Vesicles Shed by Pathological Murine Adipocytes Spread Pathology: Characterization and Functional Role of Insulin Resistant/Hypertrophied Adiposomes
Abstract
:1. Introduction
2. Results
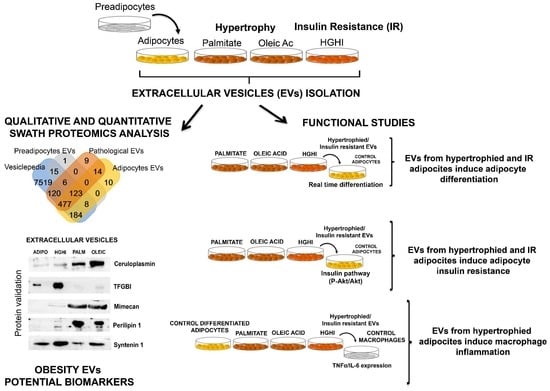
2.1. Adipocytes Shed Extracellular Vesicles That Change Their Protein Cargo with Lipid Atrophy and High Glucose/High Insulin (HGHI)-Promoted Insulin Resistance
2.2. Vesicles Shed by Hypertrophied and Insulin Resistant Adipocytes Induce Adipocyte Differentiation
2.3. Vesicles Shed by Hypertrophied and Insulin Resistant Adipocytes Induce Insulin Resistance in Healthy Cells
2.4. Vesicles Shed by Hypertrophied Adipocytes Induce Macrophage Inflammation
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Models
4.2. Immunobloting
4.3. Cells Secretome Collection and Vesicle Isolation
4.4. Immunogold and Scanning Electron Microscopy
4.5. Nanoparticle Tracking Analysis (NTA)
4.6. Mass Spectrometric Data Dependent Acquisition Qualitative Analysis (DDA) and Protein Quantification by Data Independent Acquisition (DIA) by Sequential Window Acquisition of All Theoretical Mass Spectra (SWATH)
4.7. Protein Functional Analysis
4.8. Functional Vesicle Assays
4.9. Quantitative Real-Time PCR
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EVs | Extracellular Vesicles |
| AT | Adipose Tissue |
| HGHI | High Glucose/High Insulin |
| PALM | Palmitate |
| OLEIC | Oleic acid |
| IR | Insulin Resistance |
| VAT | Visceral Adipose Tissue SAT: Subcutaneous Adipose Tissue |
| DDA | Data-Dependent Acquisition |
| SWATH | Sequential Window Acquisition of All Theoretical mass spectra |
References
- Stahl, P.D.; Raposo, G. Exosomes and extracellular vesicles: The path forward. Essays Biochem. 2018, 62, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Lo Cicero, A.; Stahl, P.D.; Raposo, G. Extracellular vesicles shuffling intercellular messages: For good or for bad. Curr. Opin. Cell Biol. 2015, 35, 69–77. [Google Scholar] [CrossRef]
- Poon, I.K.H.; Lucas, C.D.; Rossi, A.G.; Ravichandran, K.S. Apoptotic cell clearance: Basic biology and therapeutic potential. Nat. Rev. Immunol. 2014, 14, 166–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiteside, T.L. Tumor-Derived Exosomes and Their Role in Cancer Progression. Adv. Clin. Chem. 2016, 74, 103–141. [Google Scholar]
- Properzi, F.; Logozzi, M.; Fais, S. Exosomes: The future of biomarkers in medicine. Biomark. Med. 2013, 7, 769–778. [Google Scholar] [CrossRef]
- Samuelson, I.; Vidal-Puig, A.J. Fed-EXosome: Extracellular vesicles and cell–cell communication in metabolic regulation. Essays Biochem. 2018, 62, 165–175. [Google Scholar]
- Huang-Doran, I.; Zhang, C.-Y.; Vidal-Puig, A. Extracellular Vesicles: Novel Mediators of Cell Communication In Metabolic Disease. Trends Endocrinol. Metab. 2017, 28, 3–18. [Google Scholar] [CrossRef]
- Kranendonk, M.E.G.; Visseren, F.L.J.; van Balkom, B.W.M.; Nolte-’t Hoen, E.N.M.; van Herwaarden, J.A.; de Jager, W.; Schipper, H.S.; Brenkman, A.B.; Verhaar, M.C.; Wauben, M.H.M.; et al. Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity 2014, 22, 1296–1308. [Google Scholar] [CrossRef]
