y.K.; Hauser, K.F.; Knapp, P.E. Oligodendrocytes are targets of HIV-1 tat: Nmda and ampa receptor-mediated effects on survival and development. J. Neurosci. 2015, 35, 11384–11398. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, L.; Croul, S.; Morgello, S.; Amini, S.; Rappaport, J.; Khalili, K. Detection of HIV-1 tat and JCV capsid protein, VP1, in AIDS brain with progressive multifocal leukoencephalopathy. J. Neurovirol. 2000, 6, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Bellizzi, A.; Nardis, C.; Anzivino, E.; Rodio, D.; Fioriti, D.; Mischitelli, M.; Chiarini, F.; Pietropaolo, V. Human polyomavirus JC reactivation and pathogenetic mechanisms of progressive multifocal leukoencephalopathy and cancer in the era of monoclonal antibody therapies. J. Neurovirol. 2012, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hauser, K.F.; Hahn, Y.K.; Adjan, V.V.; Zou, S.; Buch, S.K.; Nath, A.; Bruce-Keller, A.J.; Knapp, P.E. HIV-1 tat and morphine have interactive effects on oligodendrocyte survival and morphology. Glia 2009, 57, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Mi, H.; Zhao, J.; Yuan, D.; Ding, J.; Li, H. White matter damage and effects of nadir CD4+ count on patients with asymptomatic HIV associated dementia complex–A DTI study. Radiol. Infect. Dis. 2014, 1, 11–16. [Google Scholar] [CrossRef]
- Bernardo, A.; Agresti, C.; Levi, G. HIV-gp120 affects the functional activity of oligodendrocytes and their susceptibility to complement. J. Neurosci. Res. 1997, 50, 946–957. [Google Scholar] [CrossRef]
- Kimura-Kuroda, J.; Nagashima, K.; Yasui, K. Inhibition of myelin formation by HIV-1 gp120 in rat cerebral cortex culture. Arch. Virol. 1994, 137, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Nukuzuma, S.; Kameoka, M.; Sugiura, S.; Nakamichi, K.; Nukuzuma, C.; Miyoshi, I.; Takegami, T. Exogenous human immunodeficiency virus-1 protein, tat, enhances replication of JC virus efficiently in neuroblastoma cell lines. J. Med. Virol. 2012, 84, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Radja, F.; Kay, D.G.; Albrecht, S.; Jolicoeur, P. Oligodendrocyte-specific expression of human immunodeficiency virus type 1 NEF in transgenic mice leads to vacuolar myelopathy and alters oligodendrocyte phenotype in vitro. J. Virol. 2003, 77, 11745–11753. [Google Scholar] [CrossRef] [PubMed]
- Correa, D.G.; Zimmermann, N.; Doring, T.M.; Wilner, N.V.; Leite, S.C.; Cabral, R.F.; Fonseca, R.P.; Bahia, P.R.; Gasparetto, E.L. Diffusion tensor mr imaging of white matter integrity in HIV-positive patients with planning deficit. Neuroradiology 2015, 57, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Gongvatana, A.; Schweinsburg, B.C.; Taylor, M.J.; Theilmann, R.J.; Letendre, S.L.; Alhassoon, O.M.; Jacobus, J.; Woods, S.P.; Jernigan, T.L.; Ellis, R.J.; et al. White matter tract injury and cognitive impairment in human immunodeficiency virus-infected individuals. J. Neurovirol. 2009, 15, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Uban, K.A.; Herting, M.M.; Williams, P.L.; Ajmera, T.; Gautam, P.; Huo, Y.; Malee, K.M.; Yogev, R.; Csernansky, J.G.; Wang, L.; et al. White matter microstructure among youth with perinatally acquired HIV is associated with disease severity. AIDS 2015, 29, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Wohlschlaeger, J.; Wenger, E.; Mehraein, P.; Weis, S. White matter changes in HIV-1 infected brains: A combined gross anatomical and ultrastructural morphometric investigation of the corpus callosum. Clin. Neurol. Neurosurg. 2009, 111, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Leite, S.C.; Correa, D.G.; Doring, T.M.; Kubo, T.T.; Netto, T.M.; Ferracini, R.; Ventura, N.; Bahia, P.R.; Gasparetto, E.L. Diffusion tensor mri evaluation of the corona radiata, cingulate gyri, and corpus callosum in HIV patients. J. Magn. Reson. Imaging 2013, 38, 1488–1493. [Google Scholar] [CrossRef] [PubMed]
- Xuan, A.; Wang, G.B.; Shi, D.P.; Xu, J.L.; Li, Y.L. Initial study of magnetic resonance diffusion tensor imaging in brain white matter of early aids patients. Chin. Med. J. (Engl.) 2013, 126, 2720–2724. [Google Scholar] [PubMed]
