Perioperative Cardioprotection by Remote Ischemic Conditioning
Abstract
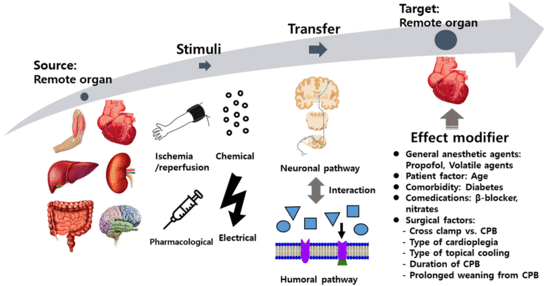
:1. Background
2. Signal Transduction
2.1. Remote Stimulus
2.2. Signal Transfer—Neuronal and Humoral Factors
2.3. Myocardial Response
2.4. Coronary Vascular Response
3. Clinical Studies Evaluating Remote Ischemic Conditioning
3.1. Percutaneous Coronary Intervention
3.2. Cardiac Surgery
3.3. Other Potential Confounding Factors for the Effect of RIPC
3.4. Future Study Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| AKI | Acute Kidney Injury |
| AUC | Area under curve |
| AVR | Aortic valve replacement |
| CABG | Coronary artery bypass graft |
| CGRP | Calcitonin gene-related peptide |
| CK-MB | Creatine kinase-myocardial band |
| eNOS | Endothelial nitric oxide synthase |
| GLP-1 | Glucagon-Like Peptide-1 |
| HIF-1α | Hypoxia-Inducible Factor-1α |
| ICU | Intensive care unit |
| I/R | Ischemia/reperfusion |
| PCI | Percutaneous Coronary Intervention |
| PKG | Protein Kinase G |
| RIPC | Remote Ischemic Preconditioning |
| SAFE | Survivor Activating Factor Enhancement |
| SDF-1α | Stromal Cell-Derived Factor-1α |
| STAT-5 | Signal Transducer and Activator of transcription-5 |
| STEMI | ST-segment Elevated Myocardial Infarction |
| TnI | Troponin I |
| TnT | Troponin T |
References
- Ibanez, B.; Heusch, G.; Ovize, M.; Van de Werf, F. Evolving therapies for myocardial ischemia/reperfusion injury. J. Am. Coll. Cardiol. 2015, 65, 1454–1471. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. Molecular basis of cardioprotection: Signal transduction in ischemic pre-, post-, and remote conditioning. Circ. Res. 2015, 116, 674–699. [Google Scholar] [CrossRef] [PubMed]
- Przyklenk, K.; Bauer, B.; Ovize, M.; Kloner, R.A.; Whittaker, P. Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 1993, 87, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Mwamure, P.K.; Venugopal, V.; Harris, J.; Barnard, M.; Grundy, E.; Ashley, E.; Vichare, S.; Di Salvo, C.; Kolvekar, S.; et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: A randomised controlled trial. Lancet 2007, 370, 575–579. [Google Scholar] [CrossRef]
- Thielmann, M.; Kottenberg, E.; Kleinbongard, P.; Wendt, D.; Gedik, N.; Pasa, S.; Price, V.; Tsagakis, K.; Neuhauser, M.; Peters, J.; et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: A single-centre randomised, double-blind, controlled trial. Lancet 2013, 382, 597–604. [Google Scholar] [CrossRef]
- Heusch, G.; Botker, H.E.; Przyklenk, K.; Redington, A.; Yellon, D. Remote ischemic conditioning. J. Am. Coll. Cardiol. 2015, 65, 177–195. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Ischaemic conditioning and reperfusion injury. Nat. Rev. Cardiol. 2016, 13, 193–209. [Google Scholar] [CrossRef]
- Basalay, M.; Barsukevich, V.; Mastitskaya, S.; Mrochek, A.; Pernow, J.; Sjoquist, P.O.; Ackland, G.L.; Gourine, A.V.; Gourine, A. Remote ischaemic pre- and delayed postconditioning—Similar degree of cardioprotection but distinct mechanisms. Exp. Physiol. 2012, 97, 908–917. [Google Scholar] [CrossRef]
- Johnsen, J.; Pryds, K.; Salman, R.; Lofgren, B.; Kristiansen, S.B.; Botker, H.E. The remote ischemic preconditioning algorithm: Effect of number of cycles, cycle duration and effector organ mass on efficacy of protection. Basic Res. Cardiol. 2016, 111, 10. [Google Scholar] [CrossRef]
- Whittaker, P.; Przyklenk, K. From ischemic conditioning to ‘hyperconditioning′: Clinical phenomenon and basic science opportunity. Dose-Response 2014, 12, 650–663. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. Remote ischemic conditioning: The enigmatic transfer of protection. Cardiovasc. Res. 2017, 113, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, R.K.; Mortensen, U.M.; White, P.A.; Kristiansen, S.B.; Schmidt, M.R.; Hoschtitzky, J.A.; Vogel, M.; Sorensen, K.; Redington, A.N.; MacAllister, R. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation 2002, 106, 2881–2883. [Google Scholar] [CrossRef] [PubMed]
- Loukogeorgakis, S.P.; Panagiotidou, A.T.; Broadhead, M.W.; Donald, A.; Deanfield, J.E.; MacAllister, R.J. Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: Role of the autonomic nervous system. J. Am. Coll. Cardiol. 2005, 46, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Botker, H.E.; Kharbanda, R.; Schmidt, M.R.; Bottcher, M.; Kaltoft, A.K.; Terkelsen, C.J.; Munk, K.; Andersen, N.H.; Hansen, T.M.; Trautner, S.; et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: A randomised trial. Lancet 2010, 375, 727–734. [Google Scholar] [CrossRef]
- Zarbock, A.; Schmidt, C.; Van Aken, H.; Wempe, C.; Martens, S.; Zahn, P.K.; Wolf, B.; Goebel, U.; Schwer, C.I.; Rosenberger, P.; et al. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: A randomized clinical trial. JAMA 2015, 313, 2133–2141. [Google Scholar] [CrossRef]
- Davidson, S.M.; Selvaraj, P.; He, D.; Boi-Doku, C.; Yellon, R.L.; Vicencio, J.M.; Yellon, D.M. Remote ischaemic preconditioning involves signalling through the SDF-1alpha/CXCR4 signalling axis. Basic Res. Cardiol. 2013, 108, 377. [Google Scholar] [CrossRef] [PubMed]
- Heyman, S.N.; Leibowitz, D.; Mor-Yosef Levi, I.; Liberman, A.; Eisenkraft, A.; Alcalai, R.; Khamaisi, M.; Rosenberger, C. Adaptive response to hypoxia and remote ischaemia pre-conditioning: A new hypoxia-inducible factors era in clinical medicine. Acta Physiol. 2016, 216, 395–406. [Google Scholar] [CrossRef]
