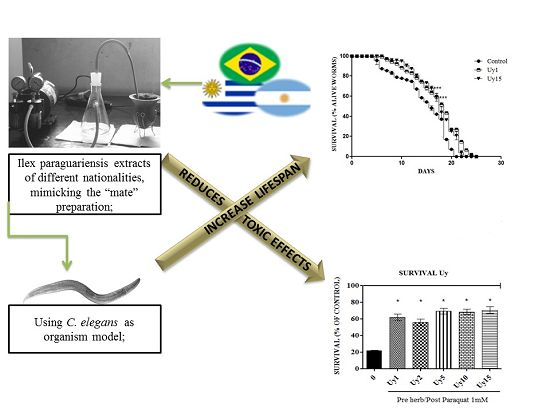
Ilex paraguariensis Extract Increases Lifespan and Protects Against the Toxic Effects Caused by Paraquat in Caenorhabditis elegans
Abstract
:1. Introduction
- (I)
- To verify whether there is some degree of toxicity of the extracts of Ilex paraguariensis, particularly their effectson the lifespan of the nematode C. elegans;
- (II)
- To analyze whether the extracts of Ilex paraguariensis can cause changes in reproduction and/or locomotor activity of C. elegans;
- (III)
- To investigate whether the extracts of Ilex paraguariensis protect from the toxic effects induced by paraquat exposure;
- (IV)
- To investigate whether the insulin/IGF-1 pathway is involved in these effects.
2. Experimental Section
2.1. Chemicals
2.2. Yerba-Mate
2.3. Extraction Method
2.4. Strains and Growth Conditions

2.5. Extract Pretreatment
2.6. Paraquat Exposure
2.7. Egg Laying
2.8. Behavior
2.9. Lifespan Experiments
2.10. ROS Measurements
2.11. Fluorescence Quantification
2.12. Statistical Analysis
3. Results and Discussion
| Herbs | I1 | I2 | I5 | I10 | I15 |
|---|---|---|---|---|---|
| Ar | 0.711 ± 0.15 | 0.967 ± 0.32 | 0.694 ± 0.16 | 0.359 ± 0.04 | 0.221 ± 0.08 |
| Br | 0.798 ± 0.19 | 0.599 ± 0.14 | 0.459 ± 0.06 | 0.338 ± 0.21 | 0.191 ± 0.05 |
| Uy | 1.033 ± 0.05 | 1.451 ± 0.21 | 1.233 ± 0.13 | 0.547 ± 0.24 | 0.514 ± 0.14 |
3.1. Ilex paraguariensis Extracts Did Not Cause Toxic Effects in C. elegans

3.2. Ilex paraguariensis Extracts Exert a Protective Activity against the Toxic Effects Induced by Paraquat in C. elegans


