The Biochemical and Pharmacological Properties of Ozone: The Smell of Protection in Acute and Chronic Diseases
Abstract
:1. Introduction
General Aspects of Ozone Mechanisms of Action and Effects on Cell Metabolism
2. Ozone Therapy and Pulmonary Diseases
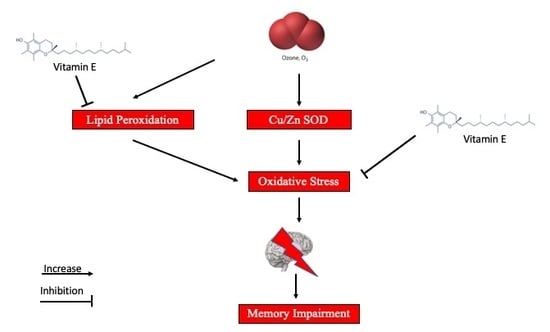
3. Ozone Therapy and CNS
4. Ozone Therapy and Skin Diseases
5. Ozone Therapy and CVD
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dewar, M.J. The structure of ozone. J. Chem. Soc. 1948, 17, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Cross, C.E.; Reznick, A.Z.; Packer, L.; Davis, P.A.; Suzuki, Y.J.; Halliwell, B. Oxidative damage to human plasma proteins by ozone. Free Radic Res. Commun. 1992, 15, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, F.N.; Dotta, L.; Sasse, A.; Teixera, M.J.; Fonoff, E.T. Ozone therapy as a treatment for low back pain secondary to herniated disc: A systematic review and meta-analysis of randomized controlled trials. Pain Physician 2012, 15, E115–E129. [Google Scholar] [PubMed]
- Smith, N.L.; Wilson, A.L.; Gandhi, J.; Vatsia, S.; Khan, S.A. Ozone therapy: An overview of pharmacodynamics, current research, and clinical utility. Med. Gas. Res. 2017, 7, 212–219. [Google Scholar] [PubMed]
- Seyam, O.; Smith, N.L.; Reid, I.; Gandhi, J.; Jiang, W.; Khan, S.A. Clinical utility of ozone therapy for musculoskeletal disorders. Med. Gas. Res. 2018, 8, 103–110. [Google Scholar] [PubMed]
- Kelly, F.J.; Mudway, I.; Krishna, M.T.; Holgate, S.T. The free radical basis of air pollution: Focus on ozone. Respir. Med. 1995, 89, 647–656. [Google Scholar] [CrossRef]
- Pryor, W.A. Mechanisms of radical formation from reactions of ozone with target molecules in the lung. Free Radic Biol. Med. 1994, 17, 451–465. [Google Scholar] [CrossRef]
- Leroy, P.; Tham, A.; Wong, H.; Tenney, R.; Chen, C.; Stiner, R.; Balmes, J.R.; Paquet, A.C.; Arjomandi, M. Inflammatory and repair pathways induced in human bronchoalveolar lavage cells with ozone inhalation. PLoS ONE 2015, 10, e0127283. [Google Scholar] [CrossRef] [PubMed]
- Zuberi, K.; Franz, M.; Rodriguez, H.; Montojo, J.; Lopes, C.T.; Bader, G.D.; Morris, Q. Genemania prediction server 2013 update. Nucleic Acids Res. 2013, 41, W115–W122. [Google Scholar] [CrossRef]
- Chang, J.T.; Nevins, J.R. Gather: A systems approach to interpreting genomic signatures. Bioinformatics 2006, 22, 2926–2933. [Google Scholar] [CrossRef]
- Mikerov, A.N.; Gan, X.; Umstead, T.M.; Miller, L.; Chinchilli, V.M.; Phelps, D.S.; Floros, J. Sex differences in the impact of ozone on survival and alveolar macrophage function of mice after klebsiella pneumoniae infection. Respir. Res. 2008, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Paz, C.; Bazan-Perkins, B. Sleep-wake disorganization in cats exposed to ozone. Neurosci. Lett. 1992, 140, 270–272. [Google Scholar] [CrossRef]
- Hackney, J.D.; Linn, W.S.; Buckley, R.D.; Pedersen, E.E.; Karuza, S.K.; Law, D.C.; Fischer, A. Experimental studies on human health effects of air pollutants: I. Design considerations. Arch. Environ. Health 1975, 30, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Arancibia, S.; Vazquez-Sandoval, R.; Gonzalez-Kladiano, D.; Schneider-Rivas, S.; Lechuga-Guerrero, A. Effects of ozone exposure in rats on memory and levels of brain and pulmonary superoxide dismutase. Environ. Res. 1998, 76, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.L.; Dorado-Martinez, C.; Rodriguez, A.; Pedroza-Rios, K.; Borgonio-Perez, G.; Rivas-Arancibia, S. Effects of vitamin e on ozone-induced memory deficits and lipid peroxidation in rats. Neuroreport 1999, 10, 1689–1692. [Google Scholar] [CrossRef]