- Rassaf, T.; Totzeck, M.; Hendgen-Cotta, U.B.; Shiva, S.; Heusch, G.; Kelm, M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circ. Res. 2014, 114, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Skyschally, A.; Gent, S.; Amanakis, G.; Schulte, C.; Kleinbongard, P.; Heusch, G. Across-species transfer of protection by remote ischemic preconditioning with species-specific myocardial signal transduction by reperfusion injury salvage kinase and survival activating factor enhancement pathways. Circ. Res. 2015, 117, 279–288. [Google Scholar] [CrossRef]
- Steensrud, T.; Li, J.; Dai, X.; Manlhiot, C.; Kharbanda, R.K.; Tropak, M.; Redington, A. Pretreatment with the nitric oxide donor SNAP or nerve transection blocks humoral preconditioning by remote limb ischemia or intra-arterial adenosine. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1598–H1603. [Google Scholar] [CrossRef] [Green Version]
- Jones, W.K.; Fan, G.C.; Liao, S.; Zhang, J.M.; Wang, Y.; Weintraub, N.L.; Kranias, E.G.; Schultz, J.E.; Lorenz, J.; Ren, X. Peripheral nociception associated with surgical incision elicits remote nonischemic cardioprotection via neurogenic activation of protein kinase C signaling. Circulation 2009, 120, S1–S9. [Google Scholar] [CrossRef]
- Liem, D.A.; Verdouw, P.D.; Ploeg, H.; Kazim, S.; Duncker, D.J. Sites of action of adenosine in interorgan preconditioning of the heart. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H29–H37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gross, E.R.; Hsu, A.K.; Urban, T.J.; Mochly-Rosen, D.; Gross, G.J. Nociceptive-induced myocardial remote conditioning is mediated by neuronal gamma protein kinase C. Basic Res. Cardiol. 2013, 108, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redington, K.L.; Disenhouse, T.; Strantzas, S.C.; Gladstone, R.; Wei, C.; Tropak, M.B.; Dai, X.; Manlhiot, C.; Li, J.; Redington, A.N. Remote cardioprotection by direct peripheral nerve stimulation and topical capsaicin is mediated by circulating humoral factors. Basic Res. Cardiol. 2012, 107, 241. [Google Scholar] [CrossRef] [PubMed]
- Kleinbongard, P.; Heusch, G. Extracellular signalling molecules in the ischaemic/reperfused heart—Druggable and translatable for cardioprotection? Br. J. Pharmacol. 2015, 172, 2010–2025. [Google Scholar] [CrossRef]
- Arroyo-Martinez, E.A.; Meaney, A.; Gutierrez-Salmean, G.; Rivera-Capello, J.M.; Gonzalez-Coronado, V.; Alcocer-Chauvet, A.; Castillo, G.; Najera, N.; Ceballos, G.; Meaney, E. Is local nitric oxide availability responsible for myocardial salvage after remote preconditioning? Arq. Bras. Cardiol. 2016, 107, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Gourine, A.; Gourine, A.V. Neural mechanisms of cardioprotection. Physiology 2014, 29, 133–140. [Google Scholar] [CrossRef]
- Basalay, M.V.; Mastitskaya, S.; Mrochek, A.; Ackland, G.L.; Del Arroyo, A.G.; Sanchez, J.; Sjoquist, P.O.; Pernow, J.; Gourine, A.V.; Gourine, A. Glucagon-like peptide-1 (GLP-1) mediates cardioprotection by remote ischaemic conditioning. Cardiovasc. Res. 2016, 112, 669–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uitterdijk, A.; Yetgin, T.; te Lintel Hekkert, M.; Sneep, S.; Krabbendam-Peters, I.; van Beusekom, H.M.; Fischer, T.M.; Cornelussen, R.N.; Manintveld, O.C.; Merkus, D.; et al. Vagal nerve stimulation started just prior to reperfusion limits infarct size and no-reflow. Basic Res. Cardiol. 2015, 110, 508. [Google Scholar] [CrossRef]
- Lu, Y.; Wong, G.T.; Zhang, Y.; Hu, J.; Dong, C. Remote intrathecal morphine preconditioning is ineffective in the presence of neuraxial blockade with lidocaine. Kaohsiung J. Med. Sci. 2014, 30, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Donato, M.; Buchholz, B.; Rodriguez, M.; Perez, V.; Inserte, J.; Garcia-Dorado, D.; Gelpi, R.J. Role of the parasympathetic nervous system in cardioprotection by remote hindlimb ischaemic preconditioning. Exp. Physiol. 2013, 98, 425–434. [Google Scholar] [CrossRef]
- Jensen, R.V.; Stottrup, N.B.; Kristiansen, S.B.; Botker, H.E. Release of a humoral circulating cardioprotective factor by remote ischemic preconditioning is dependent on preserved neural pathways in diabetic patients. Basic Res. Cardiol. 2012, 107, 285. [Google Scholar] [CrossRef] [PubMed]
- Dickson, E.W.; Reinhardt, C.P.; Renzi, F.P.; Becker, R.C.; Porcaro, W.A.; Heard, S.O. Ischemic preconditioning may be transferable via whole blood transfusion: Preliminary evidence. J. Thromb. Thrombolysis 1999, 8, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, H.A.; Kreienkamp, V.; Gent, S.; Kahlert, P.; Heusch, G.; Kleinbongard, P. Kinetics and signal activation properties of circulating factor(s) from healthy volunteers undergoing remote ischemic pre-conditioning. JACC Basic Transl. Sci. 2016, 1, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, M.; Tropak, M.; Diaz, R.J.; Suto, F.; Surendra, H.; Kuzmin, E.; Li, J.; Gross, G.; Wilson, G.J.; Callahan, J.; et al. Transient limb ischaemia remotely preconditions through a humoral mechanism acting directly on the myocardium: Evidence suggesting cross-species protection. Clin. Sci. 2009, 117, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Dickson, E.W.; Blehar, D.J.; Carraway, R.E.; Heard, S.O.; Steinberg, G.; Przyklenk, K. Naloxone blocks transferred preconditioning in isolated rabbit hearts. J. Mol. Cell. Cardiol. 2001, 33, 1751–1756. [Google Scholar] [CrossRef] [PubMed]