- Boska, M.D.; Dash, P.K.; Knibbe, J.; Epstein, A.A.; Akhter, S.P.; Fields, N.; High, R.; Makarov, E.; Bonasera, S.; Gelbard, H.A.; et al. Associations between brain microstructures, metabolites, and cognitive deficits during chronic HIV-1 infection of humanized mice. Mol. Neurodegener. 2014, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Lackner, P.; Kuenz, B.; Reindl, M.; Morandell, M.; Berger, T.; Schmutzhard, E.; Eggers, C. Antibodies to myelin oligodendrocyte glycoprotein in HIV-1 associated neurocognitive disorder: A cross-sectional cohort study. J. Neuroinflamm. 2010, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Fitting, S.; Booze, R.M.; Hasselrot, U.; Mactutus, C.F. Dose-dependent long-term effects of tat in the rat hippocampal formation: A design-based stereological study. Hippocampus 2010, 20, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J.; Miller, J.A.; Shapshak, P.; Gelman, B.; Singer, E.J.; Hinkin, C.H.; Commins, D.; Morgello, S.; Grant, I.; Horvath, S. Systems analysis of human brain gene expression: Mechanisms for HIV-associated neurocognitive impairment and common pathways with Alzheimer’s disease. BMC Med. Genom. 2013, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.R.; Aksamit, A.J.; Clifford, D.B.; Davis, L.; Koralnik, I.J.; Sejvar, J.J.; Bartt, R.; Major, E.O.; Nath, A. Pml diagnostic criteria: Consensus statement from the aan neuroinfectious disease section. Neurology 2013, 80, 1430–1438. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, L.; Pina-Oviedo, S. HIV disorders of the brain: Pathology and pathogenesis. Front. Biosci. 2006, 11, 718–732. [Google Scholar] [CrossRef] [PubMed]
- Sacktor, N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J. Neurovirol. 2002, 8, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Crossley, K.M.; Agnihotri, S.; Chaganti, J.; Rodriguez, M.L.; McNally, L.P.; Venna, N.; Turbett, S.E.; Gutman, M.; Morey, A.; Koralnik, I.J.; et al. Recurrence of progressive multifocal leukoencephalopathy despite immune recovery in two HIV seropositive individuals. J. Neurovirol. 2016, 22, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Mascarello, M.; Lanzafame, M.; Lattuada, E.; Concia, E.; Ferrari, S. Progressive multifocal leukoencephalopathy in an HIV patient receiving successful long-term haart. J. Neurovirol. 2011, 17, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Gates, T.M.; Cysique, L.A. The chronicity of HIV infection should drive the research strategy of neuroHIV treatment studies: A critical review. CNS Drugs 2016, 30, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Sharpless, N.; Gilbert, D.; Vandercam, B.; Zhou, J.M.; Verdin, E.; Ronnett, G.; Friedman, E.; Dubois-Dalcq, M. The restricted nature of HIV-1 tropism for cultured neural cells. Virology 1992, 191, 813–825. [Google Scholar] [CrossRef]
- Takahashi, K.; Wesselingh, S.L.; Griffin, D.E.; McArthur, J.C.; Johnson, R.T.; Glass, J.D. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Ann. Neurol. 1996, 39, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Albright, A.V.; Strizki, J.; Harouse, J.M.; Lavi, E.; O’Connor, M.; Gonzalez-Scarano, F. HIV-1 infection of cultured human adult oligodendrocytes. Virology 1996, 217, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Albright, A.; Lavi, E.; O’Connor, M.; González-Scarano, F. HIV-1 infection of a CD4-negative primary cell type: The oligodendrocyte. Perspect. Drug Discov. Des. 1996, 5, 43–50. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Katafiasz, B.; Fox, H.; Xiong, H. HIV-1 gp120-induced axonal injury detected by accumulation of β-amyloid precursor protein in adult rat corpus callosum. J. Neuroimmune Pharmacol. 2011, 6, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.R.; McCandless, E.E.; Dorsey, D.; Klein, R.S. CXCR4 promotes differentiation of oligodendrocyte progenitors and remyelination. Proc. Natl. Acad. Sci. USA 2010, 107, 11062–11067. [Google Scholar] [CrossRef] [PubMed]
