Gender Disparities in Epidemiology, Treatment, and Outcome for Head and Neck Cancer in Germany: A Population-Based Long-Term Analysis from 1996 to 2016 of the Thuringian Cancer Registry
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
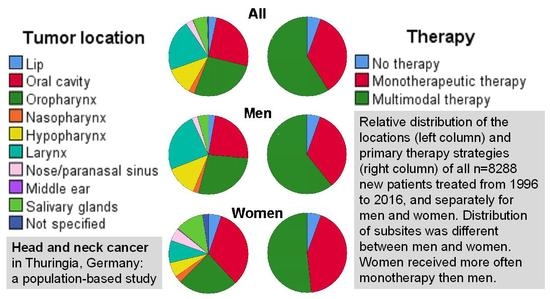
2.1. Patient’s Characteristics, Tumor Characteristics and Therapy Strategies
2.2. Epidemiology: Crude Incidences of Patients’ and Tumor Characteristics between 1996 and 2016
2.3. Treatment Strategies
2.4. Tumor Recurrence and Overall Survival
3. Discussion
4. Material and Methods
4.1. Patients
4.2. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chow, L.Q. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Rettig, E.; Zaidi, M.; Faraji, F.; Eisele, D.W.; El Asmar, M.; Fung, N.; D’Souza, G.; Fakhry, C. Oropharyngeal cancer is no longer a disease of younger patients and the prognostic advantage of Human Papillomavirus is attenuated among older patients: Analysis of the National Cancer Database. Oral Oncol. 2018, 83, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Faraji, F.; Rettig, E.M.; Ms, H.T.; El Asmar, M.; Fung, N.; Eisele, D.W.; Fakhry, C. The prevalence of human papillomavirus in oropharyngeal cancer is increasing regardless of sex or race, and the influence of sex and race on survival is modified by human papillomavirus tumor status. Cancer 2019, 125, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Rettig, E.M.; D’Souza, G. Epidemiology of Head and Neck Cancer. Surg. Oncol. Clin. N. Am. 2015, 24, 379–396. [Google Scholar] [CrossRef]
- Gatta, G.; Botta, L.; Huerta, J.M.; Anderson, L.A.; Pierannunzio, D.; Licitra, L.; Hackl, M.; Zielonke, N.; Oberaigner, W.; Van Eycken, E.; et al. Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: The EUROCARE-5 population-based study. Eur. J. Cancer 2015, 51, 2130–2143. [Google Scholar] [CrossRef]
- Thompson-Harvey, A.; Yetukuri, M.; Ba, A.R.H.; Mph, M.C.S.; Boakye, E.A.; Varvares, M.A.; Osazuwa-Peters, N. Rising incidence of late-stage head and neck cancer in the United States. Cancer 2020, 126, 1090–1101. [Google Scholar] [CrossRef]
- Taberna, M.; Oliva, M.; Mesía, R. Cetuximab-Containing Combinations in Locally Advanced and Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2019, 9, 383. [Google Scholar] [CrossRef]
- Braakhuis, B.J.; Leemans, C.R.; Visser, O. Incidence and survival trends of head and neck squamous cell carcinoma in the Netherlands between 1989 and 2011. Oral Oncol. 2014, 50, 670–675. [Google Scholar] [CrossRef] [Green Version]
- Jakobsen, K.K.; Grønhøj, C.; Jensen, D.H.; Karnov, K.K.S.; Agander, T.K.; Specht, L.; Von Buchwald, C. Increasing incidence and survival of head and neck cancers in Denmark: A nation-wide study from 1980 to 2014. Acta Oncol. 2018, 57, 1143–1151. [Google Scholar] [CrossRef] [Green Version]
- Janz, T.A.; Graboyes, E.M.; Nguyen, S.A.; Ellis, M.A.; Neskey, D.M.; Harruff, E.E.; Lentsch, E.J. A Comparison of the NCDB and SEER Database for Research Involving Head and Neck Cancer. Otolaryngol. Head Neck Surg. 2019, 160, 284–294. [Google Scholar] [CrossRef]