- Serejo, F.C.; Rodrigues, L.F.; da Silva Tavares, K.C.; de Carvalho, A.C.; Nascimento, J.H. Cardioprotective properties of humoral factors released from rat hearts subject to ischemic preconditioning. J. Cardiovasc. Pharmacol. 2007, 49, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Hepponstall, M.; Ignjatovic, V.; Binos, S.; Monagle, P.; Jones, B.; Cheung, M.H.; d’Udekem, Y.; Konstantinov, I.E. Remote ischemic preconditioning (RIPC) modifies plasma proteome in humans. PLoS ONE 2012, 7, e48284. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Rohailla, S.; Gelber, N.; Rutka, J.; Sabah, N.; Gladstone, R.A.; Wei, C.; Hu, P.; Kharbanda, R.K.; Redington, A.N. MicroRNA-144 is a circulating effector of remote ischemic preconditioning. Basic Res. Cardiol. 2014, 109, 423. [Google Scholar] [CrossRef] [PubMed]
- Hibert, P.; Prunier-Mirebeau, D.; Beseme, O.; Chwastyniak, M.; Tamareille, S.; Lamon, D.; Furber, A.; Pinet, F.; Prunier, F. Apolipoprotein a-I is a potential mediator of remote ischemic preconditioning. PLoS ONE 2013, 8, e77211. [Google Scholar] [CrossRef]
- Cabrera-Fuentes, H.A.; Niemann, B.; Grieshaber, P.; Wollbrueck, M.; Gehron, J.; Preissner, K.T.; Boning, A. RNase1 as a potential mediator of remote ischaemic preconditioning for cardioprotectiondagger. Eur. J. Cardiothorac. Surg. 2015, 48, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Mei, B.; Li, W.; Cheng, X.; Liu, X.; Gu, E.; Zhang, Y. Activating mu-opioid receptors in the spinal cord mediates the cardioprotective effect of remote preconditioning of trauma. Cardiol. J. 2017, 24, 314–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulte, G.; Sommerschild, H.; Yang, J.; Tokuno, S.; Goiny, M.; Lovdahl, C.; Johansson, B.; Fredholm, B.B.; Valen, G. Adenosine a receptors are necessary for protection of the murine heart by remote, delayed adaptation to ischaemia. Acta Physiol. Scand. 2004, 182, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Contractor, H.; Lie, R.H.; Cunnington, C.; Li, J.; Stottrup, N.B.; Manlhiot, C.; Botker, H.E.; Schmidt, M.R.; Forfar, J.C.; Ashrafian, H.; et al. Adenosine receptor activation in the “trigger” limb of remote pre-conditioning mediates human endothelial conditioning and release of circulating cardioprotective factor(s). JACC Basic Transl. Sci. 2016, 1, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.M.; Schmidt, M.R.; Barnes, G.; Botker, H.E.; Kharbanda, R.K.; Newby, D.E.; Cruden, N.L. Bradykinin does not mediate remote ischaemic preconditioning or ischaemia-reperfusion injury in vivo in man. Heart 2011, 97, 1857–1861. [Google Scholar] [CrossRef]
- Cai, Z.P.; Parajuli, N.; Zheng, X.; Becker, L. Remote ischemic preconditioning confers late protection against myocardial ischemia-reperfusion injury in mice by upregulating interleukin-10. Basic Res. Cardiol. 2012, 107, 277. [Google Scholar] [CrossRef]
- Gao, J.; Fu, W.; Jin, Z.; Yu, X. Acupuncture pretreatment protects heart from injury in rats with myocardial ischemia and reperfusion via inhibition of the beta (1)-adrenoceptor signaling pathway. Life Sci. 2007, 80, 1484–1489. [Google Scholar] [CrossRef]
- Oba, T.; Yasukawa, H.; Nagata, T.; Kyogoku, S.; Minami, T.; Nishihara, M.; Ohshima, H.; Mawatari, K.; Nohara, S.; Takahashi, J.; et al. Renal nerve-mediated erythropoietin release confers cardioprotection during remote ischemic preconditioning. Circ. J. 2015, 79, 1557–1567. [Google Scholar] [CrossRef]
- Holst, J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef]
- Olenchock, B.A.; Moslehi, J.; Baik, A.H.; Davidson, S.M.; Williams, J.; Gibson, W.J.; Chakraborty, A.A.; Pierce, K.A.; Miller, C.M.; Hanse, E.A.; et al. EGLN1 inhibition and rerouting of alpha-ketoglutarate suffice for remote ischemic protection. Cell 2016, 164, 884–895. [Google Scholar] [CrossRef]
- Martin-Puig, S.; Tello, D.; Aragones, J. Novel perspectives on the PHD-HIF oxygen sensing pathway in cardioprotection mediated by IPC and RIPC. Front. Physiol. 2015, 6, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Z.; Luo, W.; Zhan, H.; Semenza, G.L. Hypoxia-inducible factor 1 is required for remote ischemic preconditioning of the heart. Proc. Natl. Acad. Sci. USA 2013, 110, 17462–17467. [Google Scholar] [CrossRef] [Green Version]
- Chao de la Barca, J.M.; Bakhta, O.; Kalakech, H.; Simard, G.; Tamareille, S.; Catros, V.; Callebert, J.; Gadras, C.; Tessier, L.; Reynier, P.; et al. Metabolic signature of remote Ischemic Preconditioning Involving a Cocktail of Amino Acids and Biogenic Amines. J. Am. Heart Assoc. 2016, 5, e003891. [Google Scholar] [CrossRef] [PubMed]
- Donato, M.; Goyeneche, M.A.; Garces, M.; Marchini, T.; Perez, V.; Del Mauro, J.; Hocht, C.; Rodriguez, M.; Evelson, P.; Gelpi, R.J. Myocardial triggers involved in activation of remote ischaemic preconditioning. Exp. Physiol. 2016, 101, 708–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahid, M.; Tauseef, M.; Sharma, K.K.; Fahim, M. Brief femoral artery ischaemia provides protection against myocardial ischaemia-reperfusion injury in rats: The possible mechanisms. Exp. Physiol. 2008, 93, 954–968. [Google Scholar] [CrossRef] [PubMed]
- Lambert, E.A.; Thomas, C.J.; Hemmes, R.; Eikelis, N.; Pathak, A.; Schlaich, M.P.; Lambert, G.W. Sympathetic nervous response to ischemia-reperfusion injury in humans is altered with remote ischemic preconditioning. Am. J. Physiol. Heart 2016, 311, H364–H370. [Google Scholar] [CrossRef] [PubMed]
- Pickard, J.M.; Davidson, S.M.; Hausenloy, D.J.; Yellon, D.M. Co-dependence of the neural and humoral pathways in the mechanism of remote ischemic conditioning. Basic Res. Cardiol. 2016, 111, 50. [Google Scholar] [CrossRef]
- Candilio, L.; Malik, A.; Hausenloy, D.J. Protection of organs other than the heart by remote ischemic conditioning. J. Cardiovasc. Med. 2013, 14, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G.; Boengler, K.; Schulz, R. Cardioprotection: Nitric oxide, protein kinases, and mitochondria. Circulation 2008, 118, 1915–1919. [Google Scholar] [CrossRef]