- Stettner, M.R.; Nance, J.A.; Wright, C.A.; Kinoshita, Y.; Kim, W.K.; Morgello, S.; Rappaport, J.; Khalili, K.; Gordon, J.; Johnson, E.M. Smad proteins of oligodendroglial cells regulate transcription of JC virus early and late genes coordinately with the tat protein of human immunodeficiency virus type 1. J. Gen. Virol. 2009, 90, 2005–2014. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.A.; Nance, J.A.; Johnson, E.M. Effects of tat proteins and tat mutants of different human immunodeficiency virus type 1 clades on glial JC virus early and late gene transcription. J. Gen. Virol. 2013, 94, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Cinque, P.; Pierotti, C.; Vigano, M.G.; Bestetti, A.; Fausti, C.; Bertelli, D.; Lazzarin, A. The good and evil of haart in HIV-related progressive multifocal leukoencephalopathy. J. Neurovirol. 2001, 7, 358–363. [Google Scholar] [PubMed]
- Martin-Blondel, G.; Bauer, J.; Cuvinciuc, V.; Uro-Coste, E.; Debard, A.; Massip, P.; Delisle, M.B.; Lassmann, H.; Marchou, B.; Mars, L.T.; et al. In situ evidence of jc virus control by CD8+ t cells in pml-iris during HIV infection. Neurology 2013, 81, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Codazzi, F.; Menegon, A.; Zacchetti, D.; Ciardo, A.; Grohovaz, F.; Meldolesi, J. HIV-1 gp120 glycoprotein induces [Ca2+]i responses not only in type-2 but also type-1 astrocytes and oligodendrocytes of the rat cerebellum. Eur. J. Neurosci. 1995, 7, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Tsunoda, I.; Fujinami, R.S. Inside-out versus outside-in models for virus induced demyelination: Axonal damage triggering demyelination. Springer Semin. Immunopathol. 2002, 24, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Langford, T.D.; Letendre, S.L.; Marcotte, T.D.; Ellis, R.J.; McCutchan, J.A.; Grant, I.; Mallory, M.E.; Hansen, L.A.; Archibald, S.; Jernigan, T.; et al. Severe, demyelinating leukoencephalopathy in AIDS patients on antiretroviral therapy. AIDS 2002, 16, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.W.; DeGirolami, U.; Henin, D.; Bolgert, F.; Hauw, J.J. Human immunodeficiency virus (HIV) leukoencephalopathy and the microcirculation. J. Neuropathol. Exp. Neurol. 1990, 49, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.; Afonso, P.V.; Gessain, A.; Ceccaldi, P.E. Blood-brain barrier and retroviral infections. Virulence 2012, 3, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Strazza, M.; Pirrone, V.; Wigdahl, B.; Nonnemacher, M.R. Breaking down the barrier: The effects of HIV-1 on the blood-brain barrier. Brain Res. 2011, 1399, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Louboutin, J.P.; Strayer, D.S. Blood-brain barrier abnormalities caused by HIV-1 gp120: Mechanistic and therapeutic implications. ScientificWorldJournal 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Titulaer, M.J.; Dalmau, J. Antibodies to nmda receptor, blood-brain barrier disruption and schizophrenia: A theory with unproven links. Mol. Psychiatry 2014, 19, 1054. [Google Scholar] [CrossRef] [PubMed]
- Avison, M.; Nath, A.; Avison, R.; Schmitt, F.; Greenberg, R.; Berger, J. Viremia in the presence of blood-brain barrier compromise increases severity of HIV-associated neurocognitive impairment. In Annals of Neurology; Wiley-Liss Div John Wiley & Sons Inc.: New York, NY, USA, 2003; p. S49. [Google Scholar]
- Gray, F.; Belec, L.; Chretien, F.; Dubreuil-Lemaire, M.L.; Ricolfi, F.; Wingertsmann, L.; Poron, F.; Gherardi, R. Acute, relapsing brain oedema with diffuse blood-brain barrier alteration and axonal damage in the acquired immunodeficiency syndrome. Neuropathol. Appl. Neurobiol. 1998, 24, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Chintawar, S.; Cayrol, R.; Antel, J.; Pandolfo, M.; Prat, A. Blood-brain barrier promotes differentiation of human fetal neural precursor cells. Stem Cells 2009, 27, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Plane, J.M.; Andjelkovic, A.V.; Keep, R.F.; Parent, J.M. Intact and injured endothelial cells differentially modulate postnatal murine forebrain neural stem cells. Neurobiol. Dis. 2010, 37, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Relucio, J.; Menezes, M.J.; Miyagoe-Suzuki, Y.; Takeda, S.; Colognato, H. Laminin regulates postnatal oligodendrocyte production by promoting oligodendrocyte progenitor survival in the subventricular zone. Glia 2012, 60, 1451–1467. [Google Scholar] [CrossRef] [PubMed]