- Deng, Z.-B.; Poliakov, A.; Hardy, R.W.; Clements, R.; Liu, C.; Liu, Y.; Wang, J.; Xiang, X.; Zhang, S.; Zhuang, X.; et al. Adipose Tissue Exosome-Like Vesicles Mediate Activation of Macrophage-Induced Insulin Resistance. Diabetes 2009, 58, 2498–2505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Salomon, C.; Freeman, D.J. Extracellular Vesicles from Adipose Tissue-A Potential Role in Obesity and Type 2 Diabetes? Front. Endocrinol. 2017, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Koeck, E.S.; Iordanskaia, T.; Sevilla, S.; Ferrante, S.C.; Hubal, M.J.; Freishtat, R.J.; Nadler, E.P. Adipocyte exosomes induce transforming growth factor beta pathway dysregulation in hepatocytes: A novel paradigm for obesity-related liver disease. J. Surg. Res. 2014, 192, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, S.E.; Grijalva, A.; Xu, X.; Ables, E.; Nomani, A.; Ferrante, A.W. A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science 2019, 363, 989–993. [Google Scholar] [CrossRef]
- Kranendonk, M.E.G.; Visseren, F.L.J.; van Herwaarden, J.A.; Nolte-’t Hoen, E.N.M.; de Jager, W.; Wauben, M.H.M.; Kalkhoven, E. Effect of extracellular vesicles of human adipose tissue on insulin signaling in liver and muscle cells. Obesity 2014, 22, 2216–2223. [Google Scholar] [CrossRef]
- Durcin, M.; Fleury, A.; Taillebois, E.; Hilairet, G.; Krupova, Z.; Henry, C.; Truchet, S.; Trötzmüller, M.; Köfeler, H.; Mabilleau, G.; et al. Characterisation of adipocyte-derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. J. Extracell. Vesicles 2017, 6, 1305677. [Google Scholar] [CrossRef]
- Lazar, I.; Clement, E.; Dauvillier, S.; Milhas, D.; Ducoux-Petit, M.; LeGonidec, S.; Moro, C.; Soldan, V.; Dalle, S.; Balor, S.; et al. Adipocyte Exosomes Promote Melanoma Aggressiveness through Fatty Acid Oxidation: A Novel Mechanism Linking Obesity and Cancer. Cancer Res. 2016, 76, 4051–4057. [Google Scholar] [CrossRef] [Green Version]
- Mardinoglu, A.; Kampf, C.; Asplund, A.; Fagerberg, L.; Hallström, B.M.; Edlund, K.; Blüher, M.; Pontén, F.; Uhlen, M.; Nielsen, J. Defining the Human Adipose Tissue Proteome To Reveal Metabolic Alterations in Obesity. J. Proteome Res. 2014, 13, 5106–5119. [Google Scholar] [CrossRef]
- Camino, T.; Lago-Baameiro, N.; Bravo, S.B.; Molares-Vila, A.; Sueiro, A.; Couto, I.; Baltar, J.; Casanueva, F.F.; Pardo, M. Human obese white adipose tissue sheds depot-specific extracellular vesicles and reveals candidate obesity biomarkers- analysis of their role on breast cells proliferation. Transl. Res. 2020. under review. [Google Scholar]
- Derosa, G.; Ferrari, I.; D’Angelo, A.; Tinelli, C.; Salvadeo, S.A.T.; Ciccarelli, L.; Piccinni, M.N.; Gravina, A.; Ramondetti, F.; Maffioli, P.; et al. Matrix Metalloproteinase-2 and -9 Levels in Obese Patients. Endothelium 2008, 15, 219–224. [Google Scholar] [CrossRef]
- Araujo, T.F.; Cordeiro, A.V.; Vasconcelos, D.A.A.; Vitzel, K.F.; Silva, V.R.R. The role of cathepsin B in autophagy during obesity: A systematic review. Life Sci. 2018, 209, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.J.; Kuchibhotla, S.; Westfall, K.M.; Silverstein, R.L.; Morton, R.E.; Febbraio, M. A CD36-dependent pathway enhances macrophage and adipose tissue inflammation and impairs insulin signalling. Cardiovasc. Res. 2011, 89, 604–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arner, E.; Forrest, A.R.R.; Ehrlund, A.; Mejhert, N.; Itoh, M.; Kawaji, H.; Lassmann, T.; Laurencikiene, J.; Rydén, M.; Arner, P.; et al. Ceruloplasmin is a novel adipokine which is overexpressed in adipose tissue of obese subjects and in obesity-associated cancer cells. PLoS ONE 2014, 9, e80274. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.Y.; Shin, M.-J.; Moon, J.; Chung, J.H. Plasma ceruloplasmin as a biomarker for obesity: A proteomic approach. Clin. Biochem. 2011, 44, 351–356. [Google Scholar] [CrossRef]
