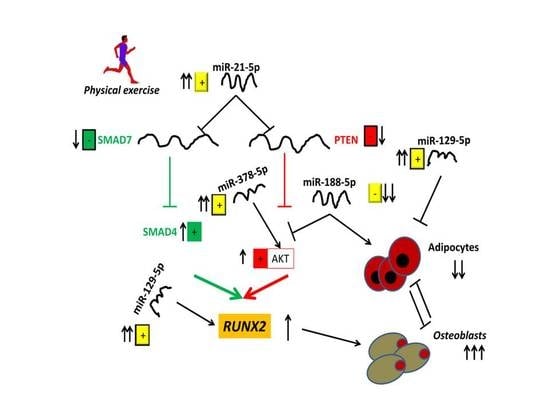
Physical Exercise Modulates miR-21-5p, miR-129-5p, miR-378-5p, and miR-188-5p Expression in Progenitor Cells Promoting Osteogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Sera Collection
2.3. In Vitro Treatments
2.4. Total RNA Extraction
2.5. Reverse Transcription
2.6. Real Time RT-PCR
2.7. Western Blotting
2.8. Alizarin Red Staining
2.9. Oil Red O Staining
2.10. Statistic Analysis
3. Results
3.1. Expression of miRNAs in Osteoprogenitor Cells
3.2. Osteogenic Differentiation of Mesenchimal Stromal Cells
3.3. MiR-21-5p Promotes Osteogenic Differentiation by Targeting PTEN and SMAD7 mRNAs
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | Serine/threonine kinase |
| COL1A2 | Collagen type I alpha 2 chain |
| ECM | Extracellular matrix |
| GADPH | Glyceraldehyde 3-phosphate dehydrogenase |
| G3BP1 | GTPase-activating protein SH3 domain-binding protein 1 |
| IBSP | Integrin Bone sialoprotein 2 |
| MSCs | Mesenchimal Stromal Cells |
| OCN | Osteocalcin |
| P13K | Phosphoinositide 3-kinase |
| PPARG | Peroxisome proliferator-activated receptor gamma |
| PTEN | Phosphatase and Tensin homolog |
| RUNX2 | Runt-related transcription factor 2 |
| SMAD | Small mother against decapentaplegic |
| SOX9 | SRY-box 9 |
| SPP1 | Osteopontin |
| SPARC | Osteonectin |
References
- Valenti, M.T.; Dalle Carbonare, L.; Mottes, M. Osteogenic Differentiation in Healthy and Pathological Conditions. Int. J. Mol. Sci. 2016, 18, 41. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Gao, D.; Gao, C.; Wei, P.; Niu, M.; Shuai, C. MicroRNAs regulate signaling pathways in osteogenic differentiation of mesenchymal stem cells (Review). Mol. Med. Rep. 2016, 14, 623–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenti, M.T.; Dalle Carbonare, L.; Mottes, M. Role of microRNAs in progenitor cell commitment and osteogenic differentiation in health and disease (Review). Int. J. Mol. Med. 2018, 41, 2441–2449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Guo, L.; Liu, Y.; Su, Y.; Xie, Y.; Du, J.; Zhou, J.; Ding, G.; Wang, H.; Bai, Y. MicroRNA-21 promotes osteogenesis of bone marrow mesenchymal stem cells via the Smad7-Smad1/5/8-Runx2 pathway. Biochem. Biophys. Res. Commun. 2017, 493, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, B.; Balagangadharan, K.; Selvamurugan, N. Syringic acid, a phenolic acid, promotes osteoblast differentiation by stimulation of Runx2 expression and targeting of Smad7 by miR-21 in mouse mesenchymal stem cells. J. Cell Commun. Signal 2018, 12, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.Z.; Gu, X.C.; Hu, B.; Liu, X.W.; Zi, Y.; Li, M. Role of microRNA-129-5p in osteoblast differentiation from bone marrow mesenchymal stem cells. Cell Mol. Biol. 2016, 62, 95–99. [Google Scholar] [PubMed]
- Hupkes, M.; Sotoca, A.M.; Hendriks, J.M.; van Zoelen, E.J.; Dechering, K.J. MicroRNA miR-378 promotes BMP2-induced osteogenic differentiation of mesenchymal progenitor cells. BMC Mol. Biol. 2014, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Cheng, P.; Liang, M.K.; Chen, Y.S.; Lu, Q.; Wang, J.Y.; Xia, Z.Y.; Zhou, H.D.; Cao, X.; Xie, H.; et al. MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J. Clin. Investig. 2015, 125, 1509–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maredziak, M.; Smieszek, A.; Chrzastek, K.; Basinska, K.; Marycz, K. Physical Activity Increases the Total Number of Bone-Marrow-Derived Mesenchymal Stem Cells, Enhances Their Osteogenic Potential, and Inhibits Their Adipogenic Properties. Stem Cells Int. 2015, 379093, 16. [Google Scholar] [CrossRef] [PubMed]
