X-Linked miRNAs Associated with Gender Differences in Rheumatoid Arthritis
Abstract
:1. Introduction
2. Results
2.1. Characteristics of Patients
2.2. miR-146a and miR-223 Expression Levels Do Not Discriminate RA Patients with Low Disease Activity Score
2.3. Sexual Dimorphism of miRNA Expression in RA
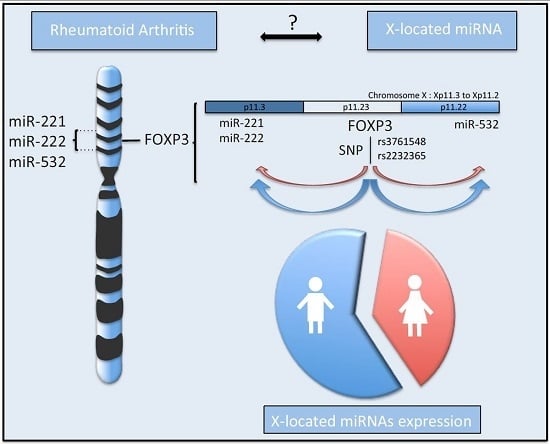
2.4. SNPs in FOXP3 Might Influence miR-221, miR-222 and miR-532 Levels of Expression
3. Discussion
4. Materials and Methods
4.1. Human Subjects
4.2. Preparation of Blood Samples
4.3. Quantification of Mature and Pri-miRNAs
4.4. SNPs Identification
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carmona, L.; Cross, M.; Williams, B.; Lassere, M.; March, L. Rheumatoid arthritis. Best Pract. Res. Clin. Rheumatol. 2010, 24, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Karlson, E.W.; Deane, K. Environmental and gene-environment interactions and risk of rheumatoid arthritis. Rheum. Dis. Clin. N. Am. 2012, 38, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.; Stubelius, A.; Karlsson, M.N.; Engdahl, C.; Erlandsson, M.; Grahnemo, L.; Lagerquist, M.K.; Islander, U. Estrogen regulates T helper 17 phenotype and localization in experimental autoimmune arthritis. Arthritis. Res. Ther. 2015, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Fairweather, D.; Frisancho-Kiss, S.; Rose, N.R. Sex differences in autoimmune disease from a pathological perspective. Am. J. Pathol. 2008, 173, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Lambert, N.C. Microchimerism and rheumatic diseases. Jt. Bone Spine 2012, 79, 563–564. [Google Scholar] [CrossRef] [PubMed]
- Rak, J.M.; Maestroni, L.; Balandraud, N.; Guis, S.; Boudinet, H.; Guzian, M.C.; Yan, Z.; Azzouz, D.; Auger, I.; Roudier, C.; et al. Transfer of the shared epitope through microchimerism in women with rheumatoid arthritis. Arthritis Rheum. 2009, 60, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Chabchoub, G.; Uz, E.; Maalej, A.; Mustafa, C.A.; Rebai, A.; Mnif, M.; Bahloul, Z.; Farid, N.R.; Ozcelik, T.; Ayadi, H. Analysis of skewed X-chromosome inactivation in females with rheumatoid arthritis and autoimmune thyroid diseases. Arthritis. Res. Ther. 2009, 11, R106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, A.; Baltimore, D. MicroRNAs as regulatory elements in immune system logic. Nat. Rev. Immunol. 2016, 16, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Chen, J.; Li, Y.; Zhang, J.; Li, D.; Huang, Z.; Cai, B.; Li, L.; Shi, Y.; Ying, B.; et al. Association of polymorphisms in pre-miRNA with inflammatory biomarkers in rheumatoid arthritis in the Chinese Han population. Hum. Immunol. 2012, 73, 101–106. [Google Scholar] [CrossRef] [PubMed]
- El-Shal, A.S.; Aly, N.M.; Galil, S.M.; Moustafa, M.A.; Kandel, W.A. Association of microRNAs genes polymorphisms with rheumatoid arthritis in Egyptian female patients. Jt. Bone Spine 2013, 80, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Brest, P.; Lapaquette, P.; Souidi, M.; Lebrigand, K.; Cesaro, A.; Vouret-Craviari, V.; Mari, B.; Barbry, P.; Mosnier, J.F.; Hébuterne, X.; et al. A synonymous variant in IRGM alters a binding site for miR-196 and causes deregulation of IRGM-dependent xenophagy in crohn’s disease. Nat. Genet. 