- Juliet, P.A.; Frost, E.E.; Balasubramaniam, J.; Del Bigio, M.R. Toxic effect of blood components on perinatal rat subventricular zone cells and oligodendrocyte precursor cell proliferation, differentiation and migration in culture. J. Neurochem. 2009, 109, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Antel, J.P.; Williams, K.; Blain, M.; McRea, E.; McLaurin, J. Oligodendrocyte lysis by CD4+ T cells independent of tumor necrosis factor. Ann. Neurol. 1994, 35, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Navikas, V.; Link, J.; Persson, C.; Olsson, T.; Hojeberg, B.; Ljungdahl, A.; Link, H.; Wahren, B. Increased mrna expression of IL-6, IL-10, TNF-α, and perforin in blood mononuclear cells in human HIV infection. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1995, 9, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Gao, J.; Taxman, D.J.; Ting, J.P.; Su, L. HIV-1 infection induces interleukin-1beta production via TLR8 protein-dependent and NLRP3 inflammasome mechanisms in human monocytes. J. Biol. Chem. 2014, 289, 21716–21726. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.L.; Giuliani, F.; Power, C.; Imai, Y.; Yong, V.W. Interleukin-1β promotes oligodendrocyte death through glutamate excitotoxicity. Ann. Neurol. 2003, 53, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Jayadev, S.; Yun, B.; Nguyen, H.; Yokoo, H.; Morrison, R.S.; Garden, G.A. The glial response to CNS HIV infection includes p53 activation and increased expression of p53 target genes. J. Neuroimmune Pharmacol. 2007, 2, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.D.; Xavier, J.M.; Steer, C.J.; Rodrigues, C.M. The role of p53 in apoptosis. Discov. Med. 2010, 9, 145–152. [Google Scholar] [PubMed]
- Liu, H.; Xiong, H.; University of Nebraska Medical Center, Omaha, NE, USA. Unpublished work. 2016.
- Remillard, C.V.; Yuan, J.X. Activation of k+ channels: An essential pathway in programmed cell death. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 286, L49–L67. [Google Scholar] [CrossRef] [PubMed]
- Hahn, Y.K.; Podhaizer, E.M.; Hauser, K.F.; Knapp, P.E. HIV-1 alters neural and glial progenitor cell dynamics in the central nervous system: Coordinated response to opiates during maturation. Glia 2012, 60, 1871–1887. [Google Scholar] [CrossRef] [PubMed]
- Buch, S.K.; Khurdayan, V.K.; Lutz, S.E.; Knapp, P.E.; El-Hage, N.; Hauser, K.F. Glial-restricted precursors: Patterns of expression of opioid receptors and relationship to human immunodeficiency virus-1 tat and morphine susceptibility in vitro. Neuroscience 2007, 146, 1546–1554. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Biswas, K.; Nag, S.; Ramachandra, S.G.; Das Sarma, J. Microglia play a major role in direct viral-induced demyelination. Clin. Dev. Immunol. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Marker, D.F.; Puccini, J.M.; Mockus, T.E.; Barbieri, J.; Lu, S.M.; Gelbard, H.A. LRRK2 kinase inhibition prevents pathological microglial phagocytosis in response to HIV-1 Tat protein. J. Neuroinflamm. 2012, 9, 261. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.E.; Marker, D.F.; Puccini, J.M.; Muly, E.C.; Lu, S.M.; Gelbard, H.A. Ultrastructure of microglia-synapse interactions in the HIV-1 Tat-injected murine central nervous system. Commun. Integr. Biol. 2013, 6, e27670. [Google Scholar] [CrossRef] [PubMed]
- Fulmer, C.G.; VonDran, M.W.; Stillman, A.A.; Huang, Y.; Hempstead, B.L.; Dreyfus, C.F. Astrocyte-derived bdnf supports myelin protein synthesis after cuprizone-induced demyelination. J. Neurosci. 2014, 34, 8186–8196. [Google Scholar] [CrossRef] [PubMed]
- Esiri, M.M.; Morris, C.S.; Millard, P.R. Fate of oligodendrocytes in HIV-1 infection. AIDS 1991, 5, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Bradl, M.; Lassmann, H. Oligodendrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Payne, S.C.; Bartlett, C.A.; Savigni, D.L.; Harvey, A.R.; Dunlop, S.A.; Fitzgerald, M. Early proliferation does not prevent the loss of oligodendrocyte progenitor cells during the chronic phase of secondary degeneration in a cns white matter tract. PLoS ONE 2013, 8, e65710. [Google Scholar] [CrossRef] [PubMed]