- Xu, A.; Wang, Y.; Xu, J.Y.; Stejskal, D.; Tam, S.; Zhang, J.; Wat, N.M.; Wong, W.K.; Lam, K.S. Adipocyte Fatty Acid–Binding Protein Is a Plasma Biomarker Closely Associated with Obesity and Metabolic Syndrome. Clin. Chem. 2006, 52, 405–413. [Google Scholar] [CrossRef]
- Fries, E.; Kaczmarczyk, A. Inter-alpha-inhibitor, hyaluronan and inflammation. Acta Biochim. Pol. 2003, 50, 735–742. [Google Scholar] [CrossRef]
- Cao, H.-M.; Ye, X.-P.; Ma, J.-H.; Jiang, H.; Li, S.-X.; Li, R.-Y.; Li, X.-S.; Guo, C.-C.; Wang, Z.-Q.; Zhan, M.; et al. Mimecan, a Hormone Abundantly Expressed in Adipose Tissue, Reduced Food Intake Independently of Leptin Signaling. EBioMedicine 2015, 2, 1718–1724. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhao, Y.; Chen, C.; Yang, L.; Lee, H.-H.; Wang, Z.; Zhang, N.; Kolonin, M.G.; An, Z.; Ge, X.; et al. The critical role of MMP14 in adipose tissue remodeling during obesity. Mol. Cell. Biol. 2020. [Google Scholar] [CrossRef]
- Zeyda, M.; Gollinger, K.; Todoric, J.; Kiefer, F.W.; Keck, M.; Aszmann, O.; Prager, G.; Zlabinger, G.J.; Petzelbauer, P.; Stulnig, T.M. Osteopontin Is an Activator of Human Adipose Tissue Macrophages and Directly Affects Adipocyte Function. Endocrinology 2011, 152, 2219–2227. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Li, Z.; Zhang, B.; He, H.; Bai, Y. PPIA is a novel adipogenic factor implicated in obesity. Obesity 2015, 23, 2093–2100. [Google Scholar] [CrossRef]
- Lancha, A.; López-Garrido, S.; Rodríguez, A.; Catalán, V.; Ramírez, B.; Valentí, V.; Moncada, R.; Silva, C.; Gil, M.J.; Salvador, J.; et al. Expression of Syntaxin 8 in Visceral Adipose Tissue Is Increased in Obese Patients with Type 2 Diabetes and Related to Markers of Insulin Resistance and Inflammation. Arch. Med. Res. 2015, 46, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Fain, J.N.; Tichansky, D.S.; Madan, A.K. Transforming Growth Factor β1 release by human adipose tissue is enhanced in obesity. Metabolism 2005, 54, 1546–1551. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Luo, H.; Raelson, J.; Huang, J.; Li, Y.; Tremblay, J.; Hu, B.; Qi, S.; Wu, J. TGFBI ( IG-H3) is a diabetes-risk gene based on mouse and human genetic studies. Hum. Mol. Genet. 2014, 23, 4597–4611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badeanlou, L.; Furlan-Freguia, C.; Yang, G.; Ruf, W.; Samad, F. Tissue factor-protease-activated receptor 2 signaling promotes diet-induced obesity and adipose inflammation. Nat. Med. 2011, 17, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Eguchi, A.; Tempaku, M.; Honda, T.; Togashi, K.; Iwasa, M.; Hasegawa, H.; Takei, Y.; Sumida, Y.; Taguchi, O. Circulating extracellular vesicles are associated with lipid and insulin metabolism. Am. J. Physiol. Metab. 2018, 315, E574–E582. [Google Scholar] [CrossRef] [PubMed]
- Mleczko, J.; Ortega, F.J.; Falcon-Perez, J.M.; Wabitsch, M.; Fernandez-Real, J.M.; Mora, S. Extracellular Vesicles from Hypoxic Adipocytes and Obese Subjects Reduce Insulin-Stimulated Glucose Uptake. Mol. Nutr. Food Res. 2018, 62, 1700917. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Li, X.; Wang, Y.; Cao, Y.; Yao, D.; Sun, L.; Qin, L.; Qiu, H.; Zhan, X. Adipocyte-derived extracellular vesicles modulate appetite and weight through mTOR signalling in the hypothalamus. Acta Physiol. 2019, 228, e13339. [Google Scholar] [CrossRef]