3.3. Ilex paraguariensis Extends C. elegans Lifespan


3.4. The Extracts Increased the Migration of DAF-16 into the Nucleus

4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bracesco, N.; Sanchez, A.G.; Contreras, V.; Menini, T.; Gugliucci, A. Recent advances on Ilex paraguariensis research: Minireview. J. Ethnopharmacol. 2011, 136, 378–384. [Google Scholar] [CrossRef]
- Berté, K.A.S. Chemical composition and antioxidant activity of yerba-mate (Ilex paraguariensis A.St.-Hil., Aquifoliaceae) extract as obtained by spray drying. J. Agric. Food Chem. 2011, 59, 5523–5527. [Google Scholar]
- Bracesco, N.; Dell, M.; Rocha, A.; Behtash, S.; Menini, T.; Gugliucci, A.; Nunes, E. Antioxidant activity of a botanical extract preparation of Ilex paraguariensis, prevention of DNA double-strand breaks in Saccharomyces cerevisiae and human low-density lipoprotein oxidation. J. Altern. Complement. Med. 2003, 9, 379–387. [Google Scholar] [CrossRef]
- Filip, R.; Lopez, P.; Giberti, G.; Coussio, J.; Ferrraro, G. Phenolic compounds in seven South American Ilex species. Fitoterapia 2001, 72, 774–778. [Google Scholar] [CrossRef]
- Matsumoto, R.L.; Bastos, D.H.; Mendonça, S.; Nunes, V.S.; Bartchewsky, W.; Ribeiro, M.L.; de Oliveira, P.C. Effects of mate tea (Ilex paraguariensis) ingestion on mRNA expression of antioxidant enzymes, lipid peroxidation, and total antioxidants status in healthy young women. J. Agric. Food Chem. 2009, 57, 1775–1780. [Google Scholar] [CrossRef]
- Marroni, N.P. Dano Oxidativo e Regulação Biológica Pelos Radicais Livres. In Estresse oxidativo e antioxidantes, 1st ed.; ULBRA: Canoas, RS, Brazil, 2002; p. 189. [Google Scholar]
- Halliwell, B.; Guterride, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: New York, NY, USA, 2007; Chapters 3–5. [Google Scholar]
- Harrington, A.J.; Hamamichil, S.; Caldwell, G.A.; Caldwell, K.A. C. elegans as a model organism to investigate molecular pathways involved with Parkinson’s disease. Dev. Dyn. 2010, 239, 1282–1295. [Google Scholar]
- Lublin, A.L.; Link, C.D. Alzheimer’s disease drug discovery: In vivo screening using Caenorhabditis elegans as a model for b-amyloid peptide-induced toxicity. Drug Discov. Today Technol. 2013, 10, e115–e119. [Google Scholar]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1994, 77, 71–94. [Google Scholar]
- Murphy, C.T.; McCarroll, S.A.; Bargmann, C.I.; Fraser, A.; Kamath, R.S.; Ahringer, J.; Li, H.; Kenyon, C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 2003, 424, 277–283. [Google Scholar] [CrossRef]
- Colpo, A.Z.C. Perfil Fitoquímico e Capacidade Antioxidante de Extratos de Erva-Mate (Ilex paraguariensis A. St. Hill.). Master’s Thesis, Federal University of Pampa, Uruguaiana, RS, Brazil, 2012. [Google Scholar]
- Ávila, D.S.; Benedetto, A.; Au, C.; Manarin, F.; Keith, E.; Soares, F.A.; Rocha, J.B.T.; Aschner, M. Organotellurium and organoselenium compounds attenuate Mn-induced toxicity in Caenorhabditis elegans by preventing oxidative stress. Free Radic. Biol. Med. 2010, 52, 1903–1910. [Google Scholar]
- Liao, V.H.; Yu, C.W.; Chu, Y.J.; Li, W.H.; Hsieh, Y.C.; Wang, T.T. Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mech. Ageing Dev. 2011, 132, 480–487. [Google Scholar] [CrossRef]
- Wilson, M.A.; Shukitt-Hale, B.; Kalt, W.; Ingram, D.K.; Joseph, J.A.; Wolkow, C.A. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell 2006, 5, 59–68. [Google Scholar] [CrossRef]
- Wiegant, F.; Surinova, S.; Ytsma, E.; Langelaar-Makkinje, M.; Wikman, G.; Post, J.A. Plant adaptogens increase lifespan and stress resistance in C. elegans. Biogeroltology 2009, 10, 27–42. [Google Scholar]