2011, 43, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Fulci, V.; Scappucci, G.; Sebastiani, G.D.; Giannitti, C.; Franceschini, D.; Meloni, F.; Colombo, T.; Citarella, F.; Barnaba, V.; Minisola, G.; et al. miR-223 is overexpressed in t-lymphocytes of patients affected by rheumatoid arthritis. Hum. Immunol. 2010, 71, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Fazi, F.; Rosa, A.; Fatica, A.; Gelmetti, V.; de Marchis, M.L.; Nervi, C.; Bozzoni, I. A minicircuitry comprised of microRNA-223 and transcription factors NFI-A and C/EBPα regulates human granulopoiesis. Cell 2005, 123, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.T.; Chen, S.Y.; Wang, C.R.; Liu, M.F.; Lin, C.C.; Jou, I.M.; Shiau, A.L.; Wu, C.L. Brief report: Amelioration of collagen-induced arthritis in mice by lentivirus-mediated silencing of microRNA-223. Arthritis Rheum. 2012, 64, 3240–3245. [Google Scholar] [CrossRef] [PubMed]
- Albertsmeier, M.; Pratschke, S.; Chaudry, I.; Angele, M.K. Gender-specific effects on immune response and cardiac function after trauma hemorrhage and sepsis. Viszeralmedizin 2014, 30, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, I.; Dejager, L.; Libert, C. X-chromosome-located micrornas in immunity: Might they explain male/female differences? The X chromosome-genomic context may affect X-located miRNAs and downstream signaling, thereby contributing to the enhanced immune response of females. Bioessays 2011, 33, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Libert, C.; Dejager, L.; Pinheiro, I. The X chromosome in immune functions: When a chromosome makes the difference. Nat. Rev. Immunol. 2010, 10, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.E.; Silman, A.J. Why are women predisposed to autoimmune rheumatic diseases? Arthritis Res. Ther. 2009, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Eghbali, M. Influence of sex differences on microRNA gene regulation in disease. Biol. Sex Differ. 2014, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.P. Sex chromosomes and brain gender. Nat. Rev. Neurosci. 2004, 5, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; O’Connell, R.M. MicroRNA control in the development of systemic autoimmunity. Arthritis Res. Ther. 2013, 15, 202. [Google Scholar] [CrossRef] [PubMed]
- Duroux-Richard, I.; Jorgensen, C.; Apparailly, F. What do micrornas mean for rheumatoid arthritis? Arthritis Rheum. 2012, 64, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.P.; Bale, T.L. Sex differences in microrna regulation of gene expression: No smoke, just miRs. Biol. Sex Differ. 2012, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Pandis, I.; Ospelt, C.; Karagianni, N.; Denis, M.C.; Reczko, M.; Camps, C.; Hatzigeorgiou, A.G.; Ragoussis, J.; Gay, S.; Kollias, G. Identification of microRNA-221/222 and microRNA-323–3p association with rheumatoid arthritis via predictions using the human tumour necrosis factor transgenic mouse model. Ann. Rheum. Dis. 2012, 71, 1716–1723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Yang, Y. Downregulation of microRNA-221 decreases migration and invasion in fibroblast-like synoviocytes in rheumatoid arthritis. Mol. Med. Rep. 2015, 12, 2395–2401. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Jin, E.H.; Kim, D.; Kim, K.Y.; Chun, C.H.; Jin, E.J. MicroRNA-222 regulates MMP-13 via targeting HDAC-4 during osteoarthritis pathogenesis. BBA Clin. 2015, 3, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.W.; Watkins, G.; Le Good, N.; Roberts, S.; Murphy, C.L.; Brockbank, S.M.; Needham, M.R.; Read, S.J.; Newham, P. The identification of differentially expressed microrna in osteoarthritic tissue that modulate the production of TNF-α and MMP13. Osteoarthr. Cartil. 