- Kennedy, A.; Martinez, K.; Chuang, C.-C.; LaPoint, K.; McIntosh, M. Saturated fatty acid-mediated inflammation and insulin resistance in adipose tissue: Mechanisms of action and implications. J. Nutr. 2009, 139, 1–4. [Google Scholar] [CrossRef]
- Lo, K.A.; Labadorf, A.; Kennedy, N.J.; Han, M.S.; Yap, Y.S.; Matthews, B.; Xin, X.; Sun, L.; Davis, R.J.; Lodish, H.F.; et al. Analysis of in vitro insulin-resistance models and their physiological relevance to in vivo diet-induced adipose insulin resistance. Cell Rep. 2013, 5, 259–270. [Google Scholar] [CrossRef] [Green Version]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef] [Green Version]
- Amosse, J.; Durcin, M.; Malloci, M.; Vergori, L.; Fleury, A.; Gagnadoux, F.; Dubois, S.; Simard, G.; Boursier, J.; Hue, O.; et al. Phenotyping of circulating extracellular vesicles (EVs) in obesity identifies large EVs as functional conveyors of Macrophage Migration Inhibitory Factor. Mol. Metab. 2018, 18, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Connolly, K.D.; Guschina, I.A.; Yeung, V.; Clayton, A.; Draman, M.S.; Von Ruhland, C.; Ludgate, M.; James, P.E.; Rees, D.A. Characterisation of adipocyte-derived extracellular vesicles released pre- and post-adipogenesis. J. Extracell. Vesicles 2015, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, S.; De Filippo, E.; Göddeke, S.; Knebel, B.; Kotzka, J.; Al-Hasani, H.; Roden, M.; Lehr, S.; Sell, H. Exosomal proteins constitute an essential part of the human adipose tissue secretome. Biochim. Biophys. Acta. Proteins Proteom. 2018, 1867, 140172. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Duan, X.; Homko, C.; Molina, E.J.; Song, W.; Perez, O.; Cheung, P.; Merali, S. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes 2008, 57, 2438–2444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heizmann, C.W.; Fritz, G.; Schäfer, B.W. S100 proteins: Structure, functions and pathology. Front. Biosci. 2002, 7, d1356–d1368. [Google Scholar] [PubMed]
- Kim, J.-M.; Jang, H.-J.; Choi, S.Y.; Park, S.-A.; Kim, I.S.; Yang, Y.R.; Lee, Y.H.; Ryu, S.H.; Suh, P.-G. DJ-1 contributes to adipogenesis and obesity-induced inflammation. Sci. Rep. 2014, 4, 4805. [Google Scholar] [CrossRef] [Green Version]
- Catalan, V.; Gomez-Ambrosi, J.; Rodriguez, A.; Ramirez, B.; Rotellar, F.; Valenti, V.; Silva, C.; Gil, M.J.; Salvador, J.; Fruhbeck, G. Increased tenascin C and Toll-like receptor 4 levels in visceral adipose tissue as a link between inflammation and extracellular matrix remodeling in obesity. J. Clin. Endocrinol. Metab. 2012, 97, E1880–E1889. [Google Scholar] [CrossRef] [Green Version]
- Samad, F.; Pandey, M.; Loskutoff, D.J. Tissue factor gene expression in the adipose tissues of obese mice. Proc. Natl. Acad. Sci. USA 1998, 95, 7591–7596. [Google Scholar] [CrossRef] [Green Version]
- Mazor, R.; Friedmann-Morvinski, D.; Alsaigh, T.; Kleifeld, O.; Kistler, E.B.; Rousso-Noori, L.; Huang, C.; Li, J.B.; Verma, I.M.; Schmid-Schönbein, G.W. Cleavage of the leptin receptor by matrix metalloproteinase–2 promotes leptin resistance and obesity in mice. Sci. Transl. Med. 2018, 10, 6324. [Google Scholar] [CrossRef] [Green Version]