- Mishra, M.; Taneja, M.; Malik, S.; Khalique, H.; Seth, P. Human immunodeficiency virus type 1 Tat modulates proliferation and differentiation of human neural precursor cells: Implication in neuroaids. J. Neurovirol. 2010, 16, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Hahn, Y.K.; Vo, P.; Fitting, S.; Block, M.L.; Hauser, K.F.; Knapp, P.E. β-chemokine production by neural and glial progenitor cells is enhanced by HIV-1 Tat: Effects on microglial migration. J. Neurochem. 2010, 114, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Sun, L.; Jia, B.; Lan, X.; Zhu, B.; Wu, Y.; Zheng, J. HIV-1-infected and immune-activated macrophages induce astrocytic differentiation of human cortical neural progenitor cells via the stat3 pathway. PLoS ONE 2011, 6, e19439. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Lee, X.; Shao, Z.; Thill, G.; Ji, B.; Relton, J.; Levesque, M.; Allaire, N.; Perrin, S.; Sands, B.; et al. Lingo-1 is a component of the nogo-66 receptor/p75 signaling complex. Nat. Neurosci. 2004, 7, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Miller, R.H.; Lee, X.; Scott, M.L.; Shulag-Morskaya, S.; Shao, Z.; Chang, J.; Thill, G.; Levesque, M.; Zhang, M.; et al. Lingo-1 negatively regulates myelination by oligodendrocytes. Nat. Neurosci. 2005, 8, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Bongarzone, E.R.; Byravan, S.; Givogri, M.I.; Schonmann, V.; Campagnoni, A.T. Platelet-derived growth factor and basic fibroblast growth factor regulate cell proliferation and the expression of notch-1 receptor in a new oligodendrocyte cell line. J. Neurosci. Res. 2000, 62, 319–328. [Google Scholar] [CrossRef]
- Kim, H.; Shin, J.; Kim, S.; Poling, J.; Park, H.C.; Appel, B. Notch-regulated oligodendrocyte specification from radial glia in the spinal cord of zebrafish embryos. Dev. Dyn. 2008, 237, 2081–2089. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Kagawa, T.; Wada, T.; Muroyama, Y.; Takada, S.; Ikenaka, K. Wnt signaling controls the timing of oligodendrocyte development in the spinal cord. Dev. Biol. 2005, 282, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-H.; Li, J.-J.; Wang, Q.-J.; Zhao, W.-Q.; Hong, J.; Lou, S.-j.; Xu, X.-H. WNK1 is involved in Nogo66 inhibition of OPC differentiation. Mol. Cell. Neurosci. 2015, 65, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Bhat, N.R.; Zhang, P.; Mohanty, S.B. P38 map kinase regulation of oligodendrocyte differentiation with creb as a potential target. Neurochem. Res. 2007, 32, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Chew, L.J.; Coley, W.; Cheng, Y.; Gallo, V. Mechanisms of regulation of oligodendrocyte development by p38 mitogen-activated protein kinase. J. Neurosci. 2010, 30, 11011–11027. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.I.; Narayanan, S.P.; Morse, E.N.; Shick, H.E.; Yin, X.; Kidd, G.; Avila, R.L.; Kirschner, D.A.; Macklin, W.B. Constitutively active akt induces enhanced myelination in the cns. J. Neurosci. 2008, 28, 7174–7183. [Google Scholar] [CrossRef] [PubMed]
- Mitew, S.; Hay, C.M.; Peckham, H.; Xiao, J.; Koenning, M.; Emery, B. Mechanisms regulating the development of oligodendrocytes and central nervous system myelin. Neuroscience 2014, 276, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Yoshida, S. Mechanisms of remyelination: Recent insight from experimental models. Biomol. Concepts 2014, 5, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.Q.; Saksena, M.M.; Soriano, V.; Vispo, E.; Saksena, N.K. Differential regulation of cytotoxicity pathway discriminating between HIV, HCV mono-and co-infection identified by transcriptome profiling of PBMCs. Virol. J. 2015, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Weiser, K.; Barton, M.; Gershoony, D.; DasGupta, R.; Cardozo, T.; Tang, S.-J. HIV’s nef interacts with β-catenin of the wnt signaling pathway in hek293 cells. PLoS ONE 2013, 8, e77865. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.H.; Narasipura, S.D.; Kim, S.; Seaton, M.S.; Lutgen, V.; Al-Harthi, L. Dynamic interaction between astrocytes and infiltrating PBMCs in context of neuroAIDS. Glia 2015, 63, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Argaw, A.T.; Gurfein, B.T.; Zameer, A.; Snyder, B.J.; Ge, C.; Lu, Q.R.; Rowitch, D.H.; Raine, C.S.; Brosnan, C.F.; et al. Notch1 signaling plays a role in regulating precursor differentiation during CNS remyelination. Proc. Natl. Acad. Sci. USA 2009, 106, 19162–19167. [Google Scholar] [CrossRef] [PubMed]