2009, 17, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Kuang, W.; Hao, Y.; Zhang, D.; Lei, M.; Du, L.; Jiao, H.; Zhang, X.; Wang, F. Downregulation of miR-27a* and miR-532–5p and upregulation of miR-146a and miR-155 in LPS-induced raw264.7 macrophage cells. Inflammation 2012, 35, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, A.L.; Schetter, A.J.; Nielsen, C.T.; Lood, C.; Knudsen, S.; Voss, A.; Harris, C.C.; Hellmark, T.; Segelmark, M.; Jacobsen, S.; et al. Circulating microRNA expression profiles associated with systemic lupus erythematosus. Arthritis Rheum. 2013, 65, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, M.; Aich, J.; Hariharan, M.; Brahmachari, S.K.; Agrawal, A.; Ghosh, B. Posttranscriptional regulation of interleukin-10 expression by HSA-miR-106a. Proc. Natl. Acad. Sci. USA 2009, 106, 5761–5766. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Song, Y.; He, L.; Wan, X.; Lai, L.; Dai, F.; Liu, Y.; Wang, Q. MicroRNA-223 promotes type i interferon production in antiviral innate immunity by targeting forkhead box protein o3 (FOXO3). J. Biol. Chem. 2016. [Google Scholar] [CrossRef] [PubMed]
- Taganov, K.D.; Boldin, M.P.; Chang, K.J.; Baltimore, D. Nf-κb-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486. [Google Scholar] [CrossRef] [PubMed]
- Pauley, K.M.; Satoh, M.; Chan, A.L.; Bubb, M.R.; Reeves, W.H.; Chan, E.K. Upregulated miR-146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Res. Ther. 2008, 10, R101. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Yoshitomi, H.; Tanida, S.; Ishikawa, M.; Nishitani, K.; Ito, H.; Nakamura, T. Plasma and synovial fluid micrornas as potential biomarkers of rheumatoid arthritis and osteoarthritis. Arthritis Res. Ther. 2010, 12, R86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Churov, A.V.; Oleinik, E.K.; Knip, M. Micrornas in rheumatoid arthritis: Altered expression and diagnostic potential. Autoimmun. Rev. 2015, 14, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Filková, M.; Aradi, B.; Senolt, L.; Ospelt, C.; Vettori, S.; Mann, H.; Filer, A.; Raza, K.; Buckley, C.D.; Snow, M.; et al. Association of circulating miR-223 and miR-16 with disease activity in patients with early rheumatoid arthritis. Ann. Rheum. Dis. 2014, 73, 1898–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allantaz, F.; Cheng, D.T.; Bergauer, T.; Ravindran, P.; Rossier, M.F.; Ebeling, M.; Badi, L.; Reis, B.; Bitter, H.; D’Asaro, M.; et al. Expression Profiling of Human Immune Cell Subsets Identifies miRNA-mRNA Regulatory Relationships Correlated with Cell Type Specific Expression. PLoS ONE 2012, 7, e29979. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.; Chai, P.S.; Chong, M.Y.; Tohit, E.R.; Ramasamy, R.; Pei, C.P.; Vidyadaran, S. Gender effect on in vitro lymphocyte subset levels of healthy individuals. Cell. Immunol. 2012, 272, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Melzer, S.; Zachariae, S.; Bocsi, J.; Engel, C.; Löffler, M.; Tárnok, A. Reference intervals for leukocyte subsets in adults: Results from a population-based study using 10-color flow cytometry. Cytom. B Clin. Cytom. 