- Bartz, R.R.; Suliman, H.B.; Piantadosi, C.A. Redox mechanisms of cardiomyocyte mitochondrial protection. Front. Physiol. 2015, 6, 291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Z.; Zhu, H.; Zhang, L.; Zhao, X.; Zweier, J.L.; Li, Y. Antioxidants and phase 2 enzymes in cardiomyocytes: Chemical inducibility and chemoprotection against oxidant and simulated ischemia-reperfusion injury. Exp. Biol. Med. 2006, 231, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Konstantinov, I.E.; Arab, S.; Li, J.; Coles, J.G.; Boscarino, C.; Mori, A.; Cukerman, E.; Dawood, F.; Cheung, M.M.; Shimizu, M.; et al. The remote ischemic preconditioning stimulus modifies gene expression in mouse myocardium. J. Thorac. Cardiovasc. Surg. 2005, 130, 1326–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdul-Ghani, S.; Heesom, K.J.; Angelini, G.D.; Suleiman, M.S. Cardiac phosphoproteomics during remote ischemic preconditioning: A role for the sarcomeric Z-disk proteins. BioMed Res. Int. 2014, 2014, 767812. [Google Scholar] [CrossRef]
- Zitta, K.; Meybohm, P.; Gruenewald, M.; Cremer, J.; Zacharowski, K.D.; Scholz, J.; Steinfath, M.; Albrecht, M. Profiling of cell stress protein expression in cardiac tissue of cardiosurgical patients undergoing remote ischemic preconditioning: Implications for thioredoxin in cardioprotection. J. Transl. Med. 2015, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G. The coronary circulation as a target of cardioprotection. Circ. Res. 2016, 118, 1643–1658. [Google Scholar] [CrossRef]
- Shimizu, M.; Konstantinov, I.E.; Kharbanda, R.K.; Cheung, M.H.; Redington, A.N. Effects of intermittent lower limb ischaemia on coronary blood flow and coronary resistance in pigs. Acta Physiol. 2007, 190, 103–109. [Google Scholar] [CrossRef]
- Kono, Y.; Fukuda, S.; Hanatani, A.; Nakanishi, K.; Otsuka, K.; Taguchi, H.; Shimada, K. Remote ischemic conditioning improves coronary microcirculation in healthy subjects and patients with heart failure. Drug Des. Dev. Ther. 2014, 8, 1175–1181. [Google Scholar]
- Grau, M.; Kollikowski, A.; Bloch, W. Remote ischemia preconditioning increases red blood cell deformability through red blood cell-nitric oxide synthase activation. Clin. Hemorheol. Microcirc. 2016, 63, 185–197. [Google Scholar] [CrossRef]
- Shimizu, M.; Saxena, P.; Konstantinov, I.E.; Cherepanov, V.; Cheung, M.M.; Wearden, P.; Zhangdong, H.; Schmidt, M.; Downey, G.P.; Redington, A.N. Remote ischemic preconditioning decreases adhesion and selectively modifies functional responses of human neutrophils. J. Surg. Res. 2010, 158, 155–161. [Google Scholar] [CrossRef]
- Pedersen, C.M.; Cruden, N.L.; Schmidt, M.R.; Lau, C.; Botker, H.E.; Kharbanda, R.K.; Newby, D.E. Remote ischemic preconditioning prevents systemic platelet activation associated with ischemia-reperfusion injury in humans. J. Thromb. Haemost. 2011, 9, 404–407. [Google Scholar] [CrossRef]
- Stazi, A.; Scalone, G.; Laurito, M.; Milo, M.; Pelargonio, G.; Narducci, M.L.; Parrinello, R.; Figliozzi, S.; Bencardino, G.; Perna, F.; et al. Effect of remote ischemic preconditioning on platelet activation and reactivity induced by ablation for atrial fibrillation. Circulation 2014, 129, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.A.; Stazi, A.; Villano, A.; Torrini, F.; Milo, M.; Laurito, M.; Flego, D.; Aurigemma, C.; Liuzzo, G.; Crea, F. Effect of remote ischemic preconditioning on platelet activation induced by coronary procedures. Am. J. Cardiol. 2016, 117, 359–365. [Google Scholar] [CrossRef] [PubMed]
- White, S.K.; Frohlich, G.M.; Sado, D.M.; Maestrini, V.; Fontana, M.; Treibel, T.A.; Tehrani, S.; Flett, A.S.; Meier, P.; Ariti, C.; et al. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc. Interv. 2015, 8, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Crimi, G.; Pica, S.; Raineri, C.; Bramucci, E.; De Ferrari, G.M.; Klersy, C.; Ferlini, M.; Marinoni, B.; Repetto, A.; Romeo, M.; et al. Remote ischemic post-conditioning of the lower limb during primary percutaneous coronary intervention safely reduces enzymatic infarct size in anterior myocardial infarction: A randomized controlled trial. JACC Cardiovasc. Interv. 2013, 6, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Sloth, A.D.; Schmidt, M.R.; Munk, K.; Kharbanda, R.K.; Redington, A.N.; Schmidt, M.; Pedersen, L.; Sorensen, H.T.; Botker, H.E.; Investigators, C. Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur. Heart J. 2014, 35, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, A.; Lourenco, A.P.; Pereira, M.A.; Azevedo, P.; Roncon-Albuquerque, R.; Marques, J.; Leite-Moreira, A.F. Randomized controlled trial of remote ischaemic conditioning in ST-elevation myocardial infarction as adjuvant to primary angioplasty (RIC-STEMI). Basic Res. Cardiol. 2018, 113, 14. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Kharbanda, R.K.; Møller, U.K.; Ramlall, M.; Aarøe, J.; Butler, R. Effect of remote ischaemic conditioning on clinical outcomes in patients with acute myocardial infarction (CONDI-2/ERIC-PPCI): A single-blind randomised controlled trial. Lancet 2019. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. Management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Fihn, S.D.; Blankenship, J.C.; Alexander, K.P.; Bittl, J.A.; Byrne, J.G.; Fletcher, B.J.; Fonarow, G.C.; Lange, R.A.; Levine, G.N.; Maddox, T.M.; et al. ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the American college of cardiology/American heart association task force on practice guidelines, and the American association for thoracic surgery, preventive cardiovascular nurses association, society for cardiovascular angiography and interventions, and society of thoracic surgeons. Circulation 2014, 130, 1749–1767. [Google Scholar] [PubMed]