- Curry, C.L.; Reed, L.L.; Golde, T.E.; Miele, L.; Nickoloff, B.J.; Foreman, K.E. Gamma secretase inhibitor blocks notch activation and induces apoptosis in kaposi's sarcoma tumor cells. Oncogene 2005, 24, 6333–6344. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, S.S.; Wu, Y.; Tuohy, T.M.; Xue, H.; Cai, J.; Back, S.A.; Sherman, L.S.; Fischer, I.; Rao, M.S. CD44 expression identifies astrocyte-restricted precursor cells. Dev. Biol. 2004, 276, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Tuohy, T.M.; Wallingford, N.; Liu, Y.; Chan, F.H.; Rizvi, T.; Xing, R.; Bebo, B.; Rao, M.S.; Sherman, L.S. CD44 overexpression by oligodendrocytes: A novel mouse model of inflammation-independent demyelination and dysmyelination. Glia 2004, 47, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Suyama, M.; Daikoku, E.; Goto, T.; Sano, K.; Morikawa, Y. Reactivation from latency displays HIV particle budding at plasma membrane, accompanying CD44 upregulation and recruitment. Retrovirology 2009, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Silverberg, M.J.; Xu, L.; Chen, L.H.; Castor, B.; Martinez-Maza, O.; Abrams, D.I.; Zha, H.D.; Haque, R.; Said, J. A comparative study of molecular characteristics of diffuse large b-cell lymphoma from patients with and without human immunodeficiency virus infection. Clin. Cancer Res. 2015, 21, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Fields, J.; Dumaop, W.; Langford, T.D.; Rockenstein, E.; Masliah, E. Role of neurotrophic factor alterations in the neurodegenerative process in HIV associated neurocognitive disorders. J. Neuroimmune Pharmacol. 2014, 9, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Peferoen, L.; Kipp, M.; van der Valk, P.; van Noort, J.M.; Amor, S. Oligodendrocyte-microglia cross-talk in the central nervous system. Immunology 2014, 141, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Erlandsson, A.; Brannvall, K.; Gustafsdottir, S.; Westermark, B.; Forsberg-Nilsson, K. Autocrine/paracrine platelet-derived growth factor regulates proliferation of neural progenitor cells. Cancer Res. 2006, 66, 8042–8048. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Boije, M.; Westermark, B.; Uhrbom, L. PDGF-B can sustain self-renewal and tumorigenicity of experimental glioma-derived cancer-initiating cells by preventing oligodendrocyte differentiation. Neoplasia 2011, 13, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Azim, K.; Butt, A.M. GSK3β negatively regulates oligodendrocyte differentiation and myelination in vivo. Glia 2011, 59, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Burke, K.; Kantor, C.; Miller, R.H.; Yang, Y. Cyclin-dependent kinase 5 mediates adult OPC maturation and myelin repair through modulation of Akt and GSK-3β signaling. J. Neurosci. 2014, 34, 10415–10429. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Yang, L.; Yao, H.; Buch, S. Platelet-derived growth factor-BB restores HIV Tat -mediated impairment of neurogenesis: Role of GSK-3β/β-catenin. J. Neuroimmune Pharmacol. 2014, 9, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Frederick, T.J.; Min, J.; Altieri, S.C.; Mitchell, N.E.; Wood, T.L. Synergistic induction of cyclin D1 in oligodendrocyte progenitor cells by IGF-I and FGF-2 requires differential stimulation of multiple signaling pathways. Glia 2007, 55, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, D.; Garcia, L.; Cartier, L.; Kettlun, A.M.; Vergara, C.; Collados, L.; Valenzuela, M.A. Trophic factors in cerebrospinal fluid and spinal cord of patients with tropical spastic paraparesis, HIV, and creutzfeldt-jakob disease. AIDS Res. Hum. Retrovir. 