- Mallin, K.; Browner, A.; Palis, B.; Gay, G.; McCabe, R.; Nogueira, L.; Yabroff, R.; Shulman, L.; Facktor, M.; Winchester, D.P.; et al. Incident Cases Captured in the National Cancer Database Compared with Those in U.S. Population Based Central Cancer Registries in 2012–2014. Ann. Surg. Oncol. 2019, 26, 1604–1612. [Google Scholar] [CrossRef]
- Guntinas-Lichius, O.; Wendt, T.G.; Kornetzky, N.; Buentzel, J.; Esser, D.; Böger, D.; Müller, A.; Schultze-Mosgau, S.; Schlattmann, P.; Schmalenberg, H. Trends in epidemiology and treatment and outcome for head and neck cancer: A population-based long-term analysis from 1996 to 2011 of the Thuringian cancer registry. Oral Oncol. 2014, 50, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weatherspoon, D.J.; Chattopadhyay, A.; Boroumand, S.; Garcia, A.I. Oral cavity and oropharyngeal cancer incidence trends and disparities in the United States: 2000–2010. Cancer Epidemiol. 2015, 39, 497–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tataru, D.; Mak, V.; Simo, R.; Davies, E.; Gallagher, J.E. Trends in the epidemiology of head and neck cancer in London. Clin. Otolaryngol. 2016, 42, 104–114. [Google Scholar] [CrossRef] [Green Version]
- Simard, E.P.; Torre, L.A.; Jemal, A. International trends in head and neck cancer incidence rates: Differences by country, sex and anatomic site. Oral Oncol. 2014, 50, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Mourad, M.; Jetmore, T.; Jategaonkar, A.A.; Moubayed, S.; Moshier, E.; Urken, M.L. Epidemiological Trends of Head and Neck Cancer in the United States: A SEER Population Study. J. Oral Maxillofac. Surg. 2017, 75, 2562–2572. [Google Scholar] [CrossRef] [Green Version]
- Leemans, C.R.; Braakhuis, B.J.M.; Brakenhoff, R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer 2011, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Tinhofer, I.; Jöhrens, K.; Keilholz, U.; Kaufmann, A.; Lehmann, A.; Weichert, W.; Stenzinger, A.; Stromberger, C.; Klinghammer, K.; Becker, E.-T.; et al. Contribution of human papilloma virus to the incidence of squamous cell carcinoma of the head and neck in a European population with high smoking prevalence. Eur. J. Cancer 2015, 51, 514–521. [Google Scholar] [CrossRef]
- Göllnitz, I.; Inhestern, J.; Wendt, T.G.; Buentzel, J.; Esser, D.; Böger, D.; Muller, A.; Piesold, J.-U.; Schultze-Mosgau, S.; Eigendorff, E.; et al. Role of comorbidity on outcome of head and neck cancer: A population-based study in Thuringia, Germany. Cancer Med. 2016, 5, 3260–3271. [Google Scholar] [CrossRef] [Green Version]
- Anantharaman, D.; Abedi-Ardekani, B.; Beachler, D.C.; Gheit, T.; Olshan, A.F.; Wisniewski, K.; Wunsch-Filho, V.; Toporcov, T.N.; Tajara, E.H.; Levi, J.E.; et al. Geographic heterogeneity in the prevalence of human papillomavirus in head and neck cancer. Int. J. Cancer 2017, 140, 1968–1975. [Google Scholar] [CrossRef] [PubMed]