- Avila-Costa, M.R.; Colin-Barenque, L.; Fortoul, T.I.; Machado-Salas, P.; Espinosa-Villanueva, J.; Rugerio-Vargas, C.; Rivas-Arancibia, S. Memory deterioration in an oxidative stress model and its correlation with cytological changes on rat hippocampus ca1. Neurosci. Lett. 1999, 270, 107–109. [Google Scholar] [CrossRef]
- Gackiere, F.; Saliba, L.; Baude, A.; Bosler, O.; Strube, C. Ozone inhalation activates stress-responsive regions of the cns. J. Neurochem. 2011, 117, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Mumaw, C.L.; Levesque, S.; McGraw, C.; Robertson, S.; Lucas, S.; Stafflinger, J.E.; Campen, M.J.; Hall, P.; Norenberg, J.P.; Anderson, T.; et al. Microglial priming through the lung-brain axis: The role of air pollution-induced circulating factors. FASEB J. 2016, 30, 1880–1891. [Google Scholar] [CrossRef]
- Rowbotham, G.F. A case of intractable pain in the head and face associated with pathological changes in the optic thalamus. Acta Neurochir. (Wien) 1960, 9, 1–8. [Google Scholar] [CrossRef]
- Bonica, J.J. Pain-basic principles of management. Northwest Med. 1970, 69, 567–568. [Google Scholar]
- Hu, B.; Zheng, J.; Liu, Q.; Yang, Y.; Zhang, Y. The effect and safety of ozone autohemotherapy combined with pharmacological therapy in postherpetic neuralgia. J. Pain Res. 2018, 11, 1637–1643. [Google Scholar] [CrossRef] [PubMed]
- Fuccio, C.; Luongo, C.; Capodanno, P.; Giordano, C.; Scafuro, M.A.; Siniscalco, D.; Lettieri, B.; Rossi, F.; Maione, S.; Berrino, L. A single subcutaneous injection of ozone prevents allodynia and decreases the over-expression of pro-inflammatory caspases in the orbito-frontal cortex of neuropathic mice. Eur. J. Pharm. 2009, 603, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Coppola, L.; Luongo, C.; Pastore, A.; Masciello, C.; Parascandola, R.R.; Mastrolorenzo, L.; Grassia, A.; Coppola, A.; De Biase, M.; Lettieri, B.; et al. Ozonized autohaemotransfusion could be a potential rapid-acting antidepressant medication in elderly patients. Int. J. Geriatr Psychiatry 2010, 25, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Frosini, M.; Contartese, A.; Zanardi, I.; Travagli, V.; Bocci, V. Selective ozone concentrations may reduce the ischemic damage after a stroke. Free Radic. Res. 2012, 46, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Salem, N.A.; Assaf, N.; Ismail, M.F.; Khadrawy, Y.A.; Samy, M. Ozone therapy in ethidium bromide-induced demyelination in rats: Possible protective effect. Cell. Mol. Neurobiol. 2016, 36, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Lu, J. Mechanisms of action involved in ozone-therapy in skin diseases. Int. Immunopharmacol. 2018, 56, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Burke, K.E. Mechanisms of aging and development-a new understanding of environmental damage to the skin and prevention with topical antioxidants. Mech. Ageing Dev. 2018, 172, 123–130. [Google Scholar] [CrossRef]
- Shpektorova, R.A. Ozone therapy of some skin diseases. Vestn. Derm. Venerol. 1964, 38, 44–46. [Google Scholar]