2006, 22, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ascherl, G.; Sgadari, C.; Bugarini, R.; Bogner, J.; Schatz, O.; Ensoli, B.; Sturzl, M. Serum concentrations of fibroblast growth factor 2 are increased in HIV type 1-infected patients and inversely related to survival probability. AIDS Res. Hum. Retrovir. 2001, 17, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Maggirwar, S.B.; Tong, N.; Ramirez, S.; Gelbard, H.A.; Dewhurst, S. HIV-1 tat-mediated activation of glycogen synthase kinase-3β contributes to Tat-mediated neurotoxicity. J. Neurochem. 1999, 73, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Sui, Z.; Sniderhan, L.F.; Fan, S.; Kazmierczak, K.; Reisinger, E.; Kovacs, A.D.; Potash, M.J.; Dewhurst, S.; Gelbard, H.A.; Maggirwar, S.B. Human immunodeficiency virus-encoded tat activates glycogen synthase kinase-3β to antagonize nuclear factor-kappab survival pathway in neurons. Eur. J. Neurosci. 2006, 23, 2623–2634. [Google Scholar] [CrossRef] [PubMed]
- Lannuzel, A.; Barnier, J.V.; Hery, C.; Huynh, V.T.; Guibert, B.; Gray, F.; Vincent, J.D.; Tardieu, M. Human immunodeficiency virus type 1 and its coat protein gp120 induce apoptosis and activate JNK and ERK mitogen-activated protein kinases in human neurons. Ann. Neurol. 1997, 42, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, F.; Benito-Muñoz, M.; Panicker, M.; Matute, C. Nmda modulates oligodendrocyte differentiation of subventricular zone cells through pkc activation. Front. Cell. Neurosci. 2013, 7, 261. [Google Scholar] [CrossRef] [PubMed]
- Lundgaard, I.; Luzhynskaya, A.; Stockley, J.H.; Wang, Z.; Evans, K.A.; Swire, M.; Volbracht, K.; Gautier, H.O.; Franklin, R.J.; Attwell, D.; et al. Neuregulin and BDNF induce a switch to nmda receptor-dependent myelination by oligodendrocytes. PLoS Biol. 2013, 11, e1001743. [Google Scholar] [CrossRef] [PubMed]
- Zeitler, M.; Steringer, J.P.; Muller, H.M.; Mayer, M.P.; Nickel, W. HIV-tat forms phosphoinositide dependent membrane pores implicated in unconventional protein secretion. J. Biol. Chem. 2015, 290, 21976–21984. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Cejudo, J.; Gutierrez-Fernandez, M.; Otero-Ortega, L.; Rodriguez-Frutos, B.; Fuentes, B.; Vallejo-Cremades, M.T.; Hernanz, T.N.; Cerdan, S.; Diez-Tejedor, E. Brain-derived neurotrophic factor administration mediated oligodendrocyte differentiation and myelin formation in subcortical ischemic stroke. Stroke 2015, 46, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Tsiperson, V.; Huang, Y.; Bagayogo, I.; Song, Y.; VonDran, M.W.; DiCicco-Bloom, E.; Dreyfus, C.F. Brain-derived neurotrophic factor deficiency restricts proliferation of oligodendrocyte progenitors following cuprizone-induced demyelination. ASN Neuro 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Vondran, M.W.; Clinton-Luke, P.; Honeywell, J.Z.; Dreyfus, C.F. BDNF+/− mice exhibit deficits in oligodendrocyte lineage cells of the basal forebrain. Glia 2010, 58, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Bachis, A.; Avdoshina, V.; Zecca, L.; Parsadanian, M.; Mocchetti, I. Human immunodeficiency virus type 1 alters brain-derived neurotrophic factor processing in neurons. J. Neurosci. 2012, 32, 9477–9484. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Tao, Y.; Greenblatt, J.; Roeder, R.G. A cofactor, tip30, specifically enhances HIV-1 tat-activated transcription. Proc. Natl. Acad. Sci. USA 1998, 95, 2146–2151. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xiao, L.; Li, C.; Liu, X.; Liu, M.; Shao, Q.; Wang, D.; Huang, A.; He, C. Tip30 inhibits oligodendrocyte precursor cell differentiation via cytoplasmic sequestration of olig1. Glia 2015, 63, 684–698. [Google Scholar] [CrossRef] [PubMed]
- Attali, B.; Wang, N.; Kolot, A.; Sobko, A.; Cherepanov, V.; Soliven, B. Characterization of delayed rectifier kv channels in oligodendrocytes and progenitor cells. J. Neurosci. 1997, 17, 8234–8245. [Google Scholar] [PubMed]
- Chittajallu, R.; Chen, Y.; Wang, H.; Yuan, X.; Ghiani, C.A.; Heckman, T.; McBain, C.J.; Gallo, V. Regulation of kv1 subunit expression in oligodendrocyte progenitor cells and their role in g1/s phase progression of the cell cycle. Proc. Natl. Acad. Sci. USA 2002, 99, 2350–2355. [Google Scholar] [CrossRef] [PubMed]