- Levine, G.N.; Bates, E.R.; Blankenship, J.C.; Bailey, S.R.; Bittl, J.A.; Cercek, B.; Chambers, C.E.; Ellis, S.G.; Guyton, R.A.; Hollenberg, S.M.; et al. ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: An update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J. Am. Coll. Cardiol. 2016, 67, 1235–1250. [Google Scholar]
- Venugopal, V.; Hausenloy, D.J.; Ludman, A.; Di Salvo, C.; Kolvekar, S.; Yap, J.; Lawrence, D.; Bognolo, J.; Yellon, D.M. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold-blood cardioplegia: A randomised controlled trial. Heart 2009, 95, 1567–1571. [Google Scholar] [CrossRef]
- Hong, D.M.; Mint, J.J.; Kim, J.H.; Sohn, I.S.; Lim, T.W.; Lim, Y.J.; Bahk, J.H.; Jeon, Y. The effect of remote ischaemic preconditioning on myocardial injury in patients undergoing off-pump coronary artery bypass graft surgery. Anaesth. Intensive Care 2010, 38, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Young, P.J.; Dalley, P.; Garden, A.; Horrocks, C.; La Flamme, A.; Mahon, B.; Miller, J.; Pilcher, J.; Weatherall, M.; Williams, J.; et al. A pilot study investigating the effects of remote ischemic preconditioning in high-risk cardiac surgery using a randomised controlled double-blind protocol. Basic Res. Cardiol. 2012, 107, 256. [Google Scholar] [CrossRef]
- Kottenberg, E.; Musiolik, J.; Thielmann, M.; Jakob, H.; Peters, J.; Heusch, G. Interference of propofol with signal transducer and activator of transcription 5 activation and cardioprotection by remote ischemic preconditioning during coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 2014, 147, 376–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candilio, L.; Malik, A.; Ariti, C.; Barnard, M.; Di Salvo, C.; Lawrence, D.; Hayward, M.; Yap, J.; Roberts, N.; Sheikh, A.; et al. Effect of remote ischaemic preconditioning on clinical outcomes in patients undergoing cardiac bypass surgery: A randomised controlled clinical trial. Heart 2015, 101, 185–192. [Google Scholar] [CrossRef]
- Walsh, M.; Whitlock, R.; Garg, A.X.; Legare, J.F.; Duncan, A.E.; Zimmerman, R.; Miller, S.; Fremes, S.; Kieser, T.; Karthikeyan, G.; et al. Effects of remote ischemic preconditioning in high-risk patients undergoing cardiac surgery (remote IMPACT): A randomized controlled trial. Can. Med. Assoc. J. 2016, 188, 329–336. [Google Scholar] [CrossRef]
- Pinaud, F.; Corbeau, J.J.; Baufreton, C.; Binuani, J.P.; De Brux, J.L.; Fouquet, O.; Angoulvant, D.; Furber, A.; Prunier, F. Remote ischemic preconditioning in aortic valve surgery: Results of a randomized controlled study. J. Cardiol. 2016, 67, 36–41. [Google Scholar] [CrossRef]
- Song, Y.; Song, J.W.; Lee, S.; Jun, J.H.; Kwak, Y.L.; Shim, J.K. Effects of remote ischemic preconditioning in patients with concentric myocardial hypertrophy: A randomized, controlled trial with molecular insights. Int. J. Cardiol. 2017, 249, 36–41. [Google Scholar] [CrossRef]
- Javaherforoosh Zadeh, F.; Moadeli, M.; Soltanzadeh, M.; Janatmakan, F. Effect of remote ischemic preconditioning on troponin I in CABG. Anesth. Pain Med. 2017, 7, e12549. [Google Scholar] [CrossRef]
- Wang, H.; Lyu, Y.; Liao, Q.; Jin, L.; Xu, L.; Hu, Y.; Yu, Y.; Guo, K. Effects of remote ischemic preconditioning in patients undergoing off-pump coronary artery bypass graft surgery. Front. Physiol. 2019, 10, 495. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wang, L.; Li, L.; Zhao, X. Protective effect of remote ischemic pre-conditioning on patients undergoing cardiac bypass valve replacement surgery: A randomized controlled trial. Exp. Ther. Med. 2019, 17, 2099–2106. [Google Scholar] [CrossRef] [PubMed]
- Meybohm, P.; Bein, B.; Brosteanu, O.; Cremer, J.; Gruenewald, M.; Stoppe, C.; Coburn, M.; Schaelte, G.; Boning, A.; Niemann, B.; et al. A multicenter trial of remote ischemic preconditioning for heart surgery. N. Engl. J. Med. 2015, 373, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Candilio, L.; Evans, R.; Ariti, C.; Jenkins, D.P.; Kolvekar, S.; Knight, R.; Kunst, G.; Laing, C.; Nicholas, J.; et al. Remote ischemic preconditioning and outcomes of cardiac surgery. N. Engl. J. Med. 2015, 373, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Coverdale, N.S.; Hamilton, A.; Petsikas, D.; McClure, R.S.; Malik, P.; Milne, B.; Saha, T.; Zelt, D.; Brown, P.; Payne, D.M. Remote ischemic preconditioning in high-risk cardiovascular surgery patients: A randomized-controlled trial. Semin. Thorac. Cardiovasc. Surg. 2018, 30, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.M.; Lee, E.H.; Kim, H.J.; Min, J.J.; Chin, J.H.; Choi, D.K.; Bahk, J.H.; Sim, J.Y.; Choi, I.C.; Jeon, Y. Does remote ischaemic preconditioning with postconditioning improve clinical outcomes of patients undergoing cardiac surgery? Remote ischaemic preconditioning with postconditioning outcome trial. Eur. Heart J. 2014, 35, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.J.; Lee, E.H.; Lee, K.; Kim, T.K.; Hong, D.M.; Chin, J.H.; Choi, D.K.; Bahk, J.H.; Sim, J.Y.; Choi, I.C.; et al. Long-term clinical outcomes of remote ischemic preconditioning and postconditioning outcome (RISPO) trial in patients undergoing cardiac surgery. Int. J. Cardiol. 2017, 231, 84–89. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, X.; Xu, J.; Zhang, Z.; Klingensmith, N.J.; Liu, S.; Pan, C.; Yang, Y.; Qiu, H. Effect of remote ischemic preconditioning on outcomes in adult cardiac surgery: A systematic review and meta-analysis of randomized controlled studies. Anesth. Analg. 2018, 127, 30–38. [Google Scholar] [CrossRef]