- Bialoszewski, D.; Kowalewski, M. Superficially, longer, intermittent ozone theraphy in the treatment of the chronic, infected wounds. Ortop. Traumatol. Rehabil. 2003, 5, 652–658. [Google Scholar]
- Bocci, V.; Borrelli, E.; Valacchi, G.; Luzzi, E. Quasi-total-body exposure to an oxygen-ozone mixture in a sauna cabin. Eur. J. Appl. Physiol. Occup. Physiol. 1999, 80, 549–554. [Google Scholar] [CrossRef]
- Bocci, V. Biological and clinical effects of ozone. Has ozone therapy a future in medicine? Br. J. Biomed. Sci. 1999, 56, 270–279. [Google Scholar] [PubMed]
- Matsumoto, K.; Aizawa, H.; Inoue, H.; Koto, H.; Nakano, H.; Hara, N. Role of neutrophil elastase in ozone-induced airway responses in guinea-pigs. Eur. Respir. J. 1999, 14, 1088–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, L.; Beaver, K.; Foy, S. Ozone treatment for radiotherapy skin reactions: Is there an evidence base for practice? Eur. J. Oncol. Nurs. 2002, 6, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Noh, S.U.; Han, Y.W.; Kim, K.M.; Kang, H.; Kim, H.O.; Park, Y.M. Therapeutic effects of topical application of ozone on acute cutaneous wound healing. J. Korean Med. Sci. 2009, 24, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Al-Dalain, S.M.; Martinez, G.; Candelario-Jalil, E.; Menendez, S.; Re, L.; Giuliani, A.; Leon, O.S. Ozone treatment reduces markers of oxidative and endothelial damage in an experimental diabetes model in rats. Pharm. Res. 2001, 44, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janic, B.; Umstead, T.M.; Phelps, D.S.; Floros, J. Modulatory effects of ozone on thp-1 cells in response to sp-a stimulation. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2005, 288, L317–L325. [Google Scholar] [CrossRef] [PubMed]
- Valacchi, G.; van der Vliet, A.; Schock, B.C.; Okamoto, T.; Obermuller-Jevic, U.; Cross, C.E.; Packer, L. Ozone exposure activates oxidative stress responses in murine skin. Toxicology 2002, 179, 163–170. [Google Scholar] [CrossRef]
- Kushmakov, R.; Gandhi, J.; Seyam, O.; Jiang, W.; Joshi, G.; Smith, N.L.; Khan, S.A. Ozone therapy for diabetic foot. Med. Gas. Res. 2018, 8, 111–115. [Google Scholar]
- Valacchi, G.; Bocci, V. Studies on the biological effects of ozone: 10. Release of factors from ozonated human platelets. Mediat. Inflamm. 1999, 8, 205–209. [Google Scholar] [CrossRef]
- Sen, C.K.; Khanna, S.; Babior, B.M.; Hunt, T.K.; Ellison, E.C.; Roy, S. Oxidant-induced vascular endothelial growth factor expression in human keratinocytes and cutaneous wound healing. J. Biol. Chem. 2002, 277, 33284–33290. [Google Scholar] [CrossRef]
- Krkl, C.; Yigit, M.V.; Ozercan, I.H.; Aygen, E.; Gulturk, B.; Artas, G. The effect of ozonated olive oil on neovascularization in an experimental skin flap model. Adv. Ski. Wound Care 2016, 29, 322–327. [Google Scholar] [CrossRef]
- Kesik, V.; Yuksel, R.; Yigit, N.; Saldir, M.; Karabacak, E.; Erdem, G.; Babacan, O.; Gulgun, M.; Korkmazer, N.; Bayrak, Z. Ozone ameliorates doxorubicine-induced skin necrosis—Results from an animal model. Int. J. Low Extrem. Wounds 2016, 15, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Guner, M.H.; Gorgulu, T.; Olgun, A.; Torun, M.; Kargi, E. Effects of ozone gas on skin flaps viability in rats: An experimental study. J. Plast Surg. Hand Surg. 2016, 50, 291–297. [Google Scholar] [CrossRef]