- Peretz, A.; Gil-Henn, H.; Sobko, A.; Shinder, V.; Attali, B.; Elson, A. Hypomyelination and increased activity of voltage-gated k+ channels in mice lacking protein tyrosine phosphatase epsilon. EMBO J. 2000, 19, 4036–4045. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, A.S.; Greenwood, K.; Wilkin, G.; Butt, A.M. Kir4.1 expression by astrocytes and oligodendrocytes in CNS white matter: A developmental study in the rat optic nerve. J. Anat. 2004, 204, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Neusch, C.; Rozengurt, N.; Jacobs, R.E.; Lester, H.A.; Kofuji, P. Kir4.1 potassium channel subunit is crucial for oligodendrocyte development and in vivo myelination. J. Neurosci. 2001, 21, 5429–5438. [Google Scholar] [PubMed]
- Tegla, C.A.; Cudrici, C.; Rozycka, M.; Soloviova, K.; Ito, T.; Singh, A.K.; Khan, A.; Azimzadeh, P.; Andrian-Albescu, M.; Niculescu, F.; et al. C5b-9-activated, k(v)1.3 channels mediate oligodendrocyte cell cycle activation and dedifferentiation. Exp. Mol. Pathol. 2011, 91, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Cheli, V.T.; Santiago Gonzalez, D.A.; Spreuer, V.; Paez, P.M. Voltage-gated Ca2+ entry promotes oligodendrocyte progenitor cell maturation and myelination in vitro. Exp. Neurol. 2015, 265, 69–83. [Google Scholar] [CrossRef] [PubMed]
- French, H.M.; Reid, M.; Mamontov, P.; Simmons, R.A.; Grinspan, J.B. Oxidative stress disrupts oligodendrocyte maturation. J. Neurosci. Res. 2009, 87, 3076–3087. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.K.; Monnerie, H.; Mannell, M.V.; Gannon, P.J.; Espinoza, C.A.; Erickson, M.A.; Bruce-Keller, A.J.; Gelman, B.B.; Briand, L.A.; Pierce, R.C.; et al. Altered oligodendrocyte maturation and myelin maintenance: The role of antiretrovirals in HIV-associated neurocognitive disorders. J. Neuropathol. Exp. Neurol. 2015, 74, 1093–1118. [Google Scholar] [CrossRef] [PubMed]
- Hoare, J.; Fouche, J.P.; Phillips, N.; Joska, J.A.; Paul, R.; Donald, K.A.; Thomas, K.G.; Stein, D.J. White matter micro-structural changes in art-naive and art-treated children and adolescents infected with HIV in south Africa. AIDS 2015, 29, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Borjabad, A.; Morgello, S.; Chao, W.; Kim, S.Y.; Brooks, A.I.; Murray, J.; Potash, M.J.; Volsky, D.J. Significant effects of antiretroviral therapy on global gene expression in brain tissues of patients with HIV-1-associated neurocognitive disorders. PLoS Pathog. 2011, 7, e1002213. [Google Scholar] [CrossRef] [PubMed]
- Heaton, R.K.; Clifford, D.B.; Franklin, D.R., Jr.; Woods, S.P.; Ake, C.; Vaida, F.; Ellis, R.J.; Letendre, S.L.; Marcotte, T.D.; Atkinson, J.H.; et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: Charter study. Neurology 2010, 75, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Baas, D.; Bourbeau, D.; Sarlieve, L.L.; Ittel, M.E.; Dussault, J.H.; Puymirat, J. Oligodendrocyte maturation and progenitor cell proliferation are independently regulated by thyroid hormone. Glia 1997, 19, 324–332. [Google Scholar] [CrossRef]
- Grinspan, J.B.; Stern, J.L.; Franceschini, B.; Pleasure, D. Trophic effects of basic fibroblast growth factor (bFGF) on differentiated oligodendroglia: A mechanism for regeneration of the oligodendroglial lineage. J. Neurosci. Res. 1993, 36, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.H. Regulation of oligodendrocyte development in the vertebrate CNS. Prog. Neurobiol. 2002, 67, 451–467. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Xu, E.; Liu, J.; Xiong, H. Oligodendrocyte Injury and Pathogenesis of HIV-1-Associated Neurocognitive Disorders. Brain Sci. 2016, 6, 23. https://doi.org/10.3390/brainsci6030023
Liu H, Xu E, Liu J, Xiong H. Oligodendrocyte Injury and Pathogenesis of HIV-1-Associated Neurocognitive Disorders. Brain Sciences. 2016; 6(3):23. https://doi.org/10.3390/brainsci6030023
Chicago/Turabian StyleLiu, Han, Enquan Xu, Jianuo Liu, and Huangui Xiong. 2016. "Oligodendrocyte Injury and Pathogenesis of HIV-1-Associated Neurocognitive Disorders" Brain Sciences 6, no. 3: 23. https://doi.org/10.3390/brainsci6030023