- Jiang, Q.; Xiang, B.; Wang, H.; Huang, K.; Kong, H.; Hu, S. Remote ischaemic preconditioning ameliorates sinus rhythm restoration rate through Cox maze radiofrequency procedure associated with inflammation reaction reduction. Basic Res. Cardiol. 2019, 114, 14. [Google Scholar] [CrossRef]
- Benstoem, C.; Stoppe, C.; Liakopoulos, O.J.; Ney, J.; Hasenclever, D.; Meybohm, P.; Goetzenich, A. Remote ischaemic preconditioning for coronary artery bypass grafting (with or without valve surgery). Cochrane Database Syst. Rev. 2017, 5, Cd011719. [Google Scholar] [CrossRef]
- Thielmann, M.; Sharma, V.; Al-Attar, N.; Bulluck, H.; Bisleri, G.; Bunge, J.J.H.; Czerny, M.; Ferdinandy, P.; Frey, U.H.; Heusch, G.; et al. ESC joint working groups on cardiovascular surgery and the cellular biology of the heart position paper: Perioperative myocardial injury and infarction in patients undergoing coronary artery bypass graft surgery. Eur. Heart J. 2017, 38, 2392–2407. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation 2017, 135, e1159–e1195. [Google Scholar] [CrossRef] [PubMed]
- Bosnjak, Z.J.; Ge, Z.D. The application of remote ischemic conditioning in cardiac surgery. F1000 Res. 2017, 6, 928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kottenberg, E.; Thielmann, M.; Bergmann, L.; Heine, T.; Jakob, H.; Heusch, G.; Peters, J. Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol—A clinical trial. Acta Anaesthesiol. Scand. 2012, 56, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Kleinbongard, P.; Peters, J.; Jakob, H.; Heusch, G.; Thielmann, M. Persistent survival benefit from remote ischemic pre-conditioning in patients undergoing coronary artery bypass surgery. J. Am. Coll. Cardiol. 2018, 71, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Landoni, G.; Greco, T.; Biondi-Zoccai, G.; Nigro Neto, C.; Febres, D.; Pintaudi, M.; Pasin, L.; Cabrini, L.; Finco, G.; Zangrillo, A. Anaesthetic drugs and survival: A Bayesian network meta-analysis of randomized trials in cardiac surgery. Br. J. Anaesth. 2013, 111, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Zangrillo, A.; Musu, M.; Greco, T.; Di Prima, A.L.; Matteazzi, A.; Testa, V.; Nardelli, P.; Febres, D.; Monaco, F.; Calabro, M.G.; et al. Additive effect on survival of anaesthetic cardiac protection and remote ischemic preconditioning in cardiac surgery: A bayesian network meta-analysis of randomized trials. PLoS ONE 2015, 10, e0134264. [Google Scholar] [CrossRef] [PubMed]
- Benstoem, C.; Goetzenich, A.; Stoppe, C. The role of propofol for remote ischaemic preconditioning in the setting of cardiac surgery—A Cochrane systematic review. Br. J. Anaesth. 2017, 119, 1234–1235. [Google Scholar] [CrossRef] [PubMed]
- Benstoem, C.; Goetzenich, A.; Autschbach, R.; Marx, G.; Stoppe, C.; Breuer, T. Volatile anesthetics versus propofol in the cardiac surgical setting of remote ischemic preconditioning: A secondary analysis of a Cochrane Systematic Review. Minerva Anestesiol. 2018, 84, 1298–1306. [Google Scholar] [CrossRef] [PubMed]
- Behmenburg, F.; van Caster, P.; Bunte, S.; Brandenburger, T.; Heinen, A.; Hollmann, M.W.; Huhn, R. Impact of anesthetic regimen on remote ischemic preconditioning in the rat heart in vivo. Anesth. Analg. 2018, 126, 1377–1380. [Google Scholar] [CrossRef]
- Cho, Y.J.; Nam, K.; Kim, T.K.; Choi, S.W.; Kim, S.J.; Hausenloy, D.J.; Jeon, Y. Sevoflurane, propofol and carvedilol block myocardial protection by limb remote ischemic preconditioning. Int. J. Mol. Sci. 2019, 20, 269. [Google Scholar] [CrossRef]
- Suematsu, Y.; Anttila, V.; Takamoto, S.; del Nido, P. Cardioprotection afforded by ischemic preconditioning interferes with chronic beta-blocker treatment. Scand. Cardiovasc. J. 2004, 38, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Smul, T.M.; Blomeyer, C.A.; Redel, A.; Klotz, K.N.; Roewer, N.; Kehl, F. Role of the beta1-adrenergic pathway in anesthetic and ischemic preconditioning against myocardial infarction in the rabbit heart in vivo. Anesthesiology 2006, 105, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Spear, J.F.; Prabu, S.K.; Galati, D.; Raza, H.; Anandatheerthavarada, H.K.; Avadhani, N.G. Beta1-adrenoreceptor activation contributes to ischemia-reperfusion damage as well as playing a role in ischemic preconditioning. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2459–H2466. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Liu, Y.; Yao, Y.; Zhou, S.; Fang, N.; Wang, W.; Li, L. Beta-blockers and volatile anesthetics may attenuate cardioprotection by remote preconditioning in adult cardiac surgery: A meta-analysis of 15 randomized trials. J. Cardiothorac. Vasc. Anesth. 2013, 27, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Kleinbongard, P.; Neuhauser, M.; Thielmann, M.; Kottenberg, E.; Peters, J.; Jakob, H.; Heusch, G. Confounders of cardioprotection by remote ischemic preconditioning in patients undergoing coronary artery bypass grafting. Cardiology 2016, 133, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Schulman, D.; Latchman, D.S.; Yellon, D.M. Effect of aging on the ability of preconditioning to protect rat hearts from ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H1630–H1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abete, P.; Ferrara, N.; Cacciatore, F.; Madrid, A.; Bianco, S.; Calabrese, C.; Napoli, C.; Scognamiglio, P.; Bollella, O.; Cioppa, A.; et al. Angina-induced protection against myocardial infarction in adult and elderly patients: A loss of preconditioning mechanism in the aging heart? J. Am. Coll. Cardiol. 1997, 30, 947–954. [Google Scholar] [CrossRef]