- Roger, V.L.; Go, A.S.; Lloyd-Jones, D.M.; Adams, R.J.; Berry, J.D.; Brown, T.M.; Carnethon, M.R.; Dai, S.; de Simone, G.; Ford, E.S.; et al. Heart disease and stroke statistics--2011 update: A report from the american heart association. Circulation 2011, 123, e18–e209. [Google Scholar] [CrossRef]
- Elahi, M.M.; Kong, Y.X.; Matata, B.M. Oxidative stress as a mediator of cardiovascular disease. Oxidative Med. Cell. Longev. 2009, 2, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Ooi, B.K.; Goh, B.H.; Yap, W.H. Oxidative stress in cardiovascular diseases: Involvement of nrf2 antioxidant redox signaling in macrophage foam cells formation. Int. J. Mol. Sci. 2017, 18, 2336. [Google Scholar] [CrossRef]
- Rao, V.; Kiran, R. Evaluation of correlation between oxidative stress and abnormal lipid profile in coronary artery disease. J. Cardiovasc. Dis. Res. 2011, 2, 57–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mason, R.P. Optimal therapeutic strategy for treating patients with hypertension and atherosclerosis: Focus on olmesartan medoxomil. Vasc. Health Risk Manag. 2011, 7, 405–416. [Google Scholar] [CrossRef]
- Bocci, V.; Travagli, V.; Zanardi, I. May oxygen-ozone therapy improves cardiovascular disorders? Cardiovasc. Hematol. Disord. Drug Targets 2009, 9, 78–85. [Google Scholar] [CrossRef]
- Bayram, H.; Sapsford, R.J.; Abdelaziz, M.M.; Khair, O.A. Effect of ozone and nitrogen dioxide on the release of proinflammatory mediators from bronchial epithelial cells of nonatopic nonasthmatic subjects and atopic asthmatic patients in vitro. J. Allergy Clin. Immunol. 2001, 107, 287–294. [Google Scholar] [CrossRef]
- Bocci, V.A.; Zanardi, I.; Travagli, V. Ozone acting on human blood yields a hormetic dose-response relationship. J. Transl. Med. 2011, 9, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pecorelli, A.; Bocci, V.; Acquaviva, A.; Belmonte, G.; Gardi, C.; Virgili, F.; Ciccoli, L.; Valacchi, G. Nrf2 activation is involved in ozonated human serum upregulation of ho-1 in endothelial cells. Toxicol. Appl. Pharmacol. 2013, 267, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V.; Aldinucci, C.; Mosci, F.; Carraro, F.; Valacchi, G. Ozonation of human blood induces a remarkable upregulation of heme oxygenase-1 and heat stress protein-70. Mediat. Inflamm. 2007, 2007, 26785. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Sanchez, G.; Delgado-Roche, L.; Diaz-Batista, A.; Perez-Davison, G.; Re, L. Effects of ozone therapy on haemostatic and oxidative stress index in coronary artery disease. Eur. J. Pharmacol. 2012, 691, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, C.; Luongo, M.; Marfella, R.; Ferraraccio, F.; Lettieri, B.; Capuano, A.; Rossi, F.; D’Amico, M. Oxygen/ozone protects the heart from acute myocardial infarction through local increase of enos activity and endothelial progenitor cells recruitment. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2010, 382, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Otero-Losada, M.; Grangeat, A.M.; Cao, G.; Azzato, F.; Rodriguez, A.; Milei, J. Ozonetherapy protects from in-stent coronary neointimal proliferation. Role of redoxins. Int. J. Cardiol. 2016, 223, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Hormetic mechanisms. Crit. Rev. Toxicol. 2013, 43, 580–606. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, M.; Xie, C.; Luo, X.; Zhang, Q.; Xue, Y. Increased growth factors play a role in wound healing promoted by noninvasive oxygen-ozone therapy in diabetic patients with foot ulcers. Oxidative Med. Cell. Longev. 2014, 2014, 273475. [Google Scholar] [CrossRef]