- Osazuwa-Peters, N.; Simpson, M.C.; Massa, S.T.; Boakye, E.A.; Antisdel, J.L.; Varvares, M.A. 40-year incidence trends for oropharyngeal squamous cell carcinoma in the United States. Oral Oncol. 2017, 74, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Halmos, G.B.; Bras, L.; Siesling, S.; Van Der Laan, B.F.; Langendijk, J.A.; Van Dijk, B.A. Age-specific incidence and treatment patterns of head and neck cancer in the Netherlands-A cohort study. Clin. Otolaryngol. 2018, 43, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Park, A.; Alabaster, A.; Shen, H.; Mell, L.K.; Katzel, J.A. Undertreatment of women with locoregionally advanced head and neck cancer. Cancer 2019, 125, 3033–3039. [Google Scholar] [CrossRef] [PubMed]
- Benchetrit, L.; Torabi, S.J.; Tate, J.P.; Mehra, S.; Osborn, H.A.; Young, M.R.; Burtness, B.; Judson, B.L. Gender disparities in head and neck cancer chemotherapy clinical trials participation and treatment. Oral Oncol. 2019, 94, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Micheli, A.; Ciampichini, R.; Oberaigner, W.; Ciccolallo, L.; De Vries, E.; Izarzugaza, I.; Zambon, P.; Gatta, G.; De Angelis, R.; EUROCARE Working Group. The advantage of women in cancer survival: An analysis of EUROCARE-4 data. Eur. J. Cancer 2009, 45, 1017–1027. [Google Scholar] [CrossRef]
- Li, H.; Li, E.Y.; Kejner, A.E. Treatment modality and outcomes in larynx cancer patients: A sex-based evaluation. Head Neck 2019, 41, 3764–3774. [Google Scholar] [CrossRef]
- Mundi, N.; Ghasemi, F.; Zeng, P.Y.; Prokopec, S.D.; Patel, K.; Kim, H.A.J.; Di Gravio, E.; MacNeil, D.; Khan, M.I.; Barrett, J.W.; et al. Sex disparities in head & neck cancer driver genes: An analysis of the TCGA dataset. Oral Oncol. 2020, 104, 104614. [Google Scholar] [CrossRef]
- Fritz, A. International Classification of Diseases for Oncology (ICD-O), 3rd ed.; 1st Revision EDN; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Available online: http://www.statistik.thueringen.de (accessed on 1 March 2020).
- European Commission. Revision of the European Standard Population—Report of Eurostat’s Task Force; European Commission: Luxembourg, 2013. [Google Scholar]




| Tumor Site | Frequency (N) | % |
|---|---|---|
| All | 8288 | 100 |
| Lips | 275 | 3.3 |
| Cavity of mouth | 2116 | 25.5 |
| Oropharynx | 2240 | 27.0 |
| Nasopharynx | 191 | 2.3 |
| Hypopharynx | 941 | 11.4 |
| Larynx | 1737 | 21.0 |
| Nose and paranasal sinus | 246 | 3.0 |
| Middle ear | 7 | 0.1 |
| Salivary glands | 490 | 5.9 |
| Not classifiable * | 45 | 0.5 |
| T classification | ||
| T1 | 2019 | 24.4 |
| T2 | 1836 | 22.2 |
| T3 | 1303 | 15.7 |
| T4 | 1941 | 23.4 |
| Tx | 1189 | 14.3 |
| N classification | ||
| N0 | 3352 | 40.4 |
| N1 | 783 | 9.4 |
| N2 | 2457 | 29.6 |
| N3 | 285 | 3.4 |
| Nx | 1411 | 17.0 |
| M classification | ||
| M0 | 7212 | 87.0 |
| M1 | 362 | 4.4 |
| Mx | 714 | 8.6 |
| Stage (AJCC 7th edition 2010) | ||
| I | 1435 | 17.3 |
| II | 911 | 11.0 |
| III | 1052 | 12.7 |
| IV | 3546 | 42.8 |
| Unstaged | 1344 | 16.2 |
| Stage (SEER) | ||
| Localized | 3294 | 39.7 |
| Regionalized | 3276 | 39.5 |
| Distant | 360 | 4.3 |
| Unstaged | 1358 | 16.4 |
| Therapy | ||
| No therapy ** | 434 | 5.2 |
| Radiotherapy alone | 567 | 6.8 |
| Radiochemotherapy or radioimmunotherapy | 985 | 11.9 |
| Surgery alone | 2193 | 26.5 |
| Surgery and chemotherapy | 92 | 1.1 |
| Surgery and radiotherapy | 1738 | 21.0 |
| Surgery and radiochemotherapy | 1755 | 21.2 |
| Chemotherapy or immunotherapy alone | 83 | 1.0 |
| Radiochemotherapy and immunotherapy | 35 | 0.4 |