- Zarse, K.; Jabin, S.; Ristow, M. L-Theanine extends lifespan of adult Caenorhabiditis elegans. Eur. J. Nutr. 2012, 51, 765–768. [Google Scholar] [CrossRef]
- Fan, D.; Hodges, D.M.; Zhangc, J.; Kirby, C.W.; Ji, X.; Lockec, S.J.; Critchleye, A.T.; Prithiviraj, B. Commercial extract of the brown seaweed Ascophyllum nodosum enhances phenolic antioxidant content of spinach (Spinaciaoleracea L.) which protects Caenorhabditis elegans against oxidative and thermal stress. Food Chem. 2011, 124, 195–202. [Google Scholar]
- Saul, N.; Pietsch, K.; Stürzenbaum, S.R.; Menzel, R.; Steinberg, C.E.W. Diversity of polyphenol action in Caenorhabditis elegans: Between toxicity and longevity. J. Nat. Prod. 2011, 74, 1713–1720. [Google Scholar] [CrossRef]
- Gems, D.; Doonan, R. Antioxidant defense and aging in C. elegans: Is the oxidative damage theory of aging wrong? Cell Cycle 2009, 8, 1681–1687. [Google Scholar] [CrossRef]
- Ristow, M.; Schmeisser, S. Extending life span by increasing oxidative stress. Free Radic. Biol. Med. 2011, 51, 327–236. [Google Scholar] [CrossRef]
- Pun, P.B.; Gruber, J.; Tang, S.Y.; Schaffer, S.; Ong, R.L.; Fong, S.; Ng, L.F.; Cheah, I.; Halliwell, B. Ageing in nematodes: Do antioxidants extend lifespan in Caenorhabditis elegans? Biogerontology 2010, 11, 17–30. [Google Scholar] [CrossRef]
- Honda, Y.; Honda, H. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999, 13, 1385–1393. [Google Scholar]
- Oh, S.K.; Mukhopadhyay, A.; Svrzikapa, N.; Jiang, F.; Davis, R.J.; Tissenbaum, H.A. JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation off orkhead transcription factor/DAF-16. Proc. Natl. Acad. Sci. USA 2006, 102, 4494–4499. [Google Scholar]
- Büchter, C.; Ackermann, D.; Havermann, S.; Honnen, S.; Chovolou, Y.; Fritz, G.; Kampkotter, A.; Wätjen, W. Myricetin-mediated lifespan extension in Caenorhabditis elegans is modulated by DAF-16. Int. J. Mol. Sci. 2013, 14, 11895–11914. [Google Scholar] [CrossRef]
- Guha, S.; Cao, M.; Kane, R.M.; Savino, A.M.; Zou, S.; Dong, Y. The longevity effect of cranberry extract in Caenorhabditis elegans is modulated by daf-16 and osr-1. Age 2012, 35, 1559–1574. [Google Scholar]
- Grunz, G.; Haas, K.; Soukup, S.; Klingenspor, M.; Kulling, S.E.; Daniel, H.; Britta, S. Structural features and bioavailability of four flavonoids and their implications for lifespan-extending and antioxidant actions in C. elegans. Mech. Ageing Dev. 2012, 133, 1–10. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, M.E.; Colpo, A.C.; Salgueiro, W.G.; Sardinha, G.E.; Ávila, D.S.; Folmer, V. Ilex paraguariensis Extract Increases Lifespan and Protects Against the Toxic Effects Caused by Paraquat in Caenorhabditis elegans. Int. J. Environ. Res. Public Health 2014, 11, 10091-10104. https://doi.org/10.3390/ijerph111010091
Lima ME, Colpo AC, Salgueiro WG, Sardinha GE, Ávila DS, Folmer V. Ilex paraguariensis Extract Increases Lifespan and Protects Against the Toxic Effects Caused by Paraquat in Caenorhabditis elegans. International Journal of Environmental Research and Public Health. 2014; 11(10):10091-10104. https://doi.org/10.3390/ijerph111010091
Chicago/Turabian StyleLima, Maria E., Ana C. Colpo, Willian G. Salgueiro, Guilherme E. Sardinha, Daiana S. Ávila, and Vanderlei Folmer. 2014. "Ilex paraguariensis Extract Increases Lifespan and Protects Against the Toxic Effects Caused by Paraquat in Caenorhabditis elegans" International Journal of Environmental Research and Public Health 11, no. 10: 10091-10104. https://doi.org/10.3390/ijerph111010091