- Hanssen, E.; Reinboth, B.; Gibson, M.A. Covalent and Non-covalent Interactions of βig-h3 with Collagen VI. J. Biol. Chem. 2003, 278, 24334–24341. [Google Scholar] [CrossRef] [Green Version]
- Roca-rivada, A.; Bravo, S.B.; Pérez-sotelo, D.; Alonso, J.; Isabel, A.; Baamonde, I.; Baltar, J.; Casanueva, F.F.; Pardo, M. CILAIR-Based Secretome Analysis of Obese Visceral and Subcutaneous Adipose Tissues Reveals Distinctive ECM Remodeling and Inflammation Mediators. Nat. Publ. Gr. 2015, 5, 12214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, P.; Abate, N. Body Fat Distribution and Insulin Resistance. Nutrients 2013, 5, 2019–2027. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, A.; Lazic, M.; Armando, A.M.; Phillips, S.A.; Katebian, R.; Maraka, S.; Quehenberger, O.; Sears, D.D.; Feldstein, A.E. Circulating adipocyte-derived extracellular vesicles are novel markers of metabolic stress. J. Mol. Med. 2016, 94, 1241. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Qiu, X.; Deis, J.; Lin, T.-Y.; Chen, X. Pentraxin 3 deficiency exacerbates lipopolysaccharide-induced inflammation in adipose tissue. Int. J. Obes. 2020, 44, 525–538. [Google Scholar] [CrossRef] [Green Version]
- Guillén, M.I.; Platas, J.; del Caz, M.D.P.; Mirabet, V.; Alcaraz, M.J. Paracrine Anti-inflammatory Effects of Adipose Tissue-Derived Mesenchymal Stem Cells in Human Monocytes. Front. Physiol. 2018, 9, 661. [Google Scholar] [CrossRef]
- Kramer, A.H.; Joos-Vandewalle, J.; Edkins, A.L.; Frost, C.L.; Prinsloo, E. Real-time monitoring of 3T3-L1 preadipocyte differentiation using a commercially available electric cell-substrate impedance sensor system. Biochem. Biophys. Res. Commun. 2014, 443, 1245–1250. [Google Scholar] [CrossRef]
- Cullberg, K.B.; Larsen, J.Ø.; Pedersen, S.B.; Richelsen, B. Effects of LPS and dietary free fatty acids on MCP-1 in 3T3-L1 adipocytes and macrophages in vitro. Nutr. Diabetes 2014, 4, e113. [Google Scholar] [CrossRef]
- Tang, Q.-Q.; Otto, T.C.; Lane, M.D. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. USA 2004, 101, 9607–9611. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Ruiz, A.; Guzmán-Ruiz, R.; Moreno, N.R.; García-Rios, A.; Delgado-Casado, N.; Membrives, A.; Túnez, I.; El Bekay, R.; Fernández-Real, J.M.; Tovar, S.; et al. Proteasome Dysfunction Associated to Oxidative Stress and Proteotoxicity in Adipocytes Compromises Insulin Sensitivity in Human Obesity. Antioxid. Redox Signal. 2015, 23, 597–612. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Sotelo, D.; Roca-Rivada, A.; Baamonde, I.; Baltar, J.; Castro, A.I.; Domínguez, E.; Collado, M.; Casanueva, F.F.; Pardo, M. Lack of Adipocyte-Fndc5/Irisin Expression and Secretion Reduces Thermogenesis and Enhances Adipogenesis. Sci. Rep. 2017, 7, 16289. [Google Scholar] [CrossRef] [Green Version]
- Lobb, R.J.; Becker, M.; Wen, S.W.; Wong, C.S.F.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef] [Green Version]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 3, 22. [Google Scholar] [CrossRef]
- Pathan, M.; Keerthikumar, S.; Ang, C.-S.; Gangoda, L.; Quek, C.Y.J.; Williamson, N.A.; Mouradov, D.; Sieber, O.M.; Simpson, R.J.; Salim, A.; et al. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics 2015, 15, 2597–2601. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Casagrande, J.T.; Thomas, P.D. Large-scale gene function analysis with the PANTHER classification system. Nat. Protoc. 2013, 8, 1551–1566. [Google Scholar] [CrossRef]