| Surgery, radiotherapy, chemo- and immunotherapy | 78 | 0.9 |
| Unknown | 328 | 4.0 |
| Histology | ||
| Squamous cell carcinoma | 7034 | 84.9 |
| Adenocarcinoma | 200 | 2.4 |
| Other carcinoma | 811 | 9.8 |
| Other neoplasia | 243 | 2.9 |
| Factor | Parameter | HR | Lower 95% CI | Upper 95% CI | p |
|---|---|---|---|---|---|
| Age | ≤60 years | 1 | Reference | ||
| >60 years | 1.405 | 1.316 | 1.502 | <0.0001 | |
| Gender | Female | 1 | Reference | ||
| Male | 1.444 | 1.317 | 1.582 | <0.0001 | |
| Tumor site | Oropharynx | 1 | Reference | ||
| Lip | 0.969 | 0.757 | 1.241 | 0.8043 | |
| Oral cavity | 1.298 | 1.188 | 1.419 | <0.0001 | |
| Nasopharynx | 0.657 | 0.519 | 0.832 | 0.0005 | |
| Hypopharynx | 1.321 | 1.192 | 1.465 | <0.0001 | |
| Larynx | 1.045 | 0.944 | 1.156 | 0.3983 | |
| Nose/paranasal | 1.022 | 0.822 | 1.271 | 0.8458 | |
| Salivary gland | 0.915 | 0.763 | 1.096 | 0.3326 | |
| Not classifiable | 8.018 | 1.124 | 57.188 | 0.0378 | |
| Stage | I | 1 | Reference | ||
| II | 1.801 | 1.572 | 2.064 | <0.0001 | |
| III | 2.258 | 1.967 | 2.593 | <0.0001 | |
| IV | 3.402 | 3.005 | 3.851 | <0.0001 | |
| Therapy | Surgery alone | 1 | Reference | ||
| Surgery and radiotherapy | 0.752 | 0.674 | 0.840 | <0.0001 | |
| Surgery and chemotherapy | 1.329 | 0.989 | 1.787 | 0.0596 | |
| Surgery and radiochemotherapy | 0.826 | 0.739 | 0.924 | 0.0008 | |
| Surgery, radiotherapy, chemo- and immunotherapy | 0.858 | 0.611 | 1.205 | 0.3771 | |
| Radiotherapy alone | 1.932 | 1.675 | 2.229 | <0.0001 | |
| Radiochemotherapy and immunotherapy | 1.623 | 1.062 | 2.478 | 0.0251 | |
| Radiochemotherapy or radioimmunotherapy | 1.382 | 1.219 | 1.566 | <0.0001 | |
| Chemotherapy or immunotherapy alone | 3.145 | 2.345 | 4.220 | <0.0001 | |
| No therapy | 2.792 | 2.370 | 3.290 | <0.0001 | |
| Year of diagnosis | 1.000 | 0.994 | 1.006 | 0.9460 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dittberner, A.; Friedl, B.; Wittig, A.; Buentzel, J.; Kaftan, H.; Boeger, D.; Mueller, A.H.; Schultze-Mosgau, S.; Schlattmann, P.; Ernst, T.; et al. Gender Disparities in Epidemiology, Treatment, and Outcome for Head and Neck Cancer in Germany: A Population-Based Long-Term Analysis from 1996 to 2016 of the Thuringian Cancer Registry. Cancers 2020, 12, 3418. https://doi.org/10.3390/cancers12113418
Dittberner A, Friedl B, Wittig A, Buentzel J, Kaftan H, Boeger D, Mueller AH, Schultze-Mosgau S, Schlattmann P, Ernst T, et al. Gender Disparities in Epidemiology, Treatment, and Outcome for Head and Neck Cancer in Germany: A Population-Based Long-Term Analysis from 1996 to 2016 of the Thuringian Cancer Registry. Cancers. 2020; 12(11):3418. https://doi.org/10.3390/cancers12113418
Chicago/Turabian StyleDittberner, Andreas, Benedikt Friedl, Andrea Wittig, Jens Buentzel, Holger Kaftan, Daniel Boeger, Andreas H. Mueller, Stefan Schultze-Mosgau, Peter Schlattmann, Thomas Ernst, and et al. 2020. "Gender Disparities in Epidemiology, Treatment, and Outcome for Head and Neck Cancer in Germany: A Population-Based Long-Term Analysis from 1996 to 2016 of the Thuringian Cancer Registry" Cancers 12, no. 11: 3418. https://doi.org/10.3390/cancers12113418