- Dalle Carbonare, L.; Manfredi, M.; Caviglia, G.; Conte, E.; Robotti, E.; Marengo, E.; Cheri, S.; Zamboni, F.; Gabbiani, D.; Deiana, M.; et al. Can half-marathon affect overall health? The yin-yang of sport. J. Proteom. 2018, 170, 80–87. [Google Scholar] [CrossRef]
- Valenti, M.T.; Dalle Carbonare, L.; Donatelli, L.; Bertoldo, F.; Zanatta, M.; Lo Cascio, V. Gene expression analysis in osteoblastic differentiation from peripheral blood mesenchymal stem cells. Bone 2008, 43, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Deiana, M.; Dalle Carbonare, L.; Serena, M.; Cheri, S.; Parolini, F.; Gandini, A.; Marchetto, G.; Innamorati, G.; Manfredi, M.; Marengo, E.; et al. New Insights into the Runt Domain of RUNX2 in Melanoma Cell Proliferation and Migration. Cells 2018, 7, 220. [Google Scholar] [CrossRef] [PubMed]
- Valenti, M.T.; Garbin, U.; Pasini, A.; Zanatta, M.; Stranieri, C.; Manfro, S.; Zucal, C.; Carbonare, L.D. Role of Ox-PAPCs in the Differentiation of Mesenchymal Stem Cells (MSCs) and Runx2 and PPAR gamma 2 Expression in MSCs-Like of Osteoporotic Patients. PLoS ONE 2011, 6, e20363. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.C.; Vossio, S.; Rougemont, J.; Gruenberg, J. TFAP2 transcription factors are regulators of lipid droplet biogenesis. Elife 2018, 7, e36330. [Google Scholar] [CrossRef] [PubMed]
- Altana, V.; Geretto, M.; Pulliero, A. MicroRNAs and Physical Activity. Microrna 2015, 4, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Akerstrom, T.; Rinnov, A.; Yfanti, C.; Scheele, C.; Pedersen, B.K.; Laye, M.J. The miRNA plasma signature in response to acute aerobic exercise and endurance training. PLoS ONE 2014, 9, e87308. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhang, L.; Gao, Y.; Ge, W.; Tang, P. The Multiple Roles of Microrna-223 in Regulating Bone Metabolism. Molecules 2015, 20, 19433–19448. [Google Scholar] [CrossRef] [PubMed]
- Sera, S.R.; Zur Nieden, N.I. microRNA Regulation of Skeletal Development. Curr. Osteoporos. Rep. 2017, 15, 353–366. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Gu, W.; Chen, L.; Pan, L.; Chen, J.; Peng, Y. MiR-378 overexpression attenuates high glucose-suppressed osteogenic differentiation through targeting CASP3 and activating PI3K/Akt signaling pathway. Int. J. Clin. Exp. Pathol. 2014, 7, 7249–7261. [Google Scholar] [PubMed]
- Luo, M.; Tan, X.; Mu, L.; Luo, Y.; Li, R.; Deng, X.; Chen, N.; Ren, M.; Li, Y.; Wang, L.; et al. MiRNA-21 mediates the antiangiogenic activity of metformin through targeting PTEN and SMAD7 expression and PI3K/AKT pathway. Sci. Rep. 2017, 7, 43427. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Z. Adrenaline inhibits osteogenesis via repressing miR-21 expression. Cell Biol. Int. 2017, 41, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, X.; Zhao, K.; Zhu, Y.; Hu, B.; Zhou, Y.; Wang, M.; Wu, Y.; Zhang, C.; Xu, J.; et al. miRNA-21 promotes osteogenesis via the PTEN/PI3K/Akt/HIF-1alpha pathway and enhances bone regeneration in critical size defects. Stem Cell Res. Ther. 2019, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Wahl, P.; Wehmeier, U.F.; Jansen, F.J.; Kilian, Y.; Bloch, W.; Werner, N.; Mester, J.; Hilberg, T. Acute Effects of Different Exercise Protocols on the Circulating Vascular microRNAs -16, -21, and -126 in Trained Subjects. Front. Physiol. 2016, 7, 643. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Hao, Y.; Han, Y.; Zhang, W.; Cong, L.; Shi, Y.; Tu, G. Role and mechanism of microRNA-21 in H2O2-induced apoptosis in bone marrow mesenchymal stem cells. J. Clin. Neurosci. 2016, 27, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Sun, B.; Yin, X.; Guo, X.; Chao, D.; Zhang, C.; Zhang, C.Y.; Chen, X.; Ma, J. Time-course responses of circulating microRNAs to three resistance training protocols in healthy young men. Sci. Rep. 2017, 7, 2203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qi, S.; Zhang, T.; Wang, A.; Liu, R.; Guo, J.; Wang, Y.; Xu, Y. miR-188-5p inhibits tumour growth and metastasis in prostate cancer by repressing LAPTM4B expression. Oncotarget 2015, 6, 6092–6104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Wu, X.; Yang, B.; Yao, X.; Cui, X.; Xu, P.; Chen, X. miR-188-5p regulates proliferation and invasion via PI3K/Akt/MMP-2/9 signaling in keloids. Acta Biochim. Biophys. Sin. (Shanghai) 2019, 51, 185–196. [Google Scholar] [CrossRef]
- Lv, S.; Ma, M.; Sun, Y.; Wang, X.; Qimuge, N.; Qin, J.; Pang, W. MicroRNA-129-5p inhibits 3T3-L1 preadipocyte proliferation by targeting G3BP1. Anim. Cells Syst. (Seoul) 2017, 21, 269–277. [Google Scholar] [CrossRef]
- Kim, J.; Okla, M.; Erickson, A.; Carr, T.; Natarajan, S.K.; Chung, S. Eicosapentaenoic Acid Potentiates Brown Thermogenesis through FFAR4-dependent Up-regulation of miR-30b and miR-378. J. Biol. Chem. 2016, 291, 20551–20562. [Google Scholar] [CrossRef] [Green Version]
- Pan, D.; Mao, C.; Quattrochi, B.; Friedline, R.H.; Zhu, L.J.; Jung, D.Y.; Kim, J.K.; Lewis, B.; Wang, Y.X. MicroRNA-378 controls classical brown fat expansion to counteract obesity. Nat. Commun. 2014, 5, 4725. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenti, M.T.; Deiana, M.; Cheri, S.; Dotta, M.; Zamboni, F.; Gabbiani, D.; Schena, F.; Dalle Carbonare, L.; Mottes, M. Physical Exercise Modulates miR-21-5p, miR-129-5p, miR-378-5p, and miR-188-5p Expression in Progenitor Cells Promoting Osteogenesis. Cells 2019, 8, 742. https://doi.org/10.3390/cells8070742
Valenti MT, Deiana M, Cheri S, Dotta M, Zamboni F, Gabbiani D, Schena F, Dalle Carbonare L, Mottes M. Physical Exercise Modulates miR-21-5p, miR-129-5p, miR-378-5p, and miR-188-5p Expression in Progenitor Cells Promoting Osteogenesis. Cells. 2019; 8(7):742. https://doi.org/10.3390/cells8070742
Chicago/Turabian StyleValenti, Maria Teresa, Michela Deiana, Samuele Cheri, Monica Dotta, Francesco Zamboni, Daniele Gabbiani, Federico Schena, Luca Dalle Carbonare, and Monica Mottes. 2019. "Physical Exercise Modulates miR-21-5p, miR-129-5p, miR-378-5p, and miR-188-5p Expression in Progenitor Cells Promoting Osteogenesis" Cells 8, no. 7: 742. https://doi.org/10.3390/cells8070742