- Fabregat, A.; Jupe, S.; Matthews, L.; Sidiropoulos, K.; Gillespie, M.; Garapati, P.; Haw, R.; Jassal, B.; Korninger, F.; May, B.; et al. The Reactome Pathway Knowledgebase. Nucleic Acids Res. 2018, 46, D649–D655. [Google Scholar] [CrossRef]
- Shevchenko, A.; Wilm, M.; Vorm, O.; Jensen, O.N.; Podtelejnikov, A.V.; Neubauer, G.; Mortensen, P.; Mann, M. A strategy for identifying gel-separated proteins in sequence databases by MS alone. Biochem. Soc. Trans. 1996, 24, 893–896. [Google Scholar] [CrossRef] [Green Version]






| EV Potential Biomarker | Protein ID | Palm EVs | Oleic EVs | HGHI EVs | Human Obese AT EVs | Pathology | Reference |
|---|---|---|---|---|---|---|---|
| 72kDa type IV collagenase (MMP-2) | MMP2_MOUSE | X↑ | VISCERAL OBESE AT | IR + obesity | Derosa et al., 2008; Mazor et al., 2018 [20]; Camino et al., 2020 under review [19] | ||
| Cathepsin B | CATB_MOUSE | X | X | obesity, inflammation, MS | Araujo et al., 2018 [21] | ||
| CD36 | CD36_MOUSE | X | X | inflammation + IR | Kennedy et al., 2011 [22] | ||
| Ceruloplasmin | CERU_MOUSE | X ↑ | X↑ | X | VISCERAL OBESE AT | obesity | Arner et al., 2014 [23]; Kim et al., 2011 [24] |
| FABP-4 | FABP4_MOUSE | X | VISCERAL OBESE AT | obesity + MS | Xu et al., 2006 [25]; Camino et al., 2020 under review [19] | ||
| ITIH3 | ITIH3_MOUSE | X | X | inflammation | Fries et al., 2003 [26] | ||
| Mimecan | MIME_MOUSE | X ↑ | X↑ | X | VISCERAL OBESE AT | adipokine | Cao et al., 2015 [27] |
| MMP-14 | MMP14_MOUSE | X | IR | Li et al., 2020 [28] | |||
| Osteopontin | OSTP_MOUSE | X | X | inflammation + IR | Zeyda et al., 2011 [29] | ||
| PARK7/DJ-1 | PARK7_MOUSE | X | X↑ | obesity-inflammation | Kim et al., 2014 [30] | ||
| Perilipin 1 | PLIN1_MOUSE | X↑ | X↑ | X | AT Ev Biomarker | Eguchi et al. 2016 [31] | |
| Syntasin-8 | STX8_MOUSE | X | VAT IR + inflammation | Lancha et al., 2015 [28] | |||
| TFGBI | BGH3_MOUSE | X↑↑ | VISCERAL OBESE AT | diabetes | Camino et al.,2020 under review [19]; Roca-Rivada et al., 2015 [32]; Fain et al., 2005 [33] | ||
| Tissue factor | TF_MOUSE | X | X | Obesity + inflammation | Samad et al., 1998 [34]; Badeanlou et al., 2011 [20] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camino, T.; Lago-Baameiro, N.; Bravo, S.B.; Sueiro, A.; Couto, I.; Santos, F.; Baltar, J.; Casanueva, F.F.; Pardo, M. Vesicles Shed by Pathological Murine Adipocytes Spread Pathology: Characterization and Functional Role of Insulin Resistant/Hypertrophied Adiposomes. Int. J. Mol. Sci. 2020, 21, 2252. https://doi.org/10.3390/ijms21062252
Camino T, Lago-Baameiro N, Bravo SB, Sueiro A, Couto I, Santos F, Baltar J, Casanueva FF, Pardo M. Vesicles Shed by Pathological Murine Adipocytes Spread Pathology: Characterization and Functional Role of Insulin Resistant/Hypertrophied Adiposomes. International Journal of Molecular Sciences. 2020; 21(6):2252. https://doi.org/10.3390/ijms21062252
Chicago/Turabian StyleCamino, Tamara, Nerea Lago-Baameiro, Susana B. Bravo, Aurelio Sueiro, Iván Couto, Fernando Santos, Javier Baltar, Felipe F. Casanueva, and María Pardo. 2020. "Vesicles Shed by Pathological Murine Adipocytes Spread Pathology: Characterization and Functional Role of Insulin Resistant/Hypertrophied Adiposomes" International Journal of Molecular Sciences 21, no. 6: 2252. https://doi.org/10.3390/ijms21062252