2015, 88, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Paradowska-Gorycka, A.; Jurkowska, M.; Felis-Giemza, A.; Romanowska-Próchnicka, K.; Manczak, M.; Maslinski, S.; Olesinska, M. Genetic polymorphisms of foxp3 in patients with rheumatoid arthritis. J. Rheumatol. 2015, 42, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Kukurba, K.R.; Parsana, P.; Balliu, B.; Smith, K.; Zappala, Z.; Knowles, D.A.; Favé, M.J.; Davis, J.R.; Li, X.; Zhu, X.; et al. Impact of the X Chromosome and sex on regulatory variation. Genome Res. 2016, 26, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Jog, N.R.; Caricchio, R. Differential regulation of cell death programs in males and females by Poly (ADP-Ribose) Polymerase-1 and 17β estradiol. Cell Death Dis. 2013, 7, e758. [Google Scholar] [CrossRef] [PubMed]
- Molloy, E.J.; O’Neill, A.J.; Grantham, J.J.; Sheridan-Pereira, M.; Fitzpatrick, J.M.; Webb, D.W.; Watson, R.W. Sex-specific alterations in neutrophil apoptosis: the role of estradiol and progesterone. Blood 2003, 102, 2653–2659. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Chen, L.; Hao, F.; Wang, G.; Liu, Y. Intron-1 rs3761548 is related to the defective transcription of Foxp3 in psoriasis through abrogating E47/c-Myb binding. J. Cell. Mol. Med. 2010, 14, 226–241. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Na, H.; Li, Y.; Qiu, Z.; Li, W. FoxP3 rs3761548 polymorphism predicts autoimmune disease susceptibility: A meta-analysis. Hum. Immunol. 2013, 74, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cell development by the transcription factor foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of cd4+cd25+ regulatory T cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Husebye, E.S. Autoimmune regulator and self-tolerance—Molecular and clinical aspects. Immunol. Rev. 2016, 271, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Grigoryev, Y.A.; Kurian, S.M.; Hart, T.; Nakorchevsky, A.A.; Chen, C.; Campbell, D.; Head, S.R.; Yates, J.R.; Salomon, D.R. MicroRNA regulation of molecular networks mapped by global microRNA, mRNA, and protein expression in activated t lymphocytes. J. Immunol. 2011, 187, 2233–2243. [Google Scholar] [CrossRef] [PubMed]
- Rüegger, S.; Großhans, H. MicroRNA turnover: When, how, and why. Trends Biochem. Sci. 2012, 37, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Baek, M.; Gusev, Y.; Brackett, D.J.; Nuovo, G.J.; Schmittgen, T.D. Systematic evaluation of microRNA processing patterns in tissues, cell lines, and tumors. RNA 2008, 14, 35–42. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | RA | HC |
|---|---|---|
| Number of samples | 21 | 22 |
| Sex, male/female (% women) | 10/11 (52.4) | 11/11 (50) |
| Age, mean ± SD (years) | 60 ± 12.0 | 54.7 ± 6.4 |
| Disease duration (years) | 16.1 ± 13.3 | NA |
| Positive ACPA, n (%) | 21 (100) | NA |
| Positive RF, n (%) | 21 (100) | NA |
| C-reactive protein (mg/L) | 14.1 ± 23.2 | NA |
| DAS28 | 2.6 ± 1.4 | NA |
| ESR | 21.8 ± 18.7 | NA |
| Drug use, n (%) | ||
| Infliximab | 7 (41.2%) | NA |
| Tocilizumab | 5 (29.5%) | NA |
| Rituximab | 5 (29.5%) | NA |
| Adalimumab | 1 (5.4%) | NA |
| Prednisolone | 5 (29.5%) | NA |
| Methotrexate | 7 (41.2%) | NA |
| miRBase ID | Chromosome | Position (pb) | Gene | Location miRNA/Gene | Distance miRNA-Gene | RA SNPs | MAF |
|---|---|---|---|---|---|---|---|