- Przyklenk, K. Efficacy of cardioprotective ‘conditioning’ strategies in aging and diabetic cohorts: The co-morbidity conundrum. Drugs Aging 2011, 28, 331–343. [Google Scholar] [CrossRef]
- Sloth, A.D.; Schmidt, M.R.; Munk, K.; Schmidt, M.; Pedersen, L.; Sorensen, H.T.; Botker, H.E.; Investigators, C. Impact of cardiovascular risk factors and medication use on the efficacy of remote ischaemic conditioning: Post hoc subgroup analysis of a randomised controlled trial. BMJ Open 2015, 5, e006923. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, T.; Chen, S.; Zhou, Q.; Li, H.; Hu, N.; Feng, Y.; Dong, N.; Yao, S.; Xia, Z. Cardiac protective effects of remote ischaemic preconditioning in children undergoing tetralogy of fallot repair surgery: A randomized controlled trial. Eur. Heart J. 2018, 39, 1028–1037. [Google Scholar] [CrossRef]
- Miki, T.; Itoh, T.; Sunaga, D.; Miura, T. Effects of diabetes on myocardial infarct size and cardioprotection by preconditioning and postconditioning. Cardiovasc. Diabetol. 2012, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Wider, J.; Undyala, V.V.R.; Whittaker, P.; Woods, J.; Chen, X.; Przyklenk, K. Remote ischemic preconditioning fails to reduce infarct size in the Zucker fatty rat model of type-2 diabetes: Role of defective humoral communication. Basic Res. Cardiol. 2018, 113, 16. [Google Scholar] [CrossRef]
- Ferdinandy, P.; Hausenloy, D.J.; Heusch, G.; Baxter, G.F.; Schulz, R. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol. Rev. 2014, 66, 1142–1174. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Perez-Polo, J.R.; Aguilar, D.; Birnbaum, Y. The potential effects of anti-diabetic medications on myocardial ischemia-reperfusion injury. Basic Res. Cardiol. 2011, 106, 925–952. [Google Scholar] [CrossRef]
- Kottenberg, E.; Thielmann, M.; Kleinbongard, P.; Frey, U.H.; Heine, T.; Jakob, H.; Heusch, G.; Peters, J. Myocardial protection by remote ischaemic pre-conditioning is abolished in sulphonylurea-treated diabetics undergoing coronary revascularisation. Acta Anaesthesiol. Scand. 2014, 58, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Epps, J.A.; Smart, N.A. Remote ischaemic conditioning in the context of type 2 diabetes and neuropathy: The case for repeat application as a novel therapy for lower extremity ulceration. Cardiovasc. Diabetol. 2016, 15, 130. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Garcia-Dorado, D.; Botker, H.E.; Davidson, S.M.; Downey, J.; Engel, F.B.; Jennings, R.; Lecour, S.; Leor, J.; Madonna, R.; et al. Novel targets and future strategies for acute cardioprotection: Position paper of the european society of cardiology working group on cellular biology of the heart. Cardiovasc. Res. 2017, 113, 564–585. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Combination therapy to target reperfusion injury after ST-segment-elevation myocardial infarction: A more effective approach to cardioprotection. Circulation 2017, 136, 904–906. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Heusch, G. Translating cardioprotection for patient benefit: The EU-CARDIOPROTECTION COST Action. J. Am. Coll. Cardiol. 2019, 73, 2001–2003. [Google Scholar] [CrossRef]
| Study | Design | Animal Type | Mediator | Stimulus/Intervention | Effect |
|---|---|---|---|---|---|
| Neuronal factors | |||||
| Basalay 2016 [28] | Animal | Rats | Vagus nerve | Vagotomy | Abolished RIC effect |
| Donato et al., 2013 [31] | Animal | Rabbits | Spinal cord | Transection at T9–10 level | Abolished RIC effect |
| Donato et al., 2013 [31] Uitterdijk et al., 2015 [29] | Animal | Pig and rabbits | Vagus nerve | Electrical stimulation | Effects similar to RIC |
| Lu et al., 2014 [30] | Animal | Rats | Spinal cord | Intrathecal lidocaine | Abolished intrathecal morphine preconditioning effect |
| Humoral factors | |||||
| Basalay 2016 [28] | Animal | Rats | GLP-1 | Blockade of GLP-1 receptors | Abolished cardioprotection by RIC |
| Hildebrandt et al., 2016 [34] | Human to animal | Healthy volunteers, mouse heart | Unspecified | Plasma dialysate | Could transfer cardioprotection, STAT3 is activated I murine myocardium. |
| Li et al., 2014 [39] | Animal | Mice | MicroRNA144 | Exogenous administration | Induced cardioprotection |
| Hepponstall et al., 2012 [38] | Human | Healthy volunteers | Apolipoprotein A1 | Following RIC | Elevated in human plasma by RIC |
| Hibert et al., 2013 [40] | Animal | Rat | Apolipoprotein A1 | Following RIC | Elevated in rat plasma by RIC |
| Cabrera-Fuentes et al., 2015 [41] | Human | Patients undergoing heart surgery | Endothelial RNase 1 | Following RIC | Elevated after RIC |
| Mei et al., 2017 [42] | Animal | Rats | adenosine or bradykinin | Exogenous administration | Confers cardioprotection |
| Schulte et al., 2004 [43] | Animal | Mice | adenosine | Brain ischemic conditioning | Adenosine increase by mice brain ischemia could transfer protection to mice heart |
| Contractor 2016 et al. [44] | Animal | Mice | adenosine | Exogenous administration | Associated with cardioprotection |
| Pedersen et al., 2011 [45] | Human | Healthy volunteers | Bradykinin | Bradykinin receptor antagonist | Had no effect on the protection by RIC |