- Coppola, L.; Giunta, R.; Verrazzo, G.; Luongo, C.; Sammartino, A.; Vicario, C.; Giugliano, D. Influence of ozone on haemoglobin oxygen affinity in type-2 diabetic patients with peripheral vascular disease: In vitro studies. Diabete Metab. 1995, 21, 252–255. [Google Scholar]
- Verrazzo, G.; Coppola, L.; Luongo, C.; Sammartino, A.; Giunta, R.; Grassia, A.; Ragone, R.; Tirelli, A. Hyperbaric oxygen, oxygen-ozone therapy, and rheologic parameters of blood in patients with peripheral occlusive arterial disease. Undersea Hyperbar. Med. J. Undersea Hyperbar. Med. Soc. Inc. 1995, 22, 17–22. [Google Scholar]
- Mehraban, F.; Seyedarabi, A.; Seraj, Z.; Ahmadian, S.; Poursasan, N.; Rayati, S.; Moosavi-Movahedi, A.A. Molecular insights into the effect of ozone on human hemoglobin in autohemotherapy: Highlighting the importance of the presence of blood antioxidants during ozonation. Int. J. Biol. Macromol. 2018, 119, 1276–1285. [Google Scholar] [CrossRef] [PubMed]
- Coppola, L.; Lettieri, B.; Cozzolino, D.; Luongo, C.; Sammartino, A.; Guastafierro, S.; Coppola, A.; Mastrolorenzo, L.; Gombos, G. Ozonized autohaemotransfusion and fibrinolytic balance in peripheral arterial occlusive disease. Blood Coagul. Fibrinol. Int. J. Haemost. Thromb. 2002, 13, 671–681. [Google Scholar] [CrossRef]
- Di Paolo, N.; Bocci, V.; Salvo, D.P.; Palasciano, G.; Biagioli, M.; Meini, S.; Galli, F.; Ciari, I.; Maccari, F.; Cappelletti, F.; et al. Extracorporeal blood oxygenation and ozonation (eboo): A controlled trial in patients with peripheral artery disease. Int. J. Artif. Organs 2005, 28, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Biedunkiewicz, B.; Tylicki, L.; Nieweglowski, T.; Burakowski, S.; Rutkowski, B. Clinical efficacy of ozonated autohemotherapy in hemodialyzed patients with intermittent claudication: An oxygen-controlled study. Int. J. Artif. Organs 2004, 27, 29–34. [Google Scholar] [CrossRef] [PubMed]
- De Monte, A.; van der Zee, H.; Bocci, V. Major ozonated autohemotherapy in chronic limb ischemia with ulcerations. J. Altern. Complement. Med. (N. Y.) 2005, 11, 363–367. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Mauro, R.; Cantarella, G.; Bernardini, R.; Di Rosa, M.; Barbagallo, I.; Distefano, A.; Longhitano, L.; Vicario, N.; Nicolosi, D.; Lazzarino, G.; et al. The Biochemical and Pharmacological Properties of Ozone: The Smell of Protection in Acute and Chronic Diseases. Int. J. Mol. Sci. 2019, 20, 634. https://doi.org/10.3390/ijms20030634
Di Mauro R, Cantarella G, Bernardini R, Di Rosa M, Barbagallo I, Distefano A, Longhitano L, Vicario N, Nicolosi D, Lazzarino G, et al. The Biochemical and Pharmacological Properties of Ozone: The Smell of Protection in Acute and Chronic Diseases. International Journal of Molecular Sciences. 2019; 20(3):634. https://doi.org/10.3390/ijms20030634
Chicago/Turabian StyleDi Mauro, Rosaria, Giuseppina Cantarella, Renato Bernardini, Michelino Di Rosa, Ignazio Barbagallo, Alfio Distefano, Lucia Longhitano, Nunzio Vicario, Daniela Nicolosi, Giacomo Lazzarino, and et al. 2019. "The Biochemical and Pharmacological Properties of Ozone: The Smell of Protection in Acute and Chronic Diseases" International Journal of Molecular Sciences 20, no. 3: 634. https://doi.org/10.3390/ijms20030634