| miR-221 | Xp11.35 | 45746157–45746266 | Foxp3 | Intergenic | ≈3.5 Mb | rs3761548, rs2232365 | 0.24, 0.41 |
| miR-222 | Xp11.3 | 45747015–45747124 | Foxp3 | Intergenic | ≈3.5 Mb | rs3761548, rs2232365 | 0.24, 0.41 |
| miR-532 | Xp11.2 | 50003148–50003238 | CLCN5 | Intragenic | - | NF | NF |
| Foxp3 | Intergenic | ≈733 Kb | rs3761548, rs2232365 | 0.24, 0.41 | |||
| miR-188 | Xp11.2 | 50003503–50003588 | CLCN5 | Intragenic Intergenic | - | NF | NF |
| Foxp3 | ≈500 Kb | rs3761548, rs2232365 | 0.24, 0.41 | ||||
| miR-98 | Xp11.2 | 53556223–53556341 | HUWE1 | Intragenic | - | NF | NF |
| let-7f-2 | Xp11.2 | 53557192–53557274 | HUWE1 | Intragenic | - | NF | NF |
| miR-223 | Xq12 | 66018870–66018979 | VSIG4 | Intergenic | ≈2868 bp | NF | NF |
| miR-652 | Xq23 | 110055329–110055426 | TMEM164 | Intragenic | - | NF | NF |
| miR-363 | Xq26 | 134169378–134169452 | Non coding gene | - | - | NF | NF |
| miR-92a-2 | Xq26 | 134169538–134169612 | Non coding gene | - | - | NF | NF |
| miR-20b | Xq26 | 134169809–134169877 | Non coding gene | - | - | NF | NF |
| miR-106a | Xq26 | 134169809–134169877 | Non coding gene | - | - | NF | NF |
| miR-3202 | Xq28 | 154019920–154019989 | TMEM187 | Intragenic | - | rs17422 | 0.41 |
| miR-718 | Xq28 | 154019920–154019989 | IRAK1 | Intragenic | - | rs1059702, rs1059703, rs1734792 | 0.37, 0.48, 0.42 |
| DAS28 | Anti-CCP | CRP | RF | Disease Duration | ESR | |
|---|---|---|---|---|---|---|
| miR-221 | p = 0.510 | p = 0.151 | p = 0.520 | p = 0.629 | p = 0.474 | p = 0.005, r = 0.65 |
| miR-222 | p = 0.255 | p = 0.246 | p = 0.267 | p = 0.280 | p = 0.800 | p = 0.004, r = 0.66 |
| miR-532 | p = 0.484 | p = 0.312 | p = 0.510 | p = 0.723 | p = 0.609 | p = 0.687 |
| miR-188 | p = 0.910 | p = 0.866 | p = 0.534 | p = 0.971 | p = 0.288 | p = 0.979 |
| miR-98 | p = 0.388 | p = 0.242 | p = 0.342 | p = 0.332 | p = 0.447 | p = 0.055 |
| let-7f-2 | p = 0.501 | p = 0.261 | p = 0.338 | p = 0.176 | p = 0.204 | p = 0.007, r = 0.62 |
| miR-223 | p = 0.659 | p = 0.004, r = 0.63 | p = 0.423 | p = 0.905 | p = 0.551 | p = 0.475 |
| miR-652 | p = 0.694 | p = 0.190 | p = 0.753 | p = 0.662 | p = 0.775 | p = 0.036, r = 0.51 |
| miR-363 | p = 0.740 | p = 0.223 | p = 0.473 | p = 0.731 | p = 0.164 | p = 0.737 |
| miR-92a-2 | p = 0.272 | p = 0.201 | p = 0.319 | p = 0.131 | p = 0.287 | p = 0.057 |
| miR-106a | p = 0.147 | p = 0.326 | p = 0.283 | p = 0.201 | p = 0.111 | p = 0.283 |
| miR-20a | p = 0.790 | p = 0.081 | p = 0.909 | p = 0.219 | p = 0.081 | p = 0.909 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalifa, O.; Pers, Y.-M.; Ferreira, R.; Sénéchal, A.; Jorgensen, C.; Apparailly, F.; Duroux-Richard, I. X-Linked miRNAs Associated with Gender Differences in Rheumatoid Arthritis. Int. J. Mol. Sci. 2016, 17, 1852. https://doi.org/10.3390/ijms17111852
Khalifa O, Pers Y-M, Ferreira R, Sénéchal A, Jorgensen C, Apparailly F, Duroux-Richard I. X-Linked miRNAs Associated with Gender Differences in Rheumatoid Arthritis. International Journal of Molecular Sciences. 2016; 17(11):1852. https://doi.org/10.3390/ijms17111852
Chicago/Turabian StyleKhalifa, Olfa, Yves-Marie Pers, Rosanna Ferreira, Audrey Sénéchal, Christian Jorgensen, Florence Apparailly, and Isabelle Duroux-Richard. 2016. "X-Linked miRNAs Associated with Gender Differences in Rheumatoid Arthritis" International Journal of Molecular Sciences 17, no. 11: 1852. https://doi.org/10.3390/ijms17111852