| Cai et al., 2012 [46] | Animal | Mice | IL-10 | Genetic knock-out of IL-10 or IL-10 receptor antibodies | Abolished infarct size reduction by RIC |
| Davidson et al., 2013 [16] | Animal | Rats | SDF-1α | Inhibiting SDF-1α chemokine 4 receptor | Attenuated the infarct size-reducing effect of RIC |
| Gao et al., 2007 [47] | Animal | Rats | Substance P, CGRP | Antagonist of substance P or CGRP | Abrogated the infarct size reduction |
| Oba et al., 2015 [48] | Animal | Mice | Erythropoietin | Exogenous administration of erythropoietin antibodies | Abrogated the infarct size reduction |
| Olenchock et al., 2016 [50] | Animal | Mice | EGLN1 gene | EGLN1 gene deletion | reduced the infarct size and decreased expression of HIF-1α. |
| Cai et al., 2013 [52] | Animal | Mice | HIF-1α | HIF-1α knockout | Abolished the infarct size reduction effect |
| Chao de la Barca et al., 2016 [53] | Animal | Rats | kynurenine and glycine | Exogenous administration | Reduced myocardial infarct size |
| Donato et al., 2016 [54], Shahid et al., 2008 [55] | Animal | Rats | nitric oxide | Inhibition of systemic nitric oxide synthase | Abolished the effect of RIC to reduce infarct size |
| Steensrud et al., 2010 [20] | Animal | Rabbits | adenosine | Femoral artery infusion of adenosine | Reduced myocardial infarct size |
| nitric oxide | Systemic nitric oxide synthase inhibitor | Did not reduce the protective effect of RIC | |||
| Arroyo-Martinez et al., 2016 [26] | Human | Patients undergoing PCI | Nitrate and nitrite | Following RIC | Nitrate was released into coronary artery blood |
| Lambert et al., 2016 [56] | Human | Human volunteers | Nitrate and nitrite | Following RIC | No release |
| Study | Number, RIPC/Control | RIPC Protocol | Surgey | Anesthetic Induction/Maintenance | Results | ||
|---|---|---|---|---|---|---|---|
| I/R Cycles | Cuff Pressure | Limb | |||||
| Cardiac biomarkers | |||||||
| Hausenloy et al., 2007 [4] | 27/30 | 3 × 5 min | 200 mmHg | Upper | CABG | Midazolam, etomidate, propofol/isoflurane, propofol | Reduced TnT during 72 h |
| Venugopal et al., 2009 [81] | 23/22 | 3 × 5 min | 200 mmHg | Upper | CABG ± aortic valve surgery | Midazolam, etomidate, propofol/volatile, propofol | Reduced 72-h AUC for TnT |
| Hong et al., 2010 [82] | 65/65 | 4 × 5 min | 200 mmHg | Upper | Off-pump CABG | Midazolam/sevoflurane | No difference in TnI during 72 h |
| Young et al., 2012 [83] | 48/48 | 3 × 5 min | 200 mmHg | Upper | High-risk cardiac surgery | Midazolam/propofol, isoflurane | No difference in 6-h and 12-h TnT |
| Thielmann et al., 2013 [5] | 162/167 | 3 × 5 min | 200 mmHg | Upper | CABG | Isoflurane or propofol | Reduced 72-h AUC for TnI |
| Kottenberg et al., 2014 [84] | 12/12 | 3 × 5 min | 200 mmHg | Upper | CABG | Etomidate/propofol | No difference in 72-h AUC for TnI |
| Candilio et al., 2015 [85] | 90/90 | 3 × 5 min | 200 mmHg | Upper + lower | CABG ± valve surgery | Midazolam, etomidate, propofol/isoflurane, sevoflurane, propofol | Reduced 72-h AUC for TnT |
| Walsh et al., 2016 [86] | 128/130 | 3 × 5 min | 300 mmHg | Lower | Cardiac surgery | Volatile, propofol | No difference in 24-h CK-MB |
| Pinaud et al., 2016 [87] | 50/49 | 3 × 5 min | 200 mmHg | Upper | AVR | Propofol/isoflurane, sevoflurane, propofol | No difference in 72-h AUC for TnI |
| Song et al., 2017 [88] | 36/36 | 3 × 5 min | 300 mmHg | Upper | AVR | Midazolam/sevoflurane | No difference in 24-h AUC for CK-MB and TnT |
| Zadeh et al., 2017 [89] | 14/14 | 3 × 5 min | 200 mmHg | Upper | CABG | Midazolam, ketamine/isoflurane | Reduced 6-h and 24-h TnI |
| Wang et al., 2019 [90] | 33/32 | 4 × 5 min | SBP + 40 mmHg | Upper | Off-pump CABG | Midazolam, etomidate/sevoflurane | Reduced 120-h TnT |
| Jin et al., 2019 [91] | 121/120 | 2 × 5 min | 200 mmHg | Upper + lower | Valve replacement surgery | Imidazole valium, propofol | Reduced 6-h and 24-h post-CPB TnT |
| Clinical outcomes | |||||||
| Thielmann et al., 2013 [5] | 162/167 | 3 × 5 min | 200 mmHg | Upper | CABG | Isoflurane or propofol | Reduced all-cause mortality at 1.5 y |
| Candilio et al., 2015 [85] | 90/90 | 3 × 5 min | 200 mmHg | Upper + lower | CABG ± valve surgery | Midazolam, etomidate, propofol / isoflurane, sevoflurane, propofol | Reduced AKI, AF, and length of ICU stay |
| Meybohm et al., 2015 [92] | 692/693 | 4 × 5 min | 200 mmHg | Upper | Cardiac surgery | Propofol | No difference in composite outcome |
| Hausenloy et al., 2015 [93] | 801/811 | 4 × 5 min | 200 mmHg | Upper | CABG ± valve surgery | Volatile, propofol | No difference in composite outcome |
| Coverdale et al., 2018 [94] | 213/215 | 3 × 5 min | 200 mmHg | Upper | High-risk cardiovascular surgery | Midazolam, propofol, etomidate / desflurane, sevoflurane, propofol | No difference in composite outcome |
| Jin et al., 2019 [91] | 121/120 | 2 × 5 min | 200 mmHg | Upper + lower | Valve replacement surgery | Imidazole valium, propofol | Reduced acute lung injury, and length of ICU and hospital stay |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.J.; Kim, W.H. Perioperative Cardioprotection by Remote Ischemic Conditioning. Int. J. Mol. Sci. 2019, 20, 4839. https://doi.org/10.3390/ijms20194839
Cho YJ, Kim WH. Perioperative Cardioprotection by Remote Ischemic Conditioning. International Journal of Molecular Sciences. 2019; 20(19):4839. https://doi.org/10.3390/ijms20194839
Chicago/Turabian StyleCho, Youn Joung, and Won Ho Kim. 2019. "Perioperative Cardioprotection by Remote Ischemic Conditioning" International Journal of Molecular Sciences 20, no. 19: 4839. https://doi.org/